Under-Sink Activated Carbon Water Filters Effectively Remove Lead from Private Well Water for over Six Months
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment of Study Participants and Baseline Pb Levels
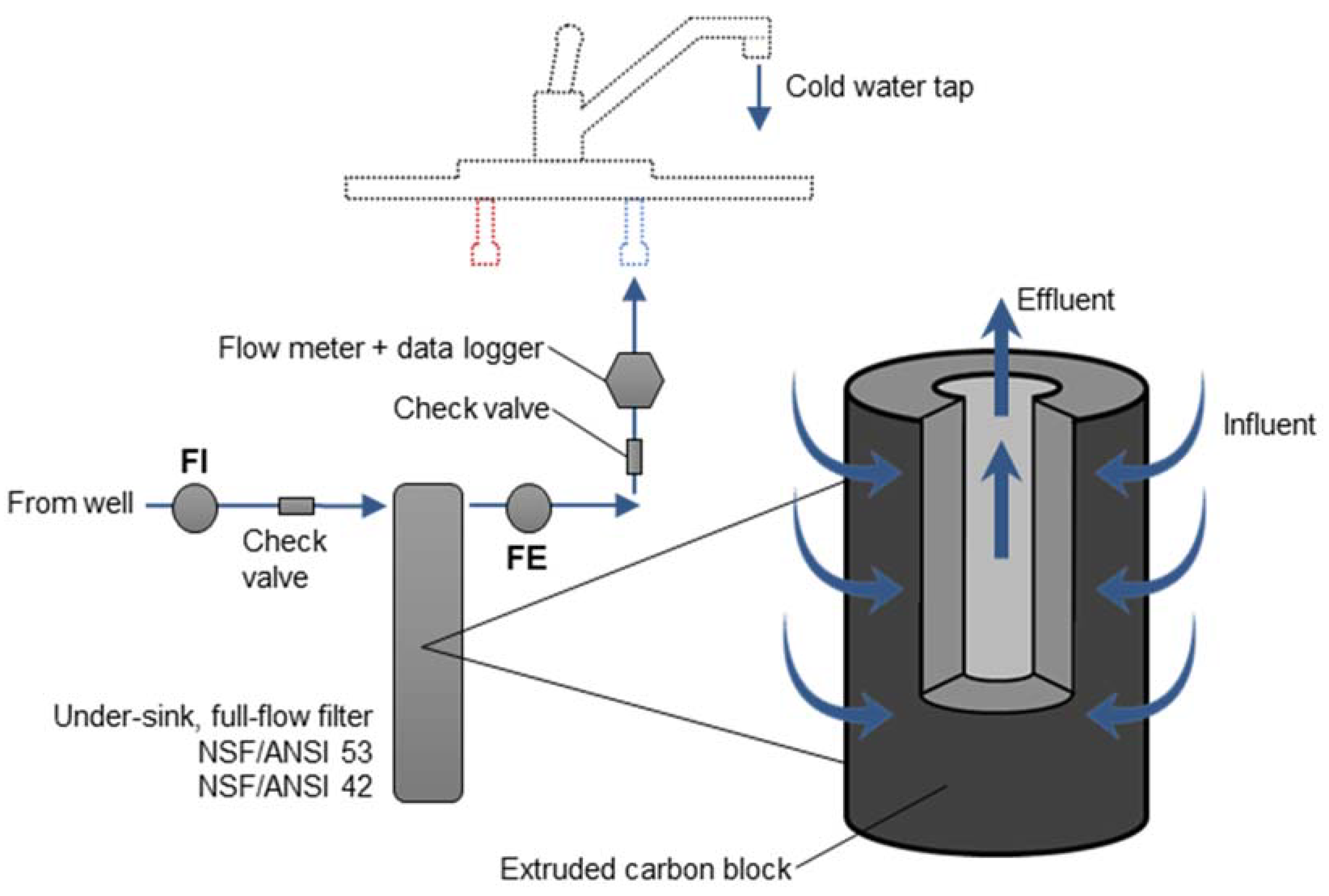
2.2. POU Intervention Design
2.3. Influent Groundwater Quality
2.4. Sampling Methods
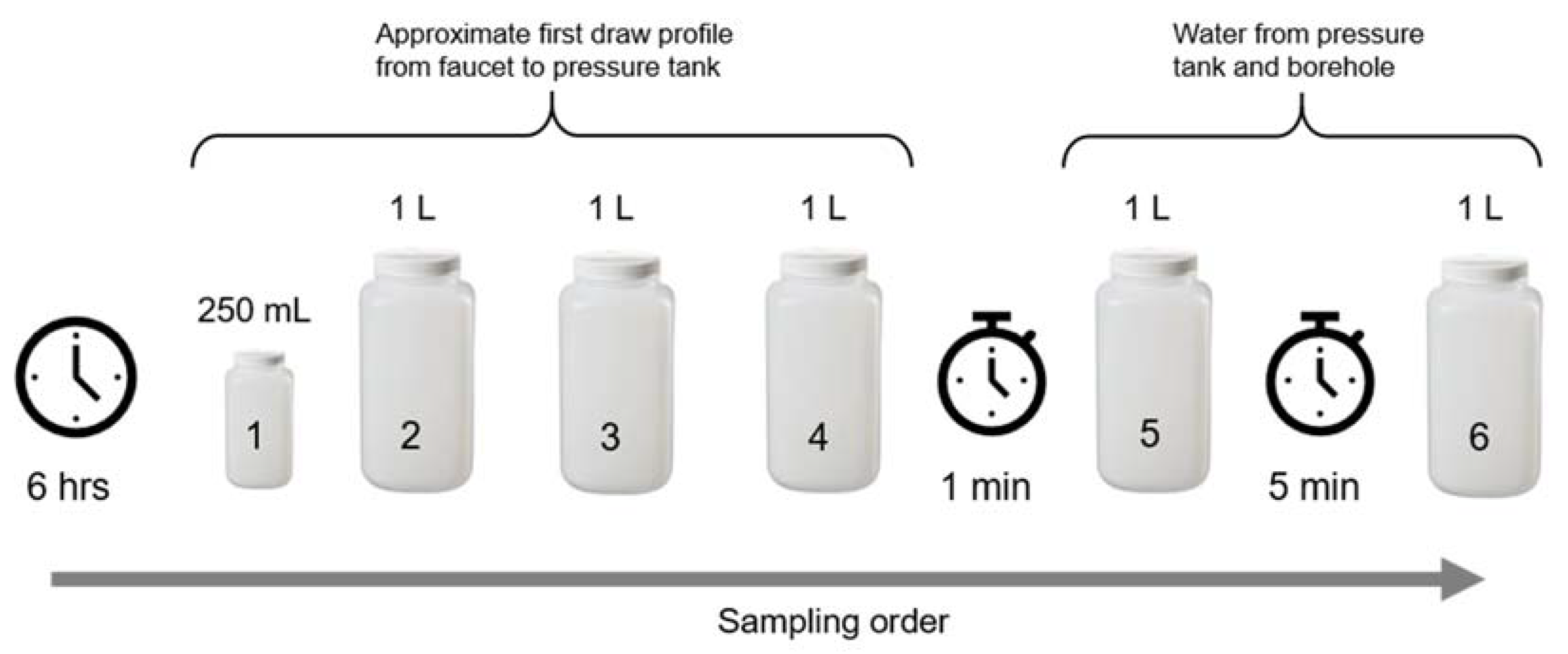
2.4.1. Monthly Sampling
2.4.2. Pb Profiling
2.5. Analytical Methods
2.6. Data Analysis
3. Results and Discussion
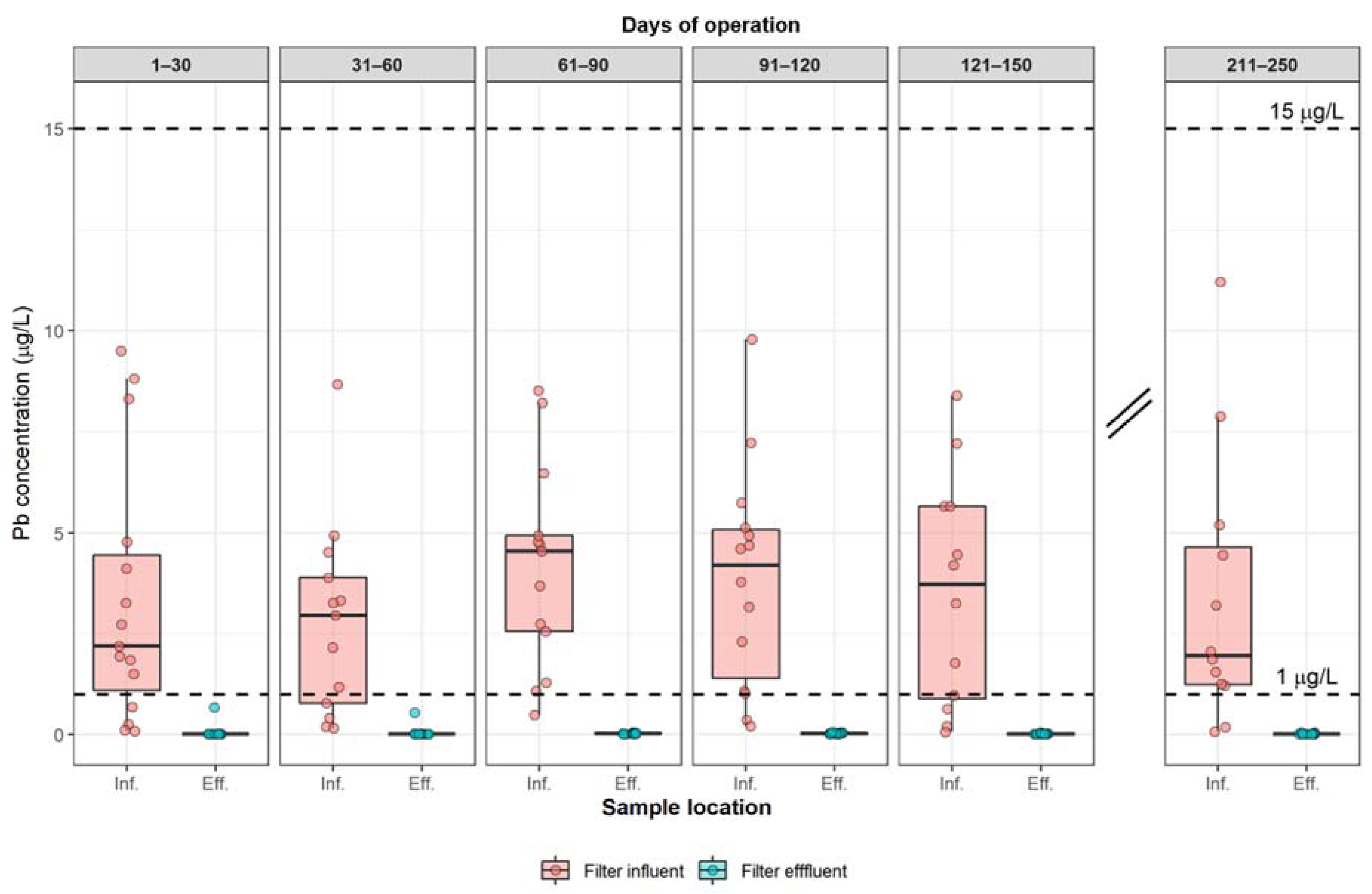
3.1. Long-Term Filter Effectiveness for Pb Removal
3.2. Filter Failure Due to Clogging
3.3. Removal of Other Metals
3.4. Contribution of the Faucet to First-Draw Pb
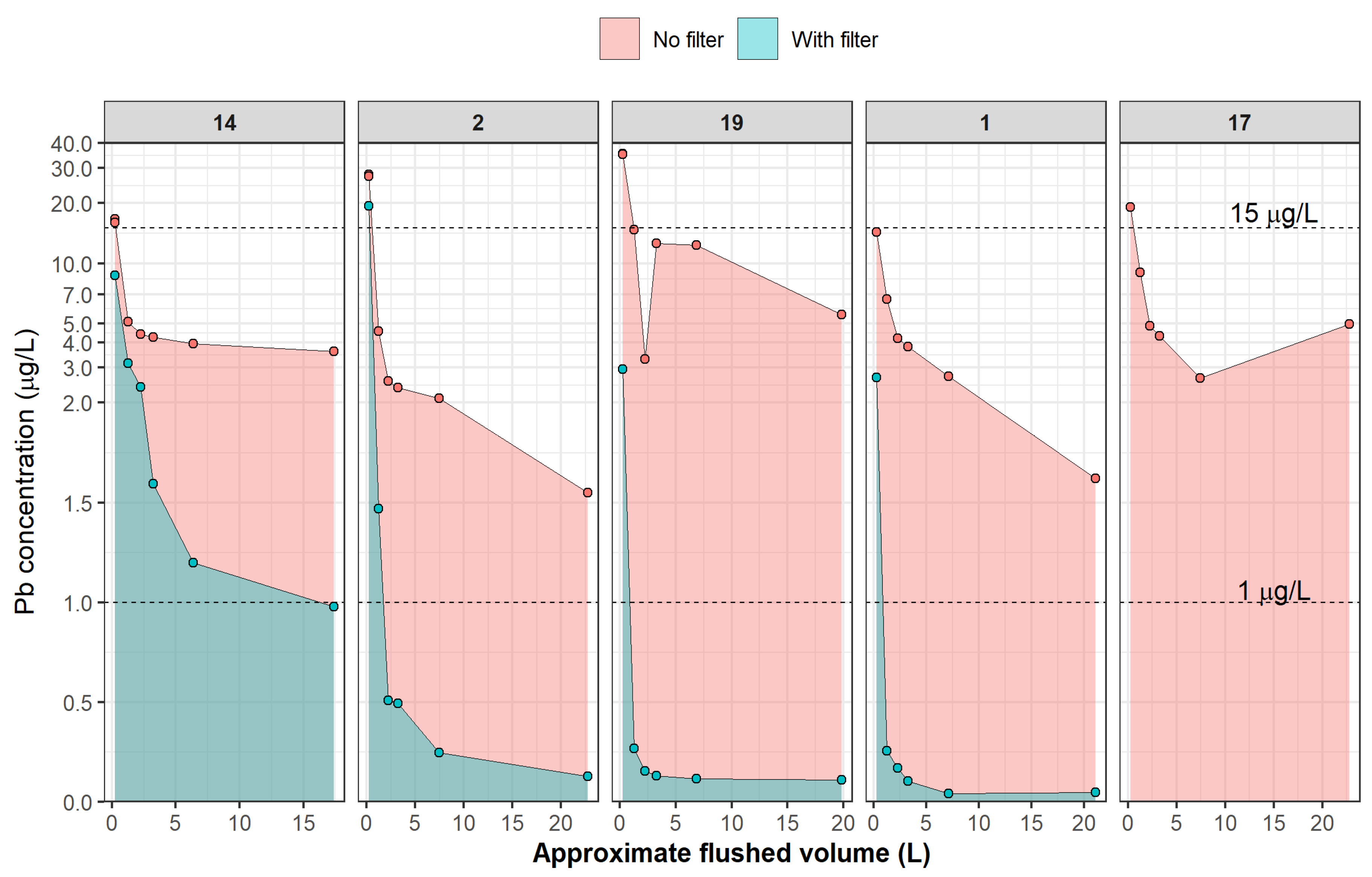
3.5. Pb Profiling Results
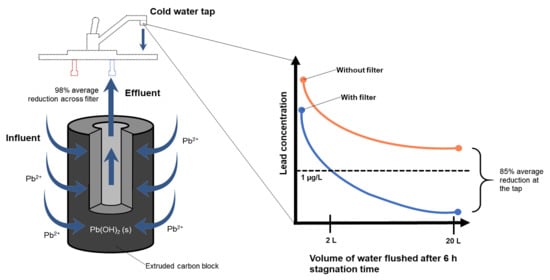
3.5.1. Flushing Effectiveness without Filter
3.5.2. Improved Flushing Effectiveness with Under-Sink Filters
3.6. Factors Influencing Filter Performance
3.6.1. Water Usage Patterns and Surface Loading
3.6.2. Influent Groundwater pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gibson, J.M.; Fisher, M.; Clonch, A.; Macdonald, J.M.; Cook, P.J. Children drinking private well water have higher blood lead than those with city water. Proc. Natl. Acad. Sci. USA 2020, 117, 16898–16907. [Google Scholar] [CrossRef] [PubMed]
