Identification of the Origins of Vadose-Zone Salinity on an Agricultural Site in the Venice Coastland by Ionic Molar Ratio Analysis
Abstract
:1. Introduction
2. Materials and Methods
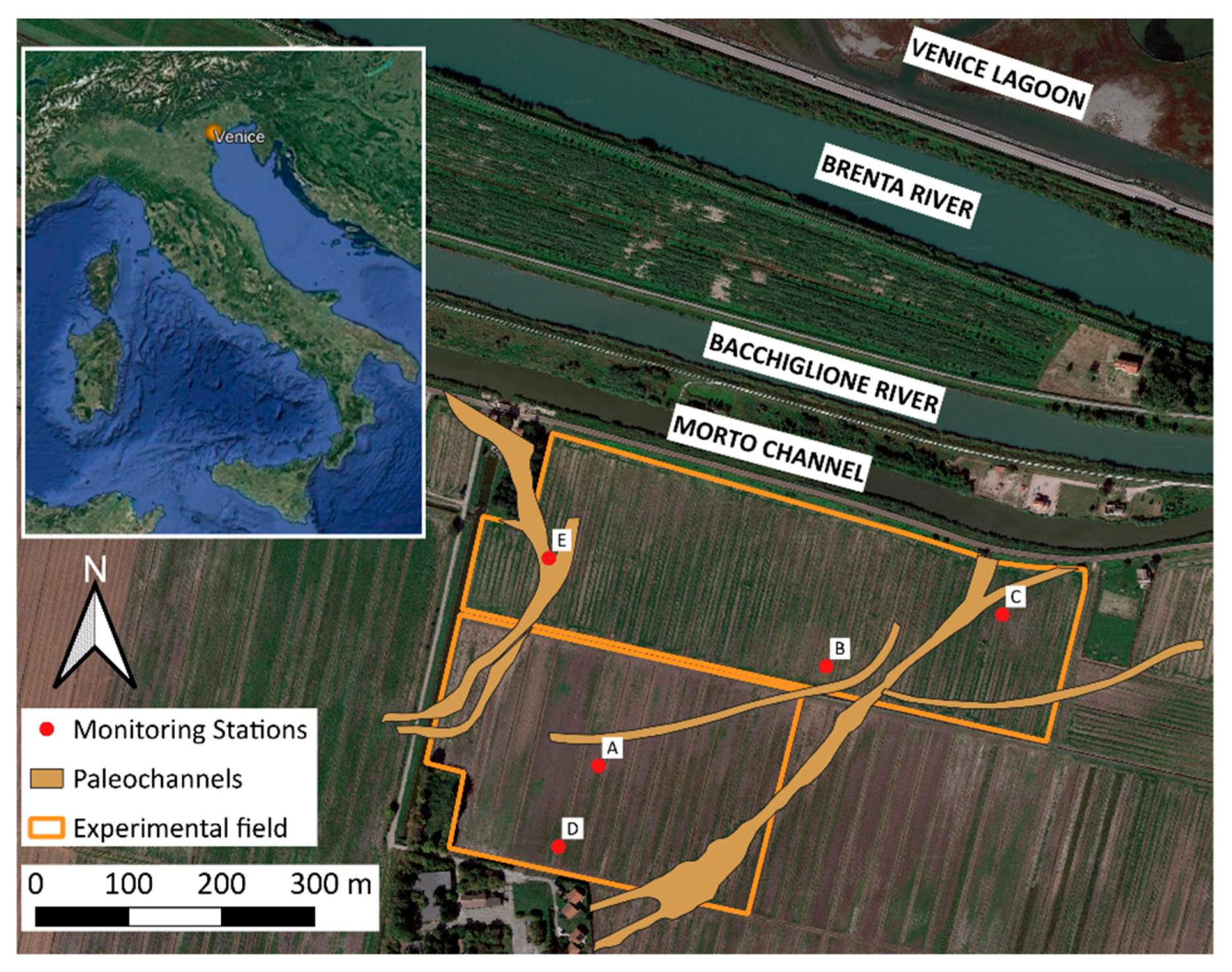
2.1. Experimental Site
2.2. Soil and Water Sampling, Analysis, and Monitoring Network
2.3. Statistical Analysis
3. Results
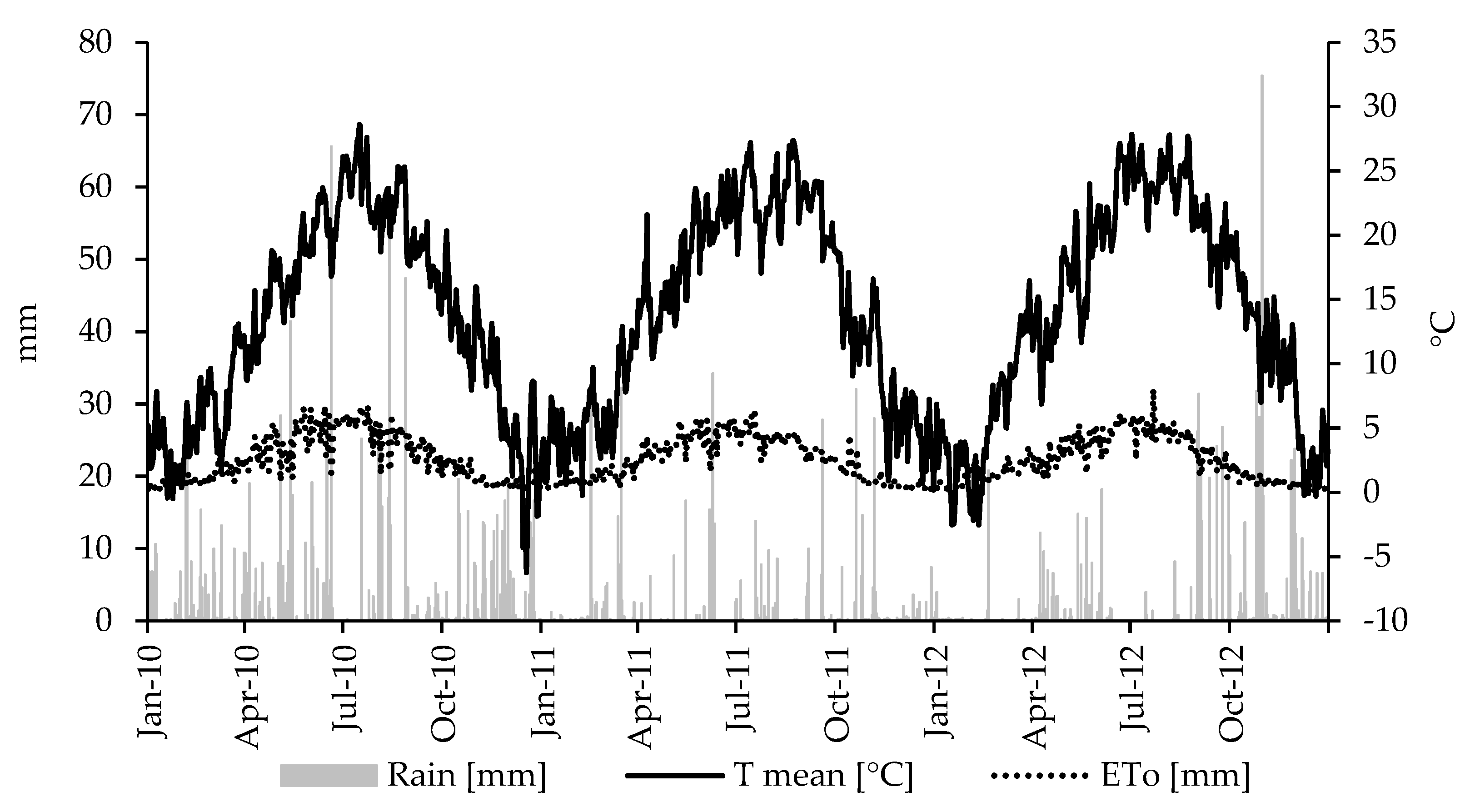
3.1. Weather and Water Table Depth Monitoring
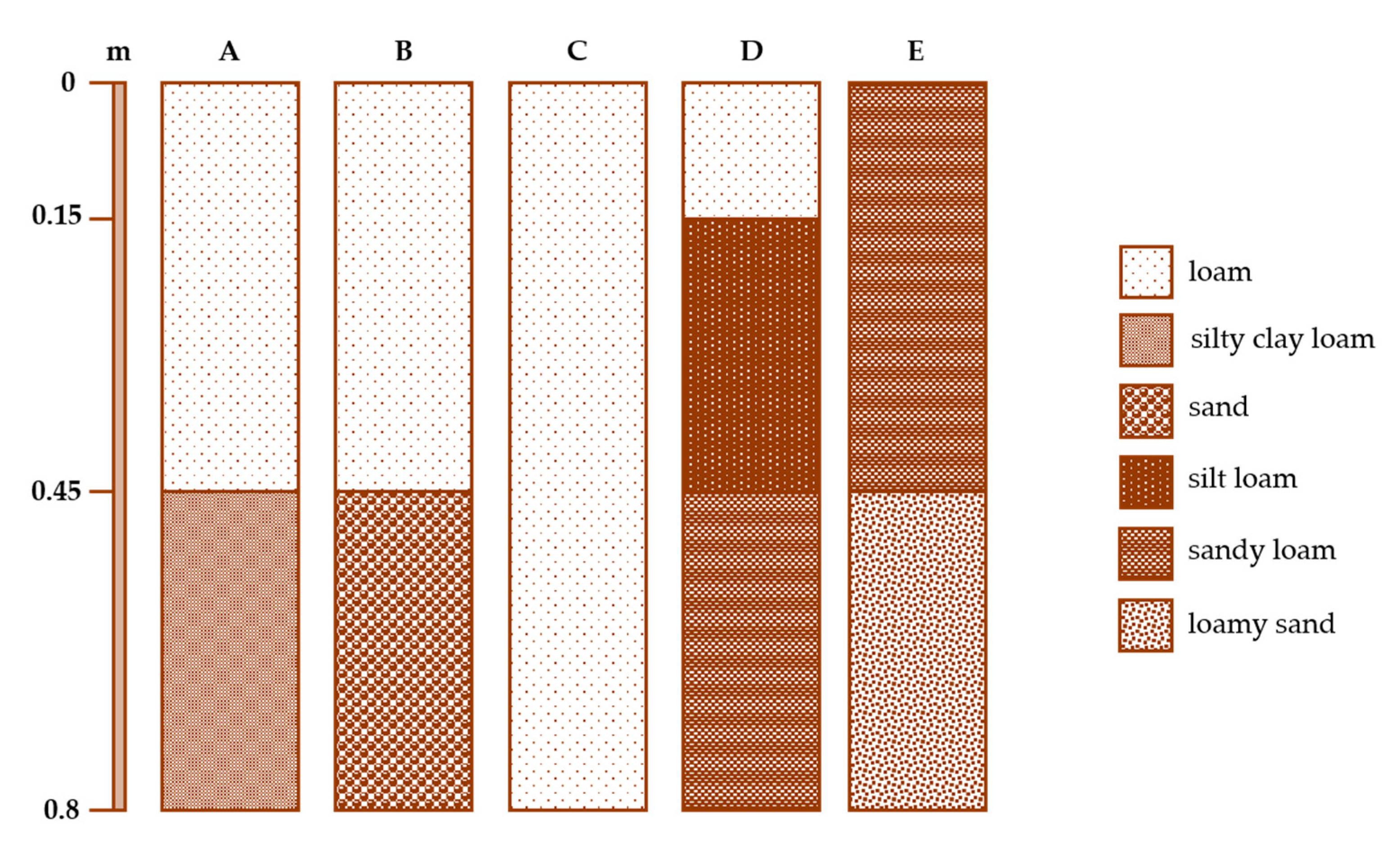
3.2. Soil Characterization
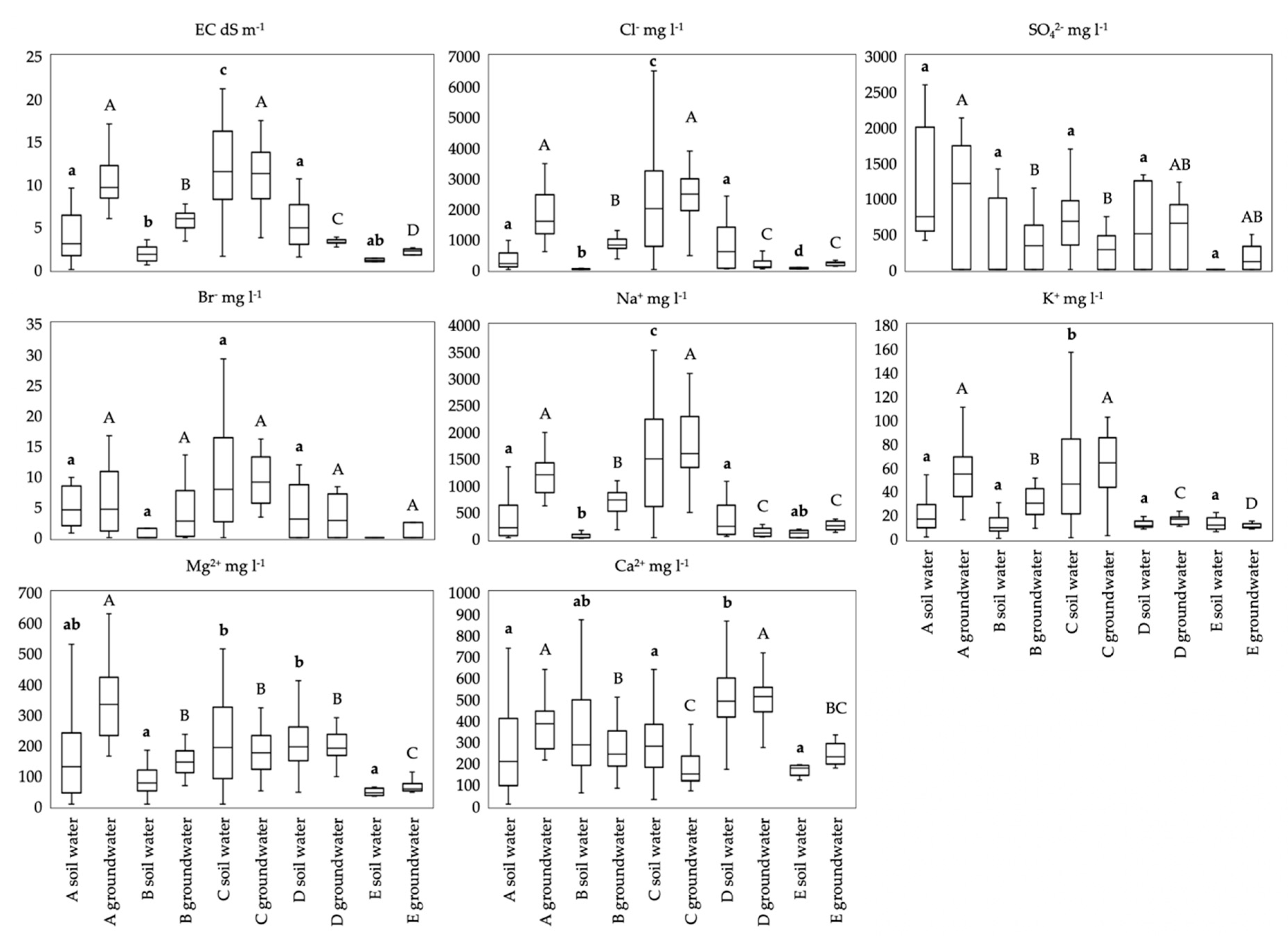
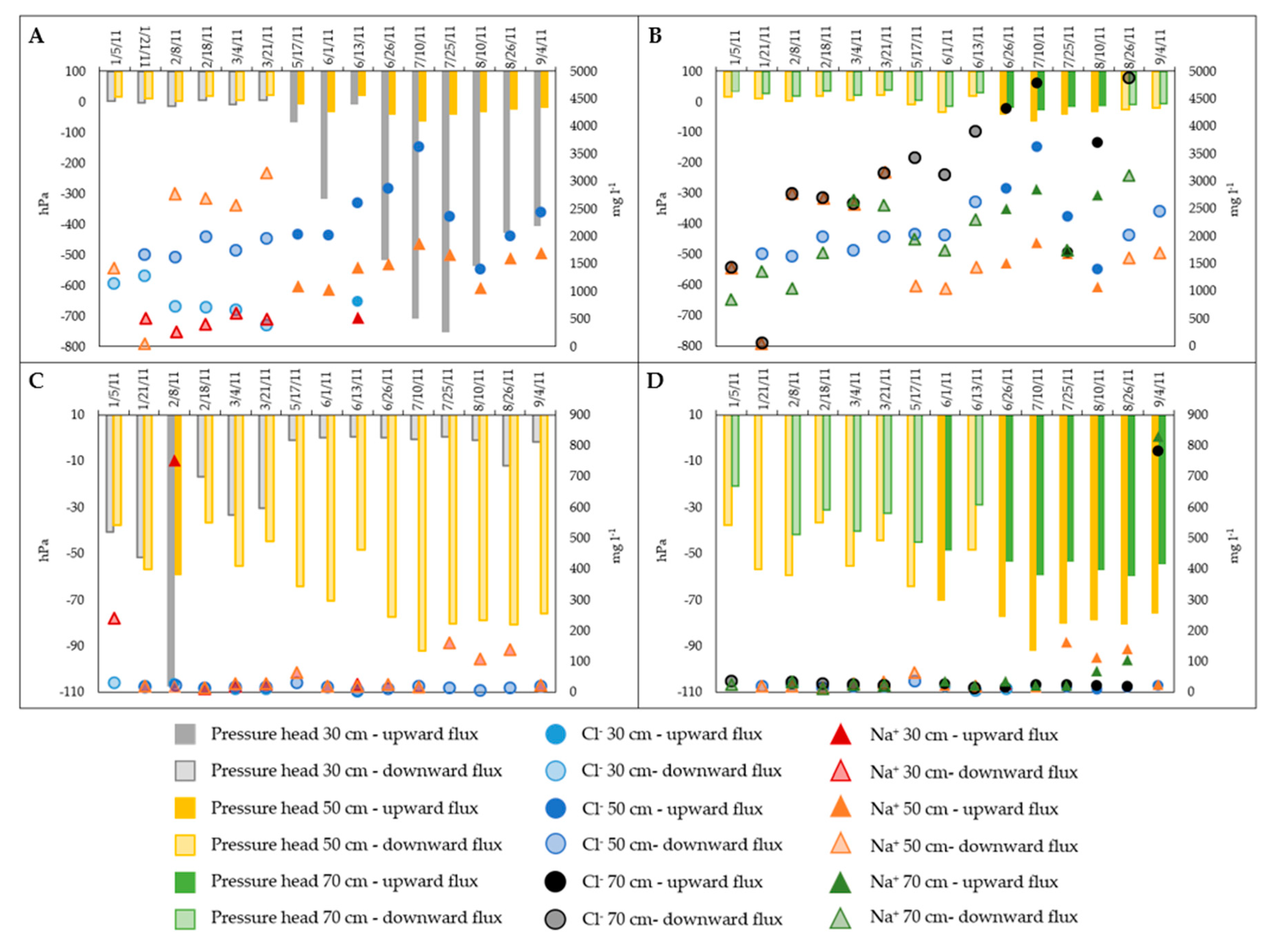
3.3. Soil Water and Groundwater Chemistry
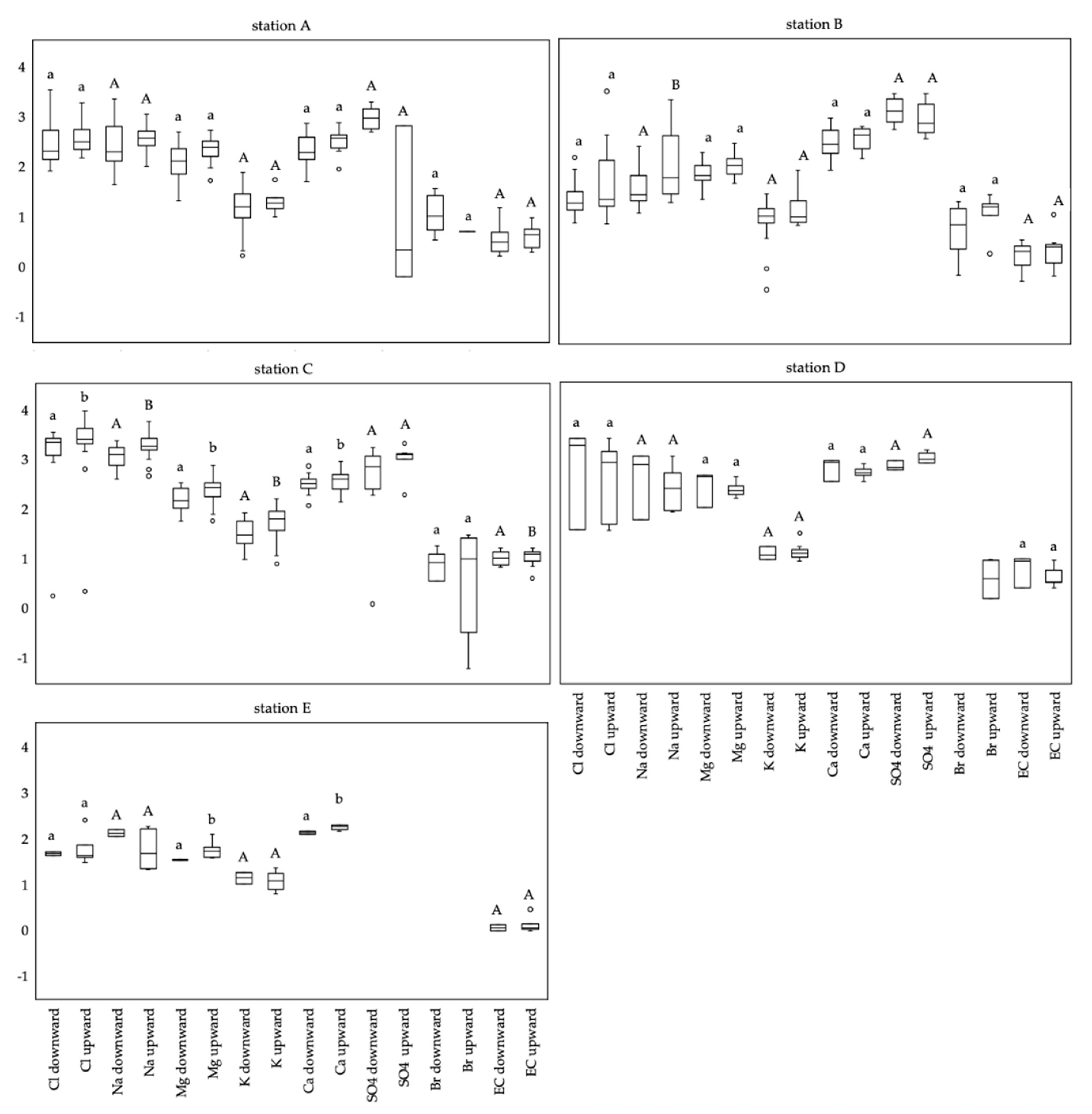
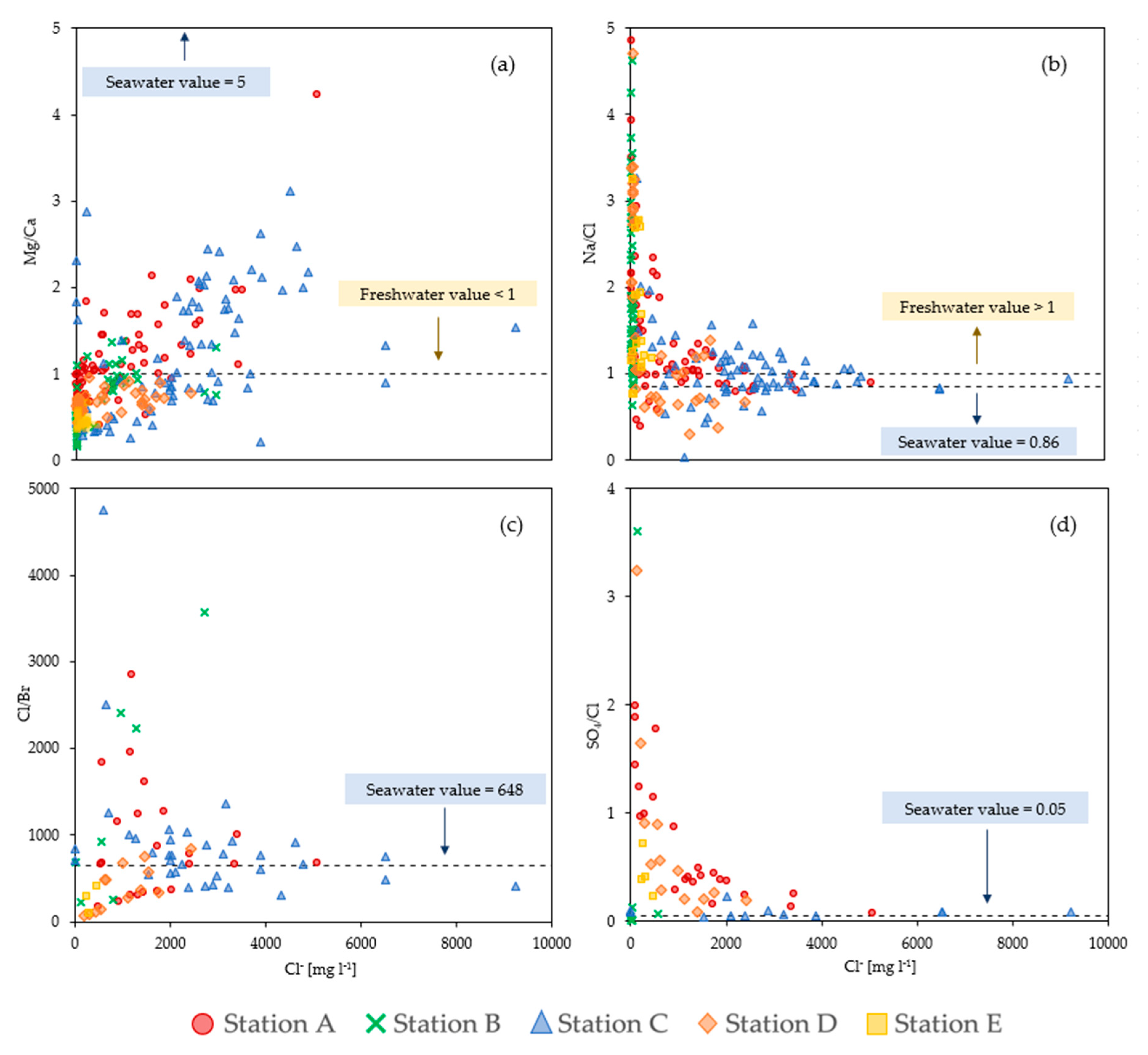
3.4. Ionic Ratios
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mondal, N.C.; Singh, V.P.; Singh, V.S.; Saxena, V.K. Determining the interaction between groundwater and saline water through groundwater major ion chemistry. J. Hydrol. Hydrol. 2010, 388, 100–111. [Google Scholar] [CrossRef]
- Carbognin, L.; Tosi, L. Il Progetto ISES per L’analisi dei Processi di Intrusione Salina e Subsidenza nei Territori Meridionali delle Province di Padova e Venezia; Istituto per lo Studio della Dinamica delle Grandi Masse, Consiglio Nazionale di Ricerca: Venice, Italy, 2003; pp. 16–88. [Google Scholar]
- Werner, A.D.; Bakker, M.; Post, V.E.A.; Vandenbohede, A.; Lu, C.; Ataie-Ashtiani, B.; Simmons, C.T.; Barry, D.A. Seawater intrusion processes, investigation and management: Recent advances and future challenges. Adv. Water Resour. 2013, 51, 3–26. [Google Scholar] [CrossRef]
- Brambati, A.; Carbognin, L.; Quaia, T.; Teatini, P.; Tosi, L. The Lagoon of Venice: Geological setting, evolution and land subsidence. Episodes 2003, 26, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Gambolati, G.; Putti, M.; Teatini, P.; Gasparetto-Stori, G. Subsidence due to peat oxidation and impacts on drainage infrastructures in a farmland catchment south of the Venice Lagoon. Environ. Geol. 2006, 49, 814–820. [Google Scholar] [CrossRef]
