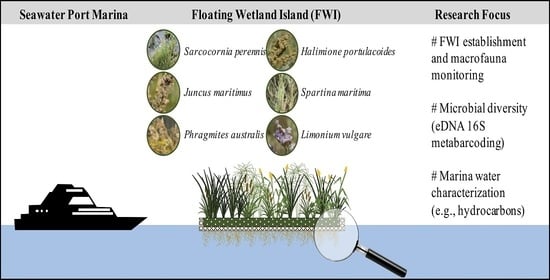
Floating Wetland Islands Implementation and Biodiversity Assessment in a Port Marina
Abstract
1. Introduction
- (i)
- resistance of the platform to the environmental conditions (salinity, tides, and waves);
- (ii)
- vegetation establishment and development;
- (iii)
- biotic (micro and macro) communities’ identification and establishment.
2. Materials and Methods
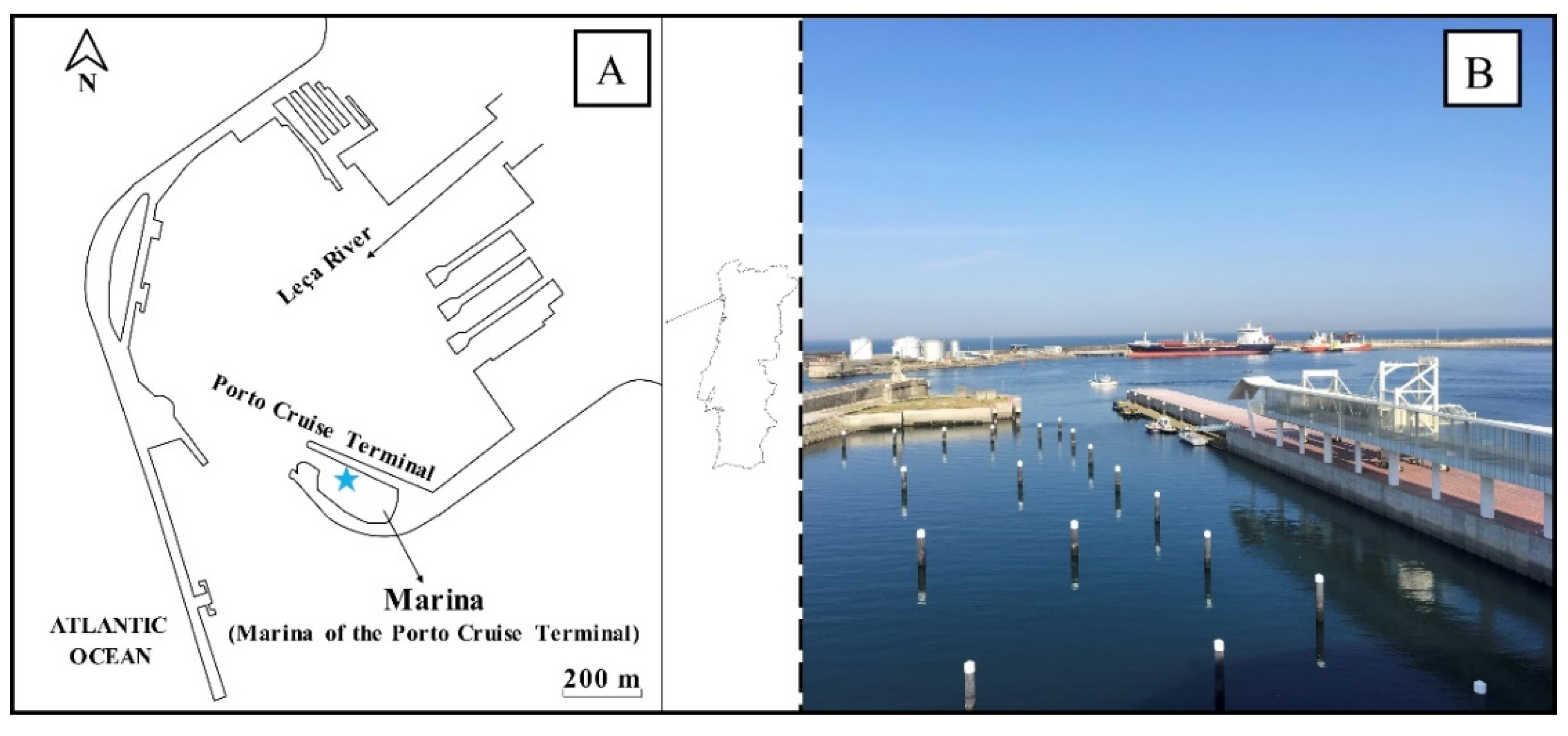
2.1. Study Area
2.2. FWI Implementation and Maintenance
2.3. Plant Selection, Planting, and Monitoring
2.4. Water Sampling Procedure and Physicochemical Analysis
2.5. Microbial Communities Sampling and Monitoring
2.5.1. Sampling Procedure
2.5.2. DNA Extraction, Quantification, and Sequencing
2.5.3. Bioinformatics Analysis
2.6. Statistical Analyses
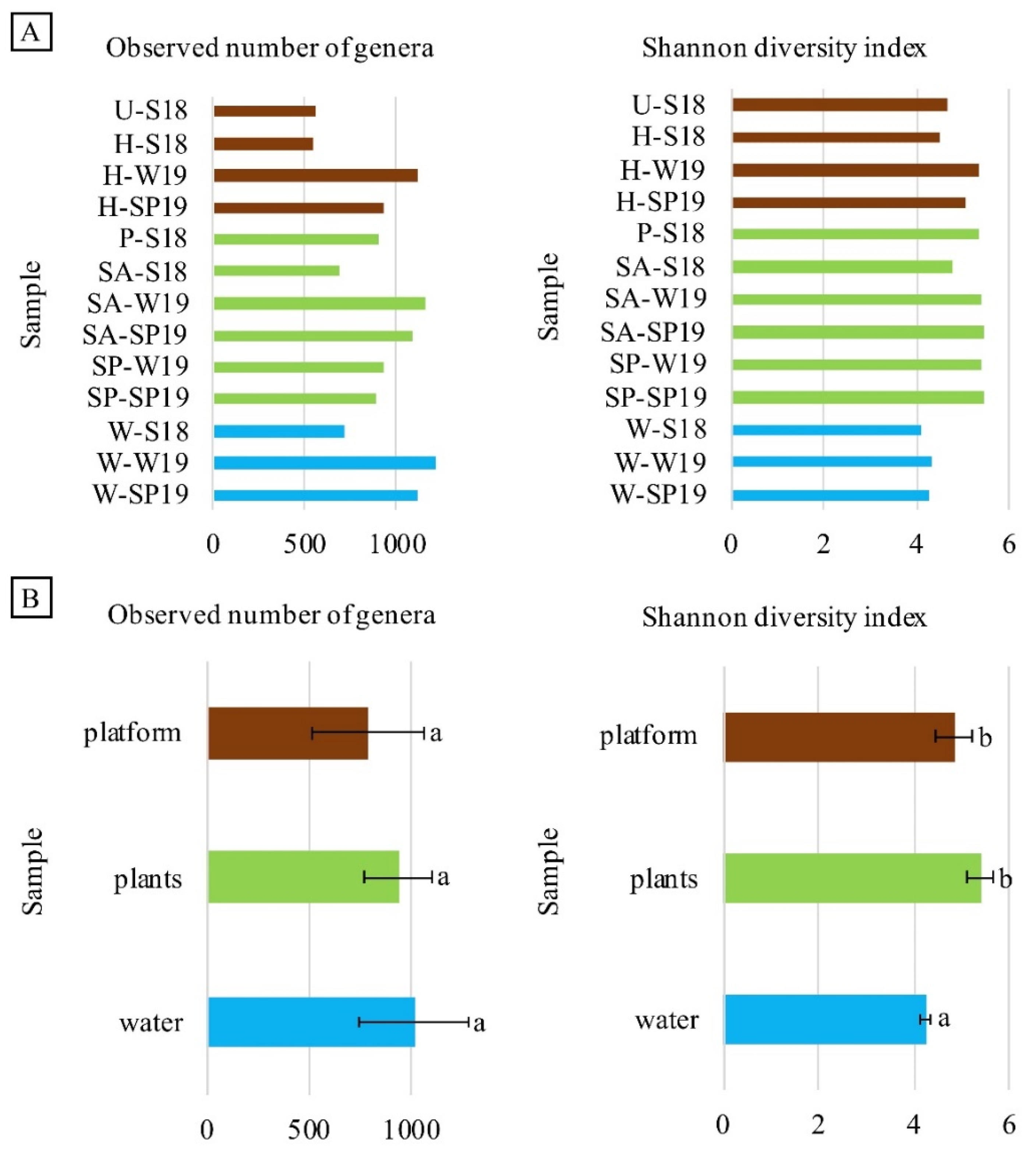
3. Results and Discussion
3.1. FWI Establishment and Macrofauna Monitoring
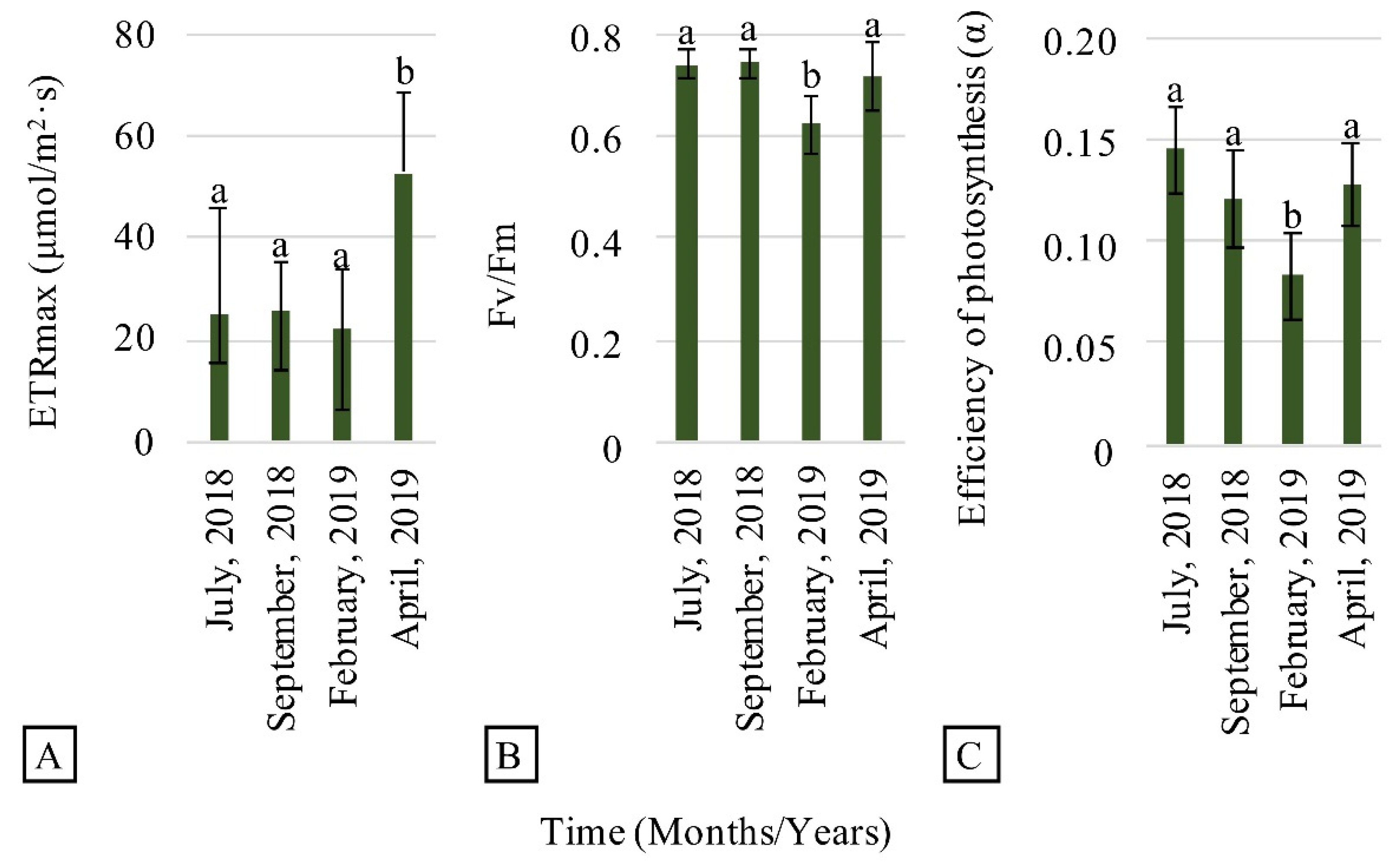
3.2. Evaluation of Plant Establishment and Development
3.3. Seawater Port Marina Characterization
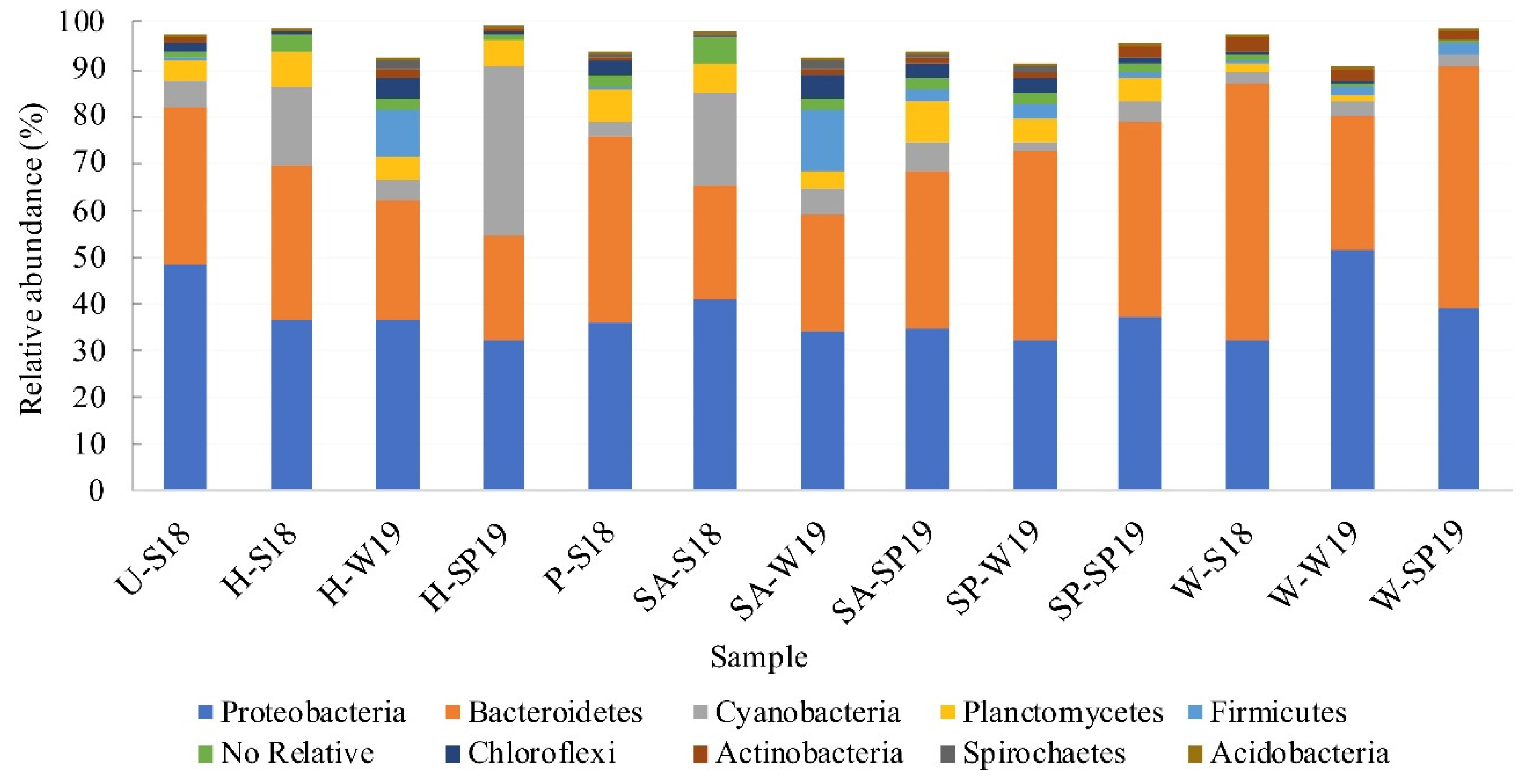
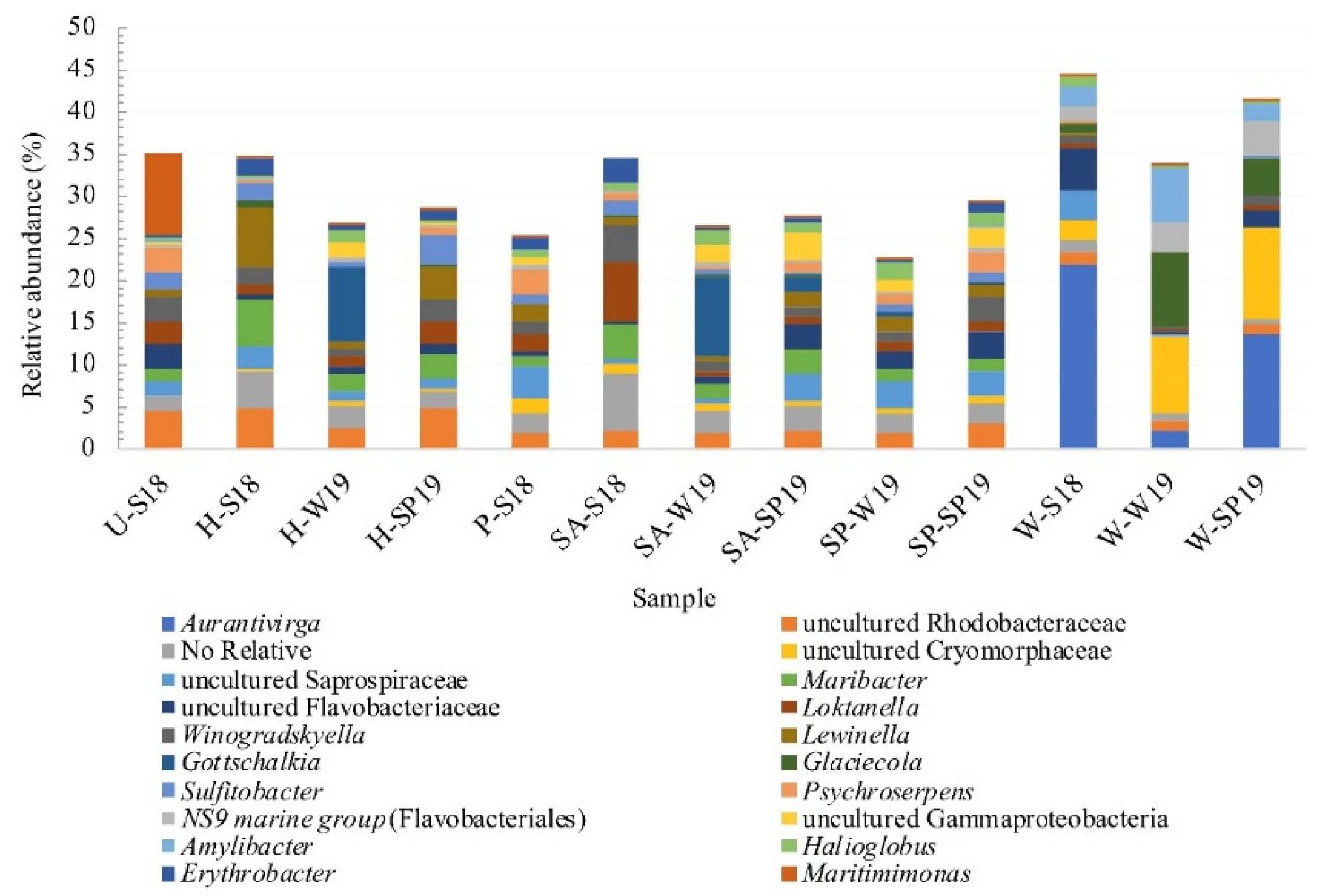
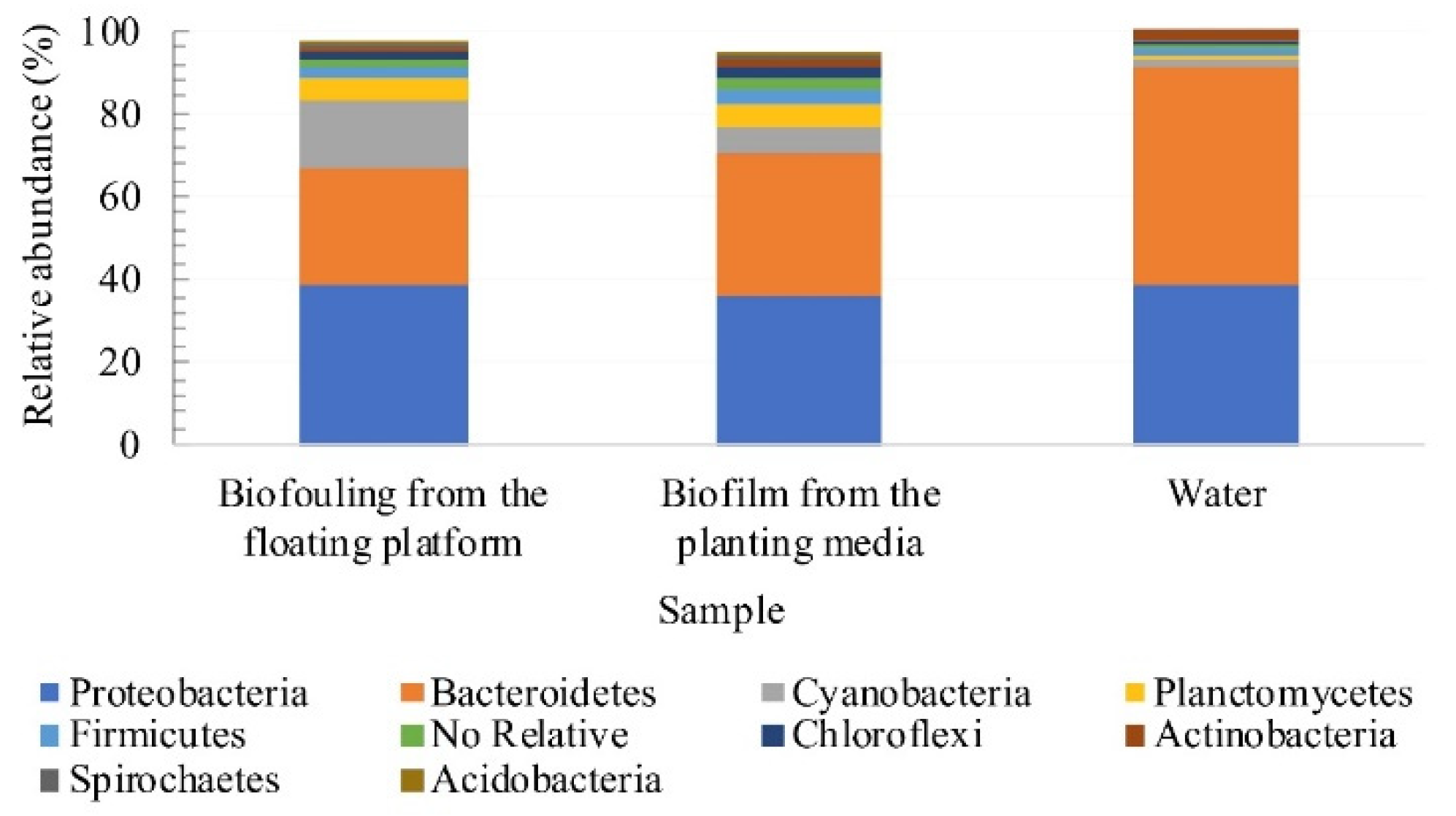
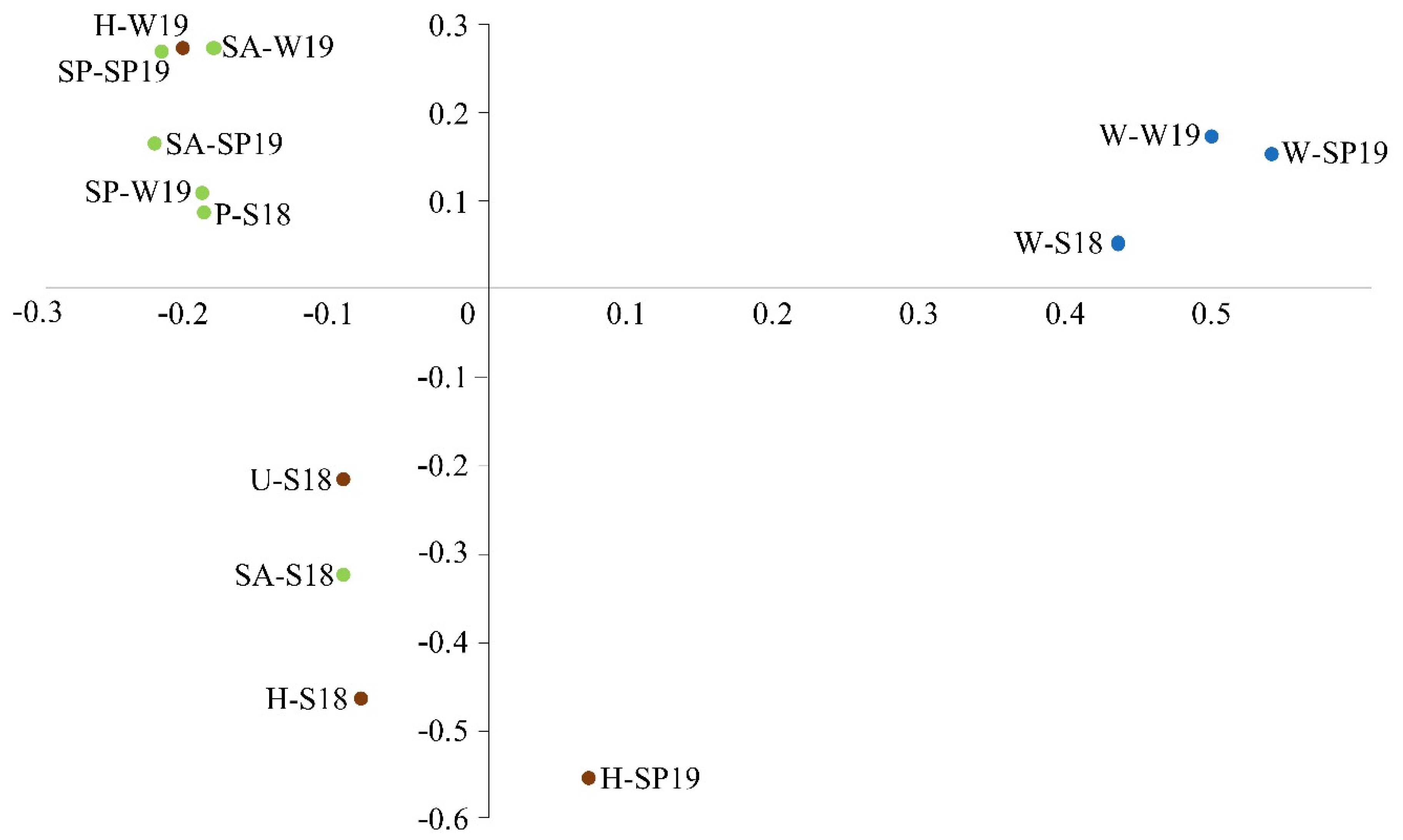
3.4. Assessment of Microbial Diversity in the FWI and Water
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Taxa (Genera) | Mean Abund. Plants (%) | Mean Abund. Water (%) | Contrib. (%) |
|---|---|---|---|
| Aurantivirga | 0.04 | 28.90 | 5.11 |
| Uncultured Cryomorphaceae | 4.85 | 19.50 | 2.57 |
| Glaciecola | 0.42 | 13.00 | 2.10 |
| Uncultured Saprospiraceae | 12.70 | 2.76 | 1.72 |
| Amylibacter | 0.14 | 9.82 | 1.63 |
| Gottschalkia | 8.95 | 0.01 | 1.56 |
| Maribacter | 8.87 | 0.02 | 1.54 |
| Lentibacter | 0.09 | 8.85 | 1.50 |
| Uncultured Gammaproteobacteria | 8.65 | 0.15 | 1.36 |
| Loktanella | 8.40 | 1.26 | 1.34 |
| Taxa (Genera) | Mean Abund. Platform (%) | Mean Abund. Water (%) | Contrib. (%) |
|---|---|---|---|
| Aurantivirga | 0.03 | 28.90 | 7.34 |
| Uncultured Cryomorphaceae | 0.99 | 19.50 | 4.43 |
| Glaciecola | 0.53 | 13.00 | 2.92 |
| Amylibacter | 0.35 | 9.82 | 2.25 |
| Lentibacter | 0.13 | 8.85 | 2.11 |
| NS9 marine group (Flavobacteriales) | 0.82 | 8.00 | 1.75 |
| Maritimimonas | 8.57 | 0.01 | 1.73 |
| Gottschalkia | 9.42 | 0.01 | 1.64 |
| Maribacter | 7.02 | 0.02 | 1.61 |
| Lewinella | 6.50 | 0.16 | 1.59 |
References
