Heterogeneous Catalytic Ozonation of Aniline-Contaminated Waters: A Three-Phase Modelling Approach Using TiO2/GAC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
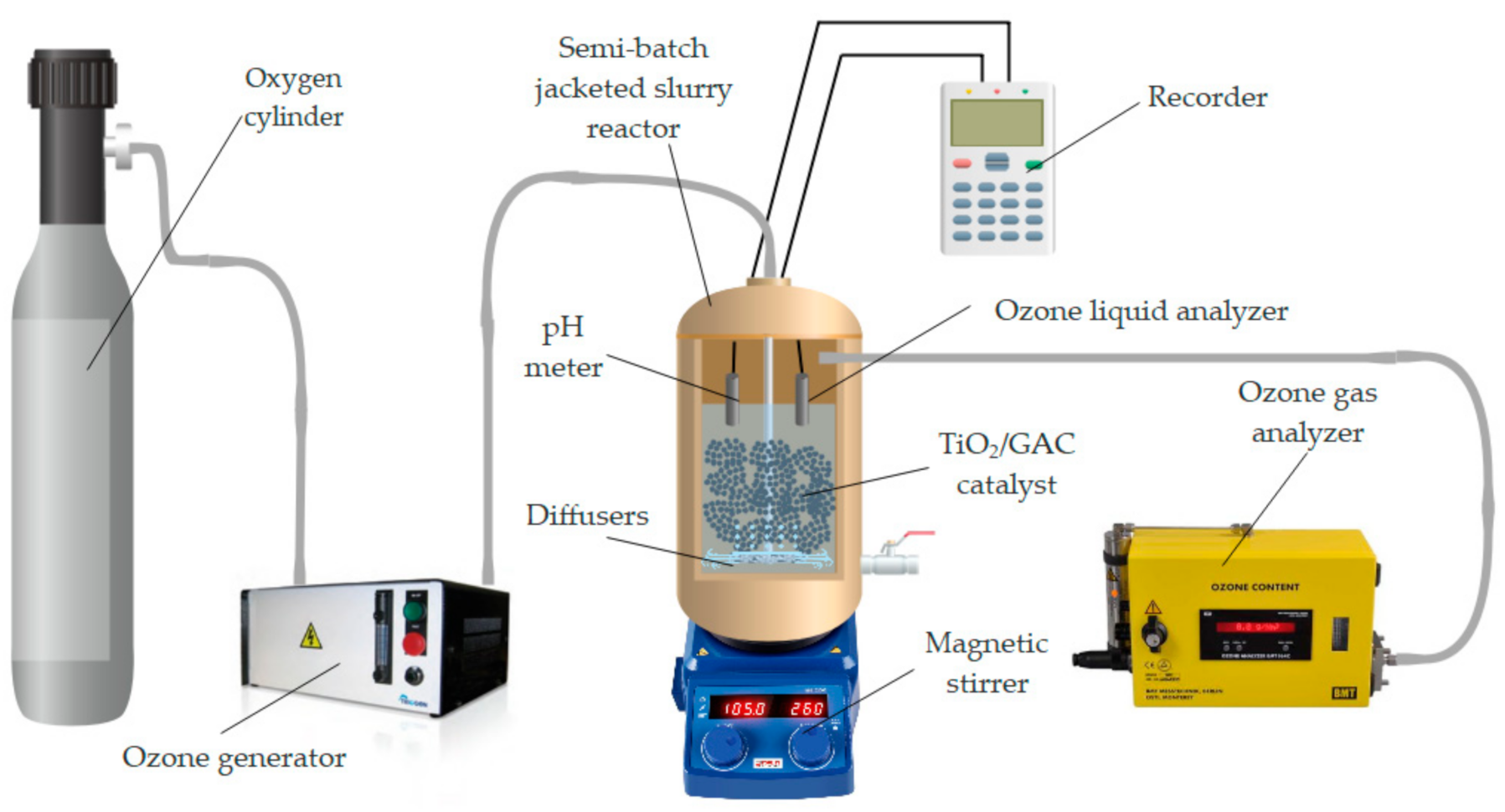
2.3. Experimental Set Up of The Catalytic Ozonation System
3. Results and Discussion
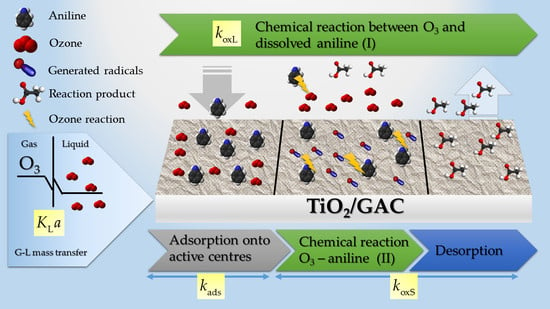
3.1. Mathematical Modelling Approach Using TiO2/GAC Catalysts
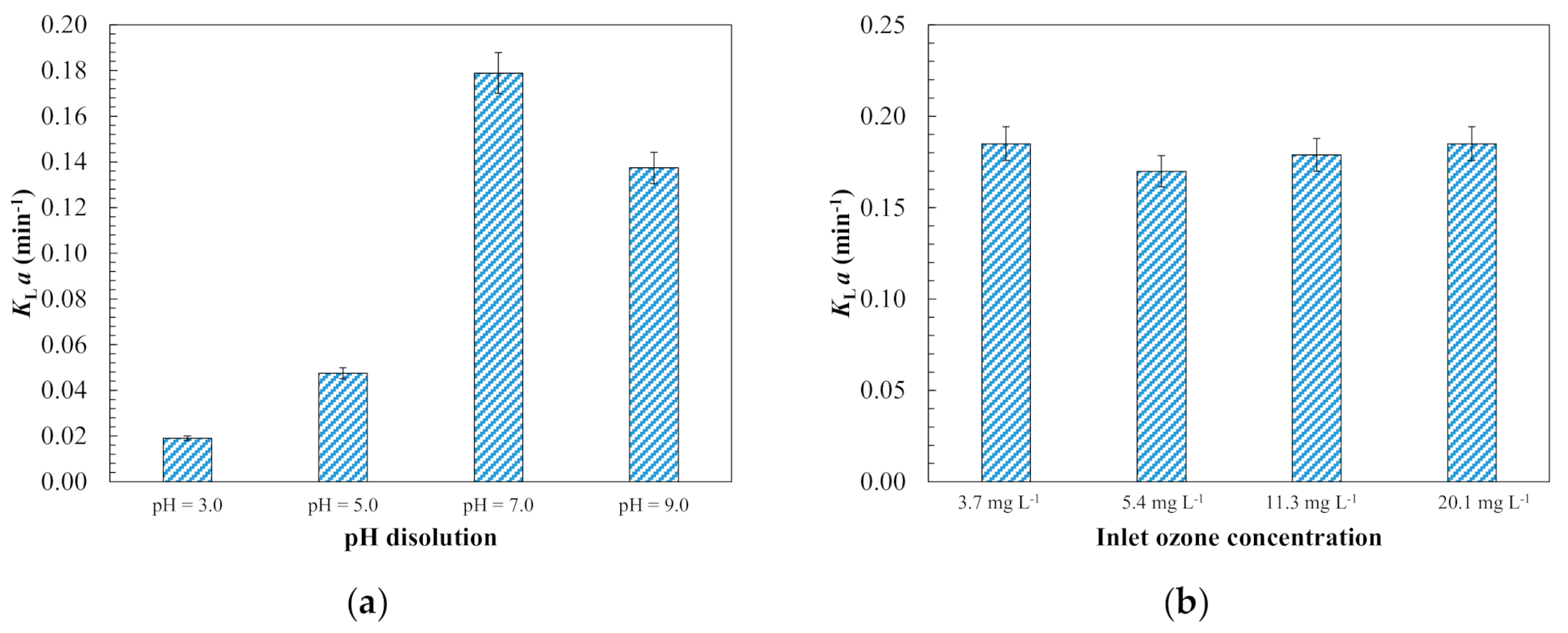
3.1.1. Ozone Kinetic Mass Transfer Modelling
3.1.2. Aniline Degradation Kinetic Modelling
- The rate of the global ozonation process, or G-L mass transfer rate, coincides with the consumption of ozone in the parallel (liquid and solid) reactive process.
- The oxidation kinetics of aniline in the liquid and in the solid, in terms of TOC, is considered as pseudo-first order.
- For the TiO2/GAC composite, it was considered a sufficiently porous material for ozone and aniline diffusion mechanisms to take place also in the internal surface of particle.
