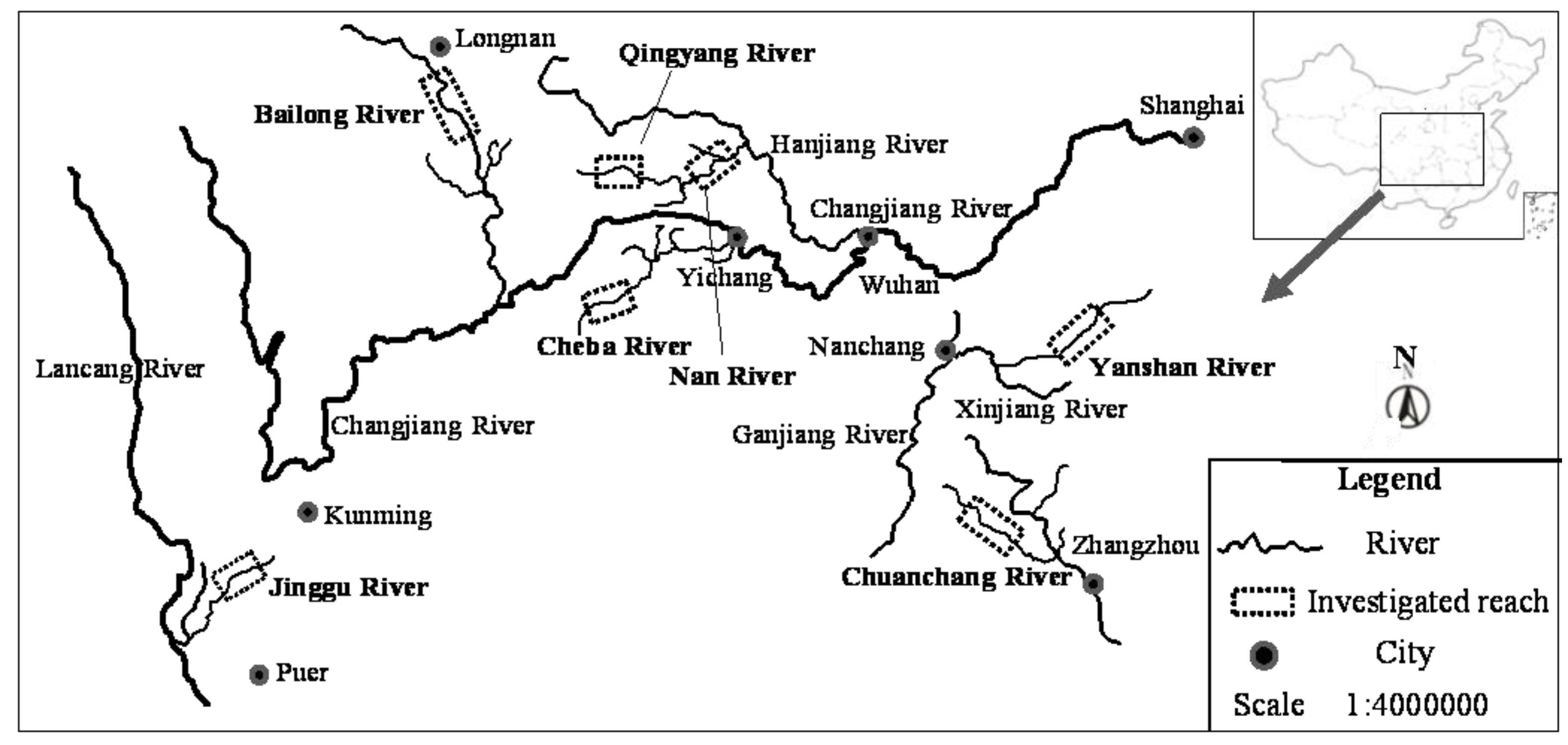
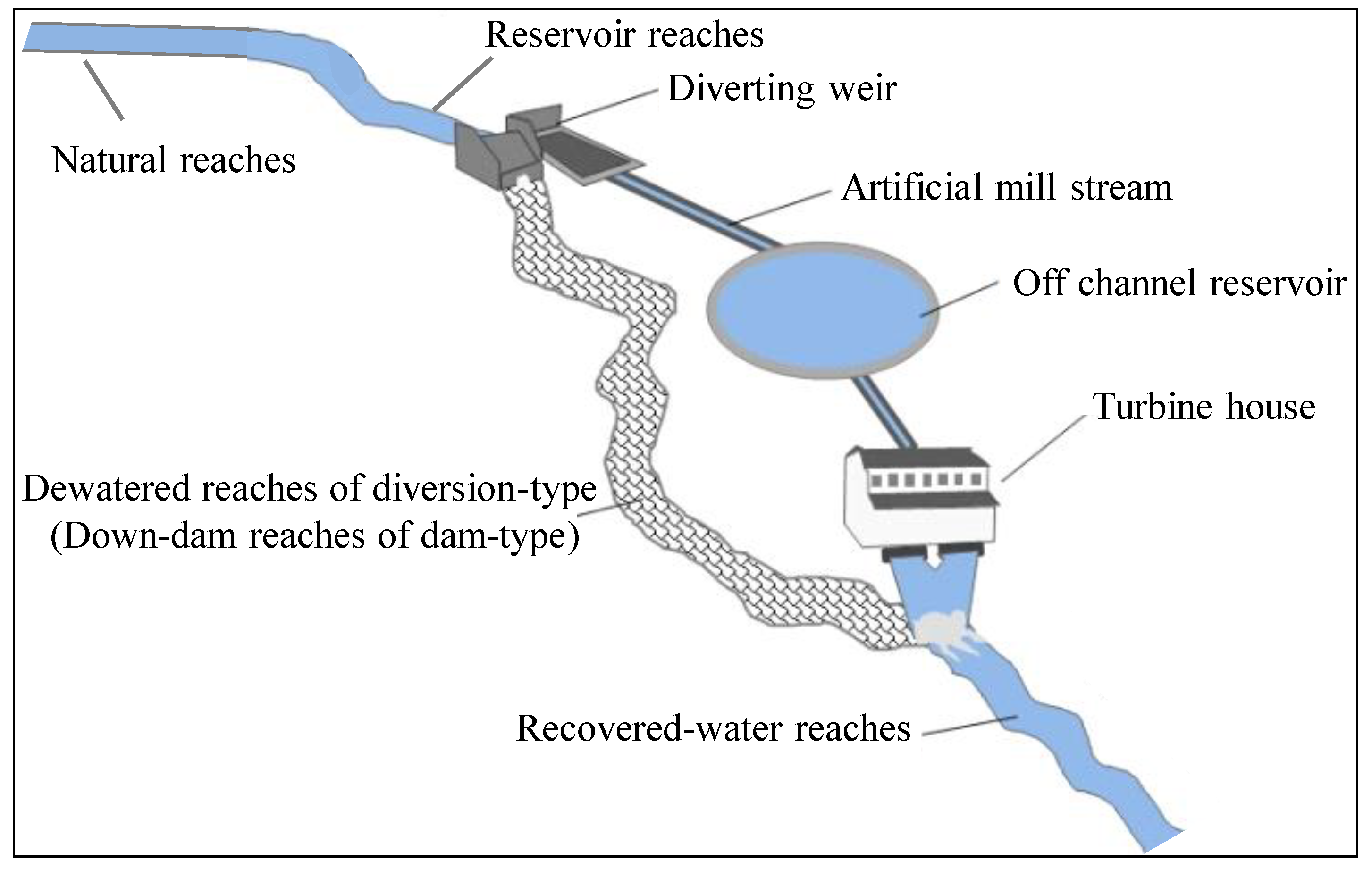
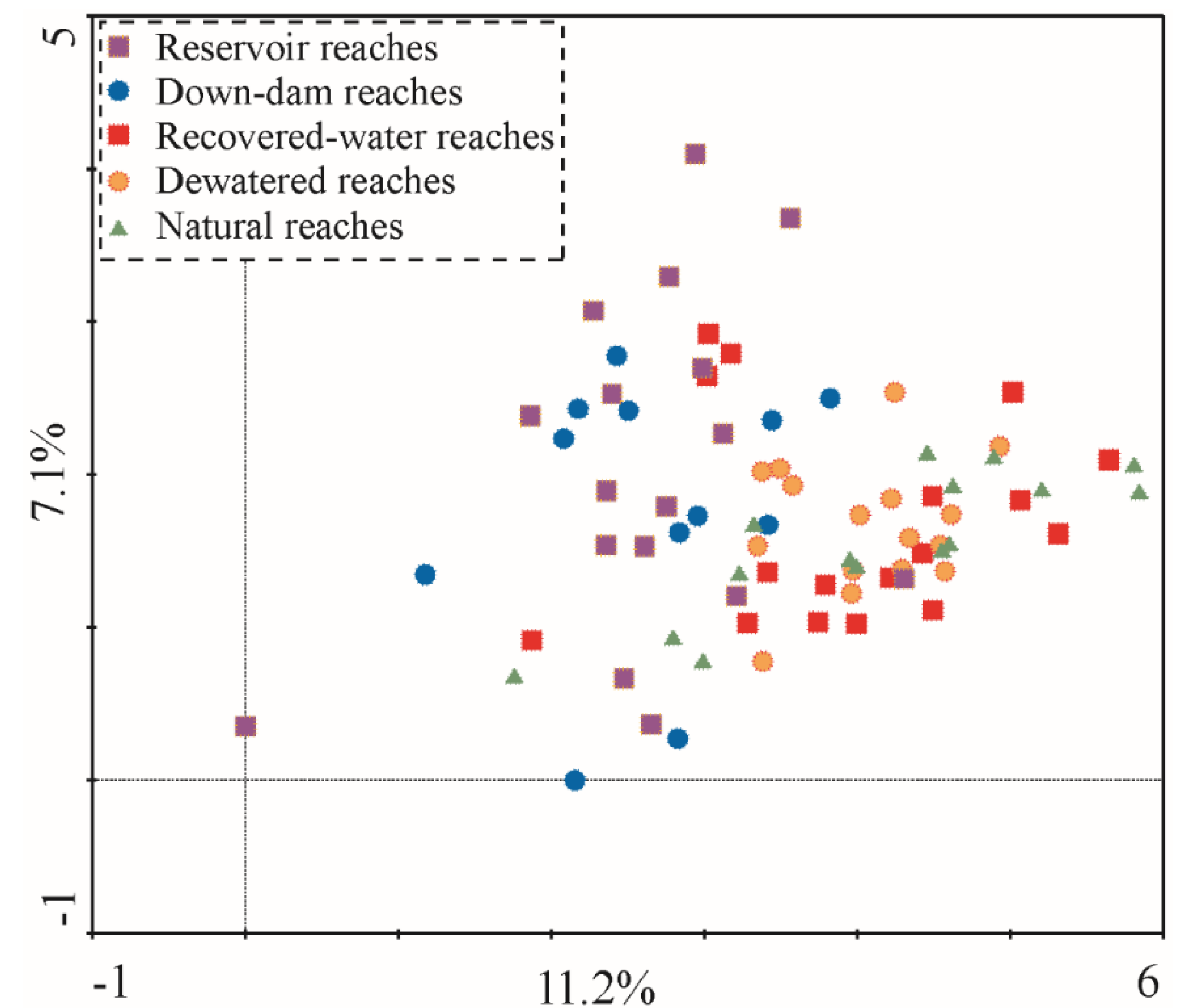
All sample sites were classified into five habitat types (reservoir reaches, down-dam reaches, dewatered reaches, recovered-water reaches, and natural reaches) according to the sampling locations. All 126 taxa collected from these five habitat types were organized into an ordination diagram (
Figure 5) through a DCA sequencing analysis. The results showed that the explanation rate for the taxa difference of Axis 1 was approximately 11.2%, the cumulative interpretation rate for Axes 1 and 2 was 18.3%, and the maximum gradient length was approximately 5.837, indicating that the taxa response to the environment exhibited a single peak pattern. The five habitat types are scattered throughout the ordination diagram, and they do not form a partition