Determination of Budesonide and Sulfasalazine in Water and Wastewater Samples Using DLLME-SFO-HPLC-UV Method
Abstract
:1. Introduction
2. Experimental
2.1. Instrumentation
2.2. Reagents and Standards
2.3. Samples Preparation
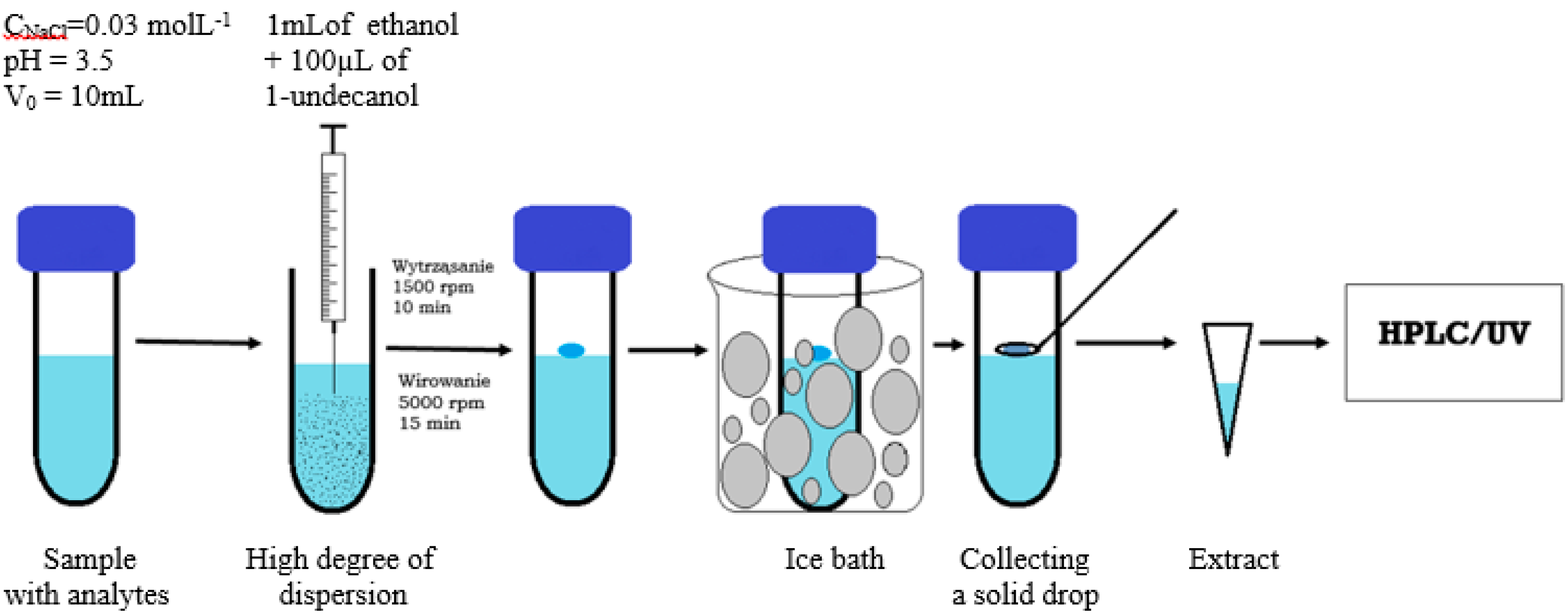
2.4. General DLLME-SFO Procedure
3. Results and Discussion
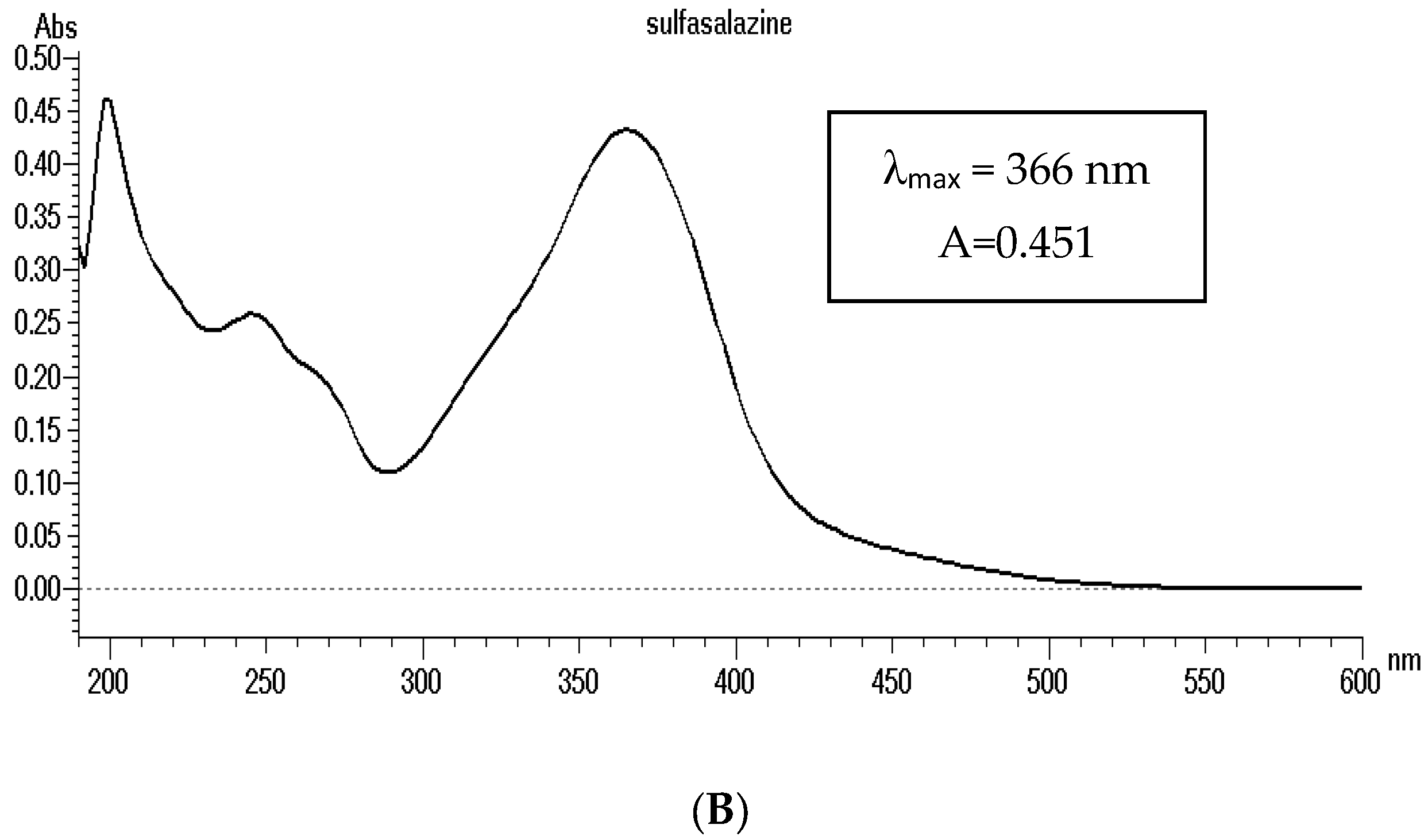
3.1. Primary Studies and HPLC Analysis
3.2. Optimization of Extraction Parameters
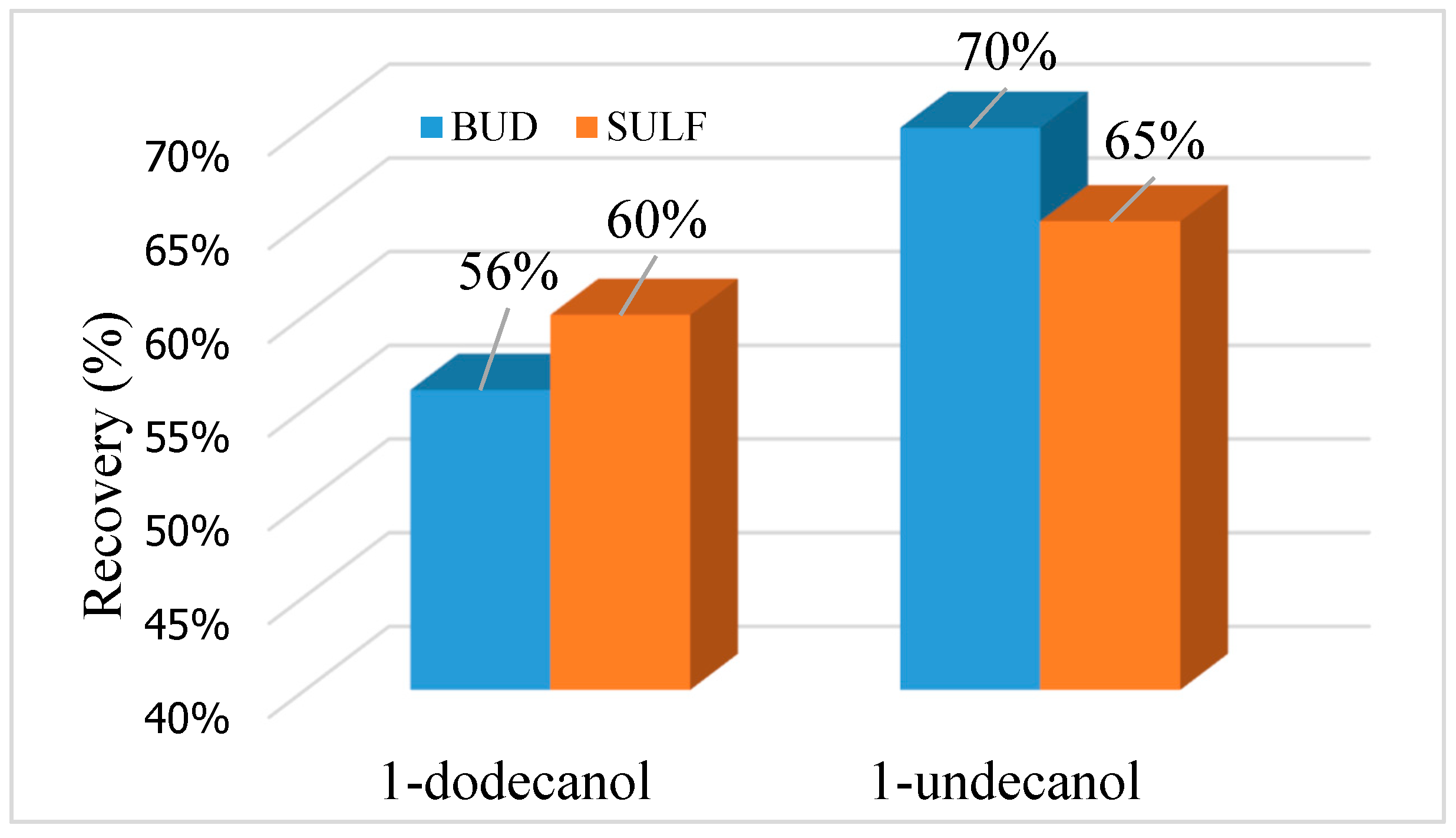
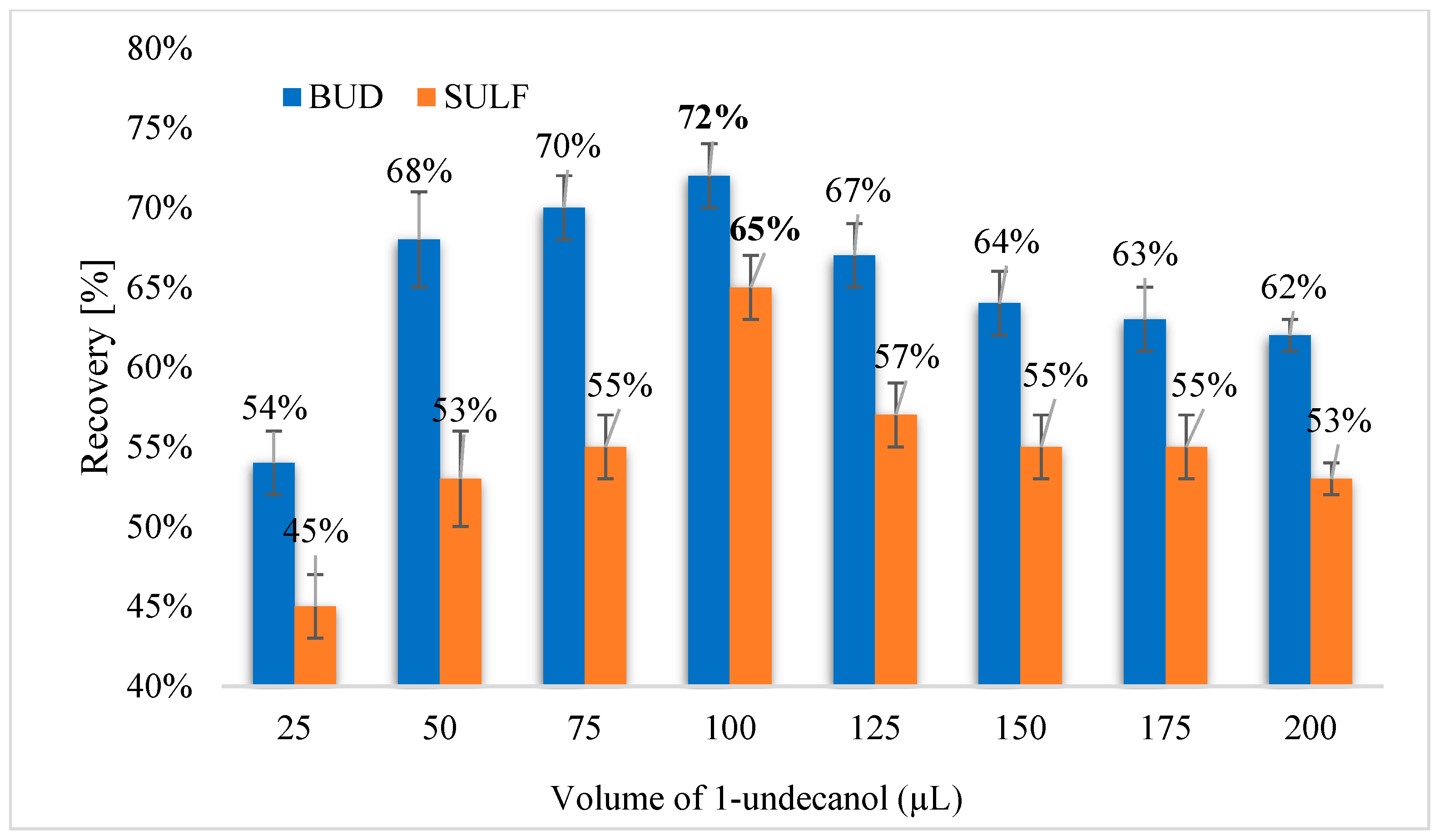
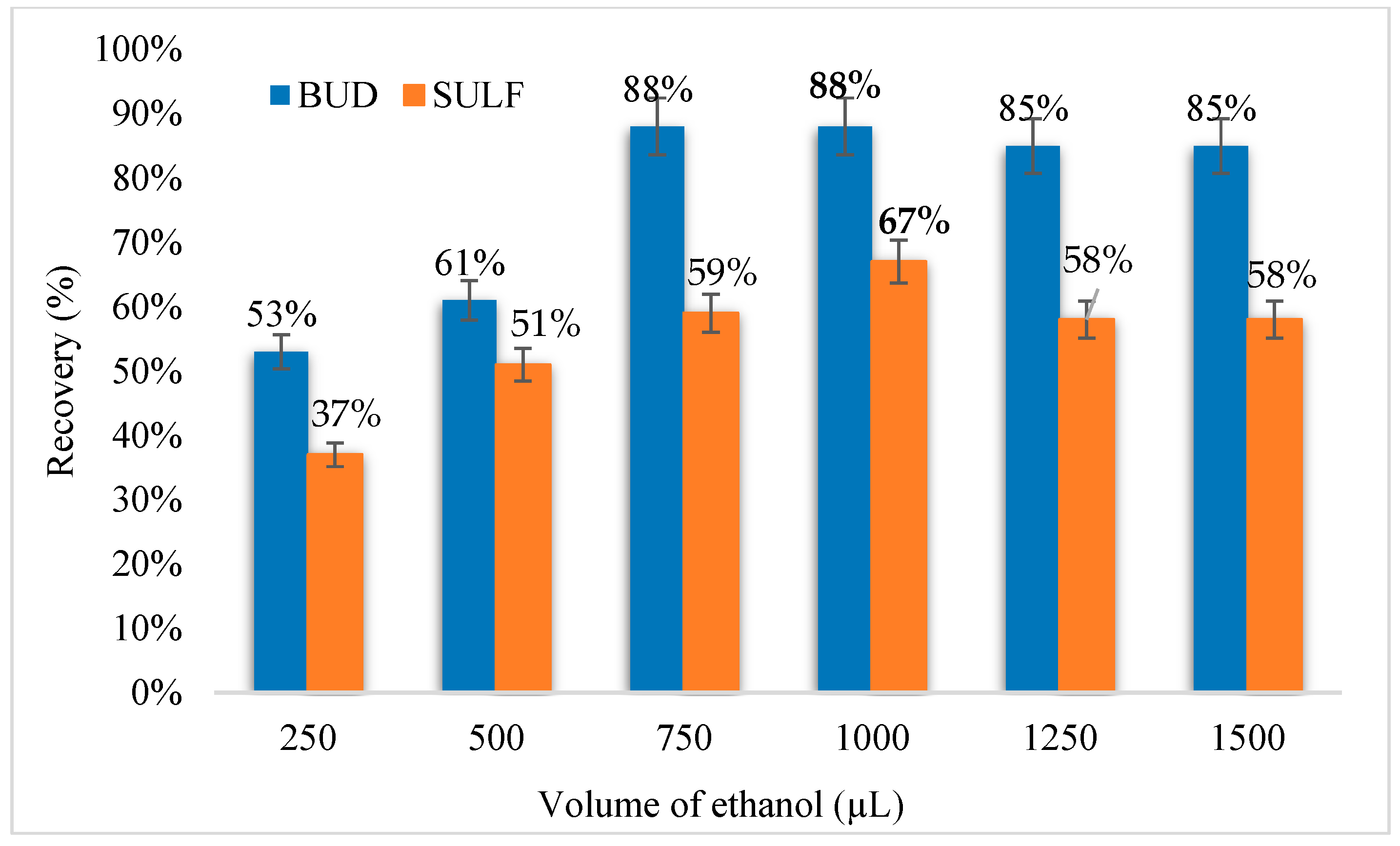
3.2.1. Selection of Extraction Solvent and Its Volume
3.2.2. Selection of Dispersant Solvent and its Volume
3.2.3. Effect of Ionic Strength
3.2.4. Influence of pH of Sample
3.2.5. Effect of the Time and Speed of Shaking and Centrifugation
3.3. Selectivity
3.4. Analytical Performance
3.5. Application to Natural Samples
4. Conclusion
Author Contributions
Conflicts of Interest
References
- Semreen, M.H.; Shanableh, A.; Semerjian, L.; Alniss, H.; Mousa, M.; Bai, X.; Acharya, K. Simultaneous Determination of Pharmaceuticals by Solid-phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry: A Case Study from Sharjah Sewage Treatment Plant. Molecules 2019, 24, 633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Y.; Moellmann, H.; Hochhaus, G. Simultaneous quantification of budesonide and its two metabolites, 6beta-hydroxybudesonide and 16alpha-hydroxyprednisolone, in human plasma by liquid chromatography negative electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2003, 17, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Gazzotti, T.; Barbarossa, A.; Zironi, E.; Roncada, P.; Pietra, M.; Pagliuca, G. An LC-MS/MS method for the determination of budesonide and 16 alpha-hydroxyprednisolone in dog plasma. Methodsx 2016, 3, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Pile, K.D. Sulfasalazine and Related Drugs; Encyclopedia of Inflammatory Diseases; Springer: New York, NY, USA, 2014; pp. 1–5. [Google Scholar]
