Brine Recycling from Industrial Textile Wastewater Treated by Ozone. By-Products Accumulation. Part 1: Multi Recycling Loop
Abstract
1. Introduction
2. Experimental
2.1. Materials
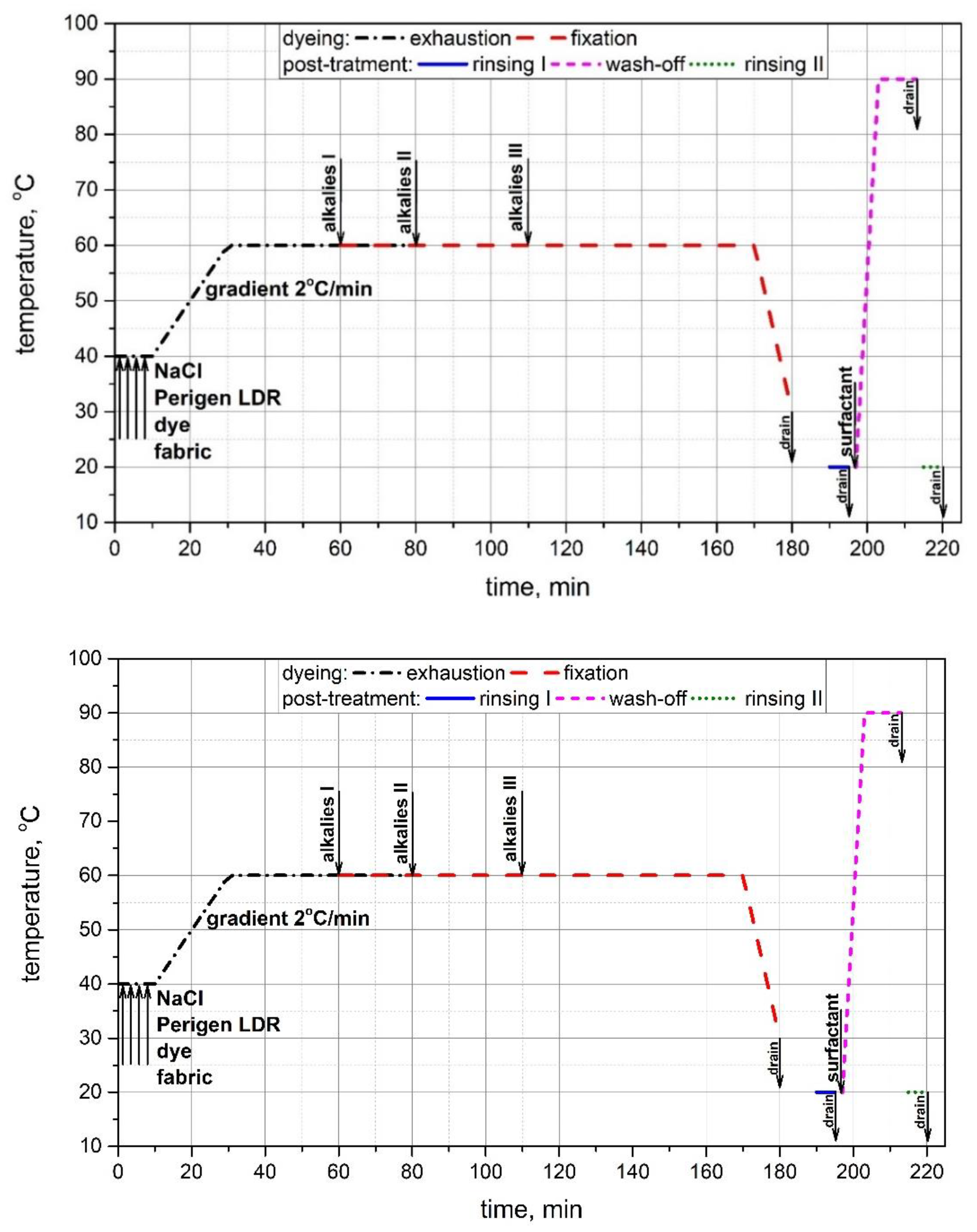
2.2. Experimental Procedure
2.3. Analytical Methods
3. Results and Discussion
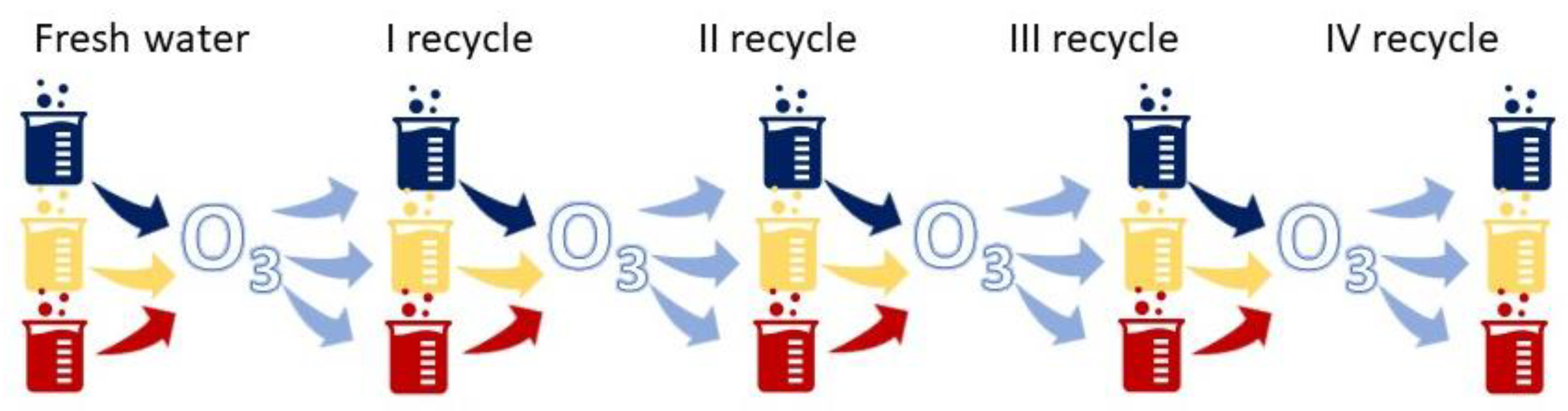
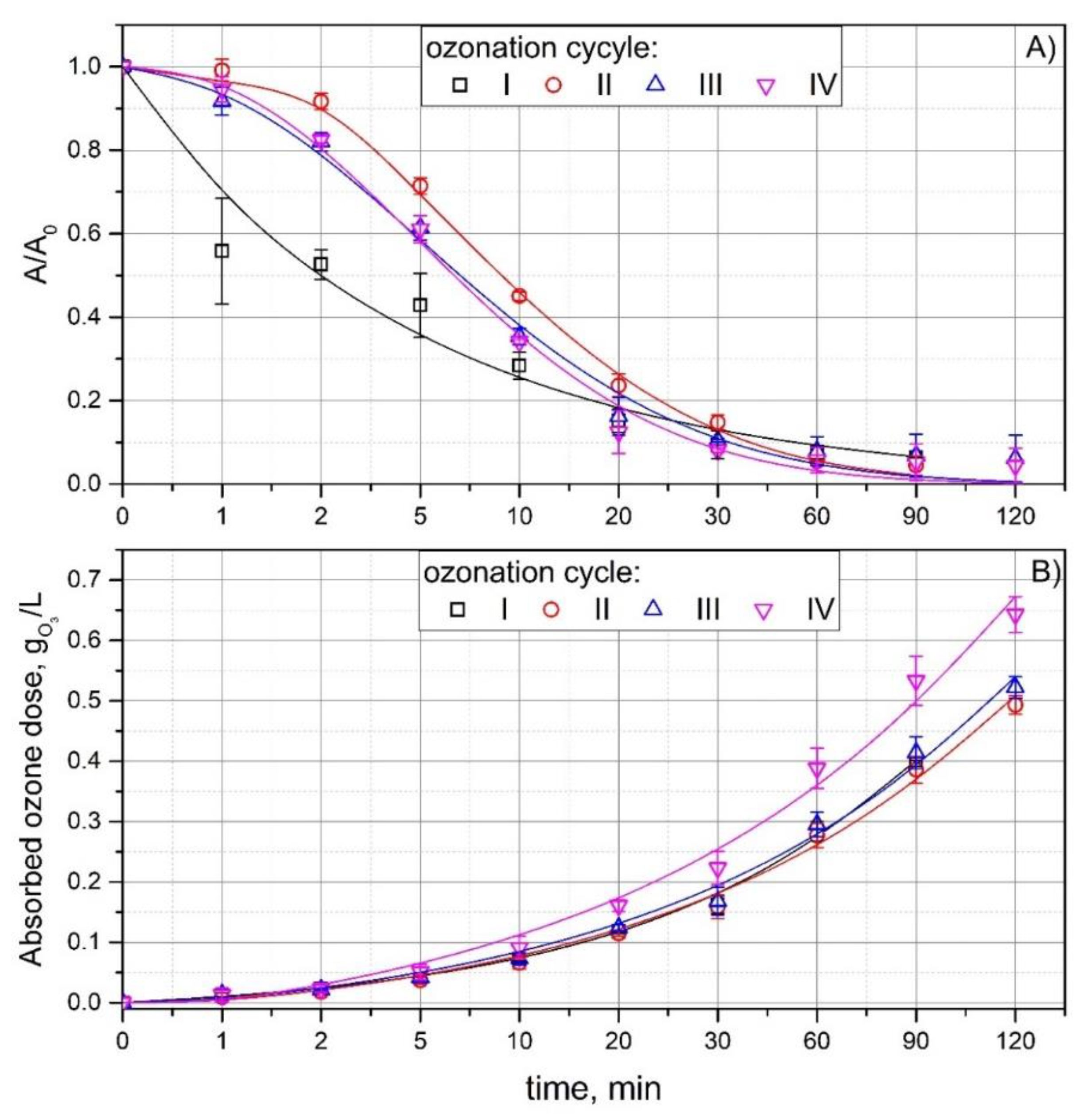
3.1. Ozonation in a Multi Recycling Loop
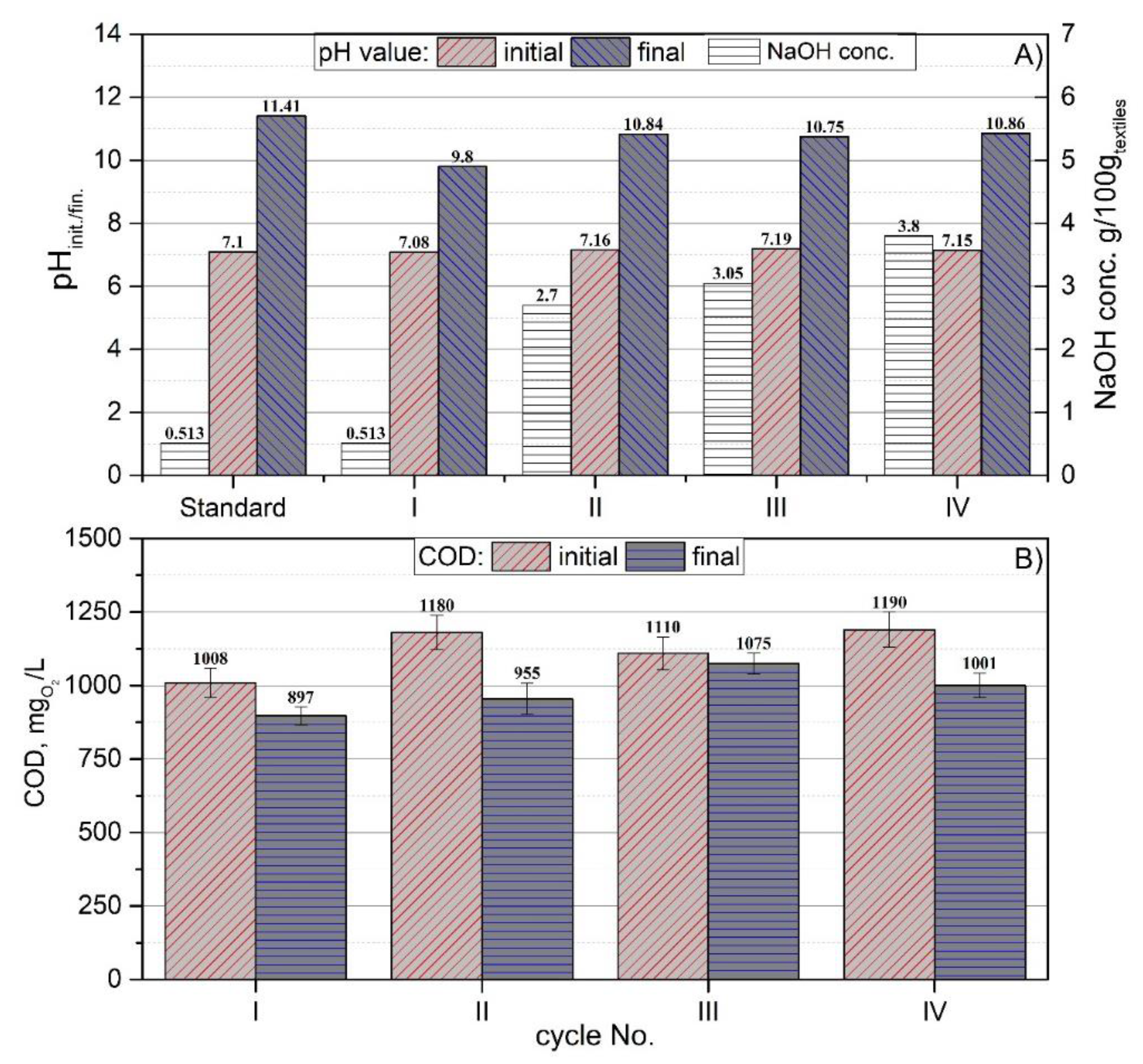
3.2. Preliminary By-Product Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- OECD. OECD Due Diligence Guidance for Responsible Supply Chains in the Garment and Footwear Sector; OECD Publishing: Paris, France, 2018. [Google Scholar] [CrossRef]
- Ghaly, A.; Ananthashankar, R.; Alhattab, M.; Ramakrishnan, V. Production, characterization and treatment of textile effluents: A critical review. J. Chem. Eng. Process Technol. 2013, 5, 1–19. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Kanwar, R.; Singh, J. Physicochemical assessment of industrial textile effluents of Punjab (India). Appl. Water Sci. 2018, 8, 83. [Google Scholar] [CrossRef]
- Allègre, C.; Moulin, P.; Maisseu, M.; Charbit, F. Treatment and reuse of reactive dyeing effluents. J. Memb. Sci. 2006, 269, 15–34. [Google Scholar] [CrossRef]
- Kalliala, E.; Talvenmaa, P. Environmental profile of textile wet processing in Finland. J. Clean. Prod. 2000, 8, 143–154. [Google Scholar] [CrossRef]
- Karcher, S.; Kornmüller, A.; Jekel, M. Anion exchange resins for removal of reactive dyes from textile wastewaters. Water Res. 2002, 36, 4717–4724. [Google Scholar] [CrossRef]
- Bisschops, I.; Spanjers, H. Literature review on textile wastewater characterization. Environ. Technol. 2003, 24, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; Wiley VCH: Weinhein, Germany, 1991. [Google Scholar]