trend. This implies that the main factors affecting the distribution of macroinvertebrates in the different rivers and hydrological periods are not a result of runoff regulation; rather, the difference among the habitat types is due to temporospatial differences.
3.2.1. Influence of Dam-Type Hydropower Stations on Macroinvertebrates
Four dam-type hydropower stations are situated along NR, making this river an effective example for illustrating the influence of dam-type hydropower on macroinvertebrates. The position of the sampling sites with respect to the hydroelectric station of NR was shown in
Figure S1 from supplementary materials. Sediment grading curves from different sampling sites of NR was shown in
Figure S3 from supplementary materials. Runoff regulation and the hydrological period (wet versus dry season) were found to be the two main flow regulation modes influencing macroinvertebrates in this river. Runoff regulation resulted in the formation of three habitat types (i.e., reservoir reaches, down-dam reaches, and natural reaches). Differences in the taxa abundance between the different habitat types were analyzed using single-factor ANOVA under two flow regulation modes (
Table 2).
The results showed that for the taxa parameters, the runoff regulation mode had a significant effect whereas the influence of the hydrologic period was relatively weak; therefore, the hydrological data for 2011 (dry season) and 2012 (wet season) could be analyzed without considering the influence of different periods. PERMANOVA of the NR basin data showed that runoff regulation differed significantly in its influence on the different macroinvertebrate community structures (
p = 0.0036, 99,999 times based on permutation test results).
