Effect of Diverse Abiotic Conditions on the Structure and Biodiversity of Ichthyofauna in Small, Natural Water Bodies Located on Agricultural Lands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods of Testing Selected Abiotic Factors
2.3. Control Catches of Fish and Ichthyological Studies
2.4. Biodiversity Indices
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choiński, A. Katalog Jezior Polski; Wydawnictwo Naukowe UAM: Poznań, Poland, 2013; p. 600. ISBN 83-232-1732-7. (In Polish) [Google Scholar]
- Hayashi, M.; van der Kamp, G.; Rudolph, D.L. Water and solute transfer between a prairie wetland and adjacent uplands, 1. Water balance. J. Hydrol. 1998, 207, 42–55. [Google Scholar] [CrossRef]
- Fiedler, M.; Antkowiak, M.; Oliskiewicz-Krzywicka, A.; Zbierska, A. Model for the volume-area-depth relations of midfield ponds using LiDAR. J. Ecol. Eng. 2017, 18, 36–42. [Google Scholar] [CrossRef]
- Pond Conservation Group. A Future for Britainś Ponds; An Agenda for Action. 1993. Available online: https://www.wildoxfordshire.org.uk/wp-content/uploads/2014/03/Ponds.pdf (accessed on 1 September 2020).
- Pieńkowski, P. Disappearance of ponds in the younger Pleistocene landscapes of Pomerania. J. Water Land Dev. 2000, 4, 55–68. [Google Scholar]
- van der Kamp, G.; Hayashi, M. Groundwater-wetland ecosystem interaction in the semiarid glaciated plains of North America. Hydrogeol. J. 2009, 17, 203–214. [Google Scholar] [CrossRef]
- Céréghino, R.; Boix, D.; Cauchie, H.M.; Martens, K.; Oertli, B. The ecological role of ponds in a changing world. Hydrobiologia 2014, 723, 1–6. [Google Scholar] [CrossRef]
- Lewis-Phillips, J.; Brooks, S.; Sayer, C.D.; McCrea, R.; Siriwardena, R.; Axmacher, J.C. Pond management enhances the local abundance and species richness of farmland bird communities. Agric. Ecosyst. Environ. 2019, 273, 130–140. [Google Scholar] [CrossRef]
- Juszczak, R.; Kędziora, A.; Olejnik, J. Assessment of water retention capacity of small ponds in Wyskoć agricultural-forest catchment in western Poland. Pol. J. Environ. Stud. 2007, 16, 685–695. [Google Scholar]
- Brysiewicz, A.; Wesołowski, P.; Bonisławska, M. Content of selected macro- and microelements in surface water of in-field ponds and in groundwater from adjacent agricultural areas. J. Elem. 2019, 24, 207–219. [Google Scholar] [CrossRef]
- Matthews, J.H. Anthropogenic climate change impacts on ponds: A thermal mass perspective. In: Ott, J. (Ed) Monitoring Climatic Change with Dragonflies. BioRisk 2010, 5, 193–209. [Google Scholar] [CrossRef]
- Biggs, J.; von Fumetti, S.; Kelly-Quinn, M. The importance of small waterbodies for biodiversity and ecosystem services: Implications for policy makers. Hydrobiologia 2017, 793, 3–39. [Google Scholar] [CrossRef]
- Riley, W.D.; Potter, E.C.E.; Biggs, J.; Collins, A.L.; Jarvie, H.P.; Jones, J.I.; Kelly-Quinn, M.; Ormerod, S.J.; Sear, D.A.; Wilby, R.L.; et al. Small water bodies in Great Britain and Ireland: Ecosystem function, human-generated degradation, and options for restorative action. Sci. Total Environ. 2018, 645, 1598–1616. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, J.H.K.; Estes, R.A.; Scott, D.E.; Whitfield Gibbons, J. Amphibian colonization and use of ponds created for trial mitigation of wetland loss. Wetlands 2001, 21, 93–112. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Joniak, T. Zooplankton diversity and macrophyte biometry in shallow water bodies of various trophic state. Hydrobiologia 2016, 774, 39–51. [Google Scholar] [CrossRef]
- Prasad, A.G.D.; Venkataramana, G.V.; Thomas, M. Fish diversity and its conservation in major wetlands of Mysore. J. Environ Biol. 2009, 30, 713–718. [Google Scholar]
- Escalera-Vázquez, L.; Zambrano, L. The effect of seasonal variation in abiotic factors on fish community structure in temporary and permanent pools in a tropical wetland. Freshwater Biol. 2010, 55, 2557–2569. [Google Scholar] [CrossRef]
- Cymes, I.; Cymes, I.; Dragańska, E.; Szymczyk, S. The influence of weather conditions on mid-field ponds situated in a reclaimed area in Sępopolska Plain. Oceanol. Hydrobiol. Stud. 2010, 39, 75–84. [Google Scholar] [CrossRef]
- Rechulicz, J.; Tarkowska-Kukuryk, M.; Pęczuła, W.; Mieczan, T. Fish community and productivity as an index of trophic state diversity in two shallow lakes. TEKA Comm. Prot. Form. Nat. Environ. 2012, 9, 181–191. [Google Scholar]
- Cuvin-Aralar, M.L.A. Impacts of aquaculture on fish biodiversity in the freshwater lake Laguna de Bay, Philippines. Lakes Reserv. Res. Manag. 2016, 21, 31–39. [Google Scholar] [CrossRef]
- Glińska-Lewczuk, K.; Burandt, P.; Kujawa, R.; Kobus, S.; Obolewski, K.; Dunalska, J.; Grabowska, M.; Lew, S.; Chormański, J. Environmental Factors Structuring Fish Communities in Floodplain Lakes of the Undisturbed System of the Biebrza River. Water 2016, 8, 146. [Google Scholar] [CrossRef]
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar] [CrossRef]
- Juszczak, R.; Kędziora, A. Threats to and deterioration of small water reservoirs located within Wyskoć catchment. Pol. J. of Environ. Stud. 2003, 12, 567–573. [Google Scholar]
- Wolnicki, J.; Kamiński, R.; Sikorska, J. Occurrence, threats and active protection of the lake minnow, Eupallasella percnurus (Pall.), in Mazowieckie Voivodeship in Poland. Arch. Pol. Fish. 2011, 19, 209–216. [Google Scholar] [CrossRef]
- Directive 92/43/EEC of 21 May 1992 on the Protection of Natural Habitats and Wild Fauna and Flora. Available online: https://natura2000.gdos.gov.pl/files/artykuly/42646/DyrektywaSiedliskowa.pdf (accessed on 1 September 2020).