- Pieper, K.J.; Krometis, L.A.H.; Gallagher, D.L.; Benham, B.L.; Edwards, M. Incidence of waterborne lead in private drinking water systems in Virginia. J. Water Health 2015, 13, 897–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieper, K.J.; Nystrom, V.E.; Parks, J.; Jennings, K.; Faircloth, H.; Morgan, J.B.; Bruckner, J.; Edwards, M.A. Elevated lead in water of private wells poses health risks: Case study in Macon county, North Carolina. Environ. Sci. Technol. 2018, 52, 4350–4357. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry (ATSDR), U.S. Department of Health and Human Services: Atlanta, GA, USA, 2007.
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect. 2005, 113, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Canfield, R.L.; Henderson, C.R.J.; Cory-Slechta, D.A.; Cox, C.; Jusko, T.A.; Lanphear, B.P. Intellectual impairment in children with blood lead concentrations below 10 µg per deciliter. N. Engl. J. Med. 2003, 348, 1517–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fizer, C.; de Bruin, W.B.; Stillo, F.; Gibson, J.M. Barriers to managing private well and septic systems in underserved communities: Mental models of homeowner decision-making. J. Environ. Health 2018, 81, 43. [Google Scholar]
- Stillo, F.; de Bruin, W.B.; Zimmer, C.; Gibson, J.M.D. Well water testing in African-American communities without municipal infrastructure: Beliefs driving decisions. Sci. Total Environ. 2019, 686, 1220–1228. [Google Scholar] [CrossRef]
- Wood, E. Promoting Private Well Testing in Underserved Minority Communities Using the Mental Models Approach to Risk Communication. Master’s Thesis, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, 2019. [Google Scholar]