- Carbognin, L.; Teatini, P.; Tosi, L. Land subsidence in the Venetian area: Known and recent aspects. G. Geol. Appl. 2005, 1, 5–11. [Google Scholar]
- Da Lio, C.; Carol, E.; Kruse, E.; Teatini, P.; Tosi, L. Saltwater contamination in the managed low-lying farmland of the Venice coast, Italy: An assessment of vulnerability. Sci. Total Environ. 2015, 533, 356–369. [Google Scholar] [CrossRef]
- Mulligan, A.E.; Evans, R.L.; Lizarralde, D. The role of paleochannels in groundwater/seawater exchange. J. Hydrol. 2007, 335, 313–329. [Google Scholar] [CrossRef]
- De Franco, R.; Biella, G.; Tosi, L.; Teatini, P.; Lozej, A.; Chiozzotto, B.; Giada, M.; Rizzetto, F.; Claude, C.; Mayer, A.; et al. Monitoring the saltwater intrusion by time lapse electrical resistivity tomography: The Chioggia test site (Venice Lagoon, Italy). J. Appl. Geophys. 2009, 69, 117–130. [Google Scholar] [CrossRef]
- Di Sipio, E.; Galgaro, A.; Zuppi, G.M. New geophysical knowledge of groundwater systems in Venice estuarine environment. Estuar. Coast. Shelf Sci. 2006, 66, 6–12. [Google Scholar] [CrossRef]
- El Moujabber, M.; Bou Samra, B.; Darwish, T.; Atallah, T. Comparison of Different Indicators for Groundwater Contamination by Seawater Intrusion on the Lebanese Coast. Water Resour. Manag. 2006, 20, 161–180. [Google Scholar] [CrossRef]
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale. Deep-Sea Res. Part I 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Sukhija, B.S.; Varma, V.N.; Nagabhushanam, P.; Reddy, D.V. Differentiation of paleomarine and modern seawater intruded salinities in coastal groundwaters (of Karaikal and Tanjavur, India) based on inorganic chemistry, organic biomarker fingerprints and radiocarbon dating. J. Hydrol. 1996, 174, 173–201. [Google Scholar] [CrossRef]