- Dafforn, K.A.; Glasby, T.M.; Airoldi, L.; Rivero, N.K.; Mayer-Pinto, M.; Johnston, E.L. Marine urbanization: An ecological framework for designing multifunctional artificial structures. Front. Ecol. Environ. 2015, 13, 82–90. [Google Scholar] [CrossRef]
- Nogales, B.; Bosch, R. Microbial communities in hydrocarbon-polluted harbors and marinas. In Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology. Handbook of Hydrocarbon and Lipid Microbiology; McGenity, T.J., Ed.; Springer: Cham, Switzerland, 2019; pp. 1–17. [Google Scholar] [CrossRef]
- Mali, M.; Dell’Anna, M.M.; Mastrorilli, P.; Damiani, L.; Piccinni, A.F. Assessment and source identification of pollution risk for touristic ports: Heavy metals and polycyclic aromatic hydrocarbons in sediments of 4 marinas of the Apulia region (Italy). Mar. Pollut. Bull. 2017, 114, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Llado, X.; Gibert, O.; Marti, V.; Diez, S.; Romo, J.; Bayona, J.M.; de Pablo, J. Distribution of polycyclic aromatic hydrocarbons (PAHs) and tributyltin (TBT) in Barcelona harbour sediments and their impact on benthic communities. Environ. Pollut. 2007, 149, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Romero, A.; Moreira, J.; Guerra-García, J.M. Marinas: An overlooked habitat for exploring the relation among polychaete assemblages and environmental factors. Mar. Pollut. Bull. 2019, 138, 584–597. [Google Scholar] [CrossRef]
- WWAP (United Nations World Water Assessment Programme)/UN-Water. The United Nations World Water Development Report 2018, Nature-Based Solutions for Water; UNESNO: Paris, France, 2018. [Google Scholar]
- Colares, G.S.; Dell’Osbel, N.; Wiesel, P.G.; Oliveira, G.A.; Lemos, P.; da Silva, H.Z.; Lutterbeck, F.P.; Kist, C.A.; Colares, L.T.; Machado, Ê.L. Floating treatment wetlands: A review and bibliometric analysis. Sci. Total Environ. 2020, 714, 136776. [Google Scholar] [CrossRef]
- Bi, R.; Zhou, C.; Jia, Y.; Wang, S.; Li, P.; Reichwaldt, E.S.; Liu, W. Giving waterbodies the treatment they need: A critical review of the application of constructed floating wetlands. J. Environ. Manag. 2019, 238, 484–498. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating wetlands: A sustainable tool for wastewater treatment. Clean Soil Air Water 2018, 46. [Google Scholar] [CrossRef]