- Flanagan, S.V.; Gleason, J.A.; Spayd, S.E.; Procopio, N.A.; Rockafellow-Baldoni, M.; Braman, S.; Chillrud, S.N.; Zheng, Y. Health protective behavior following required arsenic testing under the New Jersey private well testing act. Int. J. Hyg. Environ. Health 2018, 221, 929–940. [Google Scholar] [CrossRef]
- Morris, L.; Wilson, S.; Kelly, W. Methods of conducting effective outreach to private well owners—A literature review and model approach. J. Water Health 2016, 14, 167–182. [Google Scholar] [CrossRef]
- Colley, S.K.; Kane, P.K.M.; Gibson, J.M. Risk communication and factors influencing private well testing behavior: A systematic scoping review. Int. J. Environ. Res. Public Health 2019, 16, 4333. [Google Scholar] [CrossRef] [Green Version]
- Nigra, A.E. Environmental racism and the need for private well protections. Proc. Natl. Acad. Sci. USA 2020, 117, 17476–17478. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.W.; Gessesse, B.; Butler, L.J.; MacIntosh, D.L. Potential effectiveness of point-of-use filtration to address risks to drinking water in the United States. Environ. Health Insights 2017, 11, 117863021774699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lothrop, N.; Wilkinson, S.T.; Verhougstrate, M.; Sugeng, A.; Loh, M.M.; Klimecki, W.; Beamer, P.I.; Beamer, P.I. Home water treatment habits and effectiveness in a rural Arizona community. Water 2015, 7, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.S.; Bommarito, P.; George, A.; Yelton, S.; Cable, P.; Coyte, R.; Karr, J.; Vengosh, A.; Gray, K.M.; Fry, R.C. Assessment of inorganic contamination of private wells and demonstration of effective filter-based reduction: A pilot-study in Stokes County, North Carolina. Environ. Res. 2019, 177, 108618. [Google Scholar] [CrossRef]
- USEPA. Lead and Copper Monitoring and Reporting Guidance for Public Water Systems—EPA-816-R-02-009; U.S. Environmental Protection Agency—Office of Water: Washington, DC, USA, 2002.
- Powers, M.; Yracheta, J.; Harvey, D.; Leary, M.O.; Best, L.G.; Black, A.; Macdonald, L.; Susan, J.; Hasan, K.; Thomas, E.; et al. Arsenic in groundwater in private wells in rural North Dakota and South Dakota: Water quality assessment for an intervention trial. Environ. Res. 2019, 168, 41–47. [Google Scholar] [CrossRef]
- Spayd, S.E.; Robson, M.G.; Buckley, B.T. Whole-house arsenic water treatment provided more effective arsenic exposure reduction than point-of-use water treatment at New Jersey homes with arsenic in well water. Sci. Total Environ. 2015, 505, 1361–1369. [Google Scholar] [CrossRef] [Green Version]
- Möller, T.; Sylvester, P.; Shepard, D.; Morassi, E. Arsenic in groundwater in New England—Point-of-entry and point-of-use treatment of private wells. Desalination 2009, 243, 293–304. [Google Scholar] [CrossRef]
- NSF Joint Committee on Drinking Water Treatment Units. NSF/ANSI 53: Drinking Water Treatment Units—Health Effects; NSF International: Ann Arbor, MI, USA, 2018. [Google Scholar]