- The kinetic constant of the aniline oxidation on the solid, also in terms of TOC, includes desorption of degradation compounds.
- The adsorption process is simultaneous to the reaction process on the solid so that its kinetics are strongly affected by the ozonation conditions.
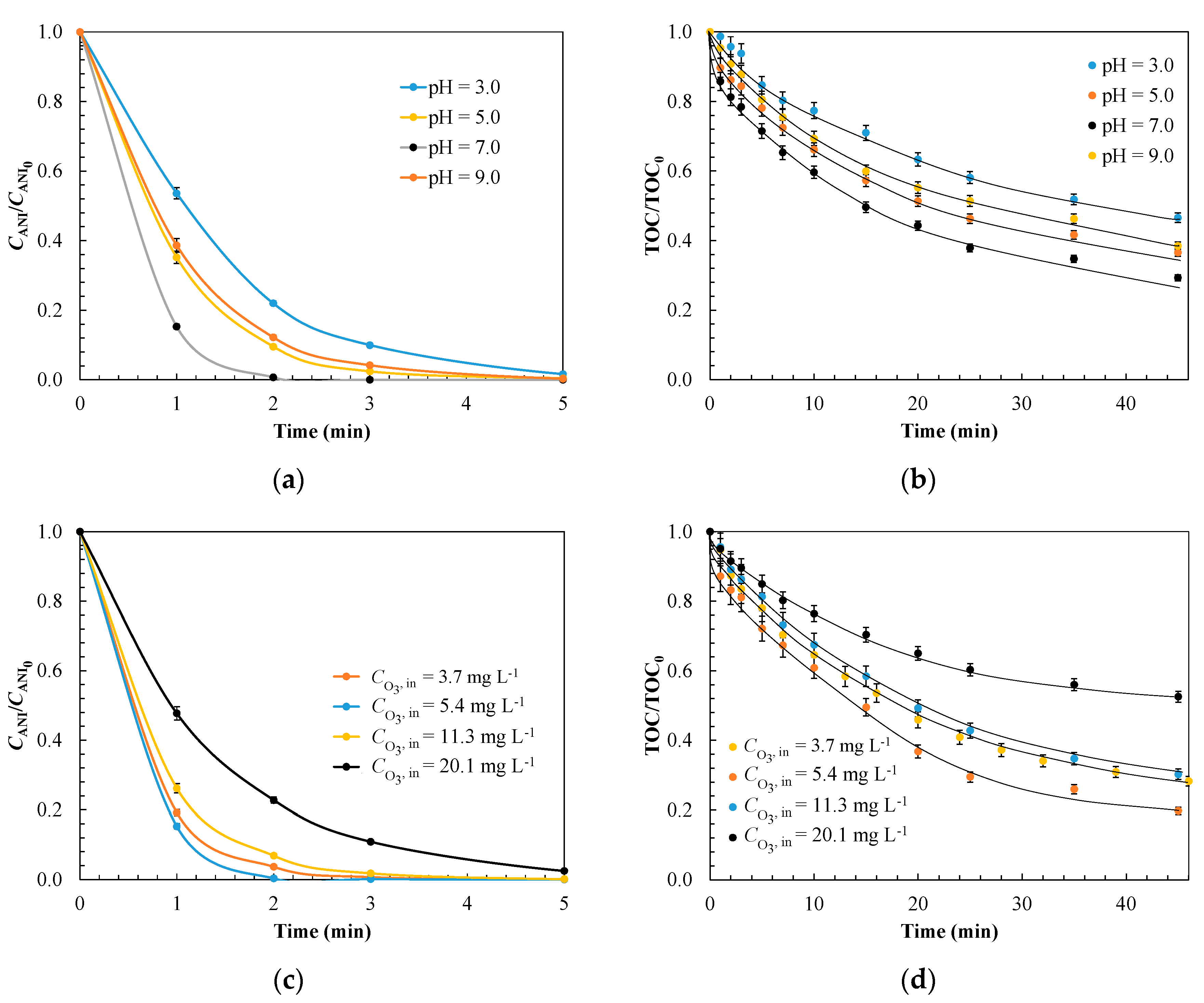
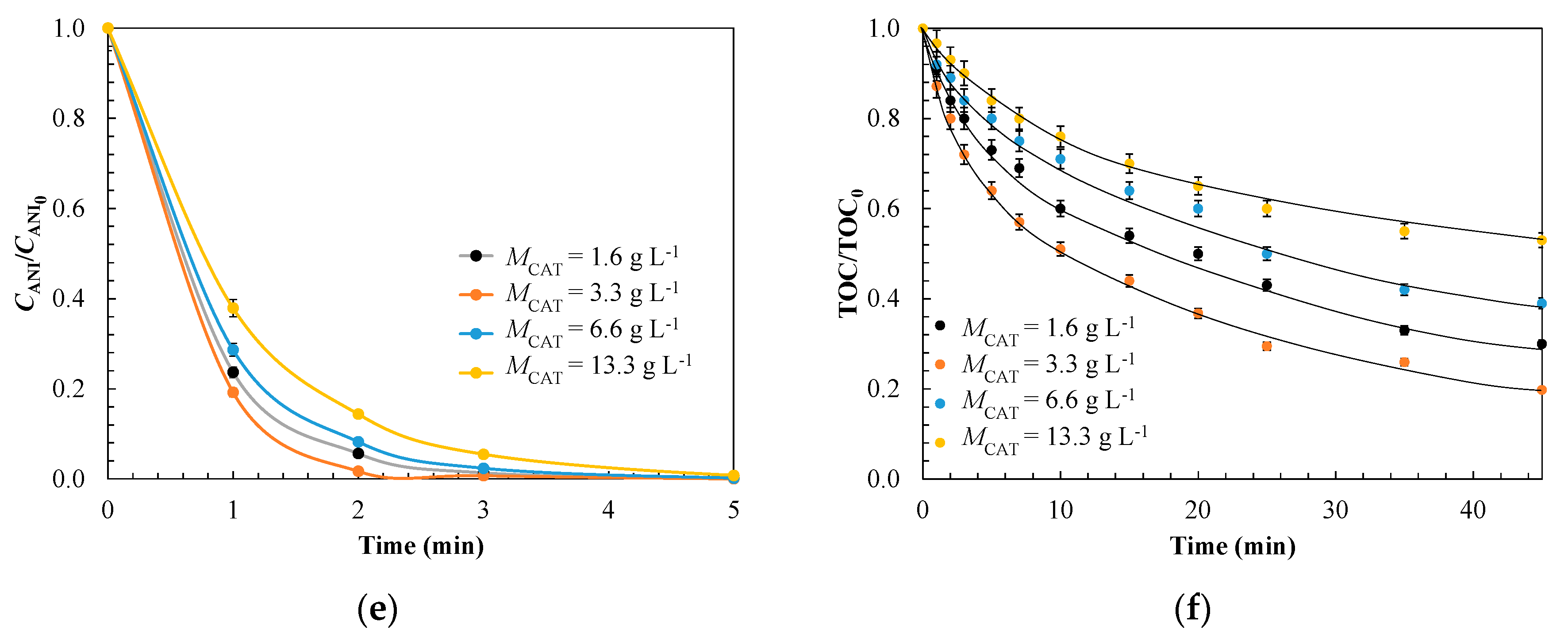
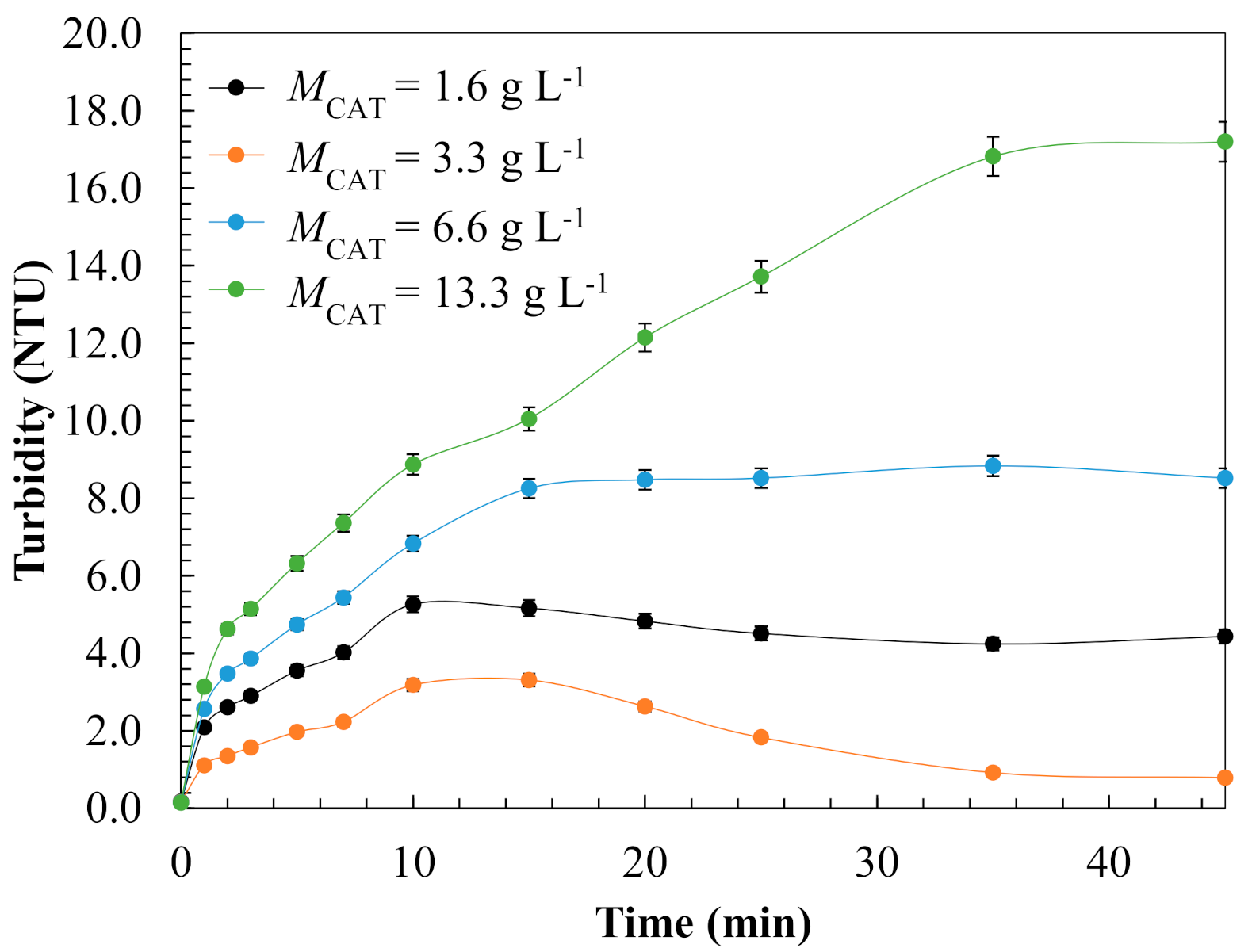
3.1.3. Evaluation of Operating Conditions for Model Validation
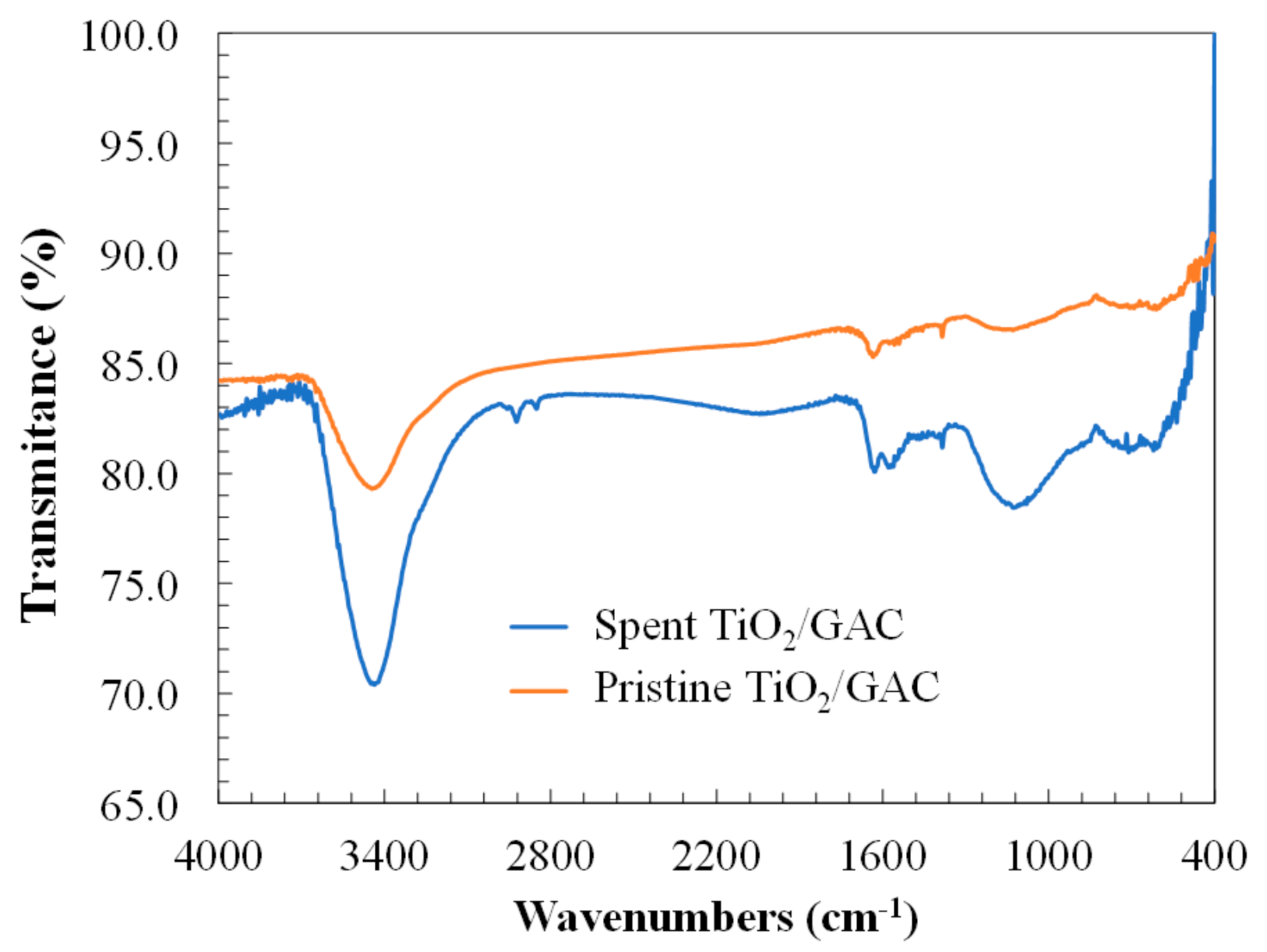
3.2. Physicochemical Surface Characterization of Spent-Granular Activated Carbon
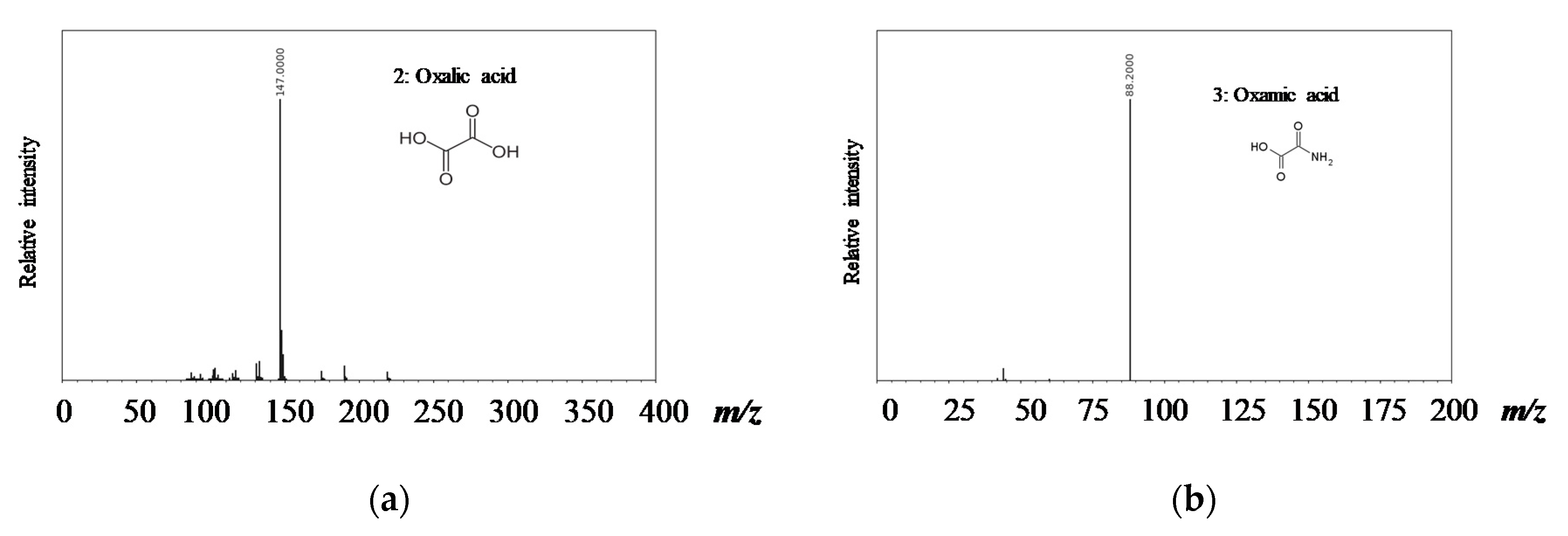
3.3. Degradation Pathway Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| σ | Weighted standard deviation |
| Concentration of ozone in the equilibrium with the ozone adsorbed on the activated carbon, mg L−1 | |
| Concentration of ozone on the catalyst in equilibrium with the liquid ozone concentration, mg L−1 | |
| Calculate pollutant concentration in the liquid in terms of total organic carbon, mg L−1 | |
| Concentration degradation products, mg L−1 | |
| Concentrations of ozone in the gas phase at the inlet, mg L−1 | |
| Ozone concentration in liquid, mg L−1 | |
| Concentration of ozone in the gas phase at the outlet, mg L−1 | |
| OH− | Concentration of hydroxyl ions, mol L−1 |
| Pollutant concentration in the liquid in terms of total organic carbon, mg L−1 | |
| Henry’s constant, bar L mg−1 | |
| kads | Kinetic constant of aniline adsorption, g mg-1 min−1 |
| kc,L | Elemental kinetic constant for the ozonation in the liquid, L mg−1 min−1 |
| kc,S | Elemental kinetic constant for the ozonation in the solid, L mg−1 min−1 |
| KF | Freundlich constant, (mg g−1) (L mg−1)1/nF |
| Overall mass transfer coefficient of ozone gas to water, min−1 | |
| Volumetric ozone mass transfer coefficient, min−1 | |
| koxL | Apparent first-order kinetic constant in the liquid in terms of TOC, min−1 |
| koxS | Apparent first-order kinetic constant over the catalyst in terms of TOC, min−1 |
| m | Slope of the equilibrium line between the liquid and solid phase |
| Concentration of catalyst, g L−1 | |
| n | Heterogeneity factor, dimensionless |
| N | Number of experimental values |
| NIIO3 | Ozone consumption in the solid, mg L−1 min−1 |
| NIO3 | Ozone consumption in the liquid, mg L−1 min−1 |
| Whole ozone consumption, mg L−1 min−1 | |
| Partial pressure of the ozone in equilibrium with the adsorbed ozone on the solid, bar | |
| Partial pressure of ozone in the gas phase, bar | |
| Ozone gas flow, L min−1 | |
| rIIp | Degradation of the pollutant on the activated carbon, mg L−1 min−1 |
| rIp | Degradation of the pollutant in the liquid, mg L−1 min−1 |
| T | Temperature, K |
| t | Time, min |
| Utilization coefficient, % | |
| Vreac | Volume of dissolution, L |
| zI | Amount of pollutant consumed in the liquid, mg TOC mg−1 O3 |
| zII | Amount of pollutant consumed on the catalyst, mg TOC mg−1 O3 |
| Concentration of pollutant in the solid, mg g−1 | |
| Amount of pollutant adsorbed in the solid in equilibrium, mg g−1 |
References
- United Nations. About the Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 27 November 2020).
