Changes in the Fish Community of a Western Caribbean Estuary after the Expansion of an Artificial Channel to the Sea
Abstract
1. Introduction
2. Materials and Methods
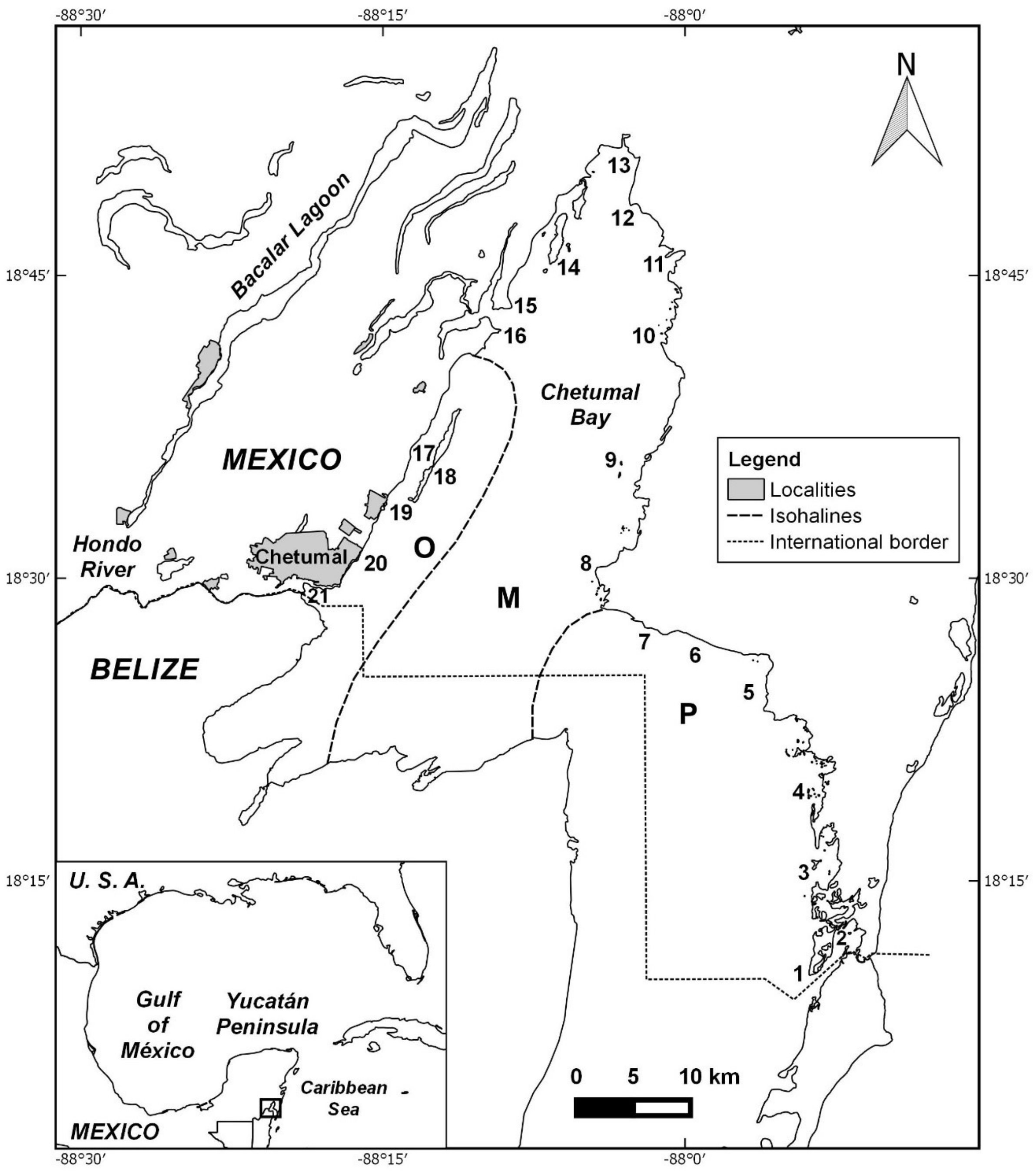
2.1. Study Area
2.2. Field and Laboratory Work
2.3. Data Analysis
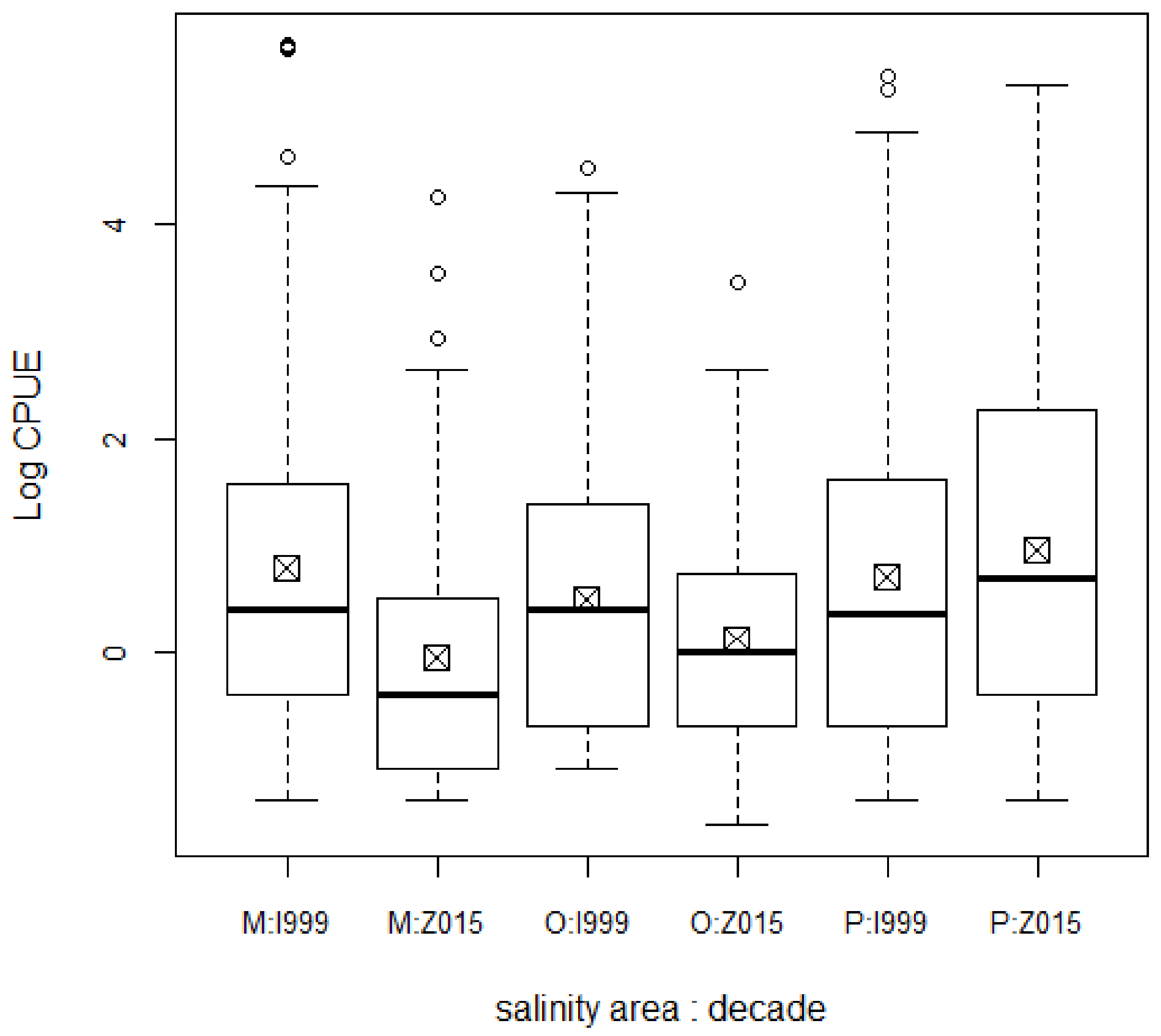
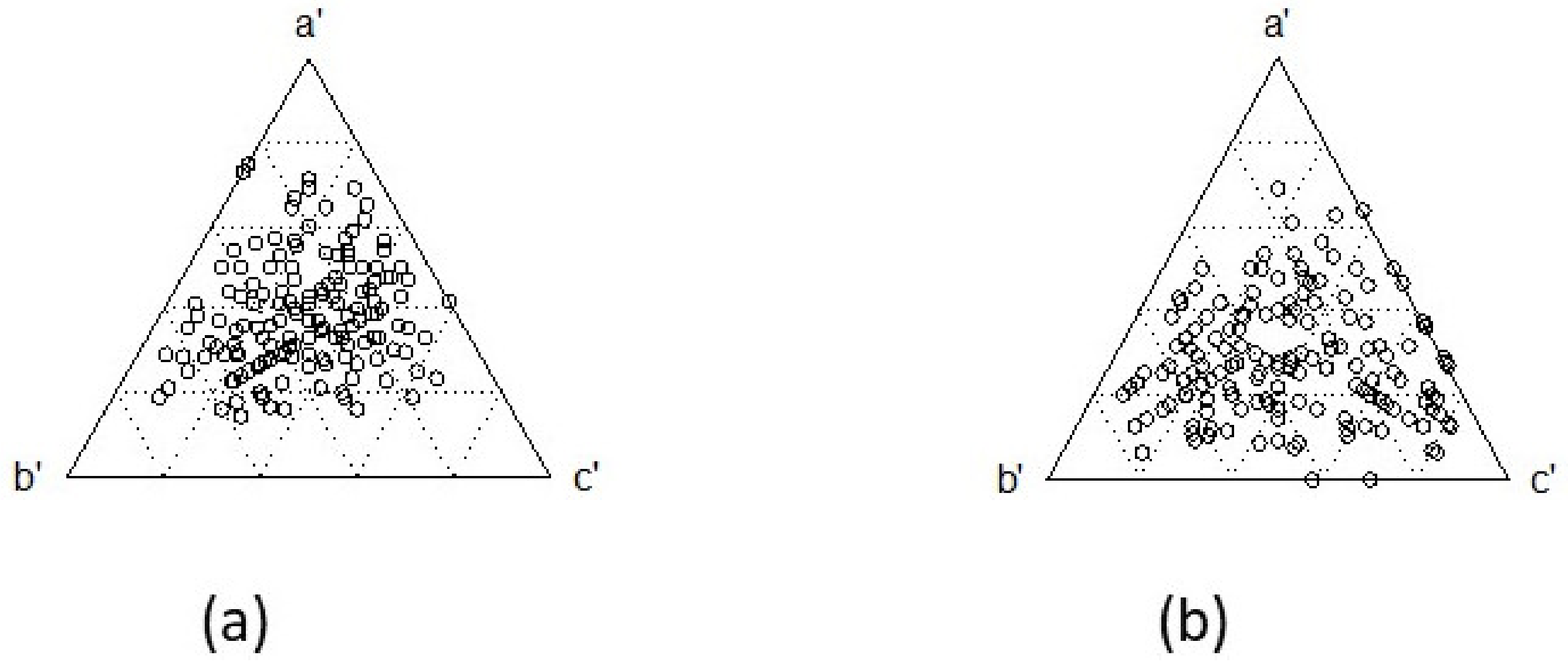
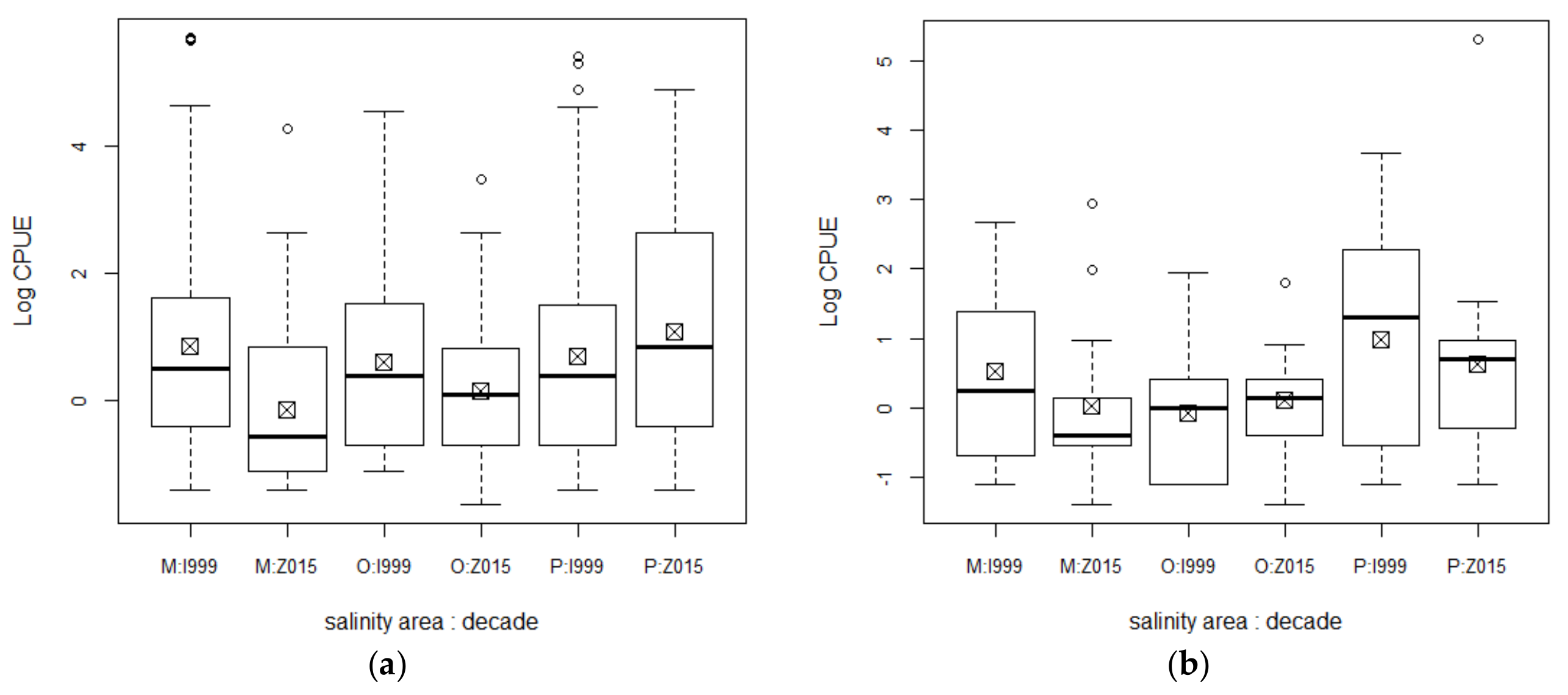
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species Zone | 1999–2001 | 2015–2018 |
| Anchoa cayorum | 0.5 | 0.5 |
| O | 0 | 0.5 |
| P | 0.5 | 0 |
| Achirus lineatus | 68.3 | 13.4 |
| M | 45.4 | 2.0 |
| O | 19.0 | 9.7 |
| P | 3.8 | 1.7 |
| Albula vulpes | 3.5 | 0 |
| P | 3.5 | 0 |
| Anchoa colonensis | 0.3 | 0 |
| M | 0.3 | 0 |
| Anchoa cubana | 19.2 | 10.0 |
| M | 3.0 | 0 |
| O | 11.7 | 0 |
| P | 4.5 | 10.0 |
| Ariopsis assimilis | 2.3 | 0 |
| M | 1.0 | 0 |
| O | 1.3 | 0 |
| Astyanax bacalarensis | 0 | 45.2 |
| M | 0 | 38.0 |
| O | 0 | 1.2 |
| P | 0 | 6.0 |
| Atherinomorus stipes | 2306.3 | 590.7 |
| M | 1542.2 | 90.8 |
| O | 167.7 | 0.3 |
| P | 596.5 | 499.5 |
| Bairdiella ronchus | 43.3 | 2.0 |
| M | 21.5 | 0 |
| O | 21.3 | 0 |
| P | 0.5 | 2 |
| Bathygobius curacao | 0 | 1.2 |
| M | 0 | 1.2 |
| Bothus ocellatus | 0.3 | 0 |
| P | 0.3 | 0 |
| Canthigaster rostrata | 0 | 0.3 |
| P | 0 | 0.3 |
| Centropomus undecimalis | 1.0 | 0 |
| M | 1.0 | 0 |
| Chriodorus atherinoides | 9.8 | 2.5 |
| M | 8.2 | 0 |
| P | 1.7 | 2.5 |
| Corvula sanctaeluciae | 0 | 0.7 |
| P | 0 | 0.7 |
| Cyprinodon artifrons | 45.2 | 6.0 |
| M | 1.5 | 0.7 |
| O | 0 | 1.5 |
| P | 43.7 | 3.8 |
| Diapterus auratus | 0 | 23.3 |
| P | 0 | 23.3 |
| Eucinostomus argenteus | 34.2 | 63.6 |
| M | 9.5 | 3.3 |
| O | 4.5 | 0.5 |
| P | 20.2 | 59.8 |
| Eucinostomus gula | 269.8 | 53.8 |
| M | 38.8 | 12.3 |
| O | 9.5 | 5.0 |
| P | 221.5 | 36.5 |
| Eucinostomus harengulus | 15.7 | 30.7 |
| M | 3.3 | 11.2 |
| O | 0.3 | 1.0 |
| P | 12 | 18.5 |
| Eucinostomus jonesii | 257.5 | 72.8 |
| M | 155.3 | 13.8 |
| O | 45.3 | 34.1 |
| P | 56.8 | 24.8 |
| Eucinostomus melanopterus | 0 | 1.7 |
| M | 0 | 0.3 |
| P | 0 | 1.3 |
| Eugerres plumieri | 78.4 | 7.8 |
| M | 23.6 | 1.0 |
| O | 52.3 | 2.9 |
| P | 2.5 | 3.8 |
| Floridichthys polyommus | 133.4 | 266.7 |
| M | 78.3 | 34.4 |
| O | 12.5 | 5.5 |
| P | 42.7 | 226.8 |
| Gerres cinereus | 1.8 | 31.2 |
| M | 1 | 4.3 |
| O | 0 | 20.3 |
