Abstract
This is the first study to carry out a laboratory-scale assay to assess the potentiality of continuous liquid–liquid extraction with dichloromethane (CLLEDCM) and high-power fractional distillation (HPFD) as a treatment to decontaminate the wastewater generated by the petroleum industry (WW). The analytical parameters of treated wastewater (TWW) evidenced a remarkable quality improvement compared to the original WW. CLLEDCM–HPFD yielded 92.4%–98.5% of the WW mass as more environmentally friendly water. Compared to the original values determined in the WW, total petroleum hydrocarbon (TPH) decreased by 95.0%–100.0%, and the chemical oxygen demand (COD) decreased by 90.5%–99.9%. Taking into account the yield of the treated water, the amount of pollutant removed, and the risks of each process, the order of the potentiality of these treatments, from highest to lowest, was HPFD > CLLEDCM–HPFD > CLLEDCM. CLLEDCM treatment alone produced TWW with poorer quality, and the CLLEDCM–HPFD sequence involved the greatest consumption of time and energy (0.390–0.905 kWh/kg). CLLEDCM-only was the least effective treatment because the TWW obtained failed to comply with the regulations of oil-producing countries.
1. Introduction
The production of crude oil (particularly heavy crude oil) involves the coproduction of process waters. In nondomestic wastewater, the petroleum industry generates a significant load of leachate (WW) with diverse compositions, depending on the geographical location of the exploitation, the crude type, and the method for the extraction of the petroleum, among other factors. The petroleum industry generates large amounts of contaminated WW (Table 1). The United States has official data for the years 2007 and 2012, and Colombia has official data for the year 2015 [1,2,3].

Table 1.
A few indicative records of the amounts of nondomestic wastewater (WW) generated by the oil industry.
The WW generated during the production of crude oil usually contains dissolved gases (CO2, H2S), salts, suspended solids, radioisotopes, hydrocarbons, and metal ions. In accordance with the guidelines in the Standard Methods for the Examination of Water and Wastewater™ [4] REF, the parameters measured to analyze WW and treated wastewater (TWW) are pH, salinity, electrical conductivity (EC), total alkalinity (TA), total suspended solids (TSSes), total dissolved solids (TDSes), chlorides, total hardness (TH), chemical oxygen demand (COD), biological oxygen demand (BOD), copper, nickel, and other compounds [4].
Different regulations in oil-producing countries establish the maximum limits of pollutants in WW that is discharged into surface water bodies (Table 2). The environment ministries and secretariats of Colombia, Mexico, Brazil, Peru, Venezuela, Asturias (Spain), and China and the World Bank Group (WBG) members’ oil-producing countries were consulted to compare differences and similarities between their maximum limits of effluents.

Table 2.
National regulations in some oil-producing countries on permissible maximum limits (PMLs) of pollutants in WW for its environmentally safe discharge.
WW can be discharged into surface water bodies and public sewage if it meets the requirements established in the regulatory norms that are in effect in a country, e.g., Spain [18], China [19], Colombia [20], Mexico [21], Brazil [22], Peru [23], Venezuela [24], and the World Bank Group (WBG) [25,26]. As a consequence, the petroleum industry has tested different strategies to regulate pollutant discharge into water bodies, marine water, and water used in households, industry, agriculture, water sports, and in power generation, among other applications.
The results of a detailed review indicated that there are a variety of methods to remove pollutants from wastewater, including (a) physical methods, such as adsorption, cyclones, enhanced flotation [16,27], flocculation [28], and activated carbons [29]; (b) chemical methods, such as precipitation, electrochemical techniques, [16,30], oxidation [27,31], photocatalytic techniques [32], and demulsifiers [33]; (c) biological methods, such as bioreactors [16,30,34]; (d) membrane techniques, such as polymeric, ceramic, or inorganic membranes, microfiltration, ultrafiltration, reverse osmosis, membrane distillation, and nanofiltration [16,27,30,35,36,37,38,39]; and (e) combined or hybrid methods, such as coagulation–flocculation and flotation, biological treatment with activated carbon and reverse osmosis (RO), and bioelectrochemical reactor and coagulation membrane processes [30,35,40,41] for reducing the COD, total petroleum hydrocarbon (TPH), and other contaminants present in petroleum WW.
Among physical treatments, the flocculation with zero-valent iron–ethylenediaminetetraacetic acid (EDTA) and air (ZEA) process and granular-activated carbon removed 92% of COD and 97% of TPH and 72.7%–88.2% of TPH, respectively [28,42,43]; and shaking extraction recovered about 60% of TPH from oil [44]. Of the chemical treatments used, electrocoagulation removed 85.81% of COD [45], and electrochemical removed 85%–96% of COD [46,47].
On the other hand, biological treatment studies have shown that rotating biological contactor (RBC) discs removed 78%–97% of COD and 95%–99% of TPH [48,49,50,51], and a membrane bioreactor (MBR) removed 96% of COD [11]. Nevertheless, new technologies have been tested, such as membrane in reverse osmosis (RO) and filtration membranes (which removed 82%–99% of COD [52,53,54,55]) and a hybrid microfiltration (MF)/ultrafiltration (UF) process (which removed 94.4%–98.8% of COD [56]).
The oil industry uses fractional distillation (FD) or extraction with solvents, and the emphasis of the technique is on obtaining the optimal benefit for the crude oil in refining or recovery operations. However, the use of distillation or extraction as a decontamination treatment of wastewater still requires further research and data. In this respect, catalytic vacuum distillation has been shown to reduce COD by 99% [57]. In addition, distillation has been investigated for the treatment of seawater. On the other hand, an extraction technique used to treat pond sludge removed 67.5% [58] and 40%–60% of COD using the solvents methyl ethyl ketone (MEK) and ethyl acetate (EA) [44].
To date, there have been no publications on the systematic use of continuous liquid–liquid extraction (CLLE) or FD, either individually or in sequence, as preliminary treatments for the decontamination of WW (including decontamination in sedimentation ponds during production).
