Abstract
Different irrigation or ventilation strategies by macrofauna may provide a competitive advantage to tolerant species invading impacted benthic systems and alter benthic-pelagic coupling. To comparatively analyze the effects of an exotic and a native polychaete burrower on sediment-water exchanges, two laboratory experiments were performed. In the first experiment, the invasive spionid polychaete Marenzelleria neglecta was added to defaunated sediments and fluxes of the inert tracer (bromide, Br−) were measured to quantify the effects of irrigation by the worm on the tracer transport. In the second experiment, M. neglecta or the native polychaete Hediste diversicolor were introduced to a relatively diverse Baltic soft-bottom macrofauna community. The effect of species on fluxes of reactive solutes (ammonium, NH4+, and phosphate, PO43−) and transport rates of Br− was estimated. The results indicate different invasion effects depending on the characteristics of the recipient habitat. In defaunated sediments, a single specimen of M. neglecta significantly enhanced originally low solute exchange rates. Total tracer flux was significantly enhanced over diffusive flux by a factor of 1.6 ± 0.14 (n = 3). In natural sediments, on the other hand, the addition of either M. neglecta or H. diversicolor had no statistically significant effects on benthic fluxes. Tracer flux estimates between control and treatment incubations differed by less than 10% on average, and both reactive solutes tended to increase by 10 to 40% after additions. One specimen of M. neglecta in cores with defaunated sediment generated approximately 20% of the tracer flux produced by the relatively diverse macrofauna community. Estimated net tracer fluxes in two experiments corresponded well with the number of adult polychaetes found in sediments (r2 = 0.73, p = 0.005, n = 12). The invasive M. neglecta produced a small effect on fluxes in biodiverse sediments, comparable to those of H. diversicolor, but it may deeply alter porewater chemistry in azoic sediment. As M. neglecta tolerates chemically reduced and sulphidic conditions, its bioirigation may favor sediment reoxidation and ultimately the recolonization by less tolerant, native species.
1. Introduction
Understanding and evaluating the functional role of individual species is linked to the growing awareness of invasion phenomena in marine ecology [1,2,3,4,5,6]. There is a need to quantify the impacts of species in recipient ecosystems [7,8,9] and to gain more knowledge regarding the extent of changes in biogeochemical cycling caused by invasions [10,11]. There is also a need to evaluate whether exotic species, whose invasion is favored by specific constraints (i.e., dystrophy, anoxia), may perform ecosystem services that contrast such constraints and ultimately reverse impacts [5,12]. While dependence between a system’s susceptibility to invasions and its diversity has been discussed extensively [8,13,14,15], the relationship between invasive species’ impact and recipient system diversity has rarely been examined [6,16]. It has been hypothesized that changes in species richness may have more profound effects in low-diversity than high-diversity ecosystems [17,18]. Moreover, the role of functional redundancy (i.e., several species performing the same functions) may be as important as or even more important than species richness [19,20]. Thus, it seems that the effect of an invasive species on the recipient ecosystem and its (biogeochemical) functions may depend on both species richness and functional diversity.
Two kinds of sediment incubations are being used to quantify species impact on the exchange of solutes. In situ studies are carried out on unmanipulated sediment, which has a natural level of transport rate variability [21,22,23,24,25]. This approach is used to characterize the complex benthic community’s role expressed as a ratio of measured total flux and estimated diffusion rate of a solute. Since manipulation of community structure is difficult in the field, assessment of the role of a particular species is limited. The second approach is based on laboratory measurements of solute exchange when a single species is introduced to partly or completely homogenized sediment [26,27,28,29,30]. Even with the manipulation of a particular species, the removal of other organisms prevents the retention of different pathways of solute transport. Therefore, the natural level of solute exchange is probably not reflected in these experiments, and treatment effects are more pronounced. Therefore, both of these approaches applied either in situ or in laboratory experiments are not suitable to quantify single species effect in a complex environment, a central issue when evaluating the role of species introductions.
In order to assess the role of an invasive species on different levels of a recipient system, macrofauna diversity was manipulated experimentally. In this respect, our study was designed to mimic the introduction of the burrowing polychaete Marenzelleria sp. into European waters in the mid-1980s [31,32,33]. This spionid species is able to tolerate low oxygen conditions and salinity gradients as well as the presence of hydrogen sulfide [34,35,36] and has the highest population production rate ever reported for polychaetes [37]. As a result, Marenzelleria became dominant within a few years in many coastal areas that had different species richness (the Baltic Sea: for review see [38]; the North Sea: [39]). Its importance in the local food web is confirmed [40,41], and the potential for significant competition with common benthic species has been reported [42,43].
The main goal of this study was to examine the role of this species on the transport of solutes in benthic habitats characterized by differences in macrofaunal diversity. We applied two different experimental approaches; (1) Marenzelleria neglecta was introduced to the defaunated sediments to mimic the species invasion into an “impoverished” type of benthic community or “azoic” state of sedimentary habitat. Similar conditions have been reported as being typical for the Baltic Sea [44,45] and clearly reflect the negligible contribution of benthic macrofauna irrigation to the net material fluxes. (2) M. neglecta or the dominant native polychaete (Hediste diversicolor) was introduced to sediment with a relatively diverse community and high natural background of solute transport rates. Here we simulated the scenario of the new species invasion and changes in abundance of the native dominant species in coastal sediment of the Baltic Sea characterized by high bottom macrofauna diversity.
2. Materials and Methods
2.1. Sediment and Macrofauna Sampling
Fifteen sediment cores (inner diameter 10 cm, length 60 cm) containing local bottom macrofauna were taken at approximately 0.6 m depth and a salinity of 15 psu in Breitling Bay, southern Baltic Sea (54°10.6′ N; 12°08.2′ E). At the same time (November, water temperature 10°C) macrofauna samples were taken in parallel (3 replicates) with hand-operated tube corer (inner diameter 10 cm) and fixed with buffered 4% formaldehyde solution. Standard macrofauna counting and weighing procedure on these samples provided general information on soft-bottom macrofauna present in the sediment sampled for the experiments.
For the experimental treatments, specimens of the nereid polychaete H. diversicolor were collected at the site where sediment sampling occurred. In the laboratory, the worms were maintained under continuous aeration in tanks which contained unsieved habitat sediment. Since Breitling Bay is known to be only occasionally invaded by very low numbers of M. neglecta [46], for experimental manipulations this species was sampled at a shallow site in the Darss-Zingst Bodden chain (54°25.1′ N; 12°46.9′ E). Worms were subjected to the stepwise salinity adaptation from 8 (natural conditions) to 15 (experimental conditions) for one-week during laboratory maintenance before the experiments. No significant effects of this salinity change on the species metabolic activity was expected, relying on the overall conclusion of the species’ ability to cope with a wide range of experimental salinity regimes (from 0.5 to 30 psu) [47].
2.2. Experimental Design
Six sediment cores were frozen after transport to the laboratory. The remaining nine cores were filled with approximately 1 L of filtered habitat water (salinity 15), aerated (air bubbling) and maintained at in situ temperature (11°C), in a dark constant-temperature room. Average water–sediment volume ratio in the cores was 1 ± 0.07:1.4 ± 0.2. Water movement inside the cores was maintained before and during incubations by slow air bubbling in order to prevent stratification. The overlying water was changed every 3 days using a peristaltic pump and avoiding sediment resuspension. Oxygen concentration did not fall below 6.5 mg/L during cores maintenance under laboratory conditions.
Two sets of experiments were carried out using defaunated (experiment 1) or natural (experiment 2) sediments.
In experiment 1, six cores with frozen sediment were thawed a week before experiment, and dead macrofauna organisms were removed from the sediment surface. Two tracer incubations, with three replicate cores each time, were carried out. Measurement of nutrients was not done in this experiment, since the freezing of sediment significantly altered fluxes of reactive solutes in preliminary experiments. Transport rates in such sediment were not comparable with those in natural cores.