- de la Hoz Franco, E.A.; Budy, P. Effects of biotic and abiotic factors on the distribution of trout and salmon along a longitudinal stream gradient. Environ. Biol. Fish. 2005, 72, 379–391. [Google Scholar] [CrossRef]
- Mahavadiya, D.; Sapra, D.; Rathod, V.; Sarman, V. Effect of biotic and abiotic factors in feeding activity in teleost fish: A review. J. Entomol. Zool. Stud. 2018, 6, 387–390. [Google Scholar]
- Woods, T.; McGarvey, D.J. Assessing the relative influences of abiotic and biotic factors on American eel Anguilla rostrata distribution using hydrologic, physical habitat, and functional trait data. Ecography 2018, 41, 2067–2079. [Google Scholar] [CrossRef]
- Mioduszewski, W. Protection of water quality in the lake situated on the agricultural area. J. Water Land Dev. 2015, 26, 65–71. [Google Scholar] [CrossRef]
- ISO 5667-24: 2016. Water Quality—Sampling—Part 24: Guidance on the Auditing of Water Quality Sampling; ISO: Geneva, Switzerland, 2016.
- European Committee for Standardisation EN 14757: 2005 (E) Water Quality—Sampling of Fish with Multi-Mesh Gillnets; European Committee for Standardisation: Brussels, Belgium, 2005.
- CEN—EN 14011, Water Quality—Sampling of Fish with Electricity; European Committee for Standardization: Brussel, Belgien, 2003.
- PN-EN 14011 Jakość Wody—Pobieranie Próbek ryb z Zastosowaniem Elektryczności; Polski Komitet Normalizacyjny (PKN): Warszawa, Ploand, 2006. (In Polish)
- Nyeste, K.; Kati, S.; Nagy, S.A.; Antal, L. Growth features of the Amur sleeper, Perccottus glenii (Actinopterygii: Perciformes: Odontobutidae), in the invaded Carpathian Basin, Hungary. Acta Ichthyol. Piscat. 2017, 47, 33–40. [Google Scholar] [CrossRef][Green Version]
- Rahman, B.; Sazedul, H.Z.; Rahman, M.; Nahar, A. Exploration of fishing gear and fisheries diversity of Agunmukha River at Galachipa Upazila in Patuakhali District of Bangladesh. Iran. J. Fish. Sci. 2017, 16, 108–126. [Google Scholar]
- Nagendra, H. Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl. Geogr. 2002, 22, 175–186. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5–3. 2018. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 1 September 2020).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Paleontol. Electr. 2001, 4, 1–9. [Google Scholar]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Schiemer, F.; Waidbacher, H. Strategies of conservation of a Danubian fish fauna. In River Conservation and Management; Boon, P.J., Calow, P., Petts, G.E., Eds.; Wiley: London, UK, 1992; pp. 365–382. [Google Scholar]
