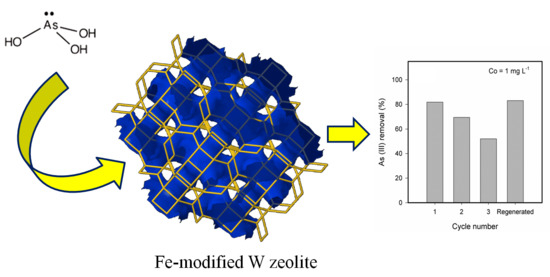
Adsorption of Aqueous As (III) in Presence of Coexisting Ions by a Green Fe-Modified W Zeolite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Modification of the W Zeolite Surface
2.3. Batch Adsorption Experiments
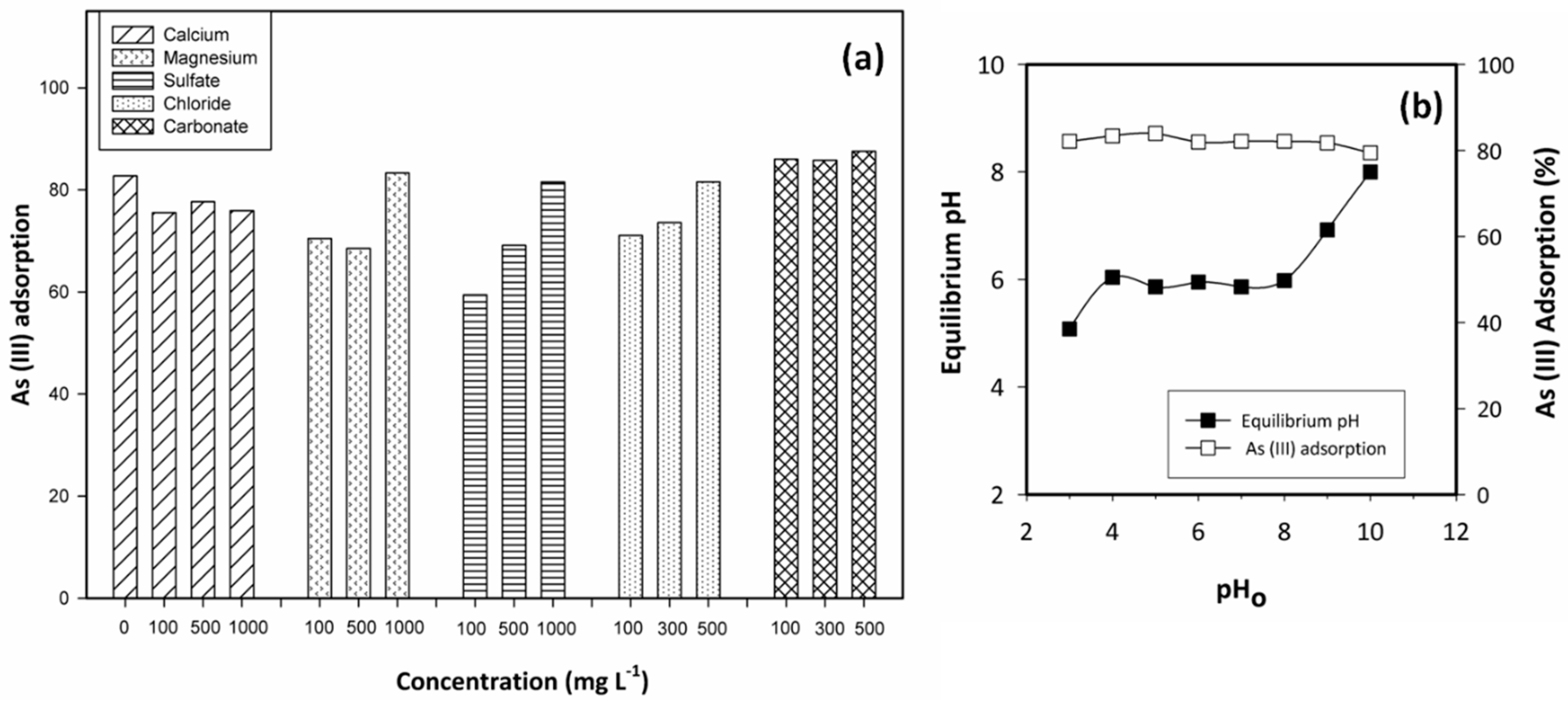
2.3.1. Competing Ions
2.3.2. Effect of Temperature, Fe-Modified W Zeolite Dose and As (III) Concentration
2.4. Ageing, Desorption and Regeneration of Fe-Modified W Zeolite
2.5. Green Metric for W Zeolite Synthesis
2.6. Characterization Techniques
3. Results and Discussion
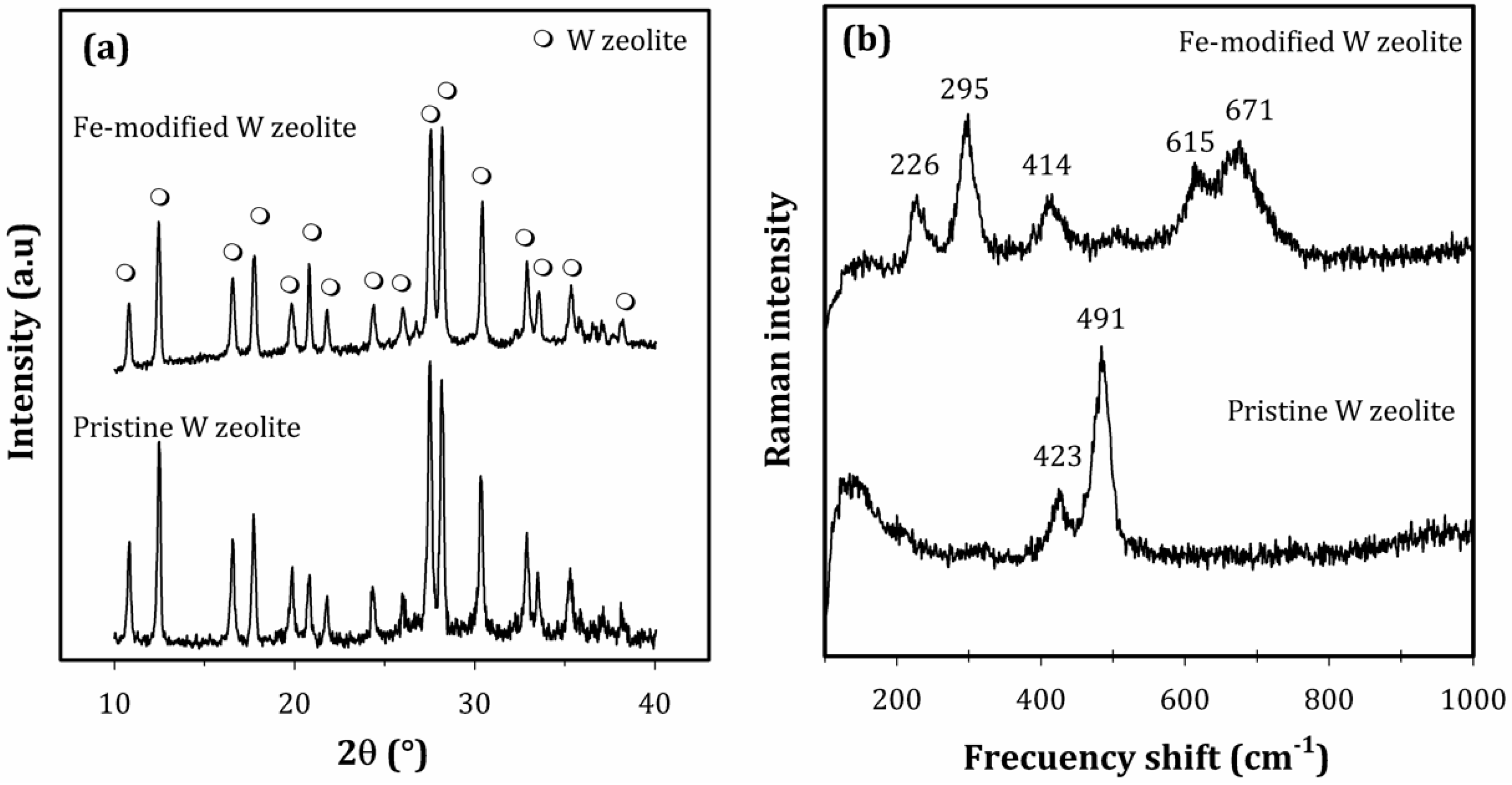
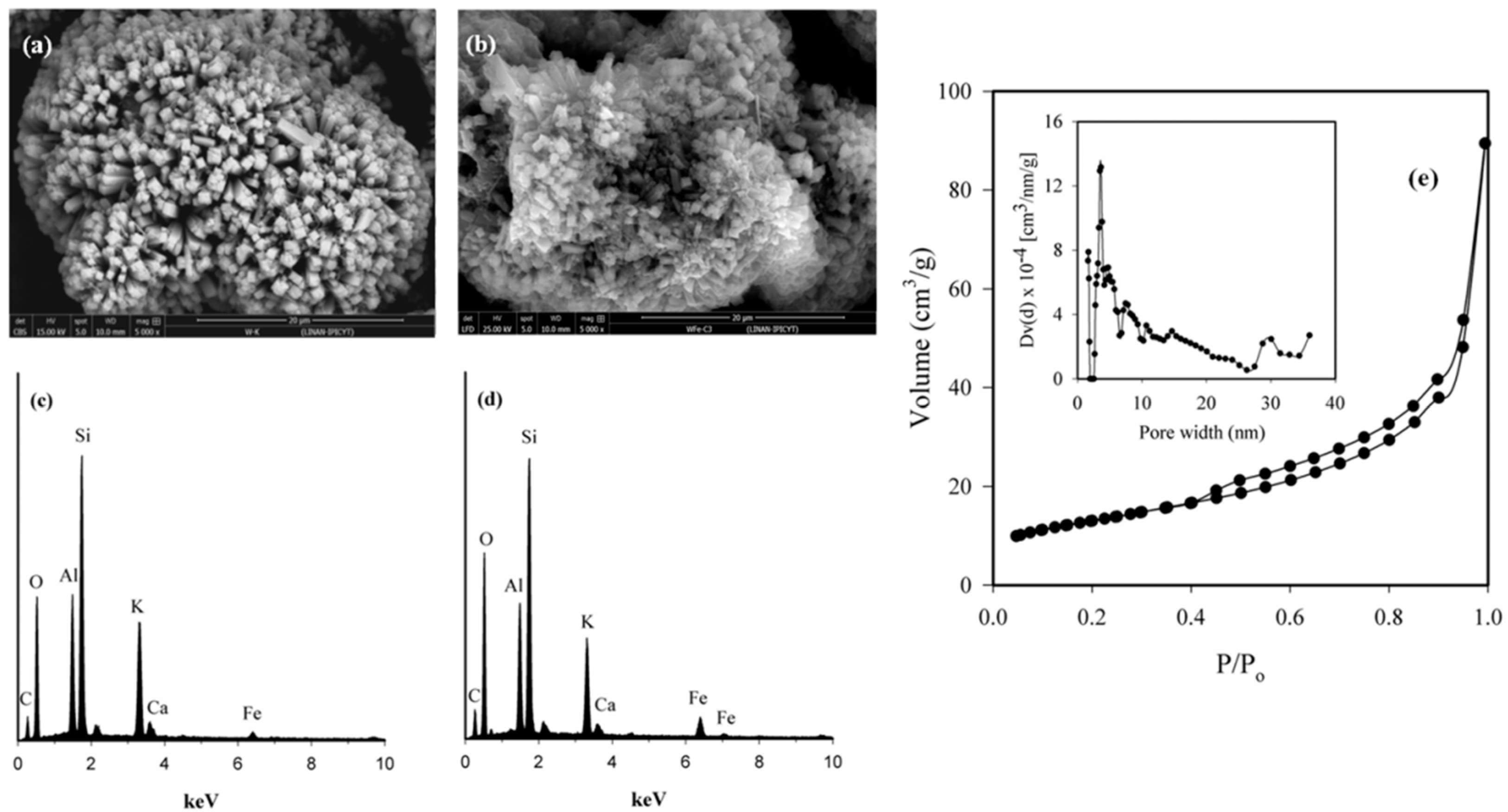
3.1. Fe-Modified W Zeolite Characterization
3.2. Adsorbent Efficiency as Function of the pH and the Presence of Coexisting Ions
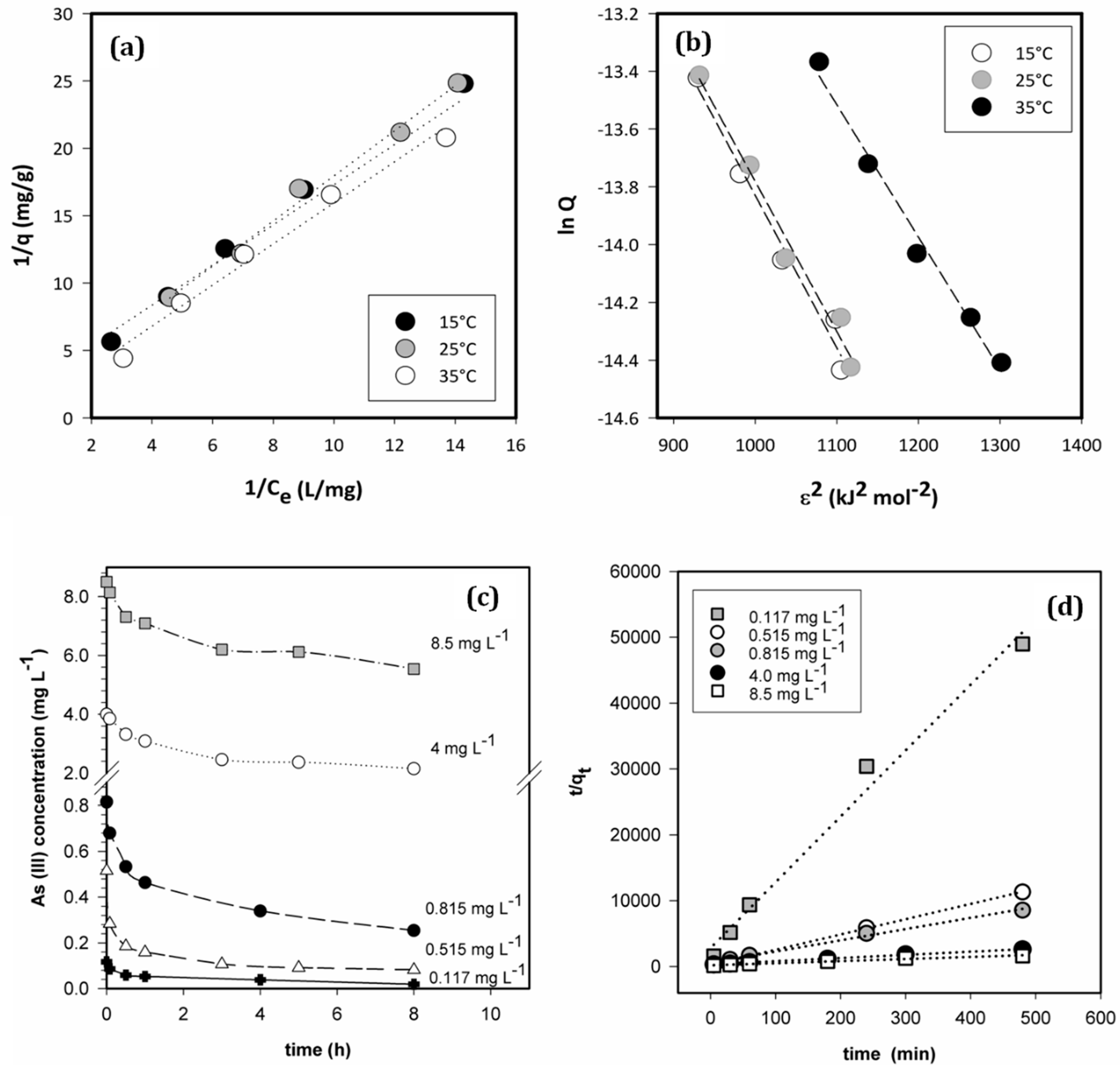
3.3. Adsorption Isotherms and Thermodynamic Parameters
3.4. Effect of As (III) Concentration
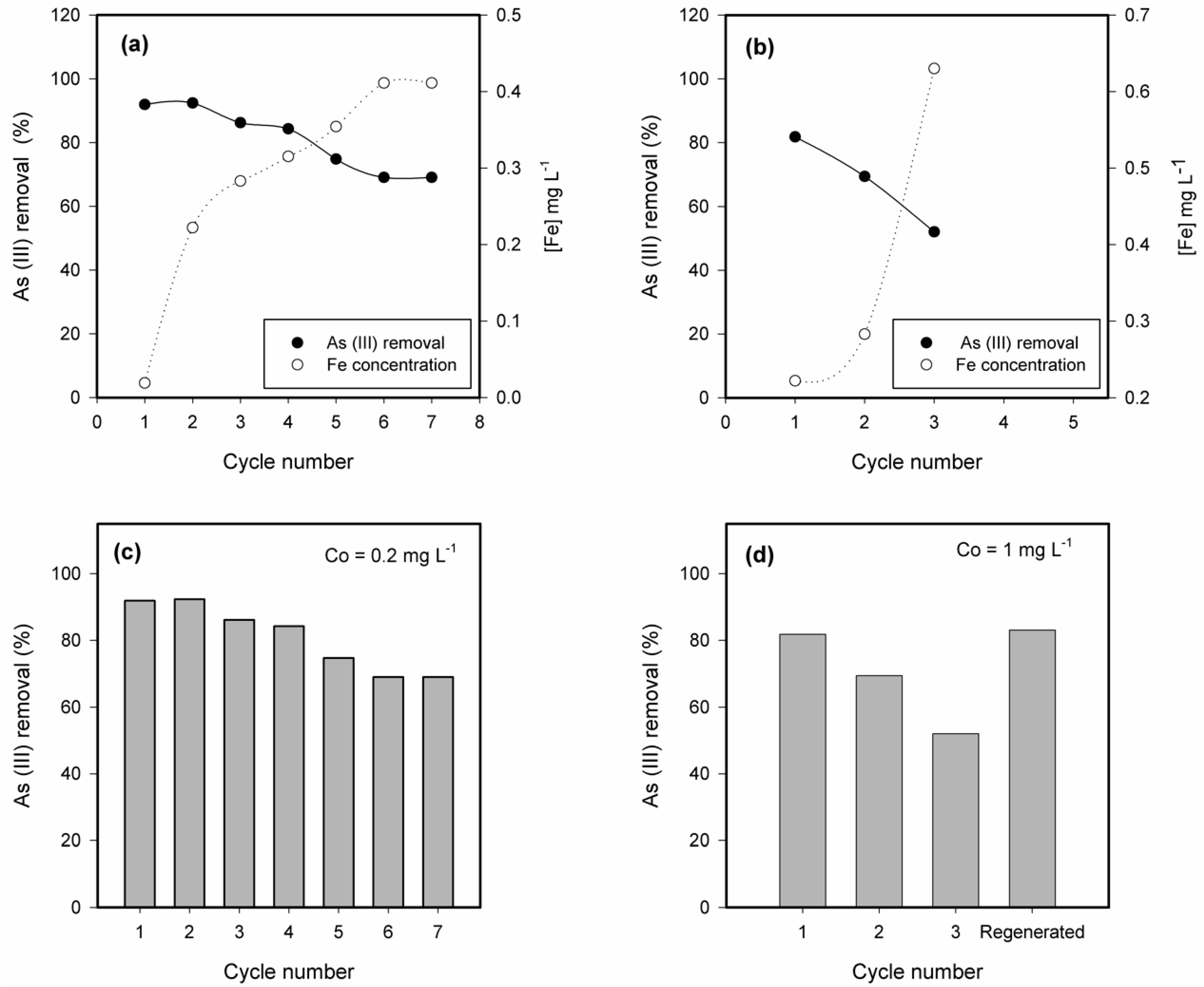
3.5. Ageing, Desorption and Regeneration of the Fe-Modified W Zeolite
3.6. Eco-Scale Applied to W Zeolite Synthesis from Fly Ash
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Wu, H.; Kuijp, T.J. The health effects of exposure to arsenic-contaminated drinking water: A review by global geographical distribution. Int. J. Environ. Health Res. 2015, 25, 432–452. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Tamás, M.J. Molecular mechanisms of metalloid transport, toxicity and tolerance. Front. Cell Dev. Biol. 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Laine, J.E.; Ilievski, V.; Richardson, D.B.; Herring, A.H.; Styblo, M.; Rubio-Andrade, M.; García-Vargas, G.; Gamble, M.V.; Fry, R.C. Maternal one carbon metabolism and arsenic methylation in a pregnancy cohort in Mexico. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 505–514. [Google Scholar] [CrossRef] [PubMed]
- González-Horta, C.; Ballinas-Casarrubias, L.; Sánchez-Ramírez, B.; Ishida, M.C.; Barrera-Hernández, A.; Gutiérrez-Torres, D.; Zacarias, O.L.; Saunders, R.S.; Drobná, Z.; Mendez, M.A.; et al. A Concurrent exposure to arsenic and fluoride from drinking water in Chihuahua, Mexico. Int. J. Environ. Res. Public Health 2015, 12, 4587–4601. [Google Scholar] [CrossRef]
- Nelson-Mora, J.; Escobar, M.L.; Rodriguez-Duran, L.; Massieu, L.; Montiel, T.; Rodríguez, V.M.; Hernandez-Mercado, K.; Gonsebatt, M.E. Gestational exposure to inorganic arsenic (iAs3+) alters glutamate disposition in the mouse hippocampus and ionotropic glutamate receptor expression leading to memory impairment. Arch. Toxicol. 2018, 92, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Del Razo, L.M.; García-Vargas, G.G.; Valenzuela, O.L.; Hernández Castellanos, E.; Sánchez-Peña, L.C.; Currier, J.M.; Drobnaa, Z.; Loomis, D.; Stýblo, M. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: A cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ. Health 2013, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Monrad, M.; ErsbØll, A.K.; SØrense, M.; Baastrup, R.; Hansen, B.; Gammelmark, A.; Tjonneland, A.; Overvad, K.; Raaschou-Nielsen, O. Low-level arsenic in drinking water and risk of incident myocardial infarction: A cohort study. Environ. Res. 2017, 154, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Susko, M.L.; Bloom, M.S.; Neamtiu, I.A.; Appleton, A.A.; Surdu, S.; Pop, C.; Fitzgerald, E.F.; Anastasiu, D.; Gurzau, E.S. Low-level arsenic exposure via drinking water consumption and female fecundity—A preliminary investigation. Environ. Res. 2017, 154, 120–125. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Huang, N.C.; Chang, Y.T.; Sung, J.M.; Shen, K.H.; Tsai, C.C.; Guo, H.R. Associations between arsenic in drinking water and the progression of chronic kidney disease: A nationwide study in Taiwan. J. Hazard. Mater. 2017, 321, 432–439. [Google Scholar] [CrossRef]
- Hussain, A.; Raveendran, V.A.; Kundu, S.; Samanta, T.; Shunmugam, R.; Pal, D.; Sarma, J.D. Mechanisms of Arsenic-Induced Toxicity with Special Emphasis on Arsenic-Binding Proteins. In Arsenic-Analytical and Toxicological Studies, 1st ed.; Stoytcheva, M., Zlatev, R., Eds.; IntechOpen: London, UK, 2018; Chapter 5; pp. 57–80. ISBN 978-1-78923-517-3. [Google Scholar]
- Aguilar-Muñiz, A.U.; Valdes-Perezgasga, F.; Garcia-Vargas, G.G. Seasonal effects in arsenic levels in drinking water in the Lagunera region. J. Phys. Conf. Ser. 2013, 421, 012017. [Google Scholar] [CrossRef]
- Ortega-Guerrero, A. Evaporative concentration of arsenic in groundwater: Health and environmental implications, La Laguna Region, Mexico. Environ. Geochem. Health 2017, 39, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Gutierrez, M.; Alarcon-Herrera, M.T.; Villalba, M.L.; Deng, S. Occurrence and treatment of arsenic in groundwater and soil in northern Mexico and southwestern USA. Chemosphere 2011, 83, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Huerta, E.A.; de la Garza Varela, A.; Gomez-Bernal, J.M.; Castillo, F.; Avalos-Borja, M.; SenGupta, B.; Martínez-Villegas, N. Arsenic contamination in irrigation water, agricultural soil and maize crop from an abandoned smelter site in Matehuala, Mexico. J. Hazard. Mater. 2017, 339, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Modificacion a la Norma Oficial Mexicana NOM-127-SSA1-1994. Available online: https://www.gob.mx/cms/uploads/attachment/file/110520/MODIFICACION_A_LA_NORMA_NOM_127_SSA1_1994_22_NOVIEMBRE_2000.pdf (accessed on 3 September 2018).