- Zouboulis, A.I.; Katsoyiannis, I.A. Recent Advances in Water and Wastewater Treatment with Emphasis in Membrane Treatment Operations. Water 2019, 11, 45. [Google Scholar] [CrossRef] [Green Version]
- Anotai, J.; Jevprasesphant, A.; Lin, Y.-M.; Lu, M.-C. Oxidation of aniline by titanium dioxide activated with visible light. Sep. Purif. Technol. 2012, 84, 132–137. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; Lombraña, J.I.; Rivero, M.J. An efficient catalytic process for the treatment of genotoxic aniline wastewater using a new granular activated carbon-supported titanium dioxide composite. J. Clean. Prod. 2019, 228, 1282–1295. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; Lombraña, J.I.; Rivero, M.J.; Zúñiga, V.; Rituerto, J.M. Analysis of a Hybrid Suspended-Supported Photocatalytic Reactor for the Treatment of Wastewater Containing Benzothiazole and Aniline. Water 2019, 11, 337. [Google Scholar] [CrossRef] [Green Version]
- Jing, Z.; Cao, S.; Yu, T.; Hu, J. Degradation Characteristics of Aniline with Ozonation and Subsequent Treatment Analysis. J. Chem. 2015, 905921. [Google Scholar] [CrossRef]
- European Commission. European Union Risk Assessment Report aniline; Office for Official Publications of the European Communities: Luxembourg, 2004; Volume 50. [Google Scholar]
- Tao, N.; Liu, G.; Bai, L.; Tang, L.; Guo, C. Genotoxicity and growth inhibition effects of aniline on wheat. Chemosphere 2017, 169, 467–473. [Google Scholar] [CrossRef]
- United State Environmental Protection Agency. Contaminant Candidate List 4-CCL 4. Available online: https://www.epa.gov/ccl/contaminant-candidate-list-4-ccl-4-0 (accessed on 9 October 2020).
- Alvárez, P.M.; Beltrán, F.J.; Masa, F.J.; Pocostales, J.P. A comparison between catalytic ozonation and activated carbon adsorption/ozone-regeneration processes for wastewater treatment. Appl. Catal. B Environ. 2009, 92, 393–400. [Google Scholar] [CrossRef]
- Cheng, W.; Quan, X.; Li, R.; Wu, J.; Zhao, Q. Ozonation of Phenol-Containing Wastewater Using O3/Ca(OH)2 System in a Micro Bubble Gas-Liquid Reactor. Ozone-Sci. Eng. 2018, 40, 173–182. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rosal, R.; Perdigón-Melón, J.A.; Mezcua, M.; Agüera, A.; Hernando, M.D.; Letón, P.; Fernández-Alba, A.R.; García-Calvo, E. Ozone-Based Technologies in Water and Wastewater Treatment. In Emerging Contaminants from Industrial and Municipal Waste. Removal Technologies; Barceló, D., Petrovic, M., Eds.; Springer-Verlag: Heidelberg, Germany, 2008; Volume 5S/2, pp. 127–175. ISBN 978-3-540-79209-3. [Google Scholar]
- Guo, Y.; Yang, L.; Wang, X. The Application and Reaction Mechanism of Catalytic Ozonation in Water Treatment. J. Environ. Anal. Toxicol. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Legube, B.; Karpel Vel Leitner, N. Catalytic ozonation: A promising advanced oxidation technology for water treatment. Catal. Today 1999, 53, 61–72. [Google Scholar] [CrossRef]