- Balon, E.K. Epigenesis of an epigeneticist: The development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph. Ichthyol. Rev. 1990, 1, 1–48. [Google Scholar]
- Mioduszewski, W. Small (natural) water retention in rural areas. J. Water Land Dev. 2014, 20, 19–29. [Google Scholar] [CrossRef]
- Davis, A.M.; Moore, A.R. Conservation potential of artificial water bodies for fish communities on a heavily modified agricultural floodplain. Aquat. Conserv. 2016, 26, 1184–1196. [Google Scholar] [CrossRef]
- Gałczyńska, M.; Mańkowska, N.; Milke, J.; Buśko, M. Possibilities and limitations of using Lemna minor, Hydro-charis morsus-ranae and Ceratophyllum demersum in removing metals with contaminated water. J. Water Land Dev. 2019, 40, 161–173. [Google Scholar] [CrossRef][Green Version]
- Mitsuoa, Y.; Tsunodab, H.; Kozawac, G.; Yuma, M. Response of the fish assemblage structure in a small farm pond to management dredging operations. Agric. Ecosyst. Environ. 2014, 188, 93–96. [Google Scholar] [CrossRef]
- Ye, S.; Li, Z.; Lek-Ang, S.; Feng, G.; Lek, S.; Cao, W. Community structure of small fishes in a shallow macrophytic lake (Niushan Lake) along the middle reach of the Yangtze River, China. Aquat. Living Resour. 2006, 19, 349–359. [Google Scholar] [CrossRef]
- Gee, J.H.R.; Smith, B.D.; Lee, K.M.; Griffiths, S.W. The ecological basis of freshwater pond management for biodiversity. Aquat. Conserv. 1997, 7, 91–104. [Google Scholar] [CrossRef]
- Bhagowati, B.; Ahamad, K.A. A review on lake eutrophication dynamics and recent developments in lake modeling. Ecohydrol. Hydrobiol. 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Puglis, H.J.; Boone, M.D. Effects of terrestrial buffer zones on amphibians on golf courses. PLoS ONE 2012, 7, e39590. [Google Scholar] [CrossRef] [PubMed]
- Stumm, W.; Stumm-Zollinger, E. The role of phosphorus in eutrofication. In Water Pollution Microbiology; Mitchel, R., Ed.; Wiley-Interscience: New York, NY, USA; London, UK; Toronto, ON, Canada, 1972. [Google Scholar]
- Chen, R.; Deng, M.; He, X.; Hou, J. Enhancing Nitrate Removal from Freshwater Pond by Regulating Carbon/Nitrogen Ratio. Front Microbiol. 2017, 8, 1712. [Google Scholar] [CrossRef]
- Boers, A.M.; Zedler, J.B. Stabilized water levels and Typha invasiveness. Wetlands 2008, 28, 676–685. [Google Scholar] [CrossRef]
- Brooks, B.W.; Chambliss, C.K.; Stanley, J.K.; Ramirez, A.; Banks, K.E.; Johnson, R.D.; Lewis, R.J. Determination of select antidepressants in fish from an effluent-dominated stream. Environ. Toxicol. Chem. 2005, 24, 464–469. [Google Scholar] [CrossRef]