This work presents and discusses the results of laboratory-scale assays using CLLE with dichloromethane (CLLEDCM) and high-power fractional distillation (HPFD), individually and in sequence, on authentic WW to produce TWW of better quality than the original water to illustrate the potentiality of these techniques for decontaminating WW. CLLEDCM and HPFD were specifically chosen due to preliminary trials showing that extraction with DCM removed more contaminants from WW and that distillation while heating low- or medium-potency WW prolonged the time of experimentation without appreciable improvement in the yield or quality of the TWW obtained.
2. Materials and Methods
2.1. Samples and Reagents
According to Protocol No. 1060 in the Standard Methods for the Examination of Water and Wastewater™ [4], 60 L of WW was collected from an oil company located in the Department of Meta (Colombia) and stored at 4 °C to perform physicochemical analyses on the initial WW and to treat it through CLLEDCM, HTFD, or CLLEDCM–HTFD to obtain the corresponding TWW. Analytical-grade dichloromethane (DCM) from Dongyue Chemical was used in the CLLE.
2.2. Sample Characterization
The initial WW and TWW obtained were analyzed according to the Standard Methods for the Examination of Water and Wastewater ™ [4] to determinate the pH (4500B), salinity (2520A), electrical conductivity EC (2510B), total alkalinity (TA) (2320B), total suspended solids (TSSes) (2540D), total dissolved solids (TDSes) (2540C), chlorides (4110B; D), total hardness (TH) (2340C), and COD (5220B, C, and D). WW and TWW were also analyzed according to the Environmental Protection Agency (EPA, Washington, DC, USA) of the United States to determinate total petroleum hydrocarbon (TPH) (EPA 8015D-EPA 3510C). The data obtained on COD, TPH, TSSes, TDSes, EC, TH, pH, TA, salinity, and chlorides were analyzed to compare water quality on the basis of the regulatory norms in force in the countries discussed in this study.
WW and TWW were also subjected to gas chromatography (GC) on a Hewlett Packard 5890 series II chromatograph operated with ultrahigh-purity nitrogen as the carrier gas, an injector (at 558.15 K), a flame ionization detector (at 593.15 K), an Hewlett-Packard (HP) 3396 series II integrator, and a reverse-phase capillary column DB5 (dimethylpolysiloxane; 30 m 0.25 mm ID, 0.25 μm) at a temperature ramp of 276.15 K/min from 558.15 to 593.15 K. Phytane and pristine were used as standards.
The estimated energy consumed by the CLLEDCM and HPFD methods was calculated in kilowatt-hours (kWh) from the specific heat of liquid water. The specific heat was determined by measuring the energy required to heat a mass of liquid water (from the initial temperature T1 to the final temperature T2) contained in the extract collector of the equipment for CLLEDCM or in the distillation flask of the HPFD assembly at identical conditions to those in the assays for WW. In particular, the heating power and losses of energy through thermal insulation were kept constant.
2.3. Experimental Design and Procedure
2.3.1. CLLEDCM
The assembly used for extraction is presented in Figure 1A. Equal and previously weighed volumes of 0.35 L of DCM and WW were subjected to processing in the extraction chamber. From the extract collector agitated at 800 min−1 at a temperature of <312.75 K (heated by a bath at 333.15 K), cold extract batches were collected during the process at 1200, 2400, 3600, 5400, 7200, 10,800, and 14,400 s after the beginning of solvent condensation on the extraction chamber after the extract collector was replenished with a volume of DCM equal to that of the collected extract. The initial WW, each batch of extract, solvent-free extract obtained by distillation in a rotary evaporator and by vacuum-drying (333.15 K, 2933.1 Pa), and the final raffinate were weighed to within a 0.0001-g accuracy. In this way, CLLEDCM and CLLEDCM–HPFD were repeated four and six times, respectively. Estimated energy consumption and operation time were measured from the onset of heating the system until the last condensation drop fell into the extraction chamber for the extract batch at 1200 s and from the first to the last drop that fell for all other batches of extract. The quality improvement in the TWW obtained was assessed through the analytical parameters of the sample.
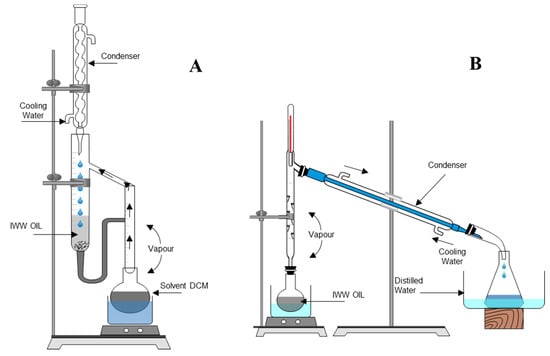
Figure 1.
(A) Laboratory assembly used for the continuous liquid–liquid extraction with dichloromethane (CLLEDCM) and (B) high-power fractional distillation (HPFD) of the wastewater (WW).
2.3.2. HPFD
Approximately 0.30 L of previously weighed WW or raffinate from CLLEDCM (TWW) was deposited into the distillation flask of the assembly (shown in Figure 1B) and distilled using a Vigreux fractionation column and a heating mantle at its highest potency. Distillate fractions were collected at 357.15–363.15 K (1–5 mL, head), 363.15–364.15 K (0.24–0.25 L, body), and 364.15–365.15 K (1–5 mL, tail). The feed, WW, or raffinate distillate fractions and each final distillation bottom were weighed to within a 0.0001-g accuracy. In this way, HPFD and CLLEDCM–HPFD were repeated 16 and 6 times, respectively. Estimated energy consumption and operation time were measured from the beginning of system heating to the collection of the last drop of distilled TWW. The quality improvement in the TWW obtained was assessed through the analytical parameters of the sample.