- Javidi, A.; Pierce, G.U.S. Households’ perception of drinking water as unsafe and its consequences: Examining alternative choices to the tap. Water Resour. Res. 2018, 54, 6100–6113. [Google Scholar] [CrossRef]
- Pieper, K.J.; Krometis, L.A.; Gallagher, D.; Benham, B.; Edwards, M. Profiling private water systems to identify patterns of waterborne lead exposure. Environ. Sci. Technol. 2015, 49, 12697–12704. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Council on Environmental Health. Prevention of childhood lead toxicity. Pediatrics 2016, 138, e20161493. [Google Scholar] [CrossRef] [Green Version]
- 99th Congress. Safe Drinking Water Act Amendments of 1986; 42 USC 300g-6; 99th Congress of the United States of America: Washington, DC, USA, 1986.
- 111th Congress. Reduction of Lead in Drinking Water Act; 124 Stat. 4131; 111th Congress of the United States of America: Washington, DC, USA, 2011.
- U.S. Census Bureau American Community Survey 5-year estimates. Available online: http://censusreporter.org (accessed on 10 October 2020).
- USEPA. Cost Evaluation of Point-of-Use and Point-of-Entry Treatment Units for Small Systems: Cost Estimating Tool and Users Guide—EPA 815-B-07-001 April; U.S. Environmental Protection Agency—Office of Water: Washington, DC, USA, 2007.
- USEPA. Point-of-Use or Point-of-Entry Treatment Options for Small Drinking Water Systems: EPA 815-R-06-010; U.S. Environmental Protection Agency: Arlington, VA, USA, 2006.