- Gonzalez, O.; Bayarri, B.; Acena, J.; Perez, S.; Barcelo, D. Treatment technologies for wastewater reuse: Fate of contaminants of emerging concern. In Advanced Treatment Technologies for Urban Wastewater Reuse; Fatta-Kassinos, D., Dionysiou, D.D., Kummerer, K., Eds.; Springer: New York, NY, USA, 2016; pp. 7–33. [Google Scholar]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Constapel, M.; Schellenträger, M.; Marzinkowski, J.M.; Gäb, S. Degradation of reactive dyes in wastewater from the textile industry by ozone: Analysis of the products by accurate masses. Water Res. 2009, 43, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, X.; Sun, D.; Ren, Y.; Xu, G. Fate and transformation of naphthylaminesulfonic azo dye Reactive Black 5 during wastewater treatment process. Environ. Sci. Pollut. Res. 2014, 21, 5713–5723. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Poznyak, T.; Chairez, I. Effect of additives on ozone-based decomposition of Reactive Black 5 and Direct Red 28 dyes. Water Environ. Res. 2013, 85, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.; Quaff, A.R.; Pandey, N.D.; Venkatesh, K. Impact of ozonation on decolorization and mineralization of azo dyes: Biodegradability enhancement, by-products formation, required energy and cost. Ozone Sci. Eng. 2015, 37, 420–430. [Google Scholar] [CrossRef]
- Meetani, M.A.; Hisaindee, S.M.; Abdullah, F.; Ashraf, S.S.; Rauf, M.A. Liquid chromatography tandem mass spectrometry analysis of photodegradation of a diazo compound: A mechanistic study. Chemosphere 2010, 80, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, Y.; Nie, J.; Ma, L.; Huang, Y.; Li, L.; Liu, Y.; Guo, Z. Pilot-scale study on catalytic ozonation of bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Colindres, P.; Yee-Madeira, H.; Reguera, E. Removal of Reactive Black 5 from aqueous solution by ozone for water reuse in textile dyeing processes. Desalination 2010, 258, 154–158. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Muthukumar, M. Studies on the possibility of recycling reactive dye bath effluent after decolouration using ozone. Dye. Pigment. 2007, 72, 251–255. [Google Scholar] [CrossRef]
- Hu, E.; Shang, S.; Tao, X.; Jiang, S.; Chiu, K. Regeneration and reuse of highly polluting textile dyeing effluents through catalytic ozonation with carbon aerogel catalysts. J. Clean. Prod. 2016, 137, 1055–1065. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Żyłła, R.; Paździor, K.; Wrębiak, J.; Sójka-Ledakowicz, J. Integration of ozonation and biological treatment of industrial wastewater from dyehouse. Ozone Sci. Eng. 2017, 39, 357–365. [Google Scholar] [CrossRef]