- García-Rico, L.; Meza-Figueroa, D.; Gandolfi, A.J.; Ibañez del Rivero, C.; Martínez-Cinco, M.A.; Meza-Montenegro, M.M. Health Risk Assessment and Urinary Excretion of Children Exposed to Arsenic through Drinking Water and Soils in Sonora, Mexico. Biol. Trace Elem. Res. 2018, 187, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Arnold, L.L.; Beck, B.D.; Lewis, A.S.; Eldan, M. Evaluation of the carcinogenicity of inorganic arsenic. Crit. Rev. Toxicol. 2013, 43, 711–752. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Balomajumder, C.; Mohanty, B. A laboratory study for the treatment of arsenic, iron, and manganese bearing ground water using Fe3+ impregnated activated carbon: Effects of shaking time, pH and temperature. J. Hazard. Mater. 2007, 144, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, M.J.; SenGupta, A.K.; Greenleaf, J.E. Arsenic removal using a polymeric/inorganic hybrid sorbent. Water Res. 2003, 37, 164–176. [Google Scholar] [CrossRef]
- Jaya, J.A.; Blute, N.K.; Hemond, H.F.; Durant, J.L. Arsenic-sulfides confound anion exchange resin speciation of aqueous arsenic. Water Res. 2004, 38, 1155–1158. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Laing, G.D. Technologies for Arsenic Removal fromWater: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef]
- Mendoza, R.M.O.; Kan, C.C.; Chuang, S.S.; Pingul-Ong, S.M.B.; Dalida, M.L.P.; Wan, M.W. Feasibility studies on arsenic removal from aqueous solutions by electrodialysis. J. Environ. Sci. Health A 2014, 49, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Neppolian, B.; Celik, E.; Choi, H. Photochemical Oxidation of Arsenic(III) to Arsenic(V) using Peroxydisulfate Ions as an Oxidizing Agent. Environ. Sci. Technol. 2008, 42, 6179–6184. [Google Scholar] [CrossRef] [PubMed]
- Kasiuliene, A.; Carabante, I.; Bhattacharya, P.; Caporale, A.G.; Adamo, P.; Kumpiene, J. Removal of metal(oid)s from contaminated water using iron-coated peat sorbent. Chemosphere 2018, 198, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Gallios, G.P.; Tolkou, A.K.; Katsoyiannis, I.O.; Stefusova, K.; Vaclavikova, M.; Deliyanni, E.A. Adsorption of Arsenate by Nano Scaled Activated Carbon Modified by Iron and Manganese Oxides. Sustainability 2017, 9, 1684. [Google Scholar] [CrossRef]
- Mahmood, T.; Aslam, M.; Naeem, A.; Siddique, T.; Din, S.U. Adsorption of As(III) from aqueous solution onto iron impregnated used tea activated carbon: Equilibrium, kinetic and thermodynamic study. J. Chil. Chem. Soc. 2018, 63, 3855–3866. [Google Scholar] [CrossRef]