- Stensløkken, K.-O.; Ellefsen, S.; Vasieva, O.; Fang, Y.; Farrell, A.P.; Olohan, L.; Vaage, J.; Nilsson, G.E.; Cossins, A.R. Life without Oxygen: Gene Regulatory Responses of the Crucian Carp (Carassius carassius) Heart Subjected to Chronic Anoxia. PLoS ONE 2014, 9, e109978. [Google Scholar] [CrossRef]
- Killgore, K.J.; Hoover, J.J. Effects of hypoxia on fish assemblages in a vegetated waterbody. J. Aquat. Plant Manag. 2001, 39, 40–44. [Google Scholar]
- Van Landeghem, M.; Wahl, D.H.; Suski, C.D. Physiological responses of largemouth bass to acute temperature and oxygen stressors. Fish. Manag. Ecol. 2010, 17, 414–425. [Google Scholar] [CrossRef]
- Bunch, A.J.; Allen, M.S.; Gwinn, D.C. Influence of macrophyte-induced hypoxia on fish communities in lakes with altered hydrology. Lake Reserv. Manag. 2015, 31, 11–19. [Google Scholar] [CrossRef]
- Tammi, J.; Lappalainen, A.; Mannio, J.; Rask, M.; Vuorenmaa, J. Effects of eutrophication on fish and fisheries in Finnish lakes: A survey based on random sampling. Fish Manag. Ecol. 1999, 6, 173–186. [Google Scholar] [CrossRef]
- Kolasa-Jamińska, B. The intensification of pond fish production and the magnitude of the waste load discharged during autumn harvesting. Arch. Pol. Fish. 2002, 10, 187–205. [Google Scholar]
- Zhao, L.; Li, W.; Lin, L.; Guo, W.; Zhao, W.; Tang, X.; Gong, D.; Li, Q.; Xu, P. Field investigation on river hydrochemical characteristics and larval and juvenile fish in the source region of the Yangtze River. Water 2019, 11, 1342. [Google Scholar] [CrossRef]
- Steidl, J.; Kalettka, T.; Bauwe, A. Nitrogen retention efficiency of a surface-flow constructed wetland receiving tile drainage water: A case study from north-eastern Germany. Agric. Ecosyst. Environ. 2019, 283, 106577. [Google Scholar] [CrossRef]
- Fisher, J.; Acreman, M.C. Wetland nutrient removal: A review of the evidence. Hydrol. Earth Syst. Sci. 2004, 8, 673–685. [Google Scholar] [CrossRef]
- Jones, J.; Brett, M.T. Lake Nutrients, Eutrophication, and Climate Change. Global Environmental Change; Springer: Berlin/Heidelberg, Germany, 2014; pp. 273–279. [Google Scholar] [CrossRef]
- Drenner, S.M.; Dodson, S.I.; Drenner, R.W.; Pinder, J.E., III. Crustacean zooplankton community structure in temporary and permanent grassland ponds. Hydrobiologia 2009, 632, 225–233. [Google Scholar] [CrossRef]
