Invasive Alien Vines Affect Leaf Traits of Riparian Woody Vegetation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Site Description
2.3. Spectral Analyses
2.4. Morphological and Anatomical Analyses
2.5. Biochemical Analyses
2.6. Statistical Analyses
3. Results
3.1. Environmental Conditions
3.2. Leaf Traits
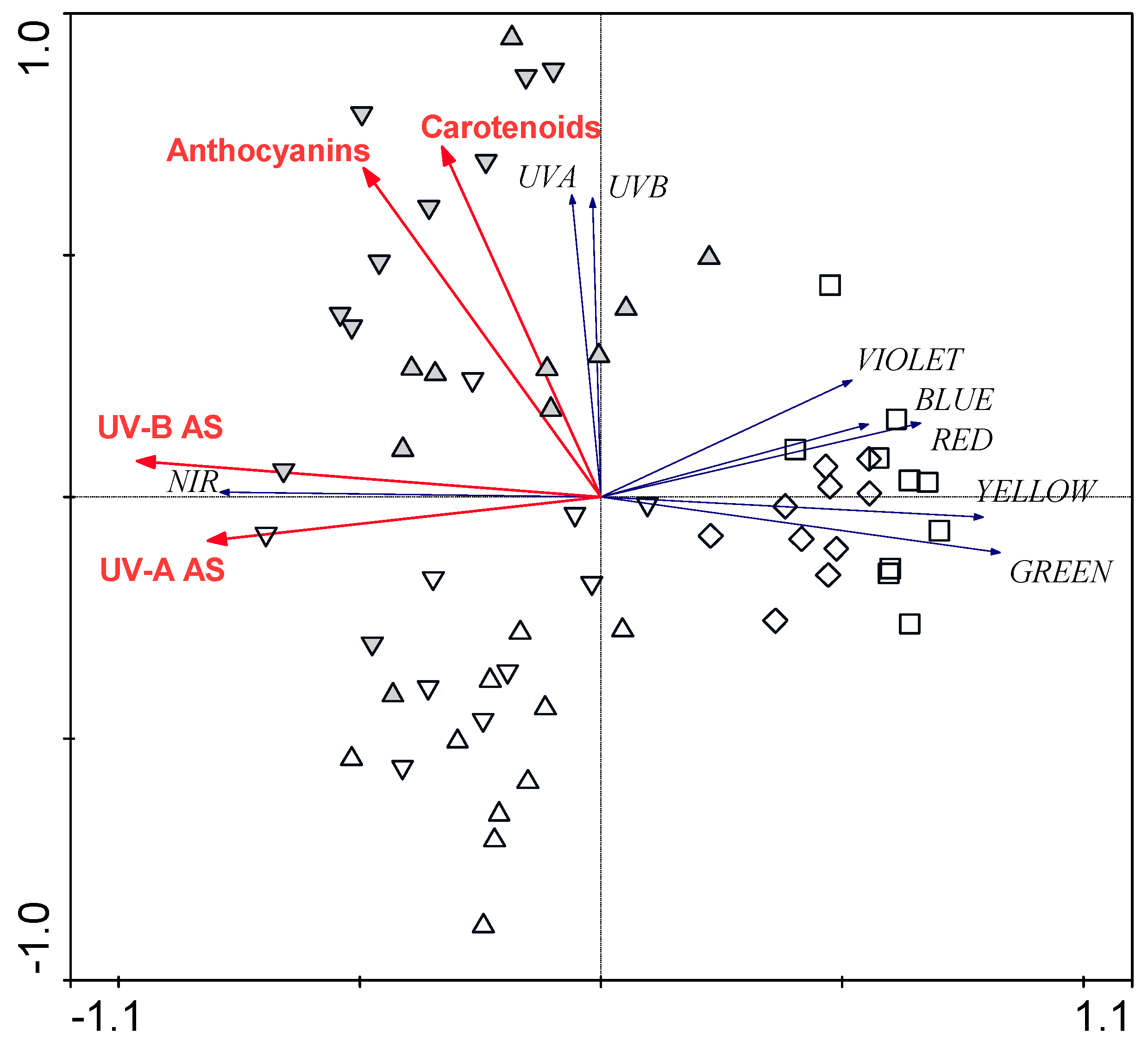
3.3. Relationships between Leaf Structural Traits and Leaf Optical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naiman, R.J.; Decamps, H.; Pollock, M.M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, Y.; Richardson, J.S. Importance of riparian zone: Effects of resource availability at land-water interface. Riparian Ecol. Conserv. 2016, 3, 1–17. [Google Scholar] [CrossRef]
- Higgisson, W.P.; Downey, P.O.; Dyer, F.J. Changes in vegetation and geomorphological condition 10 years after riparian restoration. Water 2019, 11, 1252. [Google Scholar] [CrossRef]
- Petersen, R.C. The RCE: A riparian, channel, and environmental inventory for small streams in agricultural landscape. Freshw. Biol. 1992, 27, 295–306. [Google Scholar] [CrossRef]
- Germ, M.; Gaberščik, A.; Urbanc-Berčič, O. The wider environmental assessment of river ecosystems. Acta Biol. Slov. 2000, 43, 13–19. [Google Scholar]
- Kozlowski, D.F.; Hall, R.K.; Swanson, S.R.; Heggem, D.T. Linking management and riparian physical functions to water quality and aquatic habitat. J. Water Resour. Prot. 2016, 8, 797–815. [Google Scholar] [CrossRef][Green Version]
- Pinay, G.; Bernal, S.; Abbott, B.W.; Lupon, A.; Marti, E.; Sabater, F.; Krause, S. Riparian corridors: A new conceptual framework for assessing nitrogen buffering across biomes. Front. Environ. Sci. 2018, 6, 47. [Google Scholar] [CrossRef]
- Hood, W.G.; Naiman, R.J. Vulnerability of riparian zones to invasion by exotic vascular plants. Plant Ecol. 2000, 148, 105–114. [Google Scholar] [CrossRef]
- Richardson, D.M.; Holmes, P.M.; Esler, K.J.; Galatowitsch, S.M.; Stromberg, J.C.; Kirkman, S.P.; Pyšek, P.; Hobb, R.J. Riparian vegetation: Degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 2007, 13, 126–139. [Google Scholar] [CrossRef]