| P | 0.8 | 6.5 |
| Gobiosoma yucatanum | 1.5 | 0 |
| O | 1.5 | 0 |
| Gambusia sexradiata | 0.5 | 0 |
| O | 0.5 | 0 |
| Gambusia yucatana | 9.8 | 1.5 |
| M | 8.5 | 1.0 |
| P | 1.3 | 0.5 |
| Harengula clupeola | 9 | 30 |
| P | 9 | 30 |
| Harengula humeralis | 6 | 0.3 |
| P | 6 | 0.3 |
| Harengula jaguana | 174.7 | 149.0 |
| M | 37.3 | 1.0 |
| O | 74.3 | 0 |
| P | 63.0 | 148.0 |
| Hippocampus erectus | 0.3 | 0 |
| P | 0.3 | 0 |
| Hypanus americanus | 0 | 0.3 |
| M | 0 | 0.3 |
| Hyporhamphus roberti | 1.5 | 0.25 |
| M | 0 | 0.25 |
| O | 1.5 | 0 |
| Jenkinsia lamprotaenia | 2.5 | 0 |
| P | 2.5 | 0 |
| Jordanella pulchra | 8.0 | 8.9 |
| M | 8.0 | 2.0 |
| O | 0 | 6.25 |
| P | 0 | 0.7 |
| Lophogobius cyprinoides | 13.8 | 2.8 |
| M | 7.3 | 0 |
| O | 6.5 | 2.8 |
| Lutjanus griseus | 0 | 13.3 |
| P | 0 | 13.3 |
| Mayaheros urophthalmus | 4.2 | 1.5 |
| M | 4.2 | 0 |
| O | 0 | 1.5 |
| Monacanthus tuckeri | 0 | 0.3 |
| M | 0 | 0.3 |
| Oligoplites saurus | 1.2 | 0.3 |
| M | 0.7 | 0 |
| P | 0.5 | 0.3 |
| Oostethus lineatus | 0 | 0.3 |
| M | 0 | 0.3 |
| Opisthonema oglinum | 9.8 | 0 |
| O | 5 | 0 |
| P | 4.8 | 0 |
| Opsanus beta | 1.7 | 0 |
| O | 1.7 | 0 |
| Paraclinus fasciatus | 0.5 | 0 |
| O | 0.5 | 0 |
| Poecilia mexicana | 20.2 | 0.4 |
| M | 19.2 | 0 |
| O | 2.0 | 0.4 |
| Sphoeroides testudineus | 36.0 | 47.8 |
| M | 6.0 | 27.4 |
| O | 25.2 | 11.7 |
| P | 4.8 | 8.7 |
| Sphyraena barracuda | 24.8 | 12.0 |
| M | 8.8 | 4.6 |
| O | 5.2 | 3.5 |
| P | 10.8 | 4.0 |
| Strongylura marina | 0.5 | 0 |
| P | 0.5 | 0 |
| Strongylura notata | 51.9 | 150.5 |
| M | 25.3 | 27.2 |
| O | 5.8 | 1.5 |
| P | 20.8 | 121.8 |
| Strongylura timucu | 1.5 | 0 |
| M | 1.5 | 0 |
| Syngnathus floridae | 0.25 | 0 |
| M | 0.25 | 0 |
| Syngnathus scovelli | 0.5 | 0 |
| O | 0.5 | 0 |
| Trachinotus falcatus | 0 | 1.2 |
| M | 0 | 0.3 |
| O | 0 | 0.5 |
| P | 0 | 0.3 |
| Trichromis salvini | 0 | 3.5 |
| O | 0 | 3.5 |
| Vieja melanurus | 2.0 | 0 |
| O | 2.0 | 0 |
Appendix B
| Species | O | Before 2005 M | P | O | 2006 up to Now M | P |
| Acanthurus bahianus | 4222 | |||||
| Achirus lineatus | X | X | X | X | X | X |
| Aetobatus narinari | pers. obs. | |||||
| Albula vulpes | 4221 | X | [12] | [12] | ||
| Anchoa cayorum | X | X | ||||
| A. colonensis | X | |||||
| A. cubana | X | X | X | X | ||
| Archosargus probatocephalus | 4219 | |||||
| A. rhomboidalis | 4217 | |||||