- Gupta, M.; Bhargava, H.N. Development and validation of a high-performance liquid chromatographic method for the analysis of budesonide. J. Pharm. Biomed. Anal. 2006, 40, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Naikwade, S.R.; Bajaj, A.N. Development of a validated specific HPLC method for budesonide and characterization of its alkali degradation product. Can. J. Anal. Sci. Spectrosc. 2008, 53, 113–122. [Google Scholar]
- Deventer, K.; Mikulcikova, P.; Van Hoecke, H.; Van Eenoo, P.; Del-beke, F.T. Detection of budesonide in human urine after inhalation by liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2006, 42, 474–479. [Google Scholar] [CrossRef]
- Nilsson, K.; Andersson, M.; Beck, O. Phospholipid removal combined with a semi-automated 96-well SPE application for determination of budesonide in human plasma with LC-MS/MS. J. Chrom. B-Anal. Technol. Biomed. Life Sci. 2014, 970, 31–35. [Google Scholar] [CrossRef]
- Szeitz, A.; Manji, J.; Riggs, K.W.; Thamboo, A.; Javer, A.R. Validated assay for the simultaneous determination of cortisol and budesonide in human plasma using ultra high performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 198–206. [Google Scholar] [CrossRef]
- Joseph, S.; Menon, S.; Khera, S. Simultaneous determination of methotrexate and sulfasalazine in plasma by HPLC-DAD. LC GC N. Am. 2015, 33, 122–138. [Google Scholar]
- Saini, B.; Bansal, G. Degradation study on sulfasalazine and a validated HPLC-UV method for its stability testing. Sci. Pharm. 2014, 82, 295–306. [Google Scholar] [CrossRef]