- Delmas, H.; Creanga, C.; Julcour-Lebigue, C.; Wilhelm, A.-M. AD-OX: A sequential oxidative process for water treatment-Adsorption and batch CWAO regeneration of activated carbon. Chem. Eng. J. 2009, 152, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Erol, F.; Ozbelge, T.A.; Ozbelge, H.O. Modeling of catalytic ozonation process in a three-phase reactor. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2009, 44, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, Z.R.; Chen, H.Q.; Kuo, C.H.; Zappi, E.M. Modeling of organic pollutant destruction in a stirred-tank reactor by ozonation. J. Environ. Sci. (China) 2001, 13, 449–452. [Google Scholar]
- Guelli Ulson de Souza, S.M.d.A.; de Souza, F.B.; Ulson de Souza, A.A. Application of Individual and Simultaneous Ozonation and Adsorption Processes in Batch and Fixed-Bed Reactors for Phenol Removal. Ozone-Sci. Eng. 2012, 34, 259–268. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; de Luis, A.; Lombrana, J.I. Analysis of the effect of the operational variants in a combined adsorption-ozonation process with granular activated carbon for the treatment of phenol wastewater. React. Chem. Eng. 2020, 5, 760–778. [Google Scholar] [CrossRef]
- Song, S.; He, Z.; Chen, J. US/O3 combination degradation of aniline in aqueous solution. Ultrason. Sonochem. 2007, 14, 84–88. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Orfao, J.J.M.; Pereira, M.F.R. Ozonation of aniline promoted by activated carbon. Chemosphere 2007, 67, 809–815. [Google Scholar] [CrossRef]
- Orge, C.A.; Faria, J.L.; Pereira, M.F.R. Photocatalytic ozonation of aniline with TiO2-carbon composite materials. J. Environ. Manag. 2017, 195, 208–215. [Google Scholar] [CrossRef]
- Rodriguez, C.; Ignacio Lombrana, J.; de Luis, A.; Sanz, J. Oxidizing efficiency analysis of an ozonation process to degrade the dye rhodamine 6G. J. Chem. Technol. Biotechnol. 2017, 92, 656–665. [Google Scholar] [CrossRef]
- Byun, S.; Cho, S.H.; Yoon, J.; Geissen, S.U.; Vogelpohl, A.; Kim, S.M. Influence of mass transfer on the ozonation of wastewater from the glass fiber industry. Water Sci. Technol. 2004, 49, 31–36. [Google Scholar] [CrossRef]
- Christopher, R.; Schulz, P.E.; Paul, W. Prendiville P.E. Designing High Concentration Ozone Contactors for Drinking Water Treatment Plants. Ozone Sci. Eng. 1993, 15, 245–266. [Google Scholar] [CrossRef]
- Roth, J.; Sullivan, D. Solubility of Ozone in Water. Ind. Eng. Chem. Fundam. 1981, 20, 137–140. [Google Scholar] [CrossRef]
- Egorova, G.V.; Voblikova, V.A.; Sabitova, L.V.; Tkachenko, I.S.; Tkachenko, S.N.; Lunin, V.V. Ozone Solubility in Water. Moscow Univ. Chem. Bull. 2015, 70, 207–210. [Google Scholar] [CrossRef]
- Beltran, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press: Boca Ratón, FL, USA, 2003; ISBN 978-0-203-50917-3. [Google Scholar]
- Cacace, F.; Speranza, M. Protonated Ozone: Experimental Detection of O3H+ and Evaluation of the Proton Affinity of Ozone. Science 1994, 265, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Flores-Payán, V.; Herrera-López, E.J.; Navarro-Laboulais, J.; López-López, A. Parametric sensitivity analysis and ozone mass transfer modeling in a gas–liquid reactor for advanced water treatment. J. Ind. Eng. Chem. 2015, 21, 1270–1276. [Google Scholar] [CrossRef]
- Ratnawati, R.; Kusumaningtyas, D.; Suseno, P.; Prasetyaningrum, A. Mass Transfer Coefficient of Ozone in a Bubble Column. MATEC Web Conf. 2018, 156, 02015. [Google Scholar] [CrossRef] [Green Version]
