Abstract
In this overview (introductory article to a special issue including 14 papers), we consider all main types of natural and artificial inland freshwater habitas (fwh). For each type, we identify the main biodiversity patterns and ecological features, human impacts on the system and environmental issues, and discuss ways to use this information to improve stewardship. Examples of selected key biodiversity/ecological features (habitat type): narrow endemics, sensitive (groundwater and GDEs); crenobionts, LIHRes (springs); unidirectional flow, nutrient spiraling (streams); naturally turbid, floodplains, large-bodied species (large rivers); depth-variation in benthic communities (lakes); endemism and diversity (ancient lakes); threatened, sensitive species (oxbow lakes, SWE); diverse, reduced littoral (reservoirs); cold-adapted species (Boreal and Arctic fwh); endemism, depauperate (Antarctic fwh); flood pulse, intermittent wetlands, biggest river basins (tropical fwh); variable hydrologic regime—periods of drying, flash floods (arid-climate fwh). Selected impacts: eutrophication and other pollution, hydrologic modifications, overexploitation, habitat destruction, invasive species, salinization. Climate change is a threat multiplier, and it is important to quantify resistance, resilience, and recovery to assess the strategic role of the different types of freshwater ecosystems and their value for biodiversity conservation. Effective conservation solutions are dependent on an understanding of connectivity between different freshwater ecosystems (including related terrestrial, coastal and marine systems).
1. Introduction
Global climate change now threatens all ecosystems on Earth and the species they support, and the services and resources they provide to humans [1]. Freshwater is a precious resource and its future, along with that of the species and ecosystems it supports (almost 7% of global biodiversity in spite of freshwaters being tiny in their areal extent and relative volume [2]) is uncertain. Freshwater ecosystems are among the most endangered. Threats from climate change, contamination, water harvesting, impoundment, and other stressors are widespread, and no freshwater ecosystem is secure in the face of these threats [3]. Springs have been usurped for human consumption globally, and lakes in alpine, Arctic, and boreal areas are impacted by climate warming and airborne pollutants (e.g., [4]). De Graaf et al. (2019) [5] estimate that two-thirds of the world’s developed watersheds will reach environmental flow limits due to groundwater pumping by 2050. These impacts on freshwater ecosystems call for improved understanding of these threats, how they affect biodiversity, and how to counter them. Among the key knowledge gaps are accurate and precise global lists of freshwater-dependent species. Only relatively recently have major steps been taken towards compiling such lists [6]. Addressing such gaps will bring us closer to solving these challenges to sustainable freshwater ecosystem stewardship. Each of the papers in this Virtual Special Issue (VSI) addresses knowledge gaps, with most papers focused on an individual major ecosystem type.
All kingdoms of life are found in freshwaters. At least 126,000 animal species may be dependent on freshwater ecosystems [6]. Many groups of freshwater organisms are still poorly known, particularly microbes and protists. As advocated for cyanobacteria [7], a polyphasic approach integrating information gained from morphology, molecular phylogeny, bioorganic chemistry (e.g., [8]), and ecology should be applied whenever possible to the taxonomy of freshwater taxa. Organisms do not occur in isolation outside of a biotic assemblage and ecosystem, and individual species cannot be protected unless whole systems are conserved (e.g., [9]). Some recent global initiatives have focused on this issue. For example, the Red List of Ecosystems of IUCN [10] provides a “Collapse” (CO) category, the analog of the extinct (EX) category for species proposed by IUCN [11]. The only ecosystem classified in this way was a freshwater ecosystem, the Aral Sea. A severe reduction in freshwater biodiversity there by 2050 was predicted more than 15 years ago [12]. However, there is a general bias towards terrestrial conservation in general biodiversity assessment, and a plea was recently published to include freshwater species [13]. To counter negative trends, the Alliance for Freshwater Life was recently launched [14] as a global, united call to protect freshwater biodiversity. This conservation expert network aims to provide the critical mass for effective representation of freshwater biodiversity, to develop solutions balancing the needs of development and conservation, and to more effectively communicate the important role of freshwater ecosystems.
Continental aquatic ecosystems are among the most threatened and altered natural systems. Commonly, water is considered a free resource, and wetlands are perceived by people as wastelands that should be transformed into “useful” systems [15]. Considering coastal and inland waters, there is a continuous loss of natural wetlands and a continuous increase in man-made wetlands [16]. A review by Davidson (2014) [17] showed that the reported long-term loss of natural wetlands averages between 54%–57%, but the loss may have been as high as 87% since the 18th Century. Moreover, the author notes the lack of sufficient data to obtain a comprehensive overview of changes in wetland areas worldwide, particularly for Africa, Neotropics, and Oceania. Data are missing, especially for temporal trends, and also for some of the world’s major flooded forest areas such as those in the Amazon and Congo River basins [18].
In a compendium aiming at synthesizing global freshwater biodiversity in the different types of ecosystems, biogeographical patterns must be carefully considered. Since the early 19th century, the famous explorer-naturalist Alexander Von Humboldt realized that the tropics were richer in species than the temperate zones. Over time, many scientists formulated hypotheses to explain that pattern (e.g., reviews in [19,20,21]), yet the mechanisms responsible are still debated. But are tropical freshwater ecosystems richer in biodiversity than temperate ones [22]? As noted by many experts, the Palearctic region has the highest number of recorded species (for all taxa except vertebrates), but this result might not be representative of global patterns. Many scientists point out the lack of data for Central Africa, South-East Asia, and several parts of South America: in other words, most tropical regions [6,23,24]. Considering mammals, reptiles, amphibians, fishes, crabs and crayfish, Collen et al. (2014) [25] found the greatest biodiversity for freshwater animals in the Pantropical area. The Indo-Malay region has the greatest proportion of freshwater taxa and the Palaearctic the lowest. Brazil is the most diverse single nation, with over 12% of all freshwater species, followed by the southeastern USA (sub-tropical/temperate climate), West Africa across to the Rift Valley lakes, the Ganges basin, and the Mekong basin. In that study, the distribution of restricted-range species also was highlighted as they are irregularly distributed across the tropics. Centers of endemism exist in the African Rift Valley lakes (particularly Lake Malawi and Lake Tanganyika), Thailand, Sri Lanka, and New Britain (Papua New Guinea). The diversity of fishes in tropical South America includes more than 5600 species, representing a majority of the world’s freshwater fishes [26]. In particular, the Amazon River basin is a center of fish megadiversity, with about 2500 species described and probably more than 1000 not yet described. Most of these are small-range endemic species. This high diversity is not a result of recent in situ radiations, but the effect of biogeographical events over geological time, in which high levels of geographic isolation and ecological specialization have strongly contributed to the maintenance of high biodiversity [6,27,28,29]. As mentioned before, data on insect diversity should also be interpreted with caution, as many experts report strong sampling and study biases [30]. Especially, the Holarctic insect fauna is clearly better studied than that of the Neotropical, Afrotropical, and Oriental regions [6].
However, the western Amazon Basin and South-East Asia are the regions of highest dragonfly species richness [23]. According to Crow (1993) [31], who studied the distribution of aquatic plants across North America, aquatic plants did not show an increase in diversity towards the tropics, with some families dominating in temperate latitudes (i.e., Potamogetonaceae and Juncaginaceae) and others dominating in tropical latitudes (i.e., Podostemaceae and Hydrocharitaceae). Instead, a more recent study indicates that vascular macrophyte generic diversity for the tropics is greater than for temperate regions, and also species diversity may be greater for certain tropical regions compared to temperate ones. Tropical regions are also rich in endemic species compared with high latitudes, particularly Africa, where 64% of the total species present are endemic, and Central America and South America with 61% endemism [32]. Tropical freshwater ecosystems also encompass a great number of flood-tolerant tree species, as demonstrated in South America, where, in Amazonian wetlands, there are 53% of all tree species occurring in the overall Amazon [33]. Micro-organisms, such as bacteria (s.l.), viruses, protozoans, and fungi, are drastically understudied components of aquatic biodiversity [6]. Micro-algae and cyanobacteria are well studied, especially in Europe, North-America, Australia, and several South-American countries. So far, studies of freshwater diatoms indicate that there is no uniform response of diatom species richness across latitude: some authors find no pattern or even an inverse latitudinal gradient [34,35,36,37], while others found a positive response of species richness with a decrease in latitude [38,39]. Despite the scarcity of complete data (especially critical for the least-known groups such as Arthropoda, Nematoda, and micro-organisms) of the Oriental, the Neotropical, and the Afrotropical regions, the freshwater hotspots in terms of richness and endemicity are often located in these less-studied areas [6].
In this paper, we provide an overview of these different ecosystem types as an introduction to the VSI. We describe the characteristic features of each major type of freshwater ecosystem, the species and assemblages they support, and the threats they face. We also briefly explore what can be done to protect or restore these ecosystems. We thus aim at addressing the multiplicity of still and running freshwater environments, from headwaters down to large rivers and lakes (papers published in the VSI are cited in the following): groundwater and dependent ecosystems, springs and spring-fed streams [40,41,42,43,44,45], headwaters [46,47], glacial streams [48], streams, large rivers, ancient and large lakes, high-mountain lakes, oxbow lakes, reservoirs, urban freshwater habitats (fwh) [49], mires [50], small wetland ecosystems [51], Boreal and Arctic fwh, Antarctic fwh, Mediterranean fwh, tropical fwh [52], arid-climate fwh (Table 1; the ecosystem types addressed and flagship organisms are illustrated in Figure 1 and Figure 2).

Table 1.
Main freshwater habitat types, their biodiversity, and threats, the implication for conservation issues. The papers of the VSI dealing with the ecosystem type (if any) are listed in square brackets.
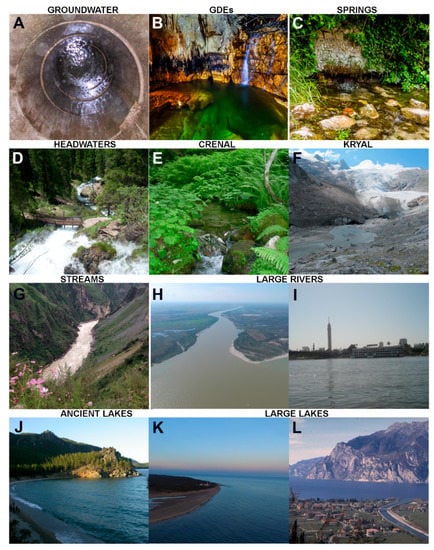


Figure 1.
Representative examples of the main different types of freshwater ecosystems considered in the present paper: (A) The unconsolidated alluvial aquifer: the view through a well (photo credit = p.c.: D.M.P.G.). (B) The saturated karst of the Stiffe Cave, central Italy (p.c.: D.M.P.G.). (C) Spring mouth in the karst: Capo Pescara spring system, central Italy (p.c.: D.M.P.G.). (D) Vallesinella headwaters (Adamello-Brenta Nature Park, southeastern Alps) fed by large karstic springs (p.c.: M.Ca.). (E) Spring-fed stream on siliceous substratum (Borzago Valley, Adamello-Brenta Nature Park) (p.c.: M.Ca.). (F) Alpine glacial stream (Schlatenbach) emerging from the glacial snout (p.c.: Leopold Füreder, Univ. Innsbruck, Austria). (G) Upper (rhithral) part of the Yangtze River (Jinsha River) (p.c.: Jianjun Wang, Nanjing Inst. Geography and Limnology, China). (H) The Danube River (p.c.: Deak Gyorgy, INCDPM). (I) The Nile River in Cairo (p.c.: A.A.S.). (J) Lake Baikal (p.c.: David M. Williams, The Natural History Museum, UK). (K) Lake Superior (p.c.: Skip Parish, USA). (L) Lake Garda, northern tip where the main inlet (Sarca River) enters into the lake (p.c.: Bruno Maiolini, MUSE). Representative examples of the main different types of freshwater ecosystems considered in the present paper: (M) A typical high-mountain lake on holocrystalline substratum: Lake Serodoli, Adamello mountain ranges (Alps), Adamello-Brenta Nature Park (p.c.: Bruno Maiolini, MUSE). (N) A typical high-mountain lake on carbonate substratum: Lake Antermoia in the Dolomites (Rosengarten/Catinaccio mountain range) (p.c.: M.Ca). (O) Ox-bow lake (p.c.: R.B). (P) Klíčava Reservoir in Central-Bohemia, Czech Republic (p.c.: P.Z.). (Q) Reschensee/Lago di Rèsia Reservoir in South Tyrol (Alps) (p.c.: M.Ca.). (R) The Schuylkill River in Philadelphia, PA, USA (p.c.: M.C.). (S) Summit bog in the Na Čihadle Nature Reserve in Jizerské (Izera) Mts, Czech Republic (p.c.: M.H.). (T) Fen close to Slussfors (Northern-Central Sweden), heterogeneous fen with alkaline spring-fed patches, more acidic patches, and a system of pools and quaking fens (p.c.: M.H.). (U) A small wetland ecosystem (p.c.: R.B.). (V) A Finnish boreal stream (p.c.: J.H.). (W) A Finnish subarctic stream (p.c.: J.H.). (X) Endorheic Lake Vanda, 85 m deep, 6 km long, and with a 3-4 m thick perennial ice cover, is one of Antarctica’s largest surface lakes (p.c.: I.H.). Representative examples of the main different types of freshwater ecosystems considered in the present paper: (Y,Z) Seasonal flow variability of Mediterranean streams and rivers: Monlleó river during flowing (Z) and dry (Y) phases (p.c.: N.C.). (AA) Mediterranean temporary rock pool (sensu EU Habitat Directive 92/43/EEC) hosting the quillwort Isoetes velata (p.c.: L.N.F.). (AB) Lake Arancio, a Mediterranean dam reservoir, filled at its maximum capacity (p.c.: L.N.-F.). (AC) Mediterranean Garcia Reservoir (p.c.: L.N.-F.). (AD) Mediterranean large near-natural lake, L. Kinneret (p.c.: Dr. Micky Cohensius, Moshavat Kinneret, Israel). (AE) Persistently stratified, deep, tropical Lake Matano, Sulawesi Island, Indonesia (p.c.: Z.L.). (AF) Aerial view of the Okavango Delta, a major subtropical wetland in north-western Botswana (p.c.: Luca Marazzi, Earthwatch Europe, Oxford, UK). (AG) Tropical stream (p.c.: S.P.). (AH) Wadi in the Eastern Desert of Egypt (p.c.: A.A.S.). (AI) Ras al Ain (p.c.: Friedhelm Krupp, Senckenberg Research Institute, Frankfurt a.M., Germany). (AJ) Waterfalls in the Wadi El Rayan Protected Area, Egypt (p.c.: A.A.S.).
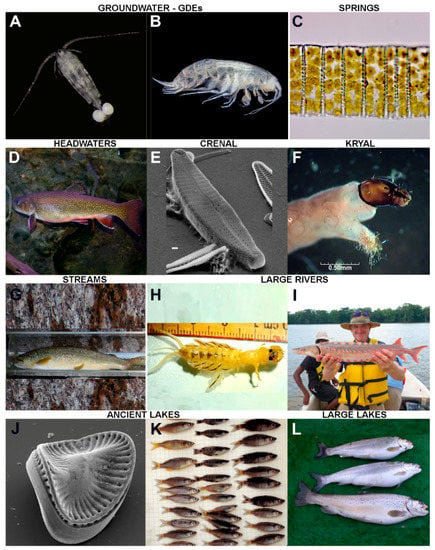

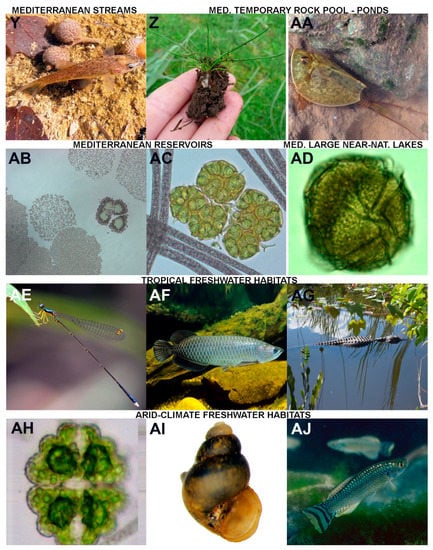
Figure 2.
Example flagship organisms of the main different types of freshwater ecosystems considered in the present paper: (A) Eudiaptomus sp., a stygobiotic calanoid copepod. (p.c.: D.M.P.G.). (B) Niphargus sp., a stygobiotic amphipod (p.c.: D.M.P.G.). (C) Band formed by several cells of the diatom Odontidium neomaximum (Borzago Valley, Adamello-Brenta Nature Park); macroscopic benthic blooms formed by Odontidium species are not rare in mountain springs on both carbonate and siliceous substratum (p.c.: M.Ca.). (D) Brook Trout (Salvelinus fontinalis) (p.c.: Brett Albanese, Georgia DNR–Wildlife Resources, “Fishes of Georgia” Photo Gallery on Flickr); this is a flagship species for headwaters in North America while it is an introduced invasive species in Europe. (E) Cymbella tridentina (scanning electron microscope = SEM), a diatom species typical of springs and spring-fed streams on carbonate mountains (modified from Cantonati et al. (2010)). (F) A midge larva of the genus Diamesa, which is typical and dominant in many glacial streams (p.c.: Leopold Füreder, Univ. Innsbruck, Austria). (G) Marble trout (Salmo marmoratus), found only in streams of a handful of drainages of the Adriatic basin (p.c.: Dott. Leonardo Pontalti, Forest and Wildlife Service of the Autonomous Province of Trento). (H) The mayfly Palingenia longicauda (p.c.: Oxana Munjiu, Academy of Sciences of Moldova). (I) Shortnose Sturgeon (Acipenser brevirostrum) (p.c.: Susan Wilde, University of Georgia, “Fishes of Georgia” Photo Gallery on Flickr). (J) SEM micrograph of Plagiodiscus glaber; this diatom is considered a flagship species of ancient Lake Ohrid (p.c.: Z.L.). (K) Endemic sailfins (Oryzias matanensis) from ancient Lake Matano (p.c.: Z.L.). (L) Salmo carpio (Ital. carpione), a salmonid fish endemic to Lake Garda in Italy (p.c.: Francesca Ciutti, Edmund Mach Foundation). Example flagship organisms of the main different types of freshwater ecosystems considered in the present paper: (M) The water flea Daphnia gr. longispina, a typical representative of the zooplankton of high-mountain lakes (p.c.: Nicola Angeli, MUSE). (N) Characteristic vertical zonation of cyanobacteria and lichens on a boulder of the rocky shores of a high-mountain lake (p.c.: M.Ca). (O) Water chestnut (Trapa natans) dominating the vegetation of an ox-bow lake (p.c.: R.B). (P) Coexistence of the threatened Potamogeton nodosus and the invasive Elodea nuttallii; in general, submerged macrophytes in reservoirs indicate suitable light conditions and water-level fluctuations, allowing for the development of a structured littoral zone (p.c.: M.Č.). (Q) Perch (Perca fluviatilis) is widespread and a sensitive indicator of eutrophication and hydromorphological modifications, and can, therefore, be considered the flagship organism of Eurasian reservoirs (p.c.: J.K.). (R) Eastern Water Dragon (Intellagama lesueurii) (p.c.: E.T.). (S) Sphagnum species (example: S. contortum) are important ecosystem engineers in acidic bogs and slightly alkaline (rich) fens (p.c.: M.H.). (T) Eriophorum latifolium indicates calcium-rich fens in a temperate climate; cotton-grasses shape the aesthetic perception of mires in many cases (p.c.: M.H.). (U) Water lily (Nymphaea alba) (p.c.: R.B.). (V) Mallard (Anas platyrhynchos), a very typical boreal species (p.c.: from Pixabay, pixabay.com). (W) Arctic grayling (Thymallus arcticus) (p.c.: from creative commons, creativecommons.org). (X) Oscillatorian cyanobacteria pinnacles at 20 m depth in L. Vanda are built over many decades and can reach heights >10 cm; perennially ice-covered lakes in Antarctica contain some of the most developed 3-D microbial mat structures on Earth (p.c.: Tyler Mackey). Example flagship organisms of the main different types of freshwater ecosystems considered in the present paper: (Y) The populations of the cyprinid fish Barbus haasi, endemic from the Iberian Peninsula, are declining in Mediterranean streams and rivers (p.c.: N.C.). (Z) Isoetes sp., a quillwort whose presence should grant protection to Mediterranean temporary ponds according to the EU Directive 92/43/EEC (p.c.: L.N.-F.). (AA) Lepidurus apus (Giara di Gesturi, Sardinia) a notostracan crustacean typically inhabiting temporary ponds (p.c.: Michael Korn). (AB) Microcystis spp., a typical representative of the phytoplankton in Sicilian dam-reservoirs (p.c.: Rossella Barone, Univ. Palermo). (AC) Cyanobacteria with gas vesicles (Planktothrix rubescens) and a buoyant green alga (Botryococcus brauni): typical representatives of the phytoplankton in Sicilian dam-reservoirs (p.c.: Rossella Barone, Univ. Palermo). (AD) The thecate dinoflagellate Peridinium gatunense from Lake Kinneret (diameter: ≈50 µm) (p.c.: Dr Alla Alster, Kinneret Limnological Laboratory, IOLR). (AE) The damselfly (male specimen) Amphicnemis platystyla (Fam. Coenagrionidae), Indonesian Borneo, peat-swamp specialist with fairly restrictive habitat requirements (p.c.: Brendan Holly, Borneo Nature Foundation). (AF) Australian Arowana (Gulf Saratoga), Scleropages jardini, found in Northern Australia and PNG (p.c.: Gunther Schmida). (AG) An American alligator (Alligator mississippiensis) in Everglades National Park, at the southern extreme of their range (p.c.: Luca Marazzi, Earthwatch Europe, Oxford, UK). (AH) The desmid Euastrum elfarafraense, described from an agricultural ditch fed by a rheocrenic, slightly-hot spring Ain El-Balad in the El-Farafra Oasis, Western Desert of Egypt (p.c.: A.A.S.). (AI) The desert-spring snail Juturnia kosteri (p.c.: Cayla Morningstar, Dept. Biology, Miami University, Oxford, Ohio, USA). (AJ) Aphanius dispar, particularly well adapted to arid environments (p.c.: Friedhelm Krupp, Senckenberg Research Institute, Frankfurt a.M., Germany).
We expect that the overview presented in this paper, together with the specific and more detailed information provided in each of the papers of this VSI, will guide scientific research and management aimed at protecting and restoring freshwater ecosystems around the world.
2. Ground Water and Groundwater-Dependent Ecosystems (GDEs)
2.1. Ground Water and Groundwater-Dependent Ecosystems
Groundwater (GW) is one of the most essential freshwater resources. As the world’s largest reservoir of freshwater (glaciers excluded), GW plays a significant role in supporting terrestrial and aquatic ecosystems and enabling human life and activities. Globally, GW is the source of one-third of all freshwater withdrawals, supplying an estimated 36%, 42%, and 27% of the water used for domestic, agricultural, and industrial purposes, respectively [53,54]. In Europe, 65% of drinking water originates from GW [55]. The baseflow of rivers, lakes, and wetlands during periods of little or no rainfall relies upon GW discharge. Hydrologists have long recognized the connectivity between GW and surface water (SW), and therefore management approaches that consider GW and SW as a joint resource are needed for effective management of water resources [56].
GW can be the main factor controlling the distribution and functionality of GDEs. GDEs are ecosystems whose species composition and ecological processes are influenced more or less directly by GW. GDEs vary in the degree of dependence on GW: some SW bodies are highly dependent on GW (for example, some SW bodies, such as GW-fed springs and upwelling sectors of streams and rivers across the hyporheic zone). Some GDEs are optionally dependent on GW when the subterranean water supports habitat conditions intermittently over time. It should also be noted that the dependence of an ecosystem on GW can be constant over time, or restricted to a particular period of the year, for example, during periods of drought (GDEs opportunistically dependent on GW). Subsurface groundwater-dependent ecosystems (SGDEs) are aquifers, also including in part and when present, their unsaturated portion (i.e., the vadose zone or the epikarst of some fractured aquifers and in caves).
The pressures acting on GDEs have significantly increased in the last decades and have determined the deterioration of their quantitative and qualitative status [57]. The database WATERBASE (the database containing data on the chemical quality and the characteristics of the GW bodies of European Union member states) revealed that 25% of GW bodies are characterized by a qualitative–quantitative state altered due to the co-occurrence of different anthropogenic factors [54,58,59]. Climate change [54], chemical pollution [60], overexploitation for irrigation [60], alteration of water flow, morphological alteration of SW bodies, drainage of wetlands, deforestation, and dam construction [59,61,62] are only a small portion of the activities that determine and have resulted in significant alterations of GDEs, with irreversible loss of biodiversity and severe changes in the composition of communities [63].
The main contaminants in groundwater are heavy metals [58,63], volatile organic compounds [63], pesticides [64], inorganic contaminants [58], and emerging organic contaminants (EOCs) such as pharmaceuticals and endocrine disruptors [65]. In GDEs, the contaminants transported by the water flow infiltrate into the subsoil, giving mixtures of toxic substances [64,65]. Mining and manufacturing industries and waste disposal activities are the main cause of contamination by heavy metals such as cadmium, lead, nickel, chromium, and copper [66]. The contamination by organic compounds originates from point sources of fuel production, refining and distribution, textile production, and the industries producing paints and pesticides [66]. Among the main organic pollutants present in GDEs, aromatic hydrocarbons (BTEX), and organochlorine hydrocarbons are widespread [66]. Pesticide contamination is mainly due to the presence of intensive agriculture, industry processes of timber, and activities of control of vegetation on road edges. Some chlorinated pesticides, organophosphates, carbamates, phenoxyacetic acids, and triazines [58,66] are persistent in groundwater, and as such, turn out to be particularly harmful to GDEs that are characterized by low-resilience communities, exposed for a long time to these pollutants.
In addition to pesticides, agricultural and agro-pastoral activities are among the main hazard centers that may determine the contamination by inorganic N-based compounds such as ammonium [63], nitrites, and nitrates [67], together with GW overexploitation for irrigation [60,68]. With regard to nitrates, the stygobionts (obligate GW dwellers) do not appear to be particularly sensitive [60], differently from what happens for ionized ammonium, which turns out to be lethal at concentrations ten times lower than the threshold value (0.5 mg L−1) established for groundwater in Italy [63].
The primary source of contamination from pharmaceutical compounds and endocrine disruptors in GW bodies is represented by urban and agricultural activities, which determine a load of wastewater in GDEs [69]. Among these hazard centers, there are hospital companies, food processing plants, municipal wastewater treatment plants, and industrial or agricultural production facilities [69,70]. As these endocrine disruptors are relatively new compounds, the ecotoxicological effects are still uncertain, even if their mode of action is known. Concern for EOCs in GW is expanding globally [71]. EOCs are natural or synthetic substances, not routinely monitored, that can induce known or suspected undesirable effects on humans and ecosystems [72,73]. EOCs’ family includes different classes of compounds such as disinfectants, industrials, pharmaceuticals, personal care products, and illicit drugs, some of which are being detected in groundwater with concentrations exceeding 0.1 μg L−1 [74,75]. Such compounds present in traces, under chronic exposure conditions (>4 days), alter the endocrine system with consequences for the development and reproduction of invertebrates living in groundwater [76].
Climate change is one of the leading factors threatening GDEs [77]. It can seriously affect the qualitative and quantitative components of GW resources, altering the interactions between GW and SW environments [62,78]. Together with GW overexploitation for many anthropic uses, climate change can help reduce seasonal water intake in the subsoil and determine an increase in the average temperature, a condition that is reflected not only on the quantitative and chemical-physical status of the GDEs but also on community composition. The effects of temperature increase due to climate change on GW fauna is still a matter of debate, even if recent studies have shown potential metabolic damage [79]. In particular, as regards the effect of climate change on the sensitivity of the invertebrates to agricultural pollutants, several studies [80,81,82] confirmed that an increase in temperature can alter the physiology of invertebrate species that live occasionally or permanently in the hyporheic habitat (for example, doubling the locomotion activity, altering the development time from the larval stages to the adult, increasing oxygen consumption rates, and even inducing death when the critical threshold is reached), increasing their sensitivity to pollutants, especially in those species with limited thermal tolerance (stenotherms) [79]. As a matter of fact, climate change may interact with many other stressors (e.g., pollutants). The toxicity of ionized ammonium to some GDE invertebrate species increases with temperature. Some other invertebrates are significantly stressed by a temperature increase of less than 3 °C relative to their thermal optimum [79]. For the GDE biota, the consequences of this double twine challenge may get worse than for SW communities. Hence, warm tolerant regional taxa may replace cold stenothermic ones, with probably complementary effects on ecosystem functioning but, at the same time, this will cause the non-substitutability extinction of one or more stenothermic species. In addition, in view of the predicted increases in water drought, pollution, marine intrusion, as is occurring in several GDEs, even the taxa most tolerant to warming will be unable to face the challenging chemical conditions or water depletion [81,82].
Increasing GW abstraction detrimentally affects coastal aquifers, leading to changes in the subsurface flow regime and inducing saltwater intrusion. Global-warming effects can increase GW salinity, altering GDE invertebrate physiology and diversity, especially for species with restricted tolerance to variation in salinity. If of prolonged duration, such stress leads to increased mortality [83] and potentially extirpation. Thus, an improved understanding of the interactions and feedbacks between GDE ecological processes and climate change is crucial for the development of appropriate solution-oriented strategies and measures for biodiversity conservation of GW and dependent ecosystems and cost-effective ecosystems-based climate change adaptation and mitigation. Multidisciplinary collaboration is needed to face these challenges [84].
SGDEs are expected to be at risk under a climate warming scenario [85]. For lotic ecosystems, vertical hydrological connectivity is crucial to conserve freshwater biodiversity and maintain ecosystem functioning. Climate change is predicted to alter flow regimes of many rivers and may disrupt these connections, with severe ecological and societal impacts (loss of their ecosystem services). However, the extents of these impacts, from a simple compositional shift in benthic and hyporheic assemblages [86] to more severe consequences on food web structure and ecosystem processes [86], have been mainly investigated in naturally intermittent streams from arid or semiarid regions, and little is known about the ecological effects of surface/subsurface flow alterations in perennial temperate rivers and streams [87]. These studies highlight the role of climate change in freshwater biodiversity loss and changes in assemblage structure in SW bodies; conversely, the effects generated in GW ecosystems and how they are reflected in GDEs are almost completely unknown. Global change can quickly trigger threshold switches between fresh and saline conditions, and water levels can be rapidly lowered to the extent that even perennial rivers, springs, ponds, and aquifers will dry.
GW harbors a unique fauna of unexpectedly high diversity that is not limited simply to the saturated zones of karst (e.g., caves) (Figure 1B) and the subsurface habitats of springs (Figure 1C). Stygobionts include bacteria, protists, invertebrates (e.g., platyhelminthes, annelids, molluscs, copepods—Figure 2A; syncarids, amphipods—Figure 2B; isopods, thermosbaenaceans, mysids, decapods), and vertebrates (amphibians and cavefish) [88,89]. Stygofaunal assemblages are commonly found in alluvial aquifers (Figure 1A) and the hyporheic zone of streams and rivers [90,91]. Stygobionts are strictly dependent on groundwater in which they complete their entire life cycle, and they have evolved traits to cope with the peculiarity of the GW environments. They generally are colorless and eyeless, small-sized, and may display a slender body [92,93]. These species have a low metabolism [82], slow development, and considerable longevity. Stygobionts have low fertility and long life-spans and are the basis of the low-resilience of GW communities [78,92,94].
A high degree of endemism characterizes stygobiotic assemblages, with most stygobionts being narrowly endemic and restricted to a single GW site, low local diversity, and truncated food webs for the absence of photoautotrophy [92], at places replaced by chemoautotrophy [95]. The total number of stygobiotic taxa worldwide is still underestimated, with many undescribed [96,97,98]. Although the ecological dimensions of GW and GDEs have been recognized for decades [77,99,100] routine biological monitoring programmes are still omitted in the current legislation at the global scale [85,101].
GW ecosystems have a biodiversity of great intrinsic conservation value, made up of rare, often endemic and particularly vulnerable species. Most taxa, at different taxonomic hierarchical levels (e.g., Crustacea Remipedia, Copepoda Gelyelloida, several genera of Copepoda Harpacticoida and Cyclopoida, Syncarida, Spelaeogriphacea, Thermosbaenacea) are phylogenetic and distributional relicts, the sole survivors of an ancient fauna whose ancestors lived in SWs and as a result of past climatic events have become extinct at the surface of the planet, thus now being the sole remnants of a fauna disappeared elsewhere. Many of these unique taxa, sometimes called “living fossils”, are considered to be at high risk of extinction at the global scale. However, to date, no tools are available to set biodiversity conservation priorities in GW habitats and the GDEs under the increasing anthropogenic pressures on the biological communities. Moreover, despite some SGDEs being Habitat Natura 2000 sites, most of these ecosystems and their outstanding biodiversity remain mostly unknown due to their "hidden" position. This, in turn, hinders efficient management planning, which, in a holistic view, includes, along with protection of the freshwater biodiversity, the GW component also. The conservation of GW biodiversity is limited by both intrinsic characteristics of taxa, and management issues. To acquire data for conservation purposes of the vast majority of invertebrates is extremely difficult, if not impossible, due to impediments such as (1) invertebrates and the high relevance of their ecological services are largely ignored by the public (social problem); (2) politicians and stakeholders are not informed about the problems of invertebrate conservation (political problem); (3) basic research and funding on invertebrates is insufficient (scientific problem); (4) most species remain unknown (Linnaean shortfall); (5) their distributions as described are poorly understood or unknown (Wallacean shortfall); (6) the number of populations and their variation in time and space is unknown (Prestonian shortfall); and (7) lifestyles and sensitivity to environmental changes are mostly unknown (Hutchinsonian shortfall) [102]. Recently the “Racovitzan shortfall” [103] has been added to explain the limits to collecting and discovering GW species. For these reasons, the development of international research programs for the less known invertebrate biodiversity is extremely important.
In 2001 the European Union granted the PASCALIS project (Protocols for the Assessment and Conservation of Aquatic Life in the Subsurface) to establish a strict protocol for the assessment of the biodiversity of aquifers, springs, and hyporheic environments at the Southern European scale, useful for defining effective sampling strategies and developing tools for conservation of subterranean fauna. GW biodiversity was mapped and analyzed [104], a few “hot spot” areas were identified by rarity, either of specific or generic richness of monotypic or exclusively European genera. The degree of endemism as a whole was high, but the results did not offer a reliable explanation as to the distribution of species or endemic taxa and underestimated stygodiversity due to the uneven availability of data. The BioFresh project [105] offered a further opportunity to refine the state of knowledge of the European stygobiotic fauna by creating a continental-scale database. It was populated with 21,700 records of a total of 1570 stygobiotic species. Analyses of those data indicated that Quaternary glaciation played a primary role in the spatial distribution of stygodiversity [106]. Stygofaunal richness increases significantly in the Balkans and other areas below 43° N [97]. However, the role of paleogeography and paleoclimatology since the Miocene [107], in the distribution of contemporary Balkan paleoendemics, remains outstanding. Robust data, such as those in the BioFresh database, should stimulate the European Union to include GW biodiversity in biomonitoring networks, to integrate the Habitat Directive by including GW habitats of priority interest, and identify rare or narrowly endemic taxa in the Annexes of the Habitat Directive 92/43/CEE [108] to develop a European network of priority sites for GDE and SGDE conservation. Given that the conservation of entire stygofaunal assemblages within aquifers is practically impossible and, in most cases, would be incompatible with socio-economic activities, identifying a network of priority sites is needed to understand long-term responses of GDEs and species to environmental changes [109]. The AQUALIFE project [110] proposed a new index for assessing priority sites of GDE biodiversity conservation based on the intrinsic conservation value of the species comprising the assemblages in each GDE site or cluster of sites. The Groundwater Biodiversity Concern (GBC) index is designed to assess the conservation value of GDE and SGDE assemblages within an aquifer by using a wide array of traits that represent different aspects of species conservation importance. The main advantage of the GBC index is its flexibility: It can be applied at any spatial scale (from single sites to an entire aquifer) and to any GDE type (e.g., springs, the hyporheic zone of streams and rivers, aquifers). To facilitate the application of the protocol, it has been fully implemented in the free software AQUALIFE available on the project website [110].
Systematic conservation planning methods [111] can also help to prioritize the protection of GW biodiversity and ecosystems. It was recently demonstrated that spatial prioritization for conservation that considers connections between rivers, wetlands, and GW ecosystems is likely to produce much more efficient conservation plans than separate prioritizations for these systems [112]. It was emphasized that these efficiency gains are conditional on integration at the planning stage but major efficiency gains are also likely if rivers, wetlands, and GW ecosystems are simultaneously considered in taking local actions.
2.2. Springs
As sites at which GW is exposed at the Earth’s surface, springs are abundant—highly individualistic [113], relatively self-contained, subsurface-surface linked, GW-dependent headwater wetland ecosystems that manifest high ecological, evolutionary, and socio-cultural interactivity, and which are globally threatened by human activities. With these characteristics, springs conservation is rapidly becoming a global concern. Although springs commonly emerge beneath the surface of streams, rivers, lakes, and the sea (subaqueous springs), our focus here is primarily upon terrestrial (subaerial) freshwater springs. Here, we review the derivation, distribution, typology, physical, biotic, eco-evolutionary, and socio-cultural characteristics and processes of spring ecosystems, as well as stewardship issues.
GW is almost entirely derived from meteoric precipitation. Depending on the regional tectonic and hydrogeological and climate setting, GW becomes altered through the infiltration process, with the emerging spring’s water quality typically related to flowpath length (ranging from <1 to hundreds of km) and duration (ranging from 0.01 to 106 y) (e.g., [114]). In karstic (limestone) aquifers, the long-term process of GW movement enlarges complex pathways that store and facilitate GW passage. Avenues to surface expression are usually driven by gravity, but sometimes by geothermal or gaseous pressures.
Estimates of springs abundance vary widely, ranging up to 50 million [115]. However, mapping of springs, even in developed countries, is incomplete and often inaccurate, and much more research is needed to better comprehend springs distribution, water production, and conservation threats. Our studies indicate that every natural stream in non-ice-dominated landscapes is sourced by springs or from lakes, many of which themselves are spring-fed [116].
The emergence environment of springs typically is where the interests of the hydrologists wane, but those of the ecologists intensify [84]. Recent attempts to classify springs have been based on physical, geographic, geochemical, geomorphological, biological, ecological, and human use variables [115,117], among which source geomorphology provides the least ambiguous typology [118,119,120,121]. Thirteen major terrestrial springs types, and several common sub-types, have been described, including cave, exposure (standing, exposed GW), fountain (artesian), geyser (geothermally or chemically driven), gushet (cliff emerging), hanging garden (seepage along a geologic contact), helocrene (floodplain and hillslope), hillslope (floodplain and upland), hypocrene (shallow subsurface flow expressed through wetland vegetation), mound-form (ice-form, chemical precipitate, and organic mounds), limnocrene (springfed pool with outflow), and rheocrene (emerging on the floor of a channel), as well as paleosprings that have not flowed in historic times [119,120]. Most GW emergence is gravity-driven, but some mineral-precipitating mound springs, geothermal, and CO2-gas driven GW flow occurs. Biological processes, such as biofilm-mediated precipitation of calcium carbonate, can influence flow emergence and the geomorphology at mineral-precipitating springs. Also, it is worth remembering that all spring types can be directly or indirectly altered or created by anthropogenic actions [120]. Several attempts have successfully distinguished within and among-taxon biological assemblage differences among springs types [84,118].
Springs ecosystems often support multiple geomorphological microhabitats including cave mouths, channels, madicolous (whitewater) flow, terraces, pools, spray zones, hyporheic habitats, peripheral riparian zones, and other microhabitats [117,120]. Each microhabitat may support its own soils or other substrata and suites of species that may or may not interact with others, but in combination, create a unique microhabitat mosaic. Springs geomorphic diversity can be calculated using the Shannon–Wiener diversity metric [122] based on the number and relative area of springs-associated microhabitats. Such calculations reveal positive correlations between springs geomorphic diversity and plant species richness at springs in arid and semi-arid landscapes across western North America [123,124]. Also, diatom species richness increases with habitat complexity in near-natural Swiss springs across elevation [45].
The disproportionately high biodiversity of springs includes a high percentage of threatened Red List species (e.g., [125]), rare and new-to-science taxa (from diatoms and invertebrates, to fishes [126,127], and many least-impaired habitat relicts, i.e., species requiring high-integrity environments and surviving only in near-natural springs in areas that are otherwise detrimentally affected by human activities [40]. We estimate that >10% of federally listed animal species in the USA are springs-dependent, at least for one phase of their life cycles. Although many springs are hotspots of biodiversity [127], arid land springs in Australia, Africa, and southwestern North America are particularly noted for supporting high levels of rare, threatened [43], and springs-endemic crenobiontic biodiversity [44].
Each springs ecosystem is subject to its own distinctive environmental disturbances and, depending on the source location, elevation, and aspect may have vastly different potential productivity. Interactions between disturbance and productivity (e.g., [128,129,130]), mediated by source geomorphology, springs type, regional climate, microclimate, elevation and latitude-dependent aspect, strongly control the shape, habitat area, and “hospitability” of springs to potentially colonizing biota. Thus, physical interactions strongly shape the physical geomorphological microhabitat mosaic on which the spring ecosystem develops, and springs should be regarded as “bottom-up” ecosystems [120]. Biological colonization involves both active and passive biogeographic colonization–extinction patterns, at least sometimes including assemblage nestedness (e.g., among desert springs fishes [131]). Trophic interactions in springs vary from simple systems with only a few, widespread species occurring in harsh or shaded settings, to highly productive (NAPP >5 kg·m−2·year−1) systems with remarkably complex trophic interactivity [132,133], and a significant amount of endemic species [134,135,136,137,138,139,140,141].
