1. Introduction
Consumption of microbially contaminated drinking water is one of the greatest threats to global public health. The World Health Organization (WHO) estimates that consumption of unsafe water causes the deaths of over 5 million people per year. More than 1.5 million of these deaths are to children under the age of 5 [
1]. Diarrheal diseases (transmitted by contaminated water) are a major contributor to these alarming numbers [
2,
3,
4]. There are additional health burdens on children who experience cognitive impairment and stunted growth as a result of gastrointestinal infections caused by consumption of water with pathogenic organisms [
5]. In many parts of sub-Saharan Africa, there is low-quality drinking water and high rates of human immunodeficiency virus (HIV) infection [
6]. People living with HIV or acquired immune deficiency syndrome (AIDS) are particularly susceptible to infections from waterborne pathogens because of their weakened immune systems [
7].
Water storage is necessary in developing countries where communities lack potable water supplies piped into their homes [
8]. It has been observed that even when water is collected from a high-quality source, the water is frequently contaminated during transport, handling, and storage [
9,
10,
11]. WHO suggests that low-cost point-of-use (POU) treatment technologies have the potential to significantly improve the microbial quality of household water and reduce the risk of diarrheal disease and death [
12]. POU water treatment can prevent the adverse health effects of low-quality source water and problems of recontamination during transport and storage. Currently, there are multiple promising POU treatment technologies (biosand filters, ceramic water filters, chlorination, etc.) available [
13]. Chlorine-based disinfection technologies have several limitations, including their ineffectiveness against protozoan pathogens, poor social acceptance due to a change in the taste of the water, the possible formation of various disinfection byproducts (DBPs), and the emergence of microorganisms that are resistant to chlorine disinfection [
14,
15,
16]. Researchers have investigated various disinfectants for chlorination, but DBP formation is an important issue. For example, in Europe during the "derogations’ period", several countries, including Italy, asked for an exemption to the quality of water distributed to the population, owing to the presence of chlorites with values that exceeded the standards [
17]. Ionic silver, by contrast, has been proven to be an effective disinfectant and works for a wide spectrum of protozoa, bacteria, and viruses [
18,
19,
20,
21,
22]. Some other technologies, which utilized antimicrobial properties of silver for disinfection of water, have been reported by other investigators [
23,
24,
25]. Nangmenyi et al. [
26] developed an Ag- modified a-Fe
2O
3 nanoparticle-impregnated fiber-glass through hydrothermal methods as an antimicrobial agent for disinfecting bacteria and viruses in water, which showed excellent antiviral efficacy.
Recently, laboratory and field testing of a new silver-ceramic-based POU water treatment technology was reported by Ehdaie et al. [
13,
27]. These researchers combined appropriate proportions of a commercial Redart pottery clay, sawdust passed through a 20-mesh sieve, water, and silver nitrate. Following mixing, these ingredients were pressed into the shape of a cylinder and fired in a kiln to a final temperature of 900 °C. During firing, the clay hardened into a ceramic, the sawdust combusted (leaving behind pore space), and the ionic silver was reduced to form metallic silver “nanopatches” throughout the ceramic pore space. Residual nitrate was not detected, suggesting that it was converted to nitrogen gas and water vapor during the firing process. Using transmission electron microscopy (TEM) and energy dispersive spectroscopy, they found that the silver nanopatches had mean diameters between 2 and 3 nm. When placed in 10 L of water, the metallic silver was gradually oxidized back to ionic silver and released into the bulk solution. Ehdaie et al. [
27] demonstrated significant laboratory disinfection of
E. coli while maintaining silver levels below the WHO drinking water guideline of 100 µg/L. They also demonstrated that the silver release is consistent day after day for about 6 months.
More recently, Ehdaie et al. [
13] reported on the performance of this silver-ceramic technology in the field in rural households in the Limpopo Province, South Africa. They observed significant reductions in coliform bacteria and
E. coli compared to blind controls. However, silver levels in the treated water were typically less than 15 µg/L, and often less than 5 µg/L. Given that silver disinfection is dependent on both contact time and silver concentration, it was noted that the technology could be improved by increasing the rate of silver ion release from the silver-ceramic matrix.
The objective of this study was to identify ways to increase the silver release rate from a silver-ceramic media prepared using methods similar to Ehdaie et al. [
13] To meet this objective, we systematically varied silver-ceramic disk manufacturing methods and materials and quantified the resultant silver release rates into 10 L of water. The design modifications studied were: (i) Modification of the firing temperature program; (ii) modification of the mixture compaction pressure; (iii) variation of the sawdust composition; (iv) modification of the mixing protocol; (v) modification of the mass of silver nitrate; and (vi) increasing the surface area per unit mass of ceramic. For the most promising design modifications,
E. coli disinfection has also been quantified. Results of these experiments are discussed in terms of mechanistic considerations for silver oxidation, sorption, and diffusion.
2. Materials and Methods
All the glassware used in this work was washed with Milli-Q deionized, organic-free water. All the reagent and chemicals used were of analytical grade. Redart clay (200 mesh) was purchased from Clayworks Supplies, Inc. Illite (Richmond, VA, USA) and kaolinite were the predominant minerals present in this clay. Sawdust used in this investigation was procured from a commercial lumberyard (Earlysville, VA, USA). Sawdust used (20 mesh sieve (0.841 mm)) in the study was first air-dried to remove moisture. Silver nitrate was purchased from Sigma Aldrich (St. Louis, MO, USA) and added in amounts that would ensure 1, 1.5, and 3 g of silver embedded per ceramic tablet for various experiments.
2.1. Ceramic Tablet Preparation
For the preparation of the ceramic tablets, clay was first mixed with sawdust at a pre-determined ratio. A known amount of silver nitrate solution (prepared in water) was added to this clay/sawdust mixture. This clay/sawdust/silver nitrate wet mixture was then transferred to a plastic mold (polyvinylchloride cylinder), and pressed for one minute at approximately 97 psi in a manually operated hydraulic power press. Pressed tablets were removed from the power press, and dried at room temperature from 8 to 72 h before transferring to a kiln (Evenflow) for firing. Air dried tablets were fired at a pre-determined temperature in a kiln for 3 h. The resulting cylindrical ceramic disks produced were 6.5 cm in diameter and 4.5 cm in height with a mass of 150 ± 1 g. This ceramic disk fabrication method is identical to the method described previously by Ehdaie et al. [
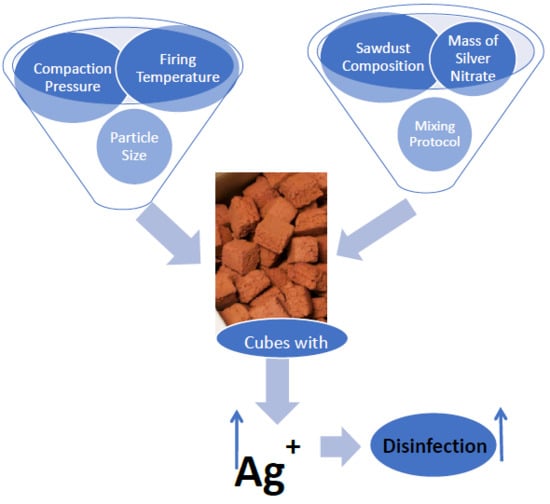
28]. Ceramic tablets were taken out of the kiln and inspected for any deformation, discoloration, or soft spots. These freshly prepared ceramic tablets were labeled and packaged immediately to avoid any environmental contamination. Quality control tablets were prepared and tested in the same way, except no silver nitrate was used in the ceramic preparation. It was hypothesized that various fabrication variables affect silver ion release through porous media. Therefore, experiments were designed to examine how different fabrication methods and silver masses affect silver release rates into water.