| Ariopsis assimilis | X | X | 4261 | |||
| Astyanax bacalarensis | 7815 | 1301 | X | X | X | |
| Atherinomorus stipes | X | X | X | X | X | X |
| Bagre marinus | 3143 | |||||
| Bairdiella ronchus | X | X | X | X | ||
| Bathygobius curacao | X | |||||
| Bothus ocellatus | X | |||||
| Canthigaster rostrata | X | |||||
| Caranx latus | 4214 | X | ||||
| Centropomus undecimalis | 4216 | X | ||||
| Chaetodipterus faber | 4213 | |||||
| Chilomycterus schopfi | 4047 | |||||
| Chriodorus atherinoides | X | X | 8020 | X | ||
| Conodon nobilis | CNPE 3221 | |||||
| Corvula sanctaeluciae | 1703 | X | ||||
| Cribroheros robertsoni | X | |||||
| Cyprinodon artifrons | X | X | X | X | X | |
| D. guttata | 4194 | |||||
| Diapterus auratus | X | |||||
| Echeneis neucratoides | 2785 | X | ||||
| Elops saurus | [38] | |||||
| Epinephelus itajara | pers. obs. | pers. obs. | ||||
| Eucinostomus argenteus | X | X | X | X | X | X |
| E. gula | X | X | X | X | X | X |
| E. harengulus | X | X | X | X | X | X |
| E. jonesii | X | X | X | X | X | X |
| E. melanopterus | X | X | ||||
| Eugerres plumieri | X | X | X | X | X | X |
| Floridichthys polyommus | X | X | X | X | X | X |
| Gambusia sexradiata | X | |||||
| G. yucatana | X | X | X | X | ||
| Gerres cinereus | X | X | X | X | X | |
| Gobiosoma yucatanum | X | |||||
| Gymnothorax funebris | pers. obs. | |||||
| Haemulon sciurus | pers. obs. | |||||
| Harengula clupeola | 2061 | X | X | |||
| H. humeralis | X | 7806 | X | |||
| H. jaguana | X | X | X | X | X | |
| Hippocampus erectus | X | |||||
| Holocentrus rufus | 4224 | |||||
| Hypanus americanus | X | |||||
| Hyporhamphus roberti | X | X | ||||
| Jenkinsia lamprotaenia | X | |||||
| Jordanella pulchra | X | X | X | X | ||
| Kyphosus incisor | pers. obs. | |||||
| Lachnolaimus maximus | pers. obs. | |||||
| Lobotes surinamensis | 4218 | |||||
| Lophogobius cyprinoides | X | X | X | |||
| Lupinoblennius vinctus | 4134 | |||||
| Lutjanus analis | [38] | |||||
| L. apodus | 4236 | |||||
| L. cyanopterus | pers. obs. | |||||
| L. griseus | 4384 | X | ||||
| L. jocu | [39] | |||||
| L. mahogoni | pers. obs. | |||||
| L. synagris | X | |||||
| Mayaheros urophthalmus | X | X | X | |||
| Megalops atlanticus | 3139 | 4381 | ||||
| Monacanthus tuckeri | 7838 | |||||
| Mugil cephalus | pers. obs. | |||||
| Narcine brasiliensis | pers. obs. | |||||
