Exploring Compatibility of Sherwood-Gilland NAPL Dissolution Models with Micro-Scale Physics Using an Alternative Volume Averaging Approach
Abstract
1. Introduction
2. Formulation
2.1. Governing Equations
2.2. Coupling Conditions
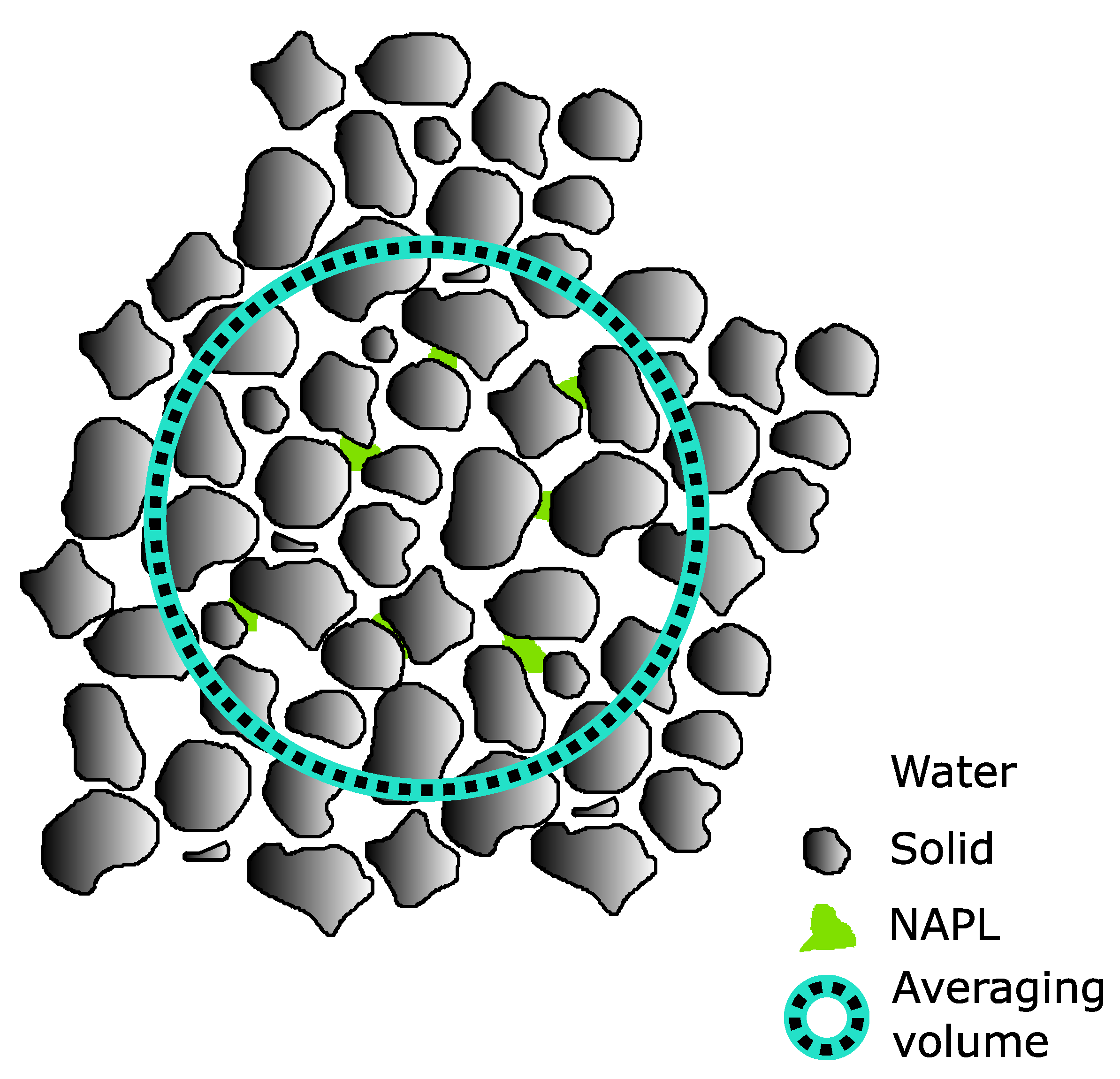
3. Simplified Volume Averaging Analysis
3.1. Accumulation Term
3.2. Advection Term
3.3. Diffusion Term
3.4. Assembly and Simplification of Governing Equation
3.5. Special Case: Advection Domination
4. Conclusions
Funding
Conflicts of Interest
Appendix A. Nomenclature
| Symbol | Dimensions | Description |
| Superficial volume average operator | ||
| Intrinsic volume average operator for i phase | ||
| Locus of interface between phases i and j within V | ||
| Spatial region with centroid at over which volume averaging occurs | ||
| Fitted constant in empirical model of mass transfer coefficient K | ||
| Fitted exponent in empirical model of mass transfer coefficient K | ||
| Fitted exponent in empirical model of mass transfer coefficient K | ||
| Fraction of V occupied by i phase | ||
| Mass transfer coefficient in (6), as derived in [13] | ||
| Density of NAPL | ||
| Mass transfer coefficient | ||
| c | Chemical concentration in the water phase | |
| Thermodynamic saturation chemical concentration in the water phase | ||
| Characteristic length of porous media | ||
| Velocity-like closure variable in (6), as derived in [13] | ||
| Effective Fickian dispersion coefficient | ||
| Dispersion-like closure variable in (6), as derived in [13] | ||
| Infinitesimal surface element on | ||
| Infinitesimal volume element | ||
| Fickian diffusion constant | ||
| Indicator function for location belonging to the i phase | ||
| k | Closure variable representing dispersive flux | |
| K | Mass transfer coefficient from NAPL to water phase | |
| L | Length scale of averaging volume; | |
| Normal vector on interface directed from i phase into j phase | ||
| Darcy velocity near NAPL source zone | ||
| Q | Strength of NAPL-to-water mass source | |
| Re | Reynolds number | |
| NAPL saturation () | ||
| t | Time | |
| Velocity-like term in (6), as derived in [13] | ||
| Pore water velocity | ||
| Physical volume of V | ||
| Spatial position vector |
Appendix B. Derivation of a Geometric Lemma
References
- Miller, C.; Poirer-McNeill, M.; Mayer, A.S. Dissolution of Trapped Nonaqueous Phase Liquids: Mass Transfer Characteristics. Water Resour. Res. 1990, 26, 2783–2796. [Google Scholar] [CrossRef]