- Khare, U.K.; Bose, P.; Vankar, P.S. Impact of ozonation on subsequent treatment of azo dye solutions. J. Chem. Technol. Biotechnol. 2007, 82, 1012–1022. [Google Scholar] [CrossRef]
- Koch, M.; Yediler, A.; Lienert, D.; Insel, G.; Kettrup, A. Ozonation of hydrolyzed azo dye reactive yellow 84 (CI). Chemosphere 2002, 46, 109–113. [Google Scholar] [CrossRef]
- Wang, C.; Yediler, A.; Lienert, D.; Wang, Z.; Kettrup, A. Ozonation of an azo dye C.I. Remazol Black 5 and toxicological assessment of its oxidation products. Chemosphere 2003, 52, 1225–1232. [Google Scholar] [CrossRef]
- Ulson, S.M.A.G.; Bonilla, K.A.S.; De Souza, A.A.U. Removal of COD and color from hydrolyzed textile azo dye by combined ozonation and biological treatment. J. Hazard. Mater. 2010, 179, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, M.T.F.; Glasser, D.; Hildebrandt, D. Wastewater treatment of reactive dyestuffs by ozonation in a semi-batch reactor. Chem. Eng. J. 2011, 166, 662–668. [Google Scholar] [CrossRef]
- Chung, J.; Kim, J.-O. Application of advanced oxidation processes to remove refractory compounds from dye wastewater. Desalin. Water Treat. 2012, 25, 233–240. [Google Scholar] [CrossRef]
- Arslan, I.; Akmehmet Balcioglu, I.; Tuhkanen, T. Advanced oxidation of synthetic dyehouse effluent by O3, H2O2/O3 and H2O2/UV processes. Environ. Technol. 1999, 20, 921–931. [Google Scholar] [CrossRef]
- Alaton, I.A.; Balcioglu, I.A.; Bahnemann, D.W. Advanced oxidation of a reactive dyebath effluent: Comparison of O3, H2O2/UV-C and TiO2/UV-A processes. Water Res. 2002, 36, 1143–1154. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Solecka, M.; Zylla, R. Biodegradation, decolourisation and detoxification of textile wastewater enhanced by advanced oxidation processes. J. Biotechnol. 2001, 89, 175–184. [Google Scholar] [CrossRef]