- Montero, J.I.Z.; Monteiro, A.S.C.; Gontijo, E.S.J.; Bueno, C.C.; de Moraes, M.A.; Rosa, A.H. High efficiency removal of As(III) from waters using a new and friendly adsorbent based on sugarcane bagasse and corncob husk Fe-coated biochars. Ecotoxicol. Environ. Saf. 2018, 162, 616–624. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Tzollas, N.M.; Tolkou, A.K.; Mitrakas, M.; Ernst, M.; Zouboulis, A.I. Use of Novel Composite Coagulants for Arsenic Removal from Waters—Experimental Insight for the Application of Polyferric Sulfate (PFS). Sustainability 2017, 9, 590. [Google Scholar] [CrossRef]
- Figueiredo, H.; Quintelas, C. Tailored zeolites for the removal of metal oxyanions: Overcoming intrinsic limitations of zeolites. J. Hazard. Mater. 2014, 274, 287–299. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Šiljeg, M.; Foglar, L.; Gudelj, I. The removal of arsenic from water with natural and modified clinoptilolite. Chem. Ecol. 2012, 28, 75–87. [Google Scholar] [CrossRef]
- Simsek, E.B.; Özdemir, E.; Beker, U. Process optimization for arsenic adsorption onto natural zeolite incorporating metal oxides by response surface methodology. Water Air Soil Pollut. 2013, 224, 1614. [Google Scholar] [CrossRef]
- Mejia-Zamudio, F.; Valenzuela-Garcia, J.; Gomez-Alvarez, A.; Meza-Figueroa, D.; Ela, W.P. Adsorption of arsenic on pre-treated zeolite at different pH levels. J. Chem. Speciat. Bioavailab. 2013, 25, 280–284. [Google Scholar] [CrossRef]
- Payne, K.B.; Abdel-Fattah, T.M. Adsorption of arsenate and arsenite by iron-treated activated carbon and zeolites: Effects of pH, temperature, and ionic strength. J. Environ. Sci. Health A 2005, 40, 723–749. [Google Scholar] [CrossRef]
- Shukla, E.A.; Johan, E.; Henmi, T.; Matsue, N. Arsenate adsorption on iron modified artificial zeolite made from coal fly ash. Procedia Environ. Sci. 2013, 17, 279–284. [Google Scholar] [CrossRef]
- Johan, E.; Shukla, E.A.; Matsue, N.; Henmi, T. Fe-treated artificial zeolite as an adsorbent for anionic and cationic pollutants. Procedia Environ. Sci. 2013, 17, 285–290. [Google Scholar] [CrossRef]
- Kong, S.; Wang, Y.; Zhan, H.; Yuan, S.; Yu, M.; Liu, M. Adsorption/oxidation of arsenic in groundwater by nanoscale Fe-Mn binary oxides loaded on zeolite. Water Environ. Res. 2014, 86, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Macedo-Miranda, M.G.; Olguin, M.T. Arsenic sorption by modified clinoptilolite–heulandite rich tuffs. J. Incl. Phenom. Macrocycl. Chem. 2007, 59, 131–142. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Medina, A.; Gamero, P.; Querol, X.; Moreno, N.; De León, B.; Almanza, M.; Vargas, G.; Izquierdo, M.; Font, O. Fly ash from a Mexican mineral coal I: Mineralogical and chemical characterization. J. Hazard. Mater. 2010, 181, 82–90. [Google Scholar] [CrossRef]
