Abstract
Hydromorphological alterations influence a wide range of environmental conditions as well as riparian vegetation and the structure of the macroinvertebrate community. We studied relationships between the structure and diversity of the macroinvertebrate community and hydromorphological and other environmental conditions in the river Gradaščica (central Slovenia). The Gradaščica river is a pre-Alpine torrential river that has been morphologically altered by humans. A selection of abiotic factors was measured, the ecomorphological status of the river was assessed, vegetation in the riparian zone was surveyed and benthic macroinvertebrates were sampled. Correlations between diversity and the structure of the macroinvertebrate community, environmental parameters and occurrence of invasive alien plant species in the riparian zone were identified. The significance of the influence of environmental parameters on the structure of the macroinvertebrate community was examined. We found that hydromorphological alterations in the river have had a significant influence on the diversity and composition of the macroinvertebrate community because of changes of flow velocity and the spread of invasive alien plant species that has followed those changes. Factors that also significantly influence the composition of macroinvertebrate community are distance from the source and conductivity. Our findings suggest minimization of further human hydromorphological changes of watercourses could prevent the loss of biodiversity of riverine ecosystems.
1. Introduction
Stream ecosystems harbor exceptional levels of biodiversity, despite their small spatial coverage of the Earth’s surface [1]. The degradation of physical habitat through hydromorphological alterations and homogenization by humans is thought to be the most significant threat to river biodiversity, ecosystem functionality and stability [2,3,4,5,6]. Morphological alterations include changes of the banks removing of riparian vegetation and various alterations of the riverbed, such as dams or reinforcements with concrete and/or rocks, all of which negatively influence the biodiversity [7].
Numerous dams have been built to manage the flow, but the subsequent accumulation of water is accompanied by accumulation of sediment, nutrients and pollutants, such as metals [8]. Dams disrupt the longitudinal pattern of riverine communities [9], however, since they impede the transport of organic matter and biota, consequently influencing the species richness and health of the river ecosystem [10,11]. In the summer, surface-release dams raise the temperature of the water downstream by increasing both the residence time of water and the surface area of water exposed to solar radiation [12]. Lessard and Hayes [13] found an increase in temperature of the water of up to 5 °C during the summer, as result of small dams on rivers and streams in Michigan (USA).
Riparian vegetation is the main source of organic matter for aquatic biota, especially in the headwaters. It minimizes erosion and provides shade and shelter [8,10,11,14], but must have sufficient width and structure [14]. Where this vegetation has been destroyed, or the bank-structure has been changed, the riparian zone is frequently invaded by alien species [10,11], which establish thick stands and change its functionality [15]. The vulnerability of riparian zones to invasion of alien plant species (IAS) depends on its structure [16,17], i.e., its width, connectivity and vegetation composition. Human alterations of the riparian zone change abiotic factors such as substrate types, concentration of oxygen, pH and water temperature [18,19] as well as interactions between organisms [15,20]. Alteration of the flow regime cause serious degradation of riverine ecosystems [21]. Removal of riparian vegetation can significantly affect the riverine food web [20] and alters the structure and diversity of the macroinvertebrate community and functional feeding groups [22,23]. Macroinvertebrates are strongly influenced by pollutants as well as by properties of the riparian vegetation [24,25] and have been used to evaluate hydromorphological changes of the river channel according to Water Framework Directive [26]. The aim of this Directive [26] is to retain or attain the good ecological status of a watercourse. Changes in the habitat structure affect the abundance of specific taxonomic groups [20]. The diversity of substrate types presents diverse interstitial spaces between particles that provide macroinvertebrates with shelter from the fast flow of the water [19], but if the stream has been recently altered and/or channelized, it usually cannot provide heterogeneous mesohabitats [27].
There are few studies on ecologically significant features associated with river morphology [28] and our understanding of the links between river ecology and hydromorphology is still incomplete [22,29]. Friberg [30] has reported that surprisingly few studies have documented clear impacts of habitat degradation on riverine communities. Only a few contributions (e.g., [27,31]) have used macroinvertebrates in attempts to quantify environmental factors changed by the channelization. Buffagni et al. [32] explain that the impacts that are discussed occur mostly in combination with other types of pressure. Invertebrate communities are unresponsive to river morphology in polluted water, but in water of moderate and good quality, morphological degradation, instream or riparian becomes a major discriminatory factor [33].
In this study, we analyzed the relationships between measured environmental variables and various diversity measures. The aims of this research include analysis of the relationships between hydromorphological alterations, structure of riparian vegetation, occurrence of invasive alien species (IAS) of plants in the riparian zone and the structure of the macroinvertebrate community. We evaluated how well stream-riparian conditions that were rapidly assessed by riparian, channel and environmental (RCE) inventory [14] and presence of IAS could explain the variations of diversity and structure of the macroinvertebrate community. We hypothesized that morphological alterations that influence the current velocity and occurrence of IAS in the riparian zone are negatively correlated with the diversity of the macroinvertebrate community, which is positively correlated with the abundance of native woody vegetation.
2. Materials and Methods
2.1. Study Area
River Gradaščica is a tributary of the Ljubljanica River in central Slovenia (Figure 1A), which is an important part of the Sava and Danube catchment area. The length of the river is 39 km, its catchment area is 181 km2, and it has a mean discharge of 2.27 m3 s−1. It originates in the Polhograjsko hribovje hills (46°3′8″ N, 14°13′21″ E) about 500 m above sea level. The bedrock of the catchment area consists of alternating layers of Paleozoic sandstones and marls, dolomite and gravel deposits. A high proportion of the catchment area, especially the valleys, is composed of impervious bedrock types, and the river has a torrential character with a high increase in its rate of discharge during rain events. The lowland part of the Gradaščica River has formed a loamy alluvial plain, which is frequently flooded, and this becomes a major problem for inhabitants of the densely populated area along the lowland section, including the western part of the city of Ljubljana. With the growth of settlements in the catchment area, the situation has deteriorated, and several measures have been taken to prevent flooding, resulting in alteration of the river channel as well as the banks in the riparian zone.