The first incubation of sediment without macrofauna was designed to test correspondence between measured and calculated diffusive fluxes (see model description below). Sodium bromide solution (tracer) was injected into the overlying water (initial Br− concentration 4.2–4.8 mM) and 1 mL samples were taken every 20–30 min during the first 3 h. Thereafter sampling frequency was incrementally decreased from once per hour to every 3 h during the daytime over the next 3 days, after which time incubation was terminated. Sediments were then immediately sectioned at 0.5 cm intervals down to 3 cm depth, and at 1 cm intervals between 3 and 10 cm depth. Subsamples of wet sediments were taken for porosity (2 cm3), density and grain size analysis (~100 g dry weight). Porewater was separated by centrifugation (800 rpm for 5 min) using a modified version of the centrifugation tube [48] and used for Br− analysis.
During the second incubation of defaunated cores, single specimens of M. neglecta were added (3 replicates). Species abundance in treated cores was equivalent to 130 ind m−2, which is in the range observed in stressed oligohaline habitats (e.g., Darss-Zingst: [49]; Curonian lagoon: [50]). After a 1-day acclimation, Br− was injected into the overlying water of experimental cores. Tracer sampling and handling of sediment were conducted as described above.
During experiment 2, three experimental runs were carried out with natural sediment cores (Figure 1). Three replicate cores with newly exchanged biotope water were used in each run. Sediment handling (slicing and macrofauna counting) and porewater extraction (centrifugation) of these three replicates at the end of each run took 10 to 12 h. Therefore, the experimental runs (1–3) were sequential and followed the same protocol.
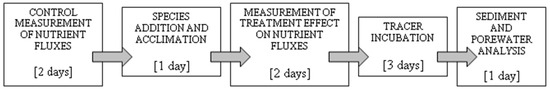
Figure 1.
Sequence of experimental procedures carried out during experimental runs with natural sediment in experiment 2 (see text for explanations).
The first run started 4 days after core collection. During the first two days, the initial control flux of NH4+ and PO43− was measured (Figure 1). Overlying water samples were taken successively every 2–3 h during the daytime. The removed water volume was replaced by adding water with known nutrient concentrations.
After the 2-day measurement of initial control flux, most of the overlying water in the cores was exchanged, and three adult worms of M. neglecta (~400 ind m−2, ~5.0–7.6 g ash free dry weight (AFDW) m−2) were added. Worms burrowed immediately, and their subsequent acclimation lasted 24 h. The duration of the acclimation period was arbitrarily selected to compromise between minimizing the time interval between the control (before) and treatment (after) measurements and maximizing the time for the added specimens to establish their burrows. After 24 h, the measurement of nutrient fluxes in treated cores was conducted for the following 2 days at regular intervals. Time intervals between samplings were the same as in control measurements.
After the nutrient flux measurements, the overlying water was exchanged, and Br− was added. Tracer sampling during the following 3 days and handling of sediment were carried out in the same way as described for experiment 1. Porewater was used for the determination of the concentrations of NH4+, PO43−, and Br−. During sectioning, large macrofauna (i.e., organisms noticed by the naked eye) were identified to the species level and counted.
The second run started on day 17 after the core collection. All experimental procedures were performed following the same scheme as in run 1 except that three adult H. diversicolor worms (~400 ind m−2, ~7.2–10.9 g AFDW m−2) were added to the experimental cores.
Since species additions in the first two runs were simultaneously coupled with water replenishment and a one-day acclimation period, run 3 was designed as an internal control to separate between these effects. All procedures during this run (27 days after sediment sampling) were performed in the same way as in the treatment runs (runs 1 and 2) but with no addition of worms.
2.3. Processing of Samples and Calculation of Solute Fluxes
Ammonium (17.5 mL samples) and PO43− (25 mL samples) were determined according to [51,52] respectively. The precision of methods was 0.02 μM for PO43− and 0.1 μM for NH4+. Bromide concentrations were measured by ion chromatography with conductivity (column: Sykam LCA A14, Sykam GmbH, Eresing, Germany) or UV/VIS detection (Sykam IBJ A3, Sykam GmbH, Eresing, Germany) with an analytical precision of 0.5% at 5 mM and 1% at <1 mM Br−. Porosity was calculated from water content data determined after drying the sediment at 60°C overnight. The grain size distribution of sediments was determined by standard dry sieving procedure for 1 cm sediment layers (down to 10 cm depth) from three randomly selected experimental cores.
Total fluxes of NH4+ and PO43− during control and post-treatment stages were estimated for the time period of the best linear fit (maximum coefficient of determination) to the initial and always highest concentration changes (Figure 2). This initial time span approached by linear fit with the coefficient of determination always greater than 0.70 was limited to 12–35 h for ammonia and to 21–35 h for phosphate flux calculation. Total fluxes across the sediment-water interface in cores were calculated according to:
where V is water volume of overlying water (l), dC/dt is the difference in NH4+ or PO43− concentrations estimated from the slope of the fitted linear regression for time period, dt, of the highest coefficient of determination and A is a surface area of the core sediments (m2). Later on, nutrient fluxes were corrected for additions of water carried out to compensate volume removed during the sampling.
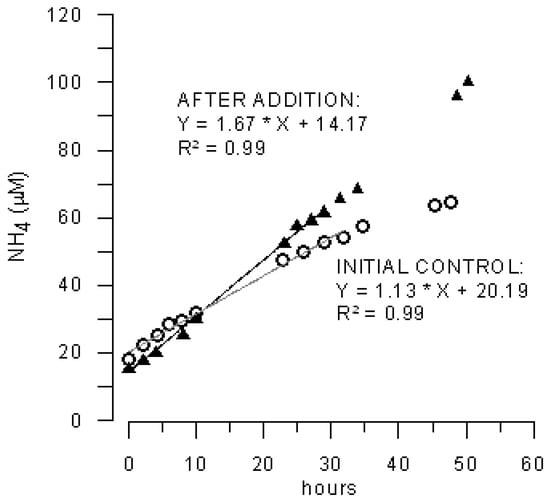
Figure 2.
Ammonium concentration before (open dots, calculated initial control flux 4.18 mmol m−2 day−1, n = 10) and after Marenzelleria neglecta addition (shaded triangles, calculated flux after addition 6.28 mmol m−2 day−1, n = 9) in experiment 2 (run 1).
Total Br− uptake from the overlying water was estimated for a whole incubation period (72 ± 0.9 h). The initial and final Br− concentrations used were derived from the logarithmic fit of concentration decrease versus incubation time.
Time-dependent diffusive decline of Br− in the overlying water and corresponding tracer profile in the sediment were simulated for all individual cores using a previously described box model [25,53]. In short, the box model constructed using software Stella® (Version 1.1, High Performance Systems, Inc., Lebanon, NH, USA, 2001) assumes a homogeneously mixed water column, a diffusive boundary layer (DBL, 350 µm thick) and 30 sediment layers of 4 mm vertical resolution. Iterating at 30 s time intervals, transport between adjacent sediment layers (boxes) is calculated from Fick’s First Law with measured porosities used to calculate the sediment diffusion coefficient according to Archie’Law [54]. The aim of the diffusion model application was to estimate an average diffusive flux during incubation. The ratio of measured (hereafter “total”) to the modeled (hereafter “diffusive”) Br− flux was then used to characterize an extent of irrigation (nonlocal effects) in each individual core. This ratio asymptotically approaches a constant value with time [25]. While it is small at the beginning of incubation due to relatively effective diffusive transport, irrigation effects increase the ratio such that a maximum is reached after roughly one day. Our tests carried out on data from the same 3-day long incubations showed that the difference of 10 h of incubation (the largest time inconsistency found in our experiments) would result in approximately a 0.005 mM difference in Br− concentration at the end of incubation. This difference is one order of magnitude smaller than the analytical error of Br− determination, and the overall effect on the total to diffusive flux ratio is negligible. It also has little effect on the flux enhancement ratio, which was changed by roughly 4%. This demonstrates that the time span of 3 days used in the calculation of fluxes effectively minimizes their dependence on the exact length of incubation and reflects the average flux, not short-term irrigation events.