- Pavlineri, N.; Skoulikidis, N.T.; Tsihrintzis, V.A. Constructed floating wetlands: A review of research, design, operation and management aspects, and data meta-analysis. Chem. Eng. J. 2017, 308, 1120–1132. [Google Scholar] [CrossRef]
- Chen, Z.; Cuervo, D.P.; Muller, J.A.; Wiessner, A.; Koser, H.; Vymazal, J.; Kästner, M.; Kuschk, P. Hydroponic root mats for wastewater treatment-a review. Environ. Sci. Pollut. Res. Int. 2016, 23, 15911–15928. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P.; Chang, Y.-H. Artificial floating islands for environmental improvement. Ren. Sustain. Energy Rev. 2015, 47, 616–622. [Google Scholar] [CrossRef]
- McCarty, M.; Ceci, J.; Streb, C. A Case Study and Recommendation for Large Scale Floating. In Proceedings of the International Conference on Sustainable Infrastructure 2017, New York, NY, USA, 26–28 October 2017; Soibelman, L., Peña-Mora, F., Eds.; American Society of Civil Engineers (ASCE): New York, NY, USA, 2017; pp. 1–12, ISBN 9780784481219. [Google Scholar]
- Sanicola, O.; Lucke, T.; Stewart, M.; Tondera, K.; Walker, C. Root and shoot biomass growth of constructed floating wetlands plants in saline environments. Int. J. Environ. Res. Public Health 2019, 16, 275. [Google Scholar] [CrossRef] [PubMed]
- Amorim Cork Composites. Technical bulletin Cork Floating Island®. ACC.851. 2018. Available online: https://amorimcorkcomposites.com/media/5627/tb-cork-floating-island.pdf (accessed on 29 May 2020).
- Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: New York, NY, USA, 2004; pp. 279–319. [Google Scholar]
- Kirk, J.T. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambrigde, UK, 1994; p. 59. [Google Scholar]
- Kitajima, M.B.W.L.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta (BBA) Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Caspers, H.; Grasshoff, K. Methods of Seawater Analysis; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; Verlag Chemie: Deerfield Beach, FL, USA, 1983. [Google Scholar]
- Jones, M.N. Nitrate reduction by shaking with cadmium: Alternative to cadmium columns. Water Res. 1984, 18, 643–646. [Google Scholar] [CrossRef]
- Almeida, C.M.; Reis, I.; Couto, M.N.; Bordalo, A.A.; Mucha, A.P. Potential of the microbial community present in an unimpacted beach sediment to remediate petroleum hydrocarbons. Environ. Sci. Pollut. Res. Int. 2013, 20, 3176–3184. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Teixeira, C.; Basto, M.C.P.; Almeida, C.M.R. PAHs levels in Portuguese estuaries and lagoons: Salt marsh plants as potential agents for the containment of PAHs contamination in sediments. Reg. Stud. Mar. Sci. 2016, 7, 211–221. [Google Scholar] [CrossRef]