2.4. Statistical Analysis
Masses, times, yields, and estimated energies that were recorded during the repetitions of CLLEDCM, HPFD, and CLLEDCM–HPFD of WW were subjected to descriptive and inferential statistical analysis using parametric and nonparametric statistics according to the results of the tests for data normality (Shapiro–Wilk test and χ2), variance homogeneity (Bartlett, Levene, or Welch test for data not adjusted to normality), and analysis of variance (ANOVA and Tukey’s test if ANOVA was significant). All analyses were performed using Statistical Package for the Social Sciences software (IBM SPSS® version 25.0) [59], at a level of significance of 5%.
2.5. Calculation of Estimated Energy Consumption
The estimated energy consumption (ΔEs) of each system for the CLLEDCM or HPFD of a determined mass of WW during the operation time (ts) was calculated from the energy (ΔE) required to heat a mass (m) of liquid water with a determined specific heat (sh) from an initial temperature (T1) to a final temperature (T2) for a measured time (t) using the same system. Operations were assumed to have identical conditions as much as possible. Thus, the following equation was used:
where ΔEs or can be expressed as kWh equivalent to 3.6 × 106 J.
3. Results and Discussion
3.1. Guidelines on the Permissible Limits of Pollutant Discharge from WW
The maximum permissible limits of COD and TPH in the norms of China, Peru, WBG members, and Colombia are stricter than those in other countries. In Colombia, the norms regulating the quality of water discharged into water bodies include more analytical parameters than those of Venezuela and Asturias (Spain), and they have less rigorous limits for TSSes, TPH, and COD than do WBG members (Table 2). Regulations in Brazil and Mexico do not require the evaluation of most of the analytical parameters included in the norms of other countries.
3.2. Physicochemical Analysis of WW
Previous studies [28,43,60,61] have presented results on the analytical characterization of WW but have not specified the type of operation or the production stage in which the samples were collected. Table 3 shows data from the physicochemical analysis of the WW sampled from the sedimentation pond of the production area of an oil company in Colombia. The values of the physicochemical parameters in the WW were analyzed and found to exceed the permissible limits for effluent discharge into surface water bodies according to any of the norms of the countries mentioned in this study.

Table 3.
Physicochemical properties of the nondomestic wastewater (WW) and the treated wastewater (TWW) obtained through CLLEDCM, HPFD, or CLLEDCM-HPFD for samples of WW from the sedimentation pond of a petroleum production area.
The peaks corresponding to aliphatic isoprenoid hydrocarbons (pristane (n-C17) and phytane (n-C18)) in the GC chromatogram of the WW (Figure 2A) are indicative of TPH of marine origin [62] (contaminants in the sample). In the GC chromatogram of WW, the unimodal distribution of peak intensities corresponded mainly to n-alkanes of low molecular weight (Cn, n ≤ 25), which ranged from n-C10 to n-C32, from n-C13 to n-C31, and from n-C17 to n-C31 [63]. These results were evidence of the presence of aliphatic and aromatic contaminants.
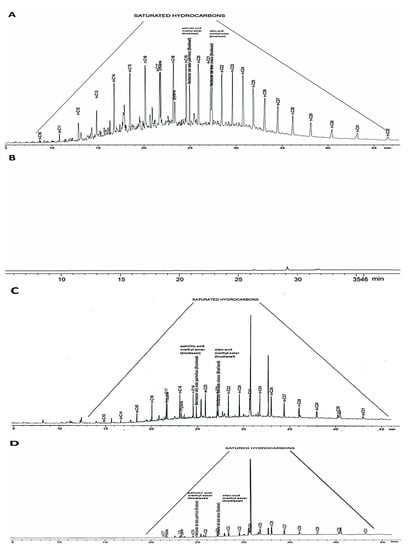
Figure 2.
Gas chromatography (GC) (A) nondomestic wastewater (WW), (B) the treated wastewater (TWW) obtained through HPFD, (C) the TWW obtained through CLLEDCM, and (D) a raffinate (partially treated water) produced through CLLEDCM–HPFD of the nondomestic wastewater (WW). Markers: n-alkanes, pristine, and phytane.
In the WW and GC chromatograms of TWW (Figure 2A–D), pentacyclic triterpenes (hopanes) (n-C27–C32) were identified. They included steranes (n-C27–C29), with a predominance of n-C27 [64], and an n-C30/n-C29 ratio higher than 1.0 [65]. All of these species were remnants of pollutants present in the original WW, and they were found in notably minor concentrations in the TWWs obtained by using any of the tested treatments (CLLEDCM, HPFD, or CLLEDCM–HPFD).
3.3. Physicochemical Analysis of TWW by CLLEDCM, HTFD, or CLLEDCM–HTFD
Columns 4–6 in Table 3 show the values of the physicochemical parameters characterizing each TWW obtained by performing CLLEDCM, HPFD, or CLLEDCM–HPFD on WW. Except for the pH, the other values of the physicochemical parameters in the analysis of the TWW generally decreased significantly. Judging by the appearance of the TWW obtained and by the decrease in TPH, COD, and chloride, the HPFD and CLLEDCM–HPFD treatments were more effective than CLLEDCM in the removal of the pollutant load from WW. Nonetheless, CLLEDCM removed most of the TPH (>99%) and diminished the COD in the WW by more than 90% (Table 4).

Table 4.
Decrease of total petroleum hydrocarbon (TPH) and chemical oxygen demand (COD) by HPFD, CLLEDCM, or CLLEDCM–HPFD (on a laboratory scale) in the nondomestic wastewater sampled in the sedimentation pond of the petroleum production area (WW).
Similar or higher decreases in COD and TPH from decontamination treatments of wastewater have been reported in the literature by only a few other studies using more expensive and sophisticated treatments that require special equipment and more controlled conditions than those of HTFD or CLLEDCM [42,66,67,68].
The pH of the TWW obtained by HTFD or CLLEDCM did not comply with the requirement of most regulations (Table 2 and Table 3). These pH values are explainable as an effect of the treatments changing the concentrations of chemical species in acid–base equilibria that were present as pollutants in the WW [69,70]. An example of such a mechanism is considered in Scheme 1.