- FAO. Seawater Intrusion in Coastal Aquifers: Guidelines for Studying, Monitoring and Control; Food and Agriculture Organization of the United Nations: Rome, Italy, 1997; pp. 5–23. [Google Scholar]
- Ghabayen, S.M.S.; McKee, M.; Kemblowski, M. Ionic and isotopic ratios for identification of salinity sources and missing data in the Gaza aquifer. J. Hydrol. 2006, 318, 360–373. [Google Scholar] [CrossRef]
- Vengosh, A.; Gill, J.; Davisson, M.L.; Hudson, G.B. A multi-isotope (B, Sr, O, H and C) and age dating (3H-3He and 14C) study of groundwater from Salinas Valley, California: Hydrogeochemistry, dynamics, and contamination processes. Water Resour. Res. 2002, 38, 1008. [Google Scholar] [CrossRef]
- Vengosh, A.; Rosenthal, E. Saline groundwater in Israel: Its bearing on the water crisis in the country. J. Hydrol. 1994, 156, 389–430. [Google Scholar] [CrossRef]
- Vengosh, A.; Spivack, A.J.; Artzi, Y.; Ayalon, A. Geochemical and boron, strontium, and oxygen isotopic constraints on the origin of the salinity in groundwater from the Mediterranean coast of Israel. Water Resour. Res. 1999, 35, 1877–1894. [Google Scholar] [CrossRef]
- Alcalá, F.J.; Custodio, E. Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal. J. Hydrol. 2008, 359, 189–207. [Google Scholar] [CrossRef]
- Abrol, I.P.; Yadav, J.S.P.; Massoud, F.I. Salt-Affected Soil and Their Management; FAO Soils Bulletin 39; Food and Agriculture organization of the United Nations: Rome, Italy, 1988. [Google Scholar]
- Kim, Y.; Lee, K.S.; Koh, D.C.; Lee, D.H.; Lee, S.G.; Park, W.B.; Koh, G.W.; Woo, N.C. Hydrogeochemical and isotopic evidence of groundwater salinization in a coastal aquifer: A case study in Jeju volcanic island, Korea. J. Hydrol. 2003, 270, 282–294. [Google Scholar] [CrossRef]
- Lee, J.Y.; Song, S.H. Evaluation of groundwater quality in coastal areas: Implications for sustainable agriculture. Environ. Geol. 2007, 52, 1231–1242. [Google Scholar] [CrossRef]