- Berry, M.J.; Taylor, C.M.; King, W.; Chew, Y.M.J.; Wenk, J. Modelling of Ozone Mass-Transfer through Non-Porous Membranes for Water Treatment. Water 2017, 9, 452. [Google Scholar] [CrossRef]
- VonSonntag, C.; VonGunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; Iwa Publishing: London, UK, 2012; ISBN 978-1-84339-313-9. [Google Scholar]
- Masel, R.I. Principles of Adsorption and Reaction on Solid Surfaces, 1st ed.; Wiley: Oak Brook, IL, USA, 1996. [Google Scholar]
- De Luis, A.; Lombraña, J.I. pH-Based Strategies for an Efficient Addition of H2O2 During Ozonation to Improve the Mineralisation of Two Contaminants with Different Degradation Resistances. Water Air Soil Pollut. 2018, 229, 372. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y.; Yu, T. Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Yu, N.; Wang, X.; Wang, Y.; Wang, L.; Li, X.; Hu, X. Adsorption Properties of Granular Activated Carbon-Supported Titanium Dioxide Particles for Dyes and Copper Ions. Sci. Rep. 2018, 8, 6463. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Valdes, H.; Sanchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of ozone treatment on surface properties of activated carbon. Langmuir 2002, 18, 2111–2116. [Google Scholar] [CrossRef]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation for the treatment of synthetic and industrial effluents—Application of mesoporous materials: A review. J. Environ. Manag. 2018, 211, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Khavanin, A.; Alizadeh, R. The investigation of catalytic ozonation and integrated catalytic ozonation/biological processes for the removal of phenol from saline wastewaters. J. Hazard. Mater. 2009, 171, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Rivero, M.J.; Ortiz, I. Kinetics of dodecylbenzenesulphonate mineralisation by TiO2 photocatalysis. Appl. Catal. B Environ. 2011, 101, 515–521. [Google Scholar] [CrossRef]
- MiarAlipour, S.; Friedmann, D.; Scott, J.; Amal, R. TiO2/porous adsorbents: Recent advances and novel applications. J. Hazard. Mater. 2018, 341, 404–423. [Google Scholar] [CrossRef] [PubMed]
- Beltran, F.J.; Rivas, F.J.; Montero-de-Espinosa, R. A TiO2/Al2O3 catalyst to improve the ozonation of oxalic acid in water. Appl. Catal. B-Environ. 2004, 47, 101–109. [Google Scholar] [CrossRef]
- Vega, E.; Valdes, H. New evidence of the effect of the chemical structure of activated carbon on the activity to promote radical generation in an advanced oxidation process using hydrogen peroxide. Microporous Mesoporous Mat. 2018, 259, 1–8. [Google Scholar] [CrossRef]
- Orge, C.A.; Sousa, J.P.S.; Gonçalves, F.; Freire, C.; Órfão, J.J.M.; Pereira, M.F.R. Development of Novel Mesoporous Carbon Materials for the Catalytic Ozonation of Organic Pollutants. Catal. Lett. 2009, 132, 1–9. [Google Scholar] [CrossRef]
- Turhan, K.; Uzman, S. The degradation products of aniline in the solutions with ozone and kinetic investigations. Ann. Chim. 2007, 97, 1129–1138. [Google Scholar] [CrossRef]