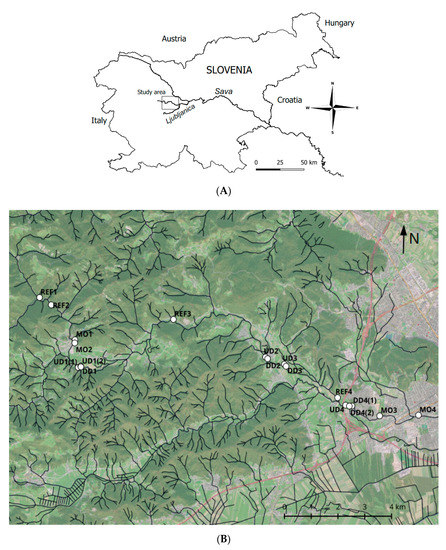
Figure 1.
Map of the study area and sampling sites on the river Gradaščica: (A) study area within Slovenia; (B) locations of the sampling sites along the river Gradaščica. Reference sites without morphological alterations: REF1, REF2, REF3, REF4; most modified sites: MO1, MO2, MO3, MO4; sites upstream dams: UD1(1), UD1(2), UD2, UD3, UD4; sites downstream dams: DD1, DD2, DD3, DD4(1), DD4(2).
On one hand, there are morphologically degraded sections of the channelized stream, and on the other hand, there are numerous small dams creating impoundments and lentic habitats within the torrential river (Figure 1B).
2.2. Sampling and Analysis
The sampling sites represent 100-m reaches, and samples were collected at the end of June 2015. We selected 18 sampling sites (Figure 1B) with contrasting morphological conditions—sites with different levels of morphological alterations (see Table 1) as well as reference sites occurring within reaches where no human alterations have been made. Sampling sites REF1–REF4 are reference sites, and MO1–MO4 refer to sampling sites where the stream has been channelized and reinforced. Sampling sites UD1(1–2), UD2–UD4, denote sites upstream of the dam, while DD1–DD3 and DD4(1–2) are sites downstream of the dam.

Table 1.
Minimal, mean and maximal values of measured abiotic factors on sampling sites in specific types of river reach, according to their rate of hydromorphological alteration. REF—reference sites, UD—sites upstream dam, DD—sites downstream dam, MO—morphologically most altered sites—channelized and paved with stones and/or concrete.
Several abiotic factors including water depth and mean flow velocity were measured. The mean flow velocity was obtained by measuring the velocity at 60 % of the total depth (Hydro-Bios, Kiel, Germany). Key parameters of water quality, including pH, oxygen saturation and concentration, temperature and conductivity were recorded on site using portable meters (Eutech Instruments, PCD 650, Singapore).
We estimated the percentage of cover and calculated the proportions of woody plant species and IAS in the riparian zone within 10 m from the wetted channel. We assessed the ecomorphological conditions in riparian zone and stream channel with an RCE inventory protocol by Petersen [14]. We surveyed 100 m of the representative reach at every sampling site. In case of upstream dam (UD) sites, we assessed a reach 100 m above the dam, and 100 m below the dam in case of downstream (DD) sites. We estimated the following RCE characteristics according to Petersen [14], where detailed descriptions are found: RCE1—Land-use pattern beyond the riparian zone (RZ), RCE2—Width of RZ, RCE3—Completeness of RZ, RCE4—Vegetation structure of RZ within a 10 m channel, RCE5—Retention devices, RCE6—Channel structure/transection, RCE7—Channel sediments, RCE8—Bank structure, RCE9—Bank undercutting, RCE10—Stony substrate appearance, RCE11—Stream bottom interstices, RCE12—riffles and pools, or meanders. In all characteristics, the highest values signifie the most preserved or near-natural status, while the lowest values characterize most human-altered or most degraded environment. Similarly, like Suriano et al. [34], we also calculated sums of scores of various groups of the estimated metrics. The sum of the RCE(2–4) scores represents the structure of riparian zone. The sum of the RCE(5–12) scores reflects the extent of alteration of the riverbed and banks.
A representative 10 m sub-reach in the lower part of the reach was selected, and the proportions of substrate in this sub-reach were estimated. Macroinvertebrate sampling included 10 sampling units (see [35,36]) and was performed using a hand net (25 × 25 cm, mesh size 0.5 mm). Sampling plots of 25 × 25 cm were allocated to representative substrata according to their cover at the sampling site, and the “kick-sampling” method was used. Invertebrate samples in each site were summarized. In case of UD sites, sampling was done 80–90 m from the start of the reach, which is 10–20 m upstream from the dam, while in DD sites sampling was undertaken 10–20 m downstream the dam. Samples were preserved in 70% ethanol. Taxonomic classification was completed to the family-level, as suggested by Monaghan and Soares [37].
Details of the physical habitat relevant to macroinvertebrates, including substrate size, depth and velocity, were recorded over a longitudinal distance of 10 m corresponding to the area of ecological data collection, in accordance with Monaghan and Soares [37]. Water depth and velocity were measured in five sub-sites except in some UD sites where velocity was too low to be measured.
2.3. Data Analysis
The Shannon–Wiener diversity index at the family level (SWI_fam.) and higher taxa above the family level (SWI_h.taxa) of macroinvertebrates was calculated for each sample. The taxonomic richness of a macroinvertebrate community is expressed as the number of families (N_fam.) and as the number of higher taxa (N_h.taxa). The proportion of taxa from the orders Ephemeroptera, Plecoptera and Trichoptera is also known as the proportion of EPT taxa or simply EPT. Average score per taxon (ASPT) values were also derived, as described in Monaghan and Soares [37] and Burdon et al. [38] to detect potential loading with organic matter, which could influence the macroinvertebrate community structure. Correlations between explanatory variables and the structure of the macroinvertebrate community were tested with a Kendall tau rank correlation coefficient. Correlations and similarity of the macroinvertebrate communities were calculated with PAST, version 2.17c [39]. Gower distance was used as similarity index. Significant differences in characteristics between the types of reaches were checked with non-parametric Kruskal–Wallis tests by rank, using SPSS statistic software, version 22.0 (IBM Corp., Armonk, NY, USA).