- Sarayu, K.; Swaminathan, K.; Sandhya, S. Assessment of degradation of eight commercial reactive azo dyes individually and in mixture in aqueous solution by ozonation. Dye. Pigment. 2007, 75, 362–368. [Google Scholar] [CrossRef]
- Bamperng, S.; Suwannachart, T.; Atchariyawut, S.; Jiraratananon, R. Ozonation of dye wastewater by membrane contactor using PVDF and PTFE membranes. Sep. Purif. Technol. 2010, 72, 186–193. [Google Scholar] [CrossRef]
- Gül, Ş.; Özcan, Ö.; Erbatur, O. Ozonation of C.I. Reactive Red 194 and C.I. Reactive Yellow 145 in aqueous solution in the presence of granular activated carbon. Dye. Pigment. 2007, 75, 426–431. [Google Scholar] [CrossRef]
- Zhang, F.; Yediler, A.; Liang, X.; Kettrup, A. Effects of dye additives on the ozonation process and oxidation by-products: A comparative study using hydrolyzed C.I. Reactive Red 120. Dye. Pigment. 2004, 60, 1–7. [Google Scholar] [CrossRef]
- Oguz, E.; Keskinler, B.; Çelik, C.; Çelik, Z. Determination of the optimum conditions in the removal of Bomaplex Red CR-L dye from the textile wastewater using O3, H2O2, HCO3− and PAC processes for the removal of Bomaplex Red CR-L dye from aqueous solution. J. Hazard. Mater. 2006, 131, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Turhan, K.; Durukan, I.; Ozturkcan, S.A.; Turgut, Z. Decolorization of textile basic dye in aqueous solution by ozone. Dye. Pigment. 2012, 92, 897–901. [Google Scholar] [CrossRef]
- Konsowa, A.H.; Ossman, M.E.; Chen, Y.; Crittenden, J.C. Decolorization of industrial wastewater by ozonation followed by adsorption on activated carbon. J. Hazard. Mater. 2010, 176, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Gül, Ş.; Özcan-Yildirim, Ö. Degradation of Reactive Red 194 and Reactive Yellow 145 azo dyes by O3 and H2O2/UV-C processes. Chem. Eng. J. 2009, 155, 684–690. [Google Scholar] [CrossRef]
- Hsing, H.J.; Chiang, P.C.; Chang, E.E.; Chen, M.Y. The decolorization and mineralization of Acid Orange 6 azo dye in aqueous solution by advanced oxidation processes: A comparative study. J. Hazard. Mater. 2007, 141, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Arslan, I.; Balcioglu, I.A. Advanced oxidation of raw and biotreated textile industry wastewater with O3, H2O2/UV-C and their sequential application. J. Chem. Technol. Biotechnol. 