- Shahamat, Y.D.; Farzadkia, M.; Nasseri, S.; Mahvi, A.H.; Gholami, M.; Esrafili, A. Magnetic heterogeneous catalytic ozonation: A new removal method for phenol in industrial wastewater. J. Environ. Health Sci. Eng. 2014, 12, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Roshani, B.; McMaster, I.; Rezaei, E.; Soltan, J. Catalytic ozonation of benzotriazole over alumina supported transition metal oxide catalysts in water. Sep. Purif. Technol. 2014, 135, 158–164. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catalysis B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Tamura, H.; Mita, K.; Tanaka, A.; Ito, M. Mechanism of Hydroxylation of Metal Oxide Surfaces. J. Colloid Interface Sci. 2001, 243, 202–207. [Google Scholar] [CrossRef]
- Valdés, H.; Sánchez-Polo, M.; Zaror, C.A. Role of oxygen-containing functional surface groups of activated carbons on the elimination of 2-hydroxybenzothiazole from waters in A hybrid heterogeneous ozonation system. J. Adv. Oxid. Technol. 2017, 20. [Google Scholar] [CrossRef]
- Orha, C.; Pode, R.; Manea, F.; Lazau, C.; Bandas, C. Titanium dioxide-modified activated carbon for advanced drinking water treatment. Process Saf. Environ. Protect. 2017, 108, 26–33. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, J.; Álvarez, P.; Montero-de-Espinosa, R. Kinetics of Heterogeneous Catalytic Ozone Decomposition in Water on an Activated Carbon. Ozone Sci. Eng. 2002, 24, 227–237. [Google Scholar] [CrossRef]
- Leyva, E.; Moctezuma, E.; Noriega, S. Photocatalytic degradation of omeprazole. Intermediates and total reaction mechanism. J. Chem. Technol. Biotechnol. 2017, 92, 1511–1520. [Google Scholar] [CrossRef]
- Bulanin, K.M.; Lavalley, J.C.; Tsyganenko, A.A. Infrared Study of Ozone Adsorption on TiO2 (Anatase). J. Phys. Chem. 1995, 99, 10294–10298. [Google Scholar] [CrossRef]
- Bodzek, M.; Rajca, M. Photocatalysis in the treatment and disinfection of water. Part I. Theoretical backgrounds. Ecol. Chem. Eng. S 2012, 19, 489–512. [Google Scholar] [CrossRef]
- Orge, C.A.; Faria, J.L.; Pereira, M.F.R. Removal of oxalic acid, oxamic acid and aniline by a combined photolysis and ozonation process. Environ. Technol. 2015, 36, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Gilarranz, M.A.; Rodríguez, J.J. Chemical Pathway and Kinetics of Phenol Oxidation by Fenton’s Reagent. Environ. Sci. Technol. 2005, 39, 9295–9302. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Activated carbon catalytic ozonation of oxamic and oxalic acids. Appl. Catal. B Environ. 2008, 79, 237–243. [Google Scholar] [CrossRef]
- Villota, N.; Lombraña, J.I.; Cruz-Alcalde, A.; Marcé, M.; Esplugas, S. Kinetic study of colored species formation during paracetamol removal from water in a semicontinuous ozonation contactor. Sci. Total Environ. 2019, 649, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, F.; Varona, F.; Villota, N. Changes in Solution Color During Phenol Oxidation by Fenton Reagent. Environ. Sci. Technol. 2006, 40, 5538–5543. [Google Scholar] [CrossRef] [PubMed]
- Villota, N.; Lomas, J.M.; Camarero, L.M. Study of the paracetamol degradation pathway that generates color and turbidity in oxidized wastewaters by photo-Fenton technology. J. Photochem. Photobiol. A Chem. 2016, 329, 113–119. [Google Scholar] [CrossRef]