- Zelnik, I.; Haler, M.; Gaberščik, A. Vulnerability of a riparian zone towards invasion by alien plants depends on its structure. Biologia 2015, 70, 869–878. [Google Scholar] [CrossRef]
- Cornejo-Denman, L.; Romo-Leon, J.R.; Castellanos, A.E.; Diaz-Caravantes, R.E.; Moreno-Vázquez, J.L.; Mendez-Estrella, R. Assessing riparian vegetation condition and function in disturbed sites of the arid northwestern Mexico. Land 2018, 7, 13. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P. What is the impact of Impatiens glandulifera on species diversity of invaded riparian vegetation? Biol. Conserv. 2006, 132, 143–152. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Alonso, Á. Effects of non-native riparian plants in riparian and fluvial ecosystems: A review for the Iberian Peninsula. Limnetica 2017, 36, 525–541. [Google Scholar] [CrossRef]
- Nobis, A.; Nowak, A.; Rola, K. Do invasive alien plants really threaten river bank vegetation? A case study based on plant communities typical for Chenopodium ficifolium—An indicator of large river valleys. PLoS ONE 2018, 13, e0194473. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, N.E.; Richardson, C.J.; Ho, M. Connecting differential responses of native and invasive riparian plants to climate change and environmental alterations. Ecol. Appl. 2015, 25, 753–767. [Google Scholar] [CrossRef]
- Ewers, F.W.; Rosell, J.A.; Olson, M.E. Lianas as structural parasites. In Functional and Ecological Xylem Anatomy; Hacke, U., Ed.; Springer: Cham, Switzerland, 2015; pp. 163–188. [Google Scholar] [CrossRef]
- Dillenburg, L.R.; Whigham, D.F.; Teramura, A.H.; Forseth, I.N. Effects of vine competition on availability of light, water, and nitrogen to a tree host (Liquidambar styraciflua). Am. J. Bot. 1993, 80, 244–252. [Google Scholar] [CrossRef]
- Toledo-Aceves, T. Above- and below-ground competition between lianas and trees. In Ecology of Lianas, 1st ed.; Schnitzer, S., Bongers, F., Burnham, R.J., Putz, F