2.4. Data Analysis
Effect of M. neglecta addition on tracer transport in defaunated sediment was tested for significance by standard Student test for dependent samples. Differences between measured total tracer fluxes and calculated by the diffusive transport model for individual cores were compared.
In order to assess the effect of species additions on the transport of non-reactive Br− in natural sediment, flux enhancement values were compared. Since the transport of Br− does not depend on changes in sediment geochemistry or microbial activity and numbers of irrigators in cores was not changed significantly during the experimental time, one-way ANOVA was applied to test differences in average flux enhancement between non-parallel experimental runs.
To estimate species impacts on nutrient fluxes, transport rates of NH4+and PO43− were measured before species addition (initial control flux) and compared with those determined after species addition (flux after addition) in the same cores using Student test for dependent samples. Prior to the comparison, the initial control flux in each run was adjusted for changes occurring during 1-day acclimation period by estimating a daily rate of flux change from the difference of initial control fluxes between consecutive runs. This daily rate was added to the initial control flux (if the control flux between runs increased) or subtracted from it (if the control flux decreased). The adjusted control values for ammonia fluxes differed from those originally measured by less than 10%, while phosphate fluxes were modified by approximately 1–2%.
3. Results
3.1. Macrofauna and Sediment Characteristics
Density of macrofauna in Breitling Bay ranged from 33,503 to 49,172 ind m−2 (Table 1), whereas biomass was much more variable ranging from 43 to 190 g AFDW m−2. In total, 31 species were found in the local community. The number of species varied from 9 to 14 in the different samples. The macrofauna was dominated by two irrigating macrofauna species, the nereid polychaete H. diversicolor, and the suspension-feeder bivalve, Mya arenaria. Both species accounted for 50–95% of the total macrofauna biomass. The density of H. diversicolor ranged from 8400 to 12,350 ind m−2 between replicates, and the number of adult specimens was approximately 10% of the species abundance or less. Individuals of M. arenaria were present in much lower numbers with a few adults per core only. The occurrence of other irrigating species, Corophium volutator, and Tubifex costatus, was highly variable. No specimens of M. neglecta were found.

Table 1.
Density and ash free dry weight (AFDW) of the most abundant species (0.5 mm mesh size) from 3 replicates taken parallel to the sediment sampling for incubation experiments at Breitling Bay (54°10.6′ N; 12°08.2′ E).
Bottom sediments were mainly formed of fine sand (integral median grain size approximately 0.16 mm, dry bulk density 1.41 g cm−3). Average sediment porosity was 0.44 ± 0.02. Variability in porosity and median grain size was low, and the pattern was similar in the first 10 cm of the sediment column.
In the natural sediment cores used for experiment 2, H. diversicolor was always present, whereas M. arenaria occurred in 4 out of 9 cores (experiment 2). The number of adult H. diversicolor was highly variable with a maximum density of about 900 ind m−2 (7 adult core−1) and a mean of 500 ind m−2. Though one core was inhabited by juvenile worms only, there was generally no regular pattern in the variability of macrofauna numbers noticed when handling sediment at the end of incubations.
The sediment from the cores was heavily bioturbated to 6–8 cm depth, and burrow constructions of the large H. diversicolor and M. arenaria were observed to 15–18 cm depth. Depth distribution of juvenile H. diversicolor was usually restricted to the surface layer (2–3 cm), whereas burrows of C. volutator extended 1 cm deep into the sediments.
The addition of the dominant burrowing polychaete H. diversicolor during experiment 2 resulted in a biomass increase of approximately 20 to 30% when compared to natural sediment. In terms of density, the addition resulted in an approximately 2 to 3% change in the total number of H. diversicolor. These figures are consistent with the small-scale heterogeneity range observed in samples taken from the field.
3.2. Effects of Marenzelleria neglecta on Bromide Fluxes in Defaunated Sediment (Experiment 1)
Total Br− flux measured in defaunated sediment corresponded well to the modeled diffusive flux (Table 2) and differed by 12%. The actual Br− distribution in the sediment at the end of the incubation was also consistent with the modeled tracer profile (Figure 3a). Tracer inventory, i.e., comparison of the actual Br– loss from the water column to its final amount measured in sediments resulted in 90% agreement (0.49 mmol loss versus 0.44 mmol in sediments).

Table 2.
Diffusive and total bromide fluxes (mmol m−2 h−1) calculated for different incubations (runs) in two experiments.
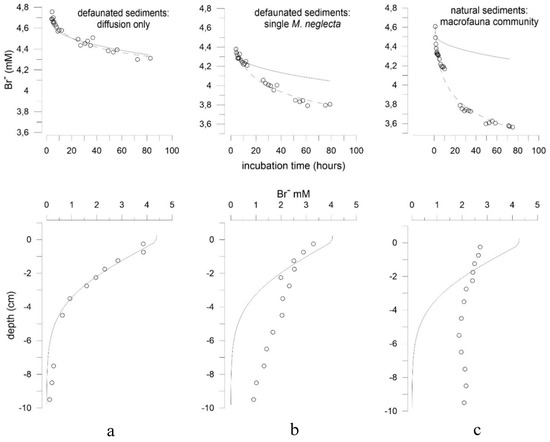
Figure 3.
Examples of tracer uptake and correspondent concentration profiles at the end of incubations of (a) defaunated sediment; (b) defaunated sediment with single M. neglecta; and (c) sediment containing natural community. Solid lines show modeled diffusive tracer uptake and resulting profile in sediment, open symbols indicate measured concentrations, dashed lines represent logarithmic fit to the measured concentrations (see “Material and methods” section for further explanations).
Results from the defaunated cores with single M. neglecta added to the sediment show on average significantly higher measured tracer flux than the modeled molecular diffusion (t = 3.43, p = 0.036) (Table 2). Correspondingly, flux enhancement was calculated as 1.60 ± 0.14.
Comparison of total and diffusive Br− distribution in sediment at the end of experiment 1 showed a nearly equal amount of tracer transported by irrigation and diffusion in the first 1.5–2.5 cm of sediment (Figure 3b). However, below this depth total and diffusive profiles of tracer distribution diverged substantially. Comparing both profiles, the amount of tracer transported by diffusive transport into the sediment depth of 3 to 4 cm, reached the deepest analyzed layer of 9 to 10 cm depth due to irrigation of a single specimen.
3.3. Effects of Polychaetes on Nutrient and Bromide Fluxes in Natural Sediment (Experiment 2)
Total Br− flux produced by the natural community in experiment 2 was higher than the exchange rate facilitated by one specimen of M. neglecta (Table 2). Total tracer uptake by the natural community was enhanced relative to the modeled diffusive flux by a factor of 3.3 ± 0.3 on average and varied from 2 to 5.6 in all experimental cores used in experiment 2. Total tracer transport showed little variation between the treated (runs 1, 2) and untreated (run 3) cores with highly consistent averages from 2.3 to 2.4 mmol m−2 h−1 between runs (Table 2). Therefore, no effect on flux enhancement was determined after the addition of either M. neglecta or H. diversicolor (one-way ANOVA, F = 0.30, p = 0.75) with the largest part of total flux variability being accounted for by variance of fluxes between replicates within experimental runs.
Measured Br− flux, i.e., total tracer transport rate, depended little on modeled diffusion (r2 = 0.22, p = 0.132, n = 12). Within experimental runs, tracer flux between replicates differed by a factor 2 to 3. However, a considerable part of observed flux variability is explained by the number of polychaetes found in the experimental cores at the end of each run (r2 = 0.73, p = 0.005, n = 12) (Figure 4a).