| Ocyurus chrysurus | [39] | |||||
| Oligoplites saurus | X | X | X | |||
| Oostethus lineatus | X | |||||
| Opisthonema oglinum | X | X | ||||
| Opsanus beta | X | |||||
| Paraclinus fasciatus | X | 3169 | ||||
| Poecilia kykesis | 1246 | |||||
| P. mexicana | X | X | X | |||
| Pristis sp. | pers. obs. | pers. obs. | ||||
| Pterois volitans | pers. obs. | |||||
| Scomberomorus maculatus | 4768 | pers. obs. | ||||
| Selene vomer | 4215 | |||||
| Sparisoma viride | pers. obs. | |||||
| Sphoeroides testudineus | X | X | X | X | X | X |
| Sphyraena barracuda | X | X | X | X | X | X |
| Strongylura marina | X | |||||
| S. notata | X | X | X | X | X | X |
| S. timucu | 5063 | X | 8025 | |||
| Styracura schmardae | 3799 | |||||
| Symphurus diomedeanus | 4137 | |||||
| Syngnathus floridae | X | |||||
| S. scovelli | X | |||||
| Trachinotus falcatus | X | X | X | |||
| Trichromis salvini | X | |||||
| Vieja melanurus | X | 4302 |
References
- Espinoza-Ávalos, J.; Islebe, G.A.; Hernández-Arana, H.A. El sistema Ecológico de la Bahía de Chetumal/Corozal, Costa Occidental del Mar Caribe; El Colegio de la Frontera Sur: Chetumal, Mexico, 2009; ISBN 9786077637134. [Google Scholar]
- Hernández-Arana, H.A.; Ameneyro-Ángeles, B. Benthic biodiversity changes due to the opening of an artificial channel in a tropical coastal lagoon (Mexican Caribbean). J. Mar. Biol. Assoc. 2011, 91, 969–978. [Google Scholar] [CrossRef]
- Perez, A.U.; Arce-Ibarra, A.M.; García-Ortega, M.; Azueta, J.O. Artisanal recreational fisheries: Using a combined approach to fishery assessment aimed at providing insights for fishery managers. Mar. Resour. Econ. 2014, 29, 89–109. [Google Scholar] [CrossRef]
- Medina-Quej, A.; Arce-Ibarra, A.M.; Herrera-Pavón, R.L.; Caballero-Pinzón, P.I.; Ortiz-León, H.; Rosas-Correa, C.O. Pesquerías: Sector Social, Recurso Base y Manejo. In El Sistema Ecológico de la Bahía de Chetumal/Corozal: Costa Occidental del Mar Caribe; Espinoza-Ávalos, J., Islebe, G.A., Hernández-Arana, H.A., Eds.; El Colegio de la Frontera Sur: Chetumal, Mexico, 2009; pp. 184–195. ISBN 9786077637134. [Google Scholar]
- Schmitter-Soto, J.J.; Vásquez-Yeomans, L.; Pimentel Cadena, E.; Herrera-Pavón, R.L.; Paz, G.; García-Téllez, N. Peces. In El Sistema Ecológico de la Bahía de Chetumal/Corozal: Costa Occidental del Mar Caribe; Espinoza-Ávalos, J., Islebe, G.A., Hernández-Arana, H.A., Eds.; El Colegio de la Frontera Sur: Chetumal, Mexico, 2009; pp. 102–114. [Google Scholar]