- Chase, J.M.; Knight, T.M. Drought-induced mosquito outbreaks in wetlands. Ecol. Lett. 2003, 6, 1017–1024. [Google Scholar] [CrossRef]
- Bastin, J.; Clark, E.; Elliott, T.; Hart, S.; Hoogen, J.V.D.; Hordijk, I.; Ma, H.; Majumder, S.; Manoli, G.; Maschler, J.; et al. Understanding climate change from a global analysis of city analogues. PLoS ONE 2019, 14, e0217592. [Google Scholar] [CrossRef]
- Oertli, B.; Joye, D.A.; Castella, E.; Juge, R.; Cambin, D.; Lachavanne, L.-B. Does size matter? The relationship between pond area and biodiversity. Biol. Conserv. 2002, 104, 59–70. [Google Scholar] [CrossRef]
- Williams, P.; Whitfield, M.; Biggs, J.; Bray, S.; Fox, G.; Nicolet, P.; Sear, D. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol. Conserv. 2003, 115, 329–341. [Google Scholar] [CrossRef]
- Fuentes-Rodri’Guez, F.; Juan, M.; Gallego, I.; Lusi, M.; Fenoy, E.; Leon, D.; Penalver, P.; Toja, J.; Casas, J. Diversity in Mediterranean farm ponds: Trade-offs and synergies between irrigation modernization and biodiversity conservation. Freshw. Biol. 2013, 58, 63–78. [Google Scholar] [CrossRef]
- Widelska, E.; Walczak, W. Restoration of ponds in the municipal park in Zduńska Wola, Poland. J. Water Land Dev. 2020, 44, 151–157. [Google Scholar] [CrossRef]
- Sawatzky, M.E.; Martin, A.E.; Fahrig, L. Landscape context is more important than wetland buffers for farmland amphibians. Agric. Ecosyst. Environ. 2019, 269, 97–106. [Google Scholar] [CrossRef]

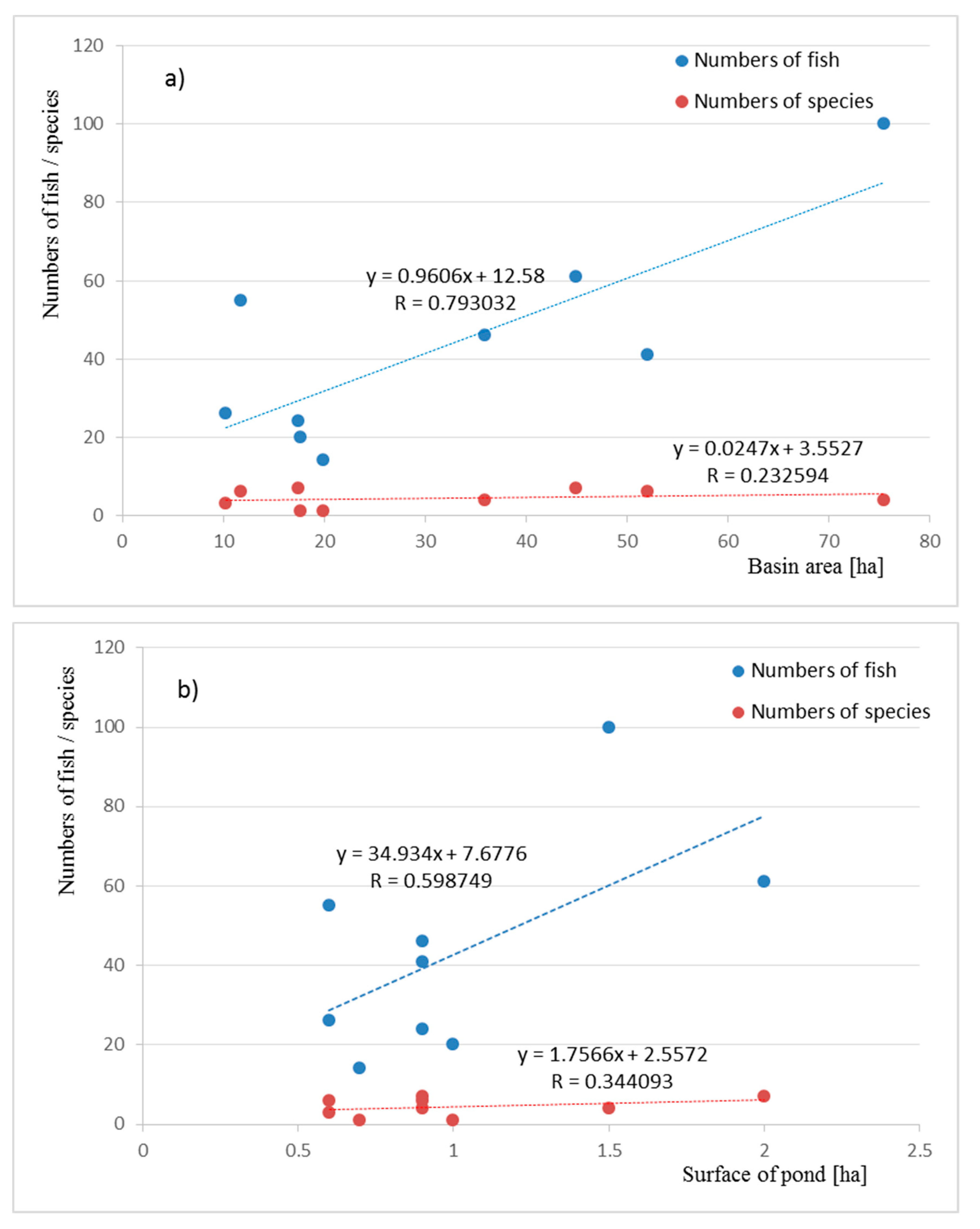
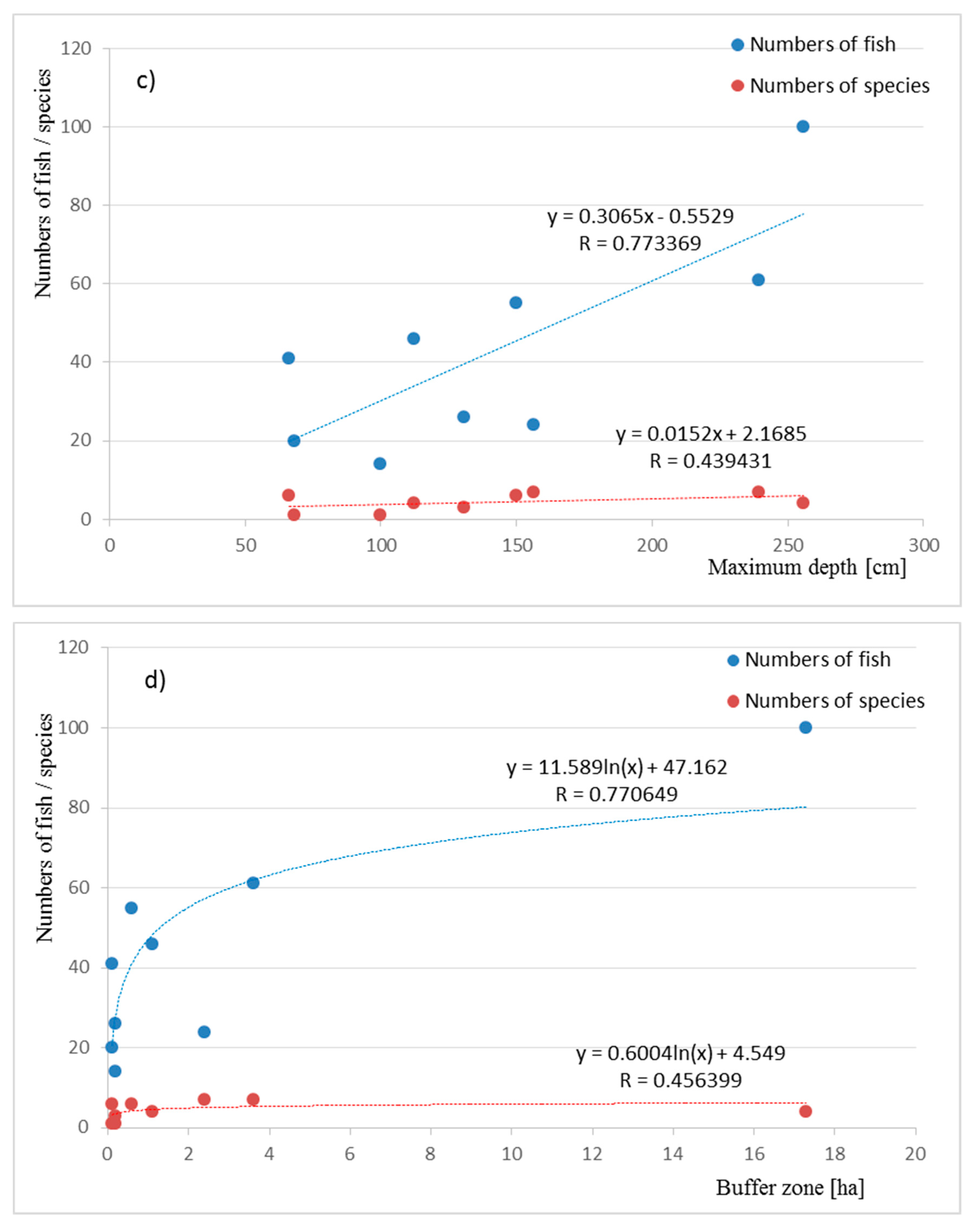
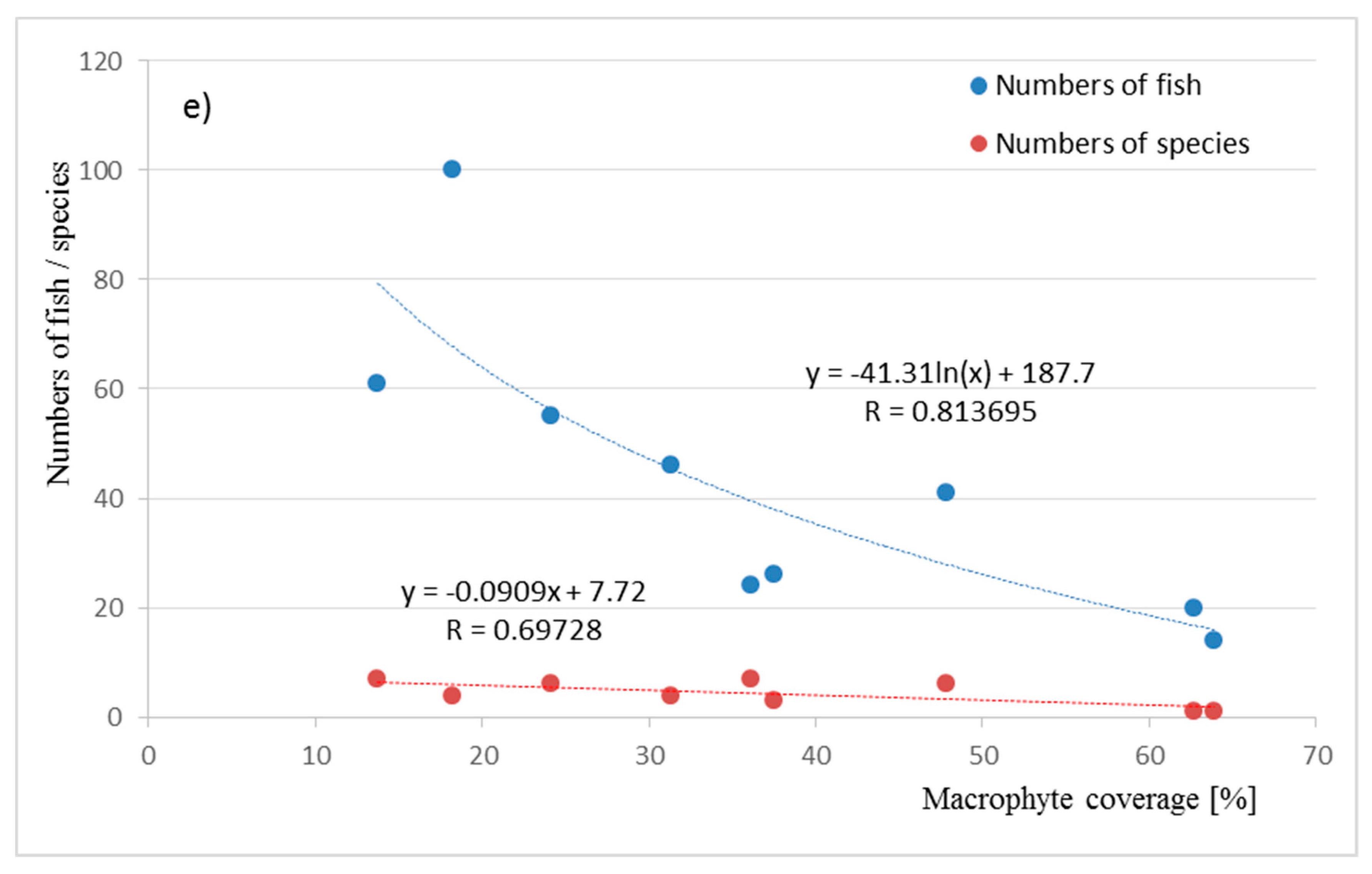
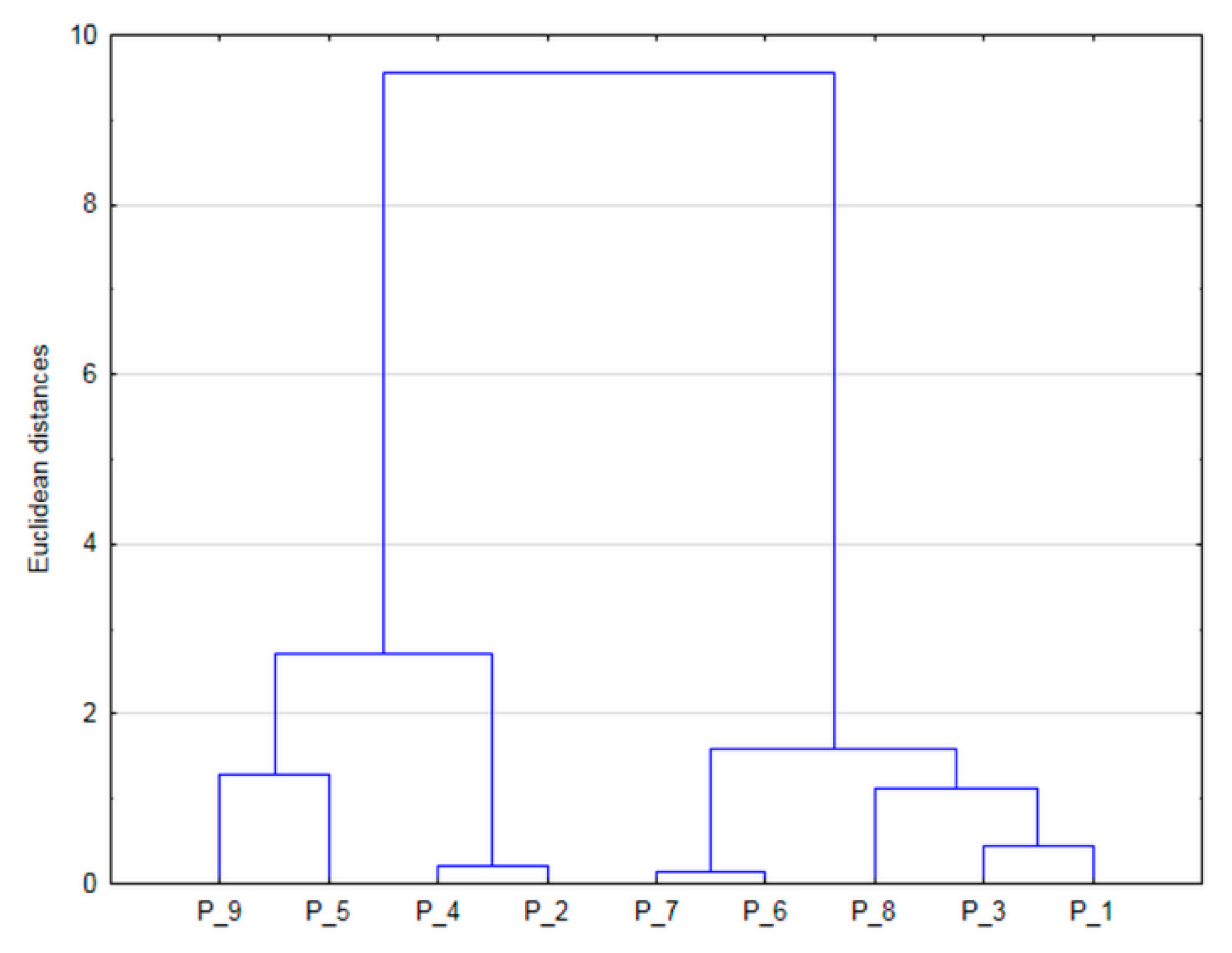

| Pond | P_1 | P_2 | P_3 | P_4 | P_5 | P_6 | P_7 | P_8 | P_9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Surface of the pond (ha) | 0.93 a | 0.17 | 1.03 a | 0.12 | 0.92 a | 0.06 | 0.72 b | 0.08 | 0.63 b | 0.12 | 0.63 b | 0.09 | 1.97c | 0.05 | 0.87 ab | 0.17 | 1.53 c | 0.12 |
| Water depth (cm) | 112.14 bc | 18.90 | 68.33 a | 12.87 | 66.29 a | 18.09 | 100.00 b | 11.55 | 130.71 cd | 8.86 | 150.00 de | 13.54 | 239.29 f | 7.87 | 156.43 e | 11.07 | 255.71 f | 21.88 |
| Macrophyte coverage (%) | 31.33 b | 4.79 | 62.70 d | 11.37 | 47.84 c | 1.51 | 63.86 d | 13.73 | 37.54 bc | 1.84 | 24.11 ab | 1.78 | 13.76 a | 2.36 | 36.13 b | 2.31 | 18.20 a | 5.07 |
| Temperature (°C) | 16.11a | 5.45 | 15.84 a | 6.41 | 16.32 a | 6.59 | 19.16 a | 2.60 | 19.54 a | 3.35 | 16.19 a | 4.09 | 17.13 a | 4.05 | 17.00 a | 4.02 | 17.22 a | 4.06 |
| Conductivity (μS/cm) | 406.00 c | 28.44 | 446.00 c | 49.70 | 128.57 a | 54.30 | 134.29 a | 15.38 | 246.43 b | 24.28 | 467.86 c | 33.34 | 575.14 d | 93.71 | 527.71 cd | 76.29 | 288.57 b | 23.97 |