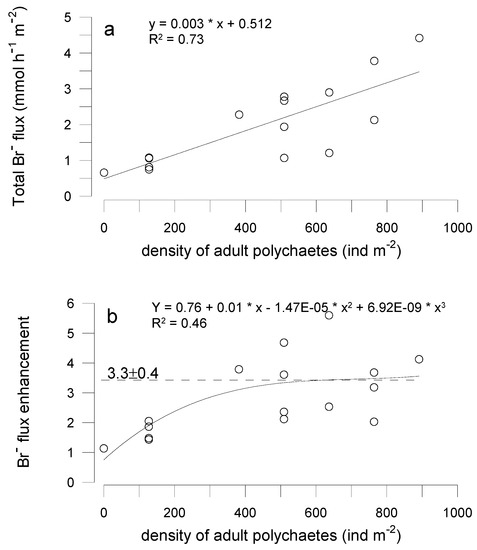
Figure 4.
Total bromide flux (a) and tracer flux enhancement (b) vs. density of polychaetes found in the sediment after incubations. The horizontal dashed line denotes average flux enhancement estimated for the unmanipulated natural sediment.
Attributing tracer flux enhancement (ratio of total to diffusive transport rate) to the number of polychaetes (Figure 4b), the linear relationship explains considerably less variation (r2 = 0.40) and poorly approaches measured values at densities larger than 400 ind m−2. Conversely, the asymptotic non-linear fit approaches an average flux enhancement of approximately 3.3, which is found at higher densities of polychaetes where no apparent increase in irrigation activity occurs.
Bromide distribution in the sediment was generally in agreement with the results on tracer exchange rates calculated from the Br− concentration decline in the overlying water. During the 3-day incubation, Br− was transported far below 10 cm depth in the natural sediment (Figure 3c). This considerably exceeds the maximal depth range of approximately 6–7 cm where Br− would be transported by diffusive transport only. Assuming a balance between the amount of tracer lost from the overlying water and distributed in the pore water of the sediment at the end of incubation, on average 40 ± 15% of tracer was transported below the diffusive transport boundary.
Ammonia and PO43− releases from sediments were increased by 10 and 14%, respectively, after M. neglecta was added to the natural community (run 1, Figure 5a). However, no significant difference in flux was found for either of the nutrients comparing control fluxes with the exchange rates after the species addition.
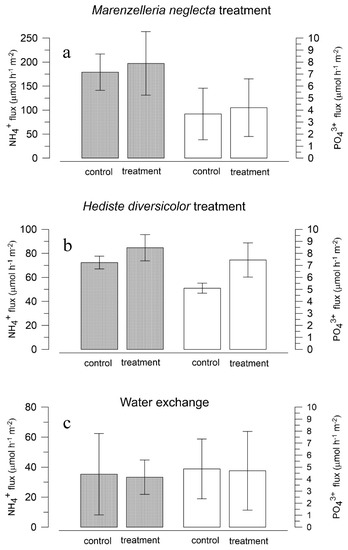
Figure 5.
Effects of species additions and water exchange on the net dissolved fluxes of ammonia (closed bars) and phosphate (open bars) in experiment 2: (a) Marenzelleria neglecta-run 1, (b) Hediste diversicolor-run 2, (c) exchange of the overlying water-run 3. Values are the mean ± standard error for 3 replicate cores.
Addition of the native species H. diversicolor (run 2) resulted in a 17% higher flux of NH4+ and a 40% increase in PO43− flux (Figure 5b). Although treatment effect on PO43− flux was substantially higher than after the addition of M. neglecta, the observed increase was not significant.
Simulated replenishment of the overlying water between control and post-treatment incubations (run 3) showed negligible effects on the total nutrient fluxes (Figure 5c). Ammonia and PO43− fluxes were reduced by up to 5% compared to the initial control flux measurements. Effects generated by water exchange in cores were an order of magnitude lower than after species additions and might be considered as unimportant for separation of polychaete effects.
4. Discussion
4.1. Effects of Polychaetes on Nutrient and Bromide Fluxes
Many experimental designs that were developed to quantify single species effects are performed with homogenized (defaunated) sediment and therefore reduced background variability of solute fluxes. Following this approach in our study, the modeled and measured diffusive fluxes in defaunated sediment agreed well, indicating errors of approximately 10–15%. Given the analytical precision of 0.025 mM and some error possibly introduced by the limited resolution of the interfacial porosity gradient, this difference is not significant.
Simulated invasion of Marenzelleria neglecta (by adding specimens to the defaunated sediments) resulted in significantly enhanced tracer fluxes. Adding a single polychaete specimen to approximately 80 cm2 of surface area facilitated the penetration of the tracer to sediment depths of 10 cm, twice exceeding molecular diffusion limits during the three-day tracer incubations. Bromide flux was on average enhanced by a factor 1.6 relative to molecular diffusion after the addition of a single M. neglecta. This is comparable to estimates revealed by oxygen flux measurements for H. diversicolor in sieved sediments [55]. Flux enhancement generated by a single polychaete is also similar to those reported for meiofauna communities [56]. Despite similar to that reported for native macrofauna or meiofauna, this result has a specific meaning for Marenzelleria, as this tolerant organism is a pioneer genus able to colonize azoic and sulphidic sediment [57]. Its burrowing activity and capacity to cope with chemically reduced conditions allow it to flush and dilute pore waters, introduce oxygen- or nitrate-rich water deep into the sediment, and permit in the long-term the creation or expansion of an oxidized horizon [5,58]. Such bioturbation activity has multiple implications, including the reoxidation of reduced metal pools (Mn2+ and Fe2+) that constitute the natural biogeochemical buffer mechanism for P, as well as for coupled nitrification-denitrification, which net remove N [5,30,59]. Recent studies revealed that Marenzelleria might promote sulphidic conditions, selecting tolerant organisms [11,60] or favoring processes as dissimilative nitrate reduction to ammonium at the expenses of denitrification [61], which is probably true in the short but not in the long term [5]. In a longer temporal perspective, the physiological capacity of Marenzelleria to cope with high sulfide concentrations in the sediment may create positive feedback for the colonization of other less tolerant and native organisms, ultimately restabilizing the biodiversity and biogeochemical functioning of the benthic system [5,58].
On the other hand, the results from treatments in sediment with a natural benthic community showed that a new species invasion (M. neglecta) or changes in abundance of a dominant native species (H. diversicolor) had no statistically significant effects on Br− fluxes. Tracer flux averages between individual runs differed by less than 10%. This result seems contrasting with differences between the two species in burrow ventilation (nearly 10 times higher in Hediste and performed at regular intervals), bioirrigation (higher by Marenzelleria), burrowing depth (higher for Marenzelleria) and physiological features (higher sulfide tolerance by Marenzelleria) [58,60,62]. It is likely that frequent ventilation of burrow with low solute concentrations produces a similar effect than less continuous ventilation of burrow with high solute concentration.
Up to now, only a few attempts have been made to manipulate the benthic community either in laboratory or field incubations [56,63] in order to study faunal impacts on the exchange of solutes. [23,27] were able to relate the abundance of H. diversicolor to the PO43− gradient in sediments in in situ incubations, yielding an R2 = 0.80–0.90 for their observation. However, neither the composition nor structure of the incubated benthic community was described. In contrast, significant relationships were not found between biotic parameters and observed fluxes in a study employing manipulated meiofauna communities [56]. Other studies [22,24,64] revealed qualitative patterns of increased exchange of solutes in habitats with more abundant bottom macrofauna than in impoverished ones, but no quantitative relationships were established. Quantitative relationships between macrofauna and meiofauna communities and process rates (denitrification), were reported likely due to the accuracy of the isotope pairing technique [30,61,62]. In our study, an abundance of adult nereids estimated from field samples (~550–1100 ind m−2, n = 3) was consistent with numbers of adults found when sectioning the experimental sediment (0–900 ind m−2, n = 12). Even though the accuracy of the later counting might be lower (meiofauna and juvenile polychaetes not considered when sectioning the experimental sediment), the density of adult polychaetes was positively related to Br-fluxes and explained 72% of the total tracer transport variability.