- Thomas, E.D.; Gittelsohn, J.; Yracheta, J.; Powers, M.; O’Leary, M.; Harvey, D.E.; Cloud, R.R.; Best, L.G.; Bear, A.B.; Navas-Acien, A.; et al. The strong heart water study: Informing and designing a multi-level intervention to reduce arsenic exposure among private well users in Great Plains Indian Nations. Sci. Total Environ. 2019, 650, 3120–3133. [Google Scholar] [CrossRef] [PubMed]
- Pratson, E.; Vengosh, A.; Dwyer, G.; Pratson, L.; Klein, E. The effectiveness of arsenic remediation from groundwater in a private home. Ground Water Monit. Remediat. 2009, 30, 85–91. [Google Scholar] [CrossRef]
- Deshommes, E.; Nour, S.; Richer, B.; Cartier, C.; Prévost, M. POU devices in large buildings: Lead removal and water quality. J. Am. Water Works Assoc. 2012, 104, 75–76. [Google Scholar] [CrossRef]
- Belitz, K.; Jurgens, B.C.; Johnson, T.D. Potential Corrosivity of Untreated Groundwater in the United States, Scientific Investigations Report 2016-5092; U.S. Geological Survey: Reston, VA, USA, 2016.
- Bosscher, V.; Lytle, D.A.; Schock, M.R.; Porter, A.; Del Toral, M. POU water filters effectively reduce lead in drinking water: A demonstration field study in flint, Michigan. J. Environ. Sci. Heal. Part A Toxic/Hazard. Subst. Environ. Eng. 2019, 54, 484–493. [Google Scholar] [CrossRef] [Green Version]
- Riblet, C.; Deshommes, E.; Laroche, L.; Prévost, M. True exposure to lead at the tap: Insights from proportional sampling, regulated sampling and water use monitoring. Water Res. 2019, 156, 327–336. [Google Scholar] [CrossRef]
- Su, F.; Luo, M.; Zhang, F.; Li, P.; Lou, K.; Xing, X. Performance of microbiological control by a point-of-use filter system for drinking water purification. J. Environ. Sci. 2009, 21, 1237–1246. [Google Scholar] [CrossRef]
- Geldreich, E.E.; Taylor, R.H.; Blannon, J.C.; Reasoner, D.J. Bacterial colonization of point-of-use water treatment devices. J. Am. Water Works Assoc. 1985, 77, 72–80. [Google Scholar] [CrossRef]
- USEPA. Method 6020B (SW-846): Inductively Coupled Plasma Mass—Spectrometry, Revision 2; U.S. Environmental Protection Agency: Washington, DC, USA, 2014.
- USEPA. Regional Guidance on Handling Chemical Concentration Data Near the Detection Limit in Risk Assessments; U.S. Environmental Protection Agency—Region 3: Philadelphia, PA, USA, 1991.
- Triantafyllidou, S.; Edwards, M. Lead (Pb) in tap water and in blood: Implications for lead exposure in the United States. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1297–1352. [Google Scholar] [CrossRef]
- Redmon, J.H.; Gibson, J.M.D.; Woodward, K.P.; Aceituno, A.M.; Levine, K.E. Safeguarding children’s health: Time to enact a health-based standard and comprehensive testing, mitigation, and communication protocol for lead in drinking water. N. C. Med. J. 2018, 79, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Deshommes, E.; Zhang, Y.; Gendron, K.; Sauvé, S.; Edwards, M.; Nour, S.; Prévost, M. Lead removal from tap water using POU devices. J. Am. Water Work. Assoc. 2010, 102, 91–105. [Google Scholar] [CrossRef]
- Boyd, G.R.; Kirmeyer, G.J.; Pierson, G.L.; Hendrickson, S.L.; Kreider, D.; English, R. Testing of point-of-use filters at Seattle schools drinking fountains. In Water Quality Technology Conference Proceedings, Proceedings of the 2005 Water Quality Technology Conference and Exposition, Quebec City, QC, Canada, 6–10 November 2005; American Water Works Association: Denver, CO, USA, 2005. [Google Scholar]
- Taylor, R.M.; Kuennen, R.W. Removing lead in drinking water with activated carbon. Environ. Prog. 1994, 13, 65–71. [Google Scholar] [CrossRef]
- Pieper, K.J.; Krometis, L.A.; Edwards, M. Quantifying lead-leaching potential from plumbing exposed to aggressive waters. J. Am. Water Works Assoc. 2016, 108, E458–E466. [Google Scholar] [CrossRef]
- Lei, I.L.; Ng, D.Q.; Sable, S.S.; Lin, Y.P. Evaluation of lead release potential of new premise plumbing materials. Environ. Sci. Pollut. Res. 2018, 25, 27971–27981. [Google Scholar] [CrossRef] [PubMed]
- Samuels, E.R.; Méranger, J.C. Preliminary studies on the leaching of some trace metals from kitchen faucets. Water R 1984, 18, 75–80. [Google Scholar] [CrossRef]
- StarCraft. Custom Builders Faucet Basics: Part 1—What are Faucets Made of? Available online: http://starcraftcustombuilders.com/sources.faucets.htm (accessed on 10 October 2020).