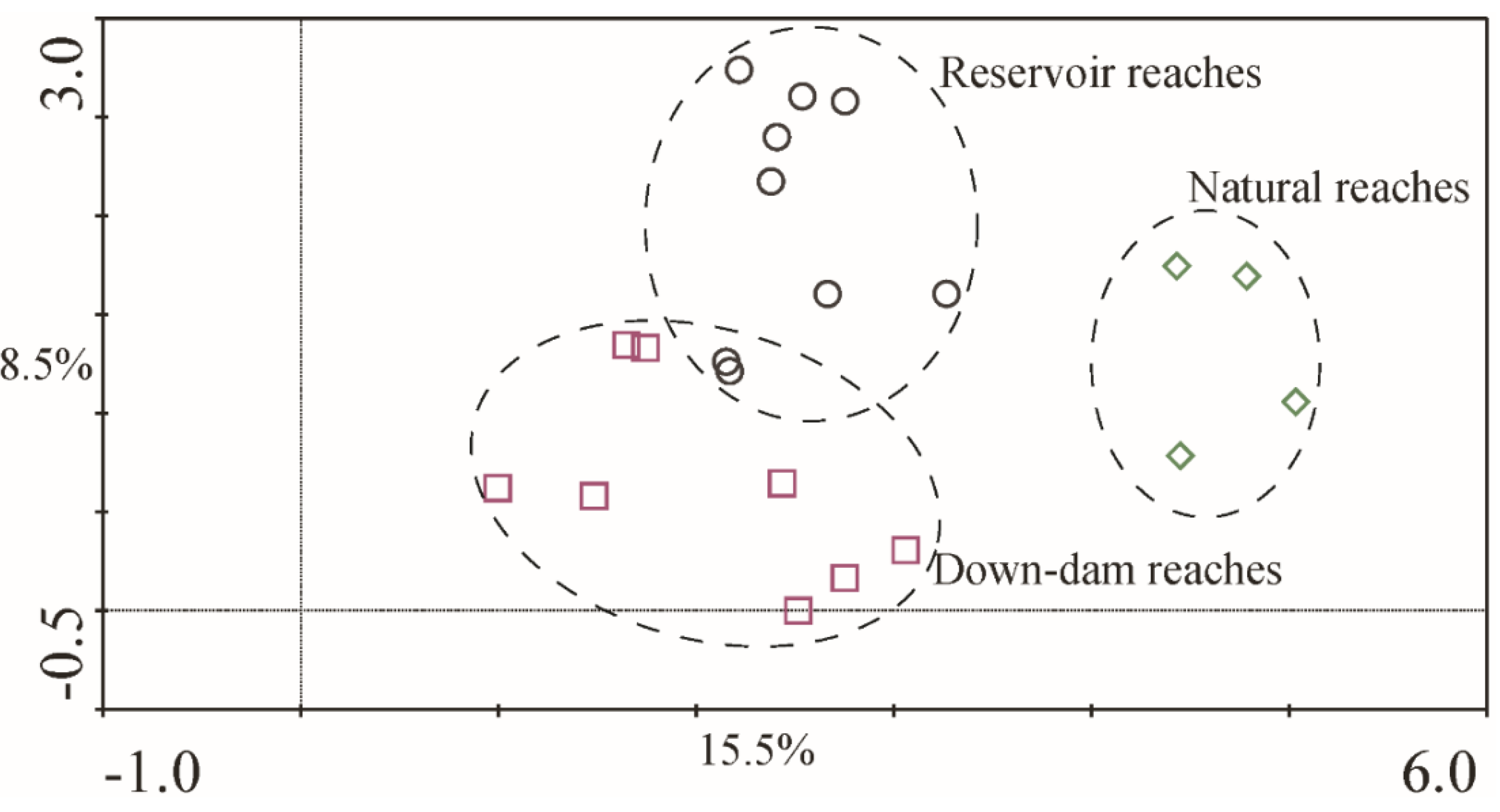
Figure 6 shows different sections of the macroinvertebrate DCA sequencing results.
The explanation rate for the taxa difference of Axis 1 was approximately 15.5%, the cumulative interpretation rate for Axes 1 and 2 was 24.0%, and the maximum gradient length was approximately 5.033, indicating that the taxa response to the environment exhibited a single peak pattern. The three sample types formed obvious partition trends in the ordination diagram, and the grouping was basically consistent with the runoff regulation model. This indicated that, regarding the distribution characteristics of taxa communities among the environmental gradient in the NR area, significant differences were observed among the sites at the dam-type hydropower station, down-dam reaches, and natural reaches. The difference in organisms between the reservoir reaches and down-dam reaches was mainly along Axis 2, and the difference between the natural reaches and unnatural reaches was mainly along Axis 1.
The structure parameters of the macroinvertebrate communities in the reservoir area, dam, and natural river reaches were classified and counted (
Table 3). The results showed small deviations and fluctuations in taxa abundance and diversity (as indicated by the Shannon–Wiener and Margalef richness indices), indicating that the three parameters for the groups at each sampling section were relatively consistent. By contrast, the volatility in deviations of density and biomass was more pronounced, indicating significant differences in these parameters between these groups. Taxa richness gradually increased from reservoirs to down-dam reaches and then to natural reaches (4.4, 6.5, and 9.5, respectively). The Shannon–Wiener index also exhibited an increasing trend in these areas (1.06, 1.48, and 1.58, respectively), whereas the biomass exhibited a decreasing trend (56.3, 25.2, and 6.0 g/m
2, respectively). According to these parameters, the characteristics of the community structure in three typical areas can be summarized as follows. In reservoirs, the number and diversity of macroinvertebrate taxa were the lowest, with higher concentrations of individual taxa, although individual animals were heavier. In down-dam reaches, the number and diversity of macroinvertebrate taxa increased slightly, but the biomass was not high because of the low weight of individual animals. In natural reaches, the number and diversity of macroinvertebrate taxa were the highest, and the weight of individual animals was the lowest.