Generally, transport rates of reactive solutes varied independently of the exchange of the inert tracer even during the same experimental runs. To some extent, this was determined by the experimental design. Sediment geochemical properties important for ammonia and phosphate transport differed between individual runs (e.g., run 1 and run 3 on day 4 and 27 after sediment sampling, respectively), whereas the tracer gradient between the sediment and overlying water was always kept comparable by adding similar tracer amounts to the cores. Another source of results divergence may come from the time lag involved in the measurement of treatment effects on inert and reactive solute fluxes. Measurement of nutrient fluxes always started one day after treatment while Br− incubation was initiated 3 days after experimental additions. The time difference between the measurements could potentially stimulate some divergence in the results even though experimental additions on both solutes fluxes tend to be insignificant. Differences in the biogeochemical nature of the solutes, however, were probably more important than specifics of experimental design. Reactive solutes would provide more a complex assessment of changes induced by fauna manipulations than inert ones, for they are involved in numerous reactions, particularly at the burrow interface. A considerable part of the inconsistency between nutrient and tracer fluxes might also be attributed to the different number of transport pathways inert and reactive solutes may follow in natural sediment. For instance, at higher PO43− concentrations in the sediment, the activity of bottom-dwelling animals may induce both its transport from sediment by irrigation of deep burrows (increasing transport from sediment) as well as adsorption to ferric oxides due to sediment aeration (reducing transport from sediment). Irrigation effect on the transport of the inert tracer from the overlying water into the sediment, however, will only result in its increased exchange along the concentration gradient. Therefore, groups of benthic organisms that differ in their behavior and vertical distribution in sediment may have opposite or compensatory effects on the fluxes of reactive solutes, while this is less possible for inert tracers. Such divergence between the transport of inert and reactive solutes is more likely in sediment with higher diversity of benthic species and increased complexity of the sediment-animal interactions. This suggestion is supported by other findings [65], which showed a decoupling between nutrient fluxes and irrigation activity at their more intensely irrigated sites.
Increasing the number of the ragworm, H. diversicolor, showed a 35% increase on Br−, 40% increase on NH4+, and 70% increase on PO43− as compared to effects induced by the non-indigenous M. neglecta. Although we applied a correction for slightly different conditions during control measurements, a clearly higher exchange was induced by the nereid worm. This can be partly explained by the larger net metabolic rate of added nereids due to their ~20–40% larger biomass compared to Marenzelleria, assuming comparable biomass normalized metabolic rates of both species at normoxic conditions according to [66]. In addition to that, higher flushing rates between 250 and 700 mL g−1 h−1, as found earlier for nereids [67], may also favor higher effects of H. diversicolor in our experiments. Under oxic conditions, H. diversicolor keeps rhythmic ventilation of its burrows, whereas M. neglecta does not show such regular irrigation [61]. The larger body width of the adult H. diversicolor [52] results in significantly large burrow diameter of 3–5 mm, while the diameter of a typical burrow of M. neglecta ranges from 1.8 to 2.2 mm. At the same time, the branched burrows of ragworms [68], as compared to the tighter I- or J-shaped structures produced by spionid M. virdis [69], may potentially result in larger sediment–water interface at comparable abundances of both species. The differing volume of sediment irrigation by these two polychaetes may lead to considerable differences between the species’ effects in longer temporal scales. In short term experiments, however, the response of microbial metabolism to the treatments is less instantaneous than compared to porewater flushing effects [10,55,70]. Therefore, in our incubations, the different extents of bacterial activity around the tubes of two polychaete species were probably less important. Similarly, the ability of M. neglecta to penetrate considerably deeper (30–35 cm depth) than Hediste diversicolor was not reflected in our experiments. Depth of experimental sediment was limited to approximately 18–20 cm and the sediment column was bioturbated by both species, while in natural conditions, M. neglecta may significantly increase the bioturbation depth and introduce qualitatively new biogeochemical reactions by flushing deep sediment with oxygenated water. This might be particularly important in habitats occupied by shallow burrowers only (e.g., Corophium species, Mesidothea entomon, chironomids, and oligochaetes), where large volumes of deep sediment are not involved in material cycling. Deep burrowing and nocturnal swimming of M. neglecta [71,72] may lead to a considerably increased number of burrows per individual if considered on the long term scale. However, specific experimental approaches would be needed in order to study these species traits in relation to the sediment-water interactions.
In summary, average flux enhancement in defaunated cores containing a single specimen of M. neglecta was about one fifth than that of the natural sediment community. Assuming averaged community’s characteristics (Table 1), one sediment core with the natural community sampled would contain roughly 80 specimens of H. diversicolor, with only 8 of them being adults. In view of this average density and probably stronger bioirrigation capability of H. diversicolor, the effect of a single M. neglecta worm appears to be dramatic. On the other hand, applying an individual irrigation rate of 0.35 mM Br− h−1 m−2 ind−1 for a single M. neglecta estimated from treated defaunated sediment (Table 2), experimental additions in the natural community would result in approximately 45% increase in tracer exchange. These results indicate that the relation between irrigation effect and macrofauna abundance may be non-linear. Similarly, fluxes of contaminants per individual from sediment were greater at a lower density of tubificids in microcosm incubations than at higher densities [73]. For higher densities of freshwater chironomid larvae, underestimated values of measured solute transport rates were reported [29] after comparison with those calculated by a model with no density effect. Density-dependent effects at the population level were recently estimated for dense clumps of mussels as well [74]. Since individuals within a clump are filtering water already cleared by individuals in the outer layer, the clearance rate of the clump is vastly overestimated if individual rates are simply multiplied with abundance [74]. In the sediment, characterising crowding effects by inter burrow distance (inversely proportional to the density) was originally explored using a cylinder model [75]. In the case of a downward tracer transport, increasing density of burrows will be coupled with the overlap of their microenvironments. This will result in a reduced total tracer flux to the sediment at the same irrigation rate than the simple arithmetic sum of the individual effects may suggest. For fluxes to the water column, however, the major regulating factor is the concentration gradient between pore and bottom water. Higher densities of burrows result in low pore water concentrations, determining low effluxes and non-linearity as compared to the stimulatory effect of a few individuals.
4.2. Implications for Experiments on New Species Impacts
In spite of numerous examples of species invasions, a general framework and criteria that allow prediction of species impacts on their environment are often lacking [9]. Even though functional redundancy was reported to be important when considering the role an invader plays in an ecosystem [17,76], and species effects seem to differ between habitat types, the phenomenon itself attained little attention in experimental ecology.
In case of the polychaete M. neglecta in the Baltic Sea, both relatively diverse coastal areas and depleted oligohaline habitats have been colonized [38]. Often the species became abundant in impoverished habitats dominated by oligochaetes and chironomids prior to the invasion. An example of the species bioturbation effect in the sediment of the brackish Curonian lagoon (south-eastern Baltic Sea) is given in Figure 6 [77]. Before the polychaete invasion, oligochaetes and chironomids exploited sediment layers down to 15 cm depth, with an approximately 20-fold decrease in the depth layer of 12–14 cm (Figure 6a). In the deposits below this depth, no macrofauna activity was found. However, as M. neglecta became the predominant burrow-constructor, this species was able to significantly extend the sediment-water interface in such environments. Relatively few polychaetes (300 ind m−2) increased sediment surface area up to three times (Figure 6b). Approximately one quarter of this contact zone was located beneath the depth of sediment reworked by oligochaetes and chironomids before invasion.