- Pulido-Leboeuf, P.; Pulido-Bosh, A.; Calvache, M.L.; Vallejos, Á.; Andreu, J.M. Strontium, SO42−/Cl− and Mg2+/Ca2+ ratios as tracers for the evolution of seawater into coastal aquifers: The example of Castell de Ferro aquifer (SE Spain). Comptes Rendus Geosci. 2003, 335, 1039–1048. [Google Scholar] [CrossRef]
- Scudiero, E.; Teatini, P.; Corwin, D.L.; Dal Ferro, N.; Simonetti, G.; Morari, F. Spatiotemporal Response of Maize Yield to Edaphic and Meteorological Conditions in a Saline Farmland. Agron. J. 2014, 106, 2163–2174. [Google Scholar] [CrossRef] [Green Version]
- Ayers, R.; Westcot, D. Water Quality for Agriculture; FAO Irrigation and Drainage Paper No. 29 Rev.1; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985. [Google Scholar]
- Volpe, V.; Manzoni, S.; Marani, M.; Katul, G. Leaf conductance and carbon gain under salt-stressed conditions. J. Geophys. Res. 2011, 116, G04035. [Google Scholar] [CrossRef]
- Viezzoli, A.; Tosi, L.; Teatini, P.; Silvestri, S. Surface water-groundwater exchange in transitional coastal environments by airborne electromagnetics: The Venice Lagoon example. Geophys. Res. Lett. 2010, 37, L01402. [Google Scholar] [CrossRef]
- FAO-UNESCO. Soil Map of the World, Revised Legend; Food and Agriculture Organization of the United Nations: Rome, Italy, 1989. [Google Scholar]
- Scudiero, E.; Teatini, P.; Corwin, D.L.; Deiana, R.; Berti, A.; Morari, F. Delineation of site-specific management units in a saline region at the Venice Lagoon margin, Italy, using soil reflectance and apparent electrical conductivity. Comput. Electron. Agric. 2013, 99, 54–64. [Google Scholar] [CrossRef]
- Morari, F. Drainage Flux Measurement and Errors Associated with Automatic Tension-controlled Suction Plates. Soil Sci. Soc. Am. J. 2006, 6, 1860–1871. [Google Scholar] [CrossRef]