2001, 60, 53–60. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Bessegato, G.G.; Zanoni, M.V.B. Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res. 2016, 98, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, I.A.; Ahmed, F.; Sahito, A.R.; Pathan, A.A. In-situ decolorization of residual dye effluent in textile jet dyeing machine by ozone. Pak. J. Anal. Envirion. Chem. 2014, 15, 72–76. [Google Scholar]
- Qi, L.; Wang, X.; Xu, Q. Coupling of biological methods with membrane filtration using ozone as pre-treatment for water reuse. Desalination 2011, 270, 264–268. [Google Scholar] [CrossRef]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dulov, A.; Dulova, N.; Trapido, M. Combined physicochemical treatment of textile and mixed industrial wastewater. Ozone Sci. Eng. 2011, 33, 285–293. [Google Scholar] [CrossRef]
- Somensi, C.A.; Simionatto, E.L.; Bertoli, S.L.; Wisniewski, A.; Radetski, C.M. Use of ozone in a pilot-scale plant for textile wastewater pre-treatment: Physico-chemical efficiency, degradation by-products identification and environmental toxicity of treated wastewater. J. Hazard. Mater. 2010, 175, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Baban, A.; Yediler, A.; Lienert, D.; Kemerdere, N.; Kettrup, A. Ozonation of high strength segregated effluents from a woollen textile dyeing and finishing plant. Dye. Pigment. 2003, 58, 93–98. [Google Scholar] [CrossRef]
- Ciardelli, G.; Ranieri, N. The treatment and reuse of wastewater in the textile industry by means of ozonation and electroflocculation. Water Res. 2001, 35, 567–572. [Google Scholar] [CrossRef]
- Perkowski, J.; Kos, L.; Zyłła, R.; Ledakowicz, S. A kinetic model of decoloration of water solution of anthraquinone dye initiated by generality hydroksyl radicals. Fibres Text. East. Eur. 2005, 13, 59–64. [Google Scholar]
- Al jibouri, A.K.H.; Wu, J.; Upreti, S.R. Continuous ozonation of methylene blue in water. J. Water Process Eng. 2015, 8, 142–150. [Google Scholar] [CrossRef]