The influence of environmental factors on the macroinvertebrate community was tested by redundancy analysis in the program package Canoco for Windows 4.5 (Microcomputer Power: Ithaca, NY, USA) [40]. The linear gradients in the matrix of taxonomic data were revealed beforehand with detrended correspondence analysis, where the eigenvalue for the first axis was 0.34 and the gradient length was 2.1 standard deviations, respectively, so redundancy analyses (RDA) were performed. Species data were log (x + 1) transformed, and centering by species was performed. We used forward selection, where 999 permutations were performed in every round to rank the relative importance of explanatory variables and to avoid co-linearity as suggested by Hudon et al. [41]. The second and third round of the analysis only involved factors for which p < 0.05 in the previous step. Statistically significant factors were used for creation of the RDA diagram.
3. Results
3.1. General Observations
RCE(2–4) scores evaluating the alteration of the riparian zone were found to be significantly higher (p < 0.029) on reference sites (REF) than on most modified sites (MO) and significantly higher than on sites upstream dams (UD) (p < 0.035) and downstream dams (DD) (p < 0.033). The RCE1 scores are significantly higher at REF sites than at sites UD (p < 0.03) and MO (p < 0.02). We used RCE(5–12) to evaluate the morphological alterations of the riverbed. The scores in reference sites are significantly higher than in sites upstream and downstream dams (p < 0.037), as well as in most altered sites (p < 0.03). The average current velocity (see Table 1) is significantly higher in the straightened channel in MO (p = 0.034) and DD (p = 0.044) sites than on UD sites.
The entire set of surveyed reaches in the riparian zone included 51 native woody species, but only four IAS were found (Fallopia japonica, Impatiens glandulifera, Rhus typhina, Robinia pseudacacia). Climax tree species are generally more abundant than pioneer species and dominate the riparian zone in most sites. When comparing the abundance and proportion of native plant species to IAS, our results showed that IAS mostly fail to reach high proportions found in the riparian zone. The highest number of IAS was found on sites around downstream dams DD4(1 and 2), which are in an urban landscape, where the IAS cover around 15% of the riparian zone. The highest proportion of IAS in the riparian zone was recorded on the thoroughly morphologically altered site MO3 (50%), which is a concrete channel. The proportion of native woody species and the number of climax species in the riparian zone are significantly lower (p = 0.029 and p = 0.015), respectively, in most altered reaches (MO) compared to reference sites, as well as sites upstream and downstream dams.
Over 56,200 individuals of aquatic macroinvertebrates were identified at the family level and found to belong to 11 orders or other higher taxa and further to 54 families of macroinvertebrates (see Table A1). The highest diversity or richness was found on the upstream dam site UD1(1), where the water velocity, depth, conductivity, the concentration of O2 and saturation with O2 were all slightly above the average values. This was also the site where the highest number of individuals was found. The lowest richness was found in site upstream dam UD4, which is the largest impoundment on the river.
The proportions of specific higher taxa differ greatly between the sites (Table A1). Ephemeroptera have the highest proportions on sites MO2 and DD4(1). Diptera is the most abundant of all the higher taxa and has the highest proportion at the site upstream the biggest dam on the river (UD4). Amphipoda are more abundant in the modified reaches of the stream. Families found in most modified (MO) and sites downstream dams (DD), but absent in sites upstream dams (UD), are Blephariceridae and Ephydridae (Diptera), whereas Gammaridae and Baetidae have average abundances at least 2.5 times higher (Table A1). On the contrary, Chironomidae were 2.5 and 4 times more common in UD sites than in DD and MO sites, respectively. Similar differences were found for the families Lumbriculidae and Haplotaxidae (Oligochaeta). We compared reference sites and sites upstream dams and recorded a decrease from 47% to 36% for EPT and an increase in the share of Oligochaeta and Chironomidae from 19% to 51%.
Significant differences between different types of reaches were calculated in case of SWI. SWI_fam. is the lowest at UD sites and is significantly higher in reference sites (p = 0.02) as well as in highly altered MO sites (p = 0.02).
3.2. Correlations between Environmental Factors and Macroinvertebrate Community
The distance from the source is positively correlated with N_h.taxa of macroinvertebrates (Table 2), but the distance from the source is negatively correlated with EPT. There is also a negative correlation between EPT and proportion of IAS in the riparian zone (τ = −0.431), but the number of IAS in riparian zone is negatively correlated with SWI_fam (τ = −0.410). A positive correlation was found between distance from the source and number of IAS plants (τ = 0.39), as was expected as a result of the increasing frequency of alterations downstream. The naturalness of the land use behind the riparian zone, evaluated with RCE1, positively correlated with SWI_h.taxa.

Table 2.
Kendall tau correlation coefficients (τ) between environmental parameters and structure of macroinvertebrate community. Statistically significant (p < 0.05) correlations are shown only. Highly significant correlations (p < 0.01) are in bold.
Amongst in-stream parameters, SWI_fam shows a positive correlation with pH value and EPT shows a negative correlation with temperature. The N_h.taxa was also positively correlated with temperature (Table 2). N_fam is positively correlated with minimal velocity, while SWI_fam is negatively correlated with water depth, both indicating lower diversity in impoundments (UD sites). The correlation of the structure of the macroinvertebrate community with abiotic factors was evident (Table 2) as highly statistically significant correlations between DCA1 scores and current velocity, water depth, as well as conductivity.
3.3. Influence of Environmental Factors on Composition of the Macroinvertebrate Community
The taxonomic composition of the macroinvertebrate community is significantly influenced by four parameters that together explain 55% of the variability of the composition of the community. The most important of these factors is maximal current velocity, which explains 22% of total variability, and is followed by distance from the source (17%), the proportion of IAS in riparian zone (9%) and the conductivity (7%). An ordination diagram (Figure 2) shows the distribution of macroinvertebrate samples according to the factors that have a statistically significant (p < 0.05) influence on the composition of macroinvertebrate community.
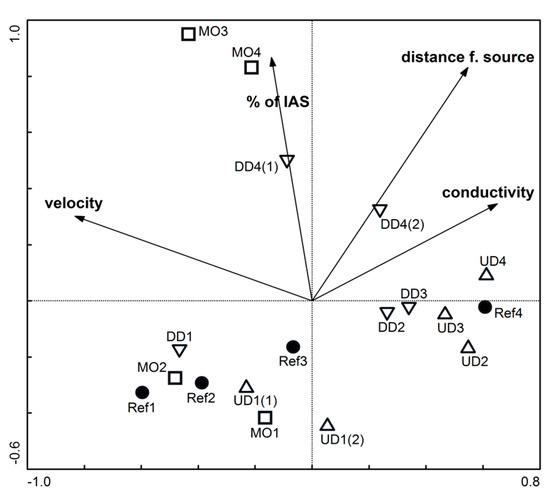
Figure 2.