- Schmitter-Soto, J.J.; Monks, S.; Vásquez-Yeomans, L.; Pimentel Cadena, E.; Herrera-Pavón, R.L.; Pulido-Flores, G.; Quintal-Lizama, C.; Valtierra-Vega, M.T. Peces, Ictioplancton y Helmintos Parásitos en la Bahía de Chetumal (Santuario del Manati); Technical Report; Conabio, Ecosur: Chetumal, Mexico, 2001. [Google Scholar]
- Carrillo, L.; Palacios-Hernández, E.; Ramírez-Manguilar, A.M.; Morales-Vela, B. Características Hidrometeorológicas y Batimétricas. In El Sistema Ecológico de la Bahía de Chetumal/Corozal: Costa Occidental del Mar Caribe; Espinoza-Avalos, J., Islebe, G.A., Hernández-Arana, H.A., Eds.; El Colegio de la Frontera Sur: Chetumal, Mexico, 2009; pp. 12–20. [Google Scholar]
- Carrillo, L.; Palacios-Hernández, E.; Yescas, M.; Ramírez-Manguilar, A.M. Spatial and seasonal patterns of salinity in a large and shallow tropical estuary of the western Caribbean. Estuaries Coasts 2009, 32, 906–916. [Google Scholar] [CrossRef]
- Granados-Sánchez, D.; López-Ríos, G.; Martínez-V, F.J.; Martínez-Castillo, J. Los manglares de Quintana Roo. Rev. Chapingo Ser. Cienc. For. Ambient. 1998, 4, 253–265. [Google Scholar]
- Álvarez-Legorreta, T. Contaminación acuática. In El Sistema Ecológico de la Bahía de Chetumal/Corozal: Costa Occidental del Mar Caribe; Espinoza-Ávalos, J., Islebe, G.A., Hernández-Arana, H.A., Eds.; El Colegio de la Frontera Sur: Chetumal, Mexico, 2009; pp. 205–217. [Google Scholar]
- Herrera-Pavón, R.L. Pesca deportivo-recreativa. In Riqueza Biológica de Quintana Roo. Un Análisis para su Conservación.Tomo 1; Pozo, C., Ed.; CONABIO: Mexico City, 2011; pp. 190–193. [Google Scholar]
- Perez, A.U.; Schmitter-Soto, J.J.; Adams, A.J.; Heyman, W.D. Connectivity mediated by seasonal bonefish (Albula vulpes) migration between the Caribbean Sea and a tropical estuary of Belize and Mexico. Environ. Biol. Fishes 2019, 102, 197–207. [Google Scholar] [CrossRef]
- Castro-Aguirre, J.L.; Espinosa-Pérez, H.; Schmitter-Soto, J.J. Ictiofauna Estuarino-Lagunar y Vicaria de México; Noriega-Limusa, IPN: Mexico City, Mexico, 1999; ISBN 9681857747. [Google Scholar]
- Ruiz-Cauich, L.E.; Schmitter-Soto, J.J.; Barba-Macías, E.; González-Solís, D. Stability vs. organization: potential of a trophic model for the management of shallow tropical streams. Food Webs 2016, 6, 38–47. [Google Scholar]