- Medina, A.; Gamero, P.; Almanza, J.M.; Vargas, A.; Montoya, A.; Vargas, G.; Izquierdo, M. Fly ash from a Mexican mineral coal II. Source of w zeolite and its effectiveness in arsenic (V.) adsorption. J. Hazard. Mater. 2010, 181, 91–104. [Google Scholar] [CrossRef]
- Jimenez-Cedillo, M.J.; Olguin, M.T.; Fall, C. Adsorption kinetic of arsenates as water pollutant on iron, manganese and iron–manganese-modified clinoptilolite-rich tuffs. J. Hazard. Mater. 2009, 163, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, D. Aqueous Environmental Geochemistry, 1st ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1997; pp. 360–364. ISBN 0-02-367412-1. [Google Scholar]
- Ayben, K.; Binay, B. Thermodynamic and kinetic investigations of uranium adsorption on amberlite IR-118H resin. Appl. Radiat. Isot. 2003, 58, 155–160. [Google Scholar] [CrossRef]
- Singh, T.S.; Pant, K.K. Equilibrium, kinetics and thermodynamic studies for adsorption of As (III) on activated alumina. Sep. Purif. Technol. 2004, 36, 139–147. [Google Scholar] [CrossRef]
- Di Natale, F.; Erto, A.; Lancia, A.; Musmarra, D. Experimental and modelling analysis of As (V) ions adsorption on granular activated carbon. Water Res. 2008, 42, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G.A. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- McKay, G.; Otterburn, M.S.; Sweeney, A.G. Surface mass transfer processes during color removal from effluent using silica. Water Res. 1981, 15, 327–331. [Google Scholar] [CrossRef]
- Van Aken, K.; Strekowski, L.; Patiny, L. EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J. Org. Chem. 2006, 2, 3. [Google Scholar] [CrossRef]
- Pini, M.; Rosa, R.; Neri, P.; Bondioli, F.; Ferrari, A.M. Environmental assessment of a bottom-up hydrolytic synthesis of TiO2 nanoparticles. Green Chem. 2015, 17, 518–531. [Google Scholar] [CrossRef]
- Gałuszka, A.; Konieczka, P.; Migaszewski, Z.M.; Namiésnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Knops-Gerrits, P.P.; De Vos, D.E.; Feijen, E.J.P.; Jacobs, P.A. Raman spectroscopy on zeolites. Microporous Mater. 1997, 8, 3–17. [Google Scholar] [CrossRef]
- Huang, Y.; Paroli, R.M.; Delgado, A.H.; Richardson, T.A. An FT-Raman study of solid-state ion exchange in zeolites. Spectrochim. Acta Part A 1998, 54, 1347–1354. [Google Scholar] [CrossRef]
- Seo, Y.H.; Prasentyanto, E.A.; Jiang, N.; Oh, S.M.; Park, S.E. Catalytic dehydration of methanol over synthetic zeolite W. Microporous Mesoporous Mater. 2010, 128, 108–114. [Google Scholar] [CrossRef]
- Yu, Y.; Xiong, G.; Li, C.; Xiao, F.S. Characterization of iron atoms in the framework of MFI-type zeolites by UV resonance Raman spectroscopy. J. Catal. 2000, 194, 487–490. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.D.; Kim, Y.C.; Kim, C.-S.; Hong, S.B. Synthesis and characterization of Ga-substituted MER-type zeolites. Microporous Mesoporous Mater. 2001, 42, 121–129. [Google Scholar] [CrossRef]
