Changes in a Fish Community in a Small River Related to the Appearance of the Invasive Topmouth Gudgeon Pseudorasbora parva (Temminck & Schlegel, 1846)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Gozlan, R.E.; Andreou, D.; Asaeda, T.; Beyer, K.; Bouhadad, R.; Burnard, D.; Caiola, N.; Cakic, P.; Djikanovic, V.; Esmaeili, H.R.; et al. Pan-continental invasion of Pseudorasbora parva: Towards a better understanding of freshwater fish invasion. Fish Fish. 2010, 11, 315–340. [Google Scholar] [CrossRef]
- Wildekamp, R.H.; Van Neer, W.; Küçük, F.; Ünlüsayin, M. First record of the eastern Asiatic gobionid fish Pseudorasbora parva from the Asiatic part of Turkey. J. Fish Biol. 1997, 51, 858–861. [Google Scholar] [CrossRef]
- Caiola, N.; De Sostoa, A. First record of the Asiatic cyprinid Pseudorasbora parva in the Iberian Peninsula. J. Fish Biol. 2002, 61, 1058–1060. [Google Scholar] [CrossRef]
- Pinder, A.C.; Gozlan, R.E.; Britton, J.R. Dispersal of the invasive topmouth gudgeon Pseudorasbora parva in the UK: A vector for an emergent infectious disease. Fish. Manag. Ecol. 2005, 12, 411–414. [Google Scholar] [CrossRef]
- Ekmekçi, F.G.; Kirankaya, S.G. Distribution of invasive fish species, Pseudorasbora parva (Temminck & Schlegel, 1846) in Turkey. Turk. J. Zool. 2006, 30, 329–334. [Google Scholar]
- Patimar, R.; Baensaf, S. Morphology, growth and reproduction of the non-indigenous topmouth gudgeon Pseudorasbora parva (Temminck et Schlegel, 1846) in the wetland of Alma-Gol, Northern Iran. Russ. J. Biol. Invasions 2012, 3, 71–75. [Google Scholar] [CrossRef]
- Záhorská, E.; Kováč, V.; Katina, S. Age and growth in a newly-established invasive population of topmouth gudgeon. Cent. Eur. J. Biol. 2010, 5, 256–261. [Google Scholar] [CrossRef]
- Rosecchi, E.; Crivelli, A.J.; Catsadorakis, G. The establishment and impact of Pseudorasbora parva, an exotic fish species introduced into Lake Mikri Prespa (North-Western Greece). Aquat. Conserv. Mar. Freshw. Ecosyst. 1993, 3, 223–231. [Google Scholar] [CrossRef]
- Xie, S.; Cui, Y.; Zhang, T.; Li, Z. Seasonal patterns in feeding ecology of three small fishes in the Biandantang Lake, China. J. Fish Biol. 2000, 57, 867–880. [Google Scholar] [CrossRef]
- Declerck, S.; Louette, G.; De Bie, T.; De Meester, L. Patterns of diet overlap between populations of non-indigenous and native fishes in shallow ponds. J. Fish Biol. 2002, 61, 1182–1197. [Google Scholar] [CrossRef]
- Hliwa, P.; Martyniak, A.; Kucharczyk, D.; Sebestyén, A. Food preferences of juvenile stages of Pseudorasbora parva (Schlegel, 1842) in the Kis-Balaton Reservoir. Arch. Pol. Fish. 2002, 10, 121–127. [Google Scholar]
- Jackson, M.C.; Britton, J.R. Variation in the trophic overlap of invasive Pseudorasbora parva and sympatric cyprinid fishes. Ecol. Freshw. Fish 2013, 22, 654–657. [Google Scholar] [CrossRef]
- Yalçın-Özdilek, S.; Kırankaya, S.G.; Ekmekçi, F.G. Feeding ecology of the Topmouth Gudgeon Pseudorasbora parva (Temminck and Schlegel, 1846) in the Gelingüllü Reservoir, Turkey. Turk. J. Fish. Aquat. Sci. 2013, 13, 87–94. [Google Scholar] [CrossRef]
- Copp, G.H.; Bianco, P.G.; Bogutskaya, N.G.; Erős, T.; Falka, I.; Ferreira, M.T.; Fox, M.G.; Freyhof, J.; Goplan, R.E.; Grabowska, J.; et al. To be, or not to be, a non-native freshwater fish? J. Appl. Ichthyol. 2005, 21, 242–262. [Google Scholar] [CrossRef]
- Grabowska, J.; Kotusz, J.; Witkowski, A. Alien invasive fish species in Polish waters: An overview. Folia Zool. 2010, 59, 73–85. [Google Scholar] [CrossRef]
- Gozlan, R.E.; Britton, J.R.; Cowx, I.; Copp, G.H. Current knowledge on non-native freshwater fish introductions. J. Fish Biol. 2010, 76, 751–786. [Google Scholar] [CrossRef]