2.1.1. Fabrication Variables—Pressure in Mold
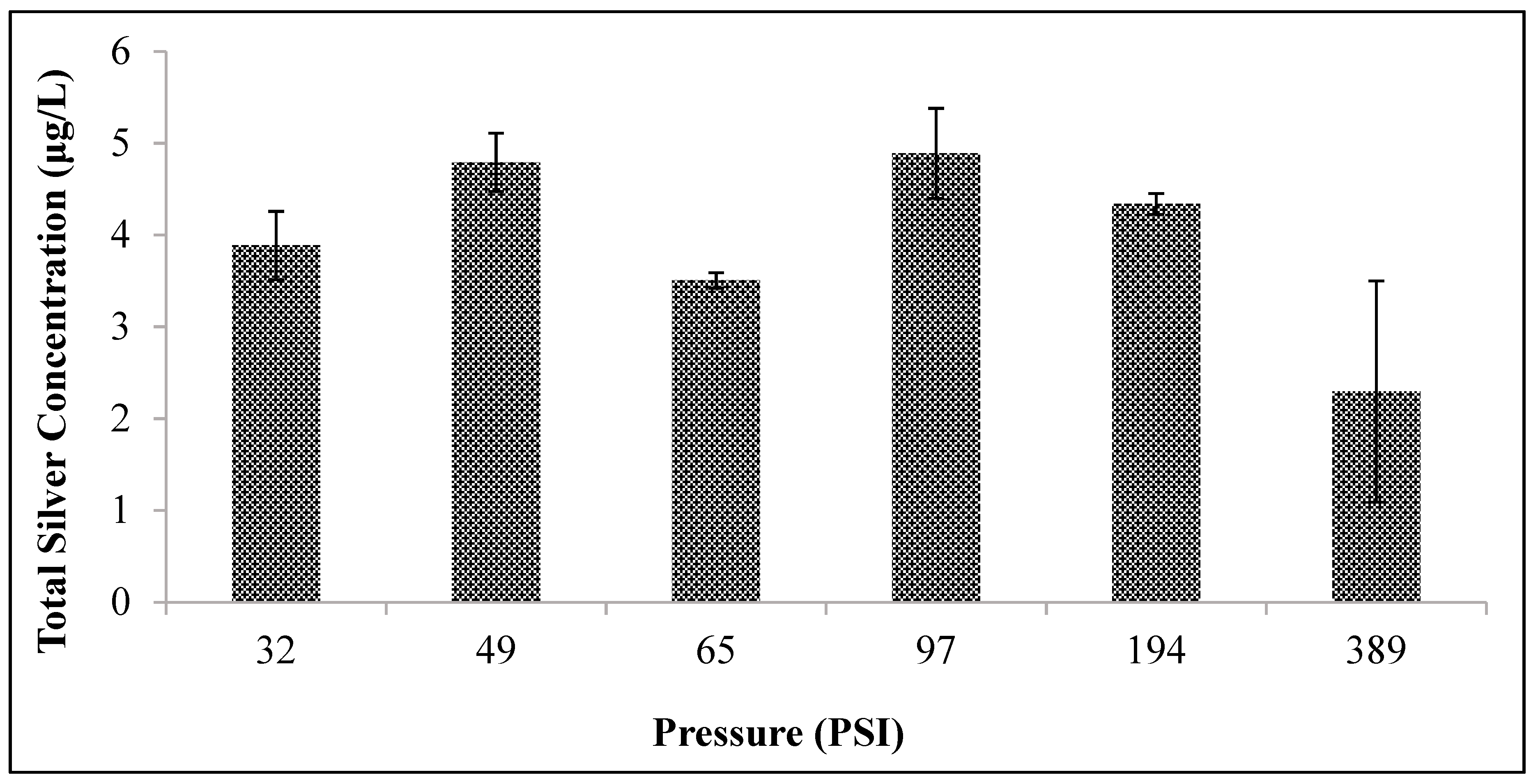
To measure the effects of pressure (compaction) on the efficacy of the silver ceramic tablet, duplicate tablets were pressed individually at different pressures (varying from 250 to 2000 pounds of force) before firing. Ceramic tablets were pressed for one minute with 2000, 1000, 500, 325, 250, and 160 pounds of force to achieve varying pressures of 389, 194, 97, 65, 49, and 32 psi, respectively.
2.1.2. Fabrication Variables—Temperature/Firing Protocol
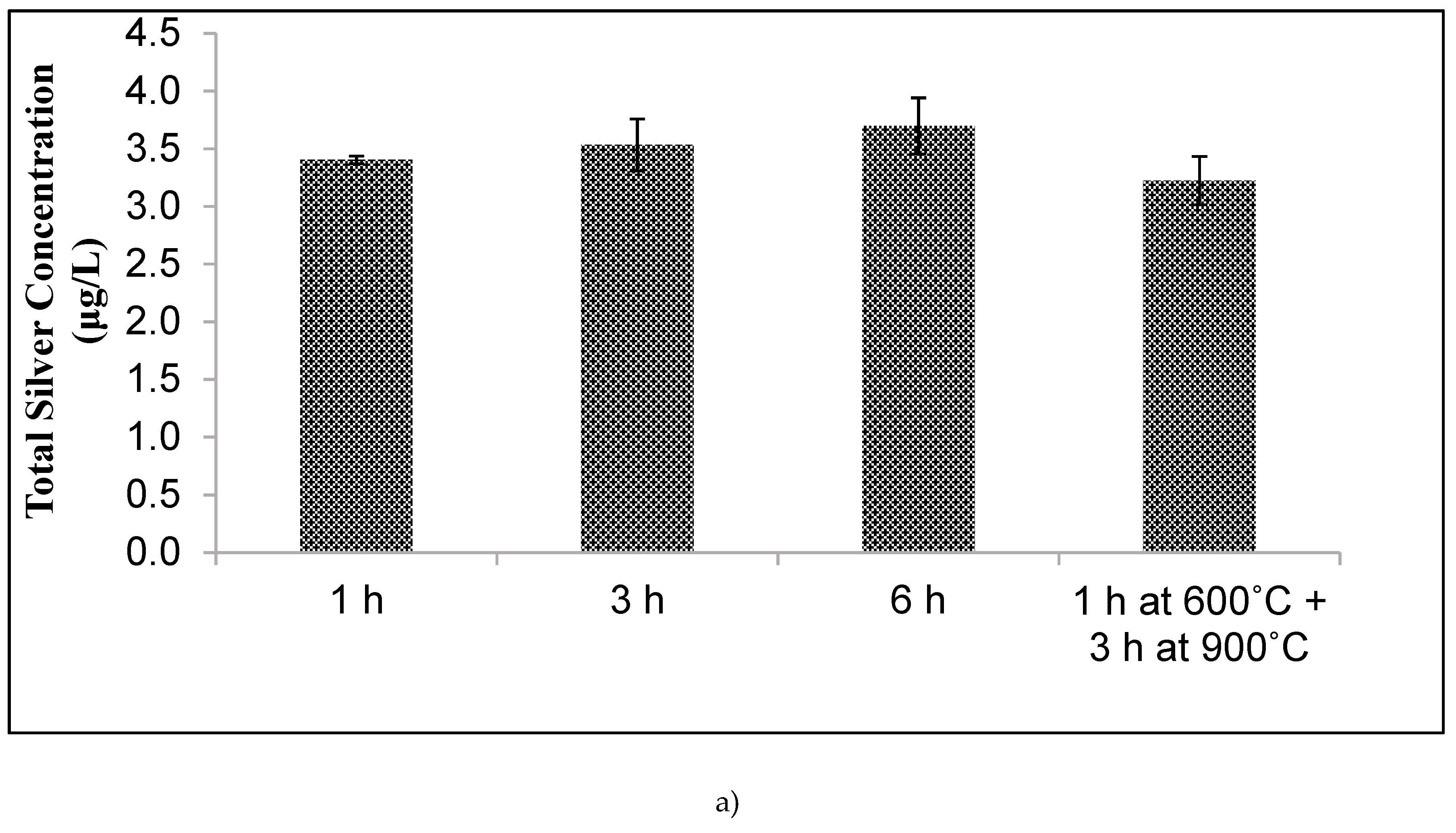
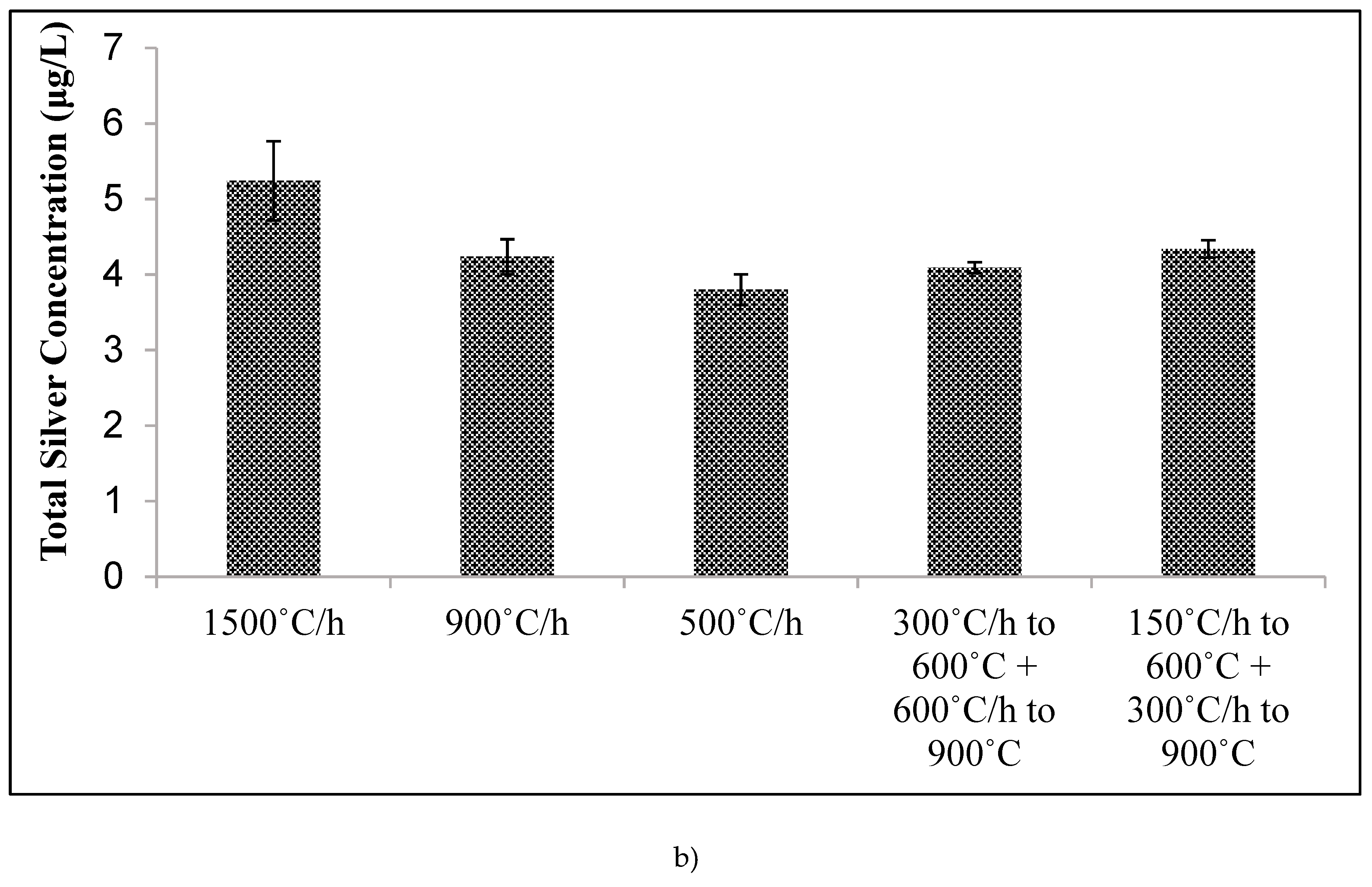