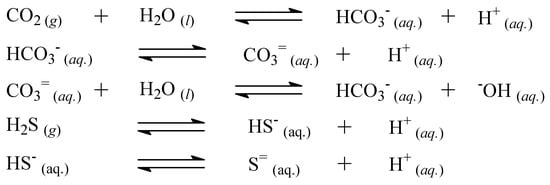
Scheme 1.
Acid–base equilibria between carbonate ions (CO3=(aq.)), bicarbonate ions (HCO3–(aq.)), and carbon dioxide (CO2(g)), and between sulfide ions (S=(aq.)), disulfide ions (S=(aq.)), and hydrogen sulfide (H2S(g)).
With the HPFD treatment, CO2(g) and H2S(g) went from the hot bottom to the steam phase. Cooling these compounds resulted in their redissolution and concentration, and the pH of the distillate decreased (pH 5.22). The solubility of CO2(g) and H2S(g) in the extract decreased as the extract warmed, and these gases were then lost from the gas phase during solvent evaporation–condensation cycles in the CLLEDCM treatment. The ions in equilibrium, as dissolved species, were concentrated in the raffinates, and the pH increased (pH 9.36).
The raffinates of the CLLEDCM treatment with scarce CO2(g) and H2S(g) products were subjected to HPFD, which produced distillate with an appropriate pH (pH 7.50). In other words, the TWW obtained by CLLEDCM–HPFD complied with the current legal requirements of the countries examined in this study [18,19,20,21,22,23,24,25,26].
The conductivity, salinity, and alkalinity of the raffinates obtained by CLLEDCM or CLLEDCM–HTFD directly related to the high concentrations of Cl–, Na+, and Ca2+ in the original WW [69,70]. These characteristics are unfavorable for the quality of the TWW yielded from these treatments.
The peak intensities in the GC chromatograms of the TWW product of CLLEDCM, HPFD, or CLLEDCM–HPFD treatments of WW (Figure 2B–D) evidenced a lower TPH content than in the starting WW (Figure 2A). The peaks between n-C13 and n-C31 in their GC chromatograms decreased (Figure 2C,D) or practically disappeared (Figure 2B). The persistence of the peaks corresponding to pristane and phytane (tR ≈ 21.7 and 23.2 min) and the increase in the pristane/phytane ratio, particularly in the GC chromatograms of the TWW obtained as raffinates (Figure 2C,D), indicated the presence of n-alkanes, as well as methyl palmitate and methyl oleate (residuals of biodiesel, tR ≈ 24.9 and 27.1 min), in the TWW.
Since their peaks were absent in the chromatogram of the extracts, the transfer of those species from the feed to the extracts in the CLLEDCM process must have been incomplete. The similarities between the GC chromatograms of WW and the raffinates confirmed the common origin of the pollutants found in those samples (Figure 2A,C,D). The prevalence of a few of such peaks in the GC chromatograms of the distillates obtained by HPFD or CLLEDCM–HPFD indicated that a tiny fraction of the contaminant load was transferred from the WW to distillates during the process. The absence of the majority of such peaks in the GC chromatogram of the distillate showed the potentiality of HPFD for decontaminating WW (Figure 2B).
3.4. Operation Time, Energy Consumption, and Performance of CLLEDCM, HPFD, and CLLEDCM–HPFD
Table 5 shows the average duration and the average mass of the TWW relative to the yields obtained. The average energy consumption (kWh) and time (h) required to obtain a unit of mass (kg) of the respective TWW are also shown, which were calculated after being subjected to n measured durations and masses according to the statistical treatment described in Section 2.4. Such measures and estimates (Table 4 and Table 5) were established at the given laboratory conditions described in Section 2.3.

Table 5.
Yields and average estimated consumption of time and energy per unit mass of treated water (TWW) obtained by CLLEDCM, HPFD, or CLLEDCM–HPFD (on a laboratory scale) of nondomestic wastewater (WW) of sedimentation ponds in the petroleum production stage.
The CLLEDCM treatment recovered 98.5% of the initial WW mass, indicating an approximate yield of 50.0 kg/kWh of TWW at a rate of 0.1 kg/h. CLLEDCM produced yellowish and opalescent water with a pH of 9.36, and TPH and COD decreased by 99% and 90.5%, respectively, compared to the starting WW: That is, CLLEDCM did not achieve percentages of removal of TPH, COD, TH, TDSes, and TSSes that would allow the TWW obtained by this method to meet the regulatory requirements of the countries in this study.
The inferential statistical analysis (Shapiro–Wilk test) of the mean weight of solvent-free extracts collected at 20, 40, 60, 90, 120, 180, and 240 min in the four CLLEDCM replicates (28 values) and six CLLEDCM–HPFD replicates (42 values) indicated that these data fit a normal distribution (p > 0.05) (Table 6A). The results of the χ2 test indicated that the mean weights of the solvent-free extracts collected at different time points in these replicates were independent (p > 0.05) (Table 7).

Table 6.
Statistics of the (A) normality test (Shapiro–Wilk) and of (B) multiple comparisons (HSD Tukey) applied to the data from the CLLEDCM and CLLEDCM–HPFD of industrial wastewater (WW).

Table 7.
Sample of results of the inferential and descriptive statistics of the application of CLLEDCM and CLLEDCM–HPFD in decontaminating the industrial wastewater (WW) of a sediment pool.
The Welch test (p < 0.05) was applied in cases of violation of the homoscedasticity assumption in the Levene and Bartlett tests. There were no significant differences in the mean weights of the extracts, and this is a requirement for the ANOVA test. The ANOVA analysis indicated that there were significant differences (p < 0.05) in the mean weight of the extracts collected at different time points (Table 7).