As a result of interactions among the above factors, shallow-aquifer springs ecosystems can be highly dynamic, changing across temporal scales of days to millennia. However, deep-aquifer/long flowpath springs can lag behind climate events by months to many (>100) years [142]. This relative ecological stability over evolutionary time permits adaptation and the evolution of complex assemblages of highly endemized taxa in harsh, constant environments, such as Montezuma Well in Arizona, Ash Meadows in Nevada, Cuatro Ciénegas in northern Mexico, Dalhousie Springs in Australia, and many other sites [120,133,137,140]. Increased incidence of spring-dependent endemism in such springs is common among aquatic invertebrates [127] but is relatively rare among vascular plants in North American, European, and Australian studies of springs. For example, there are only a few endemic, springs-dependent vascular plant species in the American Southwest, although many dozens of rare wetland plant species occur at springs. This pattern poses yet another unexplained evolutionary mystery about springs. Diatom and algae communities (e.g., [125,143]) include species that occur preferentially in springs sources (e.g., [144,145]; Figure 2C) and associated habitats, such as the peculiar desmid Oocardium stratum [146] that occurs only in springs-associated limestone deposits precipitated by petrifying springs [147]. The comprehensive study of springs diatom communities is urgently needed because of anthropogenic threats (e.g., [42]), in particular, alteration of the morphology of the springhead and increased nitrate (e.g., [148]) or orthophosphate [41] concentrations. Also, perennial, near-natural springs are stable environments that integrate indirect spatio-temporal impacts, making them invaluable natural laboratories for long-term ecological research (e.g., [149]).
Human evolution is uniquely tied to springs, as demonstrated in the paleohydrological reconstruction of the Olduvai Gorge in Kenya [150], and springs have played an important role in human history (e.g., [151,152]). Despite their value to humans, springs and the aquifers that support them are globally threatened by human activities. Springs-dependent species are among recently documented extinctions in the American Southwest (e.g., Stephan’s riffle beetle Heterelmis stephani, Pahrump killifish Empetrichthys latos, Fish Lake pyrg Pyrgulopsis ruinosa, and other species). GW pumping, livestock and other agricultural uses and pollution, mining impacts, urbanization, and the introduction of non-native species have altered most of the springs on Earth, and many marine sulfide springs now are threatened by sea-floor mining [153]. The extent of ecological impairment of springs often exceeds 70% among many landscapes in Europe, North and Central America, and Australia, and urbanization of arid regions often depletes local GW, eliminating springs altogether [40,44]. Virtually every study of spring ecosystems indicates the need for improved conservation of springs and spring-dependent species. However, the only springs currently recognized for protection by the European Union are petrifying springs [147], and national protection of springs elsewhere in the world is rare. Several conservation organizations have begun to promote improved scientific and public understanding and management of springs, including the Howard T. Odum Florida Springs Institute (floridaspringsinstitute.org) and the Museum of Northern Arizona Springs Stewardship Institute in Flagstaff (www.SpringStewardshipInstitute.org and SpringsData.org) in Florida and throughout the world, respectively. Given the great biological, cultural, and socioeconomic value of springs and the crucial role of sustainable GW for continued human existence and cultural well-being, spring ecosystems warrant far more immediate conservation attention than they have previously received.
Future basic inquiries into spring ecosystems should include mapping, inventory, and ecosystem assessment using comparable, standardized protocols; ecosystem trophic dynamics analyses on different springs types; biogeographic analyses of colonization and extinction dynamics; and improved understanding of the role of springs in relation to adjacent non-spring ecosystems and across climate variation through analysis of differential subsidy exchange [154]. In terms of applied research, far more attention should be devoted to understanding the cultural and historic significance of springs, as well as their socio-economic importance in different landscapes.
3. Running Waters
3.1. Headwaters
We distinguish headwater streams as those lacking perennial tributaries, which one could also refer to as “source streams” [47,155]. This separates the source streams from those that have their own tributaries in any network. According to the map-scale dependent system of stream orders [156], they may be smaller than first order streams, which are the first blue lines, for example, on a 1:50,000 topographic map. As the source streams in a network, and typically small habitats, they often provide refugia from larger-bodied predatory species. They also offer unique combinations of physical, chemical, and biological conditions from what is available in larger channels downstream [48]. Headwater streams may arise from several sources and may be a mixture of GW inputs, springs, and glacier or snowmelt, as described below (Figure 1D and Figure 2D).
3.1.1. Spring-Fed Streams (Crenal)
Springs can be extremely isolated (e.g., desert springs) or well connected to the surface running-water system. This connection is usually provided by spring-fed streams (e.g., [126]). Being GW fed, these streams typically have reduced fluctuations of physical and chemical factors and reduced solid transport and turbidity (Figure 1E). The diatom species Cymbella tridentina (Figure 2E) was consistently found in springs and the uppermost section of running-water systems, and thus to be typical of carbonate, high-ecological-integrity spring-fed streams [126].
3.1.2. Glacial Streams (Kryal)
Supraglacial meltwater channels [157] play a crucial role in transferring water and heat across and into ice. The supraglacial ecosystem is characterized by a diverse consortium of microbes (typically bacteria, algae, fungi, viruses, and occasional rotifers, tardigrades) in the supraglacial streams, and melt pools (cryoconite holes) [158].
In the high mountains, the most common origins of running-water systems are glaciers, springs, snowfields, surface runoff, and lakes [159]. Proglacial river systems, glacial or glacier-fed streams and rivers that flow from large alpine glaciers are extreme habitats (Figure 1F), in particular, because of the very high turbidity due to the fine suspended glacial debris and the very low temperatures (e.g., [160]). It has been highlighted that the disappearance of glaciers among the water supply sources leads to a significant reduction in the alpine invertebrate diversity (α-, β-, and γ-diversity; [161]) of proglacial river systems [162]. This is partly due to the fact that the turbid streams present extremely low-diversity and highly specialized invertebrate communities that also include a few endemic taxa. This situation does not seem to apply to benthic algae and cyanobacteria since the relatively few species that colonize turbid streams [163] are also found in high-altitude running waters of different origin. However, the genetic characteristics of these species and the possible presence of cryptic diversity have not been verified. Turbid streams fed by large, active glaciers are among the few aquatic habitats that seem to be only scarcely colonized by diatom microalgae, probably due to mechanical damage (i.e., scouring effect) and the extreme reduction in transparency caused by fine mineral detritus in suspension. They appear to be colonized only by very low-diversity and simplified diatom communities [163]. Gesierich and Rott (2012) [164] demonstrated an increase in the diversity of diatom communities with a decrease in the percentage of glacial coverage of the basins. Brown et al. (2018) [165] also found that predictable mechanisms govern river-invertebrate community responses to decreasing glacier cover globally (e.g., increase in functional diversity as glacier cover decreases, dispersal limitation as the dominant process underlying these patterns, environmental filtering evident in highly-glacierized basins). Most research on the biology of glacial streams focused on the relationship between species presence/absence and prevailing environmental conditions, while functional strategies and potentials of glacial stream specialists were hardly investigated so far [48]. However, distinct functional properties (e.g., feeding strategies, early life development, body mass and growth) of invertebrates that typically dominate glacier-fed streams (usually chironomids of the subfamily Diamesinae; Figure 2F) and show significant relationships with declining glacier cover in alpine stream catchments can be recognized (e.g., [166]).
3.2. Streams (Rhithral)
A great deal of research has focused on mid-sized streams (Figure 1G), which we define as streams beyond headwaters, i.e., second-order or larger, up to a size where they are navigable by motorized boats (see rivers below, Section 3.3.) or approximately fifth- to sixth-order. Another definition of the divide between rhithral and potamal streams would be that when they reach a size where streams are not wadeable, i.e., they are too deep, they are potamal streams.
A landmark publication in stream ecology was the River Continuum Concept, which provided a coherent, if idealized, framework for how stream networks functioned [167]. This work portrayed a gradient or continuum in process rates from headwaters to downstream. The most obvious characteristics being that as stream size increased, gradients would get lower, geomorphology would become dominated by smaller materials, canopies would open relative to channel width allowing more light and primary productivity, and the relative contribution of leaf litter and other vegetation would diminish. These characterizations were extended to considerations of food webs and ecosystem metabolism. Moving from headwaters and other small streams to larger streams, the relative contributions of inputs from the catchment (i.e., leaf litter, wood, dissolved organic carbon) decrease and downstream food webs are supported by exports of particles from upstream (i.e., organic matter, invertebrates) and more local production (autochthony). These shifts result in the characteristic organization of food webs.
Flowing waters create environments different from lentic waters. The force of flowing water, particularly at high flows associated with floods, can rearrange elements of stream channels, creating a range of habitat types (e.g., [168]). The relatively frequent rearrangement of channel morphology results in a dynamic ecosystem with heterogeneous features, providing niche space for hundreds of species across many taxonomic groups.
The unidirectional flow of water leads to some unique features of streams. Many stream organisms take advantage of flow to move around, particularly downstream, which is referred to as drift for animals [169] or hydrochory for plants [170]. Another consequence is that organic materials and nutrients being recycled actually create a spiral as the open water phase moves those particles downstream as they are released from organisms and then they are taken up [171]. The idea of spiraling has been important to the development of predictions in stream ecology. The movements of particles in open flow also results in a range of organisms that depend on flow to deliver food. A diverse set of species are known as filter-feeders, particularly within the caddisflies, flies (e.g., black flies, midges), mussels, and a few others [172]. In addition, a foraging style of fishes, known as drift-feeders, use flow to deliver food to them, of which trout (Figure 2G) is a well-known example [173].
Lakes, wetlands and reservoirs can interrupt downstream fluvial ecology through serial discontinuity [174]. This discontinuity also leads to special cases of streams, such as lake outlet streams (also applicable to reservoir outflows), where large densities of filter feeders can capitalize on high densities of high-quality plankton and often on higher concentrations of suspended organic matter caused by high densities of some fish [175,176,177].
Food webs of streams often are highly heterotrophic, but in recent decades, the significance of primary production has once again been recognized, even in the smallest of streams. A typical view of a stream network portrays small streams as dependent on particulate organic matter, such as leaf litter, seeds, wood and more, and this material is processed and exported as a range of particle sizes to downstream environments.
A general conclusion about the relation between stream size and species richness is that species richness increases with stream size, a pattern often attributed to a greater diversity of habitat types, and this pattern often peaks at intermediate stream order. Surveys often have shown a positive, and sometimes asymptotic pattern in species richness across a gradient of stream sizes (e.g., [178]). Heino et al. (2005) [179] found positive relations for macroinvertebrates, fish, and bryophytes across a large stream gradient in Finland, even though slopes were not the same. A systematic review of patterns in fish and macroinvertebrate richness through stream-size gradients taken from 165 studies across first- to eighth-order streams generally showed positive relations, but not always [180]. In some large datasets, they found that species richness increased from lower to intermediate-sized streams, then declined in the largest of streams, i.e., seventh- to eighth-order [180]. The range of explanations for this often-positive pattern, including larger habitat area and complexity, habitat stability or variability, and a greater range of trophic resources, demonstrate the difficulty in testing for responsible mechanisms [180].
Running waters are all vulnerable to anthropogenic use and alteration. Exploitation and contamination of GW and its subsequent impacts on springs and headwaters have been addressed in other articles in this series (e.g., [42,46]). Many of the same threats affect streams across the size spectrum (see Conservation Issues under Section 3.3).
3.3. Large Rivers (Potamal)
There is no simple definition of the size of stream that we call large; however, a list of the 139 largest rivers of the world on Wikipedia all have annual mean discharges of >2000 m3/s average annual discharge. Another definition would be streams with catchments of ≥10,000 km2 [181]. Large streams (rivers) (Figure 1H,I) are difficult systems to study due to their large size, lack of replication, and reliance on specialized sampling gear [182]. Their geomorphology depends on parent rock geology and the amounts and sizes of substrates available to rework, and the characteristic flows that reshape morphology [183]. Large streams typically have low gradients with fine sediments, but there are many exceptions to this with steeper gradients and boulder substrates. Another generalization is that larger streams tend to be naturally turbid, carrying suspended sediments eroded from upstream.
Downstream, rivers are often bounded by estuaries, which create a highly biodiverse transition zone or ecotone that combines features of freshwater and sea. Floodplains (alluvial) that are typically inundated seasonally along large streams can provide important habitats to freshwater species, e.g., the floodplains of the Amazon and Orinoco rivers. The energy expended by moving water can create meanders and braiding that can subsequently create oxbows (see Section 4.3), as channel avulsions occur and cut off some meanders (e.g., [183]).
Large stream food webs are typically considered as dependent on the transport of fine organic materials from their catchments, and their turbidity and light attenuation by depth limiting of primary production (e.g., [167]). However, some rivers can support plankton species, including phytoplankton, in their slower-moving sections (e.g., [184]). A combination of inputs from upstream, shoreline inputs, and autochthony provide for the energetic basis of potamal food webs (Figure 2H,I).
There is a large variety of species in large streams, especially larger-bodied species such as sturgeons (Figure 2I), paddlefish, crocodiles and caimans, freshwater dolphins, and others. Many of these large and charismatic species are seriously endangered due to heavy use of rivers by humans (e.g., [185]). In fact, large freshwater megafauna (body mass >30 kg) have declined by 88% between 1970 and 2012, which is twice the loss rate of vertebrate populations on land or in the ocean [186]. Freshwater dolphins, turtles, hippos, and large fish species such as sturgeon and paddlefish, are particularly vulnerable. Current threats to the remaining megafauna are primarily overexploitation and the loss of free-flowing large rivers.
The biodiversity patterns of these larger river systems are frequently known in terms of commercially important fish (e.g., salmon, eels, smelt) and other charismatic species, but often the other species are less well known. Fish diversity is highest in tropical river basins, especially the Amazon, Congo, Mekong, Paraná, and Orinoco, each with over 500 species of freshwater fishes (BioFresh website: http://www.freshwaterplatform.eu/). Likewise, freshwater mammal diversity is highest in these five basins. For freshwater crayfish, the highest diversity in the world is found in the Mississippi River catchment, with well over 100 species. In contrast, two of the largest rivers in Europe, the Danube (Figure 1H) and the Volga, have only about 67 to 75 native freshwater fish species in their entire catchments [187]. Most groups of freshwater organisms reach their highest species richness in tropical regions, but a large fraction of diversity in flowing waters remains unknown. This lack of information concerns especially bacteria, algae, and many groups of invertebrates.
Most large and medium-sized rivers have been anthropogenically altered or impaired [2,188]. They are harnessed for hydroelectric power production, used as transportation corridors, diked to protect infrastructure, and drained for irrigation. There are over 57,000 large (>15 m) dams and over 300 megadams (>150 m) worldwide (International Rivers Network web page). Additional 3700 large dams are planned or under construction. More than 800 of these planned dams are located in the diversity hotspots of freshwater megafauna, including Amazon, Congo, Mekong, and Ganges basins.
Conservation issues resulting from cumulative effects of dams involve declines in native freshwater biodiversity at regional scales. Many large rivers have been transformed into a series of reservoirs (dominated by introduced exotic species) connected by highly regulated flows that contain remnant populations of native riverine species. For example, of the 51.2 million km of streams in the lower 48 USA states, only 2% of the rivers remain free-flowing and are relatively undeveloped. Only 42 free-flowing rivers exist that are more than 200 km or more in length. The other 98% of USA streams have been developed [189].
The natural seasonal variability in discharge of these heavily dammed rivers has been significantly reduced, interfering with the life cycle of native freshwater organisms that are adapted to different flow regimes. Regional effects of dams in North America include extirpation/extinction of (1) native migratory taxa (salmonids, sturgeons), (2) small bodied-obligate riverine taxa (darters), (3) flood-dependent taxa, and (4) taxa dependent on freshwater inflows to estuarine habitats (e.g., delta smelt) [189]. There also have been regional increases in lentic exotic taxa, which now abound in many reservoirs and regulated reaches [189].
Cumulative impacts of dams exert other landscape-scale effects on biodiversity through (1) greenhouse gas emissions from reservoirs, (2) methylmercury mobilization and bioaccumulation in aquatic foodwebs, and (3) reduced transport of sediments and silica to coastal waters [190]. All reservoirs emit greenhouse gases for a minimum of decades and contribute significantly to climate change. The fuel for these greenhouse gases is rotting organic matter from vegetation and soils flooded when the reservoir is filled. Anaerobic sediments in reservoirs also contribute to the mobilization of methylmercury into freshwater food webs. All water bodies in the northern hemisphere are contaminated with mercury which biomagnifies within freshwater food webs, resulting in toxic effects on fish-eating wildlife and human fish consumption advisories for reservoirs throughout parts of the US (https://www.epa.gov/). Finally, the retention of sediment and silica in reservoirs behind dams results in sediment-starved beaches and reductions of silica that can discourage silicate-using diatoms and favor nuisance cyanobacteria [190].
Cumulative effects of irrigation on river ecosystems are also of great conservation concern in many regions across the globe. About 70% of the water that is abstracted from freshwater systems is used for irrigation, and irrigated areas are expected to increase rapidly due to population growth and an increase in food demands [190]. Under current conditions, 30% of irrigated crop production compromises environmental flow requirements in streams and rivers [190]. Rivers and associated wetlands in arid areas are becoming increasingly degraded and contaminated from irrigation return flow with severe negative effects on freshwater biota and associated wildlife.
Stewardship of large rivers has increasingly involved changing dam management criteria and strategies to improve flow regimes in rivers [191]. In many developed countries, the removal of obsolete dams is increasing. In 2018, eighty-two dams were removed across eighteen states within the USA (with 35 in CA). In 2019, the largest dam removal project in Europe began on the Selune River (Brussels) with the 36 m-high Vezins Dam.
Countries around the globe differ in their stewardship approaches to protect rivers and streams (e.g., [9]). As just one example, non-governmental organizations (e.g., American Rivers, Washington, DC, USA; Pacific Rivers Council, Portland, OR, USA; the Nature Conservancy, Arlington, VA, USA) in the US play a central role in the development of river conservation for natural values, ranging from developing conceptual paradigms to both collaborating with and litigating against government agencies. This is in contrast to other countries such as the UK, where strong stewardship is more centralized and characterized by relatively less NGO activity [192].
In some countries, such as Bangladesh, the case has been made that rivers should have their own rights. The first country to grant a specific river legal rights was New Zealand in 2017, followed by the Indian state of Uttarakhand. In July 2019, Bangladesh became the first country to grant all of its rivers the same legal status as humans. This landmark ruling by the Bangladeshi Supreme court is meant to protect the world’s largest delta from further pollution. Anybody accused of harming the rivers can be taken to court by the new government appointed National River Conservation Commission.
International NGOs are playing an increasingly important role in river stewardship across the globe. As just one example, International Rivers (https://www.internationalrivers.org/) protects rivers by empowering the public, stopping destructive projects (often dams) and addressing legacies, raising public awareness, and promoting sustainable alternatives/solutions.
4. Lakes
4.1. Ancient and Large Lakes
Globally, there are 253 freshwater lakes with a surface area >500 km2 and over 75 lakes with a maximum depth >100 m [193]. Out of the 14 largest lakes worldwide, five are ancient lakes. Others, like the Laurentian Great Lakes of North America, are relatively young, and their species assemblages are mainly composed of postglacial colonists [194]. Nevertheless, some endemic species are also known from postglacial large lakes (e.g., the Lake Garda (Figure 1L) salmonid fish Salmo carpio; [195], Figure 2L). Moreover, many large lakes are oligotrophic habitats which are frequently species rich (e.g., [196]). In North America, nearly 500 diatom species were found in a sample of bryophytes from deep waters of Lake Superior (Figure 1K) [197].
The term “ancient lake” is applied to any lake that existed across the last glacial cycle, >130,000 y [198]. Most lakes around the globe are younger than 10,000 y (post-glacial origin), and only a few have survived more than 100 ka [199]. Ancient lakes are considered paleorefugia (sensu [200]), qualitatively differing from younger (post-glacial) lakes because they hold relatively higher proportions of endemic species [201]. High diversity and endemism can be observed across almost all eukaryotic groups in most, if not in all, ancient lakes [202]. However, the level of endemism and diversity varies among lakes. There are many well-documented examples across biological groups (from diatoms to fish) of endemism in ancient lakes [203,204,205]. The success of endemism with elevated species diversity can be attributed to the long-term persistence and ecological stability of ancient lakes. However, all global environments are subjected to stress events including catastrophic events. For example, lakes Tanganyika, Malawi, and Victoria experienced episodes of megadroughts [206], with some evidence of complete desiccation [207]. Also, Lake Titicaca in South America went through drought and salinization during the Marine Isotope Stage (124,000–119,000 years ago) that caused significant changes in diatom species composition [208].
Most ancient lakes originate from shifting tectonic plates (tectonic graben or tectonic uplift, also called drift valleys) or impact craters which fill with the long-term storage of water [198]. The retention time of water in ancient lakes can vary from 49 to 5500 years (https://en.wikipedia.org/wiki/Lake_retention_time). Thus, the scale of impact (millennia+) will dictate a climate driver of major change with finer levels of change under local physical, chemical, and biological pressures (e.g., decadal to centuries) [209]. Trapped waterbodies with continuous atmospheric and riverine water inputs, coupled with evapotranspiration, will naturally concentrate ions. Unless other environmental stressors (override) the system (e.g., flooding events), lakes will naturally become more brackish. Lakes Titicaca and Hövsgöl are examples of waters with ionic salt accumulation [208,210]. Less abundant metals (including heavy metals) can also accumulate in ancient lakes when local geology provides erosional material. Lake Matano (Indonesia, Figure 1AE) has metal accumulation (e.g., iron) under these circumstances [211,212]. In contrast, Lake Baikal (Figure 1J) has not shown clear increases in ions and metals [213]. The volume of water (including surface area to volume ratios), lake water retention, surface flow, and northern location have buffered Lake Baikal from detectable changes over centuries [213]. The African rift lakes from Lake Malawi to Lake Victoria show a wide gradient of ion and metal accumulation (Lake Tanganyika with high conductivity to Lake Victoria with low conductivity), demonstrating both age and differentiated environmental selecting factors. Talling and Talling [214] even classified the African lakes into three classes according to total ionic concentrations. The aforementioned chain of rift lakes in Africa shows that regional climate, along with physical and chemical factors, can have a totalistic impact on ancient lakes.
Two types of ancient lakes can be distinguished: (i) lakes that did not undergo major catastrophic events during their history, and (ii) lakes that sustained major catastrophic events in relatively recent geologic time. Both types may contain lakes with a high level of biodiversity and endemism; however, these lakes evolved and function biologically differently. Species radiations can occur in both lake types but (i) mainly include taxon points of dispersal while (ii) act predominantly as taxon refugia. Extinctions caused by some catastrophic events create new ecological opportunities for colonization by alternative species changing the evolutionary path of the lake. The ultimate question is how important are these lakes in stabilizing regional and global diversity, i.e., are the lakes species sinks (refugia) or species sources (dispersal centers)?
One of the prominent features of ancient lakes is “species flocks” or closely related species that evolve sympatrically and coexist [215]. Species flocks have been documented in many groups, including invertebrates [216], fish [215,217], and photosynthetic organisms like diatoms [218]. These flocks are characterized by elevated species richness, shared origins, high levels of endemicity, and restricted geographic occurrence [219]. The most prominent hypothesis suggests that flock diversity is the result of long-term lacustrine speciation events after the colonization of ancestor species. However, the initial colonization of species may have happened at various times in a lake basin’s history, including early phases of lake formation, or after desiccation events and drainage reversals. One of the main trigger factors for intra-lacustrine speciation is the availability of empty niches, which happen after limnological changes or extinction of existing species. In both cases, stochastic factors force the evolution of novel traits, allowing organisms to exploit new niches. It is significant that temporal changes in the environment typically do not catastrophically impact the biotic community, but may alter the direction of taxa radiations. A consistent and interesting phenomenon in ancient lakes is higher endemism in littoral microhabitats [199]. Further, in most cases, littoral taxa are also intra-lacustrine (neo-) endemics, while species living in deeper parts of the lake share morphological characters and are even genetically more closely related to ancestor species (relicto-endemic). There are few examples of total extinction events in ancient lakes [202].
Here, we briefly address the organisms of ancient-lake biota. Although bacteria play an important biological function in aquatic systems, little is known about bacteria in ancient lakes. Lakes Vostok and Vida are permanently covered (ice-block lakes) in Antarctica with microbial-driven communities [220,221]. Lake Vostok has been isolated for at least 420,000 years [222]. Accretion ice in Lake Vostok contains thousands of unique gene sequences, at least half with taxonomic classifications to genus and/or species [220]. The autotrophic and heterotrophic microbial communities originate from regional inputs representing aerobic, anaerobic, psychrophilic, thermophilic, halophilic, alkaliphilic, acidophilic, and desiccation-resistant life forms [220]. Tropical Lake Matano (Figure 1AE) also sustains a significant presence of limnetic and benthic bacteria drivers [212]. Within sulfur-poor, iron-rich waters of Lake Matano, anoxic phototrophic green sulfur bacteria are prominent. The predominance of green sulfur bacteria in marine ecosystems would suggest that taxa in the freshwaters of Lake Matano are genetically different, with possible endemic species. Diatoms are an extremely diverse eukaryotic group of organisms in ancient lakes (Figure 2J) (e.g., [223]). In particular, the benthic communities show extraordinary diversity. One of the most diverse diatom genera in ancient lakes is Surirella Turpin (including Iconella Jurilj) with many endemic species [223] and/or species flocks [205]. It is also evident that more basal “primitive” organisms have higher percentages of endemism. For instance, triclads (Order: Tricladida) are diverse in Lake Baikal with 74 species recorded, and almost 100% are endemic [218]. Nematodes are species rich and characterized with high levels of endemism in Lake Baikal [224]. Oligochaetes are common in the benthos and have been well studied in ancient lakes. Gastropods are one group characterized by the highest diversity and endemism in ancient lakes. According to Strong et al. [225], the gastropod fauna from continental waters comprises ca. 4000 species and ancient lakes are considered hotspots of diversity. Crustaceans abound in lakes, and for example, Amphipoda is a diverse group in Lake Baikal, with 257 species and 74 subspecies [226]. Such a large diversity is influenced by many factors including habitat partitioning by depth, trophic differentiation, and differentiation by season of reproduction (allopatric) differentiation. The ostracod fauna in ancient lakes is very diverse and comprise about 20% of known species worldwide [227]. Freshwater sponges are much younger in origin (48–40 Mya) compared to marine species (500+ Mya) [97,228]. Morphologic and phylogenetic analysis of 25 freshwater sponge taxa from Sulawesi (Indonesia), Siberia (Russia), and South-East Europe show frequent and independent origins of species endemism to different freshwater systems through cosmopolitan founder species [229]. Possible founder species (e.g., Ephydatia spp. and Trochospongilla spp.) are freshwater taxa with cosmopolitan and neotropical origins. Fish have been the most studied in ancient lakes. From sculpins to sailfins and cichlids, endemism and radiation studies have consistently demonstrated the importance of sexual selection, body form, and niche breath within lake ecosystems. The largest and most significant ancient lake for fisheries (gross domestic product, GDP) is Lake Baikal [230]. The biological impact of fisheries (selected fish removal) on this ancient lake is likely not significant, due to the size and location of the lake, although we have no information to support this observation. Lake Baikal has 52–60 species mainly native and endemic. Endemic species flocks are observed in the sculpins (Cottidae, Comephoridae, and Abyssocottidae) [231]. The most economically important species is Omul (Salmonidae: Coregonus migratorius), the symbol of Lake Baikal since time immemorial. Sailfins (Telmatherinidae) have demonstrated the greatest levels of endemism and species radiations in the Indonesian Malili lakes (Figure 2K) [232,233]. The unique fish fauna of the African rift lakes has been recognized for more than 120 years with ca.1800 species in the five most species-rich lakes [234]. There are no cichlid species occupying more than one African Great lake [235,236].
Classic ecological principles can be used to study lake ecosystem services and functions. For example, Salzburger et al. [219] report that macroecological principles can be applied to ecological patterns found in the African Great Lakes; these functions include (i) taxonomic diversity tends to increase with area of the lake, (ii) more ecological niches allow greater taxonomic diversity, and (iii) the degree of ecosystem stability determines the periods during which adaptation can occur. However, these principles are interconnected and depend on the global location and structural morphology of the lake. Larger lakes have greater habitat diversity, are more stable and have larger resistance and resilience to disturbances [237]. Still, there are examples where large lakes such as Titicaca and Kivu have low levels of species richness and endemism [208,238]. Restricted endemism might be explained by unstable environmental conditions (physical and chemical) that are unfavorable for diverse floras and faunas. Further, poor seed populations at the origin of lake formation might provide a biotic explanation of why some lakes have low endemism.
Litoral zones are the nexus of human interactions with lakes and are often highly modified for diverse human uses [194,239]. However, littoral processes are poorly integrated into the understanding of lakes’ ecosystem functions (e.g., [240]).
Ancient and large lakes have societal and economic importance linked to drinking-water supply, commercial fisheries, subsistence fisheries, international shipping, recreation, and waste disposal [194,241]. However, they are sustaining an onslaught of anthropogenic impacts leading to the extinction of endemic species [198]. Eutrophication threatens native species and economies reliant on clean water, fisheries, and tourism [198]. However, these economies are potentially destroying the ecosystem services and biological significance of ancient lake resources. Lake Ohrid suffers from increasing anthropogenic pressure and a biodiversity crisis [242], while Lake Biwa is the best example of anthropogenic eutrophication with massive cyanobacteria blooms [243]. In another example, Lake Victoria has experienced eutrophication since the mid-1980s [244,245]. Eutrophication has also been reported in lakes Baikal, Matano, Poso, and Titicaca, although these reports are localized events in selected regions of the lakes [246,247]. Commercial fisheries are also affected. The catch for Lake Tanganyika for 1995 was estimated at 178,700 million tons (http://www.fao.org/fi/oldsite/ltr/fish.htm). Over one million people are dependent on the Lake Tanganyika fisheries. A major ten-fold decline in catch per-unit-unit-effort (CPUE) has been recorded 1983–1993 within Burundi waters (http://www.fao.org/fi/oldsite/ltr/fish.htm). Although climate warming has been cited [248] as a significant factor, anthropogenic fishing pressures are clearly having an impact; the ultimate impact on ecosystem services for Lake Tanganyika is yet to be realized. Invasive species have become a problem over the last 50 years in many ancient lakes (e.g., [249]). The introduction of rainbow trout and silversides in the 1950s (Odontesthes bonariensi) into Lake Titicaca has induced the extinction of Oestias cuveri [250]. Likewise, massive destruction of African rift lake fisheries resulted from the introduction of invasive fish [251]. The introduction of cichlids and carp into lakes Matano and Poso (Indonesia) through small-scale fish farming has altered the littoral zone composition of boned fish [252].
Hydrological alterations, including those produced through climate change, have altered lake flows, levels and niche habitats [198]. Newly constructed dams (projected massive development globally) will have significant impacts on lake ecosystem services into the next century. Lake Biwa has strict annual water level control, lower than high water level (−0.2 m) during the flooding season and higher than the high-water level (0.3 m) during the dry season. It is not clear how water-level control impacts native and endemic species, but it would be expected that a reduction in the number of stress events would affect niche habitats and natural speciation. Lake Victoria has two hydroelectric dams controlling the outflow into the Nile River. With the alteration of the natural outflow weir in 1952, discharge rates were set to mimic historical discharge levels [253]. However, since 2002, discharge levels have increased and Lake Victoria water levels have reached historically low levels [254,255]. Lower water levels, in part, will contribute to the eutrophication of water impoundments, including ancient lakes.
4.2. High-Mountain Lakes
Climate change affects glacial masses and the lakes dependent on them. Reduction and disintegration of ice impact epi- and periglacial lakes directly. Sometimes, changes in glacial mass result in sudden, catastrophic events. Surprisingly, relatively-high surface temperatures could be found even in proglacial lakes, which turned out to be discontinuous cold polymictic rather than dimictic as the typical clear alpine lakes [256]. In general, highly simplified food webs are characteristic of lakes fed by glacial meltwaters, though specific habitats are present. Climate change and glacier retreat can rapidly shift turbid periglacial lakes towards a clear-state, high-alpine lake type, with loss of the typical features of periglacial lakes. Conversely, the retreat of large glaciers will also form new glacially-fed lakes but whether this will buffer the mentioned habitat and biodiversity loss remains unclear [257].
Non-glacial alpine lakes are likely among the most comparable ecosystems across the world but the largest contrast occurs between lakes in temperate (Figure 1M,N) and tropical areas with a water-column structure based on the temperature in temperate lakes, and oxygen in tropical lakes [258]. They provide important ecosystem services ranging from the supply of resources (in particular water for various uses) to tourism and recreation.
The lake basins located above the tree line are often still poorly known environments [259] that can harbor organisms of particular interest (Figure 2M,N). A few mountain lakes are large and ancient, and host endemic species (e.g., the graben Lakes Tahoe in the USA and Hövsgöl in Mongolia; [260]). However, isolated, small post-glacial lakes can also be inhabited by populations that have interesting local genotypes. The phytoplankton of high-mountain lakes is typically dominated, both qualitatively and qualitatively, by flagellate algae (in particular, Chrysophyceae; [261]). A high genetic diversity, far beyond what is described from high-mountain lake plankton, was recorded in high-mountain lakes in the European Alps and the Himalayan mountains [262]. Mixotrophic algae usually dominate the planktic community under P-deficit and are the main factor controlling bacterioplankton [263]. Depth variation in benthic communities can be substantial and must be considered in monitoring programs and biodiversity inventories and comparisons, as was shown, e.g., for ancient mountain lake benthic invertebrate communities [260], and small, postglacial, mountain lake cyanobacteria and algal pigments [264].
Several high-mountain lakes are remote environments that are relatively far from centers of human activity and potentially subject only to broad-scale environmental changes and diffuse pollution. This kind of high-mountain lakes has thus received an important role as indicators of climate change in recent decades [265]. On the contrary, other high-altitude lakes suffer from a series of direct anthropogenic impacts. According to Catalan et al. (1993) [266], these impacts include grazing, tourism, the introduction of non-native fish species, changes to the shoreline and the basin for exploitation for hydroelectric purposes. Although this latter type of impact affects a large number of lakes in the Italian Alps, its effects on hydrochemistry and biota are still poorly known globally [267].
The introduction of non-native fishes has been recognized to be one of the main impacts on high-mountain lakes both in Europe [259,268] and in North America [269]. Most or all small high-mountain lakes were probably fishless but have been extensively stocked with domestic fish species. This was in some cases favored by the belief that some species might be native, such as the alpine charr considered a glacial relict in the Alps [268]. Especially in natural preserves, some essential conservation measures (e.g., fish-stocking ban, and protection against eutrophication) have already been taken, but efforts to restore invaded lakes through fish eradication might be necessary [259].
On holocrystalline substrates (e.g., granites) and microcrystalline substrates (e.g., porphyries), lakes with minimally-mineralized waters with alkalinity <50 µeq L−1 are relatively common (Figure 1M), and are exposed to the effects of airborne pollutants, especially acid substances [270]. The lakes with extremely low alkalinity (sentinel environments, e.g., [258]) also offer an important long-term monitoring opportunity, as they are particularly sensitive to direct and indirect anthropogenic impacts. Climate change is predicted to lead to an increase in alkalinity due to changes in the seasonal distribution of precipitation that, combined with a general increase in air temperature, lead to greater weathering in the catchment, a decrease in snow cover, and an increase in the leaching of the basin [271]. It is also possible to predict a decrease in the period of ice cover and an increase in primary production. Thies et al. (2007) [272] not only observed a substantial rise in solute concentration at two remote high mountain lakes in catchments of metamorphic rocks in the European Alps but also an unexpectedly high nickel concentration increase, and attributed these changes to solute release from the ice of an active rock glacier in the catchment as a response to climate warming. Mountain lakes are among the lakes warming the fastest: Warmer temperatures favor different species and assemblages, and greater primary production [269].
After decades of exploitation for hydroelectric purposes in recent decades, water from high-altitude lakes is now often considered with renewed interest for the production of artificial snow [273]. Climate change, resulting in an increase in temperatures and a decrease in snowfall, potentially leads to an increase in withdrawals for snow production, which changes water storage and water levels. This impact also alters the lake morphology and the induced, pronounced water-level fluctuations result in the emergence of a large part of the littoral [274]. The most common alternative to these water withdrawals is the construction from scratch of artificial reservoirs for artificial snow production. From a limnological point of view, these newly-formed environments, often located on carbonate mountains where other surface water bodies at high altitude are rare due to karst phenomena, can represent an interesting opportunity as a summer habitat for rapidly developing aquatic species with resistance forms and as reproduction sites for amphibians. For these artificial basins to perform these functions, it is important to adopt naturalistic engineering criteria in their realization, with particular attention to slope and structure of the banks (Cantonati, unpublished).
In the face of increasing rates of the exploitation of water, it is essential to protect significant numbers of high-altitude lakes in natural and semi-natural conditions. An important element in favor of their conservation could be represented by the fact that many of these lakes are included in Natural Parks (e.g., [259,274]). The illustrious ecologist Ramon Margalef wrote in 1983 [275] (translated from Spanish): “Against these threats, it is worthwhile to move in favor of the conservation of mountain lakes. Many of these are located in protected areas or parks, one more reason not to sacrifice them for hydroelectric interests”.
4.3. Oxbow Lakes
Oxbow lakes include lowland fluvial stagnant lakes, generally shallow and isolated from parent rivers (Figure 1O). They are typical of floodplains and take origin from channel shifting and associated geomorphic processes (e.g., braiding, erosion), namely, when a river bend—called meander—gets cut off, thus creating a water body that remains adjacent to their parent river [276,277]. Indeed, the rivers that flow across wide river valleys and snake across flat plains tend to create menders that slowly evolve and give rise to complex oxbow lake systems.
Due to their pivotal contribution in modulating alluvial behavior, the oxbow lakes are generally acknowledged as one of the most distinctive landforms of floodplains [278]. For instance, the presence of an oxbow lake largely improves the topographic, hydrological, and habitat diversity of river valleys [279]. Oxbow lakes rely on periodic flooding events or local rainfall and, over time, can turn into wetlands, and then into terrestrial ecosystems. Specifically, their transition rates towards terrestrial habitat are regulated by the intensity of sedimentation processes [280]. These lakes are typically connected to parent rivers during extreme floods, but the frequency, duration, and timing of the reconnection vary according to river stage, riverbank elevation, and the presence of a direct channel between the oxbow lake and river [281]. Often, they ultimately dry up in a few decades after having been cut off from the main river channel.
Both the peculiar origin of oxbow lakes and their specific hydrological regime make them dynamic, biologically diverse as well as extremely sensitive ecosystems. Several papers have clarified the key role of oxbow lakes in supporting the phyto- and zooplankton assemblages in river valleys and main streams [282]. They also are fundamental for the conservation and reproduction of fish, as they guarantee feeding and breeding habitats for many different species, and act as refugia during extreme flood events [283]. Zeug and Winemiller (2007) [284] found that the oxbow lakes held greater juvenile abundances of most fish taxa relative to the main river channel and were particularly important for the typically nest-building species. Nevertheless, their observations suggested the existence of a combined positive effect of both hydrology and habitat heterogeneity in providing optimal conditions for fish recruitment. Similarly, Bolpagni and Piotti (2015, 2016) [285,286] confirmed the pivotal contribution of riverine natural lentic sites, including oxbow lakes, to preserve high values of heterogeneity (expressed in terms of physical complexity of water bodies) and vegetation diversity (plant communities per site) in heavily exploited floodplains. This reinforces the clear dependence of aquatic plant communities on aquatic ecosystem origin, thereby confirming the intimate relationships between heterogeneity and vegetation diversity and, therefore, with the inter- and intra-annual hydrological cycles of water bodies colonized.
Oxbow lakes tend to be largely colonized by macrophytes that can act as engineer species [287]. During the growing season, the development of dense stands of submerged or free-floating plant species as Vallisneria spiralis or Trapa natans (Figure 2O) exerts a strong influence on water and sediment chemistry. In presence of pleustophytes, like Spirodela polyrhiza or Lemnaceae, frequent anoxia events can be recorded during warmest months due to the exhaustion of sediment geochemical buffers (i.e., ferric iron pool) and the associated release of nutrients (NH4+ and PO43− to the water column [288]. These processes do no more than increase the availability of nutrients in the water column, boosting the hypertrophic status of the ecosystem and stressing the relevance of trophic disservices mediated by anoxia events in aquatic ecosystems. In this context, the water level variations induced by human activities deeply affect the physical and chemical features of oxbow lakes with huge cascading effects on biota. Disturbed water regimes largely depress organisms with a full aquatic life cycle (phyto- and zooplankton, fish), whereas the biodiversity of unstable oxbow lakes (i.e., those with most marked water level variations) decreased along with increasing isolation from the river. In riverine contexts, long dry phases induced, for example, by excessive exploitation of water resources or by local effects of climate change, can stimulate the spread of terrestrial species in ecotonal and aquatic sectors of oxbow lakes [289].
Accordingly, hydrology completely controls the survival time of oxbow lakes, making them extremely vulnerable to human actions, stressing the key relevance of isolation, damming, and agricultural practices in driving oxbow dynamic trajectories. This has significant implications for the benthic and aquatic metabolism of oxbow lakes. Indeed, the denitrification efficiency was higher in connected oxbow lakes than isolated ones [290]. On the other hand, oxbow lakes play a relevant role in modulating the river C budget as a function of season and hydrology [291].
The increasing interest in levee construction, as well as in snagging, dredging and wing dike construction to control flood events and restrict meandering have essentially impaired the natural river processes [292]. As a result, riverine aquatic ecosystems are not being newly created, at least not at historical rates. This calls for urgent actions to maintain and restore existing oxbow lakes, particularly as the above-mentioned processes appear to be irreversible, or are not expected to be effectively counteracted in the short term [293].