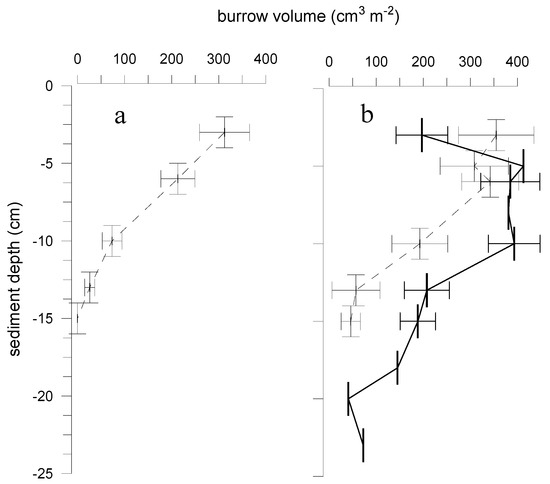
Figure 6.
Vertical distribution of macrofauna burrow volumes (vertical bars: depth range; horizontal bars: standard errors) in the soft-bottom habitats occupied by community of chironomids (1700 ± 1300 ind m−2, n = 21) and oligochaetes (950 ± 900 ind m−2, n = 21) (a) before and (b) after invasion of M. neglecta (300 ± 180 ind m−2, n = 13). Dashed lines denote burrow volumes of chironomids and oligochaetes, solid line — burrow volumes of M. neglecta. Modified after [77].
In our experiment, we simulated these different scenarios of the species invasion with respect to the diversity of the exposed habitat. Results obtained show different effects depending on the characteristics of the recipient habitat. The use of defaunated sediments was intended to mimic impoverished benthic communities like those found in the Curonian lagoon. Even single specimens contribute significantly to the originally low background of solute exchange. It is likely that in such environments, colonization/extinction processes may play a particularly important role in provoking essential changes in the functioning of an invaded ecosystem. The species’ contribution to the exchange of solutes is dampened in a habitat with originally high rates of sediment–water interaction as seen in the case of Breitling Bay where the newly contributed flux was negligible. Even though the impact of new species is increasing with abundance to some extent, it seems that intense animal–sediment interactions already present in an environment, i.e., a high background, may significantly reduce a new species’ importance in terms of its contribution to relevant processes or functions in that ecosystem.
Author Contributions
Conceptualization, D.D., S.F.; methodology, D.D., S.F.; formal analysis, D.D.; investigation, D.D., S.F.; resources, D.S., S.O., S.F., M.L.Z.; data curation, D.D., S.F., M.L.Z.; writing—original draft preparation, D.D., S.F.; writing—review and editing, M.L.Z., S.O., D.S.
Funding
This study was initiated by DAAD (Deutscher Akademischer Austauschdienst) short-term research grant to Daunys, D. Furthermore, support was provided by Baltic Seas Research Institute Warnemünde.
Acknowledgments
We would like to express our gratitude to G. Nausch and library staff of the Institute for their help throughout this work. We also thank Graf, G., Powilleit, M., and Arldt, G. for fruitful comments on experimental design and study results; and Bartoli, M. for suggestions during the final stage of the manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carlton, J.T.; Geller, J.B. Ecological roulette: The global transport of nonindigenous marine organisms. Science 1993, 261, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.T. Pattern, process and prediction in marine invasion ecology. Biol. Conserv. 1996, 78, 97–106. [Google Scholar] [CrossRef]
- Mack, R.N.; Occhipinti, A. Biotic invasion: A global perspective and ecology of invasion: Patterns and perspectives. In Perspectives in Ecology; Farina, A., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 67–74. [Google Scholar]
- Leppäkoski, E.; Gollasch, S.; Olenin, S. Alien species in European waters. In Aquatic Invasive Species of Europe—Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 1–6. [Google Scholar]
- Norkko, J.; Reed, D.C.; Timmermann, K.; Norkko, A.; Gustafsson, B.G.; Bonsdorff, E.; Slomp, C.P.; Carstensen, J.; Conley, D.J. A welcome can of worms? Hypoxia mitigation by an invasive species. Glob. Chang. Biol. 2012, 18, 422–434. [Google Scholar] [CrossRef]
- Hewitt, J.E.; Norkko, J.; Kauppi, L.; Villnäs, A.; Norkko, A.; Peters, D.P.C. Species and functional trait turnover in response to broad-scale change and an invasive species. Ecosphere 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Parker, I.M.; Simberloff, D.; Lonsdale, W.M.; Goodell, K.; Wonham, M.; Kareiva, P.M.; Williamson, M.H.; Von Holle, B.; Moyle, P.B.; Byers, J.E.; et al. Impact: Toward a framework for understanding the ecological effects of invaders. Biol. Inv. 1999, 1, 3–19. [Google Scholar] [CrossRef]
- Ruiz, G.M.; Fofonoff, P.; Hines, A.H.; Grosholz, E.D. Non-indigenous species as stressors in estuarine and marine communities: Assessing invasion impacts and interactions. Limnol. Oceanogr. 1999, 44, 950–972. [Google Scholar] [CrossRef]
- Carlton, J.T. Bioinvasion ecology: Assessing invasion impact and scale. In Aquatic Invasive Species of Europe—Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 7–19. [Google Scholar]
- Renz, J.; Forster, S. Effects of bioirrigation by the three sibling species of Marenzelleria spp. on solute fluxes and porewater nutrient profiles. Mar. Ecol. Prog. Ser. 2014, 505, 145–159. [Google Scholar] [CrossRef]
- Quintana, C.O.; Raymond, C.; Nascimento, F.; Bonaglia, S.; Forster, S.; Gunnarsson, J.S.; Kristensen, E. Functional performance of three invasive Marenzelleria species under contrasting ecological conditions within the Baltic Sea. Estuaries Coast. 2018, 41, 1766–1781. [Google Scholar] [CrossRef]
- Carstensen, J.; Conley, D.J.; Bonsdorff, E.; Gustafsson, B.G.; Hietanen, S.; Janas, U.; Jilbert, T.; Maximov, A.; Norkko, A.; Norkko, J.; et al. Hypoxia in the Baltic Sea: Biogeochemical cycles, benthic fauna, and management. AMBIO 2014, 43, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.M. Biological invasions: Lessons for ecology. Trends Ecol. Evol. 1993, 8, 133–137. [Google Scholar] [CrossRef]
- Levine, J.M.; D’Antonio, C.M. Elton revisited: A review of evidence linking diversity and invisibility. Oikos 1999, 87, 15–26. [Google Scholar] [CrossRef]
- Ruiz, G.M.; Hewitt, C.L. Toward understanding patterns of coastal marine invasions: A prospectus. In Aquatic Invasive Species of Europe—Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 529–547. [Google Scholar]
- Maximov, A.; Bonsdorff, E.; Eremina, T.; Kauppi, L.; Norkko, A.; Norkko, J. Context-dependent consequences of Marenzelleria spp. (Spionidae: Polychaeta) invasion for nutrient cycling in the Northern Baltic Sea. Oceanologia 2015, 57, 342–348. [Google Scholar] [CrossRef]
- Levin, L.A.; Boesh, D.F.; Covich, A.; Dahm, C.; Erseus, C.H.; Ewel, K.C.; Kneib, R.T.; Moldenke, A.; Palmer, M.A.; Snelgrove, P.; et al. The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 2001, 4, 430–450. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Fried, H.; Osman, R.W.; Whitlatch, R.B. Biodiversity, invasion resistance, and marine ecosystem function: Reconciling pattern and process. Ecology 2002, 83, 2575–2590. [Google Scholar] [CrossRef]
