First Captive Breeding Program for the Endangered Pyrenean Sculpin (Cottus hispaniolensis Bacescu-Master, 1964)
Abstract
1. Introduction
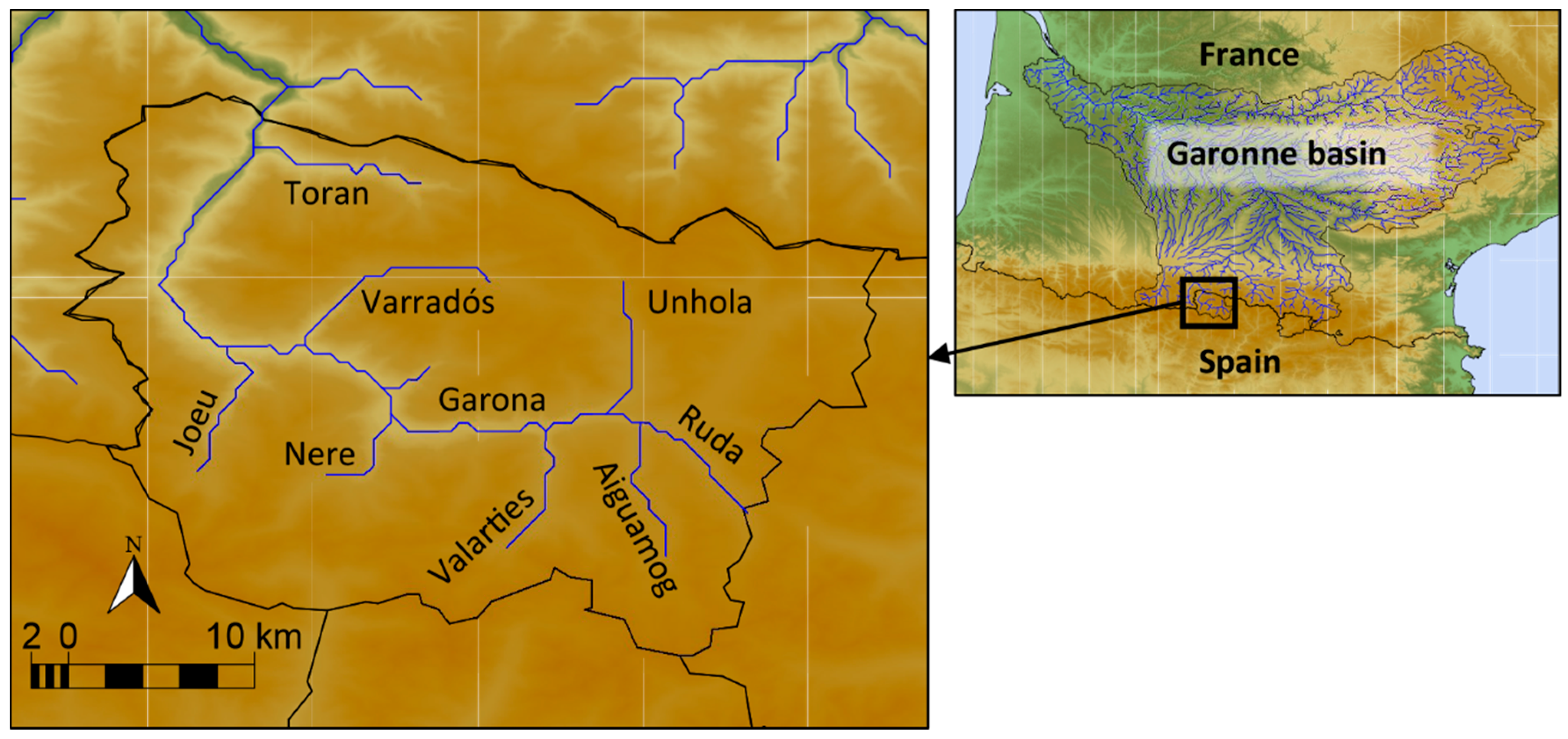
2. Materials and Methods
2.1. Brood-Stock Transportation and Maintenence
2.2. Reproductive Cycle and Data Analysis
3. Results
4. Discussion
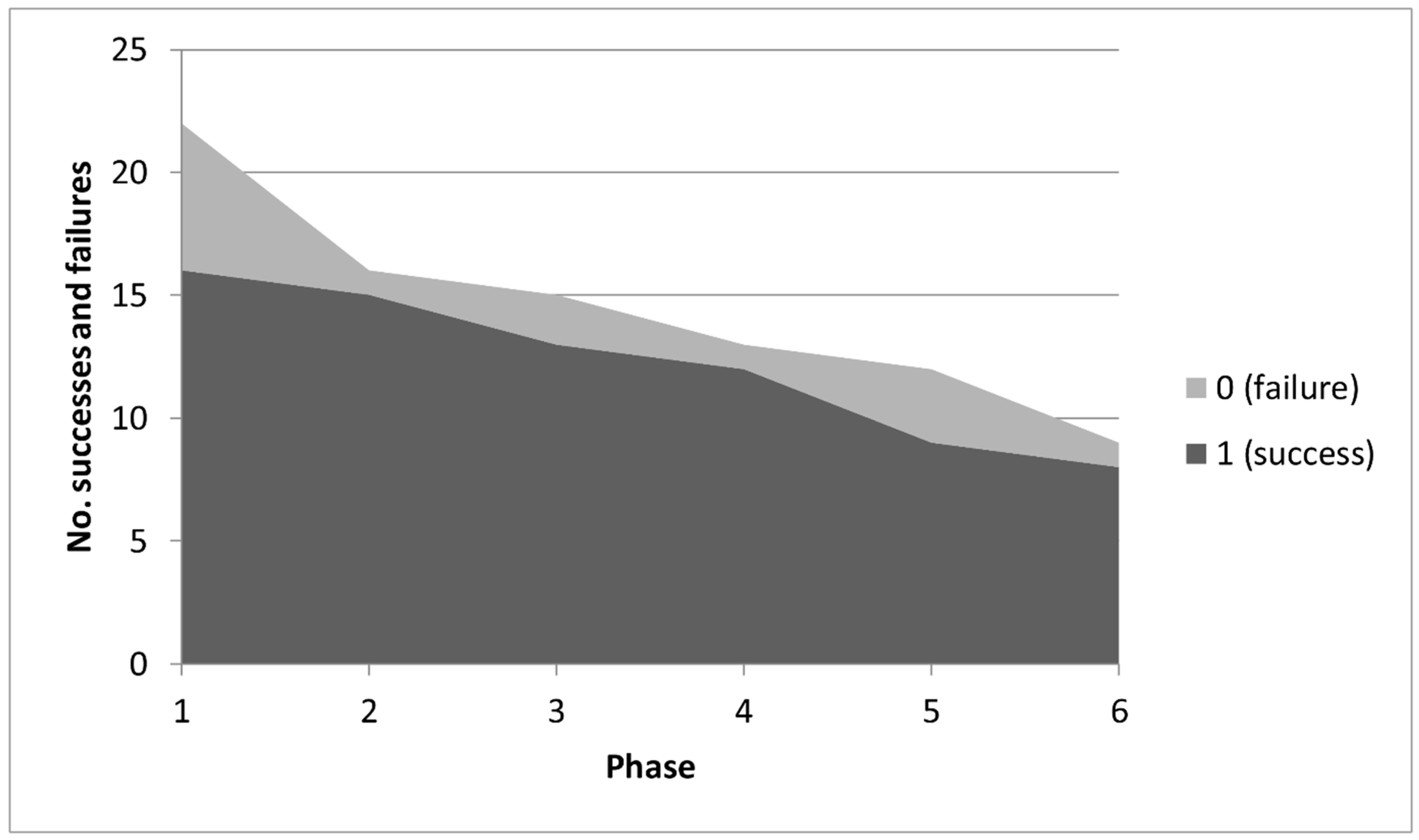
4.1. Analysis of Variation into Phases
4.2. Key Factors Affecting Success
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Freyhof, J.; Brooks, E. European Red List of Freshwater Fishes; Publications office of the European Union: Luxembourg, 2011. [Google Scholar]
- Maceda-Veiga, A. Towards the conservation of freshwater fish: Iberian Rivers as an example of threats and management practices. Rev. Fish Biol. Fish. 2013, 23, 1–22. [Google Scholar] [CrossRef]
- Miranda, R.; Pino-Del-Carpio, A. Analysing freshwater fish biodiversity records and respective conservation areas in Spain. J. Appl. Ichthyol. 2016, 32, 240–248. [Google Scholar] [CrossRef]
- Freyhof, J.; Kottelat, M.; Nolte, A. Taxonomic diversity of European Cottus with description of eight new species (Teleostei: Cottidae). Ichthyol. Explor. Freshw. 2005, 16, 107–172. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Kottelat: Cornol, Switzerland; Cornol and Freyhof: Berlin, Germany, 2007. [Google Scholar]