In summary, river valleys and floodplains have been strongly modified by human activities such as channelization, water regulations, and land reclamation and use change. All this translates into inadequate conservation status for oxbow lakes, despite their major contribution to a myriad of species as well as to fundamental ecological processes [294]. The challenge of efficient functional restoration depends not only on improving hydraulic connectivity of riverine wetlands—in order to maintain important biogeochemical functions such as nitrogen removal via denitrification—but also through providing a mosaic of diverse habitats aiming to support the widest range of species possible.
5. Man-Made Freshwater Habitats
5.1. Reservoirs
Freshwater reservoirs are artificial water bodies (Figure 1P,Q) of particular interest as they provide various ecosystem services such as the supply of drinking water, irrigation, transportation, industrial and cooling water supplies, power generation, flood control, or recreation. The purpose of the reservoir often strongly influences the hydrological regime within the reservoir. At the same time, reservoirs have a significant environmental impact since their dams disrupt the ecological connectivity of rivers, whereas reservoirs’ water storage and release patterns affect the quantity, quality, and timing of downstream flows [295]. Reservoirs differ from natural lakes in several important aspects, particularly the very common canyon-shaped reservoirs, constructed by damming a river valley and representing a transition between lotic and lentic systems [296]. Compared to natural lakes, these reservoirs have elongated morphology, shorter water residence time, pronounced water-level fluctuations and irregular water withdrawal, which can be realized from various strata [297].
Major threats to biodiversity in reservoirs can be grouped under several interacting categories: overexploitation; habitat degradation through disappearance of the littoral zone due to water level fluctuation; water pollution, including processes such as eutrophication or acidification; inappropriate management practices (e.g., fish overstocking); climate change and subsequent shifts in mixing regime, changes in precipitation and runoff patterns, wind speed, invasion by non-indigenous species, and more. Pronounced fluctuations in reservoir water level impair the development of structured macrophyte vegetation, which fulfills the vital functions in the littoral zone. Such altered hydrology has no correspondence in natural freshwater systems, as few plant species have adaptations to their specific aspects [298]. For example, the rooted submerged plants face both physiological and physical constraints because of the shifts between submergence and drainages, wave actions, ice scours, sediment erosion, and more [299,300,301]. Alterations of macrophyte communities (Figure 2P) lead to a decrease of habitat structural complexity and significant losses of the diversity of other associated organisms in the littoral zone of reservoirs (e.g., [302,303,304,305]), and have the potential to change biogeochemical processes in the whole reservoir ecosystem.
Increased discharge of domestic wastewaters and non-point pollution from agricultural practices and urban development have recently led to excessive nutrient loading into SWs and is considered to be one of the major driving forces of reservoir eutrophication [306,307,308]. Eutrophication effects on elongated reservoirs can be highly variable spatially as gradients from hyper- to oligotrophy can establish across the inflow-transition-lacustrine parts of the water body. Nutrient enrichment affects competition for light among aquatic macrophytes as well as between the macrophytes and either epiphytic and/or planktonic algae [309,310]. Initially, macrophytes are lost from the deepest and near tributary parts; as the process gets worse, all submerged plants are eventually lost from the reservoir, which becomes dominated by phytoplankton, often experiencing cyanobacterial or algal blooms. Nowadays, harmful (toxic, food-web altering, hypoxia generating) phytoplankton blooms recurrently threaten the ecological integrity and sustainability of many eutrophicated reservoirs [311].
The absence of zooplankton due to the excessive predation of planktivorous fish has been widely recognized to contribute to cyanobacterial bloom formation (top-down effect). In most Eurasian meso-eutrophic reservoirs, fish assemblages are dominated by planktivorous cyprinids like common bream (Abramis brama), roach (Rutilus rutilus), bleak (Alburnus alburnus) and white bream (Blicca bjoerkna) [312], due to their efficient foraging in the turbid (algal-dominated) and food-limited conditions [313], and high abilities to escape predators [314]. In spite of clearly the highest biomass and productivity, these cyprinid-dominated systems are not considered to be valuable from the view of biotic integrity and rather they are classified as an indicator of degraded conditions [315]. Nevertheless, the superdominant cyprinid fish could be eliminated by drying their very-shallow-laid eggs even during controlled low-range water-level fluctuation (on tenths of cm per day). Then, percids (Figure 2Q) may become dominant species in some eutrophicated lowland cascade reservoirs [316]. Interestingly, cascade reservoirs were found partly to support salmonid fish which are otherwise very scarce in lowland reservoirs. The low-range water-level fluctuation or stable water level may further increase the fish diversity since the growth of submerged macrophytes is not prevented and assemblages of strictly phytophilous fish, like pike (Esox Lucius) and tench (Tinca tinca), can fully develop. In bankside reservoirs, the pump storage water bodies with concrete or asphalt banks and relatively frequent water-level fluctuation, macrophyte growth and related cyprinid fish reproduction are prevented [317]. Like with cascade reservoirs, bankside reservoirs have less fish production but may be important hotspots of fish biodiversity in lowland nutrient-rich systems.
Reservoirs can maintain characteristic biological assemblages, but often feature novel combinations of native and introduced species. Introduced species become established, naturalized or invasive, but it is often not clear whether they have negative effects on native biota that might also be present or, alternatively, occupy niches that have been left empty due to the original habitat modification in reservoirs [318,319,320]. Obviously, invasive species thrive and often become dominant in ecosystems modified by humans (e.g., [319]), where they are largely contributing to biodiversity loss, ecosystem degradation, and impairment of ecosystem services (e.g., [321]). It has been clearly shown that reservoirs harbour more invasive species than natural lakes and rivers in the same areas [322,323]. For instance, Eichornia crassipes and many other floating weeds cover water surface in tropical and subtropical reservoirs, impairing water quality (e.g., light conditions, oxygen content, organic matter accumulation), or recreation and transport (e.g., [324,325]). Some submerged invasive plants, such as Elodea canadensis, E. nuttallii (Figure 2P), Chara vulgaris, and Myriophyllum spicatum form monospecific vegetation in hypertrophic reservoirs and play a key role in structuring and homogenization of aquatic plant communities (e.g., [325,326]).
To summarize this section on reservoirs, recent origin, intensive human use, and water-level instability often cause a lower diversity of biota of reservoirs compared to lakes. If we consider comparable undisturbed lakes as the reference condition for reservoirs, it would be desirable to minimize the disturbances and to facilitate diverse littoral development. This could be possible in less-exploited reservoirs and may lead to the balance between algal and macrophyte production as well as diverse macrophyte-associated fauna. At the same time, we should keep in mind that stable conditions can, in a high-nutrient regime, create cyprinid fish-dominated systems with high productivity, low water transparency, and low ecological and recreational value. In this respect, the value and functioning of the biota of fluctuating reservoirs is worth further investigations, as it can serve as a refugium for species outcompeted or overgrazed by the cyprinids.
5.2. Urban (Artificial) Freshwater Habitats
In some circumstances, a surprising amount of biological diversity can be found in artificial freshwaters. For instance, Verdonschot et al. (2011) [327] found a considerable fraction of the freshwater biodiversity of the Netherlands can be supported in agricultural ditches.
Urban freshwater habitats include most freshwater habitat types discussed in this paper. They also include novel freshwater ecosystems resulting from hydrological and landscape processes specific to urban settings [328]. Urban development greatly alters freshwater ecosystems, resulting in a set of attributes known as the urban stream syndrome [329]. Urban freshwater biodiversity is often poor compared with non-urban landscapes [330]. However, modifications caused by urbanization can also make it possible for new native and introduced species to colonize freshwater ecosystems resulting in an increase in species richness to levels above that of pre-urban systems [331].
How urban freshwater biodiversity conservation should be undertaken and its success measured depends on the reasons for conserving. Dearborn and Kark (2009) [332] proposed seven possible motivations for conserving urban biodiversity and placed these along a gradient from those primarily providing benefits to humans to those primarily providing benefits to nature. A range of urban freshwater ecosystems can readily be identified as providing special and tangible benefits to humans by the mere fact of being blue spaces [331]. These blue spaces can help to preserve local biodiversity in an urbanizing environment and protect important populations of local species. They can also create stepping stones or corridors for natural populations. Ponds are likely to be particularly important in preserving local biodiversity because they can be relatively isolated from one another and from other water bodies. Pollution may constitute a major threat to pond biodiversity but habitat fragmentation, a characteristic of urban landscapes, may not be a major threat to biodiversity in ponds. Increased connectivity from flooding caused by a high proportion of impervious surfaces in the catchment may be a greater threat because it could lead to colonization by invasive species. Hence, maintaining both this isolation and water quality could be important conservation goals for pond habitats. In contrast, urban streams and rivers (Figure 1R) could have an important role in creating stepping stones or corridors for natural populations in nearby, less modified, non-urban landscapes. This role may not require high water quality if the species can tolerate poor conditions for short periods during dispersal.
Assessments of urban freshwater biodiversity must use ecosystem-specific reference conditions for both natural and artificial habitats. Importantly, these methods must also be linked to specific conservation goals and the reasons for conservation. Traditional biodiversity indicators may not be particularly useful in urban environments. Widely-used methods and indicators may not be sensitive enough to demonstrate the positive effects of even quite major restoration efforts because, despite of positive changes to water quality and local habitats, the urban settings may prevent many species from reaching urban freshwater habitats or colonizing them when they get there. Secondly, many of the widely used indicators may be not be a good match for the specific conservation goals set for urban environments. For example, where connecting people with nature [332] is a high priority, the richness of benthic macroinvertebrates may have a limited role in measuring conservation success. In such circumstances, the presence and abundance of turtles and large freshwater dependent lizards (Figure 2R) may be good indicators of conservation success because they often utilize riparian and terrestrial habitats as well as instream habitats and are likely to be seen often by people [49]. Combining the use of such indicators with the established ones (e.g., macroinvertebrates), fish may help to tailor biological monitoring to meet the specific goals of urban conservation projects.
6. Mires (Peatlands): Fens and Bogs
On a global scale, peat bogs offer a wide range of ecosystem services [333], including resources and materials (drinking water, livestock forage, small fruits, reed, peat as fuel and for horticulture), carbon sequestration (peat bogs are the most important terrestrial carbon storage area on the planet), flood mitigation, erosion control, permafrost conservation, paleoecological, archaeological and paleoclimate archives, and biodiversity conservation.
All mires represent semi-terrestrial ecosystems, sometimes appearing as a mosaic of aquatic (pools), semi-aquatic (hollows), and terrestrial (lawns, hummocks) habitats. They are characterized by the accumulation of organic matter or calcareous tufa, permanently high water level and low nutrient, especially phosphorus and nitrogen, availability. They often experience extreme acidity or alkalinity [334,335,336]. The ecosystem is composed mostly of stress-tolerant habitat specialists. The variation in environmental conditions and community compositions is large and is governed mainly by the hydrology and acidity-alkalinity gradient [334,337,338].
When the ecosystem is fed largely by rainwater, the ombrotrophic, strongly acidic, bog ecosystems (Figure 1S) develop [339,340,341]. They are dominated by specialised Sphagnum mosses (Figure 2S), dwarf ericoid shrubs, and sometimes coniferous trees. On the other hand, ecosystems fed predominantly by GW, i.e., mineral and sometimes also nutrient-richer water, are called fens (Figure 1T). In the most alkaline habitats fed by carbonate-rich GW, sometimes even with precipitation of carbonates, specialized non-sphagnaceous bryophytes (often called brown mosses) dominate instead of Sphagnum mosses, together with sedges and some specialized herbs (Figure 2T). Along the acidity-alkalinity gradient, several distinct fen habitats may be recognized, from Sphagnum-dominated poor fens, through rich fens combining calcium-tolerant Sphagnum mosses and brown mosses, to extremely rich and calcareous fens dominated by brown mosses [334,337,342].
Compositional change along this gradient is great, governed by different species’ adaptations. In acidic, calcium-poor environments, non-specialized species are suppressed by iron or aluminium toxicity, and sometimes also by the deficiency of nitrogen (in bogs) and by strong competitive pressure of Sphagnum mosses preventing among others the germination of some vascular plants [343]. Calcium and magnesium shortage may play roles as well, especially for organisms forming calcareous shells, e.g., molluscs or some testate amoebae [344]. On the other hand, extremely alkaline and calcium-rich conditions are strongly selective because of phosphorus deficiency [345], the toxicity of calcium, magnesium or salts for some organisms, especially many bryophytes [346]. At both ends of the alkalinity gradient, aquatic primary producers submerged in pools or streams may be suppressed by a shortage of free carbon dioxide needed for photosynthesis [347,348]. Rich fens, occupying the middle part of the gradient, are less stressed by water chemistry, and therefore they are more prone to successional changes when water table declines or nutrient availability increases [349]; the equilibrium between calcium-tolerant peat mosses and calcicole brown mosses leads to species coexistence and hence high species richness exceeding other mire types [350,351]. Rich and calcareous fens act as important biodiversity hotspots. Many species considered as a relict from late glacial and Early Holocene times occur here [351,352,353], and they are significantly associated with old fens initiating before the Middle Holocene [354]. Such fens are richer in plant specialists than younger ones, and when they are large enough, also in snail species [355]. Differences in site ages and connectivity determine the inter-regional differences in richness of habitat specialists, with Baltic and Fennoscandian regions showing their highest concentration [351].
The colonization by organisms with specific adaptations makes mires very important for nature conservation [351,356]. Generally, they are threatened by various direct impacts (e.g., land reclamation, peat extraction, drainage, organic pollution and eutrophication; [50,357,358]) that cause their fragmentation, loss of specialized organisms [359], or even complete destruction. One per cent of the land area of the planet is covered by peat bogs, and Europe has lost about 62% of this habitat type in recent decades [360]. Indirect impacts such as climate change or increasing nitrogen deposition also represent serious threats [361,362,363,364,365,366,367]. In the current European Red List of Habitats, Janssen et al. (2016) [368] found that 85% of mire habitats are threatened in the European Union. Two habitat types of base-rich (calcareous) fens were even categorized as endangered, falling amongst 10% of the most threatened European habitats. Base-rich fens are generally less stable than poor fens or bogs [369], frequently experiencing succession towards poor fens and bogs [370], shrublands and woodlands [371,372,373], or, after anthropogenic deterioration, towards depauperate wet grasslands or reed beds [374]. All these successional developments are currently accelerating as a consequence of eutrophication, anthropogenic hydrological changes in their catchments, and climate change. Such trends have been demonstrated by many resurvey studies throughout Europe [364,374,375,376,377] or North America [378]. When natural hydrology, nutrient status, or habitat connectivity are damaged, prescribed disturbances such as mowing, grazing, or sod removal are needed to preserve rich and calcareous fens in current landscapes (e.g., [379]). On the other hand, ombrotrophic bogs do not require prescribed disturbances in most cases, but their functioning is endangered in many regions by increasing nitrogen deposition, decreasing precipitation, and increasing evaporation due to climate and land-use changes. In some regions, lime enrichment coming either by aerial liming of surrounding forests [380] or lime-rich ash deposition from industry [381] trigger successional changes in bogs. Microorganisms such as diatoms or testate amoebae respond faster to these changes than long-lived plants [340] and may act as excellent indicators [360]. However, change in microbial assemblages may alter ecosystem functioning such as decomposition patterns that affect nutrient cycling [382,383,384].
Mires experience specific temperature regimes, making them important refugia in the times of changing climate. Bog surface may experience high diurnal or seasonal fluctuation in temperature [385], determining narrow temperature niches of some invertebrates [386]. On the other hand, fens may experience thermal buffer of the GW. The importance of water and air temperature, however, changes with terrestriality. While aquatic and semi-aquatic spring-fen biota (e.g., diatoms or aquatic invertebrates) is substantially affected by water temperature [382,387], terrestrial fen biota (land snails) is affected mostly by air temperature, especially in winter. Vascular plants show intermediate response because they are rooted in permanently waterlogged peat and because water temperature affects their germination [388]. There are good reasons to assume that increasing temperatures will affect especially mires in southern Europe where their current distribution is restricted and species composition depauperate already due to previous Holocene development [351]. Southern-European bogs have a great naturalistic and biological value being relics of the Quaternary glaciations. Mires in southern Europe may be very old [389] and often harbor not only relict population of boreal species [390,391] but also endemics and specific genotypes of mire specialists [392,393]. Although disjunct occurrences and endemism of vascular plants in southern-European bogs are well known, less is known about smaller organisms such as diatoms. Diatom communities with >70% of taxa belonging to threat categories of the Red List were indeed discovered in pools of mountain mires of south-eastern Alps [394], even with species new to science [395], but not all mires of Central and Southern Europe are as rich and unique [360].
A complex nature of fen and bog ecosystems, including multiple environmental gradients and successional trajectories, crucial effects of Holocene development, the great role of ecosystem engineers belonging to less known groups of organisms such as microorganisms including testate amoebae and diatoms, or bryophytes [50,343,395,396], and high representation of habitat specialists with less known ecological preferences, makes the prediction of the future of mires difficult. Further research should disentangle relationships between individual groups of organisms, and between organisms and their environment, in order to better understand these fascinating ecosystems and to find a way how to preserve them at the largest possible geographical and environmental extent.
7. Small Standing-Water Ecosystems
Small standing-water ecosystems (SWEs) (Figure 1U) group shallow (not more than 20 m deep) and small lentic waterbodies, ranging in area between 1 and 105 m2 ha at maximum. Accordingly, SWEs embrace most small lakes, pools, ponds, wetlands, swamps, vernal pools, livestock watering tanks, and rice paddies—both perennial and temporary that are of natural or artificial origin ([397], and references therein). Despite their small size, their number makes them an essential component of catchments, able to contribute substantially to their functioning via modulating nutrient retention and recycling [398]. This is largely attributable to the reduced area/perimeter ratio of SWEs compared to their larger freshwater counterparts. Indeed, the presence of large ecotonal sectors maximizes the SWEs’ metabolic capacity, net of their reduced dimensions. As a result, SWEs are generally acknowledged as one of the most productive ecosystems at the global scale [399,400]. At the same time, however, this disproportionate perimeter/area ratio emphasizes the high impairment risk associated with SWEs, mainly due to habitat loss, land-use change, and eutrophication [401,402]. Accordingly, SWEs are counted among the ecosystems with a “sentinel behavior” due to their particular susceptibility to regional and global human impacts, including pollution, water acidification, and climate change [403,404].
The high productivity of SWEs, directly connected to their high structural heterogeneity, is also due to the high variability in the water supply sources. SWEs range from water bodies fed by atmospheric depositions only (i.e., ombrotrophic) to those with a prevalent surface feeding or with a prevalent GW contribution (e.g., GDE = groundwater dependent ecosystems). These multiple water supply modes interact intimately with each other generating manifold spatio-temporal combinations, which in turn are related to high rates of functional and compositional biodiversity. Indeed, many papers have been published concerning studies on biological diversity related to SWEs, with a particular attention to ponds. These studies recorded high degrees of plant and animal diversity, and the presence of rare or threatened species, whose representativeness notably increases with increase in submerged aquatic vegetation and/or decrease in human perturbation rates [397,403,404,405,406,407,408].
As discussed by Bolpagni et al. (2019) [51] in this VSI, SWEs are essential to maintain diverse and varied biological communities across a heterogeneous series of ecosystems, including heavily human-impacted areas in agricultural and urban settings (e.g., for hydrophytes (Figure 2U), Odonata species) [298,409,410,411,412]. Oertli and Parris (2019) [413] have recently reviewed this topic, reporting that indicator species richness is generally lower in urban ponds compared to rural ones; however, urban ponds have the potential to support greater biodiversity, if correctly managed [414]. On that subject, Hill et al. (2017) [415] recognized taxonomic richness levels in urban ponds completely comparable to that for non-urban ponds. This is possible because SWEs per se actively support diverse and wide ranges of habitat conditions, offering proper refuges to a rich array of taxa. This is a focal point for developing effective strategies for future ecological recovery actions of SWEs. Based on the current rates of urbanization, an increasingly large proportion of SWEs will find themselves engrossed by the urban environment in the next decades. This awareness should stimulate inquiry into SWEs, from a global and multi-taxon perspective. Future efforts should be directed towards quali-quantitative investigations of the roles played by SWEs in maintaining global water quality, biodiversity, and services, and in defining the priority actions for their preservation.
8. Cold-Climate Freshwater Habitats (Boreal, Arctic, Antarctic)
8.1. Boreal and Arctic Freshwater Habitats
Cold-climate regions occur on both hemispheres, but in this subsection, the focus is on the regions north of 60 N, encompassing large areas in the Nordic countries, Russia, Greenland, Canada, and Alaska. Northern regions have experienced profound effects of glaciations during ice ages, and the terrestrial and freshwater ecosystems in these regions have thus largely developed after the last glacial maximum [416]. These historical factors have moulded regions with numerous streams (Figure 1V,W), rivers, ponds and lakes scattered across landscapes. There are several types of natural lotic ecosystems in northern regions, ranging from tiny headwater streams to huge rivers in Canada and Russia, from oligotrophic streams to eutrophic streams, and from clear-water rivers to humic rivers, and from streams draining boreal coniferous forests to those draining treeless tundra [417]. Similar to lotic ecosystems, lentic ecosystems also show a lot of variability in conditions related to size, trophic status, humic status, and geographical location. All these environmental and geographical features set the stage for a high variation of biological communities between different types of freshwater ecosystems [418].
Northern freshwater ecosystems are inhabited by a wide variety of organism groups, including bacteria, algae, macrophytes, invertebrates, fish (Figure 1W), and waterfowl (Figure 1V), which show considerable biodiversity at regional and local scales (e.g., [418]). However, there are considerable differences in the levels of biodiversity among organismal groups, and while fish may show generally low numbers of species at regional and local levels in high latitudes (e.g., [419]), bacteria (e.g., [420]), algae (e.g., [421]), macrophytes [422], and invertebrates [423] may exhibit surprisingly high biodiversity considering the constraints set by historical, geographical and climatic factors. Historical factors related to the effects of last glacial maximum resulted in the elimination and subsequent recolonization of biota after the ice age over most of the northern areas (e.g., [416]). The geographical location in the North dictates that some species are on the edge of their geographical distributions, and do not occur south of the Arctic and boreal areas (e.g., [417]). The effects of geographical location on biodiversity are also directly related to climate forcing, and many cold-water fish species, for instance, show distributions restricted to the northernmost areas of the world (e.g., [419]). An example is the world’s northernmost freshwater fish species, Arctic charr (Salvelinus alpinus), which prefers cold-water environments.
Different facets (i.e., taxonomic, phylogenetic, and functional) and components (i.e., α, β, and γ; [161]) of freshwater biodiversity have recently been given increasing attention. These studies have shown that different environmental factors drive variation in taxonomic, phylogenetic, and functional biodiversity across northern freshwater ecosystems, as has been found for stream macroinvertebrates (e.g., [424]), lake macroinvertebrates (e.g., [425]), and lake fishes (e.g., [426]). Of the three facets of biodiversity, taxonomic diversity has been studied most extensively to date. These studies have found that taxonomic alpha and beta diversity are mostly driven by local environmental variables, such as ecosystem size, habitat structural features, nutrient concentration and acidity, as has been observed for multiple organismal groups (e.g., algae, macrophytes, invertebrates, and fish) in both lotic [427,428] and lentic [429,430] ecosystems. However, the effects of these local environmental variables on biodiversity may vary among different regions across the northern hemisphere, resulting from strong context-dependency in the drivers of biodiversity (e.g., [431]). This context dependency dictates that it may be difficult to make a priori predictions of the community-environment relationships beyond a particular study region.
In addition to local environmental variables, geographical position, climate and catchment features also drive biological communities in northern freshwater ecosystems. For example, geographical location determines the species pool from which local communities are assembled, and thus local communities show imprints of both regional and local factors [421,432]. Recent studies have also shown that biological communities in northern freshwater ecosystems are clearly affected by climate over broad areas, as has been observed for algae [433], macroinvertebrates [434], and fish [419]. Finally, land use, land cover, and catchment heterogeneity have been found to be associated with both alpha and beta diversity variation of macrophytes (e.g., [435]) and macroinvertebrates (e.g., [428]).
Threats to the biodiversity of freshwater ecosystems in northern regions include climate change, land-use change, eutrophication, acidification and damming [417]. Given that northern regions are projected to experience profound climate warming and increased occurrence of extreme events, their biodiversity and ecosystem functions are likely to exhibit considerable changes as well [436]. Climate warming will result in both decreases of biodiversity when cold-water species are extirpated locally or regionally, and increases of biodiversity when cool-water and warm-water species expand their distributions in the warming climate. Thus, there will certainly be “losers” and “winners” among freshwater biotas of northern regions in the face of climate change. Climate warming may also be related to land cover and land use, with vegetation changing in both terrestrial and freshwater ecosystems [437], which have repercussions for the biota dwelling in lotic and lentic ecosystems. Land use alteration may also be observed in increased coverage of agricultural areas, which also contributes to increased nutrient loads to freshwater ecosystems in northern regions. Acidification of freshwater ecosystems has been a major threat to freshwater ecosystems in parts of Fennoscandia and Canada, for example, and it typically results in impoverished faunas and floras in the affected ecosystems [438]. Finally, damming of rivers presents a formidable threat to the integrity and functioning of freshwater ecosystems, resulting in changes in biodiversity, including migratory fish populations, ecosystems functions, and ecosystem services [418]. To summarise, multiple stressors are threatening freshwater ecosystems and their biota, calling for studies within and across northern regions to understand the multiple direct and indirect effects of these stressors on biodiversity. Such information is also a prerequisite for the conservation and restoration on freshwater ecosystems in boreal and Arctic regions.
8.2. Antarctic Freshwater Habitats
Antarctic freshwater habitats are largely constrained to the patchwork of larger ice-free regions that are spread around the margin of the continent, but together make up less than 1% of its area. These areas have affinities for other geographical regions, with the extreme aridity at high latitude drawing comparisons with desert systems, the extreme cold with Arctic environments, and the patchwork of ice-free land with island systems. At the same time, the unique combination of environmental conditions and geographic isolation places Antarctica at the extreme end of many continua of biogeographic phenomena.
Our understanding of Antarctic freshwater biodiversity is based on studies that began at the beginning of the 20th century [439] and now encompass most of the major ice-free regions [440]. In each of these regions, a mixture of lakes, ponds and flowing waters are found. Many, but not all, of these were created during the ice-sheet retreat following the last glacial maximum (e.g., [441]). In addition, substantial supra-glacial [442] and subglacial [443] freshwater ecosystems are now recognized, and while both are known to support microbial ecosystems, as yet they are insufficiently surveyed to allow biogeographic inferences to be made (e.g., [444]).
Latitude plays an important role in defining Antarctic freshwater habitats [445]. The north-south gradient is accompanied by reducing precipitation, resulting in an increasing dependence on melting of glacial ice to provide meltwater, and a declining temperature, which reduces duration of free-flowing water, from several months in the northern parts to only a few days per year at 80° S. Increasing aridity at higher latitude also results in a high proportion of endorheic lakes and ponds, within which salinization becomes prevalent. Lakes (Figure 1X) and ponds in the colder inland parts of the continent can be covered with many meters of perennial ice [446], while those at lower latitudes may receive sufficient summer heat to often become seasonally ice-free [447].
Antarctic freshwaters show impoverished biodiversity compared to other cold regions. This reflects both extreme climate, particularly during past glacial maxima, and the challenges of potential colonists crossing in the Southern Ocean and locating suitable habitats [440]. All Antarctic inland waters, for example, lack vertebrates, and share depauperate macroinvertebrate communities [448]. Molluscs are absent, and the only truly aquatic insect (Parochlus steinenii) is confined to one group of islands to the north of the Antarctic Peninsula. Freshwater habitats are overwhelmingly dominated by microbes, with many large lakes and stream systems lacking invertebrates larger than nematodes and rotifers. However, the paradigm that Antarctic freshwater biota is impoverished because it comprises organisms that have colonized ice-free regions exposed following the last glacial maximum must now accommodate the observation that many groups that are present contain high levels of endemism [440,449]. This is evident at multiple levels of complexity. The cyanobacterial group Oscillatoriales are responsible for most of the benthic biomass and primary production [450] (Figure 2X). Molecular approaches show that while some stains appear to be bipolar [451], others are endemic [452,453] and may have persisted through multiple glaciations, likely in on-continent freshwater refugia [454]. Diatoms likewise have been found to contain high levels of endemism, consistent with persistence through glaciations rather than recent colonization, though due to taxonomic confusion, their regional biogeography is still uncertain [455]. At the highest level of organization, there is mounting evidence of long-term persistence, through multiple glacial cycles, of freshwater crustacea in on-continent refugia [448,456].
A key biogeographic boundary in Antarctica is the Gressitt Line [457] that separates the Antarctic Peninsula and its offshore islands from Continental Antarctica. While originally proposed based on soil organisms, it applies equally well to many groups of freshwater metazoans. In part, this may reflect the increasing severity of climate, as it is essentially a latitudinal line, though it also likely includes aspects of the ease with which freshwater habitats can be invaded from South America. There are, for example, 12 aquatic mosses in Antarctica, eight of which are confined to the north of the Gressitt Line, and three only to the south. Only Bryum pseudotriquetrum occurs in both regions [458]. Similarly, the crustacean [448] and microinvertebrate [459] faunas of sites on the continental side of the line are quite distinct from those on the peninsula side, with the latter having a more obvious affinity to South America. A full recognition of the diversity, affinities, and biogeography of organisms across Antarctica requires a much more extensive and intensive application of molecular techniques.
Threats to the unique biodiversity of Antarctica come primarily through changing climate. Warming is anticipated to fundamentally change some freshwater systems, for example, through the loss of ice cover [446], or enhanced connectivity through increased water flow [460]. Evidence of warming is already found in the Antarctic Peninsula lakes [447], and in continental Antarctica, increased meltwater generation is resulting in dramatic rises in the levels of endorheic lakes with clear examples where unique microbial communities have been lost [461]. Added to this is the risk of transport of non-native taxa to Antarctica that accompanies increased visits to the continent [457]. At present, attempts to detect invasive taxa effects in areas subject to high visitor density have shown nothing [462], though our ability to identify such organisms is constrained by our limited understanding of the current biota.
In summary, Antarctic freshwaters contain a depauperate freshwater biota restricted by the scarcity and scattered distribution of habitats, the history of glaciation and the high proportion of “new” freshwater habitats, the extreme environment, and a high level of isolation from surrounding continents. The extant biota does, however, contain high levels of endemicity and there is growing evidence to show that this reflects persistence through repeated glaciation cycles. The greatest threat to this endemic biodiversity comes from the changing climate that will ameliorate physical stressors and allow colonization by organisms from both other parts of and outside the Antarctic continent.
9. Mediterranean Freshwater Habitats
The characteristics of Mediterranean freshwater ecosystems are strictly dependent on the peculiar Mediterranean climate that prevails between 32° and 42° latitude in five regions of the world. These regions are all located on the west coast of continents and placed between temperate and tropical regions, both in the northern and in the southern hemispheres (i.e., most parts of California, Cape Province in South Africa, southwestern and South Australia, Central Chile, and the Mediterranean basin). These regions are characterized by a marked seasonality, with dry and warm summers and wet and rainy winters [463,464,465]. This seasonality results in contrasting hydrological conditions between seasons that typically lead to reduced availability of freshwater habitats and/or to complete drying during summer. Interannual variability is also a central feature of Mediterranean climate, resulting in wet and dry years. However, while seasonality is highly predictable, interannual variability is not [464]. Precipitation and temperature are also highly variable spatially, between the different Mediterranean regions and within them as a result of variability in topography. For example, mean annual precipitation ranges from 300 mm in coastal lowlands to more than 1000 mm in the mountain ranges. When comparing the different regions, Australia receives the lowest precipitation, with less than 400 mm a year [465].
Such high spatial and temporal (seasonal and interannual) variability, together with historical/biogeographical processes, plays a central role in shaping biodiversity in Mediterranean-climate regions [464]. Mediterranean regions host high species diversity, and high rates of endemisms. At the same time, they are highly threatened by anthropogenic pressures. For example, the Mediterranean basin holds the highest number of critical catchments in terms of freshwater biodiversity in Europe [466], and, according to IUCN, 56% of the freshwater fish in the Mediterranean basin are threatened. The main threats to freshwater ecosystems are water abstraction, invasive species, and pollution.
Regarding the Mediterranean basin, the largest Mediterranean area in the world, only a few natural large lakes exist in the region, and the most common type of natural freshwater ecosystem is represented by relatively small and shallow lakes, as well as ponds. The alternation between a wet and a dry period governs these water bodies and is responsible for their temporary or strongly astatic nature, as well as of the variable river discharge, strictly linked to precipitation (e.g., [467]). Moreover, in the last 70 years, thousands of agriculture lakes and hundreds of much larger dam-reservoirs have been intensively built in this area to sustain the increased irrigation needs of industrial agriculture and to provide drinking water to the growing population. The number of such man-made lentic ecosystems is nowadays much higher than that of natural ones and may have consequences on the regional biodiversity and on the spreading of alien species (e.g., [468,469]). Moreover, climate changes and the increased human exploitation of water resources profoundly influences both the water storing capacity and the dynamics of natural aquatic ecosystems, often affecting the survivorship of their rich and diversified biota [470].
9.1. Streams and Rivers
Mediterranean streams and rivers are subjected to high variability in flow (Figure 1Y,Z), following the typical seasonal and interannual patterns of precipitation described above. Bonada and Resh (2013) [464] defined Mediterranean streams and rivers as “those with sequential seasonal flooding and drying periods, with increasing loss of habitat connectivity over an annual cycle that can result in temporary habitats especially during severe droughts”. This definition excludes large rivers that, despite flowing in regions with Mediterranean climate, have a flow regime influenced by a different climate. This is the case of the Tagliamento River, which has a flashy pluvio-nival flow regime influenced by both Alpine and Mediterranean precipitation patterns [471]. Thus, here we consider as Mediterranean streams and rivers those water courses with small and intermediate sizes (e.g., low to intermediate stream orders) that show a marked seasonality, including those having perennial and temporary flow regimes.
Mediterranean rivers with a temporary flow regime are the most predominant type [464]. As with all temporary rivers worldwide, they shift between flowing and non-flowing phases, which may maintain lentic habitats (e.g., disconnected pools) or that may dry out completely. Shifts in populations and communities respond to such flow dynamics, showing changes in species abundances, richness and composition between phases [465]. To define these transitory phases, Gallart et al. (2017) [472] defined a six aquatic states based on the associated biotic changes in freshwater organisms. These are (1) hyperrheic, i.e., flood conditions; (2) eurheic, i.e., full prevalence of all the possible mesohabitats; (3) oligorheic, i.e., sequence of pools connected by flowing water threads; (4) arheic, i.e., occurrence of isolated pools; (5) hyporheic, i.e., disappearance of SW, with the wet alluvium still allowing underground aquatic life; and (6) edaphic, i.e., desiccation of the river bed and alluvium. Although these aquatic phases are typical in all temporary rivers, they are more predictable in Mediterranean-climate regions, usually finding oligorheic, arrheic, and hyporreic phases in the dry summer season, and hyperrheic and eurheic phases during the wet season. This results in clear community and population changes across seasons. For aquatic macroinvertebrates, community composition usually shifts from typical lotic taxa with predominance of EPT (i.e., Ephemeroptera, Plecoptera, and Trichoptera) during the wet season to lentic taxa with OCH (Odonata, Coleoptera, and Heteroptera) during the dry season [464]. This is shown by the high temporal turnover of aquatic macroinvertebrate assemblages between seasons [473]. If the river completely dries out for long enough, terrestrial invertebrate communities may start colonizing, shifting from aquatic to terrestrial communities. In the case of primary producers, similar assemblage shifts are observed, from lotic to lentic and eventually with drying, to sub-aerial communities [474]. However, this temporal pattern might not be true for ephemeral and episodic streams and rivers, in which the terrestrial habitats predominate throughout the year and flow depends on pulse flood events [472]. In the case of fish, assemblages are typically similar between seasons in terms of composition but fish abundances change in relation to both floods and droughts [465].
Species in Mediterranean streams and rivers display adaptations to cope with such variability in flow conditions, especially during habitat contraction and consecutive drying. Many species have resistance strategies to persist in wet remaining and/or dry riverbeds, and resilience strategies that allow them to recolonize after flow resumption [475]. In the case of macroinvertebrate communities, there is a greater proportion of taxa with resistance and resilience traits in Mediterranean streams compared to temperate climates [476]. For example, many taxa have aerial respiration to cope with lentic conditions during the state of disconnected pools, or life histories with diapause stages or resistant eggs that allow them to survive in dry riverbeds. Others possess winged adult life stages that allow them to disperse aerially and colonize different perennial aquatic habitats. In the case of fish, strategies are based on tolerance to environmental variability, high recruitment rates as a result of early sexual maturity and high fecundity, together with recolonization processes from aquatic refugia [477]. Primary producers such as algae reduce photosynthetic activity, can develop resistance structures such as crusts or spores during drying and produce carotenoids to protect cells from photooxidation [474]. Vascular plants such as macrophytes typically increase stomatal density to adjust plant physiology during drying and reduce total dry mass. Riparian plants, for example, have adaptations such as rapid root extension, reduced diameter growth, and branch abscission to cope with drying [464].
Compared with temperate-climate streams, in Mediterranean-climate regions, we may typically find higher regional diversity and beta diversity, but not alpha diversity [464]. As in other Mediterranean freshwater ecosystems, endemicity in streams and rivers (Figure 2Y) is also higher than in temperate regions. When looking at functional diversity, Bonada et al. [476] found higher functional trait-based macroinvertebrate richness and diversity in streams from the Mediterranean region compared to temperate ones, presumably as a result of the dominance of strategies to cope with drying. In a recent study comparing Mediterranean streams from California with desert streams from Arizona and oceanic-climate streams from New Zealand, Tonkin et al. [473] found that Mediterranean streams had a significantly higher temporal turnover as a result of the highly predictable seasonality that generates unique communities for each season. Another study comparing genus, species, and genetic diversity of macroinvertebrate communities from European mountain streams in Mediterranean temperate and subarctic climates [478] found a higher number of genera restricted to the Mediterranean region and that local and regional diversity increased with increasing latitude. The same pattern with latitude was also found for phylogenetic beta-diversity. When comparing perennial and intermittent/temporary streams and rivers globally, the metanalysis by Soria et al. [479] indicated that perennial rivers were generally more diverse, in terms of taxonomic richness and diversity of macroinvertebrates, fish, and diatoms than intermittent rivers. However, this was not the case for Mediterranean-climate regions, for which perennial and intermittent rivers were equally diverse.
River biomonitoring and conservation are challenging tasks in Mediterranean regions. In temporary rivers, the seasonal and interannual changes in populations and communities hamper the implementation of environmental policies such as the Water Framework Directive (WFD), which aims at assessing the ecological quality of streams and rivers. The high variability found in reference conditions (i.e., the natural or unimpaired condition of an ecosystem) and the different response to anthropogenic impacts can lead to misleading bioassessment results in such systems [480]. This means that metrics and biological indices based on taxon richness and environmental tolerances may fail in detecting anthropogenic stressors in temporary rivers. Similar results were found when checking for interannual variability, with lower values for the same biological index under reference conditions in dry years [481]. Therefore, the classification of the natural flow regime of Mediterranean streams is a crucial step prior to bioassessment [472]. For example, if a naturally perennial river shifted to temporary, biomonitoring methods for perennial rivers should be applied and restoration measures should be devoted to establish environmental flows. In contrast, if a stream is naturally temporary, methods need to be adapted and calibrated accordingly. At present, water agencies from the Mediterranean region in Spain (i.e., the Catalan Water Agency and the Júcar River Basin District) are using the software TREHS (Temporary Rivers Ecological and Hydrological Status) to overcome such difficulties in the implementation of the WFD. This software allows the input of several sources of information (i.e., gauging stations, modeling, interviews of citizens, and aerial and in situ photographs) and provides a flow regime classification together with the assessment of hydrological status. The classification of the flow regime can contribute to establishing different environmental targets for each type of river according to hydrology and associated biological communities developed. The conservation status of most freshwater species is poor, mainly as a result of water abstraction, invasive species, and pollution. In the case of strictly aquatic organisms such as freshwater molluscs and fish, most species are considered to be in a non-favourable status [465]. Regarding the protection of habitats within the Mediterranean Region, the EU Habitats Directive 92/43/EEC, Annex I recognizes several habitats of community interest [482]. Regarding temporary rivers, we find “Intermittently flowing Mediterranean rivers of the Paspalo-Agrostidion”, “Riparian formations on intermittent Mediterranean water-courses with Rhododendron ponticum, Salix and others”, “Southern riparian galleries and thickets (Nerio- Tamaricetea and Securinegion tinctoriae”, and “Platanus orientalis and Liquidambar orientalis woods (Palatanion orientalis)”. Regarding perennial rivers, we find “Constantly flowing Mediterranean rivers with Glaucium flavum”, “Constantly flowing Mediterranean rivers with Paspalo- Agrostidion species and hanging curtains of Salix and Populus alba”, “Salix alba and Populus alba galleries”, and “Tufa cascades of karstic rivers of the Dinaric Alps”. Although such habitats are well represented within the Habitats Directive and partially covered by protected areas within the Mediterranean countries, recent assessments were unfavourable in most cases.
In some Mediterranean regions, we can also find naturally saline streams [483]. Such ecosystems can be subjected to both flow intermittence and high concentrations of salts, resulting in unique biological communities. Despite receiving similar human impacts as all Mediterranean rivers and streams, naturally saline streams are also impacted by freshwater inputs typically coming from water loosed from irrigation channels. As aquatic species typical of these ecosystems are adapted to salinity, human-induced dilution can lead to biodiversity loss, affecting both regional and global biodiversity. Therefore, specific management practices are urgently needed to maintain freshwater biodiversity in such unique ecosystems. Predicted increased temperatures coupled with reduced water discharges make stream biodiversity in saline streams and rivers within Mediterranean-climate regions very vulnerable to climate change [484].