- Ciglasch, H.; Amelung, S.; Totrakool, S.; Kaupenjohann, M. Water flow patterns and pesticide fluxes in an upland soil in northern Thailand. Eur. J. Soil Sci. 2005, 56, 765–777. [Google Scholar] [CrossRef]
- APAT; CNR-IRSA. Metodi Analitici per le Acque; APAT: Rome, Italy, 2003. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon and Organic Matter. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 1201–1230. [Google Scholar]
- Hillel, D. Introduction to Environmental Soil Physics; Elsevier: San Diego, CA, USA, 2004; p. 494. [Google Scholar]
- Schabenberger, O.; Pierce, F.J. Contemporary Statistical Models for the Plant and Soil Sciences; CRC Press: Boca Raton, FL, USA, 2001; p. 725. [Google Scholar]
- Teatini, P.; Tosi, L.; Viezzoli, A.; Bardello, L.; Zecchin, M.; Silvestri, S. Understanding the hydrogeology of the Venice Lagoon subsurface with airborne electromagnetics. J. Hydrol. 2011, 411, 342–354. [Google Scholar] [CrossRef]
- Da Lio, C.; Tosi, L.; Zambon, G.; Vianello, A.; Baldin, G.; Lorenzetti, G.; Manfè, G.; Teatini, P. Long-term groundwater dynamics in the oastal confined aquifers of Venice (Italy). Estuar. Coast. Shelf Sci. 2013, 135, 248–259. [Google Scholar] [CrossRef]
- Russak, A.; Sivan, O. Hydrogeochemical Tool to Identify Salinization or Freshening of Coastal Aquifers Determined from Combined Field Work, Experiments, and Modeling. Environ. Sci. Technol. 2010, 44, 4096–4102. [Google Scholar] [CrossRef]
- Grosso, C.; Manoli, G.; Martello, M.; Chemin, Y.H.; Pons, D.H.; Teatini, P.; Piccoli, I.; Morari, F. Mapping Maize Evapotranspiration at Field Scale Using SEBAL: A Comparison with the FAO Method and Soil-Plant Model Simulations. Remote Sens. 2018, 10, 1452. [Google Scholar] [CrossRef] [Green Version]
- Dent, D. Acid Sulphate Soils: A Baseline for Research and Development; International Institute for Land Reclamation and Improvement: Wageningen, The Netherlands, 1986; pp. 22–73. [Google Scholar]