Ordination diagram of the distribution of sampling sites along the significant gradients, according to RDA. The percentage of taxon-environment relation along Axis1 is 57% and along Axis2 is 30.7%. The numbers of the sites increase downstream (see Figure 1B). Circles—reference sites; up-triangles—sites upstream dams; down-triangles—sites downstream dams; squares—most morphologically altered sites.
As the distance from the source increases, there is an increasing difference in composition of macroinvertebrate community. The similarity between the samples from different types of reaches in the upper part of the river is much larger than in those from the lowland part. The most modified MO samples are most similar to assemblages in reference sites in the headwater section (Figure 2 and Figure 3), but the situation is the opposite in lowland section, where the MO sites differ most from the reference sites. The most modified sites from the lowland part act as outliers in the sense of the composition of macroinvertebrate community (Figure 3).
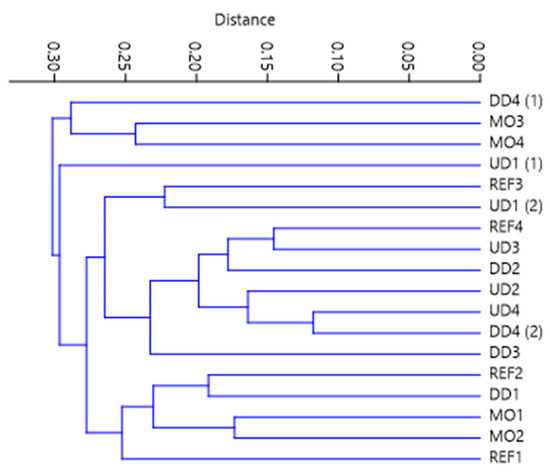
Figure 3.
Dendrogram of simmilarity of macroinvertebrate communities on different sites. Gower simmilarity index was used as a distance metric.
4. Discussion
4.1. General Observations
The proportion of native woody species and the number of climax species in the riparian zone was significantly lower in most modified reaches (MO), and is a suitable descriptor of alterations [4]. There have been many reports [27,32,42,43,44] of significantly lower taxonomic richness of macroinvertebrate community in the channelized reaches than in natural ones. Our results revealed that SWI_fam at UD sites is significantly lower than in reference sites (“natural”) as well as straightened channel (MO sites). Current velocity is significantly lower in UD sites than in straightened channel (MO sites) and in sites downstream dams (DD). This stresses worse negative influence of impoundments (UD sites) due to lower water velocity and consequent accumulation of finer sediment as reported by Eggert et al. [10]. The negative effects of accumulation of finer sediments on lotic communities have been reported by Wood and Armitage [45], Larsen et al. [46] and Lucadamo et al. [47] and include low stability [10], reduction of available space and oxygenation of substrate [48]. Chester and Norris [49] reported that the macroinvertebrate community changed from an EPT-dominated community to one dominated by Oligochaeta and Chironomidae. We compared reference sites and sites upstream dams and recorded a decrease from 47% to 36% for EPT and an increase in the share of Oligochaeta and Chironomidae from 19% to 51%.
4.2. Correlations between Environmental Factors and Diversity of the Macroinvertebrate Community
The EPT index is positively correlated with concentration of O2 and with RCE(5–12) scores, which reflects the naturalness of riverbed. Sabater et al. [44] have claimed that EPT taxa are the most sensitive to morphological alterations, and Lorenz et al. [50] emphasized the overlap of taxa indicating good morphological conditions and good oxygenation. However, evaluation of morphological alteration is difficult, because of the simultaneous influence of different stressors. A negative correlation between distance from the source and EPT is expected, since suitable conditions are decreasing downstream even in natural lotic ecosystems [9].
The number of higher taxa increases downstream, in accord with previous findings that diversity of the lotic ecosystem increases downstream, due to more diverse mesohabitats [51,52] and other resources [9,53]. On the other hand, the number of families is not increased downstream, as was recently found by Jacobsen et al. [54] in the sense of decreasing altitude, which is negatively correlated with distance from source.
The ASPT index decreases downstream and correlates with proportions of substrate types (see Table 2). Błachuta et al. [55] report that ASPT is most susceptible to water flow, while Monaghan and Soares [37] report that ASPT is positively correlated with flow velocity and substrate diversity. Surprisingly, Burdon et al. [38] found that ASPT is negatively influenced by improving riparian condition. However, we failed to find a significant correlation between ASPT and human alterations of riparian zone or morphological conditions.
The proportion of IAS of plants in the riparian zone is negatively correlated with EPT, while the number of IAS in riparian zone is negatively correlated with EPT and SWI_fam, which could be simply a consequence of increasing distance from the source. IAS in the riparian zone change the amount and type of organic matter and consequently the food for many macroinvertebrates [10,11]. We found a positive correlation between distance from the source and number of IAS plants (τ = 0.39), as was expected as result of the increasing frequency of alterations downstream. On the other hand, the structure of macroinvertebrate community represented as scores of DCA1 and DCA2 was not correlated with the distance from source.
We calculated positive correlations between macroinvertebrate taxon richness (N_fam) and diversity (SWI) and tree cover in RZ, which is related to the degree of morphological alteration [32]. Reduced input of leaf and wood litter influences trophic interaction [23]. SWI_fam was positively correlated with current velocity. Jonsson et al. [56] report the positive correlation of Diptera, Plecoptera, Trichoptera, Ephemeroptera and Coleoptera taxa with current velocity. Actually, DCA1 scores highly significantly correlated with velocity and the depth of the water (Table 2). The influence of temperature was noted in the downstream increase of macroinvertebrate diversity [2]. However, while higher temperatures favor higher species richness, they may result in the extinction of rare and endemic species [57].