Mediterranean streams and rivers are expected to be highly affected by climate change and increased water use. Many rivers that are nowadays perennial will become temporary, and many temporary rivers that today maintain dry season refugia may shift to ephemeral [485]. This will result in the disappearance of habitats at the local scale and more fragmented river networks at the catchment scale as a result of drying [486]. This will have severe effects on freshwater biodiversity and urges for innovative water management and conservation strategies able to integrate such complexity.
9.2. Ponds
Ponds, both permanent and temporary, represent the most typical freshwater ecosystem type in the Mediterranean areas. In the Mediterranean basin, permanent ponds are generally more abundant on the slopes of mountains, where higher annual precipitation and lower evaporation rates occur, whereas temporary ones are widespread and also occur in lowlands, including the rocky sea shores (freshwater rain-fed, rock pools).
Permanent ponds can be roughly divided in two groups: mountain ponds and lowland ponds. The first group includes the highest number of permanent Mediterranean ponds. These ecosystems are generally characterized by meso-eutrophic conditions and by a diverse biota. The high species richness is firstly reflected by the structure of primary producer communities, which are formed by plants, ferns, mosses, and benthic algae that, in some cases, represent glacial relicts which coexist with taxa typical of the Mediterranean area. A similar pattern was observed for planktonic algal assemblages, often characterized by taxa typical of temperate climates which are not found in lowland aquatic ecosystems [487]. This feature strongly contributes to the high biodiversity that characterizes Mediterranean permanent ponds located in the mountain ranges.
Conversely, lowland ponds, often subjected to the effects of intensive agriculture, generally show eutrophic or hypertrophic conditions and quite low species diversity. These effects are worsened by the climate-warming-driven increases in water abstraction, which lead to amplified water-level fluctuations and a rise in salinity. Unfortunately, there are just a few studies analyzing the effects that agriculture and global changes have exerted on these freshwater ecosystems in the Mediterranean regions (e.g., [470,488,489]). Another potentially negative aspect relates to the introduction of alien species. In fact, since the first half of the last century, both mountain and lowland ponds in the Mediterranean area, have been more or less accidentally stocked with alien species (e.g., the fish Gambusia holbrooki was intentionally and extensively released to fight malaria spreading mosquitos; see [469]); however, the effects of such introductions have been seldom investigated, although they can be detrimental for the autochthonous biota [490].
According to the main features of the Mediterranean climate, seasonal temporary ponds (also known as vernal pools or vernal ponds in North America) are characterized by the alternation of a water phase occurring in winter and a dry phase in summer. This extreme variability makes the environments unsuitable to fish and free from the trophic cascading effects (mainly linked to predation) that fish exert on aquatic communities. The absence of fish predation favors several species of amphibians that can find a suitable breeding habitat in such ponds [491], also thanks to their ability to adjust the rate of larval development according to the risk of pond desiccation [492]. Actually, all the aquatic organisms inhabiting these ecosystems show specific adaptations to deal with summer total desiccation and to survive the dry period (see [493] and literature therein, [494]).
Temporary ponds are generally small (from 10−2 to 107 m2) and often grouped in spatial clusters [495]. Their biotic communities should, therefore, be considered as metacommunities, and spatial factors should be included when analyzing their dynamics (e.g., [496]). Accordingly, the disappearance of ponds in a given area may weaken their connectivity and impair their biodiversity [497]. However, their dimension and spatial distribution are not the only heterogeneous feature of these ecosystems, and their variability actually spans over a number of physical, chemical and biological gradients (e.g., pH, conductivity, salinity, turbidity, nutrient concentrations, and total amount of precipitation/evaporation, hydroperiod length, macrophyte species richness and cover). This wide spectrum of heterogeneity further contributes to the very high levels of biodiversity observed in these ecosystems (e.g., [498,499]).
With regard to protection and conservation issues, temporary ponds characterized by the presence of plants belonging to the class Isoeto-Nanojuncetea (Figure 1AA and Figure 2Z) are included in the habitat typology 3170* (Mediterranean Temporary Ponds) and listed as “priority habitats” in the EU Habitat Directive 92/43/EEC. However, due to the high spatial and environmental heterogeneity, Mediterranean temporary ponds show a quite reduced cross-taxon congruence [500], and these ecosystems generally host a high number of exclusive and rare species, although lacking representatives of the plants that could grant them legal protection. As a consequence, several of these environments are disappearing since they look like mere land depressions during the dry phase. Since monitoring and on-site information are lacking for many of these ecosystems, they end up as dumpsites or filled to create human infrastructures (e.g., car parks, greenhouses, solar and wind power plants). From an ecological point of view, the disappearance of these ecosystems makes them more isolated, diminishing their connectivity and hampering those dispersal/colonization events that contribute to maintaining the integrity of their metacommunity dynamics.
The need to develop effective protection plans for these ecosystems resides in their high biodiversity. The exclusive presence of many unique species in temporary ponds make them hot-spots of biodiversity within the Mediterranean regional hot-spot of biodiversity [501] and, due to the limited cumulative surface and volume stored, they disproportionately contribute to the regional biodiversity in this area. These environments also show a strong resistance against species invasions due to their ancient, species-rich and diverse biological communities (e.g., the communities of the “Hemidiaptomus ponds”, see [469,497]), which, along with the dry summer phase, represent an effective biological hindrance against the establishment of newcomers [493]. Actually, in addition to iconic and exclusive organisms (e.g., large branchiopods (Figure 2AA), several diaptomid copepods, insects), they host a large number of endemic species [494] along with species also found in permanent ponds [502,503]. Their flora is also very diverse with regard to vascular plants, ferns [504,505], and bryophytes [506]. Species richness is also very high with regard to planktonic and metaphytic algae, with more than 450 species identified in just two temporary ponds in Sicily [507]. Many of the algal species recorded in these water bodies, including representatives of three rarely observed genera (Cyanophora, Glaucocystis, and Gloeochaete) of the phylum Glaucophyta, were exclusively found in temporary waters.
It was probably not by chance that Hutchinson was inspired by a visit to a Mediterranean temporary pond when he published his seminal paper “Homage to Santa Rosalia, or why are there so many kinds of animals” [508].
9.3. Near-Natural Lakes (L. Kinneret)
The regions subjected to Mediterranean climate listed above have relatively few natural large lakes. Of those, the majority are brackish or saline, only a few are freshwater lakes of substantial surface area (> ~100 km2) and deep enough to be stratified in summer, and thus be considered here as “large lakes”. In the Mediterranean basin, these include several lakes in Turkey (Beysehir, Aksehir), Greece (Amvrakia, Trichonida), the Balkan region (Prespa, Orhid, Skadar), and Israel (Kinneret). Other countries of the Mediterranean basin with a Mediterranean climate, like Cyprus, Syria, Lebanon, Jordan, Egypt, Tunisia, Algeria, do not have even a single natural freshwater lake of substantial size [509].
The Mediterranean climate dictates much of the ecology of Mediterranean large lakes. With all precipitation occurring only in the winter while the summers are dry and hot, Mediterranean large lakes are subject to strong seasonality. They are warm monomictic, with turnover in winter (December–January in Northern Hemisphere) and strong stratification in summer. Most nutrient loads reach the lake during the winter–spring season, when inflows are strong, and decline to low levels in the summer–fall. Dust is a substantial source of nutrients in summer, especially P [510]. This physical–chemical setting leads to strong seasonality of the biological components.
Major threats to biodiversity in natural Mediterranean lakes are similar in nature to those of reservoirs: overexploitation for supplying drinking/irrigation water, eutrophication, salinization, invasive species, habitat degradation, and inappropriate management of water levels, of fisheries (fish stocking, over-fishing), and of shores (e.g., removal of shore vegetation).
Of the large natural freshwater Mediterranean lakes, Lake Kinneret (also known as the Biblical “Sea of Galilee”) (Figure 1A,D) is by far the most studied ([511] and references therein). Hence, the majority of the discussion below uses Kinneret as an example. Being isolated from other lakes, Lake Kinneret hosts a substantial number of endemic species, including among others seven fish [512], four molluscs, and a blind shrimp that inhabits a single cave on the lake’s shore [513].
Lake Kinneret has been subjected to intense human intervention over the last nearly 100 years [514]. Its outflow was dammed in 1927–1932 for hydropower generation. Lake Hula—a natural shallow lake in its catchment that acted as a natural filter to water flowing into Lake Kinneret—was drained in the 1950s to abolish malaria. The lake has been stocked annually with fish to improve fisheries. Since 1969, the lake has been used as a major source of drinking water. This latter role led to main governmental efforts to minimize eutrophication by strict control of effluents, to prevent its salinization by diverting saline inflows, and to base the decision-making process on data and knowledge by funding and running a long-term monitoring program on the lake and its catchment [515,516], all in effort to maintain a healthy ecosystem. Being considered as a source of drinking water, the lake was initially managed for several decades to maximize its storage capacity, by pumping drinking water out of it during low-rainfall years to empty storage space for filling up in high-rainfall years. This led to multi-annual water level fluctuations 4-fold greater than natural. Over time, analysis of the accumulated monitoring data clarified that these excessive water level fluctuations destroy littoral habitats, damaging the lake’s biota and its biodiversity [517]. These insights from Lake Kinneret instigated the understanding that water level fluctuations beyond natural, previously considered harmful to shallow lakes, are also harmful to deep stratified lakes. In recent years, desalination has become Israel’s major source of drinking water, reducing the need to pump water from the lake [518]. Within several years it should be possible to manage Kinneret again at close to natural amplitudes of water level fluctuations.
Global climate change led to declining precipitation in the Kinneret watershed. Rimmer et al. (2011) [519] reported that over the period 1969–2008, epilimnion temperature has increased by ~1 °C, and epilimnion thickness has declined by ca.1.2 m, whereas the duration of stratification did not change. Interestingly, they concluded that those changes were driven mostly by the long-term decline in water levels due to excessive pumping rather than by climate change.
The invasion of alien species is another factor that has caused irreversible changes to the lake’s biodiversity, foodweb, and ecology. Until the mid 1990s, the large thecate dinoflagellate, Peridinium gatunense (Figure 2A,D), dominated the phytoplankton assemblages, blooming every spring and constituted a preferred food for the cichlid fish of the lake. In summer, the dinoflagellates were replaced by a diverse assemblage of nanoplanktonic chlorophytes and diatoms that comprised the basis for a nanoplankton–zooplankton-fish food chain [520]. This typical pattern changed in 1994, with the first appearance and major bloom of the N-fixing filamentous cyanobacterium Chrysosporum (previously Aphanizomenon) ovalisporum, shortly after hydrological changes in the catchment led to summer N-deficiency in the lake [521]. Since then, P. gatunense blooms only on high-rainfall years; in most years, toxic cyanobacteria dominate, including several species of Microcystis, C. ovalisporum, and other N-fixing cyanobacteria that also invaded the lake (Cylindrospermopsis raciborskii, Anabaena borgii) [522,523]. The filamentous green alga Mougeotia sp. also invaded Lake Kinneret in 1998, and since 2004, it is abundant, forming occasional blooms that clog fishermen’s nets [524].
The littoral zone of the lake also was dramatically altered by an invasive snail, Thiara scabra, which now constitutes >95% of all snails, and is pushing the five native snail species, four of which are endemic to the region, towards extinction [525].
9.4. Reservoirs
In the Mediterranean region, two main types of man-made reservoirs are very widespread: dam-reservoirs and agricultural lakes. Dam-reservoirs (Figure 1AB,AC) are lakes in their appearance [467]. However, they are artificial and, as underlined by Moss (2008) [526], “the purpose of the dam is deliberately to interfere with the natural characteristics of a river or former natural lake”. This statement is particularly true in several parts of the Mediterranean basin, where dam-reservoirs exhibit wide climate-driven, water-level fluctuations, which are often exacerbated by water (over)exploitation for human needs. Such fluctuations can cause a decrease in both stored volumes and maximum depths, ranging between 40% and 80% when comparing early spring and late summer values [463]. Seasonal water-level fluctuations interfere with the classical limnological paradigms that govern the dynamics of physical, chemical, and biological parameters in natural lakes, and suggest classifying dam-reservoirs in a category quite different from that of natural lakes. In recent years, these differences, mainly due to the effects of hydrological disturbance, have been widely acknowledged by limnologists both in the Mediterranean region (e.g., [527,528]) and in the rest of the world (e.g., [529] and literature therein). In particular, summer dewatering, mainly due to water use for agriculture, produces a progressive and fast reduction in the thermal stability of these water bodies, often causing an anticipated breaking of the thermocline in mid-summer. Fluctuations in water-levels, which can vary among years, have been shown to interfere with nutrient dynamics, light availability, composition and structure of phytoplankton (Figure 2AB,AC), and ultimately to influence the global structure of the reservoir’s community (e.g., [528]) also by favoring the establishment of long-lasting cyanobacterial blooms (e.g., [530]). Moreover, water-level fluctuations are generally wide enough to prevent the growth of aquatic macrophytes along their shores. The lack of biological richness and functions generally provided by the littoral zone in lakes is also important in determining the differences between natural and artificial lakes.
All these differences between natural and artificial lakes are so striking that they allow the definition of “disabled lakes” for reservoirs [526]. Actually, the human-driven functioning of man-made lakes entrusts all the primary production to the pelagic compartment, conditions the composition and structure of the aquatic biota, and favours eutrophication processes and a generalized loss of biodiversity.
Dam-reservoirs are fully considered lacustrine ecosystems within the European Water Framework Directive and, in the European part of the Mediterranean area, they were mandated to achieve a good ecological quality by 2015. Such a goal has seldom been reached due to the intrinsic problems of the Mediterranean dam-reservoirs, which reside in their original engineering planning carried out without adequate ecological knowledge [531]. Achieving a good ecological state is only possible if the planning of future reservoirs carefully considers the main features of their functioning, eventually setting limitations to water abstraction [527]. In fact, maintaining the “lacustrine” structure and functions of these ecosystems as intact as possible (through a careful and informed hydrological management) is probably the only way to improve the environmental quality of these “disabled lakes”.
Along with relatively large dam-reservoirs, a myriad of agricultural ponds was built in the last decades in the Mediterranean semi-arid areas. As a consequence, artificial lakes and ponds are nowadays much more abundant than natural ones [532]. In Sicily, these waterbodies are large tanks (stored volumes ranging between 20 and 700 m3) scattered around the lowlands and the hilly part of the territory, and forming a dense mosaic of relatively new aquatic environments. A total number of 20,000 of these agriculture lakes have been estimated to be present in the island. As shown by Naselli-Flores and Marrone (2019) [469], these artificial waterbodies generally host a species-poor biota mainly consisting of a few euryecious species and several alien, invasive taxa. The high likelihood of becoming invaded, as shown by artificial environments, is likely due to the increased target-area effect for passive dispersing organisms. Moreover, they provide a suitable environment for non-indigenous taxa due to their recent origin and to the lack of efficient biological filters against newcomers, thus acting as bridgeheads and invasion hubs favouring invasive species. In fact, the high number of these artificial aquatic ecosystems increases the spatial connectivity among these habitats, thereby facilitating the dispersal of both local euryecious and non-native, invasive species. This process can therefore enhance the risk of biological invasions in natural aquatic ecosystems from both alien and local species, and lead to the alteration of biotic community structure and richness.
The physical characteristics of artificial ponds and their placement in a given area should be carefully planned in order to facilitate colonization by autochthonous species (and thus their “naturalization”), and to effectively inhibit the establishment of alien species [532]. In order to achieve the goal of a successful coexistence of economic development and nature conservation, information about the trade-offs, risks, and opportunities of agriculture ponds should be made available to the land-owners, other stakeholders, and policy makers. In addition, the possibility of scientifically-supervised inoculation of the newly created artificial waterbodies with propagules from the local native biota should be investigated as a possible approach to accelerate “naturalization” of these man-made waterbodies through the establishment of invasion-resistant, native assemblages.
10. Tropical Freshwater Habitats
Between boundaries of the Tropic of Cancer and the Tropic of Capricorn, seasonality is strongly determined by fluctuations in rainfall through the year, while temperature and day length remain about constant. Patterns of rainfall result in hydrological seasonality, with wet and dry periods. A phase of low rainfall is followed by a phase of rainfall concentration, when discharge increases result in a flood pulse [533] in catchments, and the waters flood and rise in low-land areas and results in the most typical freshwater feature in the tropical landscape, i.e., wetlands. According to some authors [534], tropical and subtropical (Figure 1AF) freshwater wetlands (e.g., [50]) are dominated by (1) floodplains, (2) peatlands, and (3) swamps. The array of tropical freshwater habitats is completed by several (4) large river systems (e.g., [52]), and (5) lakes (Figure 1AE). Tropics are home to some of the largest river basins in the world, with dense networks of streams (Figure 1AG) and rivers, such as the River Amazon in South America, the River Congo in Central Africa, and the Rivers Mekong and Bramaputra in south-eastern Asia. These great basins are associated with vast fringing floodplains. The Amazon River system in South America drains an area of 6.3 million km2 and harbours the largest extent of floodplain forest in the world [535]. The connectivity with the river system, due to the variability in duration and timing of flooding, determines a spatial pattern through a different degree of inundation [536]. In the Amazon basin, as many as 17 different seasonally inundated wetlands have recently been classified [537]. As floodable areas, they can be considered also internal deltas, well represented by the Pantanal in South America fed by the Paranà-Paraguay rivers, and the Okavango delta in Africa (Figure 1AF) supplied by the Niger and Okavango rivers. There is a lack of a standardized definition for different types of wetlands, which can vary between authors (e.g., [534,538]) and they may depend on the researchers’ approaches [539]. However, any area characterized by an accumulation of partially decayed plants is a peatland, and this definition also comprises some kinds of swamps and marshes [538]. Recent work [540] shows a much wider extension of the tropical peat-forming wetlands compared to previous studies [541]. South America holds the largest area of tropical peatlands (46%), followed by south-eastern Asia (36%). As reported in [542], the better represented tropical lakes (Figure 1AE) are shallow (lowland lakes), old (tertiary lakes located in the African Rift Valley, such as the Vittoria, Tanganyika, and Malawi lakes), and high (high mountain lakes, such as the Titicaca in South America). Many lakes in the African Rift are saline: some of them are subjected to wide variations in water level and ion concentration, such as the Nakuru Lake in Kenya, which historically experiences sharp fluctuations in salinity [543].
All the largest tropical and subtropical freshwater ecosystems are areas of great cultural and economic importance. In wetlands, the hydraulic seasonality has also shaped the traditional practices of the indigenous populations for millennia, i.e., with agriculture and animal ranching during the low water period, and fishery throughout the year. Even today, all the great flood plains and rivers of South America, Africa, and South-East Asia are inhabited by native human communities, managing the systems in a traditional way [544,545,546]. On the other hand, in many poor countries, mostly in central Africa and South-East Asia, the river systems represent the basic resource for every daily action, so not only for specific activities including agriculture and fishing but also for drinking water, cooking, and washing. It is well known that rivers and wetlands provide a wide range of ecosystem services, including environmental, social, economic, and cultural benefits [18,547,548]. Wetlands can reduce the content of nutrients in the water by accumulation in plant tissues, and retaining sediments in the soil, as natural filters that can improve water quality [549,550,551]. Moreover, they are a natural defense against catastrophic atmospheric events since they can slow down the water flow and can reduce the height and force of floodwaters. Wetlands have the capacity to reduce flood peak magnitude by acting as natural reservoirs that can accommodate floodwater, keeping them for a variable time, and then regulate water flow by gradually releasing flood water [552]. Also, wetlands maintain biodiversity by providing habitat for many animal and plant species that can support different needs of human populations. In West and Central Africa, between 450,000 and 500,000 people are directly involved in full-time activities related to inland fisheries with a potential value of approximately USD 750 million annually [553]. In South and South-East Asia, natural wetlands sustain huge fin and shrimp fisheries: Tonle Sap, an intermittent shallow lake connected to the Mekong River, provides the highest catch of an inland fishery worldwide [15]. Tropical Asia is also characterized by the largest extension (88% of global area) of human-made wetlands in the world, i.e., paddy fields [16]. Peatlands also play a fundamental worldwide role in the carbon cycle [554,555,556]. Lal (2008) [557] estimated that about 20%–30% of C is stored in wetlands. The carbon can accumulate in the soil thanks to incomplete degradation of organic matter: wetlands are optimum natural environments for sequestering and storing carbon from the atmosphere, owing to anoxic wet conditions. Most peatlands are found in temperate and Arctic areas, and tropical peatlands have only recently received attention to be an important key in the carbon fluxes [541,558,559]. The extent, volume, and carbon content of global tropical wetlands are not well known [560]. However, a recent estimate provides a figure of 1.7 Mkm2 of tropical peatlands for a mean volume of 7268 km3 of stored peat [540].
Tropics are home to most of the developing countries and most of the world population, and tropical Asia alone harbours more than one-third of the world’s human population. As a consequence, the pressure on the environment is very high and increasing. The major threats for tropical inland waters are the growth in demand for arable land and urbanization, and therefore, the water urge for irrigation and domestic use, the increase in electricity production through the construction of dams, the introduction of exotic species, water abstractions, overexploitation of resources, and eutrophication/pollution.
Considering that most of the tropical freshwater environments are represented by seasonal wetlands, whose existence is guaranteed by periodic floods, the greatest threat is the alteration of hydrological regimes through an excessive water extraction (i.e., for agriculture), construction of dams and reservoirs, and destruction of forests in mountain areas, without considering the alteration due to the global climate change that can modify precipitation regime and evapotranspiration rates. These threats are widespread in tropical regions, especially in large river basins such as the Congo, Mekong, and Amazon [561,562]. Changes in hydrology trigger a wide range of different impacts, such as alteration of sediment dynamics, discontinuance in biogeochemical cycles, and perturbation in inundation regimes, as reported for the Amazon River basin [562]. The changes in land cover due to deforestation and conversion in agricultural areas increased erosion in the catchment of the Pantanal and have raised the sediment load of the tributaries: the Taquari River has been filled with sediments, breaking through the natural banks, and modifying the hydrology of an area of around 11,000 km2 within the Pantanal, with dramatic consequences for the environment and the local ranchers [15]. In South America, Africa, and South Asia, freshwater habitats are also impacted by ongoing mining to extract iron ore, gold, oil, gas, and bauxite. Mining and infrastructures for oil and gas extraction produce high levels of pollution and promote deforestation, dam construction, and opening of roads in remote regions [562]. Building dams and reservoirs not only impacts lowland wetlands but it is well known that dams break the natural longitudinal river connectivity [167], causing many alterations to river characteristics and function [563], such as the geomorphology [564] and the physical and chemical properties of channels, besides being barriers to the dispersal of many running-water species, i.e., macroinvertebrates and fishes, so that populations above and below dams become effectively isolated. The changes in hydrologic pattern and longitudinal connectivity also affect water quality: a comprehensive discussion of this subject, with special attention to tropical areas, can be found in [565]. Dam building is an environmental problem that today is located mainly in the tropics, considering that ongoing and proposed major dam projects are concentrated at low latitudes [566]. The situation in South America seems particularly critical, where an exceptional proliferation of dams is threatening linkages between the Andean headwaters and the lowland Amazon, with a huge river fragmentation [567], risking a start to irreversible processes, with drastic consequences on the conservation of biodiversity and for traditional and economic activities in the low lands [568,569]. A well-documented example of a dramatic impact on biodiversity and traditional activities is the construction (in 1994) of the Pak Mun Dam on the Mun river (Mekong basin), which caused the destruction of traditional fisheries and the decline of fish diversity. Roberts (2001) [570] identified the main impacts as being obstruction of breeding migrations, habitat conversion, and periodic dewatering or extreme flow variation downstream, combined with releases of oxygen-poor, warm, silty water from the reservoir. The situation is less critical in Africa but South-East Asia shows a worrying condition very similar to South America, with numerous built dams and dozens under construction or planned [571].
Another big threat for freshwater habitats is invasion by exotic species, recognized by now as the main driver of biodiversity change [572], particularly evident in lakes where non-indigenous species can rapidly become the dominant component of the ecosystem [573]. The introduction of non-native species is due to various causes, such as deliberate introduction to provide food or for sport purposes, for mosquito containment, or for ornamental purposes, or unwanted releases through the escape from aquaria, water gardens or aquacultures. An example of tremendous impact on biodiversity is Lake Victoria, where a single invader, the Nile perch (Lates niloticus), completely devastated the richest endemic fish fauna of the planet, without encountering predation or competition by native species [574,575]. Alien fishes that are not piscivores may also have large effects on their food: some authors [576] found a strong effect on aquatic invertebrate communities, via direct predation, by allochthonous Rainbow trout (Oncorhynchus mykiss) in high-Andean tropical streams. One of the most important classes of freshwater invaders includes filter-feeding molluscs, or those that feed on periphyton. These species can develop huge populations and consume so much primary production that they substantially influence the quantity and composition of primary producers. The interactions that radiate from primary producers can influence almost every part of the ecosystem [577]. The wetlands of southeastern Asia are invaded by the South American golden apple snail (Pomacea canaliculata), with severe pressure on the ecosystem through the elimination of macrophytes, causing increased concentrations of nutrients and large increases in phytoplankton [577]. The effects of herbivorous molluscs like the golden apple snail thus cause a shift in energy flow similar to that of severe eutrophication in shallow lakes [578]. Even exotic plants negatively affect ecosystems: an outstanding example is the water hyacinth (Eichhornia crassipes), a native of South America, but widespread in freshwater habitats of Africa and South-East Asia, and which is on the IUCN list of the 100 most dangerous invasive species. This species can occupy thousands of hectares of previously open water, forming extensive mats, heavily impacting the ecosystem: its rapid growth rate makes it capable of successfully competing with native plants and reducing light and oxygen resulting in a drastic change in submerged animal and plant communities. It can increase evapotranspiration, produce organic matter which increases the organic content of sediments, can favor the spread of diseases, and interferes with the use and management of water resources [579].
As mentioned before, alteration in land cover can affect sediment load but also directly impacts freshwater environments. Deforestation is particularly heavy in tropical America [562,580] and South-East Asia [581]: in Peninsular Malaysia, Sumatra, and Borneo, logging threatens peatlands at ever increasing levels, where, since 2007, industrial plantations have nearly doubled their extent [582]. So far, in these regions, managed land cover types have affected 50% of all peatlands: most of which are represented by oil palm plantation (73%), and the rest almost entirely occupied by plantations for paper pulp production, especially acacia [582]. While intact tropical peatlands can accumulate many meters of peat over thousands of years [541], degraded peatlands are susceptible to decomposition and fires [583]. This leads to soil subsidence [584], loss of biodiversity [585], and extremely high carbon dioxide emissions [586,587,588]. Finally, climate-change driven changes in temperature, total precipitation and rainfall pattern, besides increasing extreme climate events, can exacerbate all the consequences of threats outlined so far for tropical freshwater ecosystems. We can expect alterations in water temperature, in hydrology and biogeochemical cycles, in evapotranspiration rates, and shifting species distribution, altering community structures and species interactions [15]. Different authors have explored the consequences of climate change on tropical freshwater habitats and their biodiversity but much work is still needed; among others, for predicted impacts in sub-Saharan wetlands [589], for impacts on Africa’s freshwater biodiversity [590], for predicted impacts on the Brazilian Amazon wetlands [591], to investigated alterations in freshwater biodiversity in South-East Asia [592], and for impacts on tropical and subtropical Asian wetlands [593].
11. Arid-Climate Freshwater Habitats
Arid-climate freshwater habitats, including hot as well as high latitude cool- or cold-deserts are characterized by mean annual rainfall <250 mm, evaporation rates that exceed precipitation, and a variable hydrologic regime, with both droughts and flooding that vary in recurrence and severity from diel to century scales [594]. Temperature, humidity, and GW availability are among the main abiotic environmental factors influencing biodiversity and biogeography of arid freshwater groundwater, springs, streams, and lake ecosystems. However, a biocultural approach, including human population size and behavior, as has been applied in the study of oases in the Sahara and Sonoran Deserts, is needed to complement biological conservation strategies [154,595]. The casual observer might believe that arid regions support low species richness and limited biodiversity because of environmental harshness, but many taxa are well-represented in deserts, including aquatic, wetland, and riparian plants, microorganisms and aquatic faunas and assemblages (e.g., Figure 2A,J). High species richness in such habitats may be comparable to that in more mesic environments [596], and endemic diversity can be high. However, the diverse impacts of human pressures, including land-use, pollution, increased GW overexploitation, and global climatic change, pose dire threats for the sustainability of aridland-adapted freshwater species and habitats. Here we summarize arid-climate freshwater factors influencing ecology and biodiversity, with emphasis on microbial and algal ecology in desert springs, streams, and ciénegas (wet meadows).
GW and SW temperature and geochemistry strongly and non-linearly influence aridland springs biodiversity and ecology [115], and those in other freshwater ecosystems. Aridland stream nutrient dynamics are strongly influenced by SW–GW interactions, both within the stream channel and between the stream and adjacent riparian zones [597]. Nutrient transformations occur rapidly in channel floor sediments that are colonized by microbiota, and variable rates of processing result in spatial patchiness in nutrient availability in the water column. Nutrients and organic matter may accumulate along the channel margins or in riparian zones during dry periods and become remobilized during high flows, resulting in large temporal variability in nutrient dynamics with recolonization following such flows [598].
One of the most thoroughly-studied stream ecosystems in North America is Sycamore Creek in the Sonoran Desert, where warm stream temperature, high insolation and limited N availability all interact together to favour the proliferation of cyanobacterial mats, mainly including Calothrix sp. and Anabaena sp., often covering large portions of the stream bottom [599]. Freshwater stream-algal colonization following the flood succession usually starts with diatoms, then the filamentous green algae, and finally cyanobacterial mats [597]. This pattern can be related to N availability, as floods usually lead to a nitrogen pulse, thus alleviating N-limiting conditions for a brief time [600]. At early successional stages, algal standing stocks are low and mainly rely on nutrients from the water column and recycled from the sediments. As chlorophytes join the community and begin to form mats, leading to a decreased nutrient uptake from the environment and strong N limitation of this algal community, thus favoring colonization by cyanobacteria.
Freshwater organisms in arid-climate biomes have different strategic adaptations to GW and SW availability, temperature, geochemistry, and also to drying through behavior, physiology (e.g., pigment and lipid profiles), morphology, distribution (e.g., [601,602]), and biotic relationships. For example, many desert stream biota have the ability to rapidly recolonize after floods or rains and are distinctly productive and diverse due to the high light availability and warmer temperatures. In another example, the frequency and duration of flooding have decreased in response to climatic change in arid Australian wetlands; however, waterbirds are facilitating recolonization of some invertebrates, plants (e.g., Lemna, Typha, and Myriophyllum) and charophytes (e.g., Nitella) through zoochorous propagule dispersal [603].
Harsh aridlands environments promote the evolution of endemism. For example, the warm, stenothermic, highly mineralized springs in Montezuma Well, Ash Meadows, Nevada, and Cuatro Ciénegas, Mexico, support high levels of endemic biodiversity [133,140]. Similarly, 67% of the metaphyton taxa in desert springs of the Bonneville Basin, Utah appear to have restricted distributions and are endemic to some individual springs [604]. That study also revealed dispersal limitations, with the absence of some species in more isolated springs. This observation supports Foissner’s opinion (2006) [605] that algal distribution limitations exist, despite their potentially wide dispersal capacity. Dispersal limitations and adaptation similarly are responsible from much of the endemism among aridlands fauna (e.g., [606,607]).
Cantonati et al. (2015) [113] highlighted that the ambient springs in warm climate settings, including freshwater aridland ecosystems, host-diverse, species-rich cyanobacterial and algal assemblages, which, along with their adaptations to such habitats, are poorly known. The algal and cyanobacterial diversity of freshwater ponds and streams in the Central Death Valley Desert (Eastern California, USA) includes cyanoprokaryotes, green algae, and diatoms that dominate harsh thermophilic conditions there [608]. Morphotaxonomic surveys of freshwater ambient to slightly geothermal springs (ains; Figure 1AI) and drilled wells (birs) in the El-Farafra Oasis in the Western Desert of Egypt, revealed that those habitats often were impaired by human and livestock impacts (e.g., trampling and organic pollution) [609], as evidenced by the dominance by cyanoprokaryote bioindicators of eutrophic thermal freshwaters (Geitlerinema splendidum, Jaaginema geminatum, J. subtilissimum, Kamptonema jasorvense, Limnothrix redekei, Oscillatoria animalis, O. limosa, O. princeps, O. tenuis, and Phormidium terebriforme). Similarly, O2-poor, warm springs in the Cuatro Ciénegas contain unusual cm-sized waterwarts built by an Aphanothece-like unicellular cyanobacterium and were suspended within a central, conically-shaped, 6-m deep well by upwelling waters, and supported a community of other epiphytic filamentous cyanobacteria and diatoms [610]. This unique jet-suspended, calcite-ballasted, colonial cyanobacterial community was narrowly adapted to conditions in that desert spring habitat. Also, Saber et al. (2018) [611] recently noticed unusual morphological characteristics in the streptophyte Chara vulgaris growing in the Springs of Moses in the Sinai Desert (Egypt). In both freshly-collected and cultured materials, thalli were delicate and the antheridia were shed early, the latter interpreted as an environmental adaptation to this highly-isolated and selective arid-climate freshwater ecosystem. Cyanoprokaryotes have wider biogeographic distributional niches than do other eukaryotic algal groups due to their different ecophysiological adaptive mechanisms to survive in arid-climate habitats and in addition to the production of several types of resting stages, which facilitate their environmental resistance and dispersal by wind or migrating animals [612].
As a prime example of a biodiverse aridland spring type, desert wetlands, or ciénegas, spanning the borderlands of Arizona (USA) and Sonora in México, are regions of high biodiversity conservation value. These environments contain an estimated 19% of species considered for federal protection in the region. Besides being crucial refugia for plants, mollusks and other invertebrates, native fish, amphibians, and reptiles, ciénegas also constitute a critical habitat for migratory birds. The increased and targeted habitat conservation of desert wetlands can yield great benefit to the maintenance of global biodiversity [154,613]. Similarly, the endemic biodiversity of Australian desert artesian springs includes 96 species and subspecies of plants, molluscs, crustaceans, and fishes, dominated by invertebrates with limited geographical ranges [614].
Environmental variability can significantly influence the distribution and abundance of endemic aridland freshwater biota, such as gastropods, particularly when extremes occur simultaneously over sustained time periods [615]. In general, freshwater desert springs are highly threatened ecosystems, but they often are remarkably constant environments that contain unique faunas with many endemic, endangered, and cryptic aquatic taxa, like the endangered spring snails Juturnia kosteri (Figure 2AI) and Pyrgulopsis roswellensis found in springs in the Chihuahuan Desert (e.g., [616,617]), owing to their isolated, island-like nature and dispersal limitations [618]. Improved protection of these sensitive freshwater ecosystems is crucial for biodiversity conservation.
Information available on the biodiversity of arid-climate freshwater algae and cyanobacteria, as well as other freshwater springs-dependent taxa, is limited and understudied, particularly in North Africa and throughout South America. Much additional research is needed to fill these gaps in knowledge. Furthermore, the discovery of many endemic algal and cyanobacterial species in aridland freshwater ecosystems is predicted, especially as revealed through combined polyphasic approaches (i.e., morphotaxonomy, autecology, biochemistry, and genetics). For example, Saber et al. (2018) [611] recorded the new desmid species Euastrum elfarafraense (Figure 2AH) in an agricultural ditch fed by a typical inland-water rheocrenic hypothermal spring, “Ain El-Balad” in the El-Farafra Oasis, Western Desert of Egypt [619]. New and interesting cyanobacterial and green algal taxa have been recorded, but not yet described, from freshwater streams on bare rock inselbergs of wadis (Figure 1AJ) in the Eastern Desert of Egypt (Figure 1AH) (Saber A.A., unpublished data). Intensive human impacts on GW and SW, such as coarse-scale inputs of agricultural nutrients, release of domestic sewage and other pollutants, as well as the global climate change, are likely to cause severe structural and functional degradation of arid-climate freshwater habitats, with subsequent degradation of their biotic assemblages. Moreover, the resilience of these ecosystems from severe anthropogenic stress appears to be low, leading to a high risk of extirpation and extinction of many interesting and poorly-known rare and endemic species.
12. Freshwater Biodiversity Observation Network (FWBON)
The Freshwater Biodiversity Observation Network (FWBON) is a voluntary community of practice dedicated to tracking change in global freshwater biodiversity. It was established in 2016 as a biodiversity observation network affiliated with GEOBON (https://geobon.org/). FWBON promotes best practices for global freshwater biodiversity observations by helping to (1) improve the collection of harmonized data; (2) develop data standards and methodologies for data management and dissemination; (3) share biodiversity data across the world without compromising national concerns; (4) integrate biodiversity information with physical and chemical data; (5) generate products useful for sound management of rivers and their catchments, lakes, wetlands and subterranean aquatic ecosystems; and (6) integrate freshwater science and practice with terrestrial and coastal conservation objectives.
With members in over 50 countries, FWBON strives to bring freshwater biodiversity monitoring expertise and experience from across the world to global biodiversity observation platforms [620]. Its activities build on and support a multitude of projects and programs funded at local, national, and regional scales, e.g., the Circumpolar Freshwater Biodiversity Monitoring Program [418]. This enables FWBON to promote a global agenda that is firmly anchored in national and local capabilities and priorities. It also allows FWBON to facilitate the flow of knowledge on freshwater biodiversity both ways along a hierarchy of spatial scales from local to global. Many of FWBON’s activities are connected to essential biodiversity variables (EBV; [621]). FWBON has played a leading role in promoting the implementation of the EBV concept in freshwater biodiversity monitoring across the world [622]. It has also helped in developing workflows to support the generation of EBVs from primary biodiversity data [623] and the definition and development of species population EBVs [624].
In focusing on tracking global change in freshwater biodiversity, FWBON is strongly driven by a freshwater biodiversity conservation agenda, and it collaborates with others who have a similar agenda. A recent example of such collaboration is the formation of the Alliance for Freshwater Life (AFL), which is a global initiative, uniting specialists in research, data synthesis, conservation, education and outreach, and policymaking [14].
13. Discussion
Effective conservation solutions are dependent on an understanding of hydrologic connectivity between different freshwater ecosystems (including connected coastal and marine systems). This was demonstrated recently in a spatial prioritization exercise in south-eastern Australia in which rivers, wetlands and aquifers were considered simultaneously, and this greatly improved the efficiency of regional conservation planning solutions [112]. However, considering hydrological connectivity alone is not sufficient to optimize conservation planning and get the best results from local conservation actions. Different aquatic ecosystems are also connected with one another and with terrestrial ecosystems through mechanisms not explained by hydrological connectivity [625,626]. Not considering these mechanisms might result in a failure to recognize important conservation solutions and opportunities. For example, terrestrial vegetation corridors may be critical for the persistence and dispersal of aquatic species if they have the function of protecting non-aquatic stages of these species and facilitating their dispersal [627]. Similarly, hydrological connectivity alone does not account for the input of organic matter into aquatic ecosystems. Hence, the information on the structure and spatial configuration of terrestrial vegetation could complement information on hydrological connectivity.
This integrated view (Figure 3), to be achieved, might benefit from the theoretical foundations provided by approaches grounded in ecohydrogeology [84], catchment geodiversity (rock type, soil type, and geomorphological richness) as a basis to infer freshwater biodiversity facets [435], and increased recognition that healthy, diverse freshwater ecosystems provide vital natural functions at the landscape scale (e.g., [628]).
Figure 3.
Landscape perspective (schematic drawing) of the hydrologic cycle intended to focus on relationships and interactions among all freshwater ecosystem types dealt with in the present paper. Though generalized as much as possible, the depiction is placed in a specific (montane) setting for the sake of concreteness. Labels: GDEs = groundwater and dependent ecosystems, Sp = springs, SpSt = spring-fed streams, GlSt = glacial streams, St = streams, Ri = rivers, TeLa = tectonic lakes, HMLa = high-mountain lakes, ObLA = oxbow lakes, Re = reservoirs, Mi = mires, SWE = small wetland ecosystems.
Global biodiversity is challenged under the pressure of several human-induced changes of the global environment at an unprecedented rate. The extent and rate of this change is so relevant and so strongly linked to the exploitation of natural resources and to ecosystem processes that biodiversity change has to be considered an important global change in its own right [1]. Previous reviews [1,2,629,630] identified the following as the most important categories of drivers of changes in freshwater biodiversity and ecosystems at the global scale (Table 1): water pollution including eutrophication, flow modification, overexploitation, destruction or habitat degradation, biotic exchanges (invasive non-native species; e.g., [631]), changes in land use, atmospheric CO2 concentration, and nitrogen deposition and acid rain. Stendera et al. (2012) [632] highlighted that hydrological modifications are one of the main stressors of freshwater biodiversity, while Cañedo-Argüelles et al. (2016) [633] pointed out the dire consequences of salinization.
Strayer and Dudgeon (2010) [629] discussed four important challenges for freshwater conservation: (1) Climate change, which will endanger both freshwater species and human uses of fresh water, driving engineering responses that will further threaten the freshwater biota; (2) because freshwater extinctions are already ongoing, freshwater conservationists must be prepared to act now to prevent further losses, and engage more effectively with other stakeholders; (3) the gap between freshwater ecology and conservation biology needs to be bridged; (4) freshwater sciences societies and journals need to improve their historically poor record in publishing important papers and influencing practice in conservation ecology.
Climate-change effects are typically superimposed upon those of multiple stressors, and climate change is thus often regarded as a threat multiplier [634]. We thus think it is of paramount importance to favor studies and initiatives to determine and quantify freshwater-ecosystem resistance, resilience, and recovery to global climate change (e.g., [634]), because this allows assessment of their strategic role (e.g., [635]) and value for biodiversity conservation. One possibility to increase resilience is artificial aquifer recharge (e.g., [636]), but its use to support freshwater biodiversity and ecological integrity is almost unexplored. A particular facet of climate change is also extreme events that are increasing in frequency and intensity, including ever stronger heat-waves and prolonged droughts. Segadelli et al. (2019) [47] in this VSI discuss a first attempt to predict and understand the ecological effects of extreme precipitation events in small montane catchments.