- Mosley, L.M.; Zammit, B.; Jolley, A.; Barnett, L.; Fitzpatrick, R. Monitoring and assessment of surface water acidification following rewetting of oxidised acid sulfate soils. Environ. Monit. Assess. 2014, 186, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Keesstra, S.D.; Geissen, V.; Mosse, K.; Piiranen, S.; Scudiero, E.; Leistra, M.; van Schaik, L. Soil as a filter for groundwater quality. Curr. Opin. Environ. Sustain. 2012, 4, 507–516. [Google Scholar] [CrossRef]

| 2010 | 2011 | 2012 | |
|---|---|---|---|
| A | 28 July–15 October 21 December–31 December | 1 January–21 March 16 May–23 September | 26 April–26 October |
| B | 28 July–15 October 21 December–31 December | 1 January–21 March 16 May–23 September | 26 April–31 October |
| C | 28 July–15 October 21 December–31 December | 1 January–21 March 16 May–23 September | 26 April–26 October |
| D | - | 16 May–23 September | 26 April–25 October |
| E | - | 16 May–23 September | 26 April–26 October |
| A | B | C | D | E | ||
|---|---|---|---|---|---|---|
| Depth (m below soil surface) | Max | 0.94 | 1.29 | 0.67 | 1.04 | 1.50 |
| Min | 0.56 | 0.96 | 0.46 | 0.65 | 1.17 | |
| Average | 0.74 | 1.14 | 0.53 | 0.82 | 1.43 | |
| Elevation (m above msl) | Max | −3.97 | −3.21 | −2.73 | −3.72 | −3.05 |
| Min | −3.59 | −3.54 | −2.94 | −4.11 | −3.38 | |
| Average | −3.77 | −3.39 | −2.80 | −3.89 | −3.31 |
| Station * | Depth | Sand | Silt | Clay | pH1:2 | EC1:2 | SOC | CEC | CaCO3 | Mg2+ | Na+ | Ca2+ | K+ | BD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | % | dS m−1 | % | meq 100 g−1 | g cm−3 | |||||||||
| A | 0–0.15 | 39.58 | 40.97 | 19.45 | 5.66 | 0.21 | 13.72 | 5.34 | 11.5 | 4.72 | 0.22 | 11.65 | 0.21 | 0.94 |
| A | 0.15–0.45 | 42.27 | 41.65 | 16.08 | 5.54 | 0.38 | 15.84 | 30.56 | 0.50 | 4.82 | 0.30 | 12.23 | 0.26 | 0.77 |