- Imhoff, P.T.; Jaffe, P.R.; Pinder, G.F. An experimental study of complete dissolution of a nonaqueous phase liquid in saturated porous media. Water Resour. Res. 1993, 30, 307–320. [Google Scholar] [CrossRef]
- Powers, S.E.; Abriola, L.M.; Weber, W.J. An experimental investigation of nonaqueous phase liquid dissolution in saturated subsurface systems: Transient mass transfer rates. Water Resour. Res. 1994, 30, 321–332. [Google Scholar] [CrossRef]
- Saba, T.; Illangasekare, T.H. Effect of groundwater flow dimensionality on mass transfer from entrapped nonaqueous phase liquid contaminants. Water Resour. Res. 2000, 36, 971–979. [Google Scholar] [CrossRef]
- Nambi, I.M.; Powers, S.E. Mass transfer correlations for nonaqueous phase liquid dissolution from regions with high initial saturations. Water Resour. Res. 2003, 39. [Google Scholar] [CrossRef]
- Hossain, S.Z.; Mumford, K.G.; Rutter, A. Laboratory study of mass transfer from diluted bitumen trapped in gravel. Environ. Sci. Process. Impacts 2017, 19, 1583–1593. [Google Scholar] [CrossRef]
- Sherwood, T.K.; Gilliland, E.R. Diffusion of Vapors through Gas Films. Ind. Eng. Chem. 1934, 26, 1093–1096. [Google Scholar] [CrossRef]
- Kokkinaki, A.; O’Carroll, D.M.; Werth, C.J.; Sleep, B.E. An evaluation of Sherwood-Gilland models for NAPL dissolution and their relationship to soil properties. J. Contam. Hydrol. 2013, 155, 87–98. [Google Scholar] [CrossRef]
- Whitaker, S. The Method of Volume Averaging; Springer: Berlin/Heidelberg, Germany, 1999; p. 221. [Google Scholar]
- Whitaker, S. Diffusion and Dispersion in Porous Media. AIChE J. 1967, 13, 420–427. [Google Scholar] [CrossRef]
- Slattery, J.C. Flow of Viscoelastic Fluids Through Porous Media. AIChE J. 1967, 13, 1066–1071. [Google Scholar] [CrossRef]
- Wood, B.D. The role of scaling laws in upscaling. Adv. Water Resour. 2009, 32, 723–736. [Google Scholar] [CrossRef]
- Quintard, M.; Whitaker, S. Convection, dispersion, and interfacial transport of contaminants: Homogeneous porous media. Adv. Water Resour. 1994, 17, 221–239. [Google Scholar] [CrossRef]
- Porta, G.M.; Riva, M.; Guadagnini, A. Upscaling solute transport in porous media in the presence of an irreversible bimolecular reaction. Adv. Water Resour. 2012, 35, 151–162. [Google Scholar] [CrossRef]
- Porta, G.M.; Ceriotti, G.; Thovert, J.F. Comparative assessment of continuum-scale models of bimolecular reactive transport in porous media under pre-asymptotic conditions. J. Contam. Hydrol. 2016, 185–186, 1–13. [Google Scholar] [CrossRef]