In this paper, we aimed at connecting the different accounts of freshwater ecosystems presented by individual papers in this VSI. These accounts show how varied freshwater habitats are and describe current approaches to studying, monitoring, and assessing these disparate habitats. The paper also reveals major differences among experts working on different ecosystems, suggesting that the varied ways freshwater ecosystems are studied and assessed could be as much a reflection of the traditions followed by the specialists studying them and of the geographical and sociopolitical context in which they operate [9] as of physical and ecological differences among the ecosystems.
In the face of continuously shrinking resources both for freshwater-biodiversity inventories and environmental-quality assessments, the question arises whether freshwater-biodiversity inventories can be funded independently from assessment and monitoring efforts [637]. The decoupling of biodiversity studies from assessments is unfortunately most likely to cause a strong reduction in opportunities to acquire large-scale distribution data for many categories of organisms. This challenge is not unique to freshwaters. No matter how big their budget, research projects with short life-times and specific objectives cannot meet the data needs for large-scale assessments of the status of biodiversity and how this might be changing over years and decades. Hence, it is important to have a solid conceptual framework that helps to utilize data from multiple sources to fill gaps in the space–time–species space [624]. There is also a need for practical solutions, e.g., workflows [623], to convert disparate primary data into biodiversity variables that can be used to measure biodiversity change at spatial and temporal scales relevant to decision-making and policy development. Measuring the different dimensions of biodiversity in freshwaters at multiple spatial scales presents some specific challenges [622] which must be addressed in the near future.
Empirical, experimental, and theoretical research on species loss focused on the attempt to link biodiversity loss to a reduction in the capability of ecosystems to perform energy and matter cycling. Cardinale et al. (2006) [638] showed that the magnitude of these effects is ultimately determined by the identity of the species involved. They noted that a key challenge for future research is to detail more accurately how the traits that determine vulnerability to extinction are related to functional dominance in communities, and underlined that, until that time, a precautionary approach to preserving as much biodiversity as possible is justified. Biodiversity loss aroused not only concern but also interest and controversy among theoretical ecologists. The main cause of controversy was the uncertainty as to how results describing the relationship between species diversity and ecosystem processes, and identifying functionally important species, might scale up to landscape and regional levels, and generalize across ecosystem types [639]. Moreover, many recent experiments on the topic were performed using terrestrial plant-dominated systems (e.g., [640]), while the vast areas of biodiversity that involve small organisms (i.e., bacteria, archea, protists, microarthropods), which drive the bulk of ecosystem processes, are likely to be of particular importance [639]. From a strictly functional point of view, species matter so far as their individual traits and interactions contribute to maintaining the functioning and stability of ecosystems and biogeochemical cycles. However, there are a number of reasons, including cultural, economic, and aesthetic, justifying the need for biodiversity conservation (e.g., [639]). Freshwater biodiversity provides a broad variety of valuable goods and services for human societies, some of which are irreplaceable [641]. The value of this biodiversity has several components: (1) its direct contribution to economic productivity, (2) its “insurance” value in light of unexpected events, (3) its value as a storehouse of genetic information, and (4) its value in supporting the provision of ecosystem services (e.g., cleaning water) [2]. Estimates of the full value of biodiversity need to account for each of these four components. However, the precise impacts of biodiversity change will vary with the ecosystem type and with the processes and properties considered.
At smaller geographic scales, there is substantial species turnover (i.e., ß-diversity [161]) among drainage basins and water bodies, and many freshwater species have restricted ranges (e.g., [642]). These attributes combine with endemism to produce a lack of “substitutability” among freshwater habitat units. This means that protection of one or a few water bodies cannot preserve all freshwater biodiversity within a region, or even a significant proportion of it [2].
While preservation of intact freshwater bodies and their biodiversity remains a priority, it is important to recognize the potential that partly degraded habitats may have to support significant portions of their original biodiversity [2]. In an attempt to move towards such “win–win” solutions, Rosenzweig (2003) [643] advocates an approach to enhancing species richness in human-dominated landscapes termed “reconciliation ecology”. It is to such strategies that freshwater scientists should consider turning, where appropriate, rather than persisting only in attempts to preserve intact ecosystems in the face of increasing human pressure [2]. On the other hand, it is very easy to compromise freshwaters, and often almost impossible to restore [644,645].
Terrestrial conservation strategies tend to emphasize areas of high habitat quality that can be protected. This “fortress conservation” is likely to be unsuccessful for freshwaters [646] and may even be counterproductive [647] for river segments or lakes in unprotected drainage basins unless the boundaries are drawn at a catchment scale, which is virtually never the case [2,648].
The particular vulnerability of freshwater biodiversity also reflects the fact that freshwater is a resource for humans that may be used, diverted, or contaminated in ways that compromise its value as a habitat for organisms [2]. In the vast majority of disagreements over multiple uses of water, whether they are international or on a local scale, the allocation of water to maintain aquatic biodiversity is largely disregarded [649]. Humans have appropriated more than half of renewable freshwater to their own use [650], but in spite of this, nearly 80% of the world population is highly threatened as concerns water security [651]. In exploiting this resource for different uses (e.g., urban, agricultural, industrial), the vital role of water in supporting ecosystems has been widely overlooked (e.g., [652]). To protect the world’s freshwater resources, human and biodiversity perspectives on water security need to be jointly considered [651]. There has indeed been growing recognition that preserving ecological functionality and the biological complexity of freshwater ecosystems is the only way to safeguard many valuable services and benefits to society (e.g., [652]).
Author Contributions
M.C. conceived and designed the paper (including tables & figues). Authors revising and editing the whole paper: M.C., S.P., C.M.P., L.E.S., E.T., J.H., J.S.R., S.S. (Figure 3, technical editing). Section Lead Authors: M.C. (Glacial Streams, High-Mountain Lakes), L.E.S. (Springs), E.T. (Urban), J.H. (Boreal, Arctic), J.S.R. (Headwaters, Streams, Rivers), R.B. (Oxbow Lakes, SWE, lead for technical editing), A.B. (Tropical+ technical editing), N.C. (Med. Streams), M.Č. (Reservoirs), D.M.P.G. (Groundwater), M.H. (Mires), I.H. (Antarctic), Z.L. (Ancient Lakes), L.N.-F. (Mediterranean Pools, Reservoirs), A.A.S. (Arid). Section Co-Authors: M.C. (Springs, Large Lakes, Mires), C.M.P. (Streams, Rivers), M.D.C. (Groundwater, references, formatting), B.F. (Groundwater), P.B.H. (Ancient Lakes), J.K. (Reservoirs), P.Z. (Reservoirs), L.E.S. (Arid). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Tamar Zohary (IOLR & SIL, comments on an early version & photos). Nicola Angeli (MUSE; Figure 1 and Figure 2 layout), Sebastian Birk (Duisburg-Essen, literature suggestions for Large Rivers). M.H. acknowledges project number GX19-28491X. The ‘Groundwater’ section was supported by AQUALIFE LIFE12 BIO/IT/000231. M.Č., J.K. and P.Z. were supported from ERDF/ESF project No. CZ.02.1.01/0.0/0.0/16_025/0007417. L.E.S. thanks S.W. Carothers and M.E. Melray for supporting his work. Diatom part presented as plenary lecture at the North American Diatom Symposium organized by Kalina Manoylov & James Nienow (supporting M.C. for travel costs). We thank our supporting Institutions. Authors are also grateful for the very valuable suggestions provided by anonymous reviewers and the editor that helped to improve the original manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sala, O.E.; Stuart Chapin, F., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Parker, B.R.; Vinebrooke, R.D.; Schindler, D.W. Recent climate extremes alter alpine lake ecosystems. Proc. Natl. Acad. Sci. USA 2008, 105, 12927–12931. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, I.E.; Gleeson, T.; van Beek, L.P.H.; Sutanudjaja, E.H.; Bierkens, M.F.P. Environmental flow limits toglobal groundwater pumping. Nature 2019, 574, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Balian, E.V.; Segers, H.; Lévèque, C.; Martens, K. The Freshwater Animal Diversity Assessment: An overview of the results. Hydrobiologia 2008, 595, 627–637. [Google Scholar] [CrossRef]
- Komárek, J. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353. [Google Scholar] [CrossRef]
- Frassanito, R.; Cantonati, M.; Tardìo, M.; Mancini, I.; Guella, G. On-line identification of secondary metabolites in freshwater microalgae and cyanobacteria by combined liquid chromatography-photodiode array detection-mass spectrometric techniques. J. Chromatogr. A 2005, 1082, 33–42. [Google Scholar] [CrossRef]
- Boon, P.J.; Pringle, C.M. Assessing the Conservation Value of Fresh Waters; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780521848855. [Google Scholar]
- International Union for Conservation of Nature. IUCN Red List of Ecosystems Guidebook: Categories, Criteria and How to Apply Them. Version 1; Ecosystems Red List Thematic Group, Commission on EcosystemManagement (CEM) and Global Ecosystem Management Programme (GEMP); International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2016; ISBN 978-2-8317-1787-6. [Google Scholar]
- International Union for Conservation of Nature. IUCN Red List Categories and Criteria: Version 3.1; Species Survival Commission, World Conservation Union; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2001; ISBN 2-8317-0633-5. [Google Scholar]
- Jenkins, M. Prospects for Biodiversity. Science 2003, 302, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Heilpern, S. Biodiversity: Include freshwater species. Nature 2015, 518, 167. [Google Scholar] [CrossRef]
- Darwall, W.; Bremerich, V.; De Wever, A.; Dell, A.I.; Freyhof, J.; Gessner, M.O.; Grossart, H.P.; Harrison, I.; Irvine, K.; Jänig, S.C.; et al. The Alliance for Freshwater Life: A global call to unite efforts forfreshwater biodiversity science and conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1015–1022. [Google Scholar] [CrossRef]
- Junk, W.J.; An, S.; Finlayson, C.M.; Gopal, B.; Kveˇt, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current state of knowledge regarding the world’s wetlands and their future under global climate change: A synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar] [CrossRef]
- Davidson, N.C.; Finlayson, C.M. Extent, regional distribution and changes in area of different classes of wetland. Mar. Freshw. Res. 2018, 69, 1525–1533. [Google Scholar] [CrossRef]
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 2014, 65, 936–941. [Google Scholar] [CrossRef]
- Keddy, P.A.; Fraser, L.H.; Solomeshch, A.I.; Junk, W.J.; Campbell, D.R.; Arroyo, M.T.K.; Alho, C.J.R. Wet and Wonderful: The World’s Largest Wetlands Are Conservation Priorities. BioScience 2009, 59, 39–51. [Google Scholar] [CrossRef]
- Wright, S.J. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef]
- Fine, P.V.A. Ecological and evolutionary drivers of geographic variation in species diversity. Annu. Rev. Ecol. Syst. 2015, 46, 369–392. [Google Scholar] [CrossRef]
- Heino, J. A macroecological perspective of diversity patterns in the freshwater realm. Freshw. Biol. 2011, 56, 1703–1722. [Google Scholar] [CrossRef]
- Revenga, C.; Kura, Y. Status and Trends of Biodiversity of Inland Water Ecosystems; Technical Series no. 11; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2003; ISBN 92-807-2398-7. [Google Scholar]
- Dudgeon, D. (Ed.) Tropical Stream Ecology; Academic Press: London, UK, 2008; ISBN 978-0-12-088449-0. [Google Scholar]
- Collen, B.; Whitton, F.; Dyer, E.E.; Baillie, J.E.M.; Cumberlidge, N.; Darwall, W.R.T.; Pollock, C.; Richman, N.I.; Soulsby, M.; Böhm, M. Global patterns of freshwater species diversity, threat and endemism. Glob. Ecol. Biogeogr. 2014, 23, 40–51. [Google Scholar] [CrossRef]
- Albert, J.S.; Petry, P.; Reis, R.E. Major Biogeographic and Phylogenetic Patterns. In Historical Biogeography of Neotropical Freshwater Fishes; Albert, J.S., Reis, R.E., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 21–57. [Google Scholar] [CrossRef]
- Junk, W.J.; Soares, M.G.M.; Bayley, P.B. Freshwater fishes of the Amazon River basin: Their biodiversity, fisheries, and habitats. Aquat. Ecosyst. Health Manag. 2007, 10, 153–173. [Google Scholar] [CrossRef]
- Albert, J.S.; Carvalho, T.P. Neogene Assembly of Modern Faunas. In Historical Biogeography of Neotropical Freshwater Fishes; Albert, J.S., Reis, R.E., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 119–136. [Google Scholar] [CrossRef]
- Albert, J.S.; Carvalho, T.P.; Petry, P.; Holder, M.A.; Maxime, E.L.; Espino, J.; Corahua, I.; Quispe, R.; Rengifo, B.; Ortega, H.; et al. Aquatic Biodiversity in the Amazon: Habitat Specialization and Geographic Isolation Promote Species Richness. Animals 2011, 1, 205–241. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.C. Biodiversity of aquatic insects. In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; Wiley-Blackwell: Chichester, UK, 2009; pp. 165–184. ISBN 978-1-118-94557-5. [Google Scholar]
- Crow, G.E. Species diversity in aquatic angiosperms: Latitudinal patterns. Aquat. Bot. 1993, 44, 229–258. [Google Scholar] [CrossRef]
- Chambers, P.A.; Lacoul, P.; Murphy, K.J.; Thomaz, S.M. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 2007, 595, 9–26. [Google Scholar] [CrossRef]
- Luize, B.G.; Magalhães, J.L.L.; Queiroz, H.; Lopes, M.A.; Venticinque, E.M.; Leão de Moraes Novo, E.M.; Silva, T.S.F. The tree species pool of Amazonian wetland forests: Which species can assemble in periodically waterlogged habitats? PLoS ONE 2018, 13, e0198130. [Google Scholar] [CrossRef]
- Hillebrand, H.; Azovsky, A.I. Body size determines the strength of the latitudinal diversity gradient. Ecography 2001, 24, 251–256. [Google Scholar] [CrossRef]
- Passy, S.I. A distinct latitudinal gradient of diatom diversity is linked to resource supply. Ecology 2010, 91, 36–41. [Google Scholar] [CrossRef]
- Soininen, J.; Jamoneau, A.; Rosebery, J.; Passy, S.I. Global patterns and drivers of species and trait composition in diatoms. Glob. Ecol. Biogeogr. 2016, 25, 940–950. [Google Scholar] [CrossRef]
- Jyrkänkallio-Mikkola, J.; Siljander, M.; Heikinheimo, V.; Pellikka, P.; Soininen, J. Tropical stream diatom communities—The importance of headwater streams for regional diversity. Ecol. Indic. 2018, 95, 183–193. [Google Scholar] [CrossRef]
- Vyverman, W.; Verleyen, E.; Sabbe, K.; Vanhoutte, K.; Sterken, M.; Hodgson, D.A.; Mann, D.G.; Juggins, S.; Vijver, B.V.; Jones, V.; et al. Historical processes constrain patterns in global diatom diversity. Ecology 2007, 88, 1924–1931. [Google Scholar] [CrossRef]
- Benito, X.; Fritz, S.C.; Steinitz-Kannan, M.; Tapia, P.M.; Kelly, M.A.; Lowell, T.V. Geo-climatic factors drive diatom community distribution in tropical South American freshwaters. J. Ecol. 2018, 106, 1660–1672. [Google Scholar] [CrossRef]
- Taxböck, L.; Linder, H.P.; Cantonati, M. To what extent are Swiss springs refugial habitats for sensitive and endangered diatom taxa? Water 2017, 9, 967. [Google Scholar] [CrossRef]
- Zelnik, I.; Balanč, T.; Toman, M.J. Diversity and Structure of the Tychoplankton Diatom Community in the Limnocrene Spring Zelenci (Slovenia) in Relation to Environmental Factors. Water 2018, 10, 361. [Google Scholar] [CrossRef]
- Lai, G.G.; Burato, S.; Padedda, B.M.; Zorza, R.; Pizzul, E.; Delgado, C.; Lugliè, A.; Cantonati, M. Diatom biodiversity in karst springs of Mediterranean geographic areas with contrasting characteristics: Islands vs mainland. Water 2019, 11, 2602. [Google Scholar] [CrossRef]
- Rossini, R.; Fensham, R.; Walter, G. Species-specific environmental matching and patch geometry affect occupancy and persistence of threatened snails in Australian desert springs. Water. under review.
- Stevens, L.; Jenness, J.; Ledbetter, J.D. Geography, Ecology, and Human Impacts on Springs and Springs-dependent Species in the Colorado River Basin, Southwestern North America. Water 2020, 12. in press. [Google Scholar]
- Taxböck, L.; Karger, D.N.; Kessler, M.; Spitale, D.; Cantonati, M. Diatom species richness increases with elevation and habitat complexity in Swiss near-natural springs along elevational gradients. Water 2020, 12. in press. [Google Scholar]
- Richardson, J.S. Biological Diversity in Headwater Streams. Water 2019, 11, 366. [Google Scholar] [CrossRef]
- Segadelli, S.; Grazzini, F.; Adorni, M.; De Nardo, M.T.; Fornasiero, A.; Chelli, A.; Cantonati, M. Towards predicting and understanding extreme-precipitation effects on freshwater biodiversity and ecosystems in small catchments: A case study from the northern Apennines (Italy). Water 2019, 12, 79. [Google Scholar] [CrossRef]
- Füreder, L.; Niedrist, G.H. Glacial stream ecology: Structural and functional assets. Water 2020, 12. in press. [Google Scholar]
- Turak, E.; Bush, A.; DelaCruz, J.; Powell, M. Freshwater Reptile Persistence and Conservation in Cities: Insights From Species Occurrence Records. Water 2020, 12. in press. [Google Scholar]
- Marazzi, L.; Gaiser, E.; Eppinga, M.; Zhai, L.; Sah, J.; Castañeda-Moya, E.; Angelini, C. Why Do We Need to Document and Conserve Foundation Species in Freshwater Wetlands? Water 2019, 11, 265. [Google Scholar] [CrossRef]
- Bolpagni, R.; Poikane, S.; Laini, A.; Bagella, S.; Bartoli, M.; Cantonati, M. Ecological and Conservation Value of Small Standing-Water Ecosystems: A Systematic Review of Current Knowledge and Future Challenges. Water 2019, 11, 402. [Google Scholar] [CrossRef]
- Seeteram, N.A.; Hyera, P.T.; Kaaya, L.T.; Lalika, M.C.S.; Anderson, E.P. Conserving rivers and their biodiversity in Tanzania. Water 2019, 11, 2612. [Google Scholar] [CrossRef]
- Taylor, R.G.; Scanlon, B.; Döll, P.; Rodell, M.; Van Beek, R.; Wada, Y.; Longuevergne, L.; Leblanc, M.; Famiglietti, J.S.; Edmunds, M.; et al. Ground water and climate change. Nat. Clim. Chang. 2013, 3, 322–329. [Google Scholar] [CrossRef]
- Kløve, B.; Ala-Aho, P.; Bertrand, G.; Gurdak, J.J.; Kupfersberge, H.; Kværner, J.; Muotka, T.; Mykrä, H.; Preda, E.; Rossi, P.; et al. Climate change impacts on groundwater and dependent ecosystems. J. Hydrol. 2014, 518, 250–266. [Google Scholar] [CrossRef]
- Steube, C.; Richter, S.; Griebler, C. First attempts towards an integrative concept for the ecological assessment of groundwater ecosystems. Hydrogeol. J. 2009, 17, 23–35. [Google Scholar] [CrossRef]
- Miller, M.P.; Buto, S.G.; Susong, D.D.; Rumsey, C.A. The importance of base flow in sustaining surface water flow in the Upper Colorado River Basin. Water Resour. Res. 2016, 52, 3547–3562. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development—OECD. Groundwater Allocation: Managing Growing Pressures on Quantity and Quality, OECD Studies on Water; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- European Environmental Agency (EEA). 2019. Available online: https://www.eea.europa.eu/data-and-maps/data/waterbase-water-quality-2 (accessed on 21 November 2019).
- Kløve, B.; Ala-Aho, P.; Bertrand, G.; Boukalova, Z.; Ertürk, A.; Goldscheider, N.; Ilmonen, J.; Karakaya, N.; Karakaya, N.; Kupfersberger, H.; et al. Groundwater dependent ecosystems. Part I: Hydroecological status and trends. Environ. Sci. Policy 2011, 14, 770–781. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Galassi, D.M.P. Agricultural impacts on Mediterranean alluvial aquifers: Do invertebrates respond? Fund. Appl. Limnol. 2013, 182, 271–281. [Google Scholar] [CrossRef]
- Richter, B.D.; Postel, S.; Revenga, C.; Scudder, T.; Lehner, B.; Churchill, A.; Chow, M. Lost in development’s shadow: The downstream human consequences of dams. Water Alter. 2010, 3, 14–42. [Google Scholar] [CrossRef]
- Malard, F.; Tockner, K.; Dole-Olivier, M.-J.; Ward, J.V. A landscape perspective of surface–subsurface hydrological exchanges in river corridors. Freshw. Biol. 2002, 47, 621–640. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Cifoni, M.; Lombardo, P.; Fiasca, B.; Galassi, D.M.P. Ammonium threshold values for groundwater quality in the EU may not protect groundwater fauna: Evidence from an alluvial aquifer in Italy. Hydrobiologia 2015, 743, 139–150. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Cifoni, M.; Fiasca, B.; Di Cioccio, A.; Galassi, D.M.P. Ecological risk assessment of pesticide mixtures in the alluvial aquifers of central Italy: Toward more realistic scenarios for risk mitigation. Sci. Total Environ. 2018, 644, 161–172. [Google Scholar] [CrossRef]
- Di Marzio, W.D.; Cifoni, M.; Sáenz, M.E.; Galassi, D.M.P.; Di Lorenzo, T. The ecotoxicity of binary mixtures of Imazamox and ionized ammonia on freshwater copepods: Implications for environmental risk assessment in groundwater bodies. Ecotoxicol. Environ. Saf. 2018, 149, 72–79. [Google Scholar] [CrossRef]
- Olisah, C.; Okoh, O.O.; Okoh, A.I. Global evolution of organochlorine pesticides research in biological and environmental matrices from 1992 to 2018: A bibliometric approach. Emerg. Contam. 2019, 5, 157–167. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Di Marzio, W.D.; Sáenz, M.E.; Baratti, M.; Dedonno, A.A.; Iannucci, A.; Cannicci, S.; Messana, G.; Galassi, D.M.P. Sensitivity of hypogean and epigean freshwater copepods to agricultural pollutants. Environ. Sci. Pollut. Res. 2014, 21, 4643–4655. [Google Scholar] [CrossRef]
- Eamus, D.; Froend, R. Groundwater-dependent ecosystems: The where, what and why of GDEs. Aust. J. Bot. 2006, 54, 91–96. [Google Scholar] [CrossRef]
- Environmental Protection Authority—EPA. Guidance for the Assessment of Environmental Factors (In Accordance with the Environmental Protection Act 1986): Consideration of Subterranean Fauna in Groundwater and Caves During Environmental Impact Assessment in Western Australia; Environmental Protection Authority: Perth, Australia, 2004; p. 98.
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef]
- Postigo, C.; Barceló, D. Synthetic organic compounds and their transformation products in groundwater: Occurrence, fate and mitigation. Sci. Total Environ. 2015, 504, 32–47. [Google Scholar] [CrossRef]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, T.; Di Cicco, M.; Di Censo, D.; Galante, A.; Boscaro, F.; Messana, G.; Galassi, D.M.P. Environmental risk assessment of propranolol in the groundwater bodies of Europe. Environ. Pollut. 2019, 255. [Google Scholar] [CrossRef] [PubMed]
- Meffe, R.; Bustamante, I. Emerging organic contaminants in surface water and groundwater: A first overview of the situation in Italy. Sci. Total Environ. 2014, 481, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Jurado, A.; Walther, M.; Diaz-Cruz, S.M. Occurrence, fate and environmental risk assessment of the organic microcontaminants included in the Watch Lists set by EU Decisions 2015/495 and 2018/840 in the groundwater of Spain. Sci. Total Environ. 2019, 663, 258–296. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, W.D.; Castaldo, D.; Di Lorenzo, T.; Di Cioccio, A.; Sáenz, M.E.; Galassi, D.M.P. Developmental endpoints of chronic exposure to suspected endocrine-disrupting chemicals on benthic and hyporheic freshwater copepods. Ecotoxicol. Environ. Saf. 2013, 96, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Griebler, C.; Avramov, M. Groundwater ecosystem services—A review. Freshw. Sci. 2014, 34, 355–367. [Google Scholar] [CrossRef]
- Galassi, D.M.P.; Lombardo, P.; Fiasca, B.; Di Cioccio, A.; Di Lorenzo, T.; Petitta, M.; Di Carlo, P. Earthquakes trigger the loss of groundwater biodiversity. Sci. Rep. 2014, 4, 6273. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Galassi, D.M.P. Effect of temperature rising on the stygobiotic crustacean species Diacyclops belgicus: Does global warming affect groundwater populations? Water 2017, 9, 951. [Google Scholar] [CrossRef]
- Issartel, J.; Hervant, F.; Voituron, Y.; Renault, D.; Vernon, P. Behavioural, ventilatory and respiratory responses of epigean and hypogean crustaceans to different temperatures. Comp. Biochem. Physiol. 2005, 141, 1–7. [Google Scholar] [CrossRef]
- Colson-Proch, C.; Morales, A.; Hervant, F.; Konecny, L.; Moulin, C.; Douady, C.J. First cellular approach of the effects of global warming on groundwater organisms: A study of the HSP70 gene expression. Cell Stress Chaperon. 2010, 15, 259–270. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Di Marzio, W.D.; Spigoli, D.; Baratti, M.; Messana, G.; Cannicci, S.; Galassi, D.M.P. Metabolic rates of a hypogean and an epigean species of copepod in an alluvial aquifer. Freshw. Biol. 2015, 71, 426–435. [Google Scholar] [CrossRef]
- Kefford, B.J.; Palmer, C.G.; Pakhomova, L.; Nugegoda, D. Comparing test systems to measure the salinity tolerance of freshwater invertebrates. Water Sa 2004, 30, 499–507. [Google Scholar] [CrossRef][Green Version]
- Cantonati, M.; Stevens, L.E.; Segadelli, S.; Springer, A.E.; Goldscheider, N.; Celico, F.; Filippini, M.; Ogata, K.; Gargini, A. Ecohydrogeology: The interdisciplinary convergence needed to improve the study of springs and other groundwater-dependent habitats, biota, and ecosystems. Ecol. Indic. 2020, 110, 105803. [Google Scholar] [CrossRef]
- Tomlinson, M.; Boulton, A.J. Ecology and management of subsurface groundwater dependent ecosystems in Australia—A review. Mar. Freshw. Res. 2010, 61, 936–949. [Google Scholar] [CrossRef]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef]
- Stubbington, R.; Wood, P.J.; Boulton, A.J. Low flow controls on benthic and hyporheic macroinvertebrate assemblages during supra-seasonal drought. Hydrol. Proc. 2009, 23, 2253–2263. [Google Scholar] [CrossRef]
- Griebler, C.; Lueders, T. Microbial biodiversity in groundwater ecosystems. Freshw. Biol. 2009, 54, 649–677. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Culver, D.C.; Deharveng, L.; Ferreira, R.L.; Fišer, C.; Galassi, D.M.P.; Griebler, C.; Halse, S.; Humphreys, W.F.; et al. Scientists’ Warning on the Conservation of Subterranean Ecosystems. BioScience 2019, 69, 641–650. [Google Scholar] [CrossRef]
- Humphreys, W.F. Rising from Down Under: Developments in subterranean biodiversity in Australia from a groundwater fauna perspective. Invertebr. Syst. 2008, 22, 85–101. [Google Scholar] [CrossRef]
- Gibert, J.; Culver, D.C. Assessing and conserving groundwater biodiversity: An introduction. Freshw. Biol. 2009, 54, 639–648. [Google Scholar] [CrossRef]
- Gibert, J.; Deharveng, L. Subterranean Ecosystems: A Truncated Functional Biodiversity. BioScience 2002, 52, 473–481. [Google Scholar] [CrossRef]
- Galassi, D.M.P.; Huys, R.; Reid, J.W. Diversity, ecology and evolution of groundwater copepods. Freshw. Biol. 2009, 54, 691–708. [Google Scholar] [CrossRef]
- Fattorini, S.; Fiasca, B.; Di Lorenzo, T.; Di Cicco, M.; Galassi, D.M.P. A new protocol for assessing the conservation priority of groundwater dependent ecosystems. Aquat. Conserv. Mar. Freshw. Ecosyst. under review.
- Galassi, D.M.P.; Fiasca, B.; Di Lorenzo, T.; Montanari, A.; Porfirio, S.; Fattorini, S. Groundwater biodiversity in a chemoautotrophic cave ecosystem: How geochemistry regulates microcrustacean community structure. Aquat. Ecol. 2017, 51, 75–90. [Google Scholar] [CrossRef]
- Malard, F.; Boutin, C.; Camacho, A.I.; Ferreira, D.; Michel, G.; Sket, B.; Stoch, F. Diversity patterns of stygobiotic crustaceans across multiple spatial scales in Europe. Freshw. Biol. 2009, 54, 756–776. [Google Scholar] [CrossRef]
- Stoch, F.; Galassi, D.M.P. Stygobiotic crustacean species richness: A question of numbers, a matter of scale. Hydrobiologia 2010, 653, 217–234. [Google Scholar] [CrossRef]
- Gibert, J.; Culver, D. Diversity patterns in Europe. In Encyclopedia of Caves; Culver, D., White, W.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 196–201. ISBN 9780128141243. [Google Scholar]
- Boulton, A.J.; Fenwick, G.D.; Hancock, P.J.; Harvey, M.S. Biodiversity, functional roles and ecosystem services of groundwater invertebrates. Invertebr. Syst. 2008, 22, 103–116. [Google Scholar] [CrossRef]
- Batubara, B.; Batelaan, O.; Quevauviller, P. Science-policy interfacing on the issue of groundwater and groundwater-dependent ecosystems in Europe: Implications for research and policy. WIREs Water 2014, 1, 561–571. [Google Scholar] [CrossRef]
- Tomlinson, M.; Boulton, A.J.; Hancock, P.J.; Cook, P.G. Deliberate omission or unfortunate oversight: Should stygofaunal surveys be included in routine groundwater monitoring programs? Hydrogeol. J. 2007, 15, 1317–1320. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.V. The seven impediments in invertebrate conservation and how to overcome them. Biol. Cons. 2011, 144, 2647–2655. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Canedoli, C.; Stoch, F. The Racovitzan impediment and the hidden biodiversity of unexplored environments. Conserv. Biol. 2019, 33, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Deharveng, L.; Stoch, F.; Gibert, J.; Bedos, A.; Galassi, D.M.P.; Zagmajster, M.; Brancelj, A.; Camacho, A.; Fiers, F.; Martin, P.; et al. Groundwater biodiversity in Europe. Freshw. Biol. 2009, 54, 709–726. [Google Scholar] [CrossRef]
- BioFreshProject. Available online: http://project.freshwaterbiodiversity.eu/ (accessed on 21 November 2019).
- Zagmajster, M.; Eme, D.; Fišer, C.; Galassi, D.M.P.; Marmonier, P.; Stoch, F.; Cornu, J.-F.; Malard, F. Geographic variation in range size and beta diversity of groundwater crustaceans: Insights from habitats with low thermal seasonality. Glob. Ecol. Biogeogr. 2014, 23, 1135–1145. [Google Scholar] [CrossRef]
- Galassi, D.M.P.; Dole-Olivier, M.-J.; De Laurentiis, P. Phylogeny and biogeography of the genus Pseudectinosoma, and description of P. janineae sp. n. (Crustacea: Copepoda, Ectinosomatidae). Zool. Scr. 1999, 28, 289–303. [Google Scholar] [CrossRef]
- Council of the European Communities. Council Directive92⁄43⁄EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Communities 1992, 35, 7–50. [Google Scholar]
- Michel, G.; Malard, F.; Deharveng, L.; Di Lorenzo, T.; Sket, B.; De Boyer, C. Reserve selection for conserving groundwater biodiversity. Freshw. Biol. 2009, 54, 861–876. [Google Scholar] [CrossRef]
- Strona, G.; Fattorini, S.; Fiasca, B.; Di Lorenzo, T.; Di Cicco, M.; Lorenzetti, W.; Boccacci, F.; Galassi, D.M.P. AQUALIFE Software: A New Tool for a Standardized Ecological Assessment of Groundwater Dependent Ecosystems. Water 2019, 11, 2574. [Google Scholar] [CrossRef]
- Margules, C.R.; Pressey, R.L. Systematic conservation planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef]
- Linke, S.; Turak, E.; Asmyhr, M.G.; Hose, G. 3D conservation planning: Including aquifer protection in freshwater plans refines priorities without much additional effort. Aquat. Conserv. Mar. Freshw. Ecosys. 2019, 29, 1063–1072. [Google Scholar] [CrossRef]
- Cantonati, M.; Komárek, J.; Montejano, G. Cyanobacteria in ambient springs. Biodivers. Conserv. 2015, 24, 865–888. [Google Scholar] [CrossRef]
- Johnson, R.H.; DeWit, E.; Wirt, L.; Manning, A.H. Using geochemistry to identirfy the source of groundwater to Montezuma Well, a natural springs in central Arizona, USA: Part 2. Envir. Earth Sci. 2012, 67, 1837–1853. [Google Scholar] [CrossRef]
- Glazier, D.S. Springs. In Reference Module in Earth Systems and Environmental Sciences; Elias, S., Ed.; Elsevier: Waltham, MA, USA, 2014; pp. 1–78. ISBN 978-0-12-409548-9. [Google Scholar]
- Stevens, L.E.; Johnson, R.R.; Estes, C. The watershed continuum: A conceptual stream riparian ecosystem model. In Riparian Research and Management: Past, Present, and Future; General Technical Report RMRS-GTR-303; Johnson, R.R., Carothers, S.W., Finch, D.M., Kingsley, K.J., Stanley, J.R.T., Eds.; U.S. Department of Agriculture; Forest Service: Washington, DC, USA; Rocky Mountain Research Station: Fort Collins, CO, USA, 2018; Volume 2, in press. [Google Scholar]
- Springer, A.E.; Stevens, L.E.; Anderson, E.E.; Parnell, R.A.; Kreamer, D.K.; Levin, L.; Flora, S.P. A comprehensive springs classification system: Integrating geomorphic, hydrogeochemical, and ecological criteria. In Aridland Springs in North America: Ecology and Conservation; Stevens, L.E., Meretsky, V.J., Eds.; University of Arizona Press: Tucson, AZ, USA, 2008; pp. 49–75. ISBN 978-0-8165-2645-1. [Google Scholar]
- Spitale, D.; Leira, M.; Angeli, N.; Cantonati, M. Environmental classification of springs of the Italian Alps and its consistency across multiple taxonomic groups. Freshw. Sci. 2012, 31, 563–574. [Google Scholar] [CrossRef]
- Springer, A.E.; Stevens, L.E. Spheres of discharge of springs. Hydrogeol. J. 2009, 17, 83–93. [Google Scholar] [CrossRef]
- Stevens, L.E.; Schenk, E.R.; Springer, A.E. Springs ecosystem classification. Ecol. Appl. in press.
- Stevens, L.E.; Ledbetter, J.D.; Springer, A.E.; Campbell, C.; Misztal, L.; Joyce, M.; Hardwick, G. Arizona Springs Rehabilitation Handbook; Spring Stewardship Institute: Flagstaff, AZ, USA; Sky Island Alliance: Tucson, AZ, USA, 2016. [Google Scholar]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Springer, A.E.; Stevens, L.E.; Ledbetter, J.D.; Schaller, E.M.; Gill, K.; Rood, S.B. Ecohydrology and stewardship of Alberta springs ecosystems. Ecohydrology 2015, 8. [Google Scholar] [CrossRef]
- Sinclair, D.A. Springs Geomorphology Influences on Physical and Vegetation Ecosystem Characteristics, Grand Canyon Ecoregion, USA. Master’s Thesis, Northern Arizona University, Flagstaff, AZ, USA, 2018. [Google Scholar]
- Cantonati, M.; Lange-Bertalot, H. Diatom biodiversity of springs in the Berchtesgaden National Park (northern Alps, Germany), with the ecological and morphological characterization of two species new to science. Diatom Res. 2010, 25, 251–280. [Google Scholar] [CrossRef]
- Cantonati, M.; Lange-Bertalot, H.; Scalfi, A.; Angeli, N. Cymbella tridentina sp. nov. (Bacillariophyta), a crenophilous diatom from carbonate springs of the Alps. J. N. Am. Benthol. Soc. 2010, 29, 775–788. [Google Scholar] [CrossRef]
- Cantonati, M.; Füreder, L.; Gerecke, R.; Jüttner, I.; Cox, E.J. Crenic habitats, hotspots for freshwater biodiversity conservation: Toward an understanding of their ecology. Freshw. Sci. 2012, 31, 463–480. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Huston, M. A general hypothesis of species diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Huston, M.A. Biology Diversity: The Coexistence of Species on Changing Landscapes; Cambridge University Press: Cambridge, UK, 1994; ISBN 0-521-36930-4. [Google Scholar]
- Kodric-Brown, A.; Brown, L.H. Native fishes, exotic mammals, and the conservation of desert springs. Front. Ecol. Environ. 2007, 10, 549–553. [Google Scholar] [CrossRef]
- Odum, H.T. Trophic Structure and Productivity of Silver Springs, Florida. Ecol. Monogr. 1957, 27, 55–112. [Google Scholar] [CrossRef]
- Blinn, D.W. The extreme environment, trophic structure, and ecosystem dynamics of a large fishless desert spring: Montezuma Well, Arizona. In Aridland Springs in North America: Ecology and Conservation; Stevens, L.E., Meretsky, V.J., Eds.; University of Arizona Press: Tucson, AZ, USA, 2008; ISBN 978-0-8165-2645-1. [Google Scholar]
- Williams, D.D.; Danks, H.V. Arthropods of springs, with particular reference to Canada. Mem. Entomol. Soc. Can. 1991, 203–217. [Google Scholar] [CrossRef]
- Erman, N.A. Factors determining biodiversity in Sierra Nevada cold spring systems. In The History of Water: Eastern Sierra Nevada, Owens Valley, White-Inyo Mountains; Hall, C.A., Jr., Doyle-Jones, V., Widawski, B., Eds.; University of California White Mountains Research Station: Los Angeles, CA, USA, 1992; pp. 119–127. ISBN 978-1879851047. [Google Scholar]
- Botosaneanu, L. Studies in Crenobiology: The Biology of Springs and Springbrooks; Backhuys: Leiden, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Ponder, W.F. Endemic aquatic macroinvertebrates of artesian springs of the Great Artesian Basin—Progress and future directions. Rec. South Aust. Mus. Monogr. Ser. 2004, 7, 101–110. [Google Scholar]
- Fensham, R.J.; Silcock, J.L.; Kerezsy, A.; Ponder, W. Four desert waters: Setting arid zone wetland conservation priorities. Biol. Conserv. 2011, 144, 2459–2467. [Google Scholar] [CrossRef]
- Unmack, P.J. Australian Desert Fishes Descriptions. 2003. Available online: Desertfishes.org/Australia/index.html (accessed on 5 October 2019).