- Keith, L.H. The Source of U.S. EPA’s sixteen PAH priority pollutants. Polycycl. Aromat. Compd. 2014, 35, 147–160. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Schubert, M.; Lindgreen, S.; Orlando, L. AdapterRemoval v2: Rapid adapter trimming, identification, and read merging. BMC Res. Notes 2016, 9, 88. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic. Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Bragança, I.; Mucha, A.P.; Tomasino, M.P.; Santos, F.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Deltamethrin impact in a cabbage planted soil: Degradation and effect on microbial community structure. Chemosphere 2019, 220, 1179–1186. [Google Scholar] [CrossRef]
- Demertzi, M.; Paulo, J.A.; Faias, S.P.; Arroja, L.; Dias, A.C. Evaluating the carbon footprint of the cork sector with a dynamic approach including biogenic carbon flows. Int. J. Life Cycle Assess. 2018, 23, 1448–1459. [Google Scholar] [CrossRef]
- Azevedo, J.; Antunes, J.T.; Machado, A.M.; Vasconcelos, V.; Leão, P.N.; Froufe, E. Monitoring of biofouling communities in a Portuguese port using a combined morphological and metabarcoding approach. Sci. Rep. 2020, 10, 13461. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Ku, C.-R.; Yeh, N. Solar powered artificial floating island for landscape ecology and water quality improvement. Ecol. Eng. 2014, 69, 8–16. [Google Scholar] [CrossRef]
- Strosnider, W.H.; Schultz, S.E.; Strosnider, K.A.; Nairn, R.W. Effects on the underlying water column by extensive floating treatment wetlands. J. Environ. Qual. 2017, 46, 201–209. [Google Scholar] [CrossRef]
- Henriques, B.; Teixeira, A.; Figueira, P.; Reis, A.T.; Almeida, J.; Vale, C.; Pereira, E. Simultaneous removal of trace elements from contaminated waters by living Ulva lactuca. Sci. Total Environ. 2019, 652, 880–888. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Dias, J.M.; Rodrigues, M.; Leandro, S.; Morgado, F.; Oliveira, A.; Queiroga, H. Caracterização Sinóptica dos Gradientes Ambientais na Ria de Aveiro. In Parte I: Salinidade e Temperatura. Actas das Jornadas da Ria de Aveiro; 2, 3 e 4 de Maio de 2011; Almeida, A., Alves, F.L., Bernardes, C., Dias, J.M., Gomes, N.C.M., Pereira, E., Queiroga, H., Serôdio, J., Vaz, N., Eds.; Universidade de Aveiro, CESAM – Centro de Estudos do Ambiente e do Mar: Aveiro, Portugal, 2011; pp. 141–150, (In Portuguese). ISBN 9789727893379. [Google Scholar]