- Patil, A.; Raheja, V.; Damre, A. Simultaneous analysis of intestinal permeability markers, caffeine, paracetamol and sulfasalazine by reverse phase liquid chromatography: A tool for standardization of rat everted gut sac model. Asian J. Pharm. Clin. Res. 2010, 3, 204–207. [Google Scholar]
- Kwiecien, A.; Piatek, K.; Zmudzki, P.; Krzek, J. TLC-densitometric determination of sulfasalazine and its possible impurities in pharmaceutical preparations. Acta Chrom. 2015, 27, 623–635. [Google Scholar] [CrossRef]
- Su, F.; Sun, Z.Q.; Liang, X.R. Development and validation of a quantitative NMR method for the determination of the commercial tablet formulation of sulfasalazine. Curr. Pharm. Anal. 2019, 15, 39–44. [Google Scholar] [CrossRef]
- Ramezani, Z.; Dibaee, N. Determination of sulfasalazine in sulfasalazine tablets using silver nanoparticles. Iran. J. Pharm. Sci. 2012, 8, 129–134. [Google Scholar]
- Gu, G.Z.; Xia, H.M.; Pang, Z.Q.; Liu, Z.Y.; Jiang, X.G.; Chen, J. Determination of sulphasalazine and its main metabolite sulphapyridine and 5-aminosalicylic acid in human plasma by liquid chromatography/tandem mass spectrometry and its application to a pharmacokinetic study. J. Chrom. B 2011, 879, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Grabic, R.; Fick, J.; Lindberg, R.H.; Fedorova, G.; Tysklind, M. Multi-residue method for trace level determination of pharmaceuticals in environmental samples using liquid chromatography coupled to triple quadrupole mass spectrometry. Talanta 2012, 100, 183–195. [Google Scholar] [CrossRef]
- Gineys, N.; Giroud, B.; Vulliet, E. Analytical method for the determination of trace levels of steroid hormones and corticosteroids in soil, based on PLE/SPE/LC-MS/MS. Anal. Bioanal. Chem. 2010, 397, 2295–2302. [Google Scholar] [CrossRef]