| Saturation oxygen (%) | 86.87 b | 24.09 | 15.62 a | 6.91 | 28.47 a | 14.36 | 97.17 b | 14.31 | 82.19 b | 15.96 | 81.13 b | 14.46 | 73.19 b | 14.91 | 74.81 b | 12.78 | 82.11 b | 15.58 |
| pH | 7.91 d | 0.10 | 7.50 bcd | 0.31 | 6.93 a | 0.15 | 7.14 ab | 0.15 | 7.21 ac | 0.21 | 7.51 bc | 0.24 | 7.38 bc | 0.28 | 7.35 bcd | 0.20 | 7.62 d | 0.34 |
| Nitrates (mg ·dm−3) | 0.91 b | 0.03 | 0.06 a | 0.01 | 0.10 a | 0.02 | 0.33 ab | 0.14 | 0.31 ab | 0.13 | 0.63 ab | 0.34 | 0.69 ab | 0.51 | 2.11 c | 0.78 | 0.45 ab | 0.36 |
| Ammonium (mg ·dm−3) | 0.56 bc | 0.25 | 0.10 a | 0.07 | 0.11 a | 0.06 | 0.88 c | 0.36 | 0.54 bc | 0.10 | 0.33 ab | 0.10 | 0.31 ab | 0.17 | 0.36 ab | 0.25 | 0.25 ab | 0.13 |
| Phosphates (mg ·dm−3) | 0.48 bc | 0.36 | 0.49 c | 0.39 | 0.56 c | 0.32 | 0.06 ab | 0.02 | 0.05 a | 0.03 | 0.06 ab | 0.04 | 0.31 ac | 0.38 | 0.10 ac | 0.04 | 0.02 a | 0.02 |
| Cl (mg ·dm−3) | 50.61 e | 8.62 | 42.77 d | 4.30 | 9.59 a | 5.86 | 4.46 a | 0.64 | 27.50 c | 4.65 | 43.54 de | 3.16 | 31.90 c | 1.01 | 29.06 c | 1.00 | 19.75 b | 2.13 |
| Na (mg ·dm−3) | 10.58 bd | 2.69 | 11.44 cd | 7.94 | 3.76 a | 2.67 | 4.69 ab | 0.74 | 7.81 ad | 1.24 | 15.71 d | 3.21 | 13.21 d | 3.20 | 10.96 d | 2.29 | 8.04 abc | 1.69 |
| K (mg·dm−3) | 18.18 d | 1.60 | 7.48 b | 2.85 | 7.47 b | 1.66 | 1.27 a | 0.43 | 7.44 b | 1.28 | 12.54 c | 1.33 | 2.94 a | 0.47 | 2.46 a | 0.76 | 2.79 a | 0.73 |
| Mg (mg ·dm−3) | 9.26 bc | 2.35 | 11.52 cd | 2.64 | 2.18 a | 1.96 | 2.00 a | 0.50 | 6.98 b | 0.67 | 11.83 cd | 0.87 | 13.41 d | 0.64 | 13.45 d | 1.71 | 11.18 cd | 0.60 |
| Ca (mg ·dm−3) | 44.56 bd | 15.84 | 66.24 d | 7.35 | 10.73 a | 4.30 | 16.90 ab | 2.43 | 28.66 ab | 9.36 | 28.62 ab | 7.48 | 44.70 bcd | 21.17 | 49.40 cd | 30.10 | 46.89 cd | 25.35 |
| Fe (mg ·dm−3) | 0.23 bc | 0.02 | 0.06 ac | 0.06 | 0.46 d | 0.33 | 0.15 a | 0.06 | 0.31 d | 0.13 | 0.03 ab | 0.01 | 0.01 a | 0.01 | 0.09 a | 0.06 | 0.01 a | 0.01 |
| Mg (mg ·dm−3) | 0.06 a | 0.02 | 0.01 a | 0.01 | 0.02 a | 0.03 | 0.05 a | 0.02 | 0.31 b | 0.25 | 0.07 a | 0.08 | 0.03 a | 0.02 | 0.03 a | 0.02 | 0.03 a | 0.03 |
| Zn (mg ·dm−3) | 0.01 a | 0.00 | 0.01 a | 0.01 | 0.01 a | 0.00 | 0.02 a | 0.01 | 0.02 a | 0.01 | 0.02 a | 0.01 | 0.01 a | 0.00 | 0.02 a | 0.01 | 0.01 a | 0.01 |
| Species | Preferences Habitat [40] | Breeding Groups [41] | Numbers of Ponds in Which Fish Were Found | Conservation Status * |
|---|---|---|---|---|
| Percidae | ||||