According to their mode of ingestion, macroinvertebrates were classified into four functional feeding groups: scrapers, predators, filter-collectors, and shredders. The statistical results for the functional feeding groups in the reservoir, down-dam reaches, and natural river reaches indicate that the proportion of collectors in each group accounted for only a small deviation, showing that the number of individual taxa was relatively stable (
Table 4). From the reservoir to the down-dam reaches and onto the natural river reaches, the percentage of collectors exhibited a decreasing trend, indicating a single type of organism in the reservoir (collectors), which mainly fed on organic fine particles and plankton. Because of environmental changes to the habitat, shredders and scrapers accounted for a larger part in the down-dam reaches. In terms of quantity, the increase in the proportion of shredders correlated with the increase in organic fine particles. This increase in the proportion of scrapers indicated that the down-dam environment was suitable for attached algae, which contributed to its growth and development. The proportion of scrapers and predators in the natural river reaches was high, whereas that of various types of feeding organisms was relatively balanced. These results indicated that more biological types were present in the natural river reaches and that functional feeding patterns tended to be diversified.
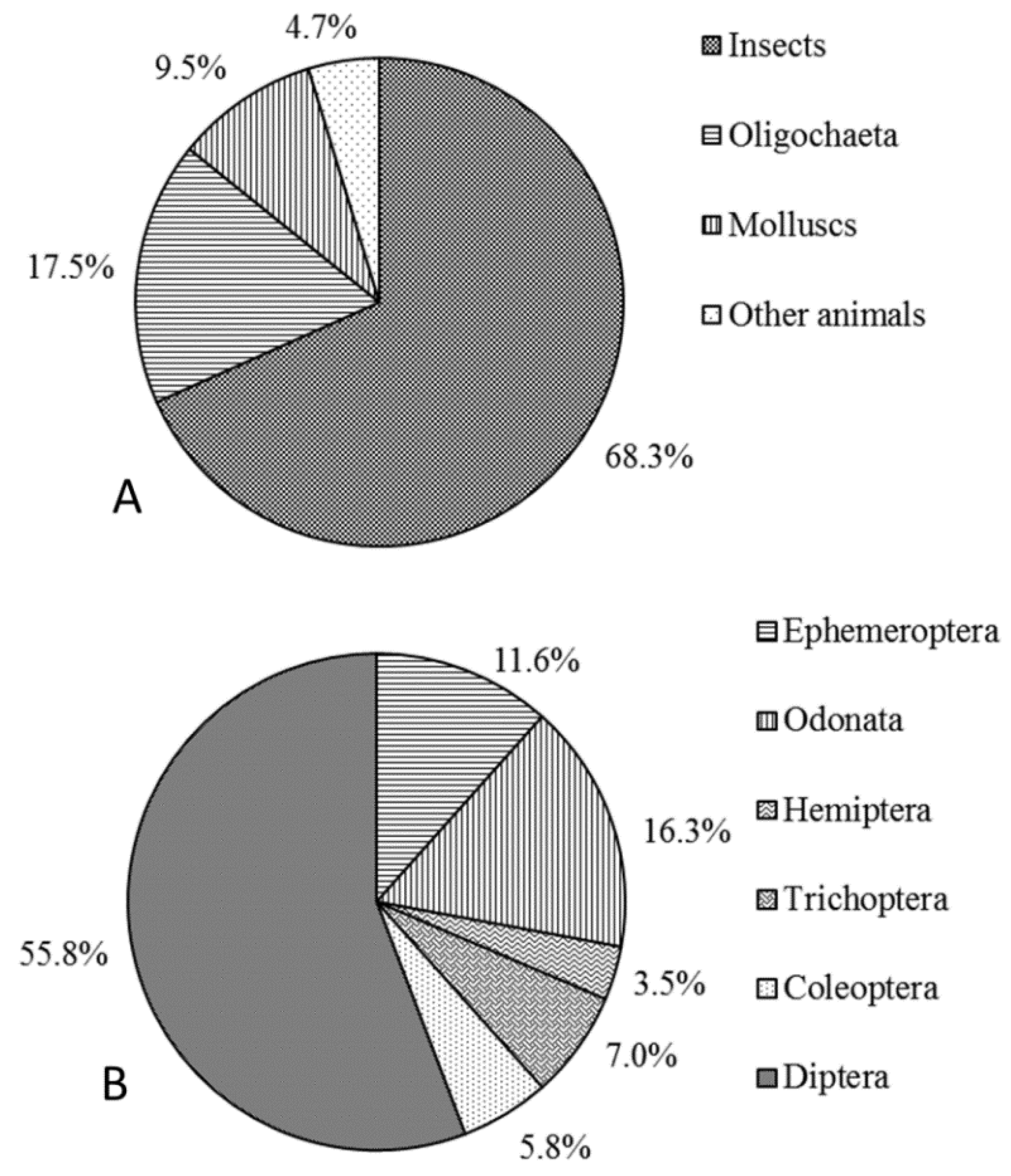
The macroinvertebrates were comprised of
Arthropoda,
Annelida, and
Mollusca in the NR. The proportion of individual
Arthropoda taxa was the highest (57.6% ± 6.8%), followed by
Mollusca taxa (30.4% ± 6.5%), and then
Annelida taxa (12.0% ± 4.4%).