- Unmack, P.J.; Minckley, W.L. The demise of desert springs. In Aridland Springs in North America: Ecology and Conservation; Stevens, L.E., Meretsky, V.J., Eds.; University of Arizona Press: Tucson, AZ, USA, 2008; pp. 11–34. ISBN 978-0-8165-2645-1. [Google Scholar]
- Martens, A.; Richter, O.; Suhling, F. The relevance of perennial springs for regional biodiversity and conservation. In Biodiversity in Southern Africa 2: Patterns and Processes at Regional Scale; Schmiedel, U., Jürgens, N., Eds.; Klaus Hess Publishers: Göttingen, Germany; Windhoek, Namibia, 2010; pp. 70–74. ISBN 978-3-933117-46-5. [Google Scholar]
- Cuthbert, M.O.; Ashley, G.M. A spring forward for hominin evolution in East Africa. PLoS ONE 2014, 9, e107358. [Google Scholar] [CrossRef]
- Cantonati, M.; Rott, E.; Spitale, D.; Angeli, N.; Komárek, J. Are benthic algae related to spring types? Freshw. Sci. 2012, 31, 481–498. [Google Scholar] [CrossRef]
- Cantonati, M.; Lange-Bertalot, H. Achnanthidium dolomiticum sp. nov. (Bacillariophyta) from oligotrophic mountain springs and lakes fed by dolomite aquifers. J. Phycol. 2006, 42, 1184–1188. [Google Scholar] [CrossRef]
- Cantonati, M.; Lange-Bertalot, H.; Angeli, N. Neidiomorpha gen. nov. (Bacillariophyta): A new freshwater diatom genus separated from Neidium Pfitzer. Bot. Stud. 2010, 51, 195–202. [Google Scholar]
- Rott, E.; Hotzy, R.; Cantonati, M.; Sanders, D. Calcification types of Oocardium stratum Nägeli and microhabitat conditions in springs of the Alps. Freshw. Sci. 2012, 31, 610–624. [Google Scholar] [CrossRef]
- Cantonati, M.; Segadelli, S.; Ogata, K.; Tran, H.; Sanders, D.; Gerecke, R.; Rott, E.; Filippini, M.; Gargini, A.; Celico, F. A global review on ambient Limestone-Precipitating Springs (LPS): Hydrogeological setting, ecology, and conservation. Sci. Total Environ. 2016, 568, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Angeli, N.; Cantonati, M.; Spitale, D.; Lange-Bertalot, H. A comparison between diatom assemblages in two groups of carbonate, low-altitude springs with different levels of anthropogenic disturbances. Fottea 2010, 10, 115–128. [Google Scholar] [CrossRef]
- Cantonati, M.; Ortler, K. Using spring biota of pristine mountain areas for long term monitoring. IAHS Publ. 1998, 248, 379–385. [Google Scholar]
- Ashley, G.M.; de Wet, C.B.; Barboni, D.; Magill, C.R. Subtle signatures of seeps: Record of groundwater in a dryland, DK, Olduvai Gorge, Tanzania. Depos. Rec. 2016, 2, 4–21. [Google Scholar] [CrossRef]
- Haynes, C.V., Jr. Quaternary cauldron springs as paleoecological archives. In Aridland Springs in North America: Ecology and Conservation; Stevens, L.E., Ed.; University of Arizona Press: Tucson, AZ, USA, 2008. [Google Scholar]
- Kreamer, D.K.; Stevens, L.E.; Ledbetter, J.D. Groundwater dependent ecosystems—Science, challenges, and policy directions. In Groundwater; Adelana, S.M., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015; ISBN 978-1-63321-759-1. [Google Scholar]
- Boschen, R.E.; Rowden, A.A.; Clark, M.R.; Gardner, J.P.A. Mining of deep-sea seafloor massive sulfides: A review of the deposits, their benthic communities, impacts from mining, regulatory frameworks and management strategies. Ocean Coast. Manag. 2013, 84, 54–67. [Google Scholar] [CrossRef]
- De Granade, R.; Stevens, L.E. Desert Oases: Aridland Springs as Diverse Biocultural Ecosystems. In Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020. (in press)
- Gomi, T.; Sidle, R.; Richardson, J.S. Headwater and channel network -understanding processes and downstream linkages of headwater systems. BioScience 2002, 52, 905–916. [Google Scholar] [CrossRef]
- Strahler, A.N. Quantitative Geomorphology of Drainage Basins and Channel Networks. In Handbook of Applied Hydrology; Chow, V.T., Ed.; McGraw Hill: New York, NY, USA, 1954; ISBN 978-0071835091. [Google Scholar]
- Pitcher, L.H.; Smith, L.C. Supraglacial streams and rivers. Annu. Rev. Earth Planet. Sci. 2019, 47, 421–452. [Google Scholar] [CrossRef]
- Hodson, A.; Anesio, A.M.; Tranter, M.; Fountain, A.; Osborn, M.; Priscu, J.; Laybourn-Parry, J.; Sattler, B. Glacial ecosystems. Ecol. Monogr. 2008, 78, 41–67. [Google Scholar] [CrossRef]
- Ward, J.V. Ecology of alpine streams. Freshw. Biol. 1994, 32, 277–294. [Google Scholar] [CrossRef]
- Brittain, J.E.; Milner, A.M. Ecology of glacier-fed rivers: Current status and concepts. Freshw. Biol. 2001, 46, 1571–1578. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Jacobsen, D.; Milner, A.M.; Brown, L.E.; Dangles, O. Biodiversity under threat in glacier-fed river systems. Nat. Clim. Chang. 2012, 2, 361–364. [Google Scholar] [CrossRef]
- Cantonati, M.; Corradini, G.; Jüttner, I.; Cox, E.J. Diatom assemblages in high mountain streams of the Alps and the Himalaya. Nova Hedwig. 2001, 123, 37–62. [Google Scholar]
- Gesierich, D.; Rott, E. Is diatom richness responding to catchment glaciation? J. Limnol. 2012, 71, 72–83. [Google Scholar] [CrossRef]
- Brown, L.E.; Khamis, K.; Wilkes, M.; Blaen, P.; Brittain, J.E.; Carrivick, J.L.; Fell, S.; Friberg, N.; Füreder, L.; Gislason, G.M.; et al. Functional diversity and community assembly of river invertebrates show globally consistent responses to decreasing glacier cover. Nat. Ecol. Evol. 2018, 2, 325–333. [Google Scholar] [CrossRef]
- Niedrist, G.H.; Cantonati, M.; Füreder, L. Environmental harshness mediates the quality of periphyton and chironomid body mass in alpine streams. Freshw. Sci. 2018, 37, 519–533. [Google Scholar] [CrossRef]
- Vannote, R.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Frissell, C.A.; Liss, W.J.; Warren, C.E.; Hurley, M.D. A hierarchical framework for stream habitat classification: Viewing streams in a watershed context. Environ. Manag. 1986, 10, 199–214. [Google Scholar] [CrossRef]
- Hayes, J.W.; Goodwin, E.O.; Shearer, K.A.; Hicks, D.M. Relationship between background invertebrate drift concentration and flow over natural flow recession and prediction with a drift transport model. Can. J. Fish. Aquat. Sci. 2019, 76, 871–885. [Google Scholar] [CrossRef]
- Nilsson, C.; Brown, R.L.; Jansson, R.; Merritt, D.M. The role of hydrochory in structuring riparian and wetland vegetation. Biol. Rev. 2010, 85, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.R.; Newbold, J.D.; Lin, L. Nutrient Spiraling and Transport in Streams: The Importance of In-Stream Biological Processes to Nutrient Dynamics in Streams. In Stream Ecosystems in a Changing Environment; Jones, J.B.E., Stanley, E., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2016; pp. 181–199. ISBN 9780124058903. [Google Scholar]
- Wallace, J.B.; Merritt, R.W. Filter-feeding ecology of aquatic insects. Annu. Rev. Entomol. 1980, 25, 103–132. [Google Scholar] [CrossRef]
- Naman, S.M.; Rosenfeld, J.S.; Richardson, J.S. Causes and consequences of invertebrate drift in running waters: From individuals to populations and trophic fluxes. Can. J. Fish. Aquat. Sci 2016, 73, 1292–1305. [Google Scholar] [CrossRef]
- Ward, J.V.; Stanford, J.A. The serial discontinuity concept—Extending the model to floodplain rivers. Regul. Rivers Res. Manag. 1995, 10, 159–168. [Google Scholar] [CrossRef]
- Richardson, J.S.; Mackay, R.J. Lake outlets and the distribution of filter feeders: An assessment of hypotheses. Oikos 1991, 62, 370–380. [Google Scholar] [CrossRef]
- Willis, T.V.; Magnuson, J.J. Patterns in fish species composition across the interface between streams and lakes. Can. J. Fish. Aquat. Sci. 2000, 57, 1042–1052. [Google Scholar] [CrossRef]
- Wotton, R.S.; Malmqvist, B.; Leonardsson, K. Expanding traditional views on suspension feeders—Quantifying their role as ecosystem engineers. Oikos 2003, 101, 441–443. [Google Scholar] [CrossRef]
- Dunn, R.R.; Colwell, R.K.; Nilsson, C. The river domain: Why are there more species halfway up the river? Ecography 2006, 29, 251–259. [Google Scholar] [CrossRef]
- Heino, J.; Paavola, R.; Virtanen, R.; Muotka, T. Searching for biodiversity indicators in running waters: Do bryophytes, macroinvertebrates, and fish show congruent diversity patterns? Biodivers. Conserv. 2005, 14, 415–428. [Google Scholar] [CrossRef]
- Vander Vorste, R.; McElmurray, P.; Bell, S.; Eliason, K.M.; Brown, B.L. Does stream size really explain biodiversity patterns in lotic systems? A call for mechanistic explanations. Diversity 2017, 9, 26. [Google Scholar] [CrossRef]
- Berg, R.; Gaumert, T.; Kämmereit, M.; Klinger, H.; Lemcke, R.; Leuner, E.; Wolter, C.; Dußling, U. The German river typology with respect to fish-faunistic reference conditions. In Handbuch Angewandte Limnologie; Steinberg, C., Calmano, W., Klapper, H., Wilken, R.D., Eds.; Ecomed-Verlag: Landsberg, Germany, 2004; pp. 12–17. ISBN 3-609-75820-1. [Google Scholar]
- Hynes, H.B.N. Keynote Address. In Proceedings of the International Large River Symposium, Honey Harbour, ON, Canada, 14–21 September 1986; Dodge, D.P., Ed.; pp. 5–10. [Google Scholar]
- Church, M. Channel stability: Morphodynamics and the morphology of rivers. In Rivers—Physical, Fluvial and Environmental Processes; Rowiński, P., Radecki-Pawlik, A., Eds.; Springer International: Cham, Switzerland, 2015; pp. 281–321. ISBN 978-3-319-17719-9. [Google Scholar]
- Chetelat, J.; Pick, F.R.; Hamilton, P.B. Potamoplankton size structure and taxonomic composition: Influence of river size and nutrient concentrations. Limnol. Oceanogr. 2006, 51, 681–689. [Google Scholar] [CrossRef]
- Pracheil, B.M.; McIntyre, P.B.; Lyons, J.D. Enhancing conservation of large-river biodiversity by accounting for tributaries. Front. Ecol. Environ. 2013, 11, 124–128. [Google Scholar] [CrossRef]
- He, F.; Zarfl, C.; Bremerich, V.; David, J.N.W.; Hogan, Z.; Kalinkat, G.; Tockner, K.; Jähnig, S.C. The global decline of freshwater megafauna. Glob. Chang. Biol. 2019, 25, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Schletterer, M.; Kuzovlev, V.V.; Zhenikov, Y.N.; Tuhtan, J.A.; Haidvogl, G.; Friedrich, T.; Górski, K.; Füreder, L. Fish fauna and fisheries of large European rivers: Examples from the Volga and the Danube. Hydrobiologia 2018, 814, 45–60. [Google Scholar] [CrossRef]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef]
- Pringle, C.M.; Freeman, M.; Freeman, B. Regional effects of hydrologic alterations on riverine macrobiota in the New World: Tropical-temperate comparisons. BioScience 2000, 50, 807–823. [Google Scholar] [CrossRef]
- Pringle, C.M. Interacting effects of altered hydrology and contaminant: Emerging ecological patterns of global concern. In Achieving Sustainable Freshwater Systems: A Web of Connections; Holland, M., Blood, E., Shaffer, L., Eds.; Island Press: Washington, DC, USA, 2003; pp. 85–107. [Google Scholar]
- Olden, J.D. Challenges and opportunities for fish conservation in dam-impacted waters. In Conservation of Freshwater Fishes; Closs, G., Krkosek, M., Olden, J., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 107–148. [Google Scholar] [CrossRef]
- Pringle, C.M.; Withrington, D. Freshwater conservation in action: Contrasting approaches in the US and UK. Chapter 2. In Assessing the Conservation Value of Fresh Waters; Boon, P.J., Pringle, C.M., Eds.; Cambridge University Press: Cambridge, UK, 2009; ISBN 978-0521613224. [Google Scholar]
- Herendorf, C.E. Distribution of the world’s large lakes. In Large Lakes: Ecological Structure and Function; Tilzer, M.M., Serruya, C., Eds.; Springer: Cham, Switzerland, 1990; pp. 3–38. ISBN 978-3-642-84077-7. [Google Scholar]
- Vadeboncoeur, Y.; McIntyre, P.B.; Vander Zanden, M.J. Borders of biodiversity: Life at the edge of the world’s large lakes. BioScience 2011, 61, 526–537. [Google Scholar] [CrossRef]
- Gratton, P.; Allegrucci, G.; Sbordoni, V.; Gandolfi, A. The evolutionary jigsaw puzzle of the surviving trout (Salmo trutta L. complex) diversity in the Italian region: A multilocus Bayesian approach. Evol. Mol. Phylogenet. Evol. 2014, 79, 292–304. [Google Scholar] [CrossRef]
- Kociolek, J.P.; Stoermer, E.F. Oligotrophy: The forgotten end of an ecological spectrum. Acta Bot. Croat. 2009, 68, 465–472. [Google Scholar]
- Stoermer, E.F. Diatoms associated with bryophyte communities growing at extreme depths in Lake Michigan. Proc. Iowa Acad. Sci. 1981, 88, 91–95. [Google Scholar]
- Hampton, S.E.; McGowan, S.; Ozersky, T.; Virdis, S.G.P.; Vu, T.T.; Spanbauer, T.L.; Kraemer, B.M.; Swann, G.; Mackay, A.W.; Powers, S.M.; et al. Recent ecological change in ancient lakes. Limnol. Oceanogr. 2018, 63, 2277–2304. [Google Scholar] [CrossRef]
- Martens, K. Speciation in ancient lakes. Trends Ecol. Evol. 1997, 12, 177–182. [Google Scholar] [CrossRef]
- Nekola, J.C. Paleorefugia and neorefugia: The influence of colonization history on community pattern and process. Ecology 1999, 80, 2459–2473. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Jaume, D. Making Waves: The Repeated Colonization Fresh Water by Copepod Crustaceans. Adv. Ecol. Res. 2000, 31, 61–79. [Google Scholar] [CrossRef]
- Cristescu, M.E.; Adamowicz, S.J.; Vaillant, J.J.; Haffner, G.D. Ancient lakes revisited: From the ecology to the genetics of speciation. Mol. Ecol. 2010, 19, 4837–4851. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.L. Speciation in ancient lakes. Q. Rev. Biol. 1950, 25, 30–176. [Google Scholar] [CrossRef]
- Martens, K.; Coulter, G.; Goddeeris, B. Speciation in ancient lakes—40 years after Brooks. Ergebn. Limnol. 1994, 44, 75–96. [Google Scholar]
- Mackay, W.; Edlund, M.B.; Khursevich, G. Diatoms in ancient lakes. In The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Smol, J.P., Stoermer, E.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 209–228. ISBN 0521509963. [Google Scholar]
- Cohen, A.S.; Stone, J.R.; Beuning, K.R.; Park, L.E.; Reinthal, P.N.; Dettman, D.; Scholz, C.A.; Johnson, T.C.; King, J.W.; Talbot, M.R.; et al. Ecological consequences of early Late Pleistocene megadroughts in tropical Africa. Proc. Natl. Acad. Sci. USA 2007, 104, 16422–16427. [Google Scholar] [CrossRef]
- Johnson, T.C.; Scholz, C.A.; Talbot, M.R.; Kelts, K.; Ricketts, R.D.; Ngobi, G.; Beuning, K.; Ssemmanda, I.I.; McGill, J.W. Late Pleistocene desiccation of Lake Victoria and rapid evolution of cichlid fishes. Science 1996, 273, 1091–1093. [Google Scholar] [CrossRef]
- Fritz, S.C.; Baker, P.A.; Tapia, P.; Spanbauer, T.; Westover, K. Evolution of the Lake Titicaca basin and its diatom flora over the last ~370,000 years. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 317–318, 93–103. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Lavoie, I.; Poulin, M. Spatial, seasonal and inter-annual variability in environmental characteristics and phytoplankton standing stock of the temperate lowland Rideau river, Ontario, Canada. River Res. Appl. 2012, 28, 1551–1566. [Google Scholar] [CrossRef]
- Goulden, C.E.; Tumurtogoo, O.; Karabanov, E.; Mongontsetseg, A. The geological history and geography of Lake Hövsgöl. In The Geology, Biodiversity and Ecology of Lake Hövsgöl (Mongolia); Goulden, C.E., Sitnikova, T., Gelhaus, J., Boldgiv, B., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2006; pp. 1–19. ISBN 90-5782-162-1. [Google Scholar]
- Sabo, E.; Roy, D.; Hamilton, P.B.; Hehanussa, P.E.; McNeely, R.; Haffner, G.D. The plankton community of Lake Matano: Factors regulating plankton composition and relative abundance in an ancient, tropical lake of Indonesia. Hydrobiologia 2008, 615, 225–235. [Google Scholar] [CrossRef]
- Crowe, S.A.; Jones, C.A.; Katsev, S.; Magen, C.; O’Neill, A.H.; Sturm, A.; Canfield, D.E.; Haffner, G.D.; Mucci, A.; Sundby, B.; et al. Photoferrotrophs thrive in an Archean Ocean analogue. Proc. Natl. Acad. Sci. USA 2008, 105, 15938–15943. [Google Scholar] [CrossRef] [PubMed]
- Granina, L. The chemical budget of Lake Baikal: A review. Limnol. Oceanogr. 1997, 42, 373–378. [Google Scholar] [CrossRef]
- Talling, J.F.; Talling, I.B. The chemical composition of African lake waters. Intern. Rev. Gesamten Hydrobiol. Hydrogr. 1965, 50, 421–463. [Google Scholar] [CrossRef]
- Greenwood, P.H. What is a species flock? In Evolution of Fish Species Flocks; Echelle, A.A., Kornfield, I., Eds.; University of Maine Press: Orono, ME, USA, 1984; pp. 13–20. ISBN 0891010572. [Google Scholar]
- Michel, E. Phylogeny of a Gastropod Species Flock: Exploring Speciation in Lake Tanganyika in a Molecular Framework. Adv. Ecol. Res. 2000, 31, 275–302. [Google Scholar] [CrossRef]
- Turner, G.G. The Nature of Species in Ancient Lakes: Perspectives from the Fishes of Lake Malawi. Adv. Ecol. Res. 2000, 31, 39–60. [Google Scholar] [CrossRef]
- Stelbrink, B.; Jovanovska, E.; Levkov, Z.; Ognjanova-Rumenova, O.; Wilke, T.; Albrecht, C. Diatoms do radiate: Evidence for a freshwater species flock. J. Evol. Biol. 2018, 31, 1969–1975. [Google Scholar] [CrossRef]
- Snoeks, J. How well known is the ichthyodiversity of the large East African lakes? Adv. Ecol. Res. 2000, 31, 17–38. [Google Scholar] [CrossRef]
- Shtarkman, Y.M.; Koçer, Z.A.; Edgar, R.; Veerapaneni, R.S.; D’Elia, T.; Morris, P.F.; Rogers, S.O. Subglacial Lake Vostok (Antarctica) Accretion Ice Contains a Diverse Set of Sequences from Aquatic, Marine and Sediment-Inhabiting Bacteria and Eukarya. PLoS ONE 2013, 8, e67221. [Google Scholar] [CrossRef]
- Doran, P.T.; Fritsen, C.H.; McKay, C.P.; Priscu, J.C.; Adams, E.E. Formation and character of an ancient 19-m ice cover and underlying trapped brine in an ‘‘ice-sealed’’ east Antarctic lake. Proc. Natl. Acad. Sci. USA 2003, 100, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Christner, B.C.; Skidmore, M.L.; Priscu, J.C.; Tranter, M.; Foreman, M. Bacteria in subglacial environments. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.-C., Gerday, C., Eds.; Springer Verlag: Berlin, Germany, 2008; pp. 51–71. ISBN 978-3-540-74335-4. [Google Scholar]
- Bramburger, A.J.; Haffner, G.D.; Hamilton, P.B.; Hinz, F.; Hehanussa, P.E. An examination of species within the genus Surirella from the Malili Lakes, Sulawesi Island, Indonesia, with descriptions of 11 new taxa. Diatom Res. 2006, 21, 1–56. [Google Scholar] [CrossRef]
- Kozhova, O.M.; Izmest’eva, L.R. Lake Baikal. Evolution and Biodiversity; Backhuys: Leiden, The Netherlands, 1998; pp. 1–447. ISBN 90-5782-001-3. [Google Scholar]
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Takhev, V.V. Trends in the Evolution of Baikal Amphipods and Evolutionary Parallels with some Marine Malcostracan Faunas. Adv. Ecol. Res. 2000, 31, 197–220. [Google Scholar] [CrossRef]
- Schön, I.; Pieri, V.; Sherbakov, D.Y.; Martens, K. Cryptic diversity and speciation in endemic Cytherissa (Ostracoda, Crustacea) from Lake Baikal. Hydrobiologia 2017, 800, 61–79. [Google Scholar] [CrossRef]
- Stoch, F.; Arteau, M.; Brancelj, A.; Galassi, D.M.P.; Malard, F. Biodiversity indicators in European groundwaters: Towards a predictive model of stygobiotic species richness. Freshw. Biol. 2009, 54, 745–755. [Google Scholar] [CrossRef]
- Meixner, K.J.; Luter, C.; Eckert, C.; Itskovich, V.; Janussen, D.; von Rintelen, T.; Bohne, A.V.; Meixner, J.M.; Hess, W.R. Phylogenetic analysis of freshwater sponges provide evidence for endemism and radiation in ancient lakes. Mol. Phylogenet. Evol. 2007, 35, 875–886. [Google Scholar] [CrossRef]
- Matveyev, A.N.; Samusenok, V.P. The fishes and fishery in Lake Baikal. Aquat. Ecosyst. Health 2015, 18, 134–148. [Google Scholar] [CrossRef]
- Kontula, T.; Kirilchik, S.V.; Väinölä, R. Endemic diversification of the monophyletic cottoid fish species flock in Lake Baikal explored with mtDNA sequencing. Mol. Phylogenet. Evol. 2003, 27, 143–155. [Google Scholar] [CrossRef]
- Herder, F.; Schwarzer, J.; Pfaender, J.; Hadiaty, R.K.; Schliewen, U.K. Preliminary checklist of sailfin silversides (Teleostei: Telmatherinidae) in the Malili Lakes of Sulawesi (Indonesia), with a synopsis of systematics and threats. Verb. Ges. Icthyol. 2006, 5, 139–163. [Google Scholar]
- Roy, D.; Paterson, G.; Hamilton, P.B.; Heath, D.D.; Haffner, G.D. Resource-based adaptive divergence in the freshwater fish Telmatherina from Lake Matano, Indonesia. Mol. Ecol. 2007, 16, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Salzburger, W.; Van Bocxlaer, B.; Cohen, A.S. Ecology and evolution of the African great lakes and their faunas. Annu. Rev. Ecol. Evol. Syst. 2014, 445, 519–545. [Google Scholar] [CrossRef]
- Fryer, G.; Iles, T.D. The Cichlid Fishes of the Great Lakes of Africa: Their Biology and Evolution; Oliver & Boyd: Edinburgh, UK, 1972; pp. 1–641. ISBN 0050023470. [Google Scholar]
- Salzburger, W.; Meyer, A. The species flocks of East African cichlid fishes: Recent advances in molecular phylogenetics and population genetics. Naturwissenschaften 2004, 91, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Jovanovska, E.; Cvetkoska, A.; Hauffe, T.; Levkov, Z.; Wagner, B.; Sulpizio, R.; Francke, A.; Albrecht, C.; Wilke, T. Differential resilience of ancient sister lakes Ohrid and Prespa to environmental disturbances during the Late Pleistocene. Biogeosciences 2016, 13, 1149–1161. [Google Scholar] [CrossRef]
- Pavlov, A.; Levkov, Z.; Williams, D.M.; Edlund, M.B. Observations on Hippodonta (Bacillariophyceae) in selected ancient lakes. Phytotaxa 2013, 90, 1–53. [Google Scholar] [CrossRef]
- Schmieder, K. European lake shores in danger concepts for a sustainable development. Limnologica 2004, 34, 3–14. [Google Scholar] [CrossRef]
- Schindler, D.E.; Scheurell, M. Habitat coupling in lake ecosystems. Oikos 2002, 98, 177–189. [Google Scholar] [CrossRef]
- Beeton, A.M. Large freshwater lakes: Present state, trends and future. Environ. Conserv. 2002, 29, 21–38. [Google Scholar] [CrossRef]
- Albrecht, C.; Wilke, T. Ancient Lake Ohrid: Biodiversity and evolution. Hydrobiologia 2008, 615, 103–140. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Ishikawa, K.; Sakai, Y.; Ishikawa, T.; Ichise, S.; Yamamoto, Y.; Kuo, T.C.; Park, H.D.; Yamamura, N.; Kumagai, M. Phytoplankton community reorganization driven by eutrophication and warming in Lake Biwa. Aquat. Sci. 2010, 72, 467–483. [Google Scholar] [CrossRef]
- Ochumba, P.B.O.; Kibaara, D.I. Observations on blue-green algal blooms in the open waters of Lake Victoria, Kenya. Afr. J. Ecol. 1989, 27, 23–34. [Google Scholar] [CrossRef]
- Hecky, R.E. The eutrophication of Lake Victoria. Verh. Int. Ver. Theor. Angew. Limnol. 1993, 25, 39–48. [Google Scholar] [CrossRef]
- Lazzaro, X.; Gamarra Peralta, C. Fucionamiento limnológico y fotobiologia del Lago Titicaca. In Línea Base De Conocimientos Sobre Los Recursos Hidrológicos e Hidrobiológicos en El Sistema TDPS Con Enfoque en La Cuenca Del Lago Titicaca; Pouilly, M., Lazzaro, X., Point, D., Aguirre, M., Eds.; IRD—UICN: Quito, Ecuador, 2014; pp. 155–217. ISBN 9789997441843. [Google Scholar]
- Kobanova, G.I.; Takhteev, V.V.; Rusanovskaya, O.O.; Timofeyev, M.A. Lake Baikal Ecosystem Faces the Threat of Eutrophication. Acta Oecol. Int. J. Ecol. 2016, 6058082. [Google Scholar] [CrossRef]
- Cohen, A.S.; Gergurich, E.L.; Kraemer, B.M.; McGlue, M.M.; McIntyre, P.B.; Russell, J.M.; Simmons, J.D.; Swarzenski, P.W. Climate warming reduces fish production and benthic habitat in Lake Tanganyika, one of the most biodiverse freshwater ecosystems. Proc. Natl. Acad. Sci. USA 2016, 113, 9563–9568. [Google Scholar] [CrossRef]
- Everett, G.V. The rainbow trout Salmo gairdneri (Rich.) fishery of Lake Titicaca. J. Fish Biol. 1973, 5, 429–440. [Google Scholar] [CrossRef]
- Parenti, L.R. A taxonomic revision of the Andean Killifish Genus Orestias (Cyprinodontiformes, Cyprinodontidae). Bull. Am. Mus. Nat. Hist. 1984, 178, 107–214. [Google Scholar]
- Barel, C.D.N.; Dorit, R.; Greenwood, P.H.; Fryer, G.; Hughes, N.; Jackson, P.B.N.; Kawanabe, H.; Lowe-McConnell, R.H.; Nagoshi, M.; Ribbink, A.J.; et al. Destruction of fisheries in Africa’s lakes. Nature 1985, 315, 19–20. [Google Scholar] [CrossRef]
- Herder, F.; Nolte, A.W.; Pfaender, J.; Schwarzer, J.; Hadiaty, R.K.; Schliewen, U.K. Adaptive radiation and hybridization in Wallace’s dreamponds: Evidence from sailfin silversides in the Malili lakes of Sulawesi. Proc. R. Soc. B 2006, 273, 2209–2217. [Google Scholar] [CrossRef]
- Yin, X.; Nicholson, S.E. The water balance of Lake Victoria. Hydrol. Sci. J. 1998, 43, 789–811. [Google Scholar] [CrossRef]
- Pearce, F. Uganda pulls plug on Lake Victoria. N. Sci. 2006, 2538, 12. [Google Scholar]
- Hassan, A.A.; Jin, S. Lake level change and total water discharge in East Africa Rift Valley from satellite-based observations. Glob. Planet. Chang. 2014, 117, 79–90. [Google Scholar] [CrossRef]
- Peter, H.; Sommaruga, R. Alpine glacier-fed turbid lakes are discontinuous cold polymictic rather than dimictic. Inland Waters 2017, 7, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Tiberti, R.; Buscaglia, F.; Callieri, C.; Rogora, M.; Tartari, G.; Sommaruga, R. Food web complexity of high-mountain lakes is largely affected by glacial retreat. Ecosystems 2019. [Google Scholar] [CrossRef]
- Catalan, J.; Rondón, J.C.D. Perspective for an integrated understanding of tropical and temperate high-mountain lakes. J. Limnol. 2016, 75, 215–234. [Google Scholar] [CrossRef]
- Tiberti, R.; Buscaglia, F.; Armodi, M.; Callieri, C.; Ribelli, F.; Rogora, M.; Tartari, G.; Bocca, M. Mountain lakes of Mont Avic Natural Park: Ecological features and conservation issues. J. Limnol. 2019. [Google Scholar] [CrossRef]
- Hayford, B.L.; Caires, A.M.; Chandra, S. A Tale of Three Lakes: A Comparison of Biodiversity between Lake Tahoe and Crater Lake, USA, and Lake Hövsgöl, Mongolia. Mt. Views 2016, 10, 37–42. [Google Scholar]
- Tolotti, M.; Thies, H.; Cantonati, M.; Tait, D. Flagellate algae (Cryptophyceae, Chrysophyceae, Dinophyceae) in 48 high mountain lakes of the Northern and Southern slope of the Eastern Alps: Diversity, distribution and driving variables. Hydrobiologia 2003, 502, 331–348. [Google Scholar] [CrossRef]
- Kammerlander, B.; Breiner, H.W.; Filker, S.; Sommaruga, R.; Sonntag, B.; Stoeck, T. High diversity of protistan plankton communities in remote high mountain lakes in the European Alps and the Himalayan mountains. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef]
- Carrillo, P.; Medina-Sánchez, J.M.; Villar-Argaiz, M.; Delgado-Molina, J.A.; Bullejos, F.J. Complex interactions in microbial food webs: Stoichiometric and functional approaches. Limnetica 2006, 25, 189–204. [Google Scholar] [CrossRef]
- Cantonati, M.; Guella, G.; Komárek, J.; Spitale, D. Depth-distribution of epilithic cyanobacteria and pigments in a mountain lake characterized by marked water-level fluctuations. Freshw. Sci. 2014, 33, 537–547. [Google Scholar] [CrossRef]
- Sommaruga-Wögrath, S.; Koinig, K.A.; Schmidt, R.; Sommaruga, R.; Tessadri, R.; Psenner, R. Temperature effects on the acidity of remote alpine lakes. Nature 1997, 387, 64–67. [Google Scholar] [CrossRef]
- Catalán, J.; Ballesteros, E.; Gacia, E.; Palau, A.; Camarero, L. Chemical composition of disturbed and undisturbed high-mountain lakes in the Pyrenees: A reference for acidified sites. Wat. Res. 1993, 27, 133–141. [Google Scholar] [CrossRef]
- Leira, M.; Fillippi, M.L.; Cantonati, M. Diatom community response to extreme water-level fluctuations in two Alpine lakes: A core case study. J. Paleolimnol. 2015, 53, 289–307. [Google Scholar] [CrossRef]
- Tiberti, R.; Splendiani, A. Management of a highly unlikely native fish: The case of arctic charr Salvelinus alpinus from the Southern Alps. Aquatic Conserv. Mar. Freshw. Ecosyst. 2019, 29, 312–320. [Google Scholar] [CrossRef]
- Baron, J. The Fate of Mountain Lakes. Mt. Views 2016, 10, 2–4. [Google Scholar]
- Camarero, L.; Catalan, J.; Boggero, A.; Marchetto, A.; Mosello, R.; Psenner, R. Acidification in high mountain lakes in Central, Southwest and Southeast Europe (Alps, Pyrenees, Pirin). Limnologica 1995, 25, 141–156. [Google Scholar]
- Tait, D.; Thaler, B. Atmospheric deposition and lake chemistry trends at a high mountain site in the eastern Alps. J. Limnol. 2000, 59, 61–71. [Google Scholar] [CrossRef]
- Thies, H.; Nickus, U.; Mair, V.; Tessadri, R.; Tait, D.; Thaler, B.; Psenner, R. Unexpected response of high alpine lake waters to climate warming. Environ. Sci. Technol. 2007, 41, 7424–7429. [Google Scholar] [CrossRef]
- Marnezy, A. Alpine dams. J. Alp. Res. 2008, 96, 103–112. [Google Scholar] [CrossRef]
- Spitale, D.; Angeli, N.; Lencioni, V.; Tolotti, M.; Cantonati, M. Comparison between natural and impacted Alpine lakes six years after hydropower exploitation has ceased. Biologia 2015, 70, 1597–1605. [Google Scholar] [CrossRef]
- Margalef, R. Limnología; Editorial Omega: Barcelona, Spain, 1983; ISBN 8428207143. [Google Scholar]
- Baker, J.A.; Killgore, K.J.; Kasul, R.L. Aquatic habitats and fish communities in the lower Mississippi River. Rev. Aquat. Sci. 1991, 3, 213–356. [Google Scholar]
- Welcomme, R.L. Fisheries Ecology of Floodplain Rivers; Longman: London, UK; New York, NY, USA, 1979; ISBN 0582463106. [Google Scholar]
- Constantine, J.A.; Dunne, T.; Piegay, H.; Kandolf, G.M. Controls on the alluviation of oxbow lakes by bed-material load along the Sacramento River, California. Sedimentology 2010, 57, 389–407. [Google Scholar] [CrossRef]
- Ward, J.V. Riverine landscapes: Biodiversity patterns, disturbance regimes, and aquatic conservation. Biol. Conserv. 1998, 83, 269–278. [Google Scholar] [CrossRef]
- Piégay, H.; Bornette, G.; Citterio, A.; Hérouin, E.; Moulin, B.; Statiotis, C. Channel instability as control factor of silting dynamics and vegetation pattern within perifluvial aquatic zones. Hydrol. Process. 2000, 14, 3011–3029. [Google Scholar] [CrossRef]
- Schramm, H.L., Jr.; Eggleton, M.A.; Minnis, R.B. Spatial Analysis of Floodplain Habitat Critical to Lower Mississippi River Fishes; Mississippi Cooperative Fish and Wildlife Research Unit: Mississippi State, MS, USA, 1999. [Google Scholar]
- Dembowska, E.A.; Kubiak-Wojcicka, K. Influence of water level fluctuations on phytoplankton communities in an oxbow lake. Fundam. Appl. Limnol. 2017, 190, 221–233. [Google Scholar] [CrossRef]
- Naus, C.J.; Reid Adams, S. Fish nursery habitat function of the main channel, floodplain tributaries and oxbow lakes of a medium-sized river. Ecol. Freshw. Fish 2018, 27, 4–18. [Google Scholar] [CrossRef]
- Zeug, S.C.; Winemiller, K.O. Relationships between hydrology, spatial heterogeneity, and fish recruitment dynamics in a temperate floodplain river. River Res. Appl. 2007, 24, 90–102. [Google Scholar] [CrossRef]
- Bolpagni, R.; Piotti, A. Hydro-hygrophilous vegetation diversity and distribution patterns in riverine wetlands in an agricultural landscape: A case study from the Oglio River (Po Plain, Northern Italy). Phytocoenologia 2015, 45, 69–84. [Google Scholar] [CrossRef]
- Bolpagni, R.; Piotti, A. The importance of being natural in a human-altered riverscape: Role of wetland type in supporting habitat heterogeneity and the functional diversity of vegetation. Aquat. Conserv. Mar. Freshw. Ecosys. 2016, 26, 1168–1183. [Google Scholar] [CrossRef]
- Pierobon, E.; Bolpagni, R.; Bartoli, M.; Viaroli, P. Net primary production and seasonal CO2 and CH4 fluxes in a Trapa natans L. meadow. J. Limnol. 2010, 69, 225–234. [Google Scholar] [CrossRef]
- Bolpagni, R.; Pierobon, E.; Longhi, D.; Nizzoli, D.; Bartoli, M.; Tomaselli, M.; Viaroli, P. Diurnal exchanges of CO2 and CH4 across the water-atmosphere interface in a water chestnut meadow (Trapa natans L.). Aquat. Bot. 2007, 87, 43–48. [Google Scholar] [CrossRef]
- Bolpagni, R.; Bartoli, M.; Viaroli, P. Species and functional plant diversity in a heavily impacted riverscape: Implications for threatened hydro-hygrophilous flora conservation. Limnologica 2013, 43, 230–238. [Google Scholar] [CrossRef]
- Racchetti, E.; Bartoli, M.; Soana, E.; Longhi, D.; Christian, R.R.; Pinardi, M.; Viaroli, P. Influence of hydrological connectivity of riverine wetlands on nitrogen removal via denitrification. Biogeochemistry 2011, 103, 335–354. [Google Scholar] [CrossRef]
- Bolpagni, R.; Laini, A.; Mutti, T.; Viaroli, P.; Bartoli, M. Connectivity and habitat typology drive CO2 and CH4 fluxes across land–water interfaces in lowland rivers. Aquat. Sci. 2019, 12, e2036. [Google Scholar] [CrossRef]
- Gore, J.A.; Shields, F.D., Jr. Can large rivers be restored? Bioscience 1995, 45, 142–152. [Google Scholar] [CrossRef]
- Miranda, L.E. Fish Assemblages in Oxbow Lakes Relative to Connectivity with the Mississippi River. T. Am. Fish. Soc. 2011, 134, 1480–1489. [Google Scholar] [CrossRef]
- Pander, J.; Mueller, M.; Geist, J. Habitat diversity and connectivity govern the conservation value of restored aquatic floodplain habitats. Biol. Conserv. 2019, 217, 1–10. [Google Scholar] [CrossRef]
- Lehner, B.; Liermann, C.R.; Revenga, C.; Vörösmarty, C.; Fekete, B.; Crouzet, P.; Döll, P.; Endejan, M.; Frenken, K.; Magome, J.; et al. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar] [CrossRef]