- Fiori, J.; Andrisano, V. LC-MS method for the simultaneous determination of six glucocorticoids in pharmaceutical formulations and counterfeit cosmetic products. J. Pharm. Biomed. Anal. 2014, 91, 185–192. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multiresidue methods for the analysis of pharmaceuticals, personal care products and illicit drugs in surface water and wastewater by solid-phase extraction and ultra performance liquid chromatography-electrospray tandem mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 1293–1308. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The effect of signal suppression and mobile phase composition on the simultaneous analysis of multiple classes of acidic/neutral pharmaceuticals and personal care products in surface water by solid-phase extraction and ultra performance liquid chromatography-negative electrospray tandem mass spectrometry. Talanta 2008, 74, 1299–1312. [Google Scholar]
- Leong, M.I.; Huang, S.D. Dispersive liquid-liquid microextraction method based on solidification of floating organic drop combined with gas chromatography with electron-capture or mass spectrometry detection. J. Chrom. A 2008, 1211, 8–12. [Google Scholar] [CrossRef]
- Ahmadi-Jouibari, T.; Fattahi, N.; Shamsipur, M. Rapid extraction and determination of amphetamines in human urine samples using dispersive liquid-liquid microextraction and solidification of floating organic drop followed by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2014, 94, 145–151. [Google Scholar] [CrossRef]
- Rahimi, A.; Hashemi, P. Development of a dispersive liquid-liquid microextraction method based on solidification of a floating organic drop for the determination of beta-carotene in human serum. J. Anal. Chem. 2014, 69, 352–356. [Google Scholar] [CrossRef]