- Sheppard, L.M. Faucet. Available online: http://www.madehow.com/Volume-6/Faucet.html (accessed on 10 October 2020).
- Otunniyi, I.O.; Oluokun, O.O. A Probe into failure of selected plumbing parts: Alloy composition, microstructural condition and aqueous exposure. J. Metall. Eng. 2014, 3, 69. [Google Scholar] [CrossRef]
- Clark, B.N.; Masters, S.V.; Edwards, M.A. Lead release to drinking water from galvanized steel pipe coatings. Environ. Eng. Sci. 2015, 32, 713–721. [Google Scholar] [CrossRef]
- Pieper, K.J.; Kriss, R.; Tang, M.; Edwards, M.A.; Katner, A. Understanding lead in water and avoidance strategies: A United States perspective for informed decision-making. J. Water Health 2019, 17, 540–555. [Google Scholar] [CrossRef] [Green Version]
- Katner, A.; Pieper, K.; Brown, K.; Lin, H.Y.; Parks, J.; Wang, X.; Hu, C.Y.; Masters, S.; Mielke, H.; Edwards, M. Effectiveness of prevailing flush guidelines to prevent exposure to lead in tap water. Int. J. Environ. Res. Public Health 2018, 15, 1537. [Google Scholar] [CrossRef] [Green Version]
- Boyd, G.R.; Pierson, G.L.; Kirmeyer, G.J.; Brition, M.D.; English, R.J. Lead release from new and end-use plumbing components in Seattle Public Schools. J. Am. Water Works Assoc. 2008, 100, 105–114. [Google Scholar] [CrossRef]
- Boyd, G.R.; Pierson, G.L.; Kirmeyer, G.J.; English, R.J. Lead variability testing in Seattle Public Schools. J. Am. Water Works Assoc. 2008, 100, 53–64. [Google Scholar] [CrossRef]
- Kuennen, R.W.; Taylor, R.M.; Van Dyke, K.; Groenevelt, K. Removing lead from drinking water with a point-of-use GAC fixed-bed adsorber. J. Am. Water Works Assoc. 1992, 84, 91–101. [Google Scholar] [CrossRef]
- Mulhern, R.E.; Summers, R.S.; Dickenson, E.R.V. Evaluating and modeling the activated carbon adsorption of wastewater-derived N-nitrosodimethylamine precursors. Environ. Sci. Water Res. Technol. 2017, 3, 844–856. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead (II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Largitte, L.; Gervelas, S.; Tant, T.; Dumesnil, P.C.; Hightower, A.; Yasami, R.; Bercion, Y.; Lodewyckx, P. Removal of lead from aqueous solutions by adsorption with surface precipitation. Adsorption 2014, 20, 689–700. [Google Scholar] [CrossRef]
- Reed, B.E.; Arunachalam, S. Use of granular activated carbon columns for lead removal. J. Environ. Eng. 1994, 120, 416–436. [Google Scholar] [CrossRef]
- Reed, B.E. Identification of removal mechanisms for lead in Granular Activated Carbon (GAC) columns. Sep. Sci. Technol. 1995, 30, 101–116. [Google Scholar] [CrossRef]
- Sontheimer, H.; Crittenden, J.C.; Summers, R.S. The production and physical-chemical evaluation of activated carbon. In Activated Carbon for Water Treatment; DVGW-Forschungsstelle: Karlsruhe, Germany, 1988; pp. 51–105. ISBN 3-922671-20-9. [Google Scholar]
- Jurgens, B.C.; Parkhurst, D.L.; Belitz, K. Assessing the lead solubility potential of untreated groundwater of the United States. Environ. Sci. Technol. 2019, 53, 3095–3103. [Google Scholar] [CrossRef] [Green Version]
- Geetha, S.; Gouthami, S. Internet of things enabled real time water quality monitoring system. Smart Water 2016, 2, 1. [Google Scholar] [CrossRef]
- Hoffman, T.; Hynds, P.; Schuster-Wallace, C.; Dickson-Anderson, S.; Majury, A. Harnessing smart technology for private well risk assessment and communication. Water Secur. 2019, 6, 100026. [Google Scholar] [CrossRef]
- Gibson, J.M.; De Felice, N.; Sebastian, D.; Leker, H. Racial disparities in access to community water supply service in Wake County, North Carolina. Front. Public Health Serv. Syst. Res. 2014, 3, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Naman, J.M.; Gibson, J.M. Disparities in water and sewer services in North Carolina: An analysis of the decision-making process. Am. J. Public Health 2015, 105, e20–e26. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.S. Race as a factor in municipal underbounding race as a factor in municipal underbounding. Ann. Assoc. Am. Geogr. 1987, 77, 564–579. [Google Scholar] [CrossRef]