- Keith, P.; Persat, H.; Feunteun, É.; Allardi, J. Les Poissons d’eau douce de France. Collection Inventaires & Biodiversité; Biotope: Mèze, France, 2011. [Google Scholar]
- Sideleva, V.G. A new sculpin species Cottus sabaudicus sp. nova (Scorpaeniformes: Cottidae) from the Savoy district, France. J. Ichthyol. 2009, 49, 209–214. [Google Scholar] [CrossRef]
- Englbrecht, C.C.; Freyhof, J.; Nolte, A.; Rassmann, K.; Schliewen, U.; Tautz, D. Phylogeography of the bullhead Cottus gobio (Pisces: Teleostei: Cottidae) suggests a pre-Pleistocene origin of the major central European populations. Mol. Ecol. 2000, 9, 709–722. [Google Scholar] [CrossRef]
- Volckaert, F.A.M.; Hänfling, B.; Hellemans, B.; Carvalho, G.R. Timing of the population dynamics of bullheadCottus gobio (Teleostei: Cottidae) during the Pleistocene. J. Evol. Biol. 2002, 15, 930–944. [Google Scholar] [CrossRef]
- Knaepkens, G.; Baekelandt, K.; Eens, M. Assessment of the movement behaviour of the bullhead (Cottus gobio), an endangered European freshwater fish. Anim. Biol. 2005, 55, 219–226. [Google Scholar] [CrossRef]
- Petrova Uzunova, E. Assessment of the conservation status of endemic sculpin Cottus haemusi (Cottidae) in the river Vit (Danube Tributary), northwest Bulgaria. Knowl. Managt. Aquatic Ecosyst. 2011, 403, 10. [Google Scholar] [CrossRef]