Kiln temperature was increased from 20 °C to 900 °C with different ramp rates and temperature holding times. Temperature was raised to 600 °C at a rate of 150 °C per h and held for 1 h, then raised to 900 °C at a rate of 150 °C per h and held for 3 h. For a second batch, temperature was increased from 20 °C to 600 °C at a rate of 150 °C per h, then immediately increased from 600 °C to 900 °C at 150 °C per hour and held for 6 h.
2.1.3. Fabrication Variables—Sawdust Composition
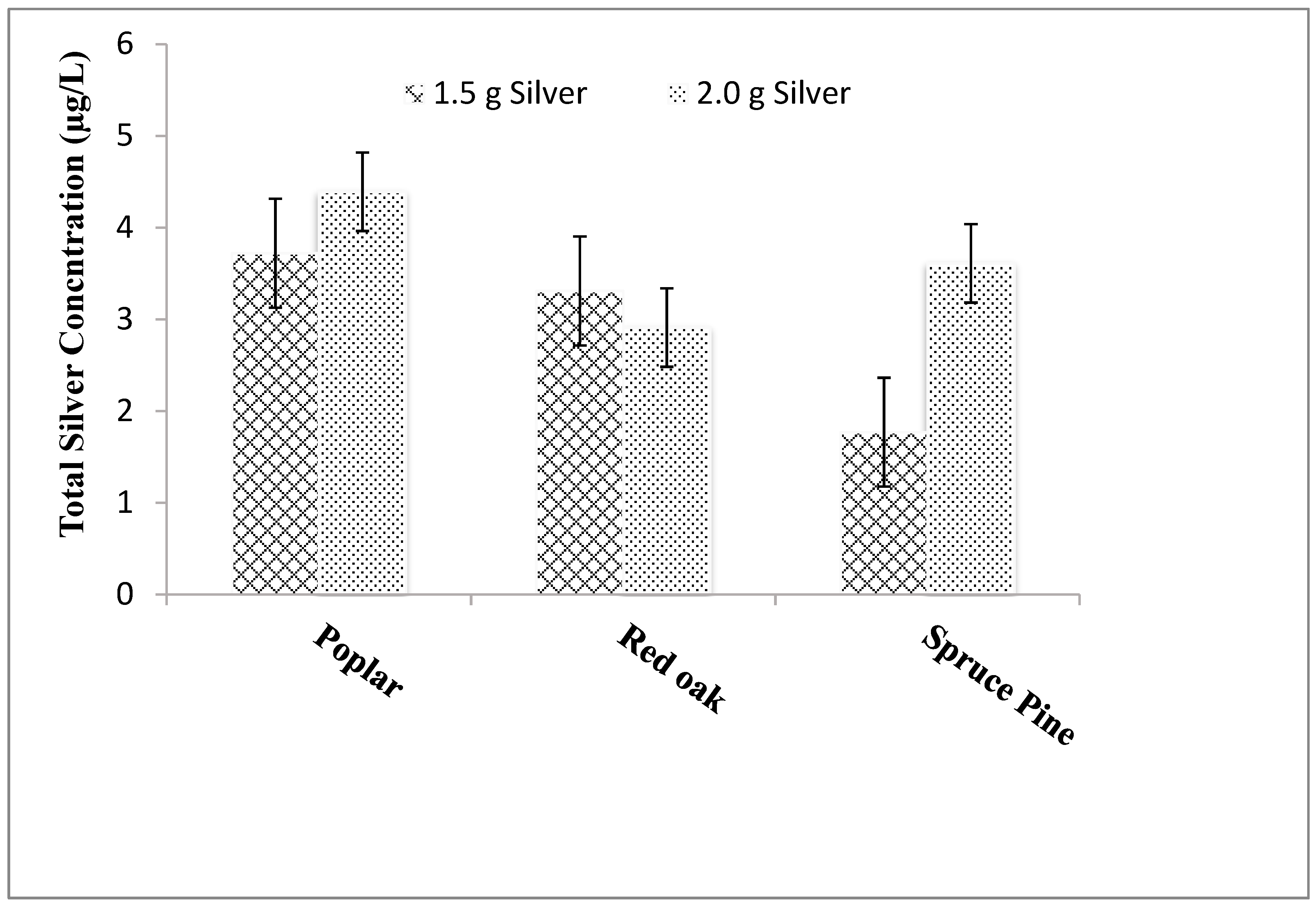
Three different wood types—poplar, red oak, and spruce pine—were used to create the sawdust for the tablets. The sawdust (20 mesh) was used as raw material to prepare tablets imbedded with 1.5 and 2 g of silver. Standard protocol, as described earlier, was used to fabricate ceramic tablets.