| European perch (Perca fluviatilis L., 1758) | Eurytopic | Phyto-litophilic | P_1, P_3, P_5, P_6, P_7, P_8 | - |
| Esocidae | ||||
| Northern pike (Esox lucius L., 1758) | Eurytopic | Phytophilic | P_1, P_3, P_6, P_7, P_8, P_9 | - |
| Cyprinidae | ||||
| Crucian carp (Carassius carassius L., 1758) | Limnophilic | Phytophilic | P_1, P_3, P_4, P_5, P_7, P_8, P_9 | - |
| Prussian carp (Carassius gibelio Bloch, 1782) | Eurytopic | Phytophilic | P_2, P_3 | - |
| Tench (Tinca tinca L., 1758) | Limnophilic | Phytophilic | P_1, P_3, P_5, P_8, P_9 | - |
| European bitterling (Rhodeus amarus Bloch, 1782) | Eurytopic | Ostracophilic | P_7 | PP; H2; B3 |
| Freshwater bream (Abramis brama L., 1758) | Eurytopic | Phyto-litophilic | P_6, P_8 | - |
| White bream (Blicca bjoerkna L., 1758) | Eurytopic | Phyto-litophilic | P_6, P_7 | - |
| Roach (Rutilus rutilus L., 1758) | Eurytopic | Phyto-litophilic | P_3, P_6, P_7, P_8 | - |
| Rudd (Scardinius erythrophthalmus L., 1758) | Eurytopic | Phytophilic | P_6, P_7, P_8 | - |
| Belica (Leucaspius delineatus Heckel, 1843) | Limnophilic | Phytophilic | P_9 | B3 |
| Index | P_1 | P_2 | P_3 | P_4 | P_5 | P_6 | P_7 | P_8 | P_9 |
|---|---|---|---|---|---|---|---|---|---|
| Number of Fish | 46 | 20 | 41 | 14 | 26 | 55 | 61 | 24 | 100 |
| Number of species | 4 | 1 | 6 | 1 | 3 | 6 | 7 | 7 | 4 |
| Margalef | 1.80 | 0.77 | 1.86 | 0.87 | 1.41 | 1.72 | 1.68 | 2.11 | 1.00 |
| Shannon | 0.51 | 0.00 | 0.51 | 0.00 | 0.19 | 0.74 | 0.71 | 0.67 | 0.38 |
| Simpson | 0.31 | 1.00 | 0.45 | 1.00 | 0.78 | 0.18 | 0.20 | 0.23 | 0.55 |
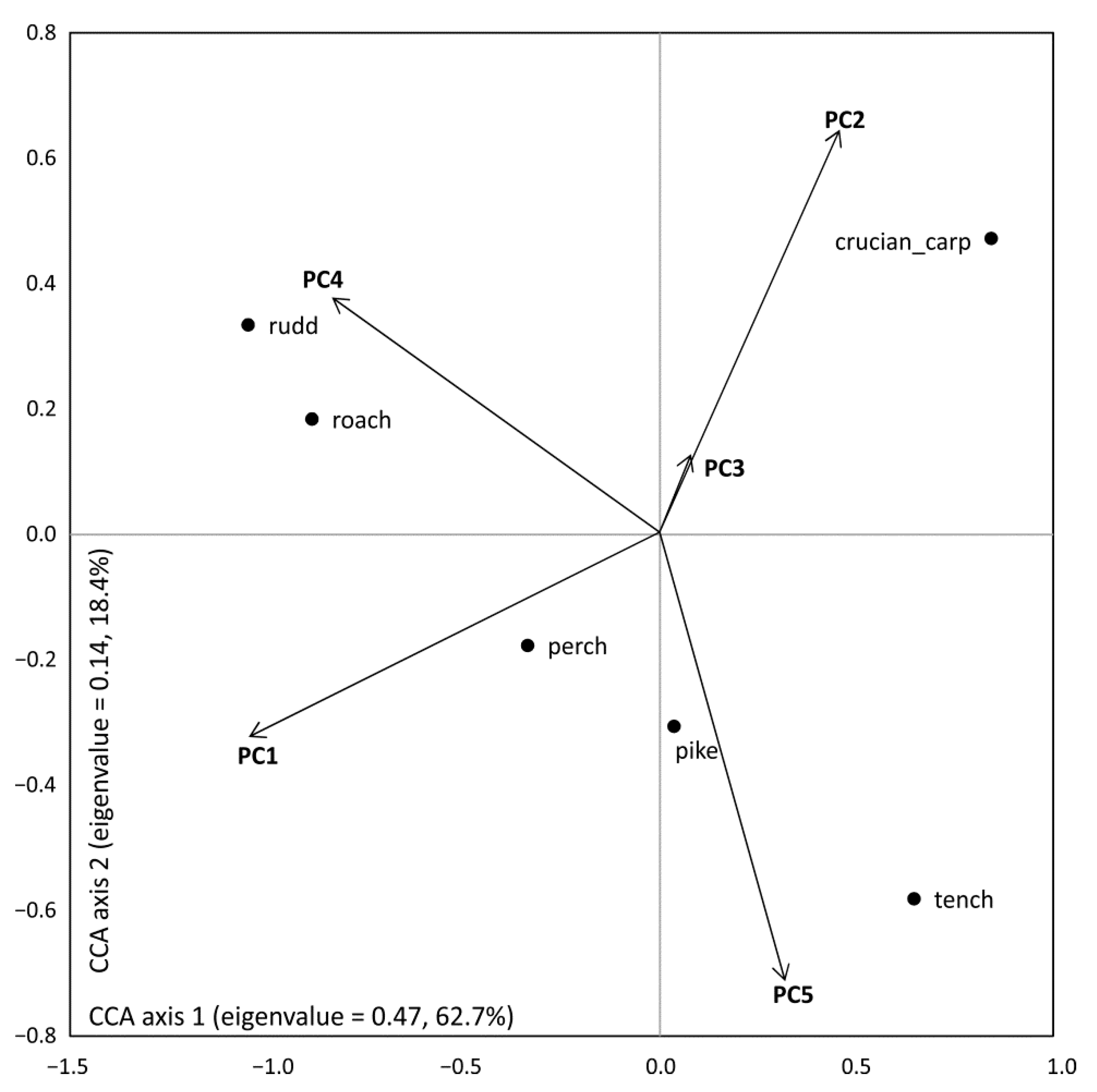
| PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|
| Eigenvalues | 7.76 | 4.49 | 2.71 | 1.47 | 1.23 |
| Percentage | 38.80 | 22.4 | 13.5 | 7.30 | 6.20 |
| Surface of the pond | 0.21 | −0.01 | −0.40 * | −0.23 | −0.16 |
| Buffer zone | 0.29 | 0.22 | −0.07 | −0.12 | 0.23 |
| Dist. to fields | 0.25 | 0.20 | −0.17 | 0.17 | −0.15 |
| Water depth | 0.25 | 0.25 | −0.21 | 0.24 | −0.19 |
| pH | 0.26 | −0.06 | 0.25 | 0.14 | 0.35 * |
| Conductivity | 0.31 * | −0.06 | 0.11 | −0.15 | 0.30 * |
| Saturation oxygen | 0.05 | 0.39 * | 0.19 | −0.23 | −0.11 |
| Temperature | –0.17 | 0.37 * | 0.01 | −0.12 | −0.37 * |
| Nitrates | 0.18 | 0.17 | 0.11 | −0.24 | 0.11 |
| Ammonium | −0.12 | 0.309 | 0.22 | 0.21 | −0.11 |
| Phosphates | −0.04 | −0.42 * | −0.07 | −0.25 | 0.14 |
| Cl | 0.23 | −0.19 | 0.39 * | 0.36 * | 0.03 |
| Na | 0.28 | −0.06 | 0.24 | −0.01 | −0.27 |
| K | 0.03 | −0.23 | 0.44 * | −0.39 * | 0.49 * |
| Mg | 0.34 * | −0.01 | 0.06 | 0.38 * | −0.05 |