- FishBase. Available online: www.fishbase.org (accessed on 31 August 2018).
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence-absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Venables, W.N.; Smith, D.M. An Introduction to R, Version 1.0; The R Development Core Team: Auckland, New Zealand, 2003. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘Vegan’: Community Ecology Package; CRAN: Oulu, Finland, 2015; p. 292. [Google Scholar]
- Dawson, C.E. Gobiosoma (Garmannia) yucatanum, a new seven-spined Atlantic goby from México. Copeia 1971, 1971, 432–439. [Google Scholar] [CrossRef]
- Greenfield, D.W.; Thomerson, J.E. Fishes of the Continental Waters of Belize; University Press of Florida: Gainesville, FL, USA, 1997; ISBN 0-8130-1497-2. [Google Scholar]
- Schmitter-Soto, J.J.; Aguilar-Perera, A.; Cruz-Martínez, A.; Herrera-Pavón, R.L.; Morales-Aranda, A.A.; Cobián-Rojas, D. Interdecadal trends in composition, density, size, and mean trophic level of fish species and guilds before and after coastal development in the Mexican Caribbean. Biodivers. Conserv. 2017, 27, 459–474. [Google Scholar] [CrossRef]
- Espinoza-Ávalos, J.; Hernández-Arana, H.A.; Álvarez-Legorreta, T.; Quan-Young, L.I.; Oliva-Rivera, J.J.; Valdez-Hernández, M.; Wórum, F.; Villegas, A.; van Tussenbroek, B.I. Vegetación acuática sumergida. In El sistema Ecológico de la bahía de Chetumal/Corozal: Costa Occidental del Mar Caribe; Espinoza-Ávalos, J., Islebe, G.A., Hernández-Arana, H.A., Eds.; El Colegio de la Frontera Sur: Chetumal, Mexico, 2009; pp. 28–40. [Google Scholar]
- Torres-Chávez, P.; Schmitter-Soto, J.J.; Mercado-Silva, N.; Valdez-Moreno, M.E. Movimiento entre hábitats de la barracuda Sphyraena barracuda, determinado por aproximaciones tróficas en el Caribe. Rev. Mex. Biodivers. 2018, 89, 865–872. [Google Scholar] [CrossRef]
- Salvadores Baledón, M.L.; Reséndez Medina, A. Modificaciones en la composición ictiofaunística del sistema lagunar El Carmen-Machona Tabasco, por la apertura de Boca de Panteones. Univ. Cienc. 1990, 7, 5–13. [Google Scholar]
- Bonaldo, R.M.; Bellwood, D.R. Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 2008, 360, 237–244. [Google Scholar] [CrossRef]