- Rabitsch, W.; Milasowszky, N.; Nehring, S.; Wiesner, C.; Wolter, C.; Essl, F. The times are changing: Temporal shifts in patterns of fish invasions in central European fresh waters. J. Fish Biol. 2013, 82, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Pollux, B.J.A.; Kőrösi, A. On the occurrence of the Asiatic cyprinid Pseudorasbora parva in the Netherlands. J. Fish Biol. 2006, 69, 1575–1580. [Google Scholar] [CrossRef]
- Balirwa, J.S.; Chapman, C.A.; Chapman, L.J.; Cowx, I.G.; Geheb, K.; Kaufman, L.; Lowe-McConnell, R.H.; Seehausen, O.; Wanink, J.H.; Welcomme, R.L.; et al. Biodiversity and fishery sustainability in the Lake Victoria Basin: An unexpected marriage? Bioscience 2003, 53, 703–715. [Google Scholar] [CrossRef]
- Gozlan, R.E.; St Hilaire, S.; Feist, S.W.; Martin, P.; Kent, M.L. Biodiversity—Disease threat to European fish. Nature 2005, 435, 1046. [Google Scholar] [CrossRef]
- McDowall, R. Crying wolf, crying foul, or crying shame: Alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Rev. Fish Biol. Fish. 2006, 16, 233–422. [Google Scholar] [CrossRef]
- Musil, M.; Novotná, K.; Potužák, J.; Hůda, J.; Pechar, L. Impact of topmouth gudgeon (Pseudorasbora parva) on production of common carp (Cyprinus carpio)—Question of natural food structure. Biologia 2014, 69, 1757–1769. [Google Scholar] [CrossRef]
- Adámek, Z.; Sukop, I. Vliv střevličky východni (Pseudorasbora parva) na parametry rybničniho prostředi. Biodiverzita ichtiofauny ČR 2000, 3, 37–43. [Google Scholar]
- Witkowski, A. Introduction of fishes into Poland: Benefaction or plague. Nat. Conserv. 2002, 59, 41–54. [Google Scholar]
- Gozlan, R.E.; Beyer, K. Hybridisation between Pseudorasbora parva and Leucaspius delineates. Folia Zool. 2006, 55, 53–60. [Google Scholar]
- Musil, J.; Adámek, Z. Piscivorous fishes diet dominated by the Asian cyprinid invader, topmouth gudgeon (Pseudorasbora parva). Biologia 2007, 62, 488–490. [Google Scholar] [CrossRef]
- Lorenzoni, M.; Ghetti, L.; Mearelli, M. Native and exotic fish species in the Tiber River watershed (Umbria–Italy) and their relationship to the longitudinal gradient. Bull. Fr. Peche Piscic. 2006, 382, 19–44. [Google Scholar] [CrossRef][Green Version]
- Gaygusuz, Ö.; Tarkan, A.S.; Gürsoy Gaygusuz, Ç. Changes in the fish community of the Ömerli reservoir (Turkey) following the introduction of non-native gibel carp Carassius gibelio (Bloch, 1782) and other human impacts. Aquat. Invasions 2007, 2, 117–120. [Google Scholar] [CrossRef]
- Rechulicz, J. Monitoring of the topmouth gudgeon, Pseudorasbora parva (Actinopterygii: Cypriniformes: Cyprinidae) in a small upland Ciemięga River, Poland. Acta Ichthyol. Piscat. 2011, 41, 193–199. [Google Scholar] [CrossRef]
- Hickley, P. Electric fishing in practice. In Fishing with Electricity—Applications in Freshwater Fisheries Management; Cowx, I.G., Lamarque, P., Eds.; Fishing News Books, Blackwell Scientific: Oxford, UK, 1990; pp. 176–187. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; pp. 87–184. [Google Scholar]
- Hurlbert, S.M. The non-concept of species diversity: A critique and alternative parameters. Ecology 1971, 52, 577–586. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 10; StatSoft, Inc.: Tulsa, OK, USA, 2011. Available online: www.statsoft.com (accessed on 1 May 2014).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package—R Package Version 2.4.0. 2016. Available online: http://CRAN.R-project.org/package=vegan. R-project org/package=vegan (accessed on 10 October 2017).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 3 March 2019).