2.1.4. Fabrication Variables—Mixing Protocol
The effect of different methods of mixing the raw materials (clay, water, 20 mesh sawdust, and silver nitrate) on silver release in 10 L of water was examined over 24 and 48 h periods. Mixing method-1 involved combining sawdust with clay followed by addition of AgNO3 solution prepared in deionized (DI)-water; mixing method-2 involved mixing sawdust in AgNO3 solution and then adding clay. Mixing method-3 was mixing clay in AgNO3 solution followed by addition of sawdust. All methods were compared in terms of silver release from the ceramic tablets over time.
2.1.5. Fabrication Variables—Granulation
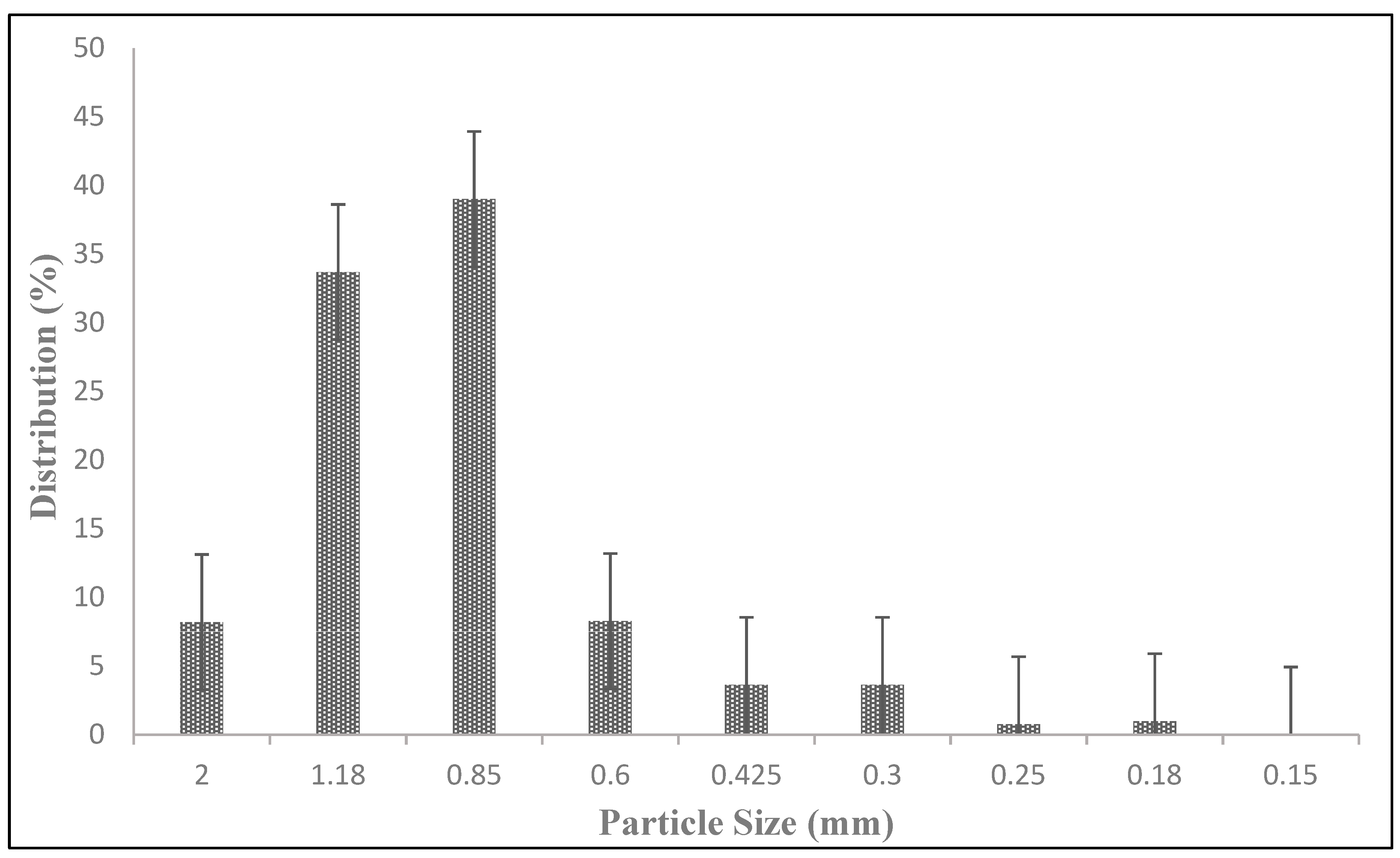
One potential limitation to the rate of silver ion release from the tablets is the kinetics of the tortuous diffusion of silver ions out of the tablet into the bulk solution. The diffusion distance can be reduced by breaking the tablet up into smaller pieces, or in the extreme, grinding it into a fine powder. To test this effect, conventional ceramic tablets were ground into relatively fine particles, and 1 cm and 2 cm ceramic cubes were synthesized.