- Naeem, S.; Knops, J.M.H.; Tilman, D.; Howe, K.M.; Kennedy, T.; Gale, S. Plant diversity increases resistance to invasion in the absence of covarying extrinsic factors. Oikos 2000, 91, 97–108. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bonner, K.I.; Barker, G.M. Stability of ecosystem properties in response to above-ground functional group richness and composition. Oikos 2000, 89, 11–23. [Google Scholar] [CrossRef]
- Hammond, D.E.; Fuller, C. The use of radon-222 to estimate benthic exchange and atmospheric exchange rates in San Francisco Bay. In San Francisco Bay: The Urbanized Estuary; Conomos, T.J., Ed.; AAAS: San Francisco, CA, USA, 1979; pp. 213–230. [Google Scholar]
- Callender, E.; Hammond, D.E. Nutrient exchange across the sediment-water interface in the Potomac River Estuary. Estuar. Coast. Shelf. Sci. 1982, 15, 395–413. [Google Scholar] [CrossRef]
- Clavero, V.; Niell, F.X.; Fernandez, J.A. Effects of Nereis diversicolor O.F. Müller abundance on the dissolved phosphate exchange between sediment and overlying water in Palmones River Estuary (southern Spain). Estuar. Coast. Shelf Sci. 1991, 33, 193–202. [Google Scholar] [CrossRef]
- Forja, J.M.; Gomez-Parra, A. Measuring nutrient fluxes across the sediment-water interface using benthic chambers. Mar. Ecol. Prog. Ser. 1998, 164, 95–105. [Google Scholar] [CrossRef]
- Forster, S.; Glud, R.N.; Gundersen, J.K.; Huettel, M. In situ study of bromide tracer and oxygen flux in coastal sediments. Estuar. Coast. Shelf. Sci. 1999, 49, 813–827. [Google Scholar] [CrossRef]
- Henriksen, K.; Hansen, J.I.; Blackburn, T.H. The influence of benthic infauna on exchange rates of inorganic nitrogen between sediment and water. Ophelia 1980, 1, 249–256. [Google Scholar]
- Clavero, V.; Niell, F.X.; Fernandez, J.A. An experimental approach to quantify the influence of Nereis diversicolor in the interchange of phosphate between sediment and water. J. Exp. Mar. Biol. Ecol. 1994, 176, 257–267. [Google Scholar] [CrossRef]
- Pelegri, S.P.; Nielsen, L.P.; Blackburn, T.H. Denitrification in estuarine sediment stimulated by the irrigation activity of the amphipod Corophium volutator. Mar. Ecol. Prog. Ser. 1994, 105, 285–290. [Google Scholar] [CrossRef]
- Matisoff, G.; Wang, X. Solute transport in sediment by freshwater infaunal bioirigators. Limnol. Oceanogr. 1998, 43, 1487–1499. [Google Scholar] [CrossRef]
- Nizzoli, D.; Bartoli, M.; Cooper, M.; Welsh, D.T.; Underwood, G.J.C.; Viaroli, P. Implications for oxygen, nutrient fluxes and denitrification rates during the early stage of sediment colonisation by the polychaete Nereis spp. in four estuaries. Est. Coast. Shelf Sci. 2007, 75, 125–134. [Google Scholar] [CrossRef]
- Essink, K.; Kleef, H.L. Marenzelleria viridis (Verrill, 1873) (Polychaeta: Spionidae): A new record from Ems estuary (The Netherlands/Federal Republic of Germany). Zool. Bijdr. 1988, 38, 1–13. [Google Scholar]
- McLusky, D.S.; Hull, S.C.; Elliott, M. Variations in the intertidal and subtidal macrofauna and sediments along a salinity gradient in the upper Forth Estuary. Neth. J. Aquat. Ecol. 1993, 27, 101–107. [Google Scholar] [CrossRef]
- Bick, A.; Burckhardt, R. Erstnachweis von Marenzelleria viridis (Polychaeta, Spionidae) für den Ostseeraum, mit einem Bestimmungsschlüssel der Spioniden der Ostsee. Mitt. Zool. Mus. Berl. 1989, 65, 237–247. [Google Scholar] [CrossRef]
- Bochert, R.; Fritzsche, D.; Burckhardt, R. Influence of salinity and temperature on growth and survival of the planktonic larvae of Marenzelleria viridis (Polychaeta, Spionidae). J. Plankton Res. 1996, 18, 1239–1251. [Google Scholar] [CrossRef]
- Bochert, A.; Richard, D.; Bochert, R. Marenzelleria cf. viridis and the sulphide regime. Aquat. Ecol. 1997, 31, 223–231. [Google Scholar] [CrossRef]
- Schiedek, D. Marenzelleria cf. viridis (Polychaeta: Spionidae)—Ecophysiological adaptations to a life in the coastal waters of the Baltic Sea. Aquat. Ecol. 1997, 31, 199–210. [Google Scholar] [CrossRef]
- Zettler, M.L. Population dynamics, growth and production of the neozoon Marenzelleria cf. viridis (Verrill, 1873) (Polychaeta: Spionidae) in a coastal water of the southern Baltic Sea. Aquat. Ecol. 1997, 31, 177–186. [Google Scholar] [CrossRef]
- Zettler, M.L.; Daunys, D.; Kotta, J.; Bick, A. History and success of an invasion into the Baltic Sea: The polychaete Marenzelleria cf. viridis development and strategies. In Aquatic Invasive Species of Europe—Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 66–75. [Google Scholar]
- Essink, K. Dispersal and development of Marenzelleria spp. (Polychaeta, Spionidae) populations in NW Europe and the Netherlands. Helgol. Meeresunters. 1999, 52, 367–372. [Google Scholar] [CrossRef]
- Zettler, M.L. Successful establishment of the spionid polychaete Marenzelleria viridis (Verrill 1873), in the Darss-Zingst estuary (southern Baltic) and its influence on the indigenous macrozoobenthos. Arch. Fish. Mar. Res. 1996, 43, 273–284. [Google Scholar]
- Burckhardt, R.; Schumann, R.; Bochert, R. Feeding biology of the pelagic larvae of Marenzelleria cf. viridis (Polychaeta: Spionidae) from the Baltic Sea. Aquat. Ecol. 1997, 31, 149–162. [Google Scholar] [CrossRef]
- Winkler, H.M.; Debus, L. Is the polychaete Marenzelleria viridis an important food item for fish? In Proceedings of the 13th BMB Symposium; Andrushaitis, A., Ed.; Institute of Aquatic Ecology, University of Latvia: Riga, Latvia, 1993; pp. 147–151. [Google Scholar]
- Kotta, J.; Orav, H.; Sandberg-Kilpi, E. Ecological consequence of the introduction of the polychaete Marenzelleria viridis into a shallow water biotope of the northern Baltic Sea. J. Sea Res. 2001, 46, 273–280. [Google Scholar] [CrossRef]
- Leppäkoski, E. Assessment of degree of pollution on the basis of macrozoobenthos in marine and brackish-water environments. Acta Academia Aboensis B 1975, 35, 37–47. [Google Scholar]
- Rumohr, H.; Bonsdorff, E.; Pearson, T.H. Zoobenthos succession in Baltic sedimentary habitats. Arch. Fish. Mar. Res. 1996, 44, 179–214. [Google Scholar]
- Zettler, M.L. Untersuchungen zum Makrozoobenthos des Breitlings (südliche Ostsee) unter besonderer Berücksichtigung der Crustacea. Rostocker Meeresbiol. Beitr. 1999, 7, 79–90. [Google Scholar]
- Schiedek, D. Ecophysiological capability of Marenzelleria populations inhabiting North Sea estuaries: An overview. Helgol. Meeresunter. 1999, 52, 373–382. [Google Scholar] [CrossRef]