- Lichter, D.T.; Parisi, D.; Grice, S.M.; Taquino, M. Municipal underbounding: Annexation and racial exclusion in small southern towns. Rural Sociol. Soc. 2007, 72, 47–68. [Google Scholar] [CrossRef] [Green Version]
- Heaney, C.D.; Wing, S.; Wilson, S.; Campbell, R.L.; Caldwell, D.; Hopkins, B.; O’Shea, S.; Yeatts, K. Public infrastructure disparities and the microbiological and chemical safety of drinking and surface water supplies in a community bordering a landfill. J. Environ. Health 2015, 75, 24–36. [Google Scholar]
- Balazs, C.L.; Ray, I. The drinking water disparities framework: On the origins and persistence of inequities in exposure. Am. J. Public Health 2014, 104, 603–611. [Google Scholar] [CrossRef]
- Seaton, P.; Garibay, V. American Recovery and Reinvestment Act of 2009 Analysis of Drinking Water and Waste Water Investment in Fresno and Stanislaus Counties; California Rural Legal Assistance, Inc.: Oakland, CA, USA, 2009. [Google Scholar]
- Van Derslice, J. Drinking water infrastructure and environmental disparities: Evidence and methodological considerations. Am. J. Public Health 2011, 101, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Colfax, R. Denied water service because of race, African Americans win $10.85 million jury verdict. Clear. Rev. J. Poverty Law Policy 2009, 43, 398–401. [Google Scholar]
- Vandewalle, E.; Jepson, W. Mediating water governance: Point-of-use water filtration devices for low-income communities along the US–Mexico border. Geo Geogr. Environ. 2015, 2, 107–121. [Google Scholar] [CrossRef]
- Zheng, Y.; Flanagan, S.V. The case for universal screening of private well water quality in the U.S. and testing requirements to achieve it: Evidence from arsenic. Environ. Health Perspect. 2017, 125, 085002. [Google Scholar] [CrossRef] [Green Version]


| Cluster A (n = 4) | Cluster B (n = 2) | Cluster C (n = 11) | ||
|---|---|---|---|---|
| Demographic Characteristics | ||||
| NC County | Orange | Robeson | Robeson | |
| Median household income | $157,422 | $27,917 | $31,971 | |
| Persons per household | 2.9 | 3.2 | 3.1 | |
| Median home value | $442,300 | $72,500 | $66,300 | |
| Housing type | Single unit | 98% | 48% | 24% |
| Multi-unit | 1% | 6% | 0% | |
| Mobile home | 1% | 46% | 74% | |
| Education | High school or higher | 97.9% | 68.8% | 71.8% |
| Bachelor’s or higher | 78.2% | 4.9% | 6.6% | |
| Race and ethnicity | White | 63% | 11% | 31% |
| Black | 9% | 17% | 28% | |
| Native | 0% | 47% | 12% | |
| Asian | 18% | 0% | 0% | |
| Two+ | 5% | 2% | 3% | |
| Hispanic | 4% | 22% | 26% | |
| Groundwater Quality Characteristics—mean (± SD) | ||||
| pH | 7.1 ± 0.4 | 5.4 ± 1.1 | 4.4 ± 0.3 | |
| Electrical conductivity (µS/cm) | 350 ± 110 | 220 ± 140 | 110 ± 35 | |
| Hardness (mg/L as CaCO3) | 110 ± 50 | 30 ± 10 | 20 ± 10 | |
| Carbonate alkalinity (mg/L as CaCO3) | 110 ± 50 | 7.3 ± 7.2 | 0.1 ± 0.1 | |
| Langelier Saturation Index | −1.0 ± 0.5 | −5.2 ± 2.1 | −7.6 ± 0.6 | |
| 250 mL first draw Pb (µg/L) * | 1.21 ± 1.19 | 17.4 ± 24 | 10.9 ± 7.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulhern, R.; MacDonald Gibson, J. Under-Sink Activated Carbon Water Filters Effectively Remove Lead from Private Well Water for over Six Months. Water 2020, 12, 3584. https://doi.org/10.3390/w12123584
Mulhern R, MacDonald Gibson J. Under-Sink Activated Carbon Water Filters Effectively Remove Lead from Private Well Water for over Six Months. Water. 2020; 12(12):3584. https://doi.org/10.3390/w12123584
Chicago/Turabian StyleMulhern, Riley, and Jacqueline MacDonald Gibson. 2020. "Under-Sink Activated Carbon Water Filters Effectively Remove Lead from Private Well Water for over Six Months" Water 12, no. 12: 3584. https://doi.org/10.3390/w12123584
APA StyleMulhern, R., & MacDonald Gibson, J. (2020). Under-Sink Activated Carbon Water Filters Effectively Remove Lead from Private Well Water for over Six Months. Water, 12(12), 3584. https://doi.org/10.3390/w12123584