The results of Tukey’s test demonstrated that the data on the mean weight of the solvent-free extracts from four CLLEDCM replicates and five CLLEDCM–HPFD replicates were significantly different (p < 0.05) and that the highest percentage of contaminants was removed in the first 40 min using CLLEDCM and in the first 20 min using CLLEDCM–HPFD (Table 6B).
The HPFD treatment recovered 94.1%–97.3% of the initial WW mass, indicating an approximate yield of 2.8 ± 0.7 kg/kWh at a rate of 0.3 ± 0.1 kg/h. HPFD produced colorless, transparent water with a pH of 5.22 (a low value), and TPH and COD decreased by 95.0%–99.9% and 98.6%–99.6%, respectively, compared to the starting WW. The TWW obtained by HPFD presented the lowest residual values of the analytical parameters for TPH, EC, TA, TH, TDSes, TSSes, chlorides, and salinity measured in the original WW analysis (Table 3 and Figure 2B).
The CLLEDCM–HPFD treatment recovered 92.4% of the initial WW mass, indicating an approximate yield of 9.1 ± 0.2 kg/kWh of TWW at a rate of 0.05 kg/h. CLLEDCM–HPFD produced colorless and transparent water with a pH of 7.50, and TPH and COD decreased by 100.0% and 99.9%, respectively, compared to the starting WW. The TWW obtained by CLLEDCM–HPFD met the requirements of current regulations in WBG countries, except for Peru and China, but presented appreciable remnants of salinity, TH, TDSes, and TSSes. Among the three treatments, this method also had the highest estimated average consumption of time and energy required.
Nonetheless, energy consumption decreased and performance slightly increased (98.5%) as the operation time of HPFD increased (Table 5). Therefore, it is still better to produce TWW with the quality achieved by individual HPFD process than wastewater with slightly better quality that meets the regulations of the analyzed countries and WBG memberˈs for effluent discharge (Table 2 and Table 3) at a relatively lower yield (92.4%), longer operation time, and higher energy consumption (using CLLEDCM–HPFD) (Table 5).
A duration of 100 min for the CLLEDCM operation, as either an individual treatment or as part of the CLLEDCM–HTFD sequence, is recommended in order to remove a relatively high fraction of the pollutants from WW with a reasonable minimal consumption of time and energy (Figure 3).
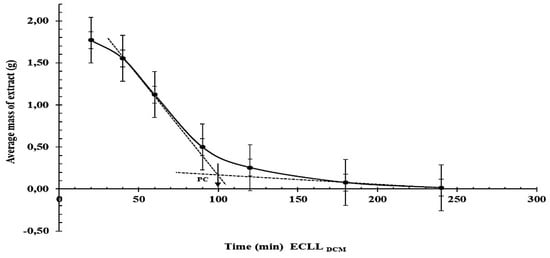
Figure 3.
Average mass of solvent-free extracts obtained in relation to the time of process during the CLLEDCM of WW.
The recommended total duration of operation for the other treatments (HPFD or CLLEDCM–HPFD) and the yields of TWW depend on the boiling regime, particularly at the end of the process. When the boiling was affected by the concentration or even the crystallization of poorly soluble contaminants in the distillation bottom, material projections could propagate from the bottom to the distillate collector.
Obviously, the yields, and probably the quality, of the TWW obtained by using any of these treatments can be optimized by using better-controlled conditions than those used in this first instance of laboratory-scale experiments. The laboratory conditions in this study that can be optimized include the control of losses due to handling, heating, the use of a vacuum, temperature control, and improvements in the energy transfer and utilization in each treatment system. Such optimization is outside the scope of this work, but it could be of some engineering interest to take advantage of the demonstrated potential of these treatments for decontaminating the WW generated by the petroleum industry or other sources.
4. Conclusions
This work constitutes the first systematic assay on a laboratory scale determining the potential for using CLLEDCM, HPFD, or CLLEDCM–HPFD as treatments to decontaminate wastewater generated in sedimentation ponds in the production stage of the petroleum industry (WW). The results of using these treatments demonstrated significant yields (94.1%–97.3% by HPFD, 98.5% by CLLEDCM, and 92.4% by CLLEDCM–HPFD) of water, in which decreases in TPH ranged from 95.0% (HPFD) to 100% (CLLEDCM–HPFD), decreases in COD ranged from 90.5% (CLLEDCM) to 99.9% (CLLEDCM–HPFD), and most of the other pollutants decreased compared to the starting WW: That is, water treated by HPFD and CLLEDCM–HPFD met the requirements of the permissible limits of TPH and COD in national and international regulations, including Colombia and China.
Among the tested treatments to decontaminate WW, HPFD was a more effective treatment than CLLEDCM–HPFD or CLLEDCM because it produced at least a 92.4% yield of water with an acceptable quality (only slightly less than the quality achieved by CLLEDCM–HPFD and higher than that obtained using CLLEDCM). Furthermore, HPFD neither demands the long process time or large energy consumption typical of CLLEDCM–HPFD nor produces the low water quality obtained by CLLEDCM, and its pH of 5.22 can be easily adjusted to meet all the requirements of the norms.
The demonstrated potential of HPFD could be attractive as an engineering optimization study. If the scarcity of water of good quality in the world becomes critical, humanity will need to meet the strictest regulations on the disposal and environmentally safe use of wastewater to satisfy water demand and thus preserve life on the planet, which will supersede concerns for economic and energy costs. The factors that currently render the use of HPFD prohibitive for the decontamination of WW will be comparatively minor problems among the others that engineers will have to solve in the future.
Author Contributions
Conceptualization, S.M.V.M. and E.A.M.; methodology, S.M.V.M. and E.A.M.; software, S.M.V.M.; validation, C.A.G.F. and R.F.M.; formal analysis, S.M.V.M., E.A.M. and C.A.G.F. investigation, S.M.V.M.; resources, S.M.V.M., E.A.M., C.A.G.F. and R.F.M. data curation, S.M.V.M. and E.A.M.; writing—original draft preparation, S.M.V.M. and E.A.M.; writing—review and editing, C.A.G.F. and R.F.M.; visualization, C.A.G.F.; supervision, C.A.G.F.; project administration, S.M.V.M.; funding acquisition, S.M.V.M.