- Saager, P.M.; Sweerts, J.-P.; Ellermeijer, H.J. A simple pore-water sampler for coarse, sandy sediments of low porosity. Limnol. Oceanogr. 1990, 35, 747–751. [Google Scholar] [CrossRef]
- Zettler, M.L. Ökologische Untersuchungen am Neozoon Marenzelleria viridis (Verrill, 1873) (Polychaeta: Spionidae) in Einem Küstengewässer der Südlichen Ostsee. Ph.D. Thesis, Rostock University, Rostock, Germany, 1996; p. 149. [Google Scholar]
- Daunys, D.; Schiedek, D.; Olenin, S. Species strategy near its boundary: The Marenzelleria cf. viridis (Polychaeta, Spionidae) case in the south-eastern Baltic Sea. Internat. Rev. Hydrobiol. 2000, 85, 639–651. [Google Scholar] [CrossRef]
- Koroleff, F.; Grasshoff, K. Determination of nutrients. In Methods of Seawater Analyses; Grasshoff, K., Ed.; Wiley: Hoboken, NY, USA, 1983; pp. 125–188. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Analyt. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Glud, R.N.; Forster, S.; Huettel, M. Influence of radial pressure gradients on solute exchange in stirred benthic chambers. Mar. Ecol. Prog. Ser. 1996, 141, 303–311. [Google Scholar] [CrossRef]
- Ullman, W.J.; Aller, R.C. Diffusion coefficients in nearshore marine sediments. Limnol. Oceanogr. 1985, 27, 552–556. [Google Scholar] [CrossRef]
- Banta, G.T.; Holmer, M.; Jensen, M.H.; Kristensen, E. Effects of two polychaete worms, Nereis diversicolor and Arenicola marina, on aerobic and anaerobic decomposition in a sandy marine sediments. Aquat. Microb. Ecol. 1999, 19, 189–204. [Google Scholar] [CrossRef]
- Aller, R.C.; Aller, J.Y. Meiofauna and solute transport in marine muds. Limnol. Oceanogr. 1992, 37, 1018–1033. [Google Scholar] [CrossRef]
- Ekeroth, N.; Blomqvist, S.; Hall, P.O.J. Nutrient fluxes from reduced Baltic Sea sediment: Effects of oxygenation and macrobenthos. Mar. Ecol. Prog. Ser. 2016, 544, 77–92. [Google Scholar] [CrossRef]
- Hahlbeck, E.; Arndt, C.; Schiedek, D. Sulphide detoxification in Hediste diversicolor and Marenzelleria viridis, two dominant polychaete worms within the shallow coastal waters of the southern Baltic Sea. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 125, 457–471. [Google Scholar] [CrossRef]
- Zilius, M.; Bartoli, M.; Bresciani, M.; Katarzyte, M.; Ruginis, T.; Petkuviene, J.; Lubiene, I.; Giardino, C.; Bukaveckas, P.A.; De Wit, R.; et al. Feedback mechanisms between cyanobacterial blooms, transient hypoxia, and benthic phosphorus regeneration in shallow coastal environments. Estuar. Coast. 2014, 37, 680–694. [Google Scholar] [CrossRef]
- Kristensen, E.; Hansen, T.; Delefosse, M.; Banta, G.T. Quintana CO Contrasting effects of the polychaetes Marenzelleria viridis and Nereis diversicolor on benthic metabolism and solute transport in sandy coastal sediment. Mar. Ecol. Prog. Ser. 2011, 425, 125–139. [Google Scholar] [CrossRef]
- Bonaglia, F.J.; Nascimento, A.; Bartoli, M.; Klawonn, I.; Brüchert, V. Meiofauna increases bacterial denitrification in marine sediments. Nat. Commun. 2014, 5, 5133. [Google Scholar] [CrossRef]
- Miron, G.; Kristensen, E. Factors influencing the distribution of nereid polychaetes: The sulfide aspect. Mar. Ecol. Prog. Ser. 1993, 93, 143. [Google Scholar] [CrossRef]
- Martin, W.R.; Banta, G.T. The measurement of sediment irrigation rates: A comparison of the Br- tracer and 222Rn/226Ra disequilibrium techniques. J. Mar. Res. 1992, 50, 125–154. [Google Scholar] [CrossRef]
- Rysgaard, S.; Christensen, P.B.; Sorensen, M.V.; Funch, P.; Berg, P. Marine meiofauna, carbon and nitrogen mineralization in sandy and soft sediments of Disko Bay, West Greenland. Aq. Microb. Ecol. 2000, 21, 59–71. [Google Scholar] [CrossRef]
- Nielsen, L.P. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol. Lett. 1992, 86, 357–362. [Google Scholar] [CrossRef]
- Fritzsche, D.; von Oertzen, J.-A. Metabolic responses to changing environmental conditions in the brackish water polychaetes Marenzelleria viridis and Hediste diversicolor. Mar. Biol. 1995, 121, 693–699. [Google Scholar] [CrossRef]
- Kristensen, E. Ventilation and oxygen uptake by three species of Nereis (Annelida: Polychaeta). II. Effects of temperature and salinity changes. Mar. Ecol. Prog. Ser. 1983, 12, 299–306. [Google Scholar] [CrossRef]
- Davey, J.T. The architecture of the burrow of Nereis diversicolor and its quantification in relation to sediment-water exchange. J. Exp. Mar. Biol. Ecol. 1994, 179, 115–129. [Google Scholar] [CrossRef]
- Zettler, M.L.; Bick, A.; Bochert, R. Röhrenbau und Vertikalverteilung von Marenzelleria viridis (Polychaeta: Spionidae) in einem inneren Küstengewässer der südlichen Ostsee. Rostocker Meeresbiol. Beitr. 1994, 2, 215–225. [Google Scholar]
- Renz, J.; Forster, S. Are similar worms different? A comparative tracer study on bioturbation in the three sibling species Marenzelleria arctia, M. viridis, and M. neglecta from the Baltic Sea. Limnol. Oceanogr. 2013, 58, 2046–2058. [Google Scholar] [CrossRef]
- Dauer, D.M.; Ewing, R.M.; Tourtellotte, G.H.; Barker, H.R., Jr. Nocturnal swimming of Scolecolepides viridis (Polychaeta: Spionidae). Estuaries 1980, 3, 148—149. [Google Scholar] [CrossRef]
- Dauer, D.M.; Ewing, R.M.; Sourbeer, J.W.; Harlan, W.T.; Stokes, T.L. Nocturnal movements of the macrobenthos of the Lafayette River, Virginia. Int. Rev. Ges. Hydrobiol. 1982, 67, 761—775. [Google Scholar]
- Reible, D.D.; Popov, V.; Valsaraj, K.T.; Thibodeaux, L.J.; Lin, F.; Dikshit, M.; Todaro, M.A.; Fleeger, J.W. Contaminant fluxes from sediment due to tubificid oligochaete bioturbation. Water Res. 1996, 30, 704–714. [Google Scholar] [CrossRef]
- Yu, N.; Culver, D.A. Effective clearance rate and refiltration estimation of zebra mussels (Dreissena polymorpha) in a stratified reservoir. Fresh. Biol. 1999, 41, 481–492. [Google Scholar] [CrossRef]
- Aller, R.C. The effects of macrobenthos on chemical properties of marine sediment and overlying water. In Animal-Sediment Relations; McCall, P.L., Tevesz, M.J.S., Eds.; Plenum Press: New York, NY, USA, 1982; pp. 53–102. [Google Scholar]
- Naeem, S.; Loreau, M.; Inchausti, P. Biodiversity and ecosystem functioning: The emergence of a synthetic ecological framework. In Biodiversity and Ecosystem Functioning; Loreau, M., Naeem, S., Inchausti, P., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 3–11. [Google Scholar]
- Daunys, D. Patterns of the bottom macrofauna variability and its role in the shallow coastal lagoon. Ph.D. Thesis, Klaipeda University, Klaipeda, Lithuania, 2001; 85p. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).