RCE1 (i.e., naturalness of land-use) is positively correlated with SWI_h.taxa, signifying that lower diversity of macroinvertebrates can be found in reaches surrounded by intensively cultivated farmland or settled area, which may cause increased nutrient input thus presenting a major problem for the health of the stream [8]. Improper land-use, for example, intensive logging, and increased sedimentation, respectively, are suggested by Zhang et al. [58] as reasons underlying the lower species richness of the macroinvertebrate community.
4.3. Influence of Environmental Factors on Composition of the Macroinvertebrate Community
The most important factor shaping taxonomic composition was current velocity, which explains 22% of total variability and reflects the influence of hydromorphological alterations, creating its wide gradient from impoundments to straightened channel. Jonsson et al. [56] and Pastuchová et al. [59] report that current velocity significantly shapes the structure of macroinvertebrate community. Hydromorphological variables such as maximal current velocity and variation of depth influence the instream community [60] and the impact of their alterations on macroinvertebrate community is documented in Hering et al. [61], Karaouzas et al. [62] and Horsák et al. [27].
The proportions of native plant species and IAS, respectively, in the riparian zone significantly explain the composition of the macroinvertebrate community (Figure 2). For instance IAS are dominant in the MO3 and MO4 site but absent from the MO1 and MO2 sites, which are in the headwater and differ greatly in the taxonomic composition of macroinvertebrate community (Figure 3). The structure of riparian vegetation, especially woody vegetation, has a large influence on the macroinvertebrate community [25,63].
The distance from the source explains 17% of the variability in the community. Lucadamo et al. [47] explain its influence in terms of substantial change of several parameters downstream, including flow velocity [64], substratum [59] and temperature [65]. Conductivity (7%) plays a minor role in shaping the composition of the macroinvertebrate community.
Significant parameters act on different scales. Current velocity and conductivity are instream parameters; the proportion of IAS in riparian zone refers to the riparian corridor, and distance from the source is a parameter on a catchment scale. Cortes et al. [66] emphasize the importance of including different scales to represent the variability of aquatic communities efficiently. Moreover, González del Tánago et al. [67] and Burdon et al. [38] emphasize that exploring hydromorphological indicators across spatial scales (e.g., the share of forest in the catchment area, the width of the riparian zone and the channel, the presence of natural retention structures) is an essential step towards the design of sustainable river management.
5. Conclusions
Our results confirm the hypothesis that morphological alterations have a negative influence on the diversity of both riparian vegetation species and the macroinvertebrate community. Alterations are also reflected in the increasing number and abundance of IAS in riparian zone, suggesting their potential use as bioindicators for a rapid and low-cost survey of the hydromorphological status of a watercourse. The diversity of the macroinvertebrate community is negatively correlated with the number of IAS in the riparian zone, but the EPT is negatively correlated with the proportion of IAS in the riparian zone, suggesting on indicative value and the applicability of IAS. Records on number and proportion of IAS in the riparian zone could complement an RCE survey in the assessment of the extent of hydromorphological alterations.
Knowledge for suitable planning of hydromorphological measures to enhance the ecological potential of a stream reach will contribute to the integrity and ecological status of the watercourses. The conditions in the studied river could be improved by restoration of meanders and wider riparian zone along the middle and lower parts of the river, which would enhance the capacity of the river for larger amounts of water in a sustainable way. When these measures are taken, the majority of the existing dams and bank reinforcements are no longer needed for mitigation of spates or high discharge events to ensure flood safety.
Author Contributions
Conceptualization, I.Z.; methodology, I.Z.; validation, I.Z. and T.M.; formal analysis, I.Z.; investigation, T.M.; data curation, T.M. and I.Z.; writing—original draft preparation, T.M.; writing—review and editing, I.Z.; visualization, I.Z.; supervision, I.Z.; project administration, I.Z.; funding acquisition, I.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the Slovenian Research Agency, grant numbers P1-0212 and 33135.
Acknowledgments
The authors thank to George W.A. Milne, who provided language help, to Dragan Abram and Matej Holcar for the assistance in the field and creation of the map. Special thanks go to Mateja Germ, for her comments that improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Appendix A

Table A1.
Average numbers of macroinvertebrate individuals per sample in four types of reaches. L—larvae, A—adulti.
Table A1.
Average numbers of macroinvertebrate individuals per sample in four types of reaches. L—larvae, A—adulti.
| Type | REF | UD | DD | MO | |
|---|---|---|---|---|---|
| Higher taxa | Family | ||||
| Gastropoda | Acroloxidae | 0.5 | 2.0 | ||
| Bithyniidae | 12.5 | 20.0 | 9.0 | 5.5 | |
| Hydrobiidae | 1.0 | 3.2 | 3.2 | ||
| Lymnaeidae | 0.4 | ||||
| Neritidae | 0.4 | ||||
| Physidae | 5.0 | 4.0 | 5.6 | ||
| Planorbidae | 0.4 | 0.4 | |||
| Viviparidae | 1.5 | ||||
| Bivalvia | Sphaeriidae | 0.4 | 0.4 | 0.5 | |
| Oligochaeta | Lumbricidae | 3.5 | 0.4 | 0.2 | 2.5 |
| Lumbriculidae | 2.0 | 7.6 | 2.0 | 4.5 | |
| Haplotaxidae | 8.0 | 42.8 | 11.2 | 25.5 | |
| Amphipoda | Gammaridae | 18.0 | 11.2 | 69.4 | 488.5 |
| Acarina | Hydrachnidia | 59.0 | 40.4 | 45.4 | 87.0 |
| Ephemeroptera | Baetidae | 205.3 | 39.8 | 97.4 | 529.8 |