- López-López, A.; Pic, J.S.; Debellefontaine, H. Ozonation of azo dye in a semi-batch reactor: A determination of the molecular and radical contributions. Chemosphere 2007, 66, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Kusvuran, E.; Gulnaz, O.; Samil, A.; Erbil, M. Detection of double bond-ozone stoichiometry by an iodimetric method during ozonation processes. J. Hazard. Mater. 2010, 175, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, F.; Yang, Y.; Tan, M.; Zhao, D. Ozonation of Cationic Red X-GRL in aqueous solution: Kinetics and modeling. J. Hazard. Mater. 2011, 187, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, T. Ozonation of aqueous azo dye in a semi-batch reactor. Water Res. 2001, 35, 1093–1099. [Google Scholar] [CrossRef]
- Gomes, A.C.; Fernandes, L.R.; Simões, R.M.S. Oxidation rates of two textile dyes by ozone: Effect of pH and competitive kinetics. Chem. Eng. J. 2012, 189–190, 175–181. [Google Scholar] [CrossRef]
- Gomes, A.C.; Nunes, J.C.; Simões, R.M.S. Determination of fast ozone oxidation rate for textile dyes by using a continuous quench-flow system. J. Hazard. Mater. 2010, 178, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tizaoui, C.; Grima, N. Kinetics of the ozone oxidation of Reactive Orange 16 azo-dye in aqueous solution. Chem. Eng. J. 2011, 173, 463–473. [Google Scholar] [CrossRef]
- Torregrosa, J.I.; Navarro-Laboulais, J.; Lopez, F.; Cardona, S.C.; Abad, A.; Capablanca, L. Study of the ozonation of a dye using kinetic information reconstruction. Ozone Sci. Eng. 2008, 30, 344–355. [Google Scholar] [CrossRef]
- Choi, I.S.; Wiesmann, U. Effect of chemical reaction and mass transfer on ozonation of the Azo Dyes Reactive Black 5 and Reactive Orange 96. Ozone Sci. Eng. 2004, 26, 539–549. [Google Scholar] [CrossRef]
- Panda, K.K.; Mathews, A.P. Ozone oxidation kinetics of Reactive Blue 19 anthraquinone dye in a tubular in situ ozone generator and reactor: Modeling and sensitivity analyses. Chem. Eng. J. 2014, 255, 553–567. [Google Scholar] [CrossRef]
- Chen, T.Y.; Kao, C.M.; Hong, A.; Lin, C.E.; Liang, S.H. Application of ozone on the decolorization of reactive dyes—Orange-13 and Blue-19. Desalination 2009, 249, 1238–1242. [Google Scholar] [CrossRef]