- Houlleberghs, M.; Breynaerta, E.; Asselmana, K.; Vaneeckhautea, E.; Radhakrishnana, S.; Anderson, M.W.; Taulellea, F.; Haouas, M.; Martens, J.A.; Kirschhock, C.E.A. Evolution of the crystal growth mechanism of zeolite W (MER) with temperature. Microporous Mesoporous Mater. 2019, 274, 379–384. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Sing, K. Adsorption by Powders and Porous Solid, Principles, Methodology and Applications, 1st ed.; Academic Press: London, UK, 1999; pp. 439–442. ISBN 978-0-12-598920-6. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density, 1st ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 26–55. ISBN 1-4020-2303-0. [Google Scholar]
- Sun, H.; Wang, L.; Zhang, R.; Sui, J.; Xu, G. Treatment of groundwater polluted by arsenic compounds by zero valent iron. J. Hazard. Mater. 2006, 129, 297–303. [Google Scholar] [CrossRef]
- Biterna, M.; Arditsoglou, A.; Tsikouras, E.; Voutsa, D. Arsenate removal by zero valent iron: Batch and column tests. J. Hazard. Mater. 2007, 149, 548–552. [Google Scholar] [CrossRef]
- Sarntanayoot, P.; Fuangswasdi, S.; Imyim, A. Iron nanoparticle-modified water treatment residues for adsorption of As(III) and As(V) and their cement-based solidification/stabilization. Int. J. Environ. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Wei, Z.; Liang, K.; Wu, Y.; Zou, Y.; Zuo, J.; Cortés Arriagada, D.; Pan, Z.; Hu, G. The effect of pH on the adsorption of arsenic(III) and arsenic(V) at the TiO2 anatase [1 0 1] surface. J. Colloid Interface Sci. 2016, 462, 252–259. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, Z.; Ma, Y.; He, X.; Zhao, Y.; Chai, Z. Adsorption and desorption characteristics of arsenic onto ceria nanoparticles. Nanoscale Res. Lett. 2012, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, A.; Ghiani, M.; Peretti, R.; Zucca, A. Red mud and fly ash for remediation of mine sites contaminated with As, Cd, Cu, Pb and Zn. J. Hazard. Mater. 2006, 134, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Polowczyk, I.; Bastrzyk, A.; Ulatowska, J.; Szczałba, E.; Koźlecki, T.; Sadowski, Z. Influence of pH on arsenic(III) removal by fly ash. Sep. Sci. Technol. 2016, 51, 2612–2619. [Google Scholar] [CrossRef]
- Li, Z.; Beachner, R.; McManama, Z.; Hanlie, H.H. Sorption of arsenic by surfactant-modified zeolite and kaolinite. Microporous Mesoporous Mater. 2007, 105, 291–297. [Google Scholar] [CrossRef]