- Gozlan, R.E. Introduction of non-native freshwater fish: Is it all bad? Fish Fish. 2008, 9, 106–115. [Google Scholar] [CrossRef]
- Kennard, M.J.; Arthington, A.H.; Pusey, B.J.; Harch, B.D. Are alien fish a reliable indicator of river health? Freshw. Biol. 2005, 50, 174–193. [Google Scholar] [CrossRef]
- Britton, J.R.; Davies, G.D.; Harrod, C. Trophic interactions and consequent impacts of the invasive fish Pseudorasbora parva in a native aquatic foodweb: A field investigation in the UK. Biol. Invasions 2010, 12, 1533–1542. [Google Scholar] [CrossRef]
- Beyer, K.; Copp, G.H.; Gozlan, R.E. Microhabitat use and interspecific associations of introduced topmouth gudgeon Pseudorasbora parva and native fishes in a small stream. J. Fish Biol. 2007, 71, 224–238. [Google Scholar] [CrossRef]
- Giannetto, D.; Carosi, A.; Franchi, E.; Ghetti, L.; Pedicillo, G.; Pompei, L.; Lorenzoni, M. Assessing the impact of non-native freshwater fishes on native species using relative weight. Knowl. Manag. Aquat. Ecosyst. 2012, 404, 3. [Google Scholar] [CrossRef][Green Version]
- Britton, J.R.; Davies, G.D.; Brazier, M.; Pinder, A.C. A case study on the population ecology of a topmouth gudgeon Pseudorasbora parva population in the UK and the implications for native fish communities. Aquat. Conserv. Mar. Freshw. Ecosyst. 2007, 17, 749–759. [Google Scholar] [CrossRef]
- Carpentier, A.; Gozlan, R.E.; Cucherousset, J.; Paillisson, J.M.; Marion, L. Is topmouth gudgeon Pseudorasbora parva responsible for the decline in sunbleak Leucaspius delineatus populations? J. Fish Biol. 2007, 71, 274–278. [Google Scholar] [CrossRef]
- Andreou, D.; Arkush, K.D.; Guégan, J.F.; Gozlan, R.E. Introduced Pathogens and Native Freshwater Biodiversity: A Case Study of Sphaerothecum destruens. PLoS ONE 2012, 7, e36998. [Google Scholar] [CrossRef]
- Thomas, C.D. The Anthropocene could raise biological diversity. Nature 2013, 502, 7. [Google Scholar] [CrossRef]