- Sousa-Santos, M.; Robalo, J.I.; Pereira, A.; Doadrio, I. Threatened fishes of the world: Cottus aturi Freyhof, Kottelat and Nolte 2005 (Cottidae). Croat. J. Fish. 2014, 72, 130–131. [Google Scholar] [CrossRef][Green Version]
- Sousa-Santos, M.; Robalo, J.I.; Pereira, A.; Doadrio, I. Threatened fishes of the world: Cottus hispaniolensis Bacescu-Mester, 1964 (Cottidae). Croat. J. Fish. 2014, 72, 132–133. [Google Scholar] [CrossRef]
- Shaffer, M.L. Minimum Population Sizes for Species Conservation. BioScience 1981, 31, 131–134. [Google Scholar] [CrossRef]
- Meffe, G.K.; Carroll, C.R. Principles of Conservation Biology; Sinauer Associates: Sunderland, MA, USA, 1997. [Google Scholar]
- IUCN/SCC. IUCN Species Survival Commission Guidelines on the Use of Ex Situ Management for Species Conservation: Version 2.0; IUCN Species Survival Commission: Gland, Switzerland, 2014. [Google Scholar]
- Rocaspana, R.; Aparicio, E. Population trends and current status of the endangered Pyrenean sculpin Cottus hispaniolensis in the Spanish part of the Garonne drainage. Know. Manag. Aquat. Ecosyst. 2017, 25. [Google Scholar] [CrossRef][Green Version]
- Freyhof, J.; Kottelat, M. Cottus Hispaniolensis. The IUCN Red List of Threatened Species 2008. Available online: https://www.iucnredlist.org/species/135573/4149912 (accessed on 19 March 2020).