- Naidoo, G.; Naidoo, Y.; Achar, P. Ecophysiological responses of the salt marsh grass Spartina maritima to salinity. Afr. J. Aqua. Sci. 2012, 37, 81–88. [Google Scholar] [CrossRef]
- Davy, A.J.; Bishop, G.F.; Mossman, H.; Redondo-Gómez, S.; Castillo, J.M.; Castellanos, E.M.; Luque, L.; Figueroa, M.E. Biological flora of the British isles: Sarcocornia perennis (Miller) A.J. Scott. J. Ecol. 2006, 94, 1035–1048. [Google Scholar] [CrossRef]
- Calheiros, C.S.; Silva, G.; Quiterio, P.V.; Crispim, L.F.; Brix, H.; Moura, S.C.; Castro, P.M. Toxicity of high salinity tannery wastewater and effects on constructed wetland plants. Int. J. Phytoremediat. 2012, 14, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.; Kandiah, A.; Mashali, A. The use of saline waters for crop production. In Food and Agriculture Organization Irrigation and Drainage Paper No 48; Food and Agriculture Organization: Rome, Italy, 1992; p. 133. [Google Scholar]
- Gouveia, V.; Almeida, C.M.R.; Almeida, T.; Teixeira, C.; Mucha, A.P. Indigenous microbial communities along the NW Portuguese Coast: Potential for hydrocarbons degradation and relation with sediment contamination. Mar. Pollut. Bull. 2018, 131, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.J.; Dores-Sousa, J.L.; Cruzeiro, C.; Rocha, E. PAHs in water and surface sediments from Douro River estuary and Porto Atlantic coast (Portugal)-impacts on human health. Environ. Monit. Assess. 2017, 189, 425. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Petroleum hydrocarbon profiles of water and sediment of algoa Bay, eastern cape, South Africa. Int. J. Environ. Res. Public Health 2017, 14, 1263. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B.; Moeinpour, A. Aliphatic and polycyclic aromatic hydrocarbons risk assessment in coastal water and sediments of Khark Island, SW Iran. Mar. Pollut. Bull. 2016, 108, 33–45. [Google Scholar] [CrossRef]
- Wattayakorn, G.; Rungsupa, S. Petroleum hydrocarbon residues in the marine environment of Koh Sichang-Sriracha, Thailand. Coast. Mar. Sci. 2012, 35, 122–128. [Google Scholar]