- Simberloff, D.; Genovesi, P. Anthropocene: Action makes sense. Nature 2013, 502, 624. [Google Scholar] [CrossRef]
- Beyer, K. Ecological Implications of Introducing Leucaspius Delineates (Heckel, 1843) and Pseudorasbora Parva (Temminck and Schlegel, 1842) into Inland Waters in England. Ph.D. Thesis, University of Hull, Hull, UK, 2008. Available online: https://hydra.hull.ac.uk/resources/hull:1002 (accessed on 15 March 2015).
- Lemmens, P.; Mergeay, J.; Vanhove, T.; De Meester, L.; Declerck, S.A.J. Suppression of invasive topmouth gudgeon Pseudorasbora parva by native pike Esox lucius in ponds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 41–48. [Google Scholar] [CrossRef]
- Kapusta, A.; Bogacka-Kapusta, E.; Czarnecki, B. The significance of stone moroko, Pseudorasbora parva (Temminck and Schlegel), in the small-sized fish assemblages in the littoral zone of the heated Lake Licheńskie. Arch. Pol. Fish. 2008, 16, 49–62. [Google Scholar] [CrossRef]
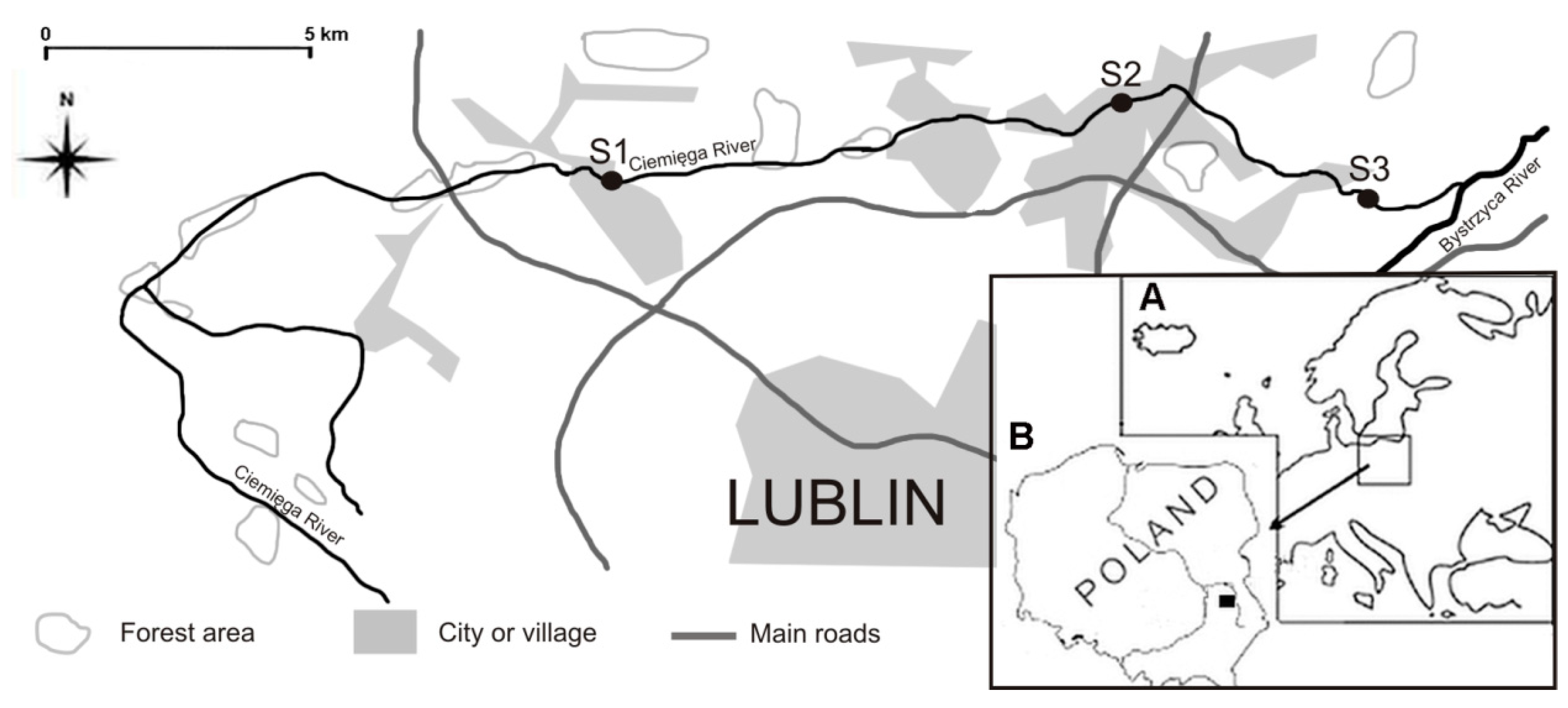
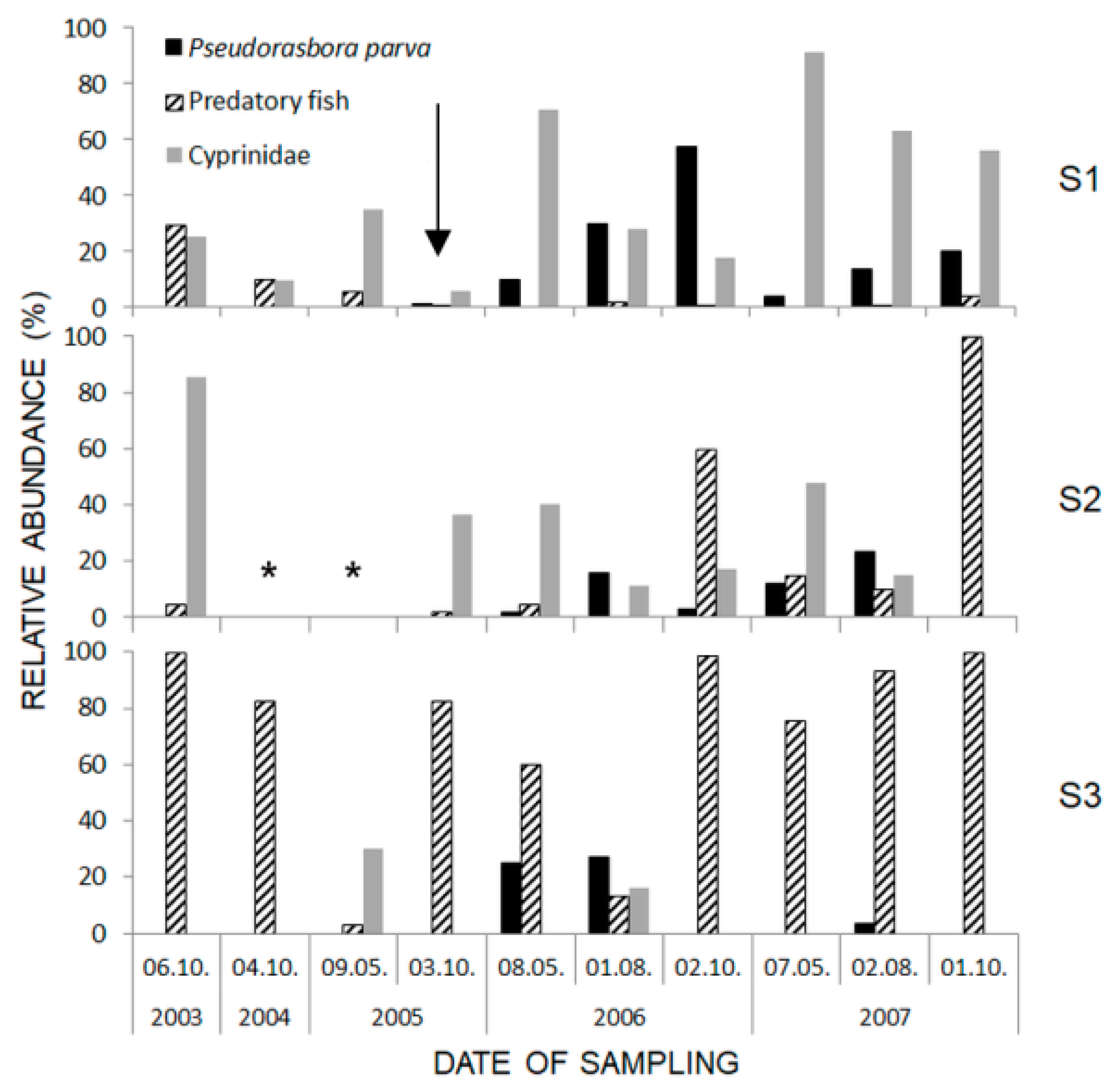
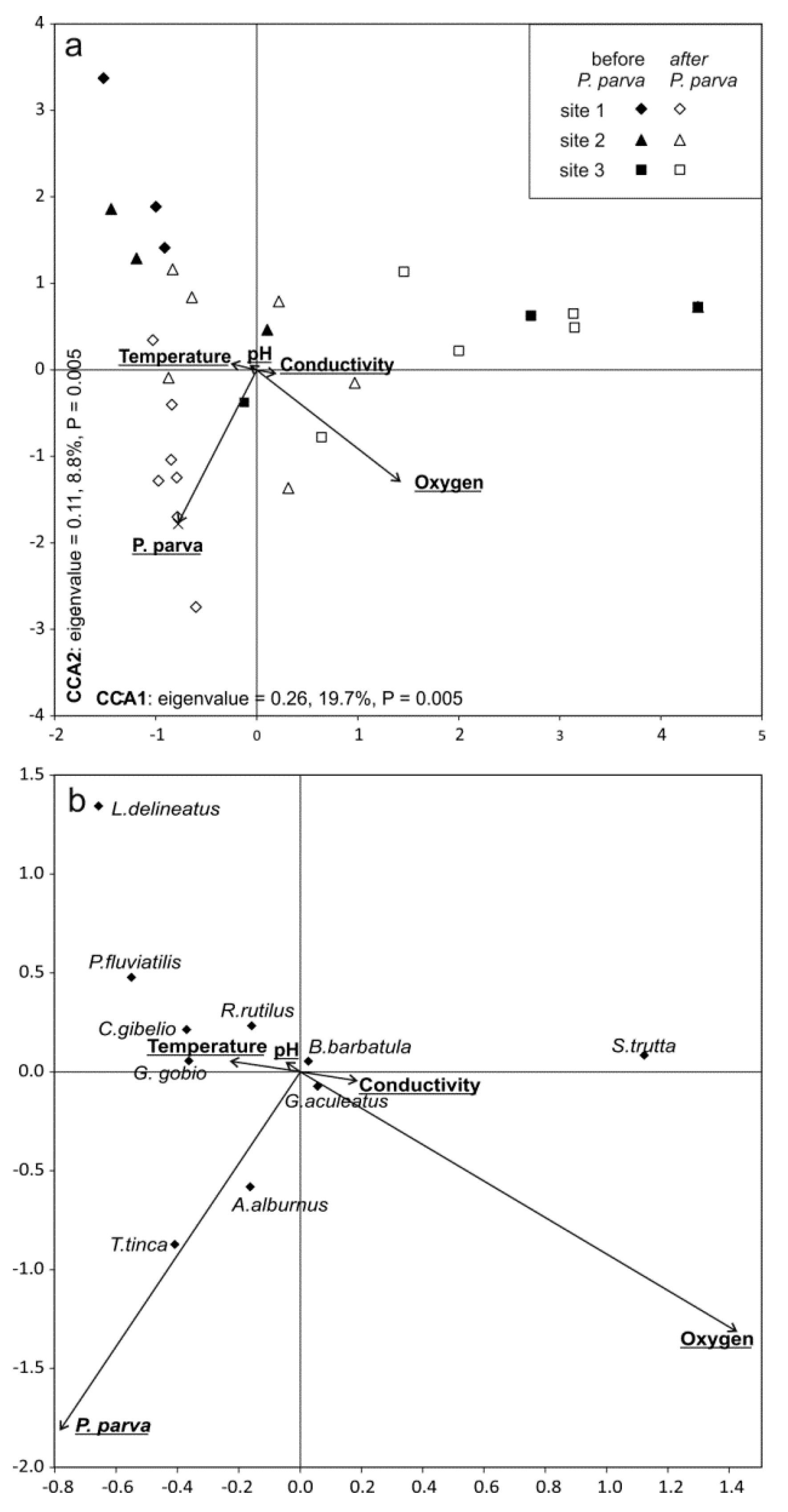
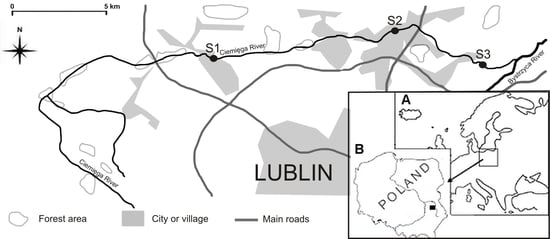
| Site Specification | S1 Jastków | S2 Dys | S3 Pliszczyn |
|---|---|---|---|
| GPS site coordinates | N 51°18’36” E 22°26’00” | N 51°18’49” E 22°33’52” | N 51°18’08” E 22°38’28” |
| Distance from the river spring (km) | 18 | 26 | 33 |
| Width of the river bed (m) | 2.50–3.00 | 3.50–4.00 | 4.50–6.00 |
| River depth (m) | 0.30–0.60 | 0.40–0.90 | 0.30–0.45 |
| Bottom characteristics | Sand and gravel bottom, many submerged macrophytes | Sand and gravel bottom, in some sections mud, many submerged macrophytes | Gravel and sand bottom, few macrophytes |
| River bed characteristics | Straight, flat banks, low banks with plants: Urtica dioica L, Lamium album L., a few trees | Irregular with a small meander, high and steep banks with plants: Urtica dioica L, Lamium album L., a few trees | Shallow, straight, flat banks, high valley, trees along the banks: Alnus glutinosa, Salix alba L. |