| A | 0.45–0.80 | 17.78 | 54.57 | 27.66 | 5.38 | 1.13 | 4.79 | 15.16 | 1.00 | 4.10 | 0.39 | 8.74 | 0.25 | 1.09 |
| B | 0–0.15 | 51.86 | 37.21 | 10.94 | 7.25 | 0.35 | 5.61 | 12.21 | 7.08 | 2.52 | 0.16 | 10.64 | 0.18 | 1.09 |
| B | 0.15–0.45 | 49.22 | 38.01 | 12.76 | 7.22 | 0.28 | 5.96 | 21.04 | 7.17 | 2.57 | 0.16 | 10.17 | 0.18 | 1.01 |
| B | 0.45–0.80 | 92.09 | 7.06 | 0.85 | 7.70 | 0.29 | 2.73 | 2.35 | 16.25 | 1.39 | 0.15 | 8.54 | 0.09 | 1.2 |
| C | 0–0.15 | 26.87 | 47.74 | 25.4 | 7.67 | 0.55 | 8.75 | 13.95 | 12.5 | 2.68 | 0.84 | 15.00 | 0.26 | 0.94 |
| C | 0.15–0.45 | 32.35 | 49.18 | 18.47 | 7.50 | 0.88 | 7.97 | 16.86 | 12.25 | 2.59 | 0.81 | 15.04 | 0.28 | 0.89 |
| C | 0.45–0.80 | 45.04 | 39.69 | 15.27 | 6.49 | 4.82 | 16.34 | 0.41 | 7.17 | 4.31 | 3.26 | 17.57 | 0.33 | 0.21 |
| D | 0–0.15 | 39.28 | 47.64 | 13.09 | 6.3 | 0.79 | 20.81 | 20.97 | - | 5.71 | 0.28 | 14.29 | 0.18 | 0.63 |
| D | 0.15–0.45 | 27.06 | 50.69 | 22.25 | 5.82 | 0.41 | 20.41 | 21.97 | 0.79 | 6.10 | 12.51 | 14.35 | 0.28 | 0.6 |
| D | 0.45–0.80 | 55.95 | 32.98 | 11.06 | 5.62 | 2.01 | 21.64 | 24.4 | 3.00 | 3.57 | 0.28 | 9.10 | 0.11 | 1.01 |
| E | 0–0.15 | 64.66 | 23.47 | 11.88 | 7.39 | 0.29 | 1.29 | 3.23 | 25.61 | 0.63 | 0.06 | 8.73 | 0.08 | 1.52 |
| E | 0.15–0.45 | 61.14 | 26.69 | 12.17 | 7.79 | 0.2 | 1.41 | 3.76 | 25.31 | 0.70 | 0.11 | 7.98 | 0.08 | 1.51 |
| E | 0.45–0.80 | 82.47 | 12.45 | 5.08 | 8.11 | 0.18 | 26.99 | 2.19 | 29.33 | 0.54 | 0.09 | 7.97 | 0.05 | 1.37 |
| ECw | Cl− | Na+ | SO42− | Mg2+ | Ca2+ | K+ | Br− | |
|---|---|---|---|---|---|---|---|---|
| ECw | 1.00 | 0.92 | 0.91 | 0.33 | 0.60 | −0.20 | 0.80 | 0.46 |
| Cl− | 0.81 | 1.00 | 0.97 | 0.18 | 0.50 | −0.19 | 0.81 | 0.56 |
| Na+ | 0.84 | 0.86 | 1.00 | 0.02 | 0.52 | −0.31 | 0.93 | 0.58 |
| SO4− | 0.38 | 0.34 | 0.24 | 1.00 | 0.56 | 0.52 | −0.06 | −0.03 |
| Mg2+ | 0.72 | 0.65 | 0.74 | 0.55 | 1.00 | 0.46 | 0.65 | 0.36 |
| Ca2+ | 0.42 | 0.32 | 0.28 | 0.59 | 0.71 | 1.00 | −0.13 | −0.15 |