Funding
The authors would like to thank the Administrative Department of Science, Technology, and Innovation (COLCIENCIAS) for the approval of her doctoral scholarship in chemistry at the National University of Colombia-Bogotá (COLCIENCIAS 647).
Acknowledgments
S.M.V.M. is grateful to the Administrative Department of Science, Technology, and Innovation (COLCIENCIAS) for the approval of her doctoral scholarship in chemistry at the National University of Colombia-Bogotá (COLCIENCIAS 647). All authors are grateful to the Chemistry Department of the National University of Colombia at Bogotá for providing the facilities and aids that allowed for the developments of this work as part of the doctoral thesis titled “Application of Continuous Liquid–Liquid Extraction and Fractional Distillation as Primary Treatments to Decontaminate Industrial Wastewaters Generated by the Petroleum Industry”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Veil, J. Produced Water Volumes and Management Practices in 2012. Available online: http://www.veilenvironmental.com/publications/pw/prod_water_volume_2012.pdf (accessed on 4 December 2017).
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.M.; Boels, L.; Lammertink, R.G.; De Vos, W.M. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Mesa, S.L.; Orjuela, J.M.; Ramírez, A.T.; Herrera, J.A. Revisión del panorama actual del manejo de agua de producción en la industria petrolera colombiana. Gestión y Ambiente 2018, 21, 87–98. [Google Scholar] [CrossRef]
- Rice, E.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF), Eds.; American Public Health Association: Washington, DC, USA, 2012; pp. 38–46. ISBN 978-08-7553-013-0. [Google Scholar]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Dana, A.D. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Dolan, F.C.; Cath, T.Y.; Hogue, T.S. Assessing the feasibility of using produced water for irrigation in Colorado. Sci. Total Environ. 2018, 640–641, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Bakke, T.; Klungsøyr, J.; Sanni, S. Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Mar. Environ. Res. 2013, 92, 154–169. [Google Scholar] [CrossRef]
- Martel-Valles, J.F.; Foroughbakchk-Pournavab, R.; Benavides-Mendoza, A. Produced waters of the oil industry as an alternative water source for food production. Rev. Int. Contam. Ambient. 2016, 32, 463–475. [Google Scholar] [CrossRef]
- Gabardo, I.T.; Platte, E.B.; Araujo, A.S.; Pulgatti, F.H. Evaluation of produced water from Brazilian offshore platforms. In Produced Water; Springer: Berlin/Heidelberg, Germany, 2011; pp. 89–113. ISBN 978-1-4614-0046-2. [Google Scholar]
- Breuer, R.; Al-Asmi, S.R. Nimr Water Treatment Project—Up Scaling A Reed Bed Trail To Industrial. In Proceedings of the SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, Rio de Janeiro, Brazil, 10 November 2018; p. 11. [Google Scholar]
- Bayat, M.; Mehrnia, M.R.; Hosseinzadeh, M.; Sheikh-Sofla, R. Petrochemical wastewater treatment and reuse by MBR: A pilot study for ethylene oxide/ethylene glycol and olefin units. J. Ind. Eng. Chem. 2015, 25, 265–271. [Google Scholar] [CrossRef]
- Kuraimid, Z.K. Treatment of Produced Water in North Rumela Oil Field for Re-Injection Application. In Proceedings of the SPE Kuwait Oil and Gas Show and Conference, Kuwait City, Kuwait, 10 November 2018; p. 12. [Google Scholar]
- AlKaabi, M.A. Enhancing Produced Water Quality using Modified Activated Carbon. Master’s Thesis, Qatar University, Doha, Qatar, 2016. [Google Scholar]
- Al Zarooni, M.; Elshorbagy, W. Characterization and assessment of Al Ruwais refinery wastewater. J. Hazard. Mater. 2006, 136, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Alsari, A.; Ibrahim, H.; Okasha, A.; Aboabboud, M.M. Treatment System of Produced Water with Supercritical Carbon Dioxide. IPCBEE 2014, 68, 40–44. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ning, Y.; Liao, L.; Yuan, B. Chapter Fourteen—Special Focus on Produced Water in Oil and Gas Fields: Origin, Management, and Reinjection Practice. In Formation Damage During Improved Oil Recovery; Yuan, B., Wood, D.A., Eds.; Gulf Professional Publishing: Houston, TX, USA, 2018; pp. 515–586. ISBN 978-0-12-813782-6. [Google Scholar]
- Official Journal of the Principality of Asturias (Boletín Oficial del Principado de Asturias). No. 5/2002, of 3 June 2002, on Discharges of Industrial Wastewater to Public Sanitation Systems. Off. J. Princ. Astur. 2002, 26180–26187. Available online: https://www.boe.es/eli/es-as/l/2002/06/03/5 (accessed on 26 December 2018).
- State Environmental Protection Administration of the People’s Republic of China. GB 8978-1996, Integrated Wastewater Discharge Standard. 1996. Available online: http://english.mee.gov.cn/Resources/standards/water_environment/Discharge_standard/200710/t20071024_111803.shtml (accessed on 26 April 2018).
- Official Journal of the Republic of Colombia (Diario Oficial de la Republica de Colombia). Resolution No. 631 of 2015; Establishes the Maximum Permitted Limits Values are Established in the Point Discharges to Surface Water Bodies and Sewage Systems Public and Other Provisions are Issued. Official Journal 49.486; 2015. Available online: http://svrpubindc.imprenta.gov.co/diario/view/diarioficial/consultarDiarios.xhtml (accessed on 26 March 2018).
- Official Journal of the United Mexican States (Diario Oficial de la Federación de los Estados Unidos Mexicanos). NOM-001-SEMARNAT-1996. Establishes the Maximum Permitted Limits of Pollutants in the Wastewater Discharges into Water and onto National Property. 2003. Available online: http://www.ordenjuridico.gob.mx/Documentos/Federal/wo69205.pdf (accessed on 26 March 2018).