- Thornton, K.W. Perspectives on reservoir limnology. In Reservoir Limnology: Ecological Perspectives; Thornton, K.W., Kimmel, B.L., Payne, F.E., Eds.; John Wiley & Sons: New York, NY, USA, 1990; pp. 1–13. ISBN 978-0471885016. [Google Scholar]
- Wetzel, R.G. Limnology. Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; ISBN 9780127447605. [Google Scholar]
- Cronk, J.K.; Fennessy, M.S. Wetland Plants, Biology and Ecology; CRC Press: Boca Raton, FL, USA, 2001; pp. 1–462. ISBN 9781566703727. [Google Scholar]
- Rørslett, B. An integrated approach to hydropower impact assesment. II. Submerged macrophytes in some Norwegian hydroelectric lakes. Hydrobiologia 1989, 175, 65–82. [Google Scholar] [CrossRef]
- Krolová, M.; Čížková, H.; Hejzlar, J.; Poláková, S. Response of littoral macrophytes to water level fluctuations in a storage reservoir. Knowl. Manag. Aquat. Ec. 2013, 408, 7. [Google Scholar] [CrossRef]
- Mjelde, M.; Hellsten, S.; Ecke, F. A water level drawdown index for aquatic macrophytes in Nordic lakes. Hydrobiologia 2013, 70, 141–151. [Google Scholar] [CrossRef]
- Wilcox, D.A.; Meeker, J.E. Implications for faunal habitat related to altered macrophyte structure in regulated lakes in northern Minnesota. Wetlands 1992, 12, 192–203. [Google Scholar] [CrossRef]
- Poff, N.L.; Olden, J.D.; Merritt, D.M.; Pepin, D.M. Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci. USA 2007, 104, 5732–5737. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, S.M.; Cunha, E.R. The role of macrophytes in habitat structuring in aquatic ecosystems: Methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnol. Bras. 2010, 22, 218–236. [Google Scholar] [CrossRef]
- Krolová, M.; Čížková, H.; Hejzlar, J. Depth limit of littoral vegetation in a storage reservoir: A case study of Lipno Reservoir (Czech Republic). Limnologica 2012, 42, 164–174. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Mainstone, C.P.; Parr, W. Phosphorus in Rivers—Ecology and Management. Sci. Total Environ. 2002, 282–283, 25–47. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems—A global problem. Environ. Sci. Pollut. R. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Hilton, J.; O’Hare, M.; Bowes, M.J.; Jones, J.I. How green is my river? A new paradigm of eutrophication in rivers. Sci. Total Environ. 2006, 365, 66–83. [Google Scholar] [CrossRef]
- Moss, B. Ecology of Freshwaters, Man and Medium, Past to Future, 3rd ed.; Blackwell Science: Oxford, UK, 1998; ISBN 978-1-444-31342-0. [Google Scholar]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Kubečka, J.; Seďa, J.; Duncan, A.; Matěna, J.; Ketelaars, H. Composition and biomass of the fish stocks in various european reservoirs and ecological consequences. Int. Rev. Hydrobiol. 1998, 83, 559–568. [Google Scholar]
- Persson, L.; Greenberg, L.A. Juvenile competitive bottlenecks: The perch (Perca fluviatilis)—Roach (Rutilus rutilus) interaction. Ecology 1990, 71, 44–56. [Google Scholar] [CrossRef]
- Šmejkal, M.; Ricard, D.; Sajdlová, Z.; Čech, M.; Vejřík, L.; Blabolil, P.; Vejříková, I.; Prchalová, M.; Vašek, M.; Souza, A.T.; et al. Can species-specific prey responses to chemical cues explain prey susceptibility to predation? Ecol. Evol. 2018, 8, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Poikane, S.; Ritterbusch, D.; Argillier, C.; Białokoz, W.; Blabolil, P.; Breine, J.; Jaarsma, N.G.; Krause, T.; Kubečka, J.; Lauridsen, T.L.; et al. Response of fish communities to multiple pressures: Development of a total anthropogenic pressure intensity index. Sci. Total Environ. 2017, 586, 502–511. [Google Scholar] [CrossRef]
- Draštík, V.; Kubečka, J.; Šovčík, P. Hydrology and angler’s catches in the Czech reservoirs. Ecohydrol. Hydrobiol. 2004, 4, 429–439. [Google Scholar]
- Duncan, A.; Kubečka, J. Land/water ecotone effects in reservoirs on the fish fauna. Hydrobiologia 1995, 303, 11–30. [Google Scholar] [CrossRef]
- Clavero, M.; Hermoso, V.; Aparicio, E.; Godinho, F.N. Biodiversity in heavily modified waterbodies: Native and introduced fish in Iberian reservoirs. Freshw. Biol. 2013, 58, 1190–1201. [Google Scholar] [CrossRef]
- Catford, J.A.; Vesk, P.A.; Richardson, D.M.; Pyšek, P. Quantifying levels of biological invasion: Towards the objective classification of invaded and invasible ecosystems. Glob. Chang. Biol. 2012, 18, 44–62. [Google Scholar] [CrossRef]
- Byers, J.E. Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 2002, 97, 449–458. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Env. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Olden, J.D.; Vander Zanden, M.J. Dam Invaders: Impoundments facilitate biological invasions into freshwaters. Front. Ecol. Environ. 2008, 6, 357–363. [Google Scholar] [CrossRef]
- Clavero, M.; Hermoso, V. Reservoirs promote the taxonomic homogenization of fish communities within river basins. Biodivers. Conserv. 2011, 20, 41–57. [Google Scholar] [CrossRef]
- Villamagna, A.M.; Murphy, B.R. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshw. Biol. 2010, 55, 282–298. [Google Scholar] [CrossRef]
- Madsen, J.D. Predicting invasion success of Eurasian watermilfoil. J. Aquat. Plant Manag. 1998, 36, 28–32. [Google Scholar]
- Van Zuidam, J.P.; Peeters, E.T.H.M. Occurrence of macrophyte monocultures in drainage ditches relates to phosphorus in both sediment and water. Springer Plus 2013, 2, 564. [Google Scholar] [CrossRef]
- Verdonschot, R.C.M.; Keizer-Vlek, H.E.; Verdonschot, P.F.M. Biodiversity value of agricultural drainage ditches; a comparative analysis of the aquatic invertebrate fauna of ditches and small lakes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 715–727. [Google Scholar] [CrossRef]
- Francis, R.A. Urban rivers: Novel ecosystems, new challenges. WIREs Water 2014, 1, 19–29. [Google Scholar] [CrossRef]
- Booth, D.B.; Roy, A.H.; Smith, B.; Capps, K.A. Global perspectives on the urban stream syndrome. Freshw. Sci. 2016, 35, 412–420. [Google Scholar] [CrossRef]
- Gál, B.; Szivák, I.; Heino, J.; Schmera, D. The effect of urbanization on freshwater macroinvertebrates—Knowledge gaps and future research directions. Ecol. Ind. 2019, 104, 357–364. [Google Scholar] [CrossRef]
- Higgins, S.L.; Thomas, F.; Goldsmith, B.; Brooks, S.J.; Hassall, C.; Harlow, J.; Stone, D.; Völker, S.; White, P. Urban freshwaters, biodiversity, and human health and well-being: Setting an interdisciplinary research agenda. Wiley Interdiscip. Rev. Water 2019, 6, e1339. [Google Scholar] [CrossRef]
- Dearborn, D.C.; Kark, S. Motivations for conserving urban biodiversity. Conserv. Biol. 2019, 24, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands. A Framework for Decision-Making; International Mire Conservation Group: Greifswald, Germany; International Peat Society: Jyväskylä, Finland; Saarijärven Offset Oy: Saarijärvi, Finland, 2002; ISBN 951-97744-8-3. [Google Scholar]
- Hájek, M.; Horsák, M.; Hájková, P.; Dítě, D. Habitat diversity of central European fens in relation to environmental gradients and an effort to standardise fen terminology in ecological studies. Perspect. Plant Ecol. 2006, 8, 97–114. [Google Scholar] [CrossRef]
- Dierssen, K.; Dierssen, B. Moore; Ökosysteme Mitteleuropas aus Geobotanischer Sicht: Stuttgart, Germany, 2001; pp. 1–230. ISBN 9783800156436. [Google Scholar]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands; Oxford University Press: Oxford, UK, 2013; pp. 1–432. ISBN 978-0199603008. [Google Scholar]
- Vitt, D.H. Peatlands: Ecosystems dominated by bryophytes. In Bryophyte Biology; Shaw, A.J., Goffinet, B., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 312–343. ISBN 9780511754807. [Google Scholar]
- Wheeler, B.D.; Proctor, M.C.F. Ecological gradients, subdivisions and terminology of north-west European mires. J. Ecol. 2000, 88, 187–203. [Google Scholar] [CrossRef]
- Proctor, M.C.; McHaffie, H.S.; Legg, C.J.; Amphlett, A. Evidence from water chemistry as a criterion of ombrotrophy in the mire complexes of Abernethy Forest, Scotland. J. Veg. Sci. 2009, 20, 160–169. [Google Scholar] [CrossRef]
- Jiroušek, M.; Poulíčková, A.; Kintrová, K.; Opravilová, V.; Hájková, P.; Rybníček, K.; Kočí, M.; Bergová, K.; Hnilica, R.; Mikulášková, E.; et al. Long-term and contemporary environmental conditions as determinants of the species composition of bog organisms. Freshw. Biol. 2013, 58, 2196–2207. [Google Scholar] [CrossRef]
- Lindsay, R. Peatland classification. In The Wetland Book: I: Structure and Function, Management, and Methods; Finlayson, C.M., Everard, M., Irvine, K., McInnes, R.J., Middleton, B.A., van Dam, A.A., Davidson, N.C., Eds.; Springer: Berlin, Germany, 2018; pp. 1515–1528. ISBN 978-94-007-1471-7. [Google Scholar]
- Peterka, T.; Hájek, M.; Jiroušek, M.; Jiménez-Alfaro, B.; Aunina, L.; Bergamini, A.; Dítě, D.; Felbaba-Klushyna, L.; Graf, U.; Hájková, P.; et al. Formalized classification of European fen vegetation at the alliance level. Appl. Veg. Sci. 2017, 20, 124–142. [Google Scholar] [CrossRef]
- Singh, P.; Těšitel, J.; Plesková, Z.; Peterka, T.; Dítě, D.; Hájková, P.; Pawlikowski, P.; Hájek, M. The ratio between bryophyte functional groups impacts vascular plants in rich fens. Appl. Veg. Sci. 2019. [Google Scholar] [CrossRef]
- Horsák, M. Mollusc community patterns and species response curves along a mineral richness gradient: A case study in fens. J. Biogeogr. 2006, 33, 98–107. [Google Scholar] [CrossRef]
- Rozbrojová, Z.; Hájek, M. Changes in nutrient limitation of spring fen vegetation along environmental gradients in the West Carpathians. J. Veg. Sci. 2008, 19, 613–620. [Google Scholar] [CrossRef]
- Vicherová, E.; Hájek, M.; Hájek, T. Calcium intolerance of fen mosses: Physiological evidence, effects of nutrient availability and successional drivers. Perspect. Plant Ecol. 2015, 17, 347–359. [Google Scholar] [CrossRef]
- Bain, J.T.; Proctor, M.C.F. The requirement of aquatic bryophytes for free CO2 as an inorganic carbon source: Some experimental evidence. N. Phytol. 1980, 86, 393–400. [Google Scholar] [CrossRef]
- Patberg, W.; Baaijens, G.J.; Smolders, A.J.; Grootjans, A.P.; Elzenga, J.T.M. The importance of groundwater carbon dioxide in the restoration of small Sphagnum bogs. Preslia 2013, 85, 389–403. [Google Scholar]
- Hájek, M.; Jiroušek, M.; Navrátilová, J.; Horodyská, E.; Peterka, T.; Plesková, Z.; Navrátil, Z.; Hájková, P.; Hájek, T. Changes in the moss layer in Czech fens indicate early succession triggered by nutrient enrichment. Preslia 2015, 87, 279–301. [Google Scholar]
- Hájková, P.; Hájek, M. Species richness and above-ground biomass of poor and calcareous spring fens in the flysch West Carpathians, and their relationships to water and soil chemistry. Preslia 2003, 75, 271–287. [Google Scholar]
- Horsáková, V.; Hájek, M.; Hájková, P.; Dítě, D.; Horsák, M. Principal factors controlling the species richness of European fens differ between habitat specialists and matrix-derived species. Divers. Distrib. 2018, 24, 742–754. [Google Scholar] [CrossRef]
- Dítě, D.; Hájek, M.; Svitková, I.; Košuthová, A.; Šoltés, R.; Kliment, J. Glacial-relict symptoms in the Western Carpathian flora. Folia Geobot. 2018, 53, 277–300. [Google Scholar] [CrossRef]
- Hájková, P.; Štechová, T.; Šoltés, R.; Šmerdová, E.; Plesková, Z.; Dítě, D.; Bradáčová, J.; Mútňanová, M.; Singh, P.; Hájek, M. Using a new database of plant macrofossils of the Czech and Slovak Republics to compare past and present distribution of hypothetically relict fen mosses. Preslia 2018, 90, 367–386. [Google Scholar] [CrossRef]
- Hájek, M.; Horsák, M.; Tichý, L.; Hájková, P.; Dítě, D.; Jamrichová, E. Testing a relict distributional pattern of fen plant and terrestrial snail species at the Holocene scale: A null model approach. J. Biogeogr. 2011, 38, 742–755. [Google Scholar] [CrossRef]
- Horsák, M.; Hájek, M.; Spitale, D.; Hájková, P.; Dítě, D.; Nekola, J.C. The age of island-like habitats impacts habitat specialist species richness. Ecology 2012, 93, 1106–1114. [Google Scholar] [CrossRef]
- Warner, B.G.; Asada, T. Biological diversity of peatlands in Canada. Aquat. Sci. 2006, 68, 240–253. [Google Scholar] [CrossRef]
- Middleton, B.A.; Holsten, B.; van Diggelen, R. Biodiversity management of fens and fen meadows by grazing, cutting and burning. Appl. Veg. Sci. 2006, 9, 307–316. [Google Scholar] [CrossRef]
- Van Diggelen, R.; Middleton, B.; Bakker, J.; Grootjans, A.; Wassen, M. Fens and floodplains of the temperate zone: Present status, threats, conservation and restoration. Appl. Veg. Sci. 2006, 9, 157–162. [Google Scholar] [CrossRef]
- Soomers, H.; Karssenberg, D.; Verhoeven, J.T.; Verweij, P.A.; Wassen, M.J. The effect of habitat fragmentation and abiotic factors on fen plant occurrence. Biodiver. Conserv. 2013, 22, 405–424. [Google Scholar] [CrossRef]
- Buczkó, K. Bryophytic diatoms from Hungary. In Proceedings of the Eighteenth International Diatom Symposium, Miedzyzdroje, Poland, 2–7 September 2004; Witkowski, A., Ed.; Biopress Limited: Bristol, UK, 2006; Volume 2006, pp. 1–15. [Google Scholar]
- Berendse, F.; Breemen, N.; Van Rydin, H.; Buttler, A.; Heijmans, M.; Hoosbeek, M.R.; Lee, J.A.; Mitchell, E.; Saarinen, T.; Vasander, H.; et al. Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and production in Sphagnum bogs. Glob. Chang. Biol. 2001, 7, 591–598. [Google Scholar] [CrossRef]
- Charman, D. Peatlands and Environmental Change; Wiley: Hoboken, NJ, USA, 2002; pp. 1–301. ISBN 978-0-470-84410-6. [Google Scholar]
- Essl, F.; Dullinger, S.; Moser, D.; Rabitsch, W.; Kleinbauer, I. Vulnerability of mires under climate change: Implications for nature conservation and climate change adaptation. Biodivers. Conserv. 2012, 21, 655–669. [Google Scholar] [CrossRef]
- Moradi, H.; Fakheran, S.; Peintinger, M.; Bergamini, A.; Schmid, B.; Joshi, J. Profiteers of environmental change in the Swiss Alps: Increase of thermophilous and generalist plants in wetland ecosystems within the last 10 years. Alp. Bot. 2012, 122, 45–56. [Google Scholar] [CrossRef]
- Herrera-Pantoja, M.; Hiscock, K.M.; Boar, R.R. The potential impact of climate change on groundwater-fed wetlands in eastern England. Ecohydrology 2012, 5, 401–413. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; García-Calvo, L.; García, P.; Acebes, J.L. Anticipating extinctions of glacial relict populations in mountain refugia. Biol. Conserv. 2016, 201, 243–251. [Google Scholar] [CrossRef]
- Page, S.E.; Baird, A.J. Peatlands and global change: Response and resilience. Annu. Rev. Env. Resour. 2016, 41, 35–57. [Google Scholar] [CrossRef]
- Janssen, J.A.M.; Rodwell, J.S.; Criado, M.G.; Arts, G.H.P.; Bijlsma, R.J.; Schaminee, J.H.J. European Red List of Habitats: Part 2. Terrestrial and Freshwater Habitats; Directorate-General for the Environment: Brussels, Belgium, 2016; ISBN 978-92-79-61588-7. [Google Scholar]
- Grootjans, A.P.; Adema, E.B.; Bleuten, W.; Joosten, H.; Madaras, M.; Janáková, M. Hydrological landscape settings of base-rich fen mires and fen meadows: An overview. Appl. Veg. Sci. 2006, 9, 175–184. [Google Scholar] [CrossRef]
- Vicherová, E.; Hájek, M.; Šmilauer, P.; Hájek, T. Sphagnum establishment in alkaline fens: Importance of weather and water chemistry. Sci. Total Environ. 2017, 580, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Hájková, P.; Horsák, M.; Hájek, M.; Jankovská, V.; Jamrichová, E.; Moutelíková, J. Using multi-proxy palaeoecology to test a relict status of refugial populations of calcareous-fen species in the Western Carpathians. Holocene 2015, 25, 702–715. [Google Scholar] [CrossRef]
- Bart, D.; Davenport, T.; Yantes, A. Environmental predictors of woody plant encroachment in calcareous fens are modified by biotic and abiotic land-use legacies. J. Appl. Ecol. 2016, 53, 541–549. [Google Scholar] [CrossRef]
- Jamrichová, E.; Gálová, A.; Gašpar, A.; Horsák, M.; Frodlová, J.; Hájek, M.; Hajnalová, M.; Hájková, P. Holocene development of two calcareous spring fens at the Carpathian-Pannonian interface controlled by climate and human impact. Folia Geobot. 2018, 53, 243–263. [Google Scholar] [CrossRef]
- Koch, M.; Jurasinski, G. Four decades of vegetation development in a percolation mire complex following intensive drainage and abandonment. Plant Ecol. Divers. 2015, 8, 49–60. [Google Scholar] [CrossRef]
- Kapfer, J.; Grytnes, J.A.; Gunnarsson, U.; Birks, H.J.B. Fine-scale changes in vegetation composition in a boreal mire over 50 years. J. Ecol. 2011, 99, 1179–1189. [Google Scholar] [CrossRef]
- Pedrotti, E.; Rydin, H.; Ingmar, T.; Hytteborn, H.; Turunen, P.; Granath, G. Fine-scale dynamics and community stability in boreal peatlands: Revisiting a fen and a bog in Sweden after 50 years. Ecosphere 2014, 5, 1–24. [Google Scholar] [CrossRef]
- Seer, F.K.; Schrautzer, J. Status, future prospects, and management recommendations for alkaline fens in an agricultural landscape: A comprehensive survey. J. Nat. Conserv. 2014, 22, 358–368. [Google Scholar] [CrossRef]
- Merriam, K.E.; Markwith, S.H.; Coppoletta, M. Livestock exclusion alters plant species composition in fen meadows. Appl. Veg. Sci. 2018, 21, 3–11. [Google Scholar] [CrossRef]
- Ross, L.C.; Speed, J.D.; Øien, D.I.; Grygoruk, M.; Hassel, K.; Lyngstad, A.; Moen, A. Can mowing restore boreal rich-fen vegetation in the face of climate change? PLoS ONE 2019, 14, e0211272. [Google Scholar] [CrossRef]
- Poulíčková, A.; Hájková, P.; Kintrová, K.; Bat’ková, R.; Czudková, M.; Hájek, M. Tracing decadal environmental change in ombrotrophic bogs using diatoms from herbarium collections and transfer functions. Environ. Pollut. 2013, 179, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Paal, J.; Vellak, K.; Liira, J.; Karofeld, E. Bog recovery in northeastern Estonia after the reduction of atmospheric pollutant input. Restor. Ecol. 2010, 18, 387–400. [Google Scholar] [CrossRef]
- Friberg, N.; Dybkjaer, J.B.; Olafsson, J.S.; Gislason, G.M.; Larsen, S.E.; Lauridsen, T.L. Relationships between structure and function in streams contrasting in temperature. Freshw. Biol. 2009, 54, 2051–2068. [Google Scholar] [CrossRef]
- Gerdol, R.; Petraglia, A.; Bragazza, L.; Iacumin, P.; Brancaleoni, L. Nitrogen deposition interacts with climate in affecting production and decomposition rates in Sphagnum mosses. Glob. Chang. Biol. 2007, 13, 1810–1821. [Google Scholar] [CrossRef]
- Bragazza, L.; Siffi, C.; Iacumin, P.; Gerdol, R. Mass loss and nutrient release during litter decay in peatland: The role of microbial adaptability to litter chemistry. Soil Biol. Biochem. 2007, 39, 257–267. [Google Scholar] [CrossRef]
- Neuhäusl, R. Hochmoore am Teich Velké Dářko; Academia-Verlag: Praha, Czech Republic, 1975; pp. 1–120. [Google Scholar]
- Nørgaard, E. Environment and behaviour of Theridion saxatile. Oikos 1956, 7, 159–192. [Google Scholar] [CrossRef]
- Horsák, M.; Polášková, V.; Zhai, M.; Bojková, J.; Syrovátka, V.; Šorfová, V.; Schenková, J.; Polášek, M.; Peterka, T.; Hájek, M. Spring-fen habitat islands in a warming climate: Partitioning the effects of mesoclimate air and water temperature on aquatic and terrestrial biota. Sci. Total Environ. 2018, 634, 355–365. [Google Scholar] [CrossRef]
- Fernández-Pascual, E.; Jiménez-Alfaro, B.; Hájek, M.; Díaz, T.E.; Pritchard, H.W. Soil thermal buffer and regeneration niche may favour calcareous fen resilience to climate change. Folia Geobot. 2015, 50, 293–301. [Google Scholar] [CrossRef]
- Seymour, K.S.; Christanis, K.; Bouzinos, A.; Papazisimou, S.; Papatheodorou, G.; Moran, E.; Dénès, G. Tephrostratigraphy and tephrochronology in the Philippi peat basin, Macedonia, Northern Hellas (Greece). Quatern. Int. 2004, 121, 53–65. [Google Scholar] [CrossRef]
- Hájek, M.; Hájková, P.; Apostolova, I.; Horsák, M.; Plášek, V.; Shaw, B.; Lazarova, M. Disjunct occurrences of plant species in the refugial mires of Bulgaria. Folia Geobot. 2009, 44, 365–386. [Google Scholar] [CrossRef]
- Vukojičić, S.; Jakovljević, K.; Matevski, V.; Randjelović, V.; Niketić, M.; Lakušić, D. Distribution, Diversity and Conservation of Boreo-Montane Plant Species in the Central Part of the Balkan Peninsula and the Southern Part of the Pannonian Plain. Folia Geobot. 2014, 49, 487–505. [Google Scholar] [CrossRef]
- Hájková, P.; Hájek, M.; Apostolova, I.; Zelený, D.; Dítě, D. Shifts in the ecological behaviour of plant species between two distant regions: Evidence from the base richness gradient in mires. J. Biogeogr. 2008, 35, 282–294. [Google Scholar] [CrossRef]
- Theodoridis, S.; Nogués-Bravo, D.; Conti, E. The role of cryptic diversity and its environmental correlates in global conservation status assessments: Insights from the threatened bird’s-eye primrose (Primula farinosa L.). Divers. Distrib. 2019, 25, 1457–1471. [Google Scholar] [CrossRef]
- Cantonati, M.; Lange-Bertalot, H.; Decet, F.; Gabrieli, J. Diatoms in very-shallow pools of the site of community importance Danta di Cadore Mires (south-eastern Alps), and the potential contribution of these habitats to diatom biodiversity conservation. Nova Hedwig. 2011, 93, 475–507. [Google Scholar] [CrossRef]
- Cantonati, M.; Van de Vijver, B.; Lange-Bertalot, H. Microfissurata gen. nov. (Bacillariophyta), a new diatom genus from dystrophic and intermittently-wet terrestrial habitats. J. Phycol. 2009, 45, 732–741. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Bond-Lamberty, B.; Euskirchen, E.; Talbot, J.; Frolking, S.; McGuire, A.D.; Tuittila, E.S. The resilience and functional role of moss in boreal and arctic ecosystems. N. Phytol. 2012, 196, 49–67. [Google Scholar] [CrossRef]
- Rosset, V.; Angelibert, S.; Arthaud, F.; Bornette, G.; Robin, J.; Wezel, A.; Vallod, D.; Oertli, B. Is eutrophication really a major impairment for small waterbody biodiversity? J. Appl. Ecol. 2014, 51, 415–425. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Bergström, L.; Aronsson, H.; Bechmann, M.; Bolster, C.H.; Börling, K.; Djodjic, F.; Jarvie, H.P.; Schoumans, O.F.; Stamm, C.; et al. Future agriculture with minimized phosphorus losses to waters: Research needs and direction. Ambio 2015, 44, 163–179. [Google Scholar] [CrossRef]
- Wetzel, R.G. Land water interfaces: Metabolic and limnological regulators. Verh. Int. Ver. Limnol. 1990, 24, 6–24. [Google Scholar] [CrossRef]
- Schiemer, F.; Zalewski, M.; Thorpe, J.E. Land/Inland water ecotones: Intermediate habitats critical for conservation and management. Hydrobiologia 1995, 303, 259–264. [Google Scholar] [CrossRef]
- Hill, M.J.; Ryves, D.B.; White, J.C.; Wood, P.J. Macroinvertebrate diversity in urban and rural ponds: Implications for freshwater biodiversity conservation. Biol. Conserv. 2016, 201, 50–59. [Google Scholar] [CrossRef]
- Bagella, S.; Gascón, S.; Filigheddu, R.; Cogoni, A.; Boix, D. Mediterranean Temporary Ponds: New challenges from a neglected habitat. Hydrobiologia 2016, 782, 1–10. [Google Scholar] [CrossRef]
- Hill, M.J.; Wood, P.J. The macroinvertebrate biodiversity and conservation value of garden and field ponds along a rural-urban gradient. Fund. Appl. Limnol. 2014, 185, 107–119. [Google Scholar] [CrossRef]
- Bazzanti, M. Pond macroinvertebrates of the Presidential Estate of Castelporziano (Rome): A review of ecological aspects and selecting indicator taxa for conservation. Rend. Lincei 2015, 26, 337–343. [Google Scholar] [CrossRef]
- Rosset, V.; Simaika, J.P.; Arthaud, F.; Bornette, G.; Vallod, D.; Samways, M.J.; Oertli, B. Comparative assessment of scoring methods to evaluate the conservation value of pond and small lake biodiversity. Aquat. Conserv. Mar. Freshw. Ecosys. 2013, 23, 23–36. [Google Scholar] [CrossRef]
- Fuentes-Rodríguez, F.; Juan, M.; Gallego, I.; Lusi, M.; Fenoy, E.; León, D.; Peñalver, P.; Toja, J.; Casas, J.J. Diversity in Mediterranean farm ponds: Trade-offs and synergies between irrigation modernisation and biodiversity conservation. Freshw. Biol. 2013, 58, 63–78. [Google Scholar] [CrossRef]
- Tóth, A.; Horváth, Z.; Vad, C.F.; Zsuga, K.; Nagy, S.A.; Boros, E. Zooplankton of the european soda pans: Fauna and conservation of a unique habitat type. Int. Rev. Hydrobiol. 2014, 99, 255–276. [Google Scholar] [CrossRef]
- Briers, R.A. Invertebrate communities and environmental conditions in a series of urban drainage ponds in eastern Scotland: Implications for biodiversity and conservation value of SUDS. Clean 2014, 42, 193–200. [Google Scholar] [CrossRef]
- Williams, P.; Whitfield, M.; Biggs, J.; Bray, S.; Fox, G.; Nicolet, P.; Sear, D. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol. Conserv. 2003, 115, 329–341. [Google Scholar] [CrossRef]
- Hunter, M.L., Jr.; Acuña, V.; Bauer, D.M.; Bell, K.P.; Calhoun, A.J.K.; Felipe-Lucia, M.R.; Fitzsimons, J.A.; González, E.; Kinnison, M.; Lindenmayer, D.; et al. Conserving small natural features with large ecological roles: A synthetic overview. Biol. Conserv. 2017, 211, 88–95. [Google Scholar] [CrossRef]
- Maynou, X.; Martín, R.; Aranda, D. The role of small secondary biotopes in a highly fragmented landscape as habitat and connectivity providers for dragonflies (Insecta: Odonata). J. Insect Conserv. 2017, 21, 517–530. [Google Scholar] [CrossRef]
- Hill, M.J.; Heino, J.; White, J.C.; Ryves, D.B.; Wood, P.J. Environmental factors are primary determinants of different facets of pond macroinvertebrate alpha and beta diversity in a human-modified landscape. Biol. Conserv. 2019, 237, 348–357. [Google Scholar] [CrossRef]
- Oertli, B.; Parris, K.M. Review: Toward management of urban ponds for freshwater biodiversity. Ecosphere 2019, 10, e02810. [Google Scholar] [CrossRef]
- Johansson, F.; Bini, L.M.; Coiffard, P.; Svanbäck, R.; Wester, J.; Heino, J. Environmental variables drive differences in the beta diversity of dragonfly assemblages among urban stormwater ponds. Ecol. Indic. 2019, 106, 105529. [Google Scholar] [CrossRef]
- Hill, M.J.; Biggs, J.; Thornhill, I.; Briers, R.A.; Gledhill, D.G.; White, J.C.; Wood, P.J.; Hassall, C. Urban ponds as an aquatic biodiversity resource in modified landscapes. Glob. Chang. Biol. 2017, 23, 986–999. [Google Scholar] [CrossRef]
- Pielou, E.C. After the Ice Age: The Return of Life to Glaciated North America; University of Chicago Press: Chicago, IL, USA, 1991; ISBN 978-0226668123. [Google Scholar]
- Wrona, F.J.; Reist, J.D. Freshwater ecosystems. In Arctic Biodiversity Assessment. Status and Trends in Arctic Biodiversity; Meltofte, H., Ed.; Conservation of Arctic Flora and Fauna, Arctic Council: Akureyri, Iceland, 2013; pp. 335–377. ISBN 978-9935-431-22-6. [Google Scholar]
- Lento, J.; Goedkoop, W.; Culp, J.; Christoffersen, K.; Fefilova, E.; Guðbergsson, G.; Liljaniemi, P.; Ólafsson, J.S.; Sandøy, S.; Zimmerman, C.; et al. Circumpolar Biodiversity Monitoring Program State of Arctic Freshwater Biodiversity Report; Conservation of Arctic Flora and Fauna, Arctic Council: Akureyri, Iceland, 2019; ISBN 978-9935-431-77-6. [Google Scholar]
- Christiansen, J.S.; Reist, J.D.; Brown, R.J.; Brykov, V.A.; Christensen, G.; Christoffersen, K.; Cott, P.; Crane, P.; Dempson, J.B.; Docker, M.; et al. Fishes. Conservation of Arctic Flora and Fauna. In Arctic Biodiversity Assessment. Status and Trends in Arctic Biodiversity; Meltofte, H., Ed.; Arctic Council: Akureyri, Iceland, 2013; pp. 192–245. ISBN 978-9935-431-22-6. [Google Scholar]
- Jyrkänkallio-Mikkola, J.; Meier, S.; Heino, J.; Laamanen, T.; Pajunen, V.; Tolonen, K.T.; Tolkkinen, M.; Soininen, J. Disentangling multi-scale environmental effects on stream microbial communities. J. Biogeogr. 2017, 44, 1512–1523. [Google Scholar] [CrossRef]
- Soininen, J.; Heino, J.; Kokocinski, M.; Muotka, T. Local-regional diversity relationship varies with spatial scale in lotic diatoms. J. Biogeogr. 2009, 36, 720–727. [Google Scholar] [CrossRef]
- Alahuhta, J. Geographic patterns of lake macrophyte communities and species richness at regional scale. J. Veg. Sci. 2015, 26, 564–575. [Google Scholar] [CrossRef]
- Heino, J.; Tolonen, K.T. Untangling the assembly of littoral macroinvertebrate communities through measures of functional and phylogenetic alpha diversity. Freshw. Biol. 2017, 62, 1168–1179. [Google Scholar] [CrossRef]
- Rocha, M.P.; Bini, L.M.; Domisch, S.; Tolonen, K.T.; Jyrkänkallio-Mikkola, J.; Soininen, J.; Hjort, J.; Heino, J. Local environment and space drive multiple facets of stream macroinvertebrate beta diversity. J. Biogeogr. 2018, 45, 2744–2754. [Google Scholar] [CrossRef]
- Heino, J.; Tolonen, K.T. Ecological drivers of multiple facets of beta diversity in a lentic macroinvertebrate metacommunity. Limnol. Oceanogr. 2017, 62, 2431–2444. [Google Scholar] [CrossRef]
- Erős, T.; Heino, J.; Schmera, D.; Rask, M. Characterising functional trait diversity and trait-environment relationships in fish assemblages of boreal lakes. Freshw. Biol. 2009, 54, 1788–1803. [Google Scholar] [CrossRef]
- Sandin, L. Benthic macroinvertebrates in Swedish streams: Community structure, taxon richness, and environmental relations. Ecography 2003, 26, 269–282. [Google Scholar] [CrossRef]
- Tolonen, K.E.; Leinonen, K.; Marttila, H.; Erkinaro, J.; Heino, J. Environmental predictability of taxonomic and functional community composition in high-latitude streams. Freshw. Biol. 2017, 62, 1–16. [Google Scholar] [CrossRef]
- Johnson, R.K.; Goedkoop, W.; Sandin, L. Spatial scale and ecological relationships between the macroinvertebrate communities of stony habitats of streams and lakes. Freshw. Biol. 2004, 49, 1179–1194. [Google Scholar] [CrossRef]
- Soininen, J.; Kokocinski, M.; Estlander, S.; Kotanen, J.; Heino, J. Neutrality, niches and determinants of plankton metacommunity structure across boreal wetland ponds. Ecoscience 2007, 14, 146–154. [Google Scholar] [CrossRef]
- Grönroos, M.; Heino, J.; Siqueira, T.; Landeiro, V.L.; Kotanen, J.; Bini, L.M. Metacommunity structuring in stream networks: Roles of dispersal mode, distance type and regional environmental context. Ecol. Evol. 2013, 3, 4473–4487. [Google Scholar] [CrossRef]
- Heino, J.; Muotka, T.; Paavola, R. Determinants of macroinvertebrate diversity in headwater streams: Regional and local influences. J. Anim. Ecol. 2003, 72, 425–434. [Google Scholar] [CrossRef]
- Pajunen, V.; Luoto, M.; Soininen, J. Stream diatom assemblages as predictors of climate. Freshw. Biol. 2016, 61, 876–886. [Google Scholar] [CrossRef]
- Culp, J.; Lento, J.; Curry, R.A.; Luiker, E.; Halliwell, D. Arctic biodiversity of stream macroinvertebrates declines in response to latitudinal change in the abiotic template. Freshw. Sci. 2019. [Google Scholar] [CrossRef]
- Toivanen, M.; Hjort, J.; Heino, J.; Tukiainen, H.; Aroviita, J.; Alahuhta, J. Is catchment geodiversity a useful surrogate of aquatic plant species richness? J. Biogeogr. 2019, 46, 1–12. [Google Scholar] [CrossRef]
- Heino, J.; Virkkala, R.; Toivonen, H. Climate change and freshwater biodiversity: Detected patterns, future trends and adaptations in northern regions. Biol. Rev. 2009, 84, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Alahuhta, J.; Heino, J.; Luoto, M. Climate change and the future distribution of aquatic macrophytes across boreal catchments. J. Biogeogr. 2011, 38, 383–393. [Google Scholar] [CrossRef]
- Hildrew, A.G. Freshwater Acidification: Natural History, Ecology and Environmental Policy; International Ecology Institute: Oldendorf, Germany, 2018; ISBN 9783946729273. [Google Scholar]
- Murray, J. British Antarctic Expedition 1907–9, under the Command of Sir E.H. Shackleton. Reports on the Scientific Investigations. Volume 1 Biology; Willian Heinemann: London, UK, 1910; p. 105. [Google Scholar] [CrossRef]
- Laybourn-Parry, J.; Wadham, J.L. Antarctic Lakes; Oxford University Press: Oxford, UK, 2014; pp. 1–215. ISBN 978-0199670505. [Google Scholar]
- Roberts, D.; McMinn, A. A diatom-based palaeosalinity history of Ace Lake, Vestfold Hills, Antarctica. Holocene 1999, 9, 401–408. [Google Scholar] [CrossRef]
- Hawes, I.; Howard-Williams, C.; Fountain, A. Ice-Based freshwater ecosystems. In Polar Lakes and Rivers—Arctic and Antarctic Aquatic Ecosystems; Vincent, W.F., Laybourn-Parry, J., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 103–118. [Google Scholar] [CrossRef]
- Siegert, M.J. A 60-year international history of Antarctic subglacial lake exploration. In Exploration of Subsurface Antarctica: Uncovering Past Changes and Modern Processes; Siegert, M.J., Jamieson, S.S.R., White, D.A., Eds.; Geological Society: London, UK, 2018; Volume 461, pp. 7–22. [Google Scholar] [CrossRef]
- Christner, B.C.; Priscu, J.C.; Achberger, A.M.; Barbante, C.; Carter, S.P.; Christianson, K.; Michaud, A.B.; Mikucki, J.A.; Mitchell, A.C.; Skidmore, M.L.; et al. A microbial ecosystem beneath the West Antarctic ice sheet. Nature 2014, 512, 310–313. [Google Scholar] [CrossRef]
- Howard-Williams, C.; Hawes, I.; Doran, P.; Siegert, M.; Camacho, A.; Kaup, E. Diversity of Antarctic lakes, ponds and streams. Antarctic Environments Portal, Information Summaries. 2019. Available online: https://www.environments.aq/information-summaries/diversity-of-antarctic-lakes-ponds-and-streams/ (accessed on 15 January 2020).