- Jian, Y.H.; Hu, Y.; Wang, T.; Liu, J.L.; Zhang, C.H.; Li, Y. Dispersive liquid-liquid microextraction based on solidification of floating organic drop with high performance liquid chromatography for determination of deca brominated diphenyl ether in surficial sediments. Chin. J. Anal. Chem. 2010, 38, 62–66. [Google Scholar] [CrossRef]
- Hou, F.; Deng, X.; Jiang, X.; Yu, J. Determination of parabens in beverage samples by dispersive liquid-liquid microextraction based on solidification of floating organic droplet. J. Chrom. Sci. 2014, 52, 1332–1338. [Google Scholar] [CrossRef]
- Yamini, Y.; Rezaee, M.; Khanchi, A.; Faraji, M. Dispersive liquid-liquid microextraction based on the solidification of floating organic drop followed by inductively coupled plasma-optical emission spectrometry as a fast technique for the simultaneous determination of heavy metals. J. Chrom. A 2010, 1217, 2358–2364. [Google Scholar] [CrossRef]
- Shamsipur, M.; Fattahi, N.; Assadi, Y.; Sadeghi, M.; Sharafi, K. Speciation of As(III) and As(V) in water samples by graphite furnace atomic absorption spectrometry after solid phase extraction combined with dispersive liquid-liquid microextraction based on the solidification of floating organic drop. Talanta 2014, 130, 26–32. [Google Scholar] [CrossRef]
- Moghadam, M.R.; Shabani, A.M.H.; Dadfarnia, S. Spectrophotometric determination of iron species using a combination of artificial neural networks and dispersive liquid-liquid microextraction based on solidification of floating organic drop. J. Hazard. Mater. 2011, 197, 176–182. [Google Scholar] [CrossRef]