| Ephemerellidae | 193.8 | 418.6 | 234.0 | 145.0 | |
| Heptageniidae | 7.0 | 8.4 | 2.8 | 8.8 | |
| Polymitarcyidae | 3.3 | 0.4 | 0.4 | ||
| Plecoptera | Capniidae | 559.5 | 821.4 | 730.4 | 434.0 |
| Nemouridae | 44.0 | 23.6 | 13.6 | 42.0 | |
| Odonata | Gomphidae | 0.4 | |||
| Calopterygidae | 0.4 | ||||
| Lestidae | 1.5 | ||||
| Trichoptera | Brachycentridae | 0.5 | |||
| Calamoceratidae | 7.5 | 0.8 | 0.4 | ||
| Drusinae | 70.0 | 42.6 | 23.2 | 5.0 | |
| Glossostomatidae | 0.2 | ||||
| Hydropsychidae | 12.5 | 0.4 | 1.8 | 1.5 | |
| Hydroptillidae | 3.5 | 11.0 | 6.4 | 11.0 | |
| Lepidostomatidae | 6.0 | 5.8 | 2.0 | ||
| Leptoceridae | 0.2 | ||||
| Limnephilidae | 2.0 | 1.6 | |||
| Polycentropodidae | 6.8 | 8.4 | |||
| Psychomyiidae | 6.0 | 1.2 | 3.2 | 9.5 | |
| Rhyacophilidae | 4.0 | 3.6 | 7.4 | 7.5 | |
| Sericostomatidae | 4.0 | 9.8 | 0.8 | 4.5 | |
| Diptera | Blephariceridae | 9.5 | 0.8 | 8.5 | |
| Ceratopogonidae | 2.5 | 2.2 | 0.2 | 0.5 | |
| Chironomidae | 445.5 | 1966.4 | 464.6 | 768.5 | |
| Culicidae | 44.0 | 191.6 | 74.8 | 92.5 | |
| Empididae | 11.0 | 4.0 | 7.0 | 6.3 | |
| Ephydridae | 2.0 | 7.5 | |||
| Limoniidae | 6.0 | 12.4 | 29.2 | 156.8 | |
| Psychodidae | 0.4 | ||||
| Ptychopteridae | 3.0 | ||||
| Simuliidae | 238.5 | 34.0 | 6.2 | 333.0 | |
| Tipulidae | 3.8 | ||||
| Coleoptera | Dryopidae—L | 0.5 | 0.8 | 1.6 | |
| Dytiscidae—L | 4.5 | 8.0 | 8.4 | 1.0 | |
| Elmidae—L | 166.8 | 215.2 | 277.8 | 95.5 | |
| Noteridae—L | 3.8 | ||||
| Dytiscidae—A | 4.8 | 2.4 | 6.4 | 3.0 | |
| Elmidae—A | 189.3 | 147.0 | 282.0 | 109.8 | |
| Hydraenidae—A | 73.5 | 4.4 | 6.2 | 5.3 |
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters, 2nd ed.; Springer: Dordrecht, The Netherlands, 2007; p. 436. ISBN 978-1-4020-5582-9. [Google Scholar]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Feld, C.K. Identification and measure of hydromorphological degradation in Central European lowland streams. Hydrobiologia 2004, 516, 69–90. [Google Scholar] [CrossRef]
- Tockner, K.; Uehlinger, U.; Robinson, C.T. Rivers of Europe; Academic Press: Heidelberg, Germany, 2009; ISBN 978-0-08-091908-9. [Google Scholar]
- Gostner, W.; Alp, M.; Schleiss, A.J.; Robinson, C.T. The hydro-morphological index of diversity: A tool for describing habitat heterogeneity in river engineering projects. Hydrobiologia 2013, 712, 43–60. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Grabowski, R.C.; Gurnell, A.M. Diagnosing problems of fine sediment delivery and transfer in a lowland catchment. Aquat. Sci. 2016, 78, 95–106. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Eggert, S.L.; Wallace, J.B.; Meyer, J.L.; Webster, J.R. Storage and export of organic matter in a headwater stream: Responses to long-term detrital manipulations. Ecosphere 2012, 3. [Google Scholar] [CrossRef]
- Mineau, M.M.; Baxter, C.V.; Marcarelli, A.M.; Minshall, G.W. An invasive riparian tree reduces stream ecosystem efficiency via a recalcitrant organic matter subsidy. Ecology 2012, 93, 1501–1508. [Google Scholar] [CrossRef]
- McRae, G.; Edwards, C.J. Thermal Characteristics of Wisconsin Headwater Streams Occupied by Beaver: Implications for Brook Trout Habitat. Trans. Am. Fish. Soc. 1994, 123, 641–656. [Google Scholar] [CrossRef]
- Lessard, J.L.; Hayes, D.B. Effects of elevated water temperature on fish and macroinvertebrate communities below small dams. River Res. Appl. 2003, 19, 721–732. [Google Scholar] [CrossRef]
- Petersen, R.C. The RCE: A Riparian, Channel, and Environmental Inventory for small streams in the agricultural landscape. Freshw. Biol. 1992, 27, 295–306. [Google Scholar] [CrossRef]
- Grašič, M.; Piberčnik, M.; Zelnik, I.; Abram, D.; Gaberščik, A. Invasive Alien Vines Affect Leaf Traits of Riparian Woody Vegetation. Water 2019, 11, 2395. [Google Scholar] [CrossRef]
- Zelnik, I.; Mavrič Klenovšek, V.; Gaberščik, A. Complex Undisturbed Riparian Zones Are Resistant to Colonisation by Invasive Alien Plant Species. Water 2020, 12, 345. [Google Scholar] [CrossRef]
- Zelnik, I.; Haler, M.; Gaberščik, A. Vulnerability of a riparian zone towards invasion by alien plants depends on its structure. Biologia 2015, 70, 869–878. [Google Scholar] [CrossRef]
- Golovanova, I.L. Effects of abiotic factors (temperature, pH, heavy metals) on activities of glycosidases in invertebrate animals. J. Evol. Biochem. Phys. 2011, 47, 15. [Google Scholar] [CrossRef]
- Connolly, N.M.; Pearson, R.G.; Pearson, B.A. Riparian vegetation and sediment gradients determine invertebrate diversity in streams draining an agricultural landscape. Agric. Ecosyst. Environ. 2016, 221, 163–173. [Google Scholar] [CrossRef]
- Blanchet, S.; Loot, G.; Dodson, J.J. Competition, predation and flow rate as mediators of direct and indirect effects in a stream food chain. Oecologia 2008, 157, 93–104. [Google Scholar] [CrossRef]
- Richter, B.D.; Mathews, R.; Harrison, D.L.; Wigington, R. Ecologically Sustainable Water Management: Managing River Flows for Ecological Integrity. Ecol. Appl. 2003, 13, 206–224. [Google Scholar] [CrossRef]
- Feld, C.K.; De Bello, F.; Dolédec, S. Biodiversity of traits and species both show weak responses to hydromorphological alteration in lowland river macroinvertebrates. Freshw. Biol. 2014, 59, 233–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Richardson, J.S.; Negishi, J.N. Detritus processing, ecosystem engineering and benthic diversity: A test of predator–omnivore interference. J. Anim. Ecol. 2004, 73, 756–766. [Google Scholar] [CrossRef]
- Armitage, P.; Blackburn, J. Historic land-use and the influence of catchment characteristics on faunal communites of small streams, Dorset, UK. Freshw. Forum 2010, 28, 5–25. [Google Scholar]
- Casotti, C.G.; Kiffer, W.P.; Costa, L.C.; Rangel, J.V.; Casagrande, L.C.; Moretti, M.S. Assessing the importance of riparian zones conservation for leaf decomposition in streams. Braz. J. Nat. Conserv. 2015, 13, 178–182. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parlament and of the Council of 23 October 2000. Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj (accessed on 14 November 2019).