- Obryk, M.K.; Doran, P.T.; Priscu, J.C. Prediction of ice-free conditions for a perennially ice-covered Antarctic Lake. J. Geophys. Res. Earth Surf. 2019, 124, 686–694. [Google Scholar] [CrossRef]
- Quayle, W.C.; Peck, L.S.; Peat, H.; Ellis-Evans, J.C.; Harrigan, P.R. Extreme responses to climate change in Antarctic lakes. Science 2002, 295, 645. [Google Scholar] [CrossRef]
- Gibson, J.A.; Bayly, I.A.E. New insights into the origins of crustaceans of Antarctic lakes. Antarct. Sci. 2007, 19, 157–163. [Google Scholar] [CrossRef]
- Vyverman, W.; Verleyen, E.; Wilmotte, A.; Hodgson, D.A.; Willems, A.; Peeters, K.; Van de Vijver, B.; De Wever, A.; Leliaert, F.; Sabbe, K. Evidence for widespread endemism among Antarctic microorganisms. Polar Sci. 2008, 4, 103–113. [Google Scholar] [CrossRef]
- Pessi, I.G.; Lara, Y.; Durieu, B.; Maalouf, P.D.C.; Verleyen, E.; Wilmotte, A. Community structure and distribution of benthic cyanobacteria in Antarctic lacustrine microbial mats. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Jung, P.; Briegel-Williams, L.; Schermer, M.; Büdel, B. Strong in combination: Polyphasic approach enhances arguments for cold-assigned cyanobacterial endemism. MicrobiologyOpen 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Strunecky, O.; Elster, J.; Komárek, J. Molecular clock evidence for survival of Antarctic cyanobacteria (Oscillatoriales, Phormidium autumnale) from Paleozoic times. FEMS Microbiol. Ecol. 2012, 82, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Taton, A.; Grubisic, S.; Balthazart, P.; Hodgson, D.A.; Laybourn-Parry, J.; Wilmotte, A. Biogeographical distribution and ecological range of benthic cyanobacteria in East Antarctic lakes. FEMS Microbiol. Ecol. 2006, 57, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Broady, P.A. Diversity, distribution and dispersal of Antarctic terrestrial algae. Biodiv. Conserv. 1996, 5, 1307–1335. [Google Scholar] [CrossRef]
- Verleyen, E.; Vyverman, W.; Sterken, M.; Hodgson, D.A.; De Wever, A.; Juggins, S.; Van de Vijver, B.; Jones, V.J.; Vanormelingen, P.; Roberts, D.; et al. The importance of dispersal related and local factors in shaping the taxonomic structure of diatom metacommunities. Oikos 2009, 118, 1239–1249. [Google Scholar] [CrossRef]
- Karanovic, T.; Gibson, J.; Hawes, I.; Andersen, D.; Stevens, M. Three new species of Diacyclops (Copepods: Cyclopoida) from continental Antarctica. Antarct. Sci. 2014, 26, 250–260. [Google Scholar] [CrossRef]
- Chown, S.L.; Huiskes, A.H.L.; Gremmen, N.J.M.; Lee, J.E.; Terauds, A.; Crosbie, K.; Frenot, Y.; Hughes, K.A.; Imura, S.; Kiefer, K.; et al. Continent-wide risk assessment for the establishment of nonindigenous species in Antarctica. Proc. Natl. Acad. Sci. USA 2012, 109, 4938–4943. [Google Scholar] [CrossRef]
- Li, S.-P.; Ochyra, R.; Wu, P.-C.; Seppelt, R.D.; Cai, M.-H.; Wang, H.-Y.; Li, C.-S. Drepanocladus longifolius (Amblystegiaceae), an addition to the moss flora of King George Island, South Shetland Islands, with a review of Antarctic benthic mosses. Polar Biol. 2009, 32, 1415–1425. [Google Scholar] [CrossRef]
- Velasco-Castrillón, A.; Gibson, J.A.; Stevens, M.I. A review of current Antarctic limno-terrestrial microfauna. Polar Biol. 2014, 37, 1517–1531. [Google Scholar] [CrossRef]
- Fountain, A.G.; Levy, J.S.; Goosefff, M.N.; Van Horn, D. The McMurdo Dry Valleys: a landscape on the threshold of change. Geomorphology 2014, 225, 25–35. [Google Scholar] [CrossRef]
- Hawes, I.; Sumner, D.Y.; Andersen, D.T.; Mackey, T.J. Legacies of recent environmental change in the benthic communities of Lake Joyce, a perennially ice covered, Antarctic lake. Geobiology 2011, 9, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Jungblut, A.D.; Hawes, I. Using Captain Scott’s Discovery specimens to unlock the past: Has Antarctic cyanobacterial diversity changed over the last 100 years? Proc. Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- Naselli-Flores, L. Mediterranean climate and eutrophication of reservoirs: Limnological skills to improve management. In Eutrophication: Causes, Consequences and Control; Ansari, A., Singh, G.S., Lanza, G., Rast, W., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 131–142. [Google Scholar] [CrossRef]
- Bonada, N.; Resh, V.H. Mediterranean-climate streams and rivers: Geographically separated but ecologically comparable freshwater systems. Hydrobiologia 2013, 719, 1–29. [Google Scholar] [CrossRef]
- Cid, N.; Bonada, N.; Carlson, S.M.; Grantham, T.E.; Gasith, A.; Resh, V.H. High Variability Is a Defining Component of Mediterranean-Climate Rivers and Their Biota. Water 2017, 9, 52. [Google Scholar] [CrossRef]
- Carrizo, S.F.; Lengyel, S.; Kapusi, F.; Szabolcs, M.; Kasperidus, H.D.; Scholz, M.; Markovic, D.; Freyhof, J.; Cid, N.; Cardoso, A.C.; et al. Critical catchments for freshwater biodiversity conservation in Europe: identification, prioritisation and gap analysis. J. Appl. Ecol. 2017, 54, 1209–1218. [Google Scholar] [CrossRef]
- Naselli-Flores, L. Man-made lakes in Mediterranean semi-arid climate: The strange case of Dr Deep Lake and Mr Shallow Lake. Hydrobiologia 2003, 506, 13–21. [Google Scholar] [CrossRef]
- Ferreira, M.; Beja, P. Mediterranean amphibians and the loss of temporary ponds: Are there alternative breeding habitats? Biol. Cons. 2013, 165, 179–186. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Marrone, F. Different invasibility of permanent and temporary water bodies in a semi-arid Mediterranean island. Inland Waters 2019, 9, 411–421. [Google Scholar] [CrossRef]
- Barone, R.; Castelli, G.; Naselli-Flores, L. Red sky at night cyanobacteria delight: The role of climate in structuring phytoplankton assemblages in a shallow Mediterranean lake (Biviere di Gela, southeastern Sicily). Hydrobiologia 2010, 639, 43–53. [Google Scholar] [CrossRef]
- Tockner, K.; Ward, J.V.; Arscott, D.B.; Edwards, P.J.; Kollmann, J.; Gurnell, A.M.; Petts, G.E.; Maiolini, B. The Tagliamento River: A model ecosystem of European importance. Aquat. Sci. 2003, 65, 239–253. [Google Scholar] [CrossRef]
- Gallart, F.; Cid, N.; Latron, J.; Llorens, P.; Bonada, N.; Jeuffroy, J.; Jiménez-Argudo, S.-M.; Vega, R.-M.; Solà, C.; Soria, M.; et al. TREHS: An open-access software tool for investigating and evaluating temporary river regimes as a first step for their ecological status assessment. Sci. Total Environ. 2017, 607–608, 519–540. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, J.D.; Bogan, M.T.; Bonada, N.; Rios-Touma, B.; Lytle, D.A. Seasonality and predictability shape temporal species diversity. Ecology 2017, 98, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Sabater, S.; Timoner, X.; Borrego, C.; Acuña, V. Stream biofilm responses to flow intermittency: From cells to ecosystems. Front. Environ. Sci. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Hershkovitz, Y.; Gasith, A. Resistance, resilience, and community dynamics in mediterranean-climate streams. Hydrobiologia 2012, 719, 59–75. [Google Scholar] [CrossRef]
- Bonada, N.; Dolédec, S.; Statzner, B. Taxonomic and biological trait differences of stream macroinvertebrate communities between mediterranean and temperate regions: Implications for future climatic scenarios. Glob. Chang. Biol. 2007, 13, 1658–1671. [Google Scholar] [CrossRef]
- Kerezsy, A.; Gido, K.; Magalhaes, M.F.; Skelton, P.H. The biota of intermittent rivers and ephemeral streams: Fishes. In Intermittent Rivers and Ephemeral Streams; Datry, T., Bonada, N., Boulton, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 273–298. [Google Scholar] [CrossRef]
- Múrria, C.; Bonada, N.; Vellend, M.; Zamora-Muñoz, C.; Alba-Tercedor, J.; Sainz-Cantero, C.E.; Garrido, J.; Acosta, R.; El Alami, M.; Barquín, J.; et al. Local environment rather than past climate determines community composition of mountain stream macroinvertebrates across Europe. Mol. Ecol. 2017, 26, 6085–6099. [Google Scholar] [CrossRef]
- Soria, M.; Leigh, C.; Datry, T.; Bini, L.M.; Bonada, N. Biodiversity in perennial and intermittent rivers: A meta-analysis. Oikos 2017, 126, 1078–1089. [Google Scholar] [CrossRef]
- Soria, M.; Gutiérrez-Cánovas, C.; Bonada, N.; Acosta, R.; Rodríguez-Lozano, P.; Fortuño, P.; Burgazzi, G.; Vinyoles, D.; Gallart, F.; Latron, J.; et al. Natural disturbances can produce misleading bioassessment results: Identifying metrics to detect anthropogenic impacts in intermittent rivers. J. Appl. Ecol. 2019, in press. [Google Scholar] [CrossRef]
- Munné, A.; Prat, N. Effects of Mediterranean climate annual variability on stream biological quality assessment using macroinvertebrate communities. Ecol. Indic. 2011, 11, 651–662. [Google Scholar] [CrossRef]
- Fritz, K.; Cid, N.; Autrey, B. Governance, Legislation, and Protection of Intermittent Rivers and Ephemeral Streams. In Intermittent Rivers and Ephemeral Streams; Datry, T., Bonada, N., Boulton, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 477–507. [Google Scholar] [CrossRef]
- Millán, A.; Velasco, J.; Gutiérrez-Cánovas, C.; Arribas, P.; Picazo, F.; Sánchez-Fernández, D.; Abellán, P. Mediterranean saline streams in southeast Spain: What do we know? J. Arid Environ. 2011, 75, 1352–1359. [Google Scholar] [CrossRef]
- Arribas, P.; Abellán, P.; Velasco, J.; Bilton, D.T.; Millán, A.; Sánchez-Fernández, D. Evaluating drivers of vulnerability to climate change: A guide for insect conservation strategies. Glob. Chang. Biol. 2012, 18, 2135–2146. [Google Scholar] [CrossRef]
- Döll, P.; Schmied, H.M. How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A global-scale analysis. Environ. Res. Lett. 2012, 7, 014037. [Google Scholar] [CrossRef]
- Datry, T.; Bonada, N.; Heino, J. Towards understanding the organisation of metacommunities in highly dynamic ecological systems. Oikos 2016, 125, 149–159. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Barone, R. Phytoplankton dynamics and structure: A comparative analysis in natural and man-made water bodies of different trophic state. Hydrobiologia 2000, 438, 65–74. [Google Scholar] [CrossRef]
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Nõges, T.; Nõges, P.; Attayde, J.L.; Zohary, T.; Coppens, J.; et al. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to change in water level and related changes in salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Barone, R. Mixotrophic phytoplankton dynamics in a shallow Mediterranean water body: How to make a virtue out of necessity. Hydrobiologia 2019, 831, 33–41. [Google Scholar] [CrossRef]
- García-Berthou, E.; Moreno-Amich, R. Introduction of exotic fish into a Mediterranean lake over a 90-year period. Arch. Hydrobiol. 2000, 149, 271–284. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, C.; Díaz-Paniagua, C.; Serrano, L.; Florencio, M.; Portheault, A. Mediterranean temporary ponds as amphibians breeding habitats: The importance of preserving pond networks. Aquat. Ecol. 2009, 43, 1179–1191. [Google Scholar] [CrossRef]
- Griffiths, R.A. Temporary ponds as amphibian habitat. Aquat. Conserv. Mar. Freshw. Ecosys. 1997, 7, 119–126. [Google Scholar] [CrossRef]
- Incagnone, G.; Marrone, F.; Barone, R.; Robba, L.; Naselli-Flores, L. How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia 2015, 750, 103–123. [Google Scholar] [CrossRef]
- Boix, D.; Kneitel, J.; Robson, B.J.; Duchet, C.; Zúñiga, L.; Day, J.; Gascón, S.; Sala, J.; Quintana, X.D.; Blaustein, L. Invertebrates of temporary ponds in Mediterranean climates. In Invertebrates in Freshwater Wetlands. An International Perspective on Their Ecology; Batzer, D., Boix, D., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 141–189. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Termine, R.; Barone, R. Phytoplankton colonization patterns. Is species richness depending on distance among freshwaters and on their connectivity? Hydrobiologia 2016, 764, 103–113. [Google Scholar] [CrossRef]
- Stoch, F.; Korn, M.; Turki, S.; Naselli-Flores, L.; Marrone, F. The role of spatial environmental factors as determinants of large branchiopod distribution in Tunisian temporary ponds. Hydrobiologia 2016, 782, 37–51. [Google Scholar] [CrossRef]
- Sahuquillo, M.; Miracle, M.R. The role of historic and climatic factors in the distribution of crustacean communities in Iberian Mediterranean ponds. Freshw. Biol. 2013, 58, 1251–1266. [Google Scholar] [CrossRef]
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar]
- Bolgovics, A.; Viktoria, B; Várbíró, G.; Krasznai, E.A.; Ács, É.; Kiss, K.T.; Borics, G. Groups of small lakes maintain larger microalgal diversity than large ones. Sci. Total Environ. 2019, 678, 162–172. [Google Scholar] [CrossRef]
- Bagella, S.; Gascón, S.; Caria, M.C.; Sala, J.; Boix, D. Cross-taxon congruence in Mediterranean temporary wetlands: Vascular plants, crustaceans, and coleopterans. Community Ecol. 2011, 12, 40–50. [Google Scholar] [CrossRef]
- Marrone, F.; Alfonso, G.; Stoch, F.; Pieri, V.; Alonso, M.; Dretakis, M.; Naselli-Flores, L. An account on the non-malacostracan crustacean fauna from the inland waters of Crete, Greece, with the synonymization of Arctodiaptomus piliger Brehm, 1955 with Arctodiaptomus alpinus (Imhof, 1885). Limnetica 2019, 38, 167–187. [Google Scholar] [CrossRef]
- Marrone, F.; Barone, R.; Naselli-Flores, L. Cladocera (Branchiopoda: Anomopoda, Ctenopoda and Onychopoda) from Sicilian inland waters: An updated inventory. Crustaceana 2005, 78, 1025–1039. [Google Scholar] [CrossRef]
- Marrone, F.; Castelli, G.; Barone, R.; Naselli-Flores, L. Ecology and distribution of calanoid copepods in Sicilian inland waters (Italy). Verh. Internat. Verein. Limnol. 2006, 29, 2150–2156. [Google Scholar] [CrossRef]
- Bagella, S.; Caria, M.C. Diversity and ecological characteristics of vascular flora in Mediterranean temporary pools. C. R. Biol. 2012, 335, 69–76. [Google Scholar] [CrossRef]
- Sciandrello, S.; Privitera, M.; Puglisi, M.; Minissale, P. Diversity and spatial patterns of plant communities in volcanic temporary ponds of Sicily (Italy). Biologia 2016, 71, 793–803. [Google Scholar] [CrossRef]
- Cogoni, A.; Filippino, G.; Marignani, M. Small-scale pattern of bryoflora in Mediterranean temporary ponds: Hints for monitoring. Hydrobiologia 2016, 782, 81–95. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Barone, R. Phytoplankton dynamics in permanent and temporary Mediterranean waters: Is the game hard to play because of hydrological disturbance? Hydrobiologia 2012, 698, 147–159. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 1959, 93, 145–159. [Google Scholar] [CrossRef]
- Zohary, T.; Yacobi, Y.Z.; Alster, A.; Fishbein, T.; Lippman, S.; Tibor, G. Phytoplankton. In Lake Kinneret: Ecology and Management; Chapter 10; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Heidelberg, Germany, 2014; pp. 161–190. [Google Scholar] [CrossRef]
- Nishri, A.; Leibovici, E. External sources of nutrients. In Lake Kinneret: Ecology and Management; Chapter 19; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Heidelberg, Germany, 2014; pp. 329–346. [Google Scholar] [CrossRef]
- Zohary, T.; Sukenik, A.; Berman, T.; Nishri, A. Lake Kinneret: Ecology and Management; Springer: Heidelberg, Germany, 2014; p. 683. [Google Scholar] [CrossRef]
- Ostrovsky, I.; Goren, M.; Shapiro, J. Fish Biology and Ecology. In Lake Kinneret: Ecology and Management; Chapter 16; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Heidelberg, Germany, 2014; pp. 273–292. [Google Scholar] [CrossRef]
- Tsurnamal, M. The biology and ecology of the blind prawn, Typhlocaris galilea Calman (Decapoda, Caridea). Crustaceana 1978, 34, 195–213. [Google Scholar] [CrossRef]
- Berman, T.; Zohary, T.; Nishri, A.; Sukenik, A. General background. In Lake Kinneret: Ecology and Management; Chapter 1; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Heidelberg, Germany, 2014; pp. 1–15. [Google Scholar] [CrossRef]
- Markel, D.; Shamir, U.; Green, P. Operational Management of Lake Kinneret and its watershed. In Lake Kinneret: Ecology and Management; Chapter 31; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Heidelberg, Germany, 2014; pp. 541–560. [Google Scholar] [CrossRef]
- Sukenik, A.; Zohary, T.; Markel, D. The monitoring program. In Lake Kinneret: Ecology and Management; Chapter 32; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Heidelberg, Germany, 2014; pp. 561–575. [Google Scholar] [CrossRef]
- Zohary, T.; Ostrovsky, I. Ecological impacts of excessive water level fluctuations in stratified freshwater lakes. Inland Water 2011, 1, 47–59. [Google Scholar] [CrossRef]
- Dreizin, Y.; Tenne, A.; Hoffman, D. Integrating large scale seawater desalination plants within Israel’s water supply system. Desalination 2008, 220, 132–149. [Google Scholar] [CrossRef]
- Rimmer, A.; Gal, G.; Opher, T.; Lechinsky, Y.; Yacobi, Y.Z. Mechanisms of long-term variations of the thermal structure in a warm lake. Limnol. Oceanogr. 2011, 56, 974–988. [Google Scholar] [CrossRef]
- Pollingher, U. Phytoplankton periodicity in a subtropical lake (Lake Kineret, Israel). Hydrobiologia 1986, 138, 127–138. [Google Scholar] [CrossRef]
- Zohary, T. Changes to the phytoplankton assemblage of Lake Kinneret after decades of a predictable, repetitive pattern. Freshw. Biol. 2004, 49, 1355–1371. [Google Scholar] [CrossRef]
- Zohary, T.; Sukenik, A.; Nishri, A. Overview. In Lake Kinneret: Ecology and Management; Chapter 37; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Heidelberg, Germany, 2014; pp. 657–671. [Google Scholar] [CrossRef]
- Sukenik, A.; Hadas, O.; Kaplan, A. Cyanobacteria. In Lake Kinneret: Ecology and Management; Chapter 12; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Heidelberg, Germany, 2014; pp. 213–226. [Google Scholar] [CrossRef]
- Zohary, T.; Alster, A.; Hadas, O.; Obertegger, U. There to stay: Opportunistic and versatile filamentous green alga Mougeotia in Lake Kinneret, Israel. Hydrobiologia 2019, 831, 87–100. [Google Scholar] [CrossRef]
- Heller, J.; Dolev, A.; Zohary, T.; Gal, G. Invasion dynamics of the snail Pseudoplotia scabra in Lake Kinneret. Biological Invasions 2014, 16, 7–12. [Google Scholar] [CrossRef]
- Moss, B. The kingdom of the shores: Achievement of good ecological potential in reservoirs. Freshw. Rev. 2008, 1, 29–42. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Barone, R. Water-level fluctuations in Mediterranean reservoirs: Setting a dewatering threshold as a management tool to improve water quality. Hydrobiologia 2005, 548, 85–99. [Google Scholar] [CrossRef]
- Naselli-Flores, L. Morphological analysis of phytoplankton as a tool to assess ecological state of aquatic ecosystems: The case of Lake Arancio, Sicily, Italy. Inland Waters 2014, 4, 15–26. [Google Scholar] [CrossRef]
- Gownaris, N.J.; Rountos, K.J.; Kaufman, L.; Kolding, J.; Lwiza, K.M.M.; Pikitch, E.K. Water level fluctuations and the ecosystem functioning of lakes. J. Great Lakes Res. 2018, 44, 1154–1163. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Barone, R. Steady-state assemblages in a Mediterranean hypertrophic reservoir. The role of Microcystis ecomorphological variability in maintaining an apparent equilibrium. Hydrobiologia 2003, 502, 133–143. [Google Scholar] [CrossRef]
- Moss, B. The Water Framework Directive: Total environment or political compromise? Sci. Total Environ. 2008, 400, 32–41. [Google Scholar] [CrossRef]
- Oertli, B. Editorial: Freshwater biodiversity conservation: The role of artificial ponds in the 21st century. Aquat. Conserv. Mar. Freshw. Ecosys. 2018, 28, 264–269. [Google Scholar] [CrossRef]
- Junk, W.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Aselmann, I.; Crutzen, P.J. Global distribution of natural freshwater wetlands and rice paddies, their net primary productivity, seasonality and possible methane emissions. J. Atmos. Chem. 1989, 8, 307–358. [Google Scholar] [CrossRef]
- Fraser, L.H.; Keddy, P.A. The World’s Largest Wetlands: Ecology and Conservation. Ecoscience 2005, 13, 559–560. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Junk, W.J. The importance of stream-wetland-systems for biodiversity: A tropical perspective. In Biodiversity in Wetlands: Assessment, Function and Conservation; Gopal, B., Junk, W.J., Davies, J.A., Eds.; Backhuys: Leiden, The Netherlands, 2000; pp. 11–34. ISBN 90-5782-059-5. [Google Scholar]
- Reis, V.; Hermoso, V.; Hamilton, S.K.; Bunn, S.E.; Fluet-Chouinard, E.; Venables, B.; Linke, S. Characterizing seasonal dynamics of Amazonian wetlands for conservation and decision making. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1073–1082. [Google Scholar] [CrossRef]
- Ramsar Convention Secretariat. The Ramsar Convention Manual: A Guide to the Convention on Wetlands (Ramsar, Iran, 1971), 6th ed.; Ramsar Convention Secretariat: Gland, Switzerland, 2013. [Google Scholar]
- Sieben, E.J.J.; Khubeka, S.P.; Sithole, S.; Job, N.M.; Kotze, D.C. The classification of wetlands: Integration of top-down and bottom-up approaches and their significance for ecosystem service determination. Wetl. Ecol. Manag. 2017, 26, 441–458. [Google Scholar] [CrossRef]
- Gumbricht, T.; Roman-Cuesta, R.M.; Verchot, L.; Herold, M.; Wittmann, F.; Householder, F.; Herold, N.; Murdiyarso, D. An expert system model for mapping tropical wetlands and peatlands reveals South America as the largest contributor. Glob. Chang. Biol. 2017, 23, 3581–3599. [Google Scholar] [CrossRef]
- Page, S.E.; Rieley, J.O.; Banks, C.J. Global and regional importance of the tropical peatland carbon pool. Glob. Chang. Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Nilssen, J.P. Tropical lakes—Functional ecology and future development. The need for a process-orientated approach. Hydrobiologia 1984, 113, 231–242. [Google Scholar] [CrossRef]
- Jenkins, M.W.; McCord, S.; Edebe, J. Sustaining Lake Levels in Lake Nakuru, Kenya: Development of a Water Balance Mode for Decision Making. Research Brief 09-05-SUMAWA; University of California Global Livestock Collaborative Research Support Program: Davis, CA, USA, 2009; p. 4. [Google Scholar]
- Junk, W.J. Long-term environmental trends and the future of tropical wetlands. Environ. Conserv. 2002, 29, 414–435. [Google Scholar] [CrossRef]
- Gopal, B.; Junk, W.J.; Finlayson, C.M.; Breen, C.M. Present state and future of tropical wetlands. In Aquatic Ecosystems; Polunin, N., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 142–154. ISBN 9780521833271. [Google Scholar]
- Oviedo, G.; Kenza, A.M. Indigenous Peoples, Local Communities and Wetland Conservation; Ramsar Convention Secretariat: Gland, Switzerland, 2018. [Google Scholar]
- Clarkson, R.B.E.; Ausseil, A.; Gerbeaux, P. Wetland ecosystem services. In Ecosystem Services in New Zealand; Dymond, J.R., Ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2014. [Google Scholar]
- Mitsch, W.J.; Blanca Bernal, B.; Hernandez, M.E. Ecosystem services of wetlands. IJBESM 2015, 11, 1–4. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Knight, R.L. Treatment Wetlands; Lewis: New York, NY, USA, 1996; ISBN 978-1-56670-526-4. [Google Scholar]
- Mitsch, W.J.; Day, J.W., Jr.; Gilliam, J.W.; Groffman, P.M.; Hey, D.L.; Randall, G.W.; Wang, N. Reducing nitrogen loading to the Gulf of Mexico from the Mississippi River Basin: Strategies to counter a persistent ecological problem. BioScience 2001, 51, 373–388. [Google Scholar] [CrossRef]
- Day, J.W.; Boesch, D.F.; Clairain, E.J.; Kemp, G.P.; Laska, S.B.; Mitsch, W.J.; Orth, K.; Mashriqui, H.S.; Reed, D.J.; Shabman, L.A.; et al. Restoration of the Mississippi Delta: Lessons from Hurricanes Katrina and Rita. Science 2007, 315, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Jackson, R. Hydrology of wetlands. In Freshwaters of New Zealand; Harding, J., Mosley, P., Pearson, C., Sorrell, B., Eds.; Caxton Press for the New Zealand Hydrological Society and New Zealand Limnological Society: Christchurch, New Zealand, 2004; pp. 1–20. ISBN 0-476-00708-9. [Google Scholar]
- Béné, C. Contribution of inland fisheries to rural livelihoods and food security in Africa: An overview. In Freshwater Ecoregions of Current State of Knowledge Regarding the World’s Wetlands 165 Africa and Madagascar: A Conservation Assessment; Thieme, M.L., Abell, R., Stiassny, M.L.J., Skelton, P., Eds.; Island Press: Washington, DC, USA, 2005; pp. 6–11. ISBN 9781559633659. [Google Scholar]
- Matthews, E.; Fung, I. Methane emissions from natural wetlands: Global distribution, area, and environmental characteristics of sources. Glob. Biogeochem. Cycles 1987, 1, 61–86. [Google Scholar] [CrossRef]
- Petrescu, A.M.; van Beek, L.; van Huissteden, J.; Prigent, C.; Sachs, T.; Corradi, C.; Parmentier, F.J.V.; Dolman, A.J. Modelling regional to global CH4 emissions from boreal and arctic wetlands. Glob. Biogeochem. Cycles 2010, 24, GB4009. [Google Scholar] [CrossRef]
- Petrescu, A.M.; Lohila, A.; Tuovinen, J.; Baldocchi, D.D.; Desai, A.R.; Roulet, N.T.; Vesala, T.; Dolman, A.J.; Oechel, W.C.; Marcolla, B.; et al. The uncertain climate footprint of wetlands under human pressure. Proc. Natl. Acad. Sci. USA 2015, 112, 4594–4599. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Lähteenoja, O.; Ruokoleinen, K.; Schulman, L.; Oinonen, M. Amazonian peatlands: An ignored C sink and potential source. Glob. Chang. Biol. 2009, 15, 2311–2320. [Google Scholar] [CrossRef]
- Sjögersten, S.; Black, C.R.; Evers, S.; Hoyos-Santillan, J.; Wright, E.L.; Turner, B.L. Tropical wetlands: A missing link in the global carbon cycle? Glob. Biogeochem. Cycles 2014, 28, 1371–1386. [Google Scholar] [CrossRef]
- Gumbricht, T. Mapping Global Tropical Wetlands from Earth Observing Satellite Imagery; Working Paper 103; CIFOR: Bogor, Indonesia, 2012. [Google Scholar] [CrossRef]
- Wohl, E.; Barros, A.; Brunsell, N.; Chappell, N. The hydrology of the humid tropics. Nat. Clim. Chang. 2012, 2, 655–662. [Google Scholar] [CrossRef]
- Castello, L.; Macedo, M.N. Large-scale degradation of Amazonian freshwater ecosystems. Glob. Chang. Biol. 2016, 22, 990–1007. [Google Scholar] [CrossRef]
- Mergou, F.E.; Lazaridou-Dimitriadou, M.; Albanakis, K. The effets of dams on rivers “continuum”. Conference paper. In Proceedings of the Protection and Restoration of the Environment XI, Thessaloniki, Greece, 3–6 July 2012. [Google Scholar]
- Childs, M. Literature Survey: The Impacts of Dams on River Channel Geomorphology; MSc research at The University of Hull; The University of Hull: Hull, UK, 2010. [Google Scholar]
- Winton, R.S.; Calamita, E.; Wehrli, B. Reviews and syntheses: Dams, water quality and tropical reservoir stratification. Biogeosciences 2019, 16, 1657–1671. [Google Scholar] [CrossRef]
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A global boom in hydropower dam construction. Aquat. Sci. 2014, 77, 161–170. [Google Scholar] [CrossRef]
- Anderson, E.P.; Jenkins, C.N.; Heilpern, S.; Maldonado-Ocampo, J.A.; Carvajal-Vallejos, F.M.; Encalada, A.C.; Rivadeneira, J.F.; Hidalgo, M.; Cañas, C.M.; Ortega, H.; et al. Fragmentation of Andes-to-Amazon connectivity by hydropower dams. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.C.; Peres, C.A.; Fearnside, P.M.; Schneider, M.; Zuanon, J.A.S. Hydropower and the future of Amazonian biodiversity. Biodivers. Conserv. 2016, 25, 451–466. [Google Scholar] [CrossRef]
- Winemiller, K.O.; McIntyre, P.B.; Castello, L.; Fluet-Chouinard, E.; Giarrizzo, T.; Nam, S.; Baird, I.G.; Darwall, W.; Lujan, N.K.; Harrison, I.; et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 2016, 351, 128–129. [Google Scholar] [CrossRef]
- Roberts, T.R. On the river of no returns: Thailand’s Pak Mun Dam and its fish ladder. Nat. Hist. Bull. Siam. Soc. 2001, 49, 189–230. [Google Scholar]
- Dudgeon, D. Threats to freshwater biodiversity globally and in the Indo-Burma Biodiversity Hotspot. In The Status and Distribution of Freshwater Biodiversity in Indo-Burma; Allen, D.J., Smith, K.G., Darwall, W.R.T., Eds.; IUCN: Cambridge, UK; Gland, Switzerland, 2012; pp. 1–28. ISBN 978-2-8317-1424-0. [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystem and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005; ISBN 9781569735886. [Google Scholar]
- Lodge, D.M. Lakes. In Global Biodiversity in a Changing Environment: Scenarios for the 21st Century; Chapin, F.S., III, Sala, O.E., Huber-Sannwald, E., Eds.; Springer: New York, NY, USA, 2001; pp. 277–313. ISBN 978-1-4613-0157-8. [Google Scholar]
- Craig, J.F. Human induced changes in the composition of fish communities in the African Great Lakes. Rev. Fish Biol. Fisher. 1992, 2, 93–124. [Google Scholar] [CrossRef]
- Lowe-McConnell, R.H. Fish faunas of the African Great Lakes: Origins, diversity, and vulnerability. Conserv. Biol. 1993, 7, 634–643. [Google Scholar] [CrossRef]
- Vimos, D.J.; Encalada, A.C.; Ríos-Touma, B.; Suárez, E.; Prat, N. Effects of exotic trout on benthic communities in high-Andean tropical streams. Freshw. Sci. 2015, 34, 770. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Carlsson, N.O.L.; Brönmark, C.; Hansson, L. Invading herbivory: The golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 2004, 85, 1575–1580. [Google Scholar] [CrossRef]
- Global Invasive Species Database. Species Profile: Eichhornia crassipes. 2019. Available online: http://www.iucngisd.org/gisd/species.php?sc=70 (accessed on 3 September 2019).
- Dos Santos, J.R.; Galvão, L.S.; Oliveira, L.E.; de Aragão, C. Remote sensing of amazonian forests: Monitoring structure, phenology and responses to environmental changes. Rev. Bras. Cartogr. 2014, 3, 1413–1436. [Google Scholar]
- Miettinen, J.; Hooijer, A.; Shi, C.; Tollenaar, D.; Vernimmen, R.; Liew, S.C.; Malins, C.; Page, S.E. Extent of industrial plantations on Southeast Asian peatlands in 2010 with analysis of historical expansion and future projections. GCB Bioenergy 2012, 4, 908–918. [Google Scholar] [CrossRef]
- Miettinen, J.; Shi, C.; Liew, S.C. Land cover distribution in the peatlands of Peninsular Malaysia, Sumatra and Borneo in 2015 with changes since 1990. Glob. Ecol. Conserv. 2016, 6, 67–78. [Google Scholar] [CrossRef]
- Langner, A.; Miettinen, J.; Siegert, F. Land cover change 2002–2005 in Borneo and the role of fire derived from MODIS imagery. Glob. Chang. Biol. 2007, 13, 2329–2340. [Google Scholar] [CrossRef]
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef]
- Harrison, M.E.; Ripoll Capilla, B.; Thornton, S.A.; Cattau, M.E.; Page, S.E. Impacts of the 2015 fire season on peat-swamp forest biodiversity in Indonesian Borneo. In Peatlands in Harmony—Agriculture, Industry & Nature: Oral Presentations, Proceedings of the 15th International Peat Congress, Sarawak, Malaysia, 15–19 August 2016; International Peat Society and Malaysian Peat Society: Kuching, Malaysia, 2016; pp. 713–717. [Google Scholar]
- Page, S.E.; Siegert, F.; Rieley, J.O.; Boehm, H.D.V.; Jaya, A.; Limin, S. The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 2002, 420, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Segah, H.; Harada, T.; Limin, S.; June, T.; Hirata, R.; Osaki, M. Carbon dioxide balance of a tropical peat swamp forest in Kalimantan, Indonesia. Glob. Chang. Biol. 2006, 13, 412–425. [Google Scholar] [CrossRef]
- Moore, S.; Evans, C.D.; Page, S.E.; Garnett, M.H.; Jones, T.G.; Freeman, C.; Hooijer, A.; Wiltshire, A.J.; Limin, S.H.; Gauci, V. Deep instability of deforested tropical peatlands revealed by fluvial organic carbon fluxes. Nature 2013, 493, 660–663. [Google Scholar] [CrossRef]
- Mitchell, S.A. The status of wetlands, threats and the predicted effect of global climate change: The situation in Sub-Saharan Africa. Aquat. Sci. 2013, 75, 95–112. [Google Scholar] [CrossRef]
- Thieme, M.L.; Lehner, B.; Abell, R.; Matthews, J. Exposure of Africa’s freshwater biodiversity to a changing climate. Conserv. Lett. 2010, 3, 324–331. [Google Scholar] [CrossRef]
- Barros, D.F.; Albernaz, A.L.M. Possible impacts of climate change on wetlands and its biota in the Brazilian Amazon. Braz. J. Biol. 2014, 74, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Grumbine, R.E.; Shrestha, A.; Eriksson, M.; Yang, X.; Wang, Y.; Wilkes, A. The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conserv. Biol. 2009, 23, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Gopal, B. Future of wetlands in tropical and subtropical Asia, especially in the face of climate change. Aquat. Sci. 2013, 75, 39–61. [Google Scholar] [CrossRef]
- Jafari, M.; Tavili, A.; Panahi, F.; Esfahan, E.Z.; Ghorbani, M. Characteristics of arid and desert ecosystems. In Reclamation of Arid Lands; Jafari, M., Tavili, A., Panahi, F., Esfahan, E.Z.M., Ghorbani, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 21–91. [Google Scholar] [CrossRef]
- Tydecks, L.; Jeschke, J.M.; Zarfl, C.; Böhning-Gaese, K.; Schütt, B.; Bremerich, V.; Tockner, K. Oases in the Sahara Desert—Linking Biological and Cultural Diversity. People Nat. 2020, in press. [Google Scholar]
- Holzapfel, C. Deserts. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2008; pp. 879–898. [Google Scholar]
- Fisher, S.G.; Gray, L.J.; Grimm, N.B.; Busch, D.E. Temporal succession in a desert stream ecosystem following flash flooding. Ecol. Monogr. 1982, 52, 93–110. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Cohen, Z. The effect of phosphate starvation on the lipid and fatty acid composition of the freshwater eustigmatophyte Monodus subterraneus. Phytochemistry 2006, 67, 696–701. [Google Scholar] [CrossRef]
- Grimm, N.B.; Petrone, K.C. Nitrogen fixation in a desert stream ecosystem. Biogeochemistry 1997, 37, 33–61. [Google Scholar] [CrossRef]
- Scott, J.T.; Marcarelli, A.M. Cyanobacteria in freshwater benthic environments. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer Science+Business Media: Dordrecht, The Netherlands, 2012; pp. 271–289. [Google Scholar] [CrossRef]
- Harms, T.K.; Sponseller, R.A.; Grimm, N.B. Desert Streams. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 871–879. ISBN 9780444520333. [Google Scholar]
- Saber, A.A.; Mareš, J.; Guella, G.; Anesi, A.; Štenclová, L.; Cantonati, M. Polyphasic approach to a characteristic Ulva population from a limno-rheocrenic, mineral (chloride, sodium, sulphate) spring in the Siwa Oasis (Western Desert of Egypt). Fottea 2018, 18, 227–242. [Google Scholar] [CrossRef]
- Green, A.J.; Jenkins, K.M.; Bells, D.; Morris, P.J.; Kingsford, R.T. The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshw. Biol. 2008, 53, 380–392. [Google Scholar] [CrossRef]
- Keleher, M.J.; Rader, R.B. Dispersal limitations and history explain community composition of metaphyton in desert springs of the Bonneville Basin, Utah: A multiscale analysis. Limnol. Oceanogr. 2008, 53, 1604–1613. [Google Scholar] [CrossRef]
- Foissner, W. Biogeography and dispersal of microorganisms: A review emphasizing protists. Acta Protozool. 2006, 45, 111–136. [Google Scholar]
- Witt, J.D.; Threloff, D.L.; Hebert, P.D. DNA barcoding reveals extraordinary cryptic diversity in an amphipod genus: Implications for desert spring conservation. Mol. Ecol. 2006, 15, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Sluys, R.; Grant, L.J.; Blair, D. Freshwater planarians from artesian springs in Queensland, Australia (Platyhelminthes, Tricladida, Paludicola). Contrib. Zool. 2007, 76, 9–19. [Google Scholar] [CrossRef]
- Brown, C.M. 1965. Freshwater algae of the Central Death Valley Desert. Ohio J. Sci. 1965, 65, 11–28. [Google Scholar]
- Shaaban, A.S.; Mansour, H.A.; Saber, A.A. Unveiling algal biodiversity of El-Farafra Oasis (Western Desert, Egypt) and potential relevance of its use in water bio-assessment: Special interest on springs and drilled wells. Egypt. J. Phycol. 2015, 16, 47–75. [Google Scholar]
- Garcia-Pichel, F.; Wade, B.D.; Farmer, J.D. Jet-suspended, calcite-ballasted cyanobacterial waterwarts in a desert spring. J. Phycol. 2002, 38, 420–428. [Google Scholar] [CrossRef]
- Saber, A.A.; Ballot, A.; Schneider, S.; Cantonati, M. Morphological and molecular features of a Chara vulgaris population from desert springs on the Sinai Peninsula (Springs of Moses, Egypt). Bot. Lett. 2018, 165, 77–89. [Google Scholar] [CrossRef]
- Dvořák, P.; Casamatta, D.A.; Hašler, P.; Jahodářová, E.; Norwich, A.R.; Poulíčková, A. Diversity of the cyanobacteria. In Modern topics in the phototrophic prokaryotes: Environmental and applied Aspects; Hallenbeck, P.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–46. [Google Scholar]
- Minckley, T.A.; Turner, D.S.; Weinstein, S.R. The relevance of wetland conservation in arid regions: A re-examination of vanishing communities in the American Southwest. J. Arid Environ. 2013, 88, 213–221. [Google Scholar] [CrossRef]
- Rossini, R.A.; Fensham, R.J.; Stewart-Koster, B.; Gotch, T.; Kennard, M.J. Biogeographical patterns of endemic diversity and its conservation in Australia’s artesian desert springs. Divers. Distrib. 2018, 24, 1199–1216. [Google Scholar] [CrossRef]
- Rossini, R.A.; Tibbetts, H.L.; Fensham, R.J.; Walter, G.H. Can environmental tolerances explain convergent patterns of distribution in endemic spring snails from opposite sides of the Australian arid zone? Aquat. Ecol. 2017, 51, 605–624. [Google Scholar] [CrossRef]
- Holste, D.R.; Inoue, K.; Lang, B.K.; Berg, D.J. Identification of microsatellite loci and examination of genetic structure for the endangered springsnails Juturnia kosteri and Pyrgulopsis roswellensis in the Chihuahuan Desert. Aquat. Conserv. Mar. Freshw. Ecosys. 2016, 26, 715–723. [Google Scholar] [CrossRef]
- Stanislawczyk, K.; Walters, A.D.; Haan, T.J.; Sei, M.; Lang, B.K.; Berg, D.J. Variation among macroinvertebrate communities suggests the importance of conserving desert springs. Aquat. Conserv. Mar. Freshw. Ecosys. 2018, 28, 944–953. [Google Scholar] [CrossRef]
- Murphy, N.P.; Pavlova, A.L.; Thompson, R.; Davis, J.; Sunnucks, P. Swimming through sand: Connectivity of aquatic fauna in deserts. Ecol. Evol. 2015, 5, 5252–5264. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.A.; Kouwets, F.A.C.; Haworth, E.Y.; Cantonati, M. A new Euastrum species (Conjugatophyceae, Streptophyta) from the Western Desert of Egypt. Cryptogam. Algol. 2018, 39, 215–226. [Google Scholar] [CrossRef]
- Navarro, L.M.; Fernández, N.; Guerra, C.; Guralnick, R.; Kissling, W.D.; Londoño, M.C.; Muller-Karger, F.; Turak, E.; Balvanera, P.; Costello, M.J.; et al. Monitoring biodiversity change through effective global coordination. Curr. Opin. Env. Sust. 2017, 29, 158–169. [Google Scholar] [CrossRef]
- Pereira, H.M.; Ferrier, S.; Walters, M.; Geller, G.N.; Jongman, R.H.; Scholes, R.J.; Bruford, M.W.; Brummitt, N.; Butchart, S.H.; Cardoso, A.C.; et al. Essential biodiversity variables. Science 2013, 339, 277–278. [Google Scholar] [CrossRef]
- Turak, E.; Harrison, I.; Dudgeon, D.; Abell, R.; Bush, A.; Darwall, W.; Finlayson, C.M.; Ferrier, S.; Freyhof, J.; Hermoso, V.; et al. Essential Biodiversity Variables for measuring change in global freshwater biodiversity. Biol. Conserv. 2017, 213, 272–279. [Google Scholar] [CrossRef]
- Kissling, W.D.; Ahumada, J.A.; Bowser, A.; Fernandez, M.; Fernández, N.; García, E.A.; Guralnick, R.P.; Isaac, N.J.; Kelling, S.; Los, W.; et al. Building essential biodiversity variables (EBV s) of species distribution and abundance at a global scale. Biol. Rev. 2018, 93, 600–625. [Google Scholar] [CrossRef]
- Jetz, W.; McGeoch, M.; Guralnick, R.; Ferrier, S.; Beck, J.; Costello, M.; Fernandez, M.; Geller, G.; Keil, P.; Cory Merow, C.; et al. Essential biodiversity variables for mapping and monitoring species populations. Nat. Ecol. Evol. 2019, 3. [Google Scholar] [CrossRef]
- Richardson, J.S.; Sato, T. Resource flows across freshwater-terrestrial boundaries and influence on processes linking adjacent ecosystems. Ecohydrology 2015, 8, 406–415. [Google Scholar] [CrossRef]
- Soininen, J.; Bartels, P.; Heino, J.; Luoto, M.; Hillebrand, H. Toward more integrated ecosystem research in aquatic and terrestrial environments. BioScience 2015, 65, 174–182. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Altermatt, F.; Finn, D.; Heino, J.; Olden, J.D.; Pauls, S.U.; Lytle, D.A. The role of dispersal in river network metacommunities: Patterns, processes, and pathways. Freshw. Biol. 2018, 63, 141–163. [Google Scholar] [CrossRef]
- Hughes, J.M.R.; Bond, H.; Knight, C.; Stanley, K. Challenges for Freshwater Ecosystems. In Water Science, Policy, and Management; Dadson, S.J., Garrick, D.E., Penning-Rowsell, C., Hall, J.W., Hope, R., Hughes, J., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Downing, J.A. Limnology and oceanography: Two estranged twins reuniting by global change. Inland Waters 2014, 4, 215–232. [Google Scholar] [CrossRef]
- Allan, J.D.; Flecker, A.S. Biodiversity conservation in running waters. BioScience 1993, 43, 32–43. [Google Scholar] [CrossRef]
- Stendera, S.; Adrian, R.; Bonada, N.; Cañedo-Argüelles, M.; Hugueny, B.; Januschke, K.; Plettenbuer, F.; Hering, D. Drivers and stressors of freshwater biodiversity patterns across different ecosystems and scales: A review. Hydrobiologia 2012, 696, 1–28. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Hawkins, C.P.; Kefford, B.J.; Schäfer, R.B.; Dyack, B.J.; Brucet, S.; Buchwalter, D.; Dunlop, J.; Frör, O.; Lazorchak, J.; et al. Saving freshwater from salts. Science 2016, 351, 914–916. [Google Scholar] [CrossRef]
- Kernan, M.; Battarbee, R.W.; Moss, B. Climate Change, Freshwater Ecosystems. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Richter, B.D.; Powell, E.M.; Lystash, T.; Faggert, M. Protection and Restoration of Freshwater Ecosystems. In Water Policy and Planning in a Variable and Changing Climate; Miller, K.A., Hamlet, A.F., Kenney, D.S., Redmond, K.T., Eds.; Taylor & Francis Group, LLC: London, UK, 2016; pp. 82–103. ISBN 9781138490864. [Google Scholar]
- Maliva, R.G. ASR and Aquifer Recharge Using Wells. In Anthropogenic Aquifer Recharge; Maliva, R.G., Ed.; Springer: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Poikane, S.; Kelly, M.G.; Cantonati, M. Benthic algal assessment of ecological status in European lakes and rivers: Challenges and opportunities. Sci. Total Environ. 2016, 568, 603–613. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Tilman, D.; Downing, J.A. Biodiversity and stability in grasslands. Nature 1994, 367, 363–365. [Google Scholar] [CrossRef]
- Covich, A.P.; Austen, M.C.; Bärlocher, F.; Chauvet, E.; Cardinale, B.J.; Biles, C.L.; Inchausti, P.; Dangles, O.; Solan, M.; Gessner, M.O.; et al. The role of biodiversity in the functioning of freshwater and marine benthic ecosystems. BioScience 2004, 54, 767–775. [Google Scholar] [CrossRef]
- Strayer, D.; Downing, J.A.; Haag, W.R.; King, T.L.; Layer, J.B.; Newton, T.J.; Nichols, S. Changing perspectives on pearly mussels, North America’s most imperiled animals. BioScience 2004, 54, 429–439. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Reconciliation ecology and the future of species diversity. Oryx 2003, 37, 194–205. [Google Scholar] [CrossRef]
- Palmer, M.A.; Hondula, K.L.; Koch, B.J. Ecological Restoration of Streams and Rivers: Shifting Strategies and Shifting Goals. Annu. Rev. Ecol. Evol. S. 2014, 45, 247–269. [Google Scholar] [CrossRef]
- Nilsson, C.; Polvi, L.E.; Gardeström, J.; Hasselquist, E.M.; Lind, L.; Sarneel, J.M. Riparian and in-stream restoration of boreal streams and rivers: Success or failure? Ecohydrology 2015, 8, 753–764. [Google Scholar] [CrossRef]
- Boon, P.J.; Davies, B.R.; Petts, G.E. Global Perspectives on River Conservation: Science, Policy and Practice; John Wiley: Chichester, UK, 2000; p. 548. ISBN 978-0-471-96062-1. [Google Scholar]
- Moss, B. Biodiversity in fresh waters: An issue of species preservation or system functioning? Environ. Conserv. 2000, 27, 1–4. [Google Scholar] [CrossRef]
- Herbert, M.E.; Mcintyre, P.B.; Doran, P.J.; Allan, J.D.; Abell, R. Terrestrial Reserve Networks Do Not Adequately Represent Aquatic Ecosystems. Conserv. Biol. 2010, 24, 1002–1011. [Google Scholar] [CrossRef]
- Poff, N.L.; Allan, J.D.; Palmer, M.A.; Hart, D.D.; Richter, B.D.; Arthington, A.H.; Rogers, K.H.; Meyer, J.L.; Stanford, J.A. River flows and water wars: Emerging science for environmental decision-making. Front. Ecol. Environ. 2003, 1, 298–306. [Google Scholar] [CrossRef]
- Postel, S.L.; Daily, G.C.; Ehrlich, P.R. Human appropriation of renewable fresh water. Science 1996, 271, 785–788. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, C.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.S.; LeRoy Poff, N.; Angermeier, P.L.; Dahm, C.N.; Gleick, P.H.; Hairston, N.G., Jr.; Jackson, R.B.; Johnston, C.A.; Richter, B.D.; Steinman, A.D. Meeting ecological and societal needs for freshwater. Ecol. Appl. 2002, 12, 1247–1260. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).