| K+ | 0.59 | 0.66 | 0.80 | 0.10 | 0.64 | 0.18 | 1.00 | 0.60 |
| Br− | 0.75 | 0.80 | 0.81 | −0.20 | 0.61 | 0.22 | 0.72 | 1.00 |
| Soil Water | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | Mg2+ | K+ | Ca2+ | Cl− | SO42− | Br− | ECw | pH | ||
| Soil | Na+ | 0.70 | 0.47 | 0.50 | 0.06 | 0.58 | 0.31 | 0.14 | 0.52 | −0.19 |
| Mg2+ | 0.30 | 0.26 | −0.04 | −0.02 | 0.21 | 0.28 | 0.38 | 0.13 | −0.63 | |
| K+ | 0.59 | 0.26 | 0.58 | −0.13 | 0.47 | 0.28 | 0.11 | 0.36 | −0.12 | |
| Ca2+ | 0.52 | 0.13 | 0.49 | −0.18 | 0.43 | 0.04 | 0.35 | 0.23 | 0.11 | |
| CaCO3 | −0.14 | −0.21 | −0.01 | −0.04 | −0.17 | −0.42 | −0.18 | −0.12 | 0.61 | |
| EC1:2 | 0.81 | 0.84 | 0.36 | 0.59 | 0.87 | 0.18 | 0.78 | 0.89 | −0.18 | |
| pH | −0.28 | −0.30 | −0.08 | −0.14 | −0.26 | −0.45 | −0.13 | −0.23 | 0.79 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zancanaro, E.; Teatini, P.; Scudiero, E.; Morari, F. Identification of the Origins of Vadose-Zone Salinity on an Agricultural Site in the Venice Coastland by Ionic Molar Ratio Analysis. Water 2020, 12, 3363. https://doi.org/10.3390/w12123363
Zancanaro E, Teatini P, Scudiero E, Morari F. Identification of the Origins of Vadose-Zone Salinity on an Agricultural Site in the Venice Coastland by Ionic Molar Ratio Analysis. Water. 2020; 12(12):3363. https://doi.org/10.3390/w12123363
Chicago/Turabian StyleZancanaro, Ester, Pietro Teatini, Elia Scudiero, and Francesco Morari. 2020. "Identification of the Origins of Vadose-Zone Salinity on an Agricultural Site in the Venice Coastland by Ionic Molar Ratio Analysis" Water 12, no. 12: 3363. https://doi.org/10.3390/w12123363
APA StyleZancanaro, E., Teatini, P., Scudiero, E., & Morari, F. (2020). Identification of the Origins of Vadose-Zone Salinity on an Agricultural Site in the Venice Coastland by Ionic Molar Ratio Analysis. Water, 12(12), 3363. https://doi.org/10.3390/w12123363