- Patil, N.N.; Shukla, S.R. Decolorization of Reactive Blue 171 dye using ozonation and UV/H2O2 and elucidation of the degradation mechanism. Environ. Prog. Sustain. Energy 2015, 1–10. [Google Scholar] [CrossRef]
- Chu, W.; Ma, C.W. Quantitative prediction of direct and indirect dye ozonation kinetics. Water Res. 2000, 34, 3153–3160. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Gonera, M. Optimisation of oxidants dose for combined chemical and biological treatment of textile wastewater. Water Res. 1999, 33, 2511–2516. [Google Scholar] [CrossRef]
- Paździor, K.; Wrębiak, J.; Klepacz-Smółka, A.; Gmurek, M.; Bilińska, L.; Kos, L.; Sójka-Ledakowicz, J.; Ledakowicz, S. Influence of ozonation and biodegradation on toxicity of industrial textile wastewater. J. Environ. Manag. 2017, 195, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Eremektar, G.; Selcuk, H.; Meric, S. Investigation of the relation between COD fractions and the toxicity in a textile finishing industry wastewater: Effect of preozonation. Desalination 2007, 211, 314–320. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 105-J03:2009—Textiles—Tests for Colour Fastness—Part J03: Calculation of Colour Differences, 2009. Available online: https://www.iso.org/standard/51385.html (accessed on 16 December 2018).
- International Organization for Standardization (ISO). ISO 105-C06:2010—Textiles—Tests for Colour Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering, n.d. Available online: https://www.iso.org/standard/51276.html (accessed on 16 December 2018).
- International Organization for Standardization (ISO). ISO 105-E04:2013—Textiles—Tests for Colour Fastness—Part E04: Colour Fastness to Perspiration, n.d. Available online: https://www.iso.org/standard/57973.html (accessed on 20 December 2018).
- International Organization for Standardization (ISO). ISO 105-X12:2016—Textiles—Tests for Colour Fastness—Part X12: Colour Fastness to Rubbing, n.d. Available online: https://www.iso.org/standard/65207.html (accessed on 20 December 2018).