- Li, Y.; Zhao, Y.; Peng, S.; Zhou, Q.; Ma, L.Q. Temporal and spatial trends of total petroleum hydrocarbons in the seawater of Bohai Bay, China from 1996 to 2005. Mar. Pollut. Bull. 2010, 60, 238–243. [Google Scholar] [CrossRef]
- Wang, D.G.; Alaee, M.; Byer, J.; Liu, Y.J.; Tian, C.G. Fugacity approach to evaluate the sediment-water diffusion of polycyclic aromatic hydrocarbons. J. Environ. Monit. 2011, 13, 1589–1596. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Wharmby, C.; Castillo, J.M.; Mateos-Naranjo, E.; Luque, C.J.; de Cires, A.; Luque, T.; Davy, A.J.; Figuero, M.E. Growth and photosynthetic responses to salinity in an extreme halophyte, Sarcocornia fruticose. Physiol. Plant. 2006, 128, 116–124. [Google Scholar] [CrossRef]
- Grigore, M.N.; Villanueva, M.; Boscaiu, M.; Vicente, O. Do halophytes really require salts for their growth and Development? An experimental approach. Not. Sci Biol. 2012, 4, 23–29. [Google Scholar] [CrossRef]
- Von Ammon, U.; Wood, S.A.; Laroche, O.; Zaiko, A.; Tait, L.; Lavery, S.; Inglis, G.; Pochon, X. The impact of artificial surfaces on marine bacterial and eukaryotic biofouling assemblages: A high-throughput sequencing analysis. Mar. Environ. Res. 2018, 133, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lucke, T.; Walker, C.; Beecham, S. Experimental designs of field-based constructed floating wetland studies: A review. Sci. Total Environ. 2019, 660, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.T.; Sousa, A.G.G.; Azevedo, J.; Rego, A.; Leão, P.N.; Vasconcelos, V. Distinct temporal succession of bacterial communities in early marine biofilms in a Portuguese Atlantic Port. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Oliveira, V.; Gomes, N.C.; Cleary, D.F.; Almeida, A.; Silva, A.M.; Simoes, M.M.; Silva, H.; Cunha, A. Halophyte plant colonization as a driver of the composition of bacterial communities in salt marshes chronically exposed to oil hydrocarbons. FEMS Microbiol. Ecol. 2014, 90, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, W.; Li, Y.X.; Tam, C.; Bougouff, S.; Wang, R.; Pei, B.; Chiang, H.; Leung, P.; Lu, Y. Marine biofilms constitute a bank of hidden microbial diversity and functional potential. Nat. Commun. 2019, 10, 517. [Google Scholar] [CrossRef]