| River modifications | Partially regulated | Partially regulated, banks with concrete blocks near a bridge | Natural river, without modifications, with a weir above the study site |
| pH | 7.5 ± 0.3 | 7.57 ± 0.32 | 7.5 ± 0.5 |
| Conductivity (μS cm−1) | 611.2 ± 159.2 | 638.6 ± 49.6 | 581.4 ± 150.8 |
| Temperature (°C) | 11.2 ± 5.8 | 10.8 ± 5.0 | 10.2 ± 3.7 |
| Oxygen saturation (%) | 80.0 ± 6.9 | 79.5 ± 25.0 | 93.1 ± 5.9 |
| Dissolved oxygen (mg L−1) | 8.2 ± 1.4 | 8.9 ± 1.9 | 9.3 ± 2.0 |
| Fish Species | Family | Status | Before Finding P. parva | After Finding P. parva | ||||
|---|---|---|---|---|---|---|---|---|
| N | Tl | W | N | Tl | W | |||
| Gudgeon (Gobio gobio) | Cyprinidae | NS | 106 | 11.0 ± 1.4 | 12.4 ± 5.4 | 146 | 11.2 ± 2.8 | 14.2 ± 7.5 |
| Roach (Rutilus rutilus) | Cyprinidae | NS | 34 | 12.4 ± 4.2 | 28.5 ± 28.2 | 98 | 11.3 ± 1.8 | 14.2 ± 9.9 |
| Tench (Tinca tinca) | Cyprinidae | NS | 1 | 5.9 ± 0.0 | 2.5 ± 0.0 | 34 | 8.1 ± 3.0 | 9.6 ± 13.8 |
| Bleak (Alburnus alburnus) | Cyprinidae | NS | 3 | 4.0 ± 0.5 | 0.9 ± 0.2 | 714 | 4.7 ± 0.9 | 1.2 ± 0.4 |
| Spirilin (Alburnoides bipunctatus) | Cyprinidae | NS | 1 | 6.8 ± 0.0 | 6.0 ± 0.0 | 0 | - | - |
| Sunbleak (Leucaspius delineatus) | Cyprinidae | NS | 7 | 5.8 ± 1.0 | 2.3 ± 1.4 | 0 | - | - |
| Ide (Leuciscus idus) | Cyprinidae | NS | 0 | - | - | 4 | 13.1 ± 3.3 | 34.8 ± 19.0 |
| Gibel carp (Carassius gibelio) | Cyprinidae | NNS | 31 | 9.0 ± 5.4 | 30.3 ± 42.8 | 79 | 9.0 ± 3.6 | 19.2 ± 22.2 |
| Topmouth gudgeon (Pseudorasbora parva) | Cyprinidae | NNS | 0 | - | - | 316 | 6.2 ± 1.0 | 2.2 ± 1.3 |
| Brown trout (Salmo trutta) | Salmonidae | NS | 53 | 27.2 ± 3.9 | 235.0 ± 84.9 | 549 | 17.0 ± 5.5 | 67.2 ± 72.8 |
| Northen pike (Esox lucius) | Esocidae | NS | 2 | 22.0 ± 1.4 | 62.1 ± 5.5 | 1 | 27.8 ± 0.0 | 151.0 ± 0.0 |