- Das, B.; Devi, R.R.; Umlong, I.M.; Borah, K.; Banerjee, S.; Talukdar, A.K. Arsenic (III) adsorption on iron acetate coated activated alumina: Thermodynamic, kinetics and equilibrium approach. J. Environ. Health Sci. Eng. 2013, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.R.; Umlong, I.O.; Das, B.; Borah, K.; Thakur, A.J.; Raul, P.K.; Banerjee, S.; Singh, L. Removal of iron and arsenic (III) from drinking water using iron oxide-coated sand and limestone. Appl. Water Sci. 2014, 4, 175–182. [Google Scholar] [CrossRef]
- Ananta, S.; Banerjee, S.; Vijay, V. Adsorption Isotherm, Thermodynamic and kinetic study of arsenic (III) on iron oxide coated granular activated charcoal. Int. Res. J. Environ. Sci. 2015, 4, 64–77. [Google Scholar]
- Suzuki, T.M.; Bomani, J.O.; Matsunaga, H.; Yokoyama, T. Preparation of porous resin loaded with crystalline hydrous zirconium oxide and its application to the removal of arsenic. React. Funct. Polym. 2000, 43, 165–172. [Google Scholar] [CrossRef]
- Banerjee, K.; Amy, G.L.; Prevost, M.; Nour, S.; Jekel, M.; Gallagher, P.M.; Blumenschein, C.D. Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar] [CrossRef]
- Kuriakose, S.; Singh, T.S.; Pant, K.K. Adsorption of As(III) from aqueous solution onto iron oxide impregnated activated alumina. Water Qual. Res. J. 2004, 39, 258–266. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
| Langmuir. | Dubinin Raduskevich | |||||||
|---|---|---|---|---|---|---|---|---|
| T (K) | qm (mg g−1) | b (L mg−1) | R2 | K × 103 (mol2 kJ−2) | Qmax (mg g−1) | r | E (kJ mol−1) | R2 |
| 288 | 0.315 | 2.230 | 0.9589 | −5.285 | 14.588 | 0.3837 | 9.7266 | 0.9768 |
| 298 | 0.311 | 2.418 | 0.9681 | −5.197 | 14.016 | 0.3648 | 9.8086 | 0.9808 |
| 308 | 0.293 | 2.668 | 0.9847 | −4.583 | 15.646 | 0.3422 | 10.4450 | 0.9874 |
| As (III) Concentration (mg L−1) | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | q1 (mg L−1) | R2 | k2 (g mg−1 min−1) | q2 (mg L−1) | R2 | |
| 0.117 | 0.005 | 0.0641 | 0.760 | 4.8476 | 0.0100 | 0.986 |
| 0.515 | 0.011 | 0.0206 | 0.845 | 2.5626 | 0.0431 | 0.999 |
| 0.815 | 0.007 | 0.0414 | 0.902 | 0.5145 | 0.0586 | 0.994 |
| 4.0 | 0.007 | 0.1580 | 0.950 | 0.0701 | 0.2082 | 0.997 |
| 8.5 | 0.005 | 0.2386 | 0.902 | 0.0523 | 0.3163 | 0.982 |
| W zeolite Synthesis (from Fly Ash) | W Zeolite Synthesis (from Analytical Reagents) | ||||||
|---|---|---|---|---|---|---|---|
| Reagent | Amount (g) | Amount (mL) | Total Mass (g) | Reagent | Amount (g) | Amount (mL) | Total Mass (g) |
| Fly ash | 160 | 160 | Ludox | 232.68 | 302.48 | ||
| KOH | 53.20 | 53.20 | Al2Na2O4 | 41.45 | 41.45 | ||
| Water 1 | 1980 | 1980 | KOH | 154.99 | 154.99 | ||
| Water 1 | 2700 | 2700 | |||||
| Total wastes | 2193.20 | Total wastes | 3198.93 | ||||
| W zeolite (g) | 197.5 | W zeolite (g) | 187.87 | ||||
| E-Factor | (2193.20 − 197.5)/197.5 = 10.10 | E-Factor | (3198.93 − 187.87)/187.87 = 16.03 | ||||