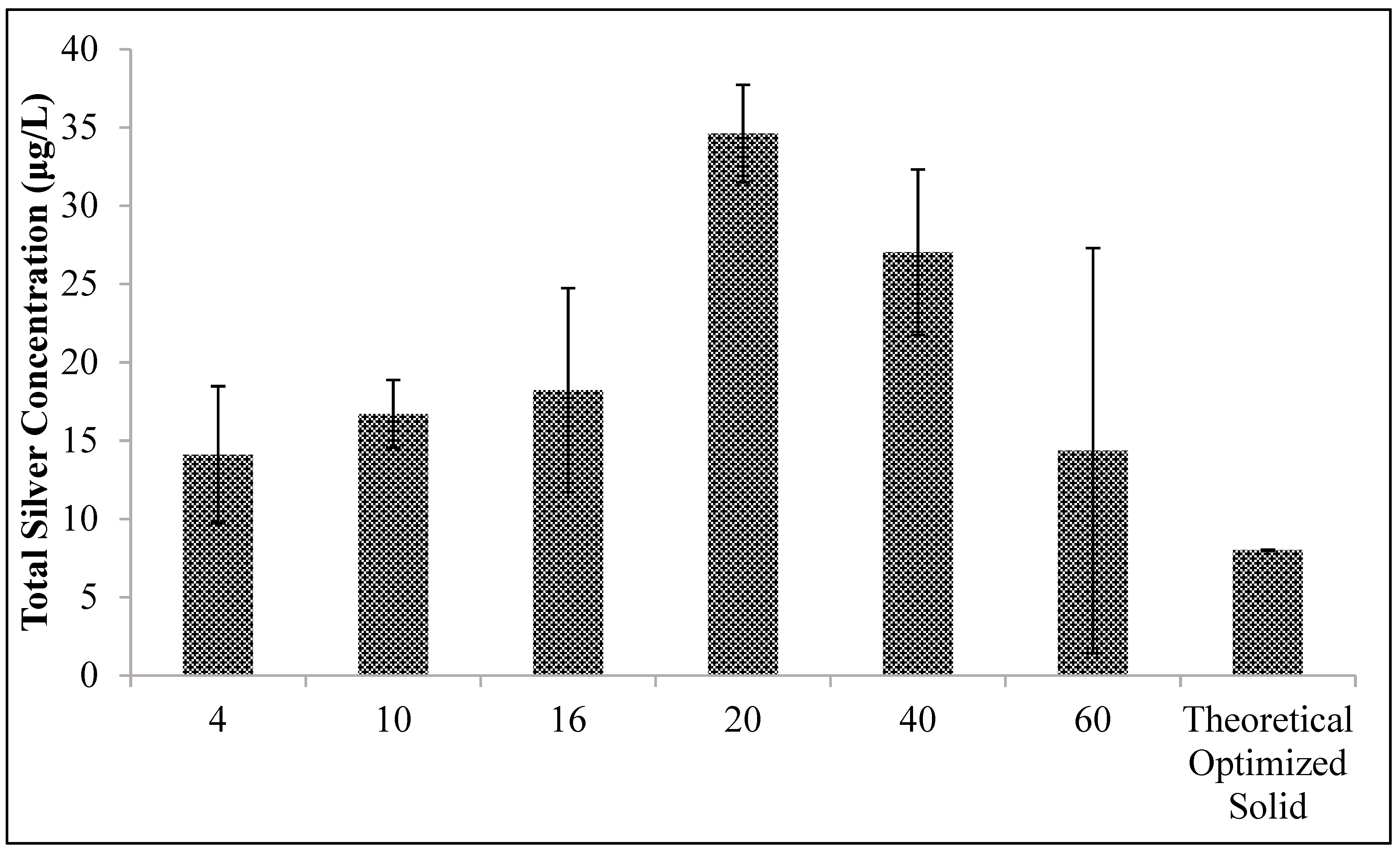
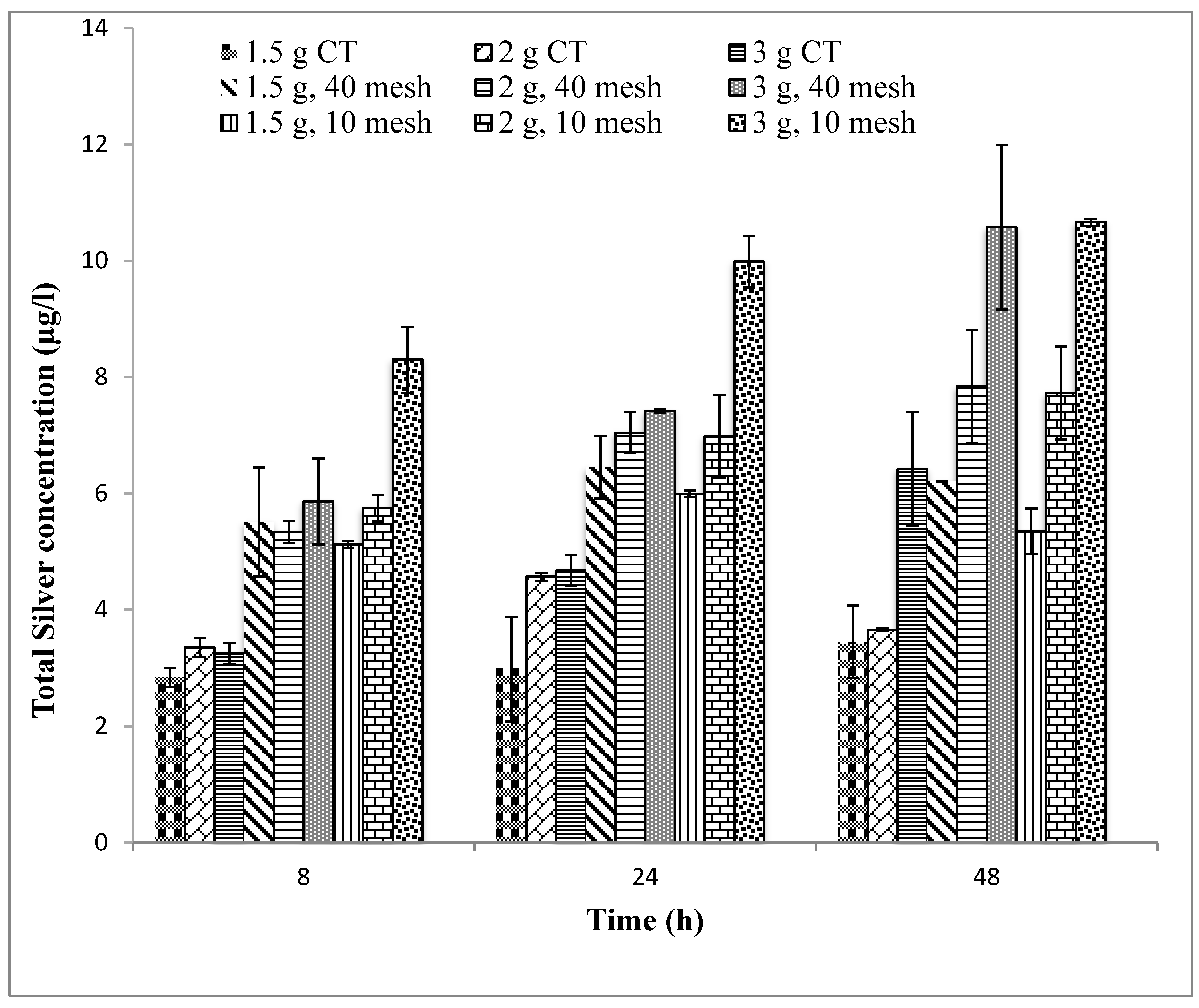
Ceramic tablets were crushed using a jar mill. Crushed ceramic material was sieved to obtain different particle size fractions (4, 10, 16, 20, 40, and 60 mesh). Whole ceramic tablets containing 1.5, 2.0, and 3.0 g of silver in different mesh sizes were studied to compare silver release.
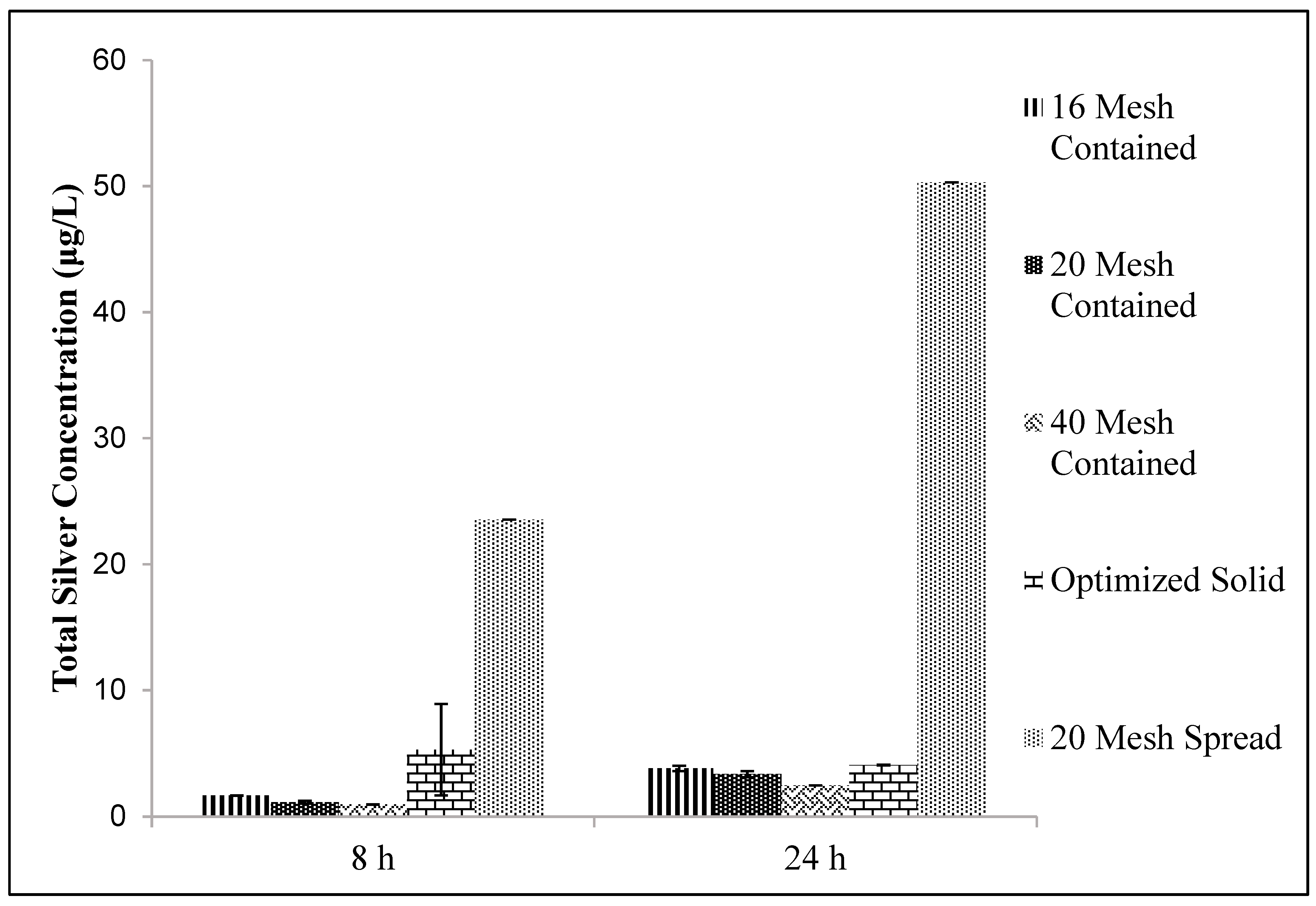
The granulation material was tested for silver release in two forms: Dispersed and contained. Contained particles were put in either a porous fabric bag or a perforated plastic cylindrical container (12 cm diameter by 1 cm thickness). An experiment with 10 g of ceramic in 300 mL of tap water was run to determine the effects of sorption and clumping. The first condition had only 16 mesh particles spread along the bottom of the container. The second and third conditions had 16 mesh particles spread at the bottom of the container accompanied by the porous fabric. The last conditions had different particle sizes contained inside pouches made from the porous fabric.