- Doadrio, I.; Perea, S.; Garzón-Heydt, P.; González, J. Ictiofauna continental española. Bases para su seguimiento; DG Medio Natural y Política Forestal. MARM: Madrid, Spain, 2011. [Google Scholar]
- European Commission. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, L 206, 7–50. [Google Scholar]
- Gobierno de España, Ministerio de Medio Ambiente, y Medio Rural y Marino. Real Decreto 139/2011, de 4 de febrero, para el desarrollo del Listado de Especies Silvestres en Régimen de Protección Especial y del Catálogo Español de Especies Amenazadas. BOE 2011, 46, 20912–20951. [Google Scholar]
- Pineda, N.; Prohom, M.; Serra, A.; Martí, G.; Garcia, C.; Velasco, E.; Gracia, A. Causes que van provocar la riuada a la Val d’Aran el 18 de juny 2013. In Proceedings of the Jornada La Gestió de les Inundacions, Barcelona, Spain, 27–28 November 2013; pp. 120–125. [Google Scholar]
- Confederación Hidrográfica del Ebro. Informe de la Avenida del 17 al 20 de Junio de 2013 en la Cuenca del río Garona (689/377); Confederación Hidrográfica del Ebro: Zaragoza, Spain, 2014. [Google Scholar]
- Victoriano, A.; García-Silvestre, M.; Furdada, G.; Bordonau, J. Long-term entrenchment and consequences for present flood hazard in the Garona River (Val d’Aran, Central Pyrenees, Spain). Nat. Hazards Earth Syst. Sci. 2016, 16, 2055–2070. [Google Scholar] [CrossRef]
- Knaepkens, G.; Bervoets, L.; Verheyen, E.; Eens, M. Relationship between population size and genetic diversity in endangered populations of the European bullhead (Cottus gobio): Implications for conservation. Biol. Conserv. 2004, 115, 403–410. [Google Scholar] [CrossRef]
- Snyder, N.F.R.; Derrickson, S.R.; Beissinger, S.R.; Wiley, J.W.; Smith, T.B.; Toone, W.D.; Miller, B. Limitations of Captive Breeding in Endangered Species Recovery. Conserv. Biol. 1996, 10, 338–348. [Google Scholar] [CrossRef]
- Robert, A. Captive breeding genetics and reintroduction success. Biol. Conserv. 2009, 142, 2915–2922. [Google Scholar] [CrossRef]
- McGowan, P.J.K.; Traylor-Holzer, K.; Leus, K. IUCN Guidelines for Determining When and How Ex Situ Management Should Be Used in Species Conservation. Conserv. Lett. 2016, 10, 361–366. [Google Scholar] [CrossRef]
- Philippart, J.C. Is captive breeding an effective solution for the preservation of endemic species? Biol. Conserv. 1995, 72, 281–295. [Google Scholar] [CrossRef]
- Dolman, P.M.; Collar, N.J.; Scotland, K.M.; Burnside, R.J. Ark or park: The need to predict relative effectiveness of ex situ and in situ conservation before attempting captive breeding. J. Appl. Ecol. 2015, 52, 841–850. [Google Scholar] [CrossRef]
- Araki, H.; Cooper, B.; Blouin, M.S. Genetic Effects of Captive Breeding Cause a Rapid, Cumulative Fitness Decline in the Wild. Science 2007, 318, LP100–LP103. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.A.; Pavajeau, L. Captive Breeding, Reintroduction, and the Conservation of Amphibians. Conserv. Biol. 2008, 22, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.J.; Eldridge, M.D.B.; Morris, K.; Thomas, N.; Herbert, C.A. Captive management and the maintenance of genetic diversity in a vulnerable marsupial, the greater bilby. Aust. Mammal. 2015, 37. [Google Scholar] [CrossRef]
- ADEFFA. El cavilat. Conèixer-lo per conservar-lo [Documentary]; ADEFFA: Barcelona, Spain, 2016. [Google Scholar]
- Thoen, E.; Evensen, Ø.; Skaar, I. Pathogenicity of Saprolegnia spp. to Atlantic salmon, Salmo salar L., eggs. J. Fish Dis. 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Vught, I.; De Charleroy, D.; Van Liefferinge, C.; Coenen, E.; Coeck, J. Conservation of bullhead Cottus perifretum in the Demer River (Belgium) basin using re?introduction. J. Appl. Ichthyol. 2011, 27, 60–65. [Google Scholar] [CrossRef]
- Piccinini, A.; Bilò, F.; Stefani, L.; Marzano, F.N. Artificial breeding of bullhead Cottus gobio in two full recirculation systems. Ital. J. Freshw. Ichthyol. 2017, 1. Available online: http://www.aiiad.it/ijfi/index.php/ijfi/article/view/35 (accessed on 19 June 2020).