- Rasalingam, S.; Kibombo, H.S.; Wu, C.-M.; Peng, R.; Baltrusaitis, J.; Koodali, R.T. Competitive role of structural properties of titania–silica mixed oxides and a mechanistic study of the photocatalytic degradation of phenol. Appl. Catal. B Environ. 2014, 148–149, 394–405. [Google Scholar] [CrossRef]
- Anpo, M.; Kamat, P.V. (Eds.) Environmentally Benign Photocatalysts: Applications of Titanium Oxide-based Materials; Nanostructure Science and Technology; Springer: New York, NY, USA, 2010; ISBN 978-0-387-48441-9. [Google Scholar]
- Brillas, E.; Bastida, R.M.; Llosa, E.; Casado, J. Electrochemical Destruction of Aniline and 4-Chloroaniline for Wastewater Treatment Using a Carbon-PTFE O2—Fed Cathode. J. Electrochem. Soc. 1995, 142, 1733. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, A.; Montejano, A.; Casas, J.A.; Bahamonde, A. Elucidation of the photocatalytic-mechanism of phenolic compounds. J. Environ. Chem. Eng. 2018, 6, 5712–5719. [Google Scholar] [CrossRef]
- Sánchez, L.; Peral, J.; Domènech, X. Photocatalyzed destruction of aniline in UV-illuminated aqueous TiO2 suspensions. Electrochim. Acta 1997, 42, 1877–1882. [Google Scholar] [CrossRef]
- Comninellis, C.; Pulgarin, C. Anodic oxidation of phenol for waste water treatment. J. Appl. Electrochem. 1991, 21, 703–708. [Google Scholar] [CrossRef]
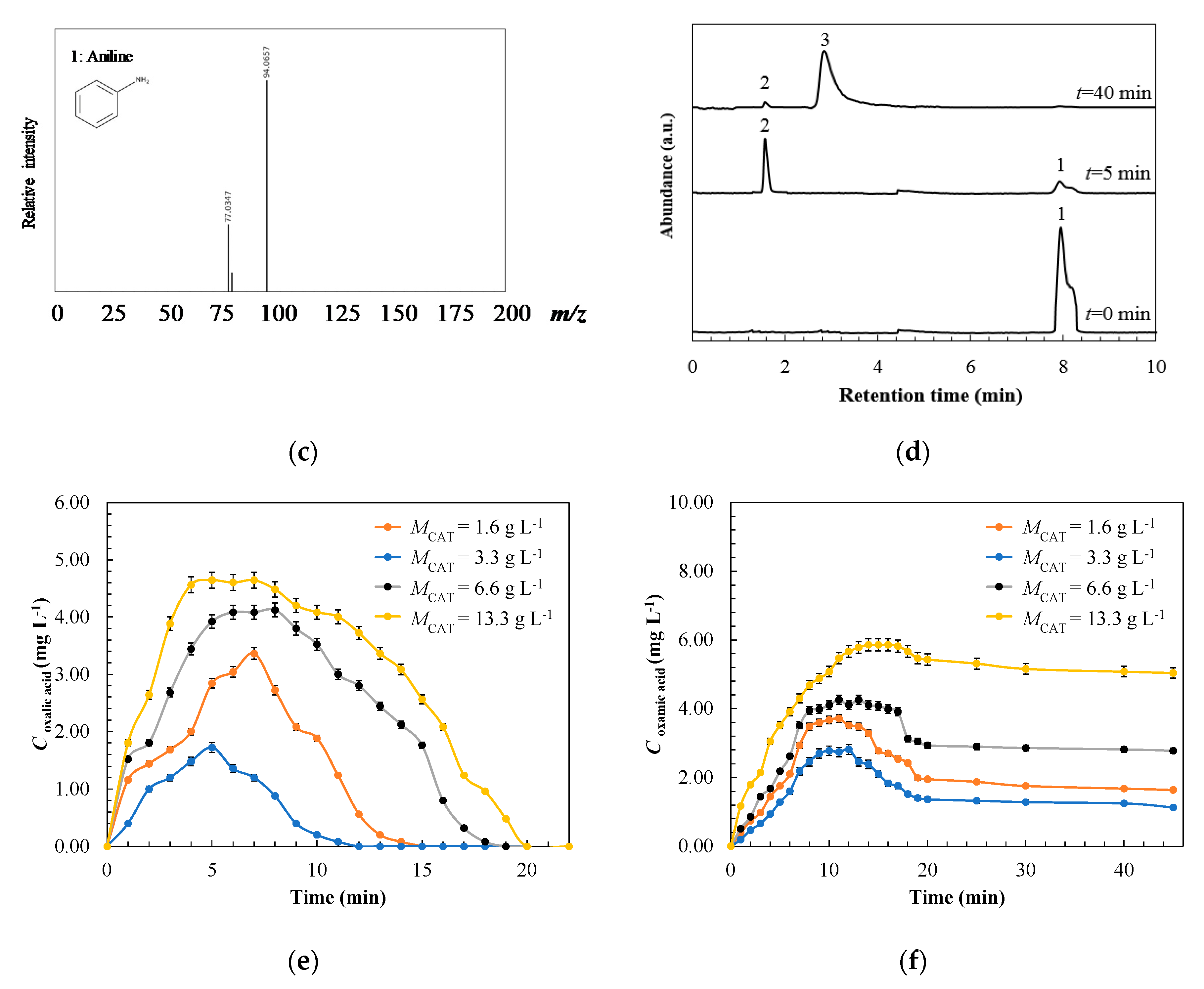
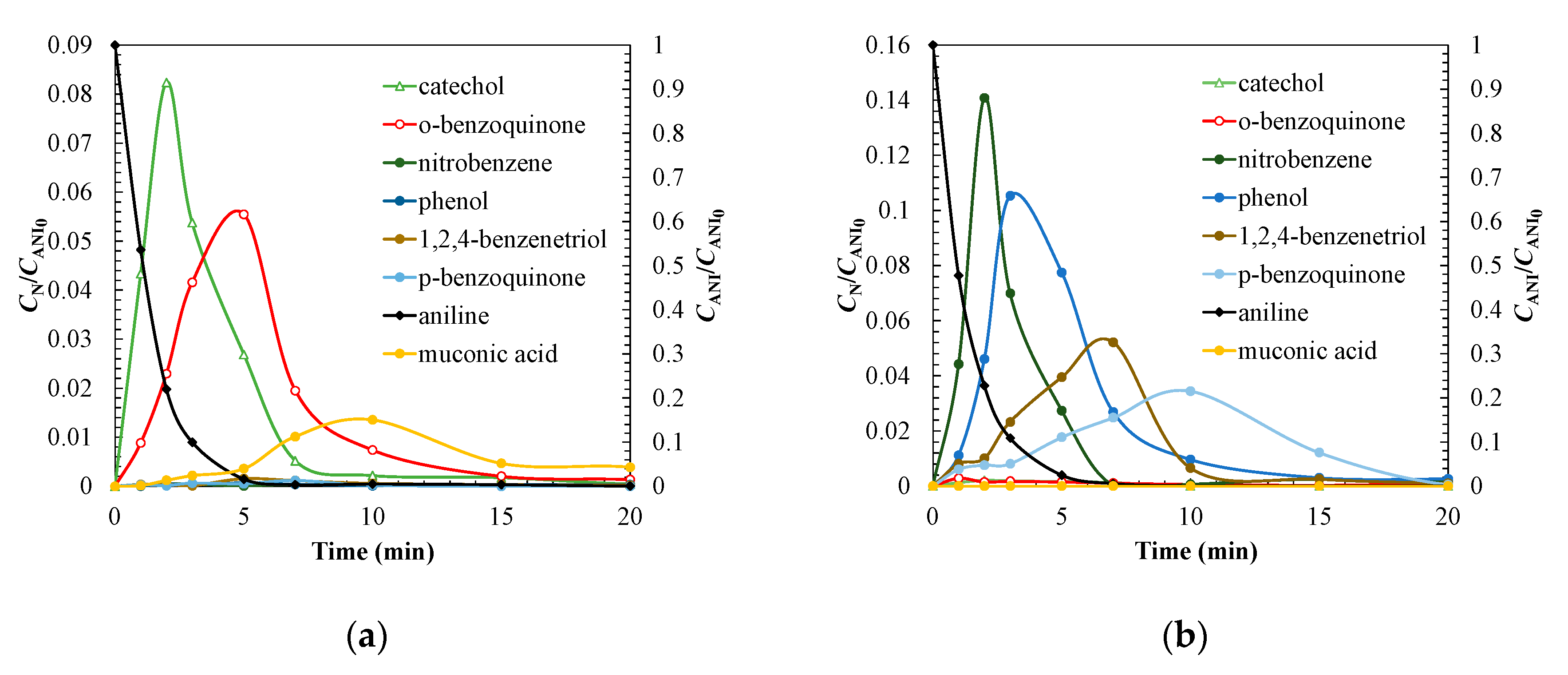
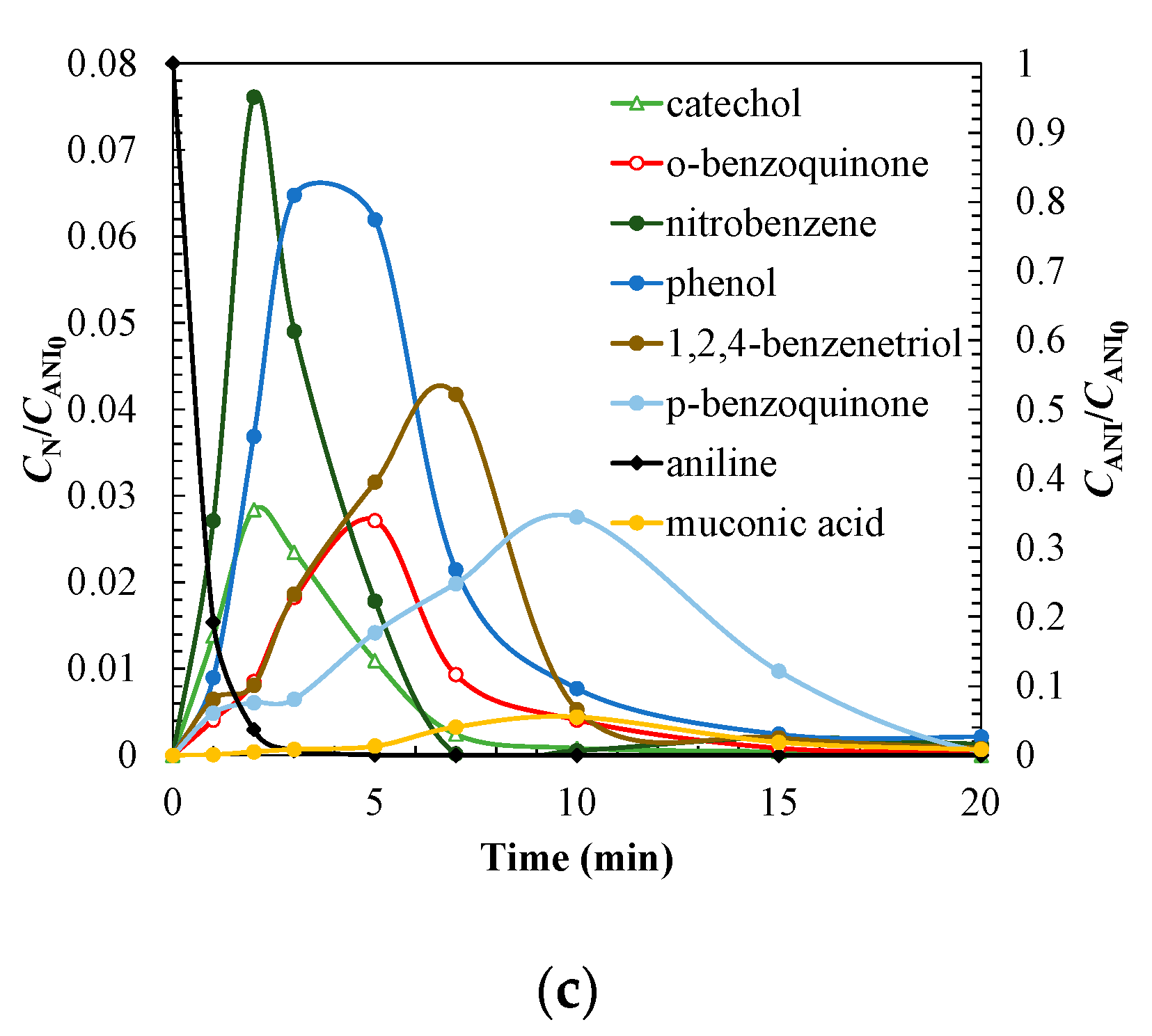
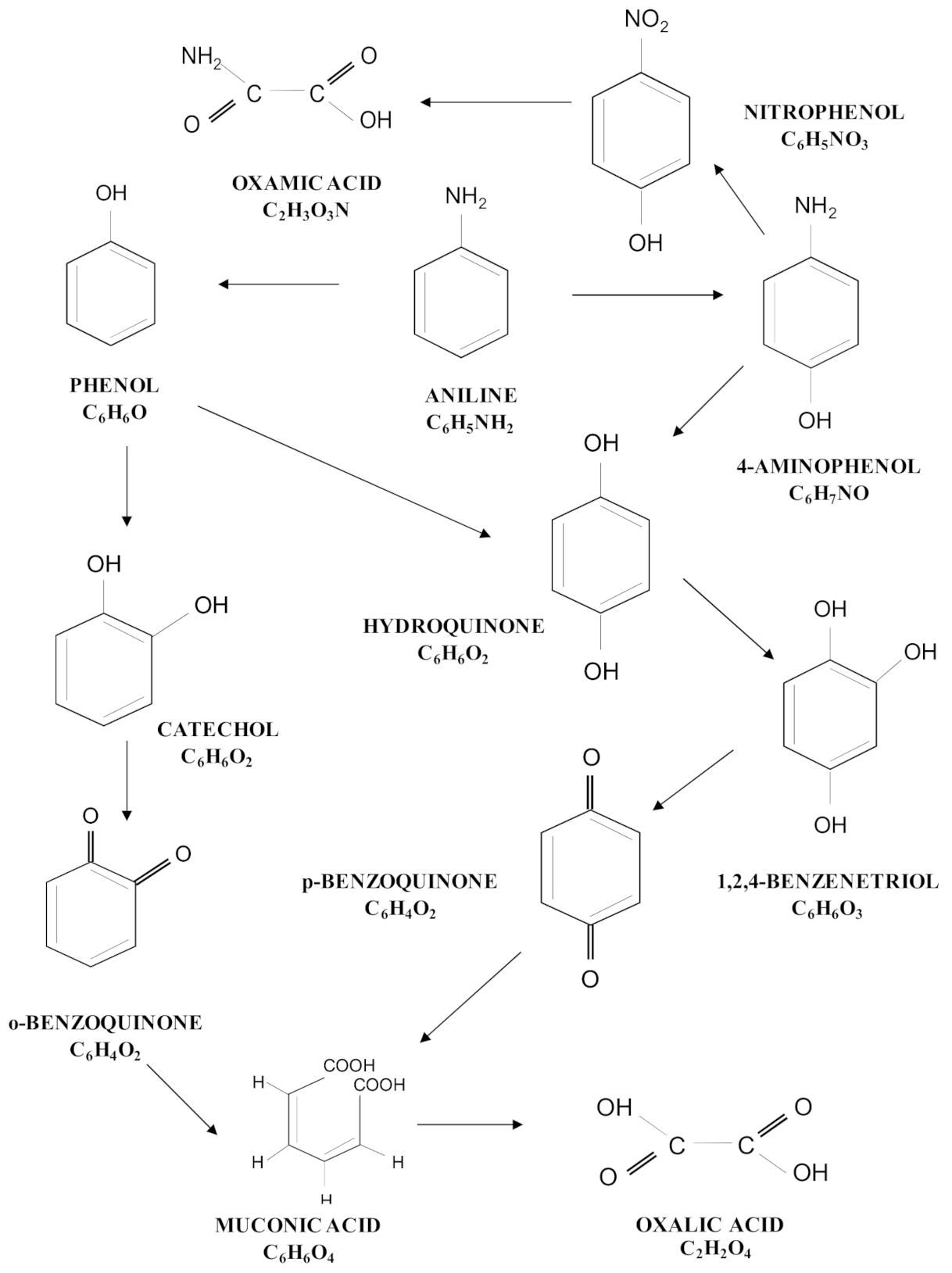
| Treatment | Catalyst | Operating Conditions | Comments | References |
|---|---|---|---|---|
| Ozone | — | (Time) = 120 min; = 103.81 mg L−1; pH = 7.0; T = 20 °C; FG = 2.5 g h−1; = 22.0 mg L−1; (%) = 93.56%; (% COD) = 31.03% | Studied the effect of operational variables on the biodegradability of aniline oxidation by-products, highlighting among them diacid butane, oxalic acid, and formic acid. | [6] |
| US/O3 | — | (Time) = 30 min; = 100 mg L−1; pH0 = 7.0; T = 25 °C; FG = 12 mg min−1; USdensity = 0.1 W mL−1; (%) = 99%; (% TOC) = 51% | The synergistic effect improved the degradation and mineralization of aniline by 64% and 110% respectively in terms of total organic carbon (TOC) compared to simple ozonation. | [20] |
| O3/GAC | GAC (Norit® 1240 Plus granular activated carbon (Cabot Norit Americas, Inc., Marshall, TX, USA)) | (Time) = 30 min; = 102.44 mg L−1; pH = 7.0; T = 25 °C; FG = 150 cm3 min−1; = 50.0 mg L−1; (%) = 100%; (% TOC) = 56%; = 500 mg L−1 | Studied the catalytic effect of GAC on the ozonisation process. Basic GACs had a higher capacity for decomposition of O3 and organics adsorption. | [21] |
| O3/TiO2-GAC | TiO2/GAC (Nanocyl® 3100 activated carbon doped with TiO2 by hydration–dehydration method (Nanocyl SA, Sambreville, Belgium)) | (Time) = 60 min; = 93.13 mg L−1; pH = 5.6; T = 25 °C; FG = 150 cm3 min−1; = 50.0 mg L−1; (%) = 100%; (% TOC) = 57%; = 500 mg L−1 | A higher mineralization was observed when doping the GAC with TiO2 oxides. The absence of NH4+ promoted a different oxidation mechanism compared to pristine GAC. | [22] |
| TiO2/GAC (Norit® 1240 Plus granular activated carbon doped with TiO2 by precipitation method (Cabot Norit Americas, Inc., Marshall, TX, USA)) | (Time) = 45 min; = 20.0 mg L−1; pH = 7.0; T = 18 °C; FG = 2.5 g h−1; = 5.4 mg L−1; (%) = 100%; (% TOC) = 80.24%; = 3.33 g L−1 | Through a novel method of synthesis by precipitation, a high yield was obtained in terms of degradation and mineralization. | [4] | |