- Beltran, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; Lewis Publishers: Boca Raton, FL, USA, 2004. [Google Scholar]
- Mezzanotte, V.; Fornaroli, R.; Canobbio, S.; Zoia, L.; Orlandi, M. Colour removal and carbonyl by production in high dose ozonation for effluent polishing. Chemosphere 2013, 91, 629–634. [Google Scholar] [CrossRef] [PubMed]
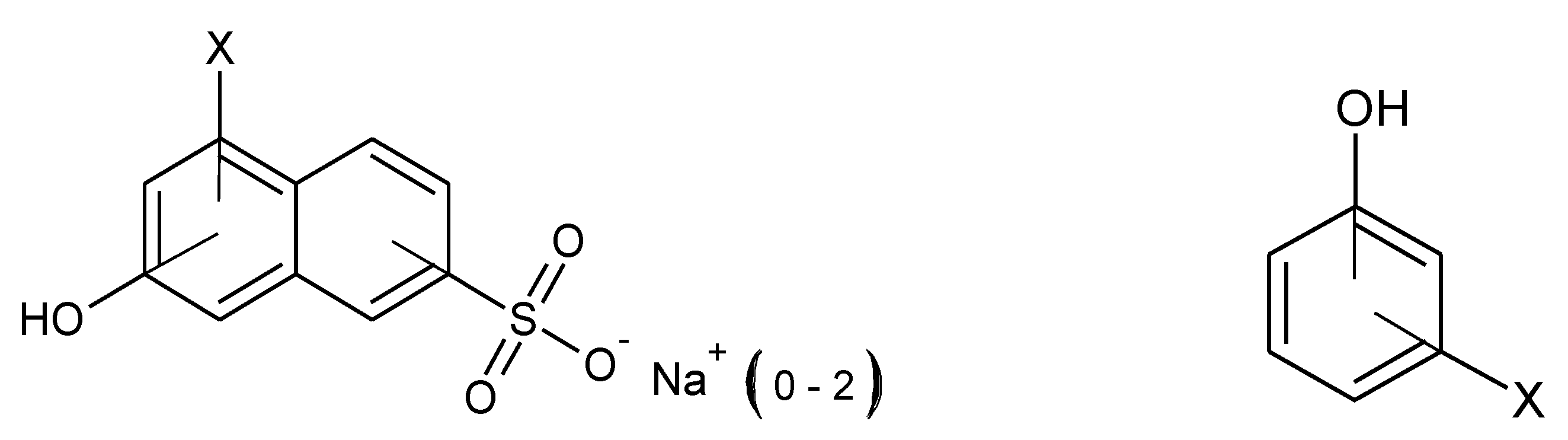
| Research Goal | Textile Wastewater Type | References |
|---|---|---|
| Color, COD, TOC or BOD removal | Simulated | [17,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] |
| Industrial | [19,39,40,41,42,43,44,45,46,47] | |
| Kinetic study | Simulated | [25,26,27,30,31,33,35,37,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] |
| Products, degradation mechanism | Simulated | [11,12,13,14,15] |
| Salt or alkaline influence | Simulated | [13,17,31,34] |
| Cost evaluation | Simulated | [27,28] |
| Industrial | [40,43,44] | |
| Recycling | Simulated | [17] |
| Industrial | [18,19,20] | |
| Toxicity | Simulated | [23,24,27,29,61,63] |
| Industrial | [44,45,46,64,65] | |
| Scale upgradation | Industrial | [16] |
| Re-Dying Type of the Dye | Cycle No. | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Synozol Yellow KHL | 0.50 | 1.71 | 1.46 | 2.22 |
| Synozol Red K3BS150% | 0.50 | 1.02 | 0.64 | 1.41 |
| Setazol Black DPT | 1.16 | 1.92 | 1.73 | 1.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilińska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S. Brine Recycling from Industrial Textile Wastewater Treated by Ozone. By-Products Accumulation. Part 1: Multi Recycling Loop. Water 2019, 11, 460. https://doi.org/10.3390/w11030460
Bilińska L, Blus K, Gmurek M, Ledakowicz S. Brine Recycling from Industrial Textile Wastewater Treated by Ozone. By-Products Accumulation. Part 1: Multi Recycling Loop. Water. 2019; 11(3):460. https://doi.org/10.3390/w11030460
Chicago/Turabian StyleBilińska, Lucyna, Kazimierz Blus, Marta Gmurek, and Stanisław Ledakowicz. 2019. "Brine Recycling from Industrial Textile Wastewater Treated by Ozone. By-Products Accumulation. Part 1: Multi Recycling Loop" Water 11, no. 3: 460. https://doi.org/10.3390/w11030460
APA StyleBilińska, L., Blus, K., Gmurek, M., & Ledakowicz, S. (2019). Brine Recycling from Industrial Textile Wastewater Treated by Ozone. By-Products Accumulation. Part 1: Multi Recycling Loop. Water, 11(3), 460. https://doi.org/10.3390/w11030460