- Briand, J.F.; Djeridi, I.; Jamet, D.; Coupe, S.; Bressy, C.; Molmeret, M.; Le Berre, B.; Rimet, F.; Bouchez, A.; Blache, Y. Pioneer marine biofilms on artificial surfaces including antifouling coatings immersed in two contrasting French Mediterranean coast sites. Biofouling 2012, 28, 453–463. [Google Scholar] [CrossRef]
- Muthukrishnan, T.; Abed, R.M.; Dobretsov, S.; Kidd, B.; Finnie, A.A. Long-term microfouling on commercial biocidal fouling control coatings. Biofouling 2014, 30, 1155–1164. [Google Scholar] [CrossRef]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal dynamics of bacterial and fungal colonization on plastic debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef]
- Poehlein, A.; Yutin, N.; Daniel, R.; Galperin, M.Y. Proposal for the reclassification of obligately purine-fermenting bacteria Clostridium acidurici (Barker 1938) and Clostridium purinilyticum (Durre et al. 1981) as Gottschalkia acidurici gen. nov. comb. nov. and Gottschalkiapurinilytica comb. nov. and of Eubacterium angustum (Beuscher and Andreesen 1985) as Andreesenia angusta gen. nov. comb. nov. in the family Gottschalkiaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 2711–2719. [Google Scholar]
- Nedashkovskaya, O.I.; Kim, S.B.; Han, S.K.; Lysenko, A.M.; Rohde, M.; Rhee, M.S.; Frolova, G.M.; Falsen, E.; Mikhailov, V.V.; Bae, K.S. Maribacter gen. nov., a new member of the family Flavobacteriaceae, isolated from marine habitats, containing the species Maribacter sedimenticola sp. nov., Maribacter aquivivus sp. nov., Maribacter orientalis sp. nov. and Maribacter ulvicola sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.W.; Weiss, A.; Kuegler, S.; Hermes, C.; Wichard, T. Macroalgal-bacterial interactions: Role of dimethylsulfoniopropionate in microbial gardening by Ulva (Chlorophyta). Mol. Ecol. 2018, 27, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Fukunaga, Y.; Nakagawa, Y.; Harayama, S. Emended descriptions of the genus Lewinella and of Lewinella cohaerens, Lewinella nigricans and Lewinella persica, and description of Lewinella lutea sp. nov. and Lewinella marina sp. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2946–2951. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, S.J.; Nielsen, P.H. The family saprospiraceae. The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: New York, NY, USA, 2014; pp. 863–889. [Google Scholar]