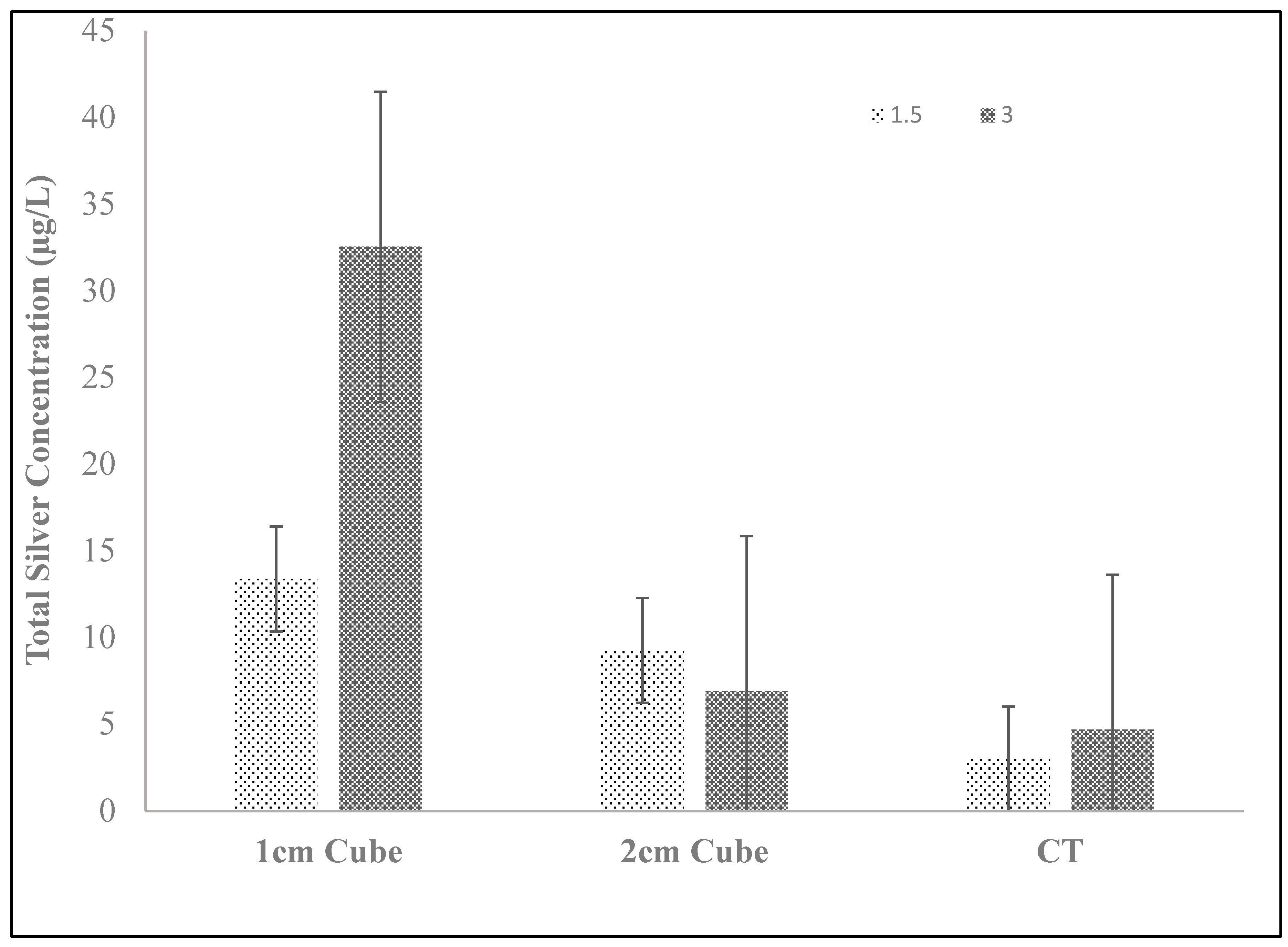
Finally, 1 cm and 2 cm ceramic cubes were manufactured using standard tablet mixing protocol. Raw material was mixed to get a mass of the clay-water-sawdust-silver-nitrate mix equivalent to that used to manufacture 3 ceramic tablets. The material was compressed into a 1 or 2 cm flat sheets using a conventional table-top slab roller (Brent SR-36). Wax paper on both sides was used to roll each batch. This flat-rolled ceramic mixture was then cut into 1 and 2 cm ceramic cubes using a metal cutter. The cubes were air dried for 8 h and fired in a kiln using the standard firing protocol. Cubes equivalent in mass to one ceramic tablet (150 g) were used in each bucket reactor for silver release experiments.
2.2. Characterization Studies
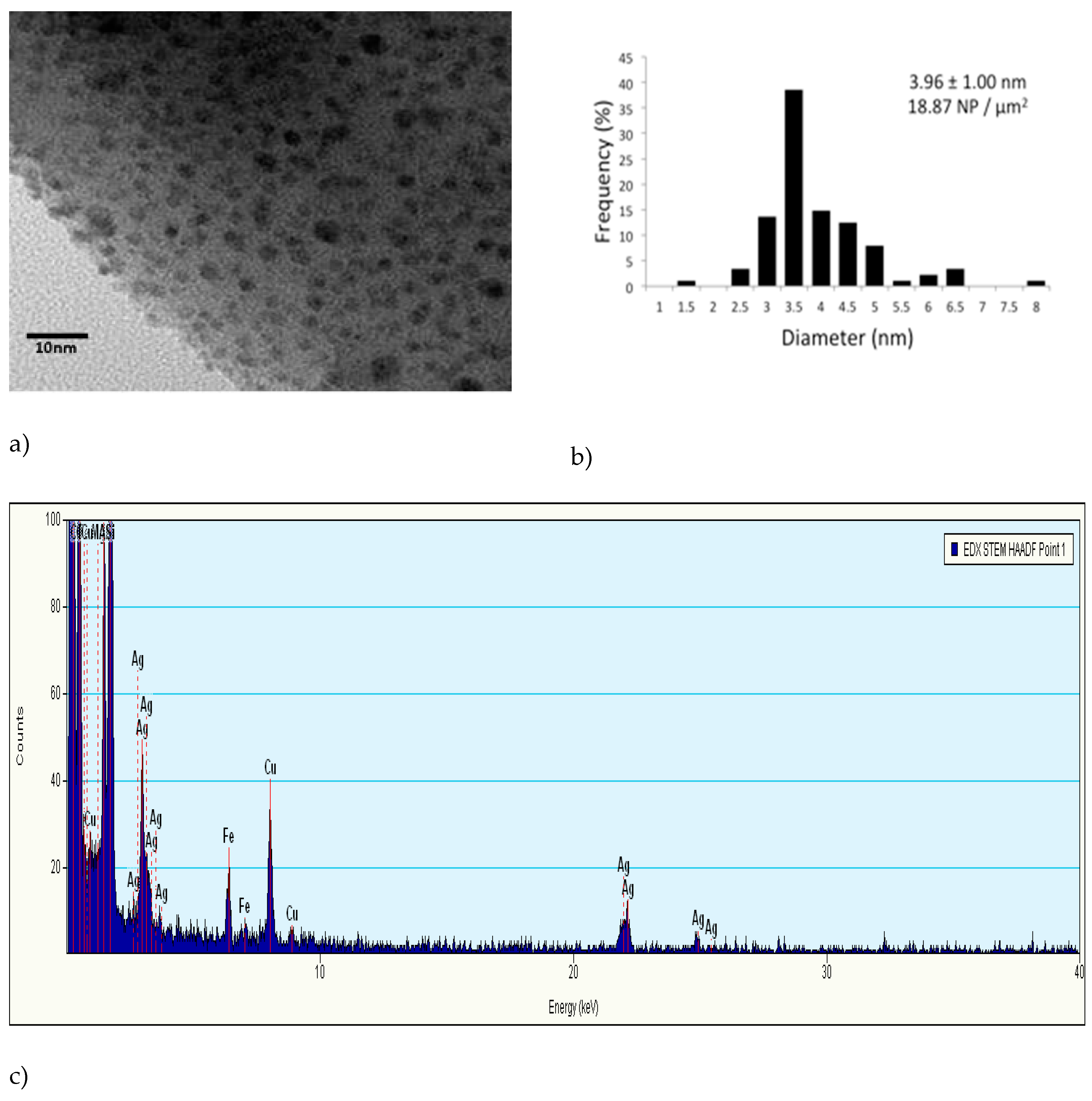
Transmission electron microscopy (TEM) was used to characterize the silver nanopatches in ceramic media. Sample preparation involved lightly scraping the ceramic surface in DI water. A few drops of the suspension were added to a TEM grid (Tedd Pella Item no. 01822 Ultrathin Carbon A) and were left to dry in a laboratory fume hood prior to imaging. High resolution TEM images were obtained on TEM (FEI titan) at 300 Kv. Silver was chemically verified using STEM mode and micrographs were created using a charged coupled device (CCD) digital camera. Size distribution and density of silver nanopatches were quantified using image processing software. This method was identical to the method described by [
28]. Sawdust particle size distribution was performed using various screens ranging from 10 to 100 (US mesh size). A particle size of 20 mesh and smaller were used throughout the study.