- Horsák, M.; Bojková, J.; Zahrádková, S.; Omesová, M.; Helešic, J. Impact of reservoirs and channelization on lowland river macroinvertebrates: A case study from Central Europe. Limnologica 2009, 39, 140–151. [Google Scholar] [CrossRef]
- Belmar, O.; Bruno, D.; Martínez-Capel, F.; Barquín, J.; Velasco, J. Effects of flow regime alteration on fluvial habitats and riparian quality in a semiarid Mediterranean basin. Ecol. Indic. 2013, 30, 52–64. [Google Scholar] [CrossRef]
- Vaughan, I.P.; Diamond, M.; Gurnell, A.M.; Hall, K.A.; Jenkins, A.; Milner, N.J.; Naylor, L.A.; Sear, D.A.; Woodward, G.; Ormerod, S.J. Integrating ecology with hydromorphology: A priority for river science and management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 113–125. [Google Scholar] [CrossRef]
- Friberg, N. Impacts and indicators of change in lotic ecosystems. WIREs Water 2014, 1, 513–531. [Google Scholar] [CrossRef]
- Martínez Fernández, V.; González del Tánago, M.; Garcia de Jalon, D. Assessment of macroinvertebrates communities responses across regulated and non regulated sites. In Proceedings of the XIX Conference of the Iberian Association of Limnology Inland Waters and XXI Century Challenges: From Scientific Knowledge to Environmental Management, Coimbra, Portugal, 24–29 June 2018; p. 141. [Google Scholar]
- Buffagni, A.; Tenchini, R.; Cazzola, M.; Erba, S.; Balestrini, R.; Belfiore, C.; Pagnotta, R. Detecting the impact of bank and channel modification on invertebrate communities in Mediterranean temporary streams (Sardinia, SW Italy). Sci. Total Environ. 2016, 565, 1138–1150. [Google Scholar] [CrossRef]
- Buffagni, A.; Erba, S.; Cazzola, M.; Kemp, J.L. The AQEM multimetric system for the southern Italian Apennines: Assessing the impact of water quality and habitat degradation on pool macroinvertebrates in Mediterranean rivers. Hydrobiologia 2004, 516, 313–329. [Google Scholar] [CrossRef]
- Suriano, M.T.; Fonseca-Gessner, A.A.; Roque, F.O.; Froehlich, C.G. Choice of macroinvertebrate metrics to evaluate stream conditions in Atlantic Forest, Brazil. Environ. Monit. Assess. 2011, 175, 87–101. [Google Scholar] [CrossRef]
- Traversetti, L.; Scalici, M. Assessing the influence of source distanceand hydroecoregion on the invertebrate assemblagesimilarity in central Italy streams. Knowl. Manag. Aquat. Ecosyst. 2014, 414. [Google Scholar] [CrossRef]
- Erba, S.; Cazzola, M.; Belfiore, C.; Buffagni, A. Macroinvertebrate metrics responses to morphological alteration in Italian rivers. Hydrobiologia 2020, 847, 2169–2191, . [Google Scholar] [CrossRef]
- Monaghan, K.A.; Soares, A.M.V.M. The bioassessment of fish and macroinvertebrates in a Mediterranean–Atlantic climate: Habitat assessment and concordance between contrasting ecological samples. Ecol. Indic. 2010, 10, 184–191. [Google Scholar] [CrossRef]
- Burdon, F.J.; Ramberg, E.; Sargac, J.; Forio, M.A.E.; De Saeyer, N.; Mutinova, P.T.; Moe, T.F.; Pavelescu, M.O.; Dinu, V.; Cazacu, C.; et al. Assessing the Benefits of Forested Riparian Zones: A Qualitative Index of Riparian Integrity is Positively Associated with Ecological Status in European Streams. Water 2020, 12, 1178. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer PowerPublisher: Ithaca, NY, USA, 2002. [Google Scholar]
- Hudon, C.; Gagnon, P.; Amyot, J.-P.; Létourneau, G.; Jean, M.; Plante, C.; Rioux, D.; Deschênes, M. Historical changes in herbaceous wetland distribution induced by hydrological conditions in Lake Saint-Pierre (St. Lawrence River, Quebec, Canada). Hydrobiologia 2005, 539, 205–224. [Google Scholar] [CrossRef]
- Negishi, J.N.; Inoue, M.; Nunokawa, M. Effects of channelisation on stream habitat in relation to a spate and flow refugia for macroinvertebrates in northern Japan. Freshw. Biol. 2002, 47, 1515–1529. [Google Scholar] [CrossRef]
- Wyżga, B.; Amirowicz, A.; Oglęcki, P.; Hajdukiewicz, H.; Radecki-Pawlik, A.; Zawiejska, J.; Mikuś, P. Response of fish and benthic invertebrate communities to constrained channel conditions in a mountain river: Case study of the Biała, Polish Carpathians. Limnologica 2014, 46, 58–69. [Google Scholar] [CrossRef]
- Sabater, S.; Bregoli, F.; Acuña, V.; Barceló, D.; Elosegi, A.; Ginebreda, A.; Marcé, R.; Muñoz, I.; Sabater-Liesa, L.; Ferreira, V. Effects of human-driven water stress on river ecosystems: A meta-analysis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.J.; Armitage, P.D. Biological Effects of Fine Sediment in the Lotic Environment. Environ. Manag. 1997, 21, 203–217. [Google Scholar] [CrossRef]
- Larsen, S.; Vaughan, I.P.; Ormerod, S.J. Scale-dependent effects of fine sediments on temperate headwater invertebrates. Freshw. Biol. 2009, 54, 203–219. [Google Scholar] [CrossRef]
- Lucadamo, L.; Mezzotero, A.; Voelz, N.J.; Gallo, L. Seasonal changes of abiotic and biotic gradients downstream a multiple use reservoir in a Mediterranean River. River Res. Appl. 2012, 28, 103–117. [Google Scholar] [CrossRef]