Arthropoda proportions of individual macroinvertebrate taxa in the reservoirs, down-dam reaches, and natural reaches were 37.2% ± 9.0%, 69.3% ± 7.3%, and 95.5% ± 2.1%, respectively,
Mollusca were 9.0% ± 43.4%, 7.3% ± 24.7%, and 2.1% ± 2.6%, respectively, and
Annelida were 43.4% ± 10.5%, 24.7% ± 7.7%, and 2.6% ± 2.6%, as shown in
Table 5. In reservoirs,
Arthropoda taxa accounted for the lowest proportion among the three habitat types, whereas the proportion of
Mollusca taxa was the highest (although the overall proportion was still low). In the down-dam reaches, the proportion of
Arthropoda taxa was higher relative to that in the reservoirs, whereas the proportion of
Mollusca and
Annelida taxa was lower. In the natural reaches, the proportion of
Arthropoda taxa was the highest among the three habitat types, and those of the
Annelida and
Mollusca taxa were the lowest among the habitat types.
Among the Arthropoda taxa, aquatic insects accounted for a high proportion of individual taxa, with the arthropod organisms in most samples comprising entirely aquatic insects. In the reservoirs and down-dam reaches, the midges (Diptera, Chironomidae) were the absolute dominant taxa among the aquatic insects, accounting for nearly 100% of Diptera taxa. In the natural reaches, the presence of aquatic insects from orders other than Diptera, including Ephemeroptera (76% ± 5.8%), Odonata (1.5% ± 1.1%), Trichoptera (6.6% ± 2.1%), and Coleoptera (1.5% ± 0.9%), taken together with those from Diptera (14.3% ± 4.9%), also confirmed that diverse organism types were present in the natural reaches. Concurrently, the process of artificial reservoir flow regulation altered the environmental conditions (e.g., flow regime and sediment composition) and habitat factors, causing considerable changes in the taxa composition and community structure of aquatic insects.
3.2.2. Influence of Diversion-Type Hydropower Stations on Macroinvertebrates
To analyze the influence of diversion hydropower stations on macroinvertebrates community structure, data on the dewatered reaches, recovered-water reaches and natural reaches for 2011 (wet season) and 2012 (dry season) in QYR, where there are many diversion-type hydropower stations, were selected for analysis. The position of the sampling sites with respect to the hydroelectric station of QYR was shown in
Figure S2 from supplementary materials. Sediment grading curves from different sampling sites of QYR was shown in
Figure S4 from supplementary materials. One-way ANOVA was adopted to test for significant differences in taxa abundance (at the 0.05 level) under the two types of classification, which were runoff regulation and hydrological period. The results showed that the effect of the hydrological period was more significant than that of runoff regulation, which was relatively weak (
Table 6).
Table 7 presents the macroinvertebrate structure parameters for QYR for the wet and dry seasons. The table data show an increase in macroinvertebrate taxa abundance (5.2 to 8.3), density (322.2 to 1170.5 ind/m
2), and biomass (24.6 to 40.1 g/m
2), as well as increases in Shannon–Wiener index (1.23 to 2.08) and Margalef richness index (1.66 to 3.51).