2.3. Silver Release Studies
Silver release studies were conducted using 300 mL and 10 L of tap water. Containers were pre-cleaned and filled with a pre-determined volume of water (300 mL and/or 10 L). Before placing the ceramic tablet in water, an initial water sample (~6 mL) was collected. Containers were covered with a top lid, and incubated at room temperature for 24 h. After 24 h, ceramic tablets were carefully removed, and a final sample was taken for analysis of silver. All the samples were taken in duplicates and acidified with 1% HNO3 before analysis. Control experiments were conducted where no ceramic tablets were added. Samples were then analyzed for total silver content using a graphite furnace atomic absorption (GFAA) spectrometer (Perkin-Elmer model AAnalyst 200).
2.4. Disinfection Studies
E. coli was selected and used as a test organism because it is a widely used indicator of fecal contamination of drinking water. A nonpathogenic strain of E. coli was prepared from frozen stock (IDEXX Laboratories) in Luria-Bertani (LB) broth. Fifty µL of thawed (15 min) E. coli stock was added to 50 mL of LB broth and incubated for 12 h at 37 °C. After 12 h incubation and mixing in an orbital shaker (VWR scientific), the culture was centrifuged for 20 min at 1400 rpm using a laboratory centrifuge (Thermo Fisher Scientific model Sorvall Legend XTR). The E. coli pellet from the bottom of the centrifuge tube was re-suspended in 50 mL of 10 mM phosphate buffer (PB) solution (0.4949 g/L K2HPO4 and 0.212 g/L KH2PO4) and stored at 4 °C to preserve the viability of E. coli in solution while preventing growth, prior to any disinfection experiments. Sodium thiosulphate (60 g/L) was prepared by dissolving anhydrous sodium thiosulphate (Fisher Scientific) in deionized water. All solutions and containers used in disinfection studies were autoclaved for sterilization.
Water samples were collected at 8 and 24 h to quantify viable E. coli. To stop further disinfection in the sample, sodium thiosulphate was used. The United State Environmental Protection Agency (US-EPA) approved Colilert test was used to quantify viable E. coli in samples. This test uses Defined Substrate Technology (DST) to detect E. coli. Ortho-Nitrophenyl β-galactocide ONPG and 4-methylumbelliferone glucuronide (MUG) are the major sources of carbon in Colilert and can be metabolized by the coliform enzyme, β-galactosidase, and the E. coli enzyme, β-glucuronidase, respectively. E. coli use β-glucuronidase to metabolize MUG and create fluorescence. Since most non-coliforms do not have these enzymes, they are unable to grow and interfere. Colilert media was added to 100 mL of sample and mixed until dissolved, completely avoiding bubble formation while shaking. The solution was poured in IDEXX Quanti trays, and sealed and incubated at 34 °C for 24 h. Trays were scanned using a fluorescent UV lamp to count fluorescent wells positive for E. coli concentration and counted with a most probable number (MPN) table provided by IDEXX laboratories.
2.5. Analytical
Acidified samples were analyzed for total silver using a graphite furnace atomic absorption (GFAA) spectrometer using a hollow cathode lamp (Perkin Elmer A Analyst 200). Slit width used was 2.7/0.8 at a wavelength of 328.07 nm. Samples were transferred into GFAA sample vials using a micropipette and were introduced into GFAA using an autosampler (AS-900). Samples were analyzed in triplicate, and the average concentration has been reported in the study. Wherever the sample concentration exceeded the calibration range, an auto-dilution was prepared by the autosampler. Percent standard deviation during samples analysis was within ±6%. Check standards (10%) and blanks were used for quality control during analysis. GFAA was calibrated with a silver standard solution with a purity of 99.5% procured from Acros Chemicals.
4. Discussion
The fabrication of a porous ceramic media embedded with silver nanoparticles is an important innovation for point-of-use water treatment [
27]. Ionic silver is a broad-spectrum disinfectant that significantly reduces the concentrations of waterborne pathogens, often by factors of 10
3 or greater [
21,
27,
28,
29,
30]. Optimization of this technology to produce higher concentrations of ionic silver in household water can further increase its effectiveness and potentially lower the contact time required for effective disinfection.
Ionic silver release from the silver ceramic media is likely a function of three potentially rate-limiting processes. First, metallic silver on the ceramic surface must be oxidized to ionic silver. Second, the ionic silver must diffuse through the porous ceramic tablet into the bulk solution. During diffusion, the ionic silver transport may be retarded by sorption onto the negatively charged pore walls of the ceramic. However, this latter effect will likely only retard silver ion transport until a steady-state sorption condition is reached and therefore will not significantly affect long-term performance. It is likely that the first two mechanisms (silver oxidation and the tortuous diffusion of silver ions from the tablet to the bulk solution) will be rate-limiting steps.
In this optimization study, it appears that two approaches have improved the release of silver ions into the bulk aqueous solution. First, increasing the mass of silver nitrate used to fabricate the silver-ceramic media generally increases silver release, regardless of the form factor of the ceramic (e.g., ground, cubes, or tablet) (
Figure 7,
Figure 8,
Figure 10, and
Figure 11). This is consistent with the findings of Ehdaie et al. [
23], who demonstrated that increases in silver mass in a given ceramic tablet increased silver release rates. Furthermore, Edhaie et al. [
23] showed that the density of silver nanopatches on the ceramic surface increased with silver nitrate addition, even while the size distribution of the nanopatches stayed relatively constant. Therefore, using higher masses of silver nitrate to synthesize the silver-ceramic media will increase the density of nanopatches formed. This increases the silver-water interfacial area, which in turn increases the silver oxidation rate. The antimicrobial mechanism of Ag nanoparticles is due to an induction of oxidative stress that results from the formation of reactive oxygen species (ROS), which may cause degradation of the membrane of the bacterial cell; it depends on the amount of silver that can be oxidized to Ag
1+ and released into solution in the presence of dissolved oxygen [
31,
32,
33,
34]. Some other studies reported that the antimicrobial mechanism of MgO could be attributed to the formation of ROS [
35].