- Morris, D. The reproductive behaviour of the river bull-head (Cottus gobio L.), with special reference to the fanning activity. Behaviour 1954, 7, 1–32. [Google Scholar] [CrossRef]
- Smith, S.; Armstrong, R.A.; Springate, J.; Barker, G. Infection and colonization of trout eggs by Saprolegniaceae. Trans. Br. Mycol. Soc. 1985, 85, 719–723. [Google Scholar] [CrossRef]
- Khoo, L. Fungal diseases in fish. Semin. Avian Exot. Pet Med. 2000, 9, 102–111. [Google Scholar] [CrossRef]
- Fregeneda-Grandes, J.M.; Rodríguez-Cadenas, F.; Aller-Gancedo, J.M. Fungi isolated from cultured eggs, alevins and broodfish of brown trout in a hatchery affected by saprolegniosis. J. Fish Biol. 2007, 71, 510–518. [Google Scholar] [CrossRef]
- Bisazza, A.; Marconato, A. Female mate choice, male-male competition and parental care in the river bullhead, Cottus gobio L. (Pisces, Cottidae). Anim. Behav. 1988, 36, 1352–1360. [Google Scholar] [CrossRef]
- Marconato, A.; Bisazza, A. Mate choice, egg cannibalism and reproductive success in the river bullhead, Cottus gobio L. J. Fish Biol. 1988, 33, 905–916. [Google Scholar] [CrossRef]
- Goto, A. Male mating success and female mate choice in the river sculpin, Cottus nozawae (Cottidae). Environ. Biol. Fishes 1993, 37, 347–353. [Google Scholar] [CrossRef]
- Abdoli, A.; Pont, D.; Sagnes, P. Influence of female age, body size and environmental conditions on annual egg production of the bullhead. J. Fish. Biol. 2005, 67, 1327–1341. [Google Scholar] [CrossRef]
- Natsumeda, T.; Mori, S.; Yuma, M. Size-mediated dominance and aggressive behavior of male Japanese fluvial sculpin Cottus pollux (Pisces: Cottidae) reduce nest-site abundance and mating success of conspecific rivals. J. Ethol. 2012, 30, 239–245. [Google Scholar] [CrossRef]
- Shumway, C.A. A neglected science: Applying behavior to aquatic conservation. Environ. Biol. Fishes 1999, 55, 183–201. [Google Scholar] [CrossRef]
- Brown, L. Patterns of female choice in mottled sculpins (Cottidae, teleostei). Anim. Behav. 1981, 29, 375–382. [Google Scholar] [CrossRef]
- Teixeira, C.P.; De Azevedo, C.S.; Mendl, M.; Cipreste, C.F.; Young, R.J. Revisiting translocation and reintroduction programmes: The importance of considering stress. Anim. Behav. 2007, 73, 1–13. [Google Scholar] [CrossRef]
- Raghavan, R.; Philip, S.; Ali, A.; Katwate, U.; Dahanukar, N. Fishery, biology, aquaculture and conservation of the threatened Asian Sun catfish. Rev. Fish Biol. Fish. 2016, 26, 169–180. [Google Scholar] [CrossRef]
- Oláh, J.; Farkas, J. Effect of temperature, pH, antibiotics, formalin and malachite green on the growth and survival of Saprolegnia and Achlya parastic on fish. Aquaculture 1978, 13, 273–288. [Google Scholar] [CrossRef]
- Heikkinen, J.; Tiirola, M.; Mustonen, S.M.; Eskelinen, P.; Navia-Paldanius, D.; Von Wright, A. Suppression of Saprolegnia infections in rainbow trout (Oncorhynchus mykiss) eggs using protective bacteria and ultraviolet irradiation of the hatchery water. Aquac. Res. 2016, 47, 925–939. [Google Scholar] [CrossRef]