- Official Journal of the Federative Republic of Brazil (Diário Oficial da República Federativa do Brasil). Resolution No. 430 of 2011. Establishes the Conditions and Patterns of Effluent Release, Complements and Alters Resolution No. 357, of March 17, 2005, of the National Council of the Environment-CONAMA. Official Journal No. 92. 2011. Available online: https://www.jusbrasil.com.br/diarios/26738562/pg-89-secao-1-diario-oficial-da-uniao-dou-de-16-05-2011 (accessed on 12 April 2018).
- Official Journal of the Republic of Peru (Diario Oficial de la Republica de Perú). Supreme Decree No. 004-2017, Establishes the Environmental Quality Standards (ECA) for Water and Establishes Complementary Provisions. 2017. Available online: https://busquedas.elperuano.pe/normaslegales/aprueban-estandares-de-calidad-ambiental-eca-para-agua-y-e-decreto-supremo-n-004-2017-minam-1529835-2/ (accessed on 12 April 2018).
- Official Journal of the Republic of Venezuela (Gaceta Oficial de la República de Venezuela). Decree No. 883 of the Republic of Venezuela, Pertaining to the Classification and Quality Control of Water Bodies and Contaminating Spillage. Federal Law Journal No. 5.021 of 18th December 1995. Available online: http://www.mvh.gob.ve/fabricadeinsumos27f/index.php?option=com_phocadownload&view=category&download=31:decreto-n-883-normas-para-la-clasificacion-y-el-control-de-la-calidad-de-los-cuerpos-de-agua-y-vertidos-o-efluentes-liquidos&id=11:leyes&Itemid=654 (accessed on 23 December 2018).
- World Bank Group. Environmental, Health, and Safety Guidelines for Onshore oil and Gas Development (English); IFC E&S; World Bank Group: Washington, DC, USA, 2007; Available online: http://documents.worldbank.org/curated/en/858751486372860509/Environmental-health-and-safety-guidelines-for-onshore-oil-and-gas-development (accessed on 26 March 2018).
- World Bank Group. Environmental, Health, and Safety Guidelines for Petroleum Refining (English); World Bank Group: Washington, DC, USA, 2016; Available online: http://documents.worldbank.org/curated/en/522801489581711256/Environmental-health-and-safety-guidelines-for-petroleum-refining (accessed on 26 March 2018).
- Zheng, J.; Chen, B.; Thanyamanta, W.; Hawboldt, K.; Zhang, B.; Liu, B. Offshore produced water management: A review of current practice and challenges in harsh/Arctic environments. Mar. Pollut. Bull. 2016, 104, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chen, Y.; Chen, D.; Dong, Y.; Zhang, Z.; Long, Y. Oil removal in tight-emulsified petroleum waste water by flocculation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 042005. [Google Scholar] [CrossRef]
- Zapata Acosta, K.; Carrasco-Marin, F.; Cortés, F.B.; Franco, C.A.; Lopera, S.H.; Rojano, B.A. Immobilization of P. stutzeri on Activated Carbons for Degradation of Hydrocarbons from Oil-in-Saltwater Emulsions. Nanomaterials 2019, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Khan, W.Z.; Najeeb, I.; Tuiyebayeva, M.; Makhtayeva, Z. Refinery wastewater degradation with titanium dioxide, zinc oxide, and hydrogen peroxide in a photocatalytic reactor. Process Saf. Environ. Prot. 2015, 94, 479–486. [Google Scholar] [CrossRef]
- Chen, D.; Li, F.; Gao, Y.; Yang, M. Pilot Performance of Chemical Demulsifier on the Demulsification of Produced Water from Polymer/Surfactant Flooding in the Xinjiang Oilfield. Water 2018, 10, 1874. [Google Scholar] [CrossRef]
- Karadag, D.; Köroğlu, O.E.; Ozkaya, B.; Cakmakci, M. A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem. 2015, 50, 262–271. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chung, T.-S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Siyal, M.I.; Lee, C.-K.; Park, C.; Khan, A.A.; Kim, J.-O. A review of membrane development in membrane distillation for emulsified industrial or shale gas wastewater treatments with feed containing hybrid impurities. J. Environ. Manag. 2019, 243, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Mohammadtabar, F.; Khorshidi, B.; Hayatbakhsh, A.; Sadrzadeh, M. Integrated Coagulation-Membrane Processes with Zero Liquid Discharge (ZLD) Configuration for the Treatment of Oil Sands Produced Water. Water 2019, 11, 1348. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Al-Raoush, R.I.; Abu-Reesh, I.M.; Aljaml, K. Removal of petroleum hydrocarbons and sulfates from produced water using different bioelectrochemical reactor configurations. Sci. Total Environ. 2019, 665, 820–827. [Google Scholar] [CrossRef]
- Benito-Alcázar, C.; Vincent-Vela, M.; Gozálvez-Zafrilla, J.; Lora-García, J. Study of different pretreatments for reverse osmosis reclamation of a petrochemical secondary effluent. J. Hazard. Mater. 2010, 178, 883–889. [Google Scholar] [CrossRef]
- Lu, M.; Wei, X. Treatment of oilfield wastewater containing polymer by the batch activated sludge reactor combined with a zerovalent iron/EDTA/air system. Bioresour. Technol. 2011, 102, 2555–2562. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Hou, H. A combination of solvent extraction and freeze thaw for oil recovery from petroleum refinery wastewater treatment pond sludge. J. Hazard. Mater. 2015, 283, 832–840. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, G.; Cheng, G.; Wang, Y.; Fu, H. Hardness, COD and turbidity removals from produced water by electrocoagulation pretreatment prior to Reverse Osmosis membranes. Desalination 2014, 344, 454–462. [Google Scholar] [CrossRef]