- Ryan, P.A. Environmental effects of sediment on New Zealand streams: A review. N. Z. J. Mar. Freshw. 1991, 25, 207–221. [Google Scholar] [CrossRef]
- Chester, H.; Norris, R. Dams and Flow in the Cotter River, Australia: Effects on Instream Trophic Structure and Benthic Metabolism. Hydrobiologia 2006, 572, 275–286. [Google Scholar] [CrossRef]
- Lorenz, A.; Hering, D.; Feld, C.K.; Rolauffs, P. A new method for assessing the impact of hydromorphological degradation on the macroinvertebrate fauna of five German stream types. Hydrobiologia 2004, 516, 107–127. [Google Scholar] [CrossRef]
- Mormul, R.P.; Thomaz, S.M.; Takeda, A.M.; Behrend, R.D. Structural Complexity and Distance from Source Habitat Determine Invertebrate Abundance and Diversity. Biotropica 2011, 43, 738–745. [Google Scholar] [CrossRef]
- Hitchman, S.M.; Mather, M.E.; Smith, J.M.; Fencl, J.S. Identifying keystone habitats with a mosaic approach can improve biodiversity conservation in disturbed ecosystems. Glob. Chang. Biol. 2018, 24, 308–321. [Google Scholar] [CrossRef]
- Minshall, G.W.; Cummins, K.W.; Petersen, R.C.; Cushing, C.E.; Bruns, D.A.; Sedell, J.R.; Vannote, R.L. Developments in Stream Ecosystem Theory. Can. J. Fish. Aquat. Sci. 1985, 42, 1045–1055. [Google Scholar] [CrossRef]
- Jacobsen, D.; Wiberg-Larsen, P.; Brodersen, K.P.; Hansen, S.B.; Lindegaard, C.; Friberg, N.; Dall, P.C.; Kirkegaard, J.; Skriver, J.; Toman, M. Macroinvertebrate communities along the main stem and tributaries of a pre-Alpine river: Composition responds to altitude, richness does not. Limnologica 2020, 84, 125816. [Google Scholar] [CrossRef]
- Błachuta, J.; Szoszkiewicz, K.; Gebler, D.; Schneider, S.C. How Do Environmental Parameters Relate to Macroinvertebrate Metrics?—Prospects for River Water Quality Assessment. Pol. J. Ecol. 2014, 62, 111–122. [Google Scholar] [CrossRef]
- Jonsson, M.; Burrows, R.M.; Lidman, J.; Fältström, E.; Laudon, H.; Sponseller, R.A. Land use influences macroinvertebrate community composition in boreal headwaters through altered stream conditions. Ambio 2017, 46, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, F.; Soininen, J.; Heino, J.; Shen, J. Nutrient enrichment modifies temperature-biodiversity relationships in large-scale field experiments. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Richardson, J.S.; Pinto, X. Catchment-Scale Effects of Forestry Practices on Benthic Invertebrate Communities in Pacific Coastal Streams. J. Appl. Ecol. 2009, 46, 1292–1303. [Google Scholar] [CrossRef]
- Pastuchová, Z.; Grešková, A.; Lehotský, M. Spatial distribution pattern of macroinvertebrates in relation to morphohydraulic habitat structure: Perspectives for ecological stream assessment. Pol. J. Ecol. 2010, 58, 347–360. [Google Scholar]
- Feld, C.K.; Martins da Silva, P.; Sousa, J.P.; Bello, F.D.; Bugter, R.; Grandin, U.; Hering, D.; Lavorel, S.; Mountford, O.; Pardo, I.; et al. Indicators of biodiversity and ecosystem services: A synthesis across ecosystems and spatial scales. Oikos 2009, 118, 1862–1871. [Google Scholar] [CrossRef]
- Hering, D.; Johnson, R.K.; Kramm, S.; Schmutz, S.; Szoszkiewicz, K.; Verdonschot, P.F.M. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw. Biol. 2006, 51, 1757–1785. [Google Scholar] [CrossRef]
- Karaouzas, I.; Gritzalis, K.; Skoulikidis, N. Land use effects on macroinvertebrate assemblages and stream quality along an agricultural river basin. Fresenius Environ. Bull. 2007, 16, 645–653. [Google Scholar]
- Martínez, A.; Larrañaga, A.; Basaguren, A.; Pérez, J.; Mendoza-Lera, C.; Pozo, J. Stream regulation by small dams affects benthic macroinvertebrate communities: From structural changes to functional implications. Hydrobiologia 2013, 711, 31–42. [Google Scholar] [CrossRef]
- Statzner, B.; Higler, B. Stream hydraulics as a major determinant of benthic invertebrate zonation patterns. Freshw. Biol. 1986, 16, 127–139. [Google Scholar] [CrossRef]
- Vannote, R.L.; Sweeney, B.W. Geographic Analysis of Thermal Equilibria: A Conceptual Model for Evaluating the Effect of Natural and Modified Thermal Regimes on Aquatic Insect Communities. Am. Nat. 1980, 115, 667–695. [Google Scholar] [CrossRef]
- Cortes, R.M.V.; Hughes, S.J.; Varandas, S.G.P.; Magalhães, M.; Ferreira, M.T. Habitat variation at different scales and biotic linkages in lotic systems: Consequences for monitorization. Aquat. Ecol. 2009, 43, 1107–1120. [Google Scholar] [CrossRef]
- González del Tánago, M.; Gurnell, A.M.; Belletti, B.; García de Jalón, D. Indicators of river system hydromorphological character and dynamics: Understanding current conditions and guiding sustainable river management. Aquat. Sci. 2016, 78, 35–55. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).