The other design change that increased silver release rate was the form factor of the tablet. The highest silver release rates were observed after grinding the ceramic tablet into a 20-mesh particle size (
Figure 5). However, to maximize silver release, these particles had to be spread across the bottom of a water storage container. Gathering the particles together in a porous fiber bag decreased the silver ion release rate (
Figure 6). These observations are directly linked to the diffusion mechanism of silver ion release from the porous substrate. Grinding the silver-ceramic tablet into powder form decreases the diffusion lengths for the release of silver ions into the bulk solution. Collecting the particles together again increases the diffusion length again and largely negates the effect of the grinding process. It has been shown that for an AgNPs suspension, the diffusion rate of Ag
1+ ions into the bulk solution is faster than their dissolution from the AgNPs [
36].
These observations are also consistent with the data from
Figure 11, where the form factor of the silver-ceramic porous media was changed to 1 and 2 cm silver-ceramic cubes. Despite having the same total mass of silver and ceramic as a full-sized tablet, the silver released by the cubes increased relative to the whole tablet. Changing the form factor to the cubes reduced diffusion distances and thereby increased the silver ion diffusion flux from the tablet to the bulk solution. Unlike the grinding process, the cubes are a more practical form factor for a point-of-use water treatment intervention.
Unfortunately, other design changes had little effect on the silver ion release. Changes in tablet compaction pressure, firing protocol, mixing protocol, and sawdust composition resulted in negligible changes. It was hypothesized that one or more of these manufacturing changes would either increase silver oxidation rate (by changing the nanopatch morphology) or reduce the tortuosity. However, changes in silver ion release were not affected. The experiments performed spanned a range of conditions, but were not, of course, exhaustive. Therefore, it is possible that other manufacturing variations not considered in this work might improve silver ion release rates.
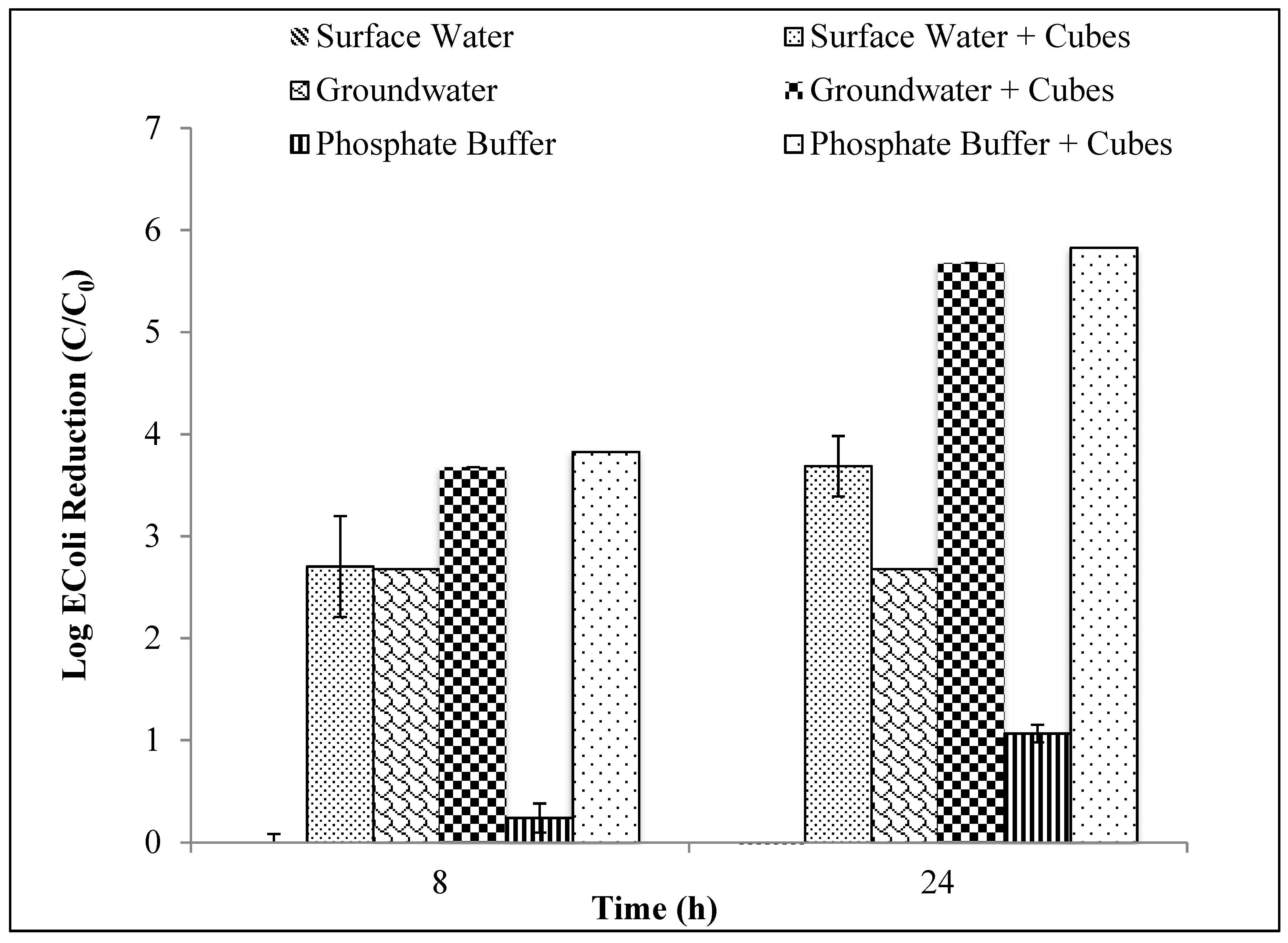
Finally, the best silver-ceramic designs resulted in significant bacterial disinfection. One cm ceramic cubes embedded with 3 g of silver resulted in a 5-log E. coli reduction in a phosphate buffer aqueous solution and a 3.6-log reduction in surface water after 24 h. For an 8-h contact time, a 2.7-log reduction was observed in surface water samples and a 3.8-log reduction was observed in the phosphate buffer solution.
The results from this study suggest that this novel silver impregnated ceramic technology is effective, safe, socially acceptable, and commercially viable. Kahler et al. [
37] reported the economic viability of this product elsewhere. They also conducted a field study in South Africa, and examined a cost model based on a “willingness to pay” study. Currently, there is a commercial product on the market called the MadiDrop+. This product is a silver-ceramic tablet that releases silver ions into household water storage containers. This product has a retail price of
$15 and can treat more than 7000 L of water over a 12-month period (20 L/d). Therefore, there is practical evidence that the silver ceramic technology described here can be commercially viable.
5. Conclusions
Silver-ceramic tablets continue to show promise for point-of-use water treatment. Silver ions are highly effective disinfectants for a broad spectrum of waterborne microbial pathogens. Silver-ceramic tablets can be synthesized by a relatively simple, low-cost manufacturing method, and herein, we presented data to better optimize silver release for household water treatment. Silver-ceramic water-treatment systems should be designed to provide sufficient ionic silver levels for disinfection, but still be below the WHO drinking water guideline of 100 µg/L. The technology described herein delivers a dose of silver which is well below this guideline. Also, research on animal toxicity has been limited and mainly focused on silver nanoparticles. Silver nanoparticles in our silver- impregnated ceramic technology are bonded to the ceramic matrix, which makes them unavailable for any direct human/animal consumption. The low-concentrations of silver used for disinfection do not affect the taste or odor of the natural water, unlike chlorine-based disinfectants, which improves the social acceptability of this technology. Silver has become a common disinfectant for water treatment; it is currently used in a variety of commercial point-of-use water treatment products, including the Folia Water filtration system, the MadiDrop+, SilverDyne, pot-shaped ceramic water filters promoted by nonprofit organizations, such as Potters for Peace, PureMadi, and Wine to Water, candle-style filters sold by Katadyn, and pitcher-style filters sold by Brita and Aquaphor. Finally, the ease of use of silver-ceramic tablets minimizes user behavior modification, which in turn improves the likelihood of consistent and continued use.