- Martínez-Palacios, C.A.; Morte, J.C.; Tello-Ballinas, J.A.; Toledo-Cuevas, M.; Ross, L.G. The effects of saline environments on survival and growth of eggs and larvae of Chirostoma estor estor Jordan 1880 (Pisces: Atherinidae). Aquaculture 2004, 238, 509–522. [Google Scholar] [CrossRef]
- Matthews, R.A. Ichthyophthirius multifiliis Fouquet and Ichthyophthiriosis in Freshwater Teleosts. Adv. Parasitol. 2005, 59, 159–241. [Google Scholar] [CrossRef] [PubMed]
- Marconato, A.; Bisazza, A.; Fabris, M. The cost of parental care and egg cannibalism in the river bullhead, Cottus gobio L. (Pisces, Cottidae). Behav. Ecol. Sociobiol. 1993, 32, 229–237. [Google Scholar] [CrossRef]
- Willis, K.; Willis, R.E. How many founders, how large a population? Zoo Biol. 2010, 29, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Valbuena-Ureña, E.; Soler-Membrives, A.; Steinfartz, S.; Alonso, M.; Carbonell, F.; Larios-Martín, R.; Obon, E.; Carranza, S. Getting off to a good start? Genetic evaluation of the ex situ conservation project of the Critically Endangered Montseny brook newt (Calotriton arnoldi). PeerJ 2017, 5, e3447. [Google Scholar] [CrossRef]
- Duchesne, P.; Bernatchez, L. An analytical investigation of the dynamics of inbreeding in multi-generation supportive breeding. Conserv. Genet. 2002, 3, 45–58. [Google Scholar] [CrossRef]
- Williams, S.E.; Hoffman, E.A. Minimizing genetic adaptation in captive breeding programs: A review. Biol. Conserv. 2009, 142, 2388–2400. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and conservation biology. Comptes Rendus Biol. 2003, 326, 22–29. [Google Scholar] [CrossRef]
| Phase Name | Description | Success Criteria |
|---|---|---|
| Nesting behaviour | In males: when the yellow margin of the first dorsal fin contrasts against the black overall coloration. | If male constructs and guards the gravel cavity (the cave) under the stones or tiles. |
| In females: when ovulation process occurs. | If female is gravid (full of completed eggs). | |
| Courtship (sexual behaviour) | When the male attracts the female to the cave and ‘hugs’ her during hours until laying finishes. | If sexual behaviour occurs (external and internal courtship) and the eggs are fertilized. |
| Fixation | While female is laying, eggs are fixed by gelatinous matrix in an oval compact clutch. | If the clutch is fixed on the ceiling of the cave (fixation substrate is indicated to evaluate this phase). |
| Parental care (incubation) | The male ventilates (fanning activity) and guards the eggs from predators and infections. | If the male takes care of the eggs at least some days (number of days are indicated to assess this success). |
| Hatching | When larvae go out of the egg (it takes hours). They are little mobile and feed on the vitelline sack few days until they become juveniles. | If at least one individual hatches. It is assessed with the hatching percentage. (Nlaid eggs/Nlive hatched alevins) |
| Survival | Survival during the juvenile development, 3 months after the hatching. | If at least one juvenile survives. It is assessed with the survival rate. |