| Eurasian perch (Perca fluviatilis) | Percidae | NS | 19 | 10.6 ± 5.3 | 27.0 ± 33.5 | 14 | 9.3 ± 2.1 | 10.5 ± 8.8 |
| Threespine stickleback (Gasterosteus aculeatus) | Gasterosteidae | NS | 43 | 5.7 ± 1.2 | 2.3 ± 1.8 | 255 | 4.6 ± 0.9 | 1.4 ± 0.7 |
| Stone loach (Barbatula barbatula) | Nemacheilidae | NS | 33 | 8.9 ± 1.9 | 6.5 ± 4.3 | 89 | 9.7 ± 2.2 | 9.1 ± 5.2 |
| Brown bullhead (Ameiurus nebulosus) | Ictaluridae | NNS | 1 | 15.0 ± 0.0 | 47.0 ± 0.0 | 0 | - | - |
| Groups and Fish Species | Before Finding P. parva | After Finding P. parva | U | Z | p Value | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | ||||
| Native fish species | |||||||
| Cyprinidae | 8.4 ± 6.7 | 0.0–35.0 | 23.7 ± 25.3 | 0.0–187.5 | 73.0 | −0.97 | 0.33 |
| Gudgeon (Gobio gobio) | 5.9 ± 5.4 | 0.0–35.0 | 3.5 ± 2.5 | 0.0–16.5 | 86.0 | −0.38 | 0.70 |
| Roach (Rutilus rutilus) | 1.9 ± 2.2 | 0.0–13.0 | 2.3 ± 2.5 | 0.0–23.0 | 83.0 | −0.52 | 0.60 |
| Tench (Tinca tinca) | 0.1 ± 0.1 | 0.0–0.5 | 0.8 ± 0.8 | 0.0–7.0 | 75.0 | −0.88 | 0.38 |
| Bleak (Alburnus alburnus) | 0.2 ± 0.3 | 0.0–1.5 | 17.0 ± 26.0 | 0.0–187.5 | 52.0 | −1.92 | 0.05 |
| Spirilin (Alburnoides bipunctatus) | 0.2 ± 0.1 | 0.0–0.5 | – | – | – | − | – |
| Sunbleak (Leucaspius delineatus) | 0.4 ± 0.5 | 0.0–3.0 | – | – | – | − | – |
| Ide (Leuciscus idus) | – | – | 0.1 ± 0.2 | 0.0–1.0 | – | − | – |
| Predatory fish | 4.1 ± 2.8 | 0.0–14.0 | 14.1 ± 11.2 | 0.0–80.5 | 79.0 | −0.70 | 0.48 |
| Brown trout (Salmo trutta) | 2.9 ± 2.4 | 0.0–14.0 | 13.7 ± 11.3 | 0.0–80.5 | 68.5 | −1.18 | 0.24 |
| Northen pike (Esox lucius) | 0.1 ± 0.2 | 0.0–1.0 | 0.1 ± 0.1 | 0.0–0.5 | 88.0 | 0.29 | 0.77 |
| Eurasian perch (Perca fluviatilis) | 1.1 ± 0.4 | 0.0–3.5 | 0.3 ± 0.2 | 0.0–2.0 | 76.0 | 0.84 | 0.40 |
| Other fish | 4.2 ± 3.3 | 0.0–15.5 | 8.3 ± 5.5 | 0.0–48.5 | 68.0 | −1.20 | 0.23 |