- Li, Y.; Peng, G.; He, Q.; Zhu, H.; Al-Hamadani, S.M.Z.F. Dispersive liquid-liquid microextraction based on the solidification of floating organic drop followed by ICP-MS for the simultaneous determination of heavy metals in wastewaters. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2015, 140, 156–161. [Google Scholar] [CrossRef]
- Al-Saidi, H.M.; Emara Adel, A.A. The recent developments in dispersive liquid–liquid microextraction for preconcentration and determination of inorganic analytes. J. Saudi Chem. Soc. 2014, 18, 745–761. [Google Scholar] [CrossRef]
- Wang, H.M.; Jiang, X.H.; Lin, S.; Yi, H. Studies on determination of sulfasalazine and sulfapyridine in human plasma by HPLC and pharmacokinetics in human volunteers. Chin. J. Antibiot. 2013, 38, 223–226. [Google Scholar]



| Extractant | Chemical Formula | Density (g cm−3) | Temperature of Solidification (°C) |
|---|---|---|---|
| n-hexadecane | CH3(CH2)14CH3 | 0.77 | 18 |
| 2-dodecanol | CH3(CH2)9CH(OH)CH3 | 0.80 | 17–18 |
| 1-decanol | CH3(CH2)9OH | 0.83 | 6.4 |
| 1-dodecanol | CH3(CH2)11OH | 0.83 | 22–24 |
| 1-undecanol | CH3(CH2)10OH | 0.83 | 13–15 |
| 1-chlorooctadecane | CH3(CH2)16CH2Cl | 0.85 | 20–24 |
| 1-bromohexadecane | CH3(CH2)15Br | 0.99 | 16–18 |
| 1,10-dichlorodecane | Cl(CH2)10Cl | 0.99 | 14–16 |
| Type of Electrolyte | Recovery (%) | |
|---|---|---|
| BUD | NaCl | 90 |
| KCl | 57 | |
| CaCl2 | 72 | |
| SULF | NaCl | 78 |
| KCl | 64 | |
| CaCl2 | 73 |
| Interferent | BUD (5 × 10−5 mol L−1) | SULF (5 × 10−5 mol L−1) |
|---|---|---|
| Diclofenac | 5 | 15 |
| Ibuprofen | 5 | 5 |
| Metronidazole | 10 | 15 |
| Caffeine | 10 | 10 |
| Acetylsalicylic acid | 20 | 3 |
| Ascorbic acid | 15 | 15 |
| Levomepromazine | 20 | 5 |
| Naproxen | 5 | 2 |
| Ranitidine | 20 | 10 |
| Mg2+ | 20 | 15 |
| Ca2+ | 20 | 20 |
| Fe3+ | 3 | 2 |