- Herrera-Silveira, J.A.; Arreola-Lizárraga, J.A.; Ramírez, J. Cambios hidrológicos y de estado trófico entre los años 2000 y 2006. In El Sistema Ecológico de la Bahía de Chetumal/Corozal: Costa Occidental del Mar Caribe; Espinoza-Ávalos, J., Islebe, G.A., Hernández-Arana, H.A., Eds.; El Colegio de la Frontera Sur: Chetumal, Mexico, 2009; pp. 21–27. [Google Scholar]
- Sheppard, C.; Rioja-Nieto, R. Sea surface temperature 1871–2099 in 38 cells in the Caribbean region. Mar. Environ. Res. 2005, 60, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Martínez, V.M.; Aguirre-Macedo, M.L.; Gold-Bouchot, G.; Caballero-Pinzón, P.I. Potential interactions between metazoan parasites of the Mayan catfish Ariopsis assimilis and chemical pollution in Chetumal Bay, Mexico. J. Helminthol. 2003, 77, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Melgar Tísoc, E.R. La explotación de recursos marinos en Oxtankah, Quintana Roo. Actual. Arqueol. 2006, 1, 5–29. [Google Scholar]
- Netto, S.A.; Domingos, A.M.; Kurtz, M.N. Effects of artificial breaching of a temporarily open/closed estuary on benthic macroinvertebrates (Camacho Lagoon, Southern Brazil). Estuaries Coasts 2012, 35, 1069–1081. [Google Scholar] [CrossRef]
- Layman, C.A.; Jud, Z.R.; Nichols, P.K. Lionfish alter benthic invertebrate assemblages in patch habitats of a subtropical estuary. Mar. Biol. 2014, 161, 2179–2182. [Google Scholar] [CrossRef]
- Santangelo, J.M.; Rocha, A.M.; Bozelli, R.L.; Carneiro, L.S.; Esteves, F.A. Zooplankton responses to sandbar opening in a tropical eutrophic coastal lagoon. Estuar. Coast. Shelf Sci. 2007, 71, 657–668. [Google Scholar] [CrossRef]
- Schmitter-Soto, J.J. Catálogo de Los Peces Continentales de Quintana Roo; El Colegio de la Frontera Sur: San Cristóbal de Las Casas, Mexico, 1998; ISBN 9687555041. [Google Scholar]
- Rahel, F.J. Homogenization, differentiation, and the widespread alteration of fish faunas. Am. Fish. Soc. Symp. 2010, 73, 311–326. [Google Scholar]
- Villéger, S.; Blanchet, S.; Beauchard, O.; Oberdorff, T.; Brosse, S. Homogenization patterns of the world’s freshwater fish faunas. Proc. Natl. Acad. Sci. USA 2011, 108, 18003–18008. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Filip, L.; Millet-Encalada, M.; Reyes-Bonilla, H. Impact of hurricanes Emily and Wilma on the coral community of Cozumel island, Mexico. Bull. Mar. Sci. 2009, 84, 295–306. [Google Scholar]