- Wang, M.; Chen, J.-K.; Li, B. Characterization of bacterial community structure and diversity in rhizosphere soils of three plants in rapidly changing salt marshes using 16S rDNA. Pedosphere 2007, 17, 545–556. [Google Scholar] [CrossRef]



| Date (year/month) | Water Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Cond. (mS/cm) | Salinity (ppt) | pH | PO43− (mg/L) | NH4+ (mg/L) | NO2− (mg/L) | NO3− (mg/L) | TPHs (mg/L) | COD (mg/L) | ||
| 2018 | April | 15.4 | 47.4 | 30.7 | 8.03 | 0.09 | 0.14 | 0.05 | 1.35 | <d.l. | 135 |
| May | 14.0 | 49.3 | 32.1 | 7.86 | 0.13 | 0.19 | 0.08 | 2.20 | <d.l. | 210 | |
| June | 15.3 | 47.3 | 30.4 | 7.93 | 0.11 | 0.07 | 0.05 | 1.00 | <d.l. | 270 | |
| July | 19.8 | 44.9 | 28.8 | 8.25 | 0.07 | 0.06 | 0.03 | 0.83 | <d.l. | 150 | |
| August | 18.5 | 46.6 | 30.0 | 8.21 | 0.10 | 0.05 | 0.03 | 0.38 | <d.l. | 210 | |
| September | 17.2 | 48.8 | 31.6 | 7.89 | 0.53 | 0.34 | 0.03 | 1.27 | <d.l. | 375 | |
| 2019 | January | 13.8 | 51.8 | 33.5 | 8.37 | 0.12 | <d.l. | 0.04 | 1.31 | 6 | 630 |
| February | 13.1 | 51.7 | 33.3 | 8.33 | 0.11 | 0.16 | 0.04 | 0.93 | 5 | 225 | |
| March | 13.5 | 54.2 | 35.1 | 8.09 | 0.13 | 0.14 | 0.04 | 1.36 | <d.l. | 435 | |
| April | 14.1 | 44.3 | 28.1 | 8.10 | 0.17 | 0.59 | 0.31 | 3.91 | <d.l. | 360 | |
| May | 15.2 | 49.7 | 32.0 | 7.97 | 0.31 | 0.68 | 0.22 | 3.03 | <d.l. | 330 | |
| June | 16.3 | 53.2 | 34.6 | 8.05 | 0.15 | 0.30 | 0.08 | 1.13 | 4 | 375 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calheiros, C.S.C.; Carecho, J.; Tomasino, M.P.; Almeida, C.M.R.; Mucha, A.P. Floating Wetland Islands Implementation and Biodiversity Assessment in a Port Marina. Water 2020, 12, 3273. https://doi.org/10.3390/w12113273
Calheiros CSC, Carecho J, Tomasino MP, Almeida CMR, Mucha AP. Floating Wetland Islands Implementation and Biodiversity Assessment in a Port Marina. Water. 2020; 12(11):3273. https://doi.org/10.3390/w12113273
Chicago/Turabian StyleCalheiros, Cristina S. C., João Carecho, Maria P. Tomasino, C. Marisa R. Almeida, and Ana P. Mucha. 2020. "Floating Wetland Islands Implementation and Biodiversity Assessment in a Port Marina" Water 12, no. 11: 3273. https://doi.org/10.3390/w12113273
APA StyleCalheiros, C. S. C., Carecho, J., Tomasino, M. P., Almeida, C. M. R., & Mucha, A. P. (2020). Floating Wetland Islands Implementation and Biodiversity Assessment in a Port Marina. Water, 12(11), 3273. https://doi.org/10.3390/w12113273