- Dos Santos, E.V.; Rocha, J.H.B.; de Araújo, D.M.; de Moura, D.C.; Martínez-Huitle, C.A. Decontamination of produced water containing petroleum hydrocarbons by electrochemical methods: A minireview. Environ. Sci. Pollut. Res. 2014, 21, 8432–8441. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, B.; Gargouri, O.D.; Gargouri, B.; Trabelsi, S.K.; Abdelhedi, R.; Bouaziz, M. Application of electrochemical technology for removing petroleum hydrocarbons from produced water using lead dioxide and boron-doped diamond electrodes. Chemosphere 2014, 117, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Aljuboury, D.; Palaniandy, P.; Abdul Aziz, H.; Feroz, S. Treatment of petroleum wastewater by conventional and new technologies-A review. Glob. Nest J. 2017, 19, 439–452. [Google Scholar]
- Jafarinejad, S. Treatment of Oily Wastewater. In Petroleum Waste Treatment and Pollution Control; Jafarinejad, S., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 185–267. ISBN 978-0-12-809243-9. [Google Scholar]
- Nasiri, M.; Jafari, I. Produced Water from Oil-Gas Plants: A Short Review on Challenges and Opportunities. Periodica Polytech. Chem. Eng. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Nonato, T.C.M.; Alves, A.A.D.A.; Sens, M.L.; Dalsasso, R.L. Produced water from oil—A review of the main treatment technologies. J. Environ. Toxicol. 2018, 2, 23–27. [Google Scholar]
- Ahmad, N.; Goh, P.; Abdul Karim, Z.; Ismail, A. Thin Film Composite Membrane for Oily Waste Water Treatment: Recent Advances and Challenges. Membranes 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Karhu, M.; Kuokkanen, T.; Rämö, J.; Mikola, M.; Tanskanen, J. Performance of a commercial industrial-scale UF-based process for treatment of oily wastewaters. J. Environ. Manag. 2013, 128, 413–420. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Eslamifard, M.R. Recycle unit wastewater treatment in petrochemical complex using reverse osmosis process. J. Hazard. Mater. 2010, 174, 404–409. [Google Scholar] [CrossRef]
- Wan Ikhsan, S.N.; Yusof, N.; Aziz, F.; Nurasyikin, M. A review of oilfield wastewater treatment using membrane filtration over conventional technology. MJAS 2017, 21, 643–658. [Google Scholar] [CrossRef]
- Masoudnia, K.; Raisi, A.; Aroujalian, A.; Fathizadeh, M. A hybrid microfiltration/ultrafiltration membrane process for treatment of oily wastewater. Desalin. Water Treat. 2014, 55, 1–12. [Google Scholar] [CrossRef]
- Yan, L.; Ma, H.; Wang, B.; Mao, W.; Chen, Y. Advanced purification of petroleum refinery wastewater by catalytic vacuum distillation. J. Hazard. Mater. 2010, 178, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, E.A.; Otolorin, J.A. Oil Recovery from Petroleum Sludge by Solvent Extraction. Petrol. Sci. Technol. 2009, 27, 836–844. [Google Scholar] [CrossRef]
- SPSS. Computer Software SPSS; SPSS, Inc.: Chicago, IL, USA, 2015. [Google Scholar]
- Zhang, S.; Wang, P.; Fu, X.; Chung, T.-S. Sustainable water recovery from oily wastewater via forward osmosis-membrane distillation (FO-MD). Water Res. 2014, 52, 112–121. [Google Scholar] [CrossRef] [PubMed]
- El-Naas, M.H.; Alhaija, M.A.; Al-Zuhair, S. Evaluation of a three-step process for the treatment of petroleum refinery wastewater. J. Environ. Chem. Eng. 2014, 2, 56–62. [Google Scholar] [CrossRef]
- Jeng, W.-L.; Huh, C.-A. A comparison of sedimentary aliphatic hydrocarbon distribution between the southern Okinawa Trough and a nearby river with high sediment discharge. Estuar. Coast. Shelf Sci. 2006, 66, 217–224. [Google Scholar] [CrossRef]
- Mijaylova Nacheva, P.; Birkle, P.; Ramírez Camperos, E.; Sandoval Yoval, L. Tratamiento de aguas de la desalación del petróleo para su aprovechamiento en inyección al subsuelo. Revista AIDIS de Ing y Cienc Amb Invest des y prac 2007, 1, 1–16. [Google Scholar] [CrossRef]
- Cortes, J.E.; Rincon, J.M.; Jaramillo, J.M.; Philp, R.P.; Allen, J. Biomarkers and compound-specific stable carbon isotope of n-alkanes in crude oils from Eastern Llanos Basin, Colombia. J. S. Am. Earth Sci. 2010, 29, 198–213. [Google Scholar] [CrossRef]
- Peters, K.; Walters, C.; Moldowan, J. The Biomarker Guide: Biomarkers and Isotopes in the Environment and Human History, 2nd ed.; Cambridge University Press: New York, NY, USA; Cambridge, UK, 2005; Volume 1, p. 471. ISBN 978-05-1152-486-8. [Google Scholar]
- Chavan, A.; Mukherji, S. Treatment of hydrocarbon-rich wastewater using oil degrading bacteria and phototrophic microorganisms in rotating biological contactor: Effect of N:P ratio. J. Hazard. Mater. 2008, 154, 63–72. [Google Scholar] [CrossRef]
- Koo, C.H.; Mohammad, A.W. Recycling of oleochemical wastewater for boiler feed water using reverse osmosis membranes—A case study. Desalination 2011, 271, 178–186. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.; Lau, W.; Ng, B.; Mataram, A.; Kassim, M. Effects of process conditions in submerged ultrafiltration for refinery wastewater treatment: Optimization of operating process by response surface methodology. Desalination 2012, 287, 350–361. [Google Scholar] [CrossRef]
- Al-Deffeeri, N.S. Chemical analysis of distilled water: A case study. Desalin. Water Treat. 2013, 51, 1936–1940. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2012, 9, 157–177. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).