| Threespine stickleback (Gasterosteus aculeatus) | 2.4 ± 2.5 | 0.0–15.5 | 6.1 ± 5.3 | 0.0–48.5 | 68.5 | −1.18 | 0.24 |
| Stone loach (Barbatula barbatula) | 1.8 ± 2.0 | 0.0–6.5 | 2.3 ± 1.5 | 0.0–10.0 | 88.0 | −0.29 | 0.77 |
| Non-native fish species | |||||||
| Topmouth gudgeon (Pseudorasbora parva) | – | – | 7.5 ± 9.1 | 0.0–81.0 | – | − | – |
| Gibel carp (Carassius gibelio) | 1.7 ± 1.0 | 0.0–8.0 | 1.9 ± 1.9 | 0.0–12.0 | 85.5 | 0.83 | 0.69 |
| Brown bullhead (Ameiurus nebulosus) | 0.1 ± 0.1 | 0.0–0.5 | – | – | – | − | – |
| Total (excluding P. parva) | 18.6 ± 2.8 | 0.0–35.0 | 48.0 ± 32.5 | 0.0–73.2 | 3.00 | −1.59 | 0.11 |
| Total (including P. parva) | 18.6 ± 2.8 | 0.0–35.0 | 55.5 ± 35.5 | 0.0–187.5 | 40.5 | −2.4 | 0.01 * |
| Effect | df | SS | MS | F | Pr (>F) | |
|---|---|---|---|---|---|---|
| Two-way analysis | Site | 2 | 2.21 | 1.11 | 4.11 | 0.001 * |
| P. parva | 1 | 0.39 | 0.39 | 1.46 | 0.168 | |
| Site × P. parva | 2 | 0.96 | 0.48 | 1.78 | 0.045 * | |
| Residuals | 24 | 6.46 | 0.27 | 0.64 | ||
| Separate 1-way analyses for particular sites | ||||||
| Site 1 | P. parva | 1 | 0.46 | 0.46 | 1.72 | 0.093 |
| Residuals | 8 | 2.14 | 0.27 | 0.82 | ||
| Site 2 | P. parva | 1 | 0.64 | 0.64 | 2.03 | 0.045 * |
| Residuals | 8 | 2.51 | 0.31 | 0.80 | ||
| Site 3 | P. parva | 1 | 0.25 | 0.25 | 1.11 | 0.357 |
| Residuals | 8 | 1.81 | 0.23 | 0.88 | ||
| Indices | Before Pseudorasbora parva | After Pseudorasbora parva | U | Z | p Value | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | ||||
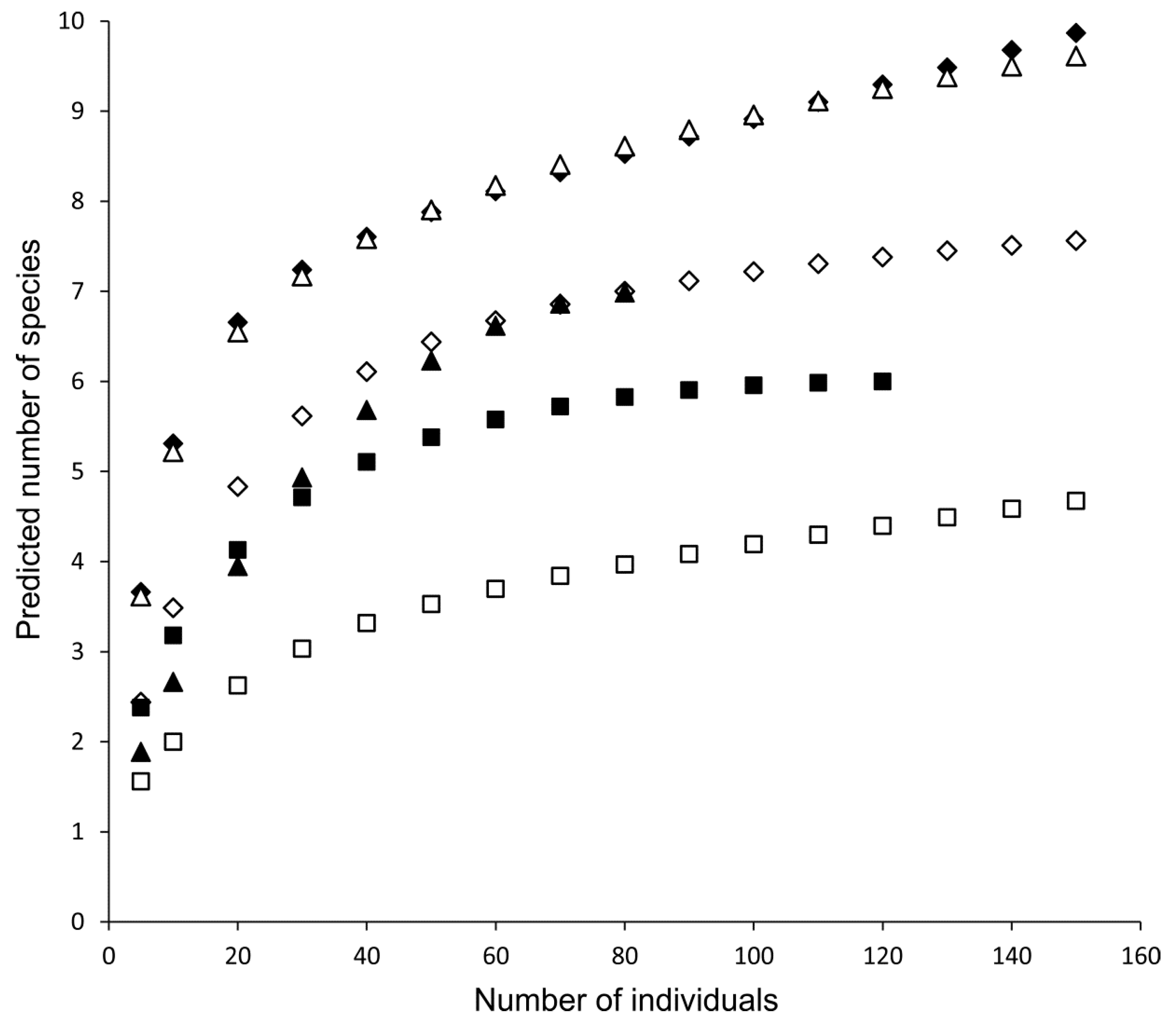
| Estimated Species richness | 7.5 ± 2.1 | 4.4–9.8 | 10.1 ± 2.0 | 4.4–12.0 | 35.0 | −2.7 | 0.01 * |
| Rarefacted species richness (for 20 individuals) | 3.3 ± 2.1 | 1.0–5.7 | 3.7 ± 1.7 | 1.0–6.2 | 83.0 | −0.5 | 0.60 |
| Margalef index (R) | 1.3 ± 1.4 | 0.0–3.4 | 1.6 ± 1.3 | 0.0–3.6 | 82.0 | −0.6 | 0.57 |
| Variables | r | t (N − 2) | p Value |
|---|---|---|---|
| Total density of fish | 0.29 | 1.34 | 0.20 |