- Ma, Y.; Mohebbi, R.; Rashidi, M.M.; Manca, O.; Yang, Z. Numerical investigation of MHD effects on nanofluid heat transfer in a baffled U-shaped enclosure using lattice Boltzmann method. J. Therm. Anal. Calorim. 2019, 135, 3197–3213. [Google Scholar] [CrossRef]
- Quintard, M.; Whitaker, S. Dissolution of an Immobile Phase during Flow in Porous Media. Ind. Eng. Chem. Res. 1999, 38, 833–844. [Google Scholar] [CrossRef]
- Bahar, T.; Golfier, F.; Oltéan, C.; Benioug, M. An Upscaled Model for Bio-Enhanced NAPL Dissolution in Porous Media. Transp. Porous Media 2016, 113, 653–693. [Google Scholar] [CrossRef]
- Bahar, T.; Golfier, F.; Oltéan, C.; Lefevre, E.; Lorgeoux, C. Comparison of theory and experiment for NAPL dissolution in porous media. J. Contam. Hydrol. 2018, 211, 49–64. [Google Scholar] [CrossRef]
- Kechagia, P.E.; Tsimpanogiannis, I.N.; Yortsos, Y.C.; Lichtner, P.C. On the upscaling of reaction-transport processes in porous media with fast or finite kinetics. Chem. Eng. Sci. 2002, 57, 2565–2577. [Google Scholar] [CrossRef]
- Golfier, F.; Wood, B.D.; Orgogozo, L.; Quintard, M.; Buès, M. Biofilms in porous media: Development of macroscopic transport equations via volume averaging with closure for local mass equilibrium conditions. Adv. Water Resour. 2009, 32, 463–485. [Google Scholar] [CrossRef]
- Orgogozo, L.; Golfier, F.; Buès, M.; Quintard, M. Upscaling of transport processes in porous media with biofilms in non-equilibrium conditions. Adv. Water Resour. 2010, 33, 585–600. [Google Scholar] [CrossRef]
- Rubin, Y. Applied Stochastic Hydrogeology; Oxford University Press: New York, NY, USA, 2003; p. 391. [Google Scholar]
- Gelhar, L.W. Stochastic Subsurface Hydrology; Prentice-Hall: Englewood Cliffs, NJ, USA, 1993; p. 390. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, S.K. Exploring Compatibility of Sherwood-Gilland NAPL Dissolution Models with Micro-Scale Physics Using an Alternative Volume Averaging Approach. Water 2019, 11, 1525. https://doi.org/10.3390/w11071525
Hansen SK. Exploring Compatibility of Sherwood-Gilland NAPL Dissolution Models with Micro-Scale Physics Using an Alternative Volume Averaging Approach. Water. 2019; 11(7):1525. https://doi.org/10.3390/w11071525
Chicago/Turabian StyleHansen, Scott K. 2019. "Exploring Compatibility of Sherwood-Gilland NAPL Dissolution Models with Micro-Scale Physics Using an Alternative Volume Averaging Approach" Water 11, no. 7: 1525. https://doi.org/10.3390/w11071525
APA StyleHansen, S. K. (2019). Exploring Compatibility of Sherwood-Gilland NAPL Dissolution Models with Micro-Scale Physics Using an Alternative Volume Averaging Approach. Water, 11(7), 1525. https://doi.org/10.3390/w11071525