- Pérez-Ruzafa, Á.; García-Charton, J.A.; Barcala, E.; Marcos, C. Changes in benthic fish assemblages as a consequence of coastal works in a coastal lagoon: the Mar Menor (Spain, Western Mediterranean). Mar. Pollut. Bull. 2006, 53, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine invasive alien species: A threat to global biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
| Species | F | Difference |
|---|---|---|
| Achirus lineatus | 12.70 | Decreased by decade at all zones |
| Achirus lineatus | 3.41 | Greater abundance in O than in P |
| Atherinomorus stipes | 4.97 | Decreased by decade in M |
| Eugerres plumieri | 6.11 | Decreased by decade in O and M, not in P |
| Gerres cinereus | 3.44 | Decreased by decade in M |
| Harengula jaguana | 7.97 | Greater abundance in P in 2015–2018 |
| Strongylura notata | 2.47 | Greater abundance in P in 2015–2018 |
| Guild | 1999–2001 | 2015–2018 |
|---|---|---|
| Large benthivores | 0 | 4 |
| Middle-sized benthivores | 63 | 46 |
| Small benthivores | 157 | 97 |
| Medium-sized detritivores | 8 | 1 |
| Small detritivores | 8 | 6 |
| Medium-sized herbivores | 1 | 0 |
| Small herbivores | 9 | 3 |
| Medium-sized omnivores | 3 | 2 |
| Small omnivores | 11 | 12 |
| Large piscivores | 1 | 0 |
| Medium-sized piscivores | 45 | 42 |
| Small piscivores | 1 | 6 |
| Medium-sized planktivores | 1 | 0 |
| Small planktivores | 63 | 26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitter-Soto, J.J.; Herrera-Pavón, R.L. Changes in the Fish Community of a Western Caribbean Estuary after the Expansion of an Artificial Channel to the Sea. Water 2019, 11, 2582. https://doi.org/10.3390/w11122582
Schmitter-Soto JJ, Herrera-Pavón RL. Changes in the Fish Community of a Western Caribbean Estuary after the Expansion of an Artificial Channel to the Sea. Water. 2019; 11(12):2582. https://doi.org/10.3390/w11122582
Chicago/Turabian StyleSchmitter-Soto, Juan J., and Roberto L. Herrera-Pavón. 2019. "Changes in the Fish Community of a Western Caribbean Estuary after the Expansion of an Artificial Channel to the Sea" Water 11, no. 12: 2582. https://doi.org/10.3390/w11122582
APA StyleSchmitter-Soto, J. J., & Herrera-Pavón, R. L. (2019). Changes in the Fish Community of a Western Caribbean Estuary after the Expansion of an Artificial Channel to the Sea. Water, 11(12), 2582. https://doi.org/10.3390/w11122582