| TiO2/UV | Hybrid Suspended-Supported TiO2 | (Time) = 4.73 h; = 22 mg L−1; = 60 mg L−1; (Supp. Cat.) = 2.3 mg cm−2; pH = 12.0; T = 25 °C; (%) = 99% | Under favourable operating conditions, using a hybrid system with suspended TiO2 catalyst, a 23% improvement in the elimination of aniline was observed compared to supported catalyst. | [5] |
| Samples | SBET (m2 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Dp (Å) | pHpzc |
|---|---|---|---|---|---|
| Norit® GAC 1240 Plus | 967.0 | 0.32 | 0.16 | 36.8 | 7.4 |
| TiO2/GAC | 985.0 | 0.29 | 0.16 | 33.9 | 6.4 |
| Catalyst Comparison | ||||
|---|---|---|---|---|
| Kinetic Parameter | Norit® GAC 1240 Plus 1,2 | TiO2/GAC 1,2 | ||
| kads × 10−4, g mg−1 min−1 | 2.4 | 3.5 | ||
| koxL × 101, min−1 | 8.1 | 5.9 | ||
| koxS × 101, min−1 | 0.0030 | 2.3 | ||
| σ | 0.050 | 0.046 | ||
| TiO2/GAC Composite Analysis | ||||
| Kinetic Parameter | Effect of pH | |||
| 3.0 | 5.0 | 7.0 | 9.0 | |
| kads × 10−4, g mg−1 min−1 | 3.6 | 3.1 | 2.9 | 2.5 |
| koxL × 101, min−1 | 3.4 | 6.5 | 8.3 | 8.6 |
| koxS × 101, min−1 | 0.20 | 1.1 | 1.2 | 0.90 |
| σ | 0.061 | 0.053 | 0.059 | 0.048 |
| Kinetic Parameter | Effect of Ozone Inlet Concentration, mg L−1 | |||
| 3.7 | 5.4 | 11.3 | 20.1 | |
| kads × 10−4, g mg−1 min−1 | 3.2 | 3.5 | 2.9 | 1.8 |
| koxL × 101, min−1 | 4.2 | 5.9 | 8.3 | 11.5 |
| koxS × 101, min−1 | 1.5 | 2.3 | 1.2 | 0.50 |
| σ | 0.052 | 0.046 | 0.057 | 0.045 |
| Kinetic Parameter | Effect of Catalyst Dose, g L−1 | |||
| 1.6 | 3.3 | 6.6 | 13.3 | |
| kads × 10−4, g mg-1 min−1 | 1.8 | 3.5 | 4.4 | 8.4 |
| koxL × 101, min−1 | 3.8 | 5.9 | 2.2 | 1.0 |
| koxS × 101, min−1 | 1.9 | 2.3 | 1.5 | 0.90 |
| σ | 0.054 | 0.046 | 0.055 | 0.052 |
| Property | Pristine TiO2/GAC | Spent TiO2/GAC | |
|---|---|---|---|
| SBET, m2 g−1 | 985.0 | 980.4 | 901.2 |
| Sext, m2 g−1 | 298.9 | 289.1 | 267.5 |
| VT, cm3 g−1 | 0.45 | 0.39 | 0.32 |
| Vµ, cm3 g−1 | 0.29 | 0.25 | 0.20 |
| VM, cm3 g−1 | 0.16 | 0.14 | 0.12 |
| VM/VT, % | 35.2 | 35.9 | 37.5 |
| Vµ/VT, % | 64.8 | 64.1 | 62.5 |
| DP, Å | 33.9 | 33.0 | 29.3 |
| pHpzc | 6.4 | 6.2 | 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreiro, C.; Villota, N.; Lombraña, J.I.; Rivero, M.J. Heterogeneous Catalytic Ozonation of Aniline-Contaminated Waters: A Three-Phase Modelling Approach Using TiO2/GAC. Water 2020, 12, 3448. https://doi.org/10.3390/w12123448
Ferreiro C, Villota N, Lombraña JI, Rivero MJ. Heterogeneous Catalytic Ozonation of Aniline-Contaminated Waters: A Three-Phase Modelling Approach Using TiO2/GAC. Water. 2020; 12(12):3448. https://doi.org/10.3390/w12123448
Chicago/Turabian StyleFerreiro, Cristian, Natalia Villota, José Ignacio Lombraña, and María J. Rivero. 2020. "Heterogeneous Catalytic Ozonation of Aniline-Contaminated Waters: A Three-Phase Modelling Approach Using TiO2/GAC" Water 12, no. 12: 3448. https://doi.org/10.3390/w12123448
APA StyleFerreiro, C., Villota, N., Lombraña, J. I., & Rivero, M. J. (2020). Heterogeneous Catalytic Ozonation of Aniline-Contaminated Waters: A Three-Phase Modelling Approach Using TiO2/GAC. Water, 12(12), 3448. https://doi.org/10.3390/w12123448