| Ca | 0.27 | −0.13 | 0.02 | −0.10 | 0.07 |
| Fe | −0.27 | −0.10 | 0.06 | −0.11 | 0.18 |
| Mg | −0.12 | 0.20 | 0.31 * | −0.04 | −0.10 |
| Zn | −0.16 | 0.27 | 0.28 | 0.08 | −0.24 |
| Macrophyte | −0.25 | −0.14 | 0.02 | −0.34 * | 0.20 |
| df | MS | F | P | |||
|---|---|---|---|---|---|---|
| Abundance | Crucian carp | Model | 3 | 106.96 | 2.52 | 0.17 |
| Error | 5 | 42.40 | ||||
| Tench | Model | 3 | 8.77 | 0.21 | 0.88 | |
| Error | 5 | 41.54 | ||||
| European Perch | Model | 3 | 44.10 | 0.41 | 0.76 | |
| Error | 5 | 108.92 | ||||
| Northern Pike | Model | 3 | 11.40 | 0.65 | 0.62 | |
| Error | 5 | 17.61 | ||||
| Total length | Crucian carp | Model | 3 | 98.90 | 1.82 | 0.32 |
| Error | 3 | 54.32 | ||||
| Tench | Model | 3 | 84.13 | 0.23 | 0.87 | |
| Error | 1 | 370.33 | ||||
| European Perch | Model | 3 | 82.58 | 4.18 | 0.20 | |
| Error | 2 | 19.74 | ||||
| Northern Pike | Model | 3 | 65.77 | 0.18 | 0.90 | |
| Error | 2 | 366.51 | ||||
| Fulton index | Crucian carp | Model | 3 | 0.01 | 0.03 | 0.99 |
| Error | 3 | 0.23 | ||||
| Tench | Model | 3 | 0.10 | 1.11 | 0.59 | |
| Error | 1 | 0.09 | ||||
| European Perch | Model | 3 | 0.07 | 7.65 | 0.12 | |
| Error | 2 | 0.01 | ||||
| Northern Pike | ||||||
| Model | 3 | 0.01 | 0.74 | 0.62 | ||
| Error | 2 | 0.01 | ||||
| Species richness | Model | Model | 3 | 2.13 | 0.57 | 0.66 |
| Error | 5 | 3.71 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brysiewicz, A.; Czerniejewski, P.; Bonisławska, M. Effect of Diverse Abiotic Conditions on the Structure and Biodiversity of Ichthyofauna in Small, Natural Water Bodies Located on Agricultural Lands. Water 2020, 12, 2674. https://doi.org/10.3390/w12102674
Brysiewicz A, Czerniejewski P, Bonisławska M. Effect of Diverse Abiotic Conditions on the Structure and Biodiversity of Ichthyofauna in Small, Natural Water Bodies Located on Agricultural Lands. Water. 2020; 12(10):2674. https://doi.org/10.3390/w12102674
Chicago/Turabian StyleBrysiewicz, Adam, Przemysław Czerniejewski, and Małgorzata Bonisławska. 2020. "Effect of Diverse Abiotic Conditions on the Structure and Biodiversity of Ichthyofauna in Small, Natural Water Bodies Located on Agricultural Lands" Water 12, no. 10: 2674. https://doi.org/10.3390/w12102674
APA StyleBrysiewicz, A., Czerniejewski, P., & Bonisławska, M. (2020). Effect of Diverse Abiotic Conditions on the Structure and Biodiversity of Ichthyofauna in Small, Natural Water Bodies Located on Agricultural Lands. Water, 12(10), 2674. https://doi.org/10.3390/w12102674