| Estimated species richness (eS) | 0.77 | 5.30 | <0.01 * |
| Rarefacted species richness (for 20 ind.) | 0.68 | 4.04 | <0.01 * |
| Margalef index (R) | 0.76 | 5.18 | <0.01 * |
| Density of predatory fish (CPUE) | −0.52 | −2.63 | 0.02 * |
| Abundance of predatory fish (%) | −0.61 | −3.39 | 0.01 * |
| Density of brown trout (CPUE) | −0.62 | −3.10 | 0.01 * |
| Density of Eurasian perch (CPUE) | −0.26 | −1.25 | 0.25 |
| Density of Cyprinidae fish (excluding P. parva) (CPUE) | 0.62 | 3.47 | 0.01 * |
| Abundance of Cyprinidae fish (excluding P. parva) (%) | 0.49 | 2.46 | 0.02 * |
| Density of gudgeon (CPUE) | 0.67 | 3.94 | <0.01 * |
| Density of roach (CPUE) | 0.28 | 1.28 | 0.21 |
| Density of tench (CPUE) | 0.63 | 3.53 | 0.01 * |
| Density of bleak (CPUE) | 0.64 | 3.59 | 0.01 * |
| Density of threespine stickleback (CPUE) | 0.38 | 1.82 | 0.08 |
| Density of stone loach (CPUE) | 0.48 | 2.42 | 0.03 * |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rechulicz, J. Changes in a Fish Community in a Small River Related to the Appearance of the Invasive Topmouth Gudgeon Pseudorasbora parva (Temminck & Schlegel, 1846). Water 2019, 11, 1857. https://doi.org/10.3390/w11091857
Rechulicz J. Changes in a Fish Community in a Small River Related to the Appearance of the Invasive Topmouth Gudgeon Pseudorasbora parva (Temminck & Schlegel, 1846). Water. 2019; 11(9):1857. https://doi.org/10.3390/w11091857
Chicago/Turabian StyleRechulicz, Jacek. 2019. "Changes in a Fish Community in a Small River Related to the Appearance of the Invasive Topmouth Gudgeon Pseudorasbora parva (Temminck & Schlegel, 1846)" Water 11, no. 9: 1857. https://doi.org/10.3390/w11091857
APA StyleRechulicz, J. (2019). Changes in a Fish Community in a Small River Related to the Appearance of the Invasive Topmouth Gudgeon Pseudorasbora parva (Temminck & Schlegel, 1846). Water, 11(9), 1857. https://doi.org/10.3390/w11091857