| Year | No. Breeders | Code Couple | Reproductive Phases | No. Fitness | |||||
|---|---|---|---|---|---|---|---|---|---|
| Nesting | Courtship | Fixation | Incubation | Hatching | Survival | ||||
| 2006 | 4 | 1 | Yes | Yes | Yes (stone) | Yes (21 days) | Yes (49%) | Yes (14%) | 14 |
| 2 | No | - | - | - | - | - | - | ||
| 2009 | 8 | 3 | Yes | Yes | Yes (stone) | Yes (4 days) | No (0%) | - | - |
| 4 | Yes | Yes | Yes (stone) | Yes (3 days) | No (0%) | - | - | ||
| 5 | No | - | - | - | - | - | - | ||
| 6 (F ‡, M ‡) | Yes § | Yes | Yes (glass) | No | - | - | - | ||
| 2014 | 7 | 7 | Yes | Yes | Yes (tile) | Yes (12 days) | Yes (73%) | Yes (94%) | 170 |
| 8 | No | - | - | - | - | - | - | ||
| 9 | No | - | - | - | - | - | - | ||
| 2015 | 8 | 10 (F †, M †) | Yes | Yes | Yes (stone) | Yes (7 days) | Yes (7%) | Yes (100%) | 4 |
| 11 (F †, M †) | No | - | - | - | - | - | - | ||
| 12 (F †, M †) | Yes | Yes | No | - | - | - | - | ||
| 13 (F ‡, M †) | Yes § | Yes | No | - | - | - | - | ||
| 2017 | 9 | 14 | Yes | Yes | Yes (tile) | Yes (11 days) | Yes (36%) | No (0%) | - |
| 15 (F ‡) | Yes | Yes | Yes (tile) | Yes (11 days) | Yes (29%) | Yes (100%) | 16 | ||
| 16 | Yes | No | - | - | - | - | - | ||
| 17 | Yes | Yes | Yes (tile) | Yes (5 days) | Yes (41%) | Yes (93%) | 75 | ||
| 18 | No | - | - | - | - | - | - | ||
| 2018 | 9 | 19 (F ‡) | Yes | Yes | Yes (stone) | Yes (5 days) | No (0%) | - | - |
| 20 | Yes | Yes | Yes (stone) | Yes (9 days) | Yes (9%) | Yes (14%) | 2 | ||
| 21 | Yes | Yes | Yes (tile) | Yes (22 days) | Yes (56%) | Yes (95%) | 212 | ||
| 22 | Yes | Yes | Yes (tile) | Yes (13 days) | Yes (60%) | Yes (96%) | 115 | ||
| % initial success | 73% | 68% | 59% | 55% | 41% | 36% | |||
| % phase success | 73% | 94% | 87% | 92% | 75% | 89% | |||
| Model | p-Value | d.f. | AIC |
|---|---|---|---|
| Exit ~ Phase + Type + Year | 0.442 | 12 | 82.232 |
| Exit ~ Phase + Type | 0.817 | 8 | 77.976 |
| Exit ~ Phase + Year | 0.769 | 9 | 79.094 |
| Exit ~ Phase | 0.595 | 5 | 72.913 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manubens, J.; Comas, O.; Valls, N.; Benejam, L. First Captive Breeding Program for the Endangered Pyrenean Sculpin (Cottus hispaniolensis Bacescu-Master, 1964). Water 2020, 12, 2986. https://doi.org/10.3390/w12112986
Manubens J, Comas O, Valls N, Benejam L. First Captive Breeding Program for the Endangered Pyrenean Sculpin (Cottus hispaniolensis Bacescu-Master, 1964). Water. 2020; 12(11):2986. https://doi.org/10.3390/w12112986
Chicago/Turabian StyleManubens, Joan, Oriol Comas, Núria Valls, and Lluís Benejam. 2020. "First Captive Breeding Program for the Endangered Pyrenean Sculpin (Cottus hispaniolensis Bacescu-Master, 1964)" Water 12, no. 11: 2986. https://doi.org/10.3390/w12112986
APA StyleManubens, J., Comas, O., Valls, N., & Benejam, L. (2020). First Captive Breeding Program for the Endangered Pyrenean Sculpin (Cottus hispaniolensis Bacescu-Master, 1964). Water, 12(11), 2986. https://doi.org/10.3390/w12112986