PERMANOVA of the macroinvertebrates in QYR also showed that based on the runoff regulation grouping, the difference between macroinvertebrate community structures was nonsignificant in 2011 (
p = 0.169) and 2012 (
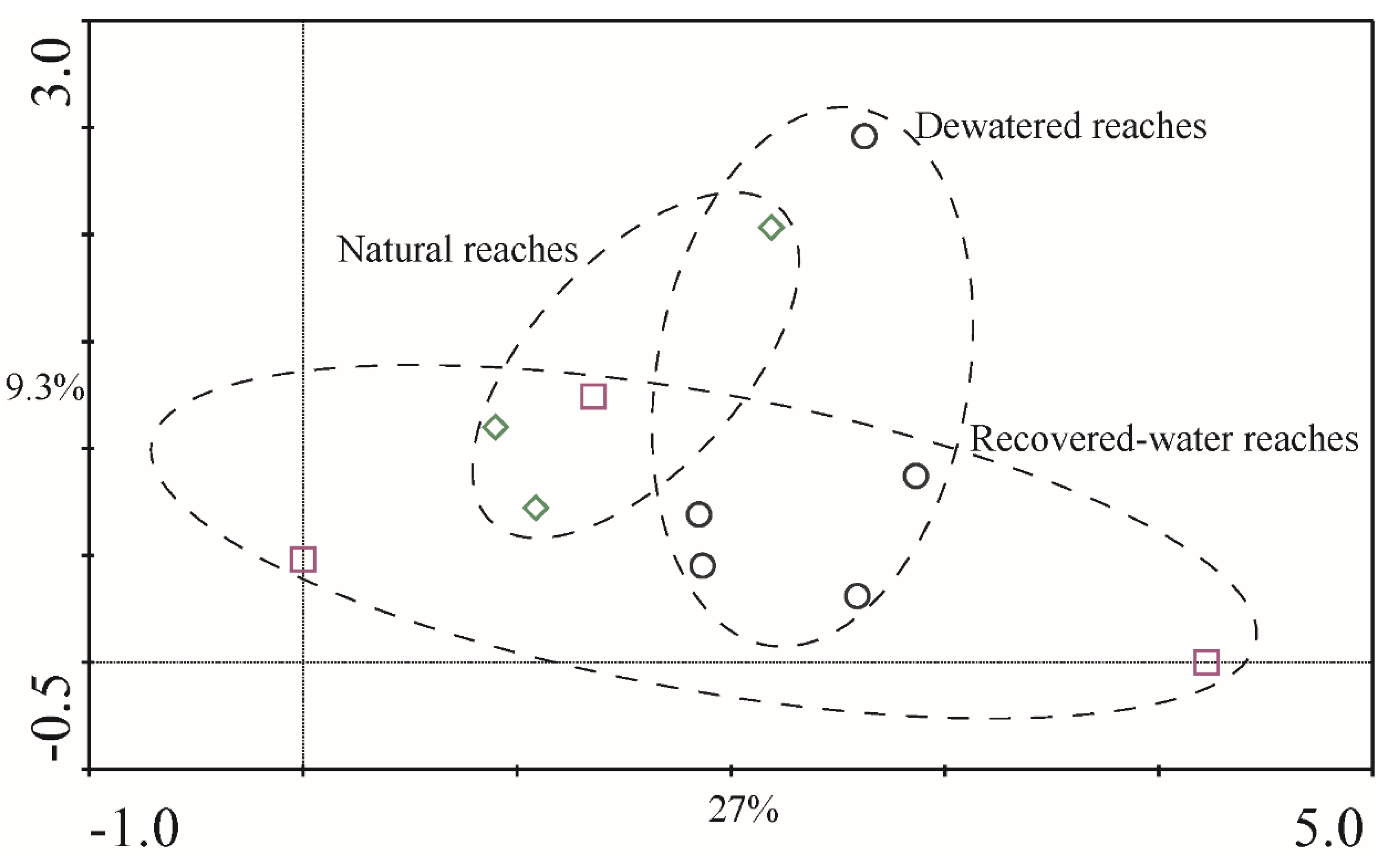
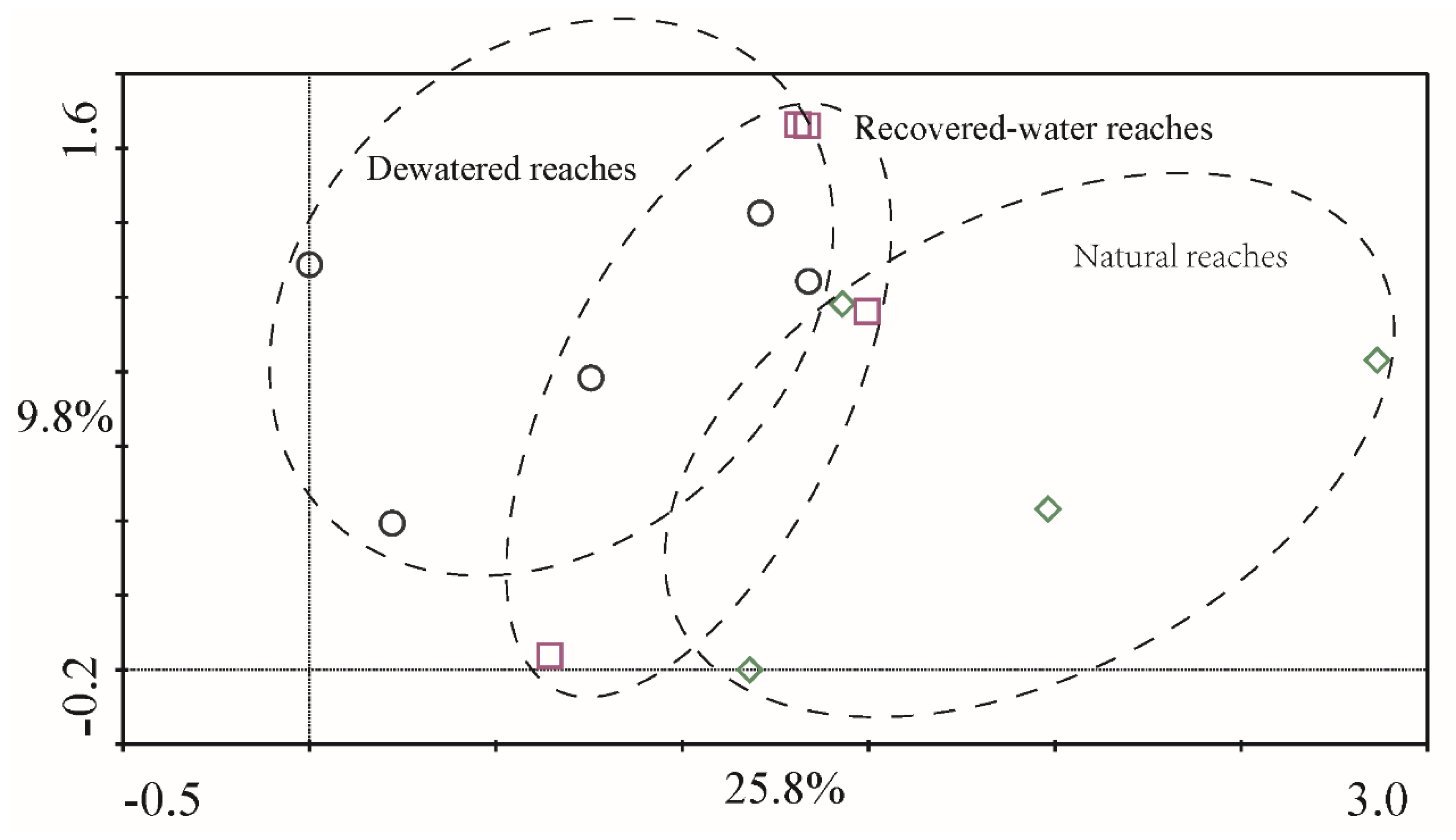
p = 0.191). The DCA results for 2011 (
Figure 7) and 2012 (
Figure 8) in QYR indicated a high degree of similarity in the samples obtained from dewatered reaches, recovered-water reaches, and natural reaches (
Figure 7 and
Figure 8). These regions overlap each other and showed no obvious distinction. This was consistent with the ANOVA and PERMANOVA results.
In summary, the influence of hydropower stations on macroinvertebrate communities exhibited obvious differences between the dam- and diversion-type power stations. For dam-type hydropower stations, a reservoir forms because of the high dam. The substrate, velocity, and depth in reservoirs (silt substrate, low velocity, and high depth) differ from those in down-dam reaches (gravel substrate, high velocity, and small depth) and natural reaches. Hence, the macroinvertebrate taxa composition, standing crops, diversity, and functional feeding groups exhibited clear differences between reservoirs and down-dam reaches. These results are similar to those reported by Ren et al. [
30]. Furthermore, the hydrological period had no influence on these factors, as evidenced by the habitat characteristics of the reservoirs and down-dam reaches exhibiting no change with the hydrological period.
For diversion-type power stations, no obvious reservoir formed because of the low dam; however, dewatered reaches and recovered-water reaches did form as a result of these power stations being installed. However, there was no significant substrate difference among dewatered reaches, recovered-water reaches, and natural reaches with the same type of gravel. Many studies have shown that sediment is one of the most crucial factors influencing macroinvertebrates [
31,
32,
33,
34]. Identical substrate types also indicate similar macroinvertebrate structures. Nevertheless, the intercepting effect of diversion-type power stations (low dam) in the wet season was not apparent. The dewatered reaches and recovered-water reaches would have been eroded as a result of flooding, which could have been linked to the low level of macroinvertebrate standing crops. Therefore, the macroinvertebrates near diversion-type power stations were susceptible to hydrological period effects [
35]. One extreme impact on macroinvertebrates near diversion-type power stations is zero flow and dry ups. This phenomenon occurs occasionally in the mountainous rivers in the dry season with a lack of a runoff supply. In this situation, most macroinvertebrate species may disappear and community structure will change in the river.