| W Zeolite Synthesis (from Fly Ash) | W Zeolite Synthesis (from Analytical Reagents) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reagent | Amount (g) | Amount (mL) | Price (USD/g) | Total (USD/g) | Reagent | Amount (g) | Amount (mL) | Price (USD/g) | Total (USD/g) |
| Fly ash | 160 | 0 | 0 | Ludox | 302.48 | 232.68 | 0.03529 | 10.6752 | |
| KOH | 53.20 | 0.044 | 2.3408 | Al2Na2O4 | 41.45 | 0.0463 | 1.9191 | ||
| Water | 1980 | 0.00082 | 1.6424 | KOH | 154.99 | 0.044 | 6.8195 | ||
| Water | 2700 | 0.00082 | 2.214 | ||||||
| Total cost 2 | 3.9832 | Total cost | 21.6278 | ||||||
| W zeolite (g) | 197.5 | W zeolite (g) | 187.87 | ||||||
| Cost W zeolite (USD/g) | 0.0201 | Cost W zeolite (USD/g) | 0.1151 | ||||||
| W Zeolite Synthesis (from Fly Ash) | W Zeolite Synthesis (from Analytical Reagents) | ||||
|---|---|---|---|---|---|
| Parameter | Penalty Points | Parameter | Penalty Points | ||
| Yield 3 | (100 − %yield)/2 | 0 | Yield 3 | (100 − %yield)/2 | 0 |
| Price to obtain 10 mmol | Inexpensive (<USD10) | 0 | Price to obtain 10 mmol | Inexpensive (<USD10) | 0 |
| Safety | KOH Fly ash | 3 1 | Safety | KOH Al2Na2O4 | 3 3 |
| Technical setup | PE > 1 atm | 3 | Technical setup | PE > 1 atm | 3 |
| Temperature/time heating | Heating > 1 h | 3 | Temperature/time heating | Heating > 1 h | 3 |
| W&P | Simple filtration | 0 | W&P | Simple filtration | 0 |
| Total penalty points (TPP) | 10 | Total penalty points (TTP) | 12 | ||
| Eco-Scale | (100 − TPP) | 90 | Eco-Scale | (100 − TPP) | 88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Ramirez, A.; Gamero-Melo, P.; Ruiz-Camacho, B.; Minchaca-Mojica, J.I.; Romero-Toledo, R.; Gamero-Vega, K.Y. Adsorption of Aqueous As (III) in Presence of Coexisting Ions by a Green Fe-Modified W Zeolite. Water 2019, 11, 281. https://doi.org/10.3390/w11020281
Medina-Ramirez A, Gamero-Melo P, Ruiz-Camacho B, Minchaca-Mojica JI, Romero-Toledo R, Gamero-Vega KY. Adsorption of Aqueous As (III) in Presence of Coexisting Ions by a Green Fe-Modified W Zeolite. Water. 2019; 11(2):281. https://doi.org/10.3390/w11020281
Chicago/Turabian StyleMedina-Ramirez, Adriana, Procoro Gamero-Melo, Beatriz Ruiz-Camacho, Jesus Isaac Minchaca-Mojica, Rafael Romero-Toledo, and Karen Yazmin Gamero-Vega. 2019. "Adsorption of Aqueous As (III) in Presence of Coexisting Ions by a Green Fe-Modified W Zeolite" Water 11, no. 2: 281. https://doi.org/10.3390/w11020281
APA StyleMedina-Ramirez, A., Gamero-Melo, P., Ruiz-Camacho, B., Minchaca-Mojica, J. I., Romero-Toledo, R., & Gamero-Vega, K. Y. (2019). Adsorption of Aqueous As (III) in Presence of Coexisting Ions by a Green Fe-Modified W Zeolite. Water, 11(2), 281. https://doi.org/10.3390/w11020281