| SO42− | 30 | 30 |
| PO43− | 50 | 40 |
| CO32− | 30 | 30 |
| BUD | SULF | |
|---|---|---|
| Beer’s low range (mol L−1) | 5 × 10−8–2 × 10−5 | 5 × 10−8–2 × 10−5 |
| Beer’s low range (µg mL−1) | 0.022–8.611 | 0.020–7.968 |
| Equation of calibration graph (n = 5) | y = 1.09 × 1011 x + 6 971 | y = 1.15 × 1011 x − 6 390 |
| Slope ± standard deviation SD | 1.09 × 1011 ± 0.88 × 1010 | 1.15 × 1011 ± 0.98 × 1010 |
| Intercept ± standard deviation SD | 6 971 ± 1 102 | 6 390 ± 989 |
| Correlation coefficient R2 ± standard deviation SD | 0.999 ± 0.004 | |
| Precision—intraday RSD (n = 10, %) | 3.75 | 3.15 |
| Precision—interday RSD (n = 10, %) | 0.66 | 2.88 |
| Limit of detection LOD (mol L−1) | 2.67 × 10−8 | 2.92 × 10−8 |
| Limit of detection LOD (µg mL−1) | 0.011 | 0.012 |
| Limit of quantification LOQ (mol L−1) | 8.10 × 10−8 | 8.84 × 10−8 |
| Limit of quantification LOQ (µg mL−1) | 0.035 | 0.035 |
| Enrichment factor EF | 145.7 | 119.5 |
| Recovery ± standard deviation SD (%) | 102 ± 7 | 84 ± 5 |
| Volume of extract (µL) | 70 ± 5 | |
| Sample | Isolation Technique | Determination Method | LOQ | EF | Lit. |
|---|---|---|---|---|---|
| BUD | |||||
| Surface water, wastewater | SPE | LC-MS/MS | 4.2–5.8 ng L−1 | nd | [17] |
| Soils | SPE (Oasis HLB sorbent) | LC-MS/MS | 2.84 ng g−1 | nd | [18] |
| Surface water, wastewater samples | DLLME-SFO | HPLC-UV | 0.035 µg mL−1 | 145.7 | This method |
| SULF | |||||
| Surface water | SPE | UPLC-MS/MS | 5 ng L−1 | nd | [21] |
| Surface water, wastewater | SPE (Oasis MCX sorbent) | UPLC-ESI/MS/MS | 1.5 ng L−1 | nd | [20] |
| Human serum | nd | HPLC-DAD | 0.1 ng µL−1 | nd | [10] |
| Human serum | nd | HPLC-UV | 0.5 µg mL−1 | nd | [32] |
| Surface water, wastewater | DLLME-SFO | HPLC-UV | 0.035 µg mL−1 | 119.5 | This method |
| Sample | Added Concentration of Analyte (mol L−1) | Concentration of Found Analyte (mol L−1) | Average Concentration of Found Analyte ± SD (n = 3) (mol L−1) | RSD (n = 3, %) | Average Recovery ± SD (%) |
|---|---|---|---|---|---|
| BUD | |||||
| Biała river | 5.00 × 10−6 | 4.68 × 10−6 | 5.11 × 10−6 ± 3.97 × 10−7 | 7.8 | 102.1 ± 7.7 |
| 5.16 × 10−6 | |||||
| 5.47 × 10−6 | |||||
| Wastewater | 5.84 × 10−6 | 5.85 × 10−6 ± 1.02 × 10−7 | 1.7 | 117.1 ± 1.7 | |
| 5.76 × 10−6 | |||||
| 5.96 × 10−6 | |||||
| SULF | |||||
| Biała river | 5.00 × 10−6 | 4.37 × 10−6 | 4.78 × 10−6 ± 4.03 × 10−7 | 8.4 | 95.5 ± 8.3 |
| 5.17 × 10−6 | |||||
| 4.79 × 10−6 | |||||
| Wastewater | 5.15 × 10−6 | 5.34 × 10−6 ± 3.06 × 10−7 | 5.7 | 106.7 ± 5.7 | |
| 5.69 × 10−6 | |||||
| 5.17 × 10−6 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hryniewicka, M.; Starczewska, B.; Gołębiewska, A. Determination of Budesonide and Sulfasalazine in Water and Wastewater Samples Using DLLME-SFO-HPLC-UV Method. Water 2019, 11, 1581. https://doi.org/10.3390/w11081581
Hryniewicka M, Starczewska B, Gołębiewska A. Determination of Budesonide and Sulfasalazine in Water and Wastewater Samples Using DLLME-SFO-HPLC-UV Method. Water. 2019; 11(8):1581. https://doi.org/10.3390/w11081581
Chicago/Turabian StyleHryniewicka, Marta, Barbara Starczewska, and Agnieszka Gołębiewska. 2019. "Determination of Budesonide and Sulfasalazine in Water and Wastewater Samples Using DLLME-SFO-HPLC-UV Method" Water 11, no. 8: 1581. https://doi.org/10.3390/w11081581
APA StyleHryniewicka, M., Starczewska, B., & Gołębiewska, A. (2019). Determination of Budesonide and Sulfasalazine in Water and Wastewater Samples Using DLLME-SFO-HPLC-UV Method. Water, 11(8), 1581. https://doi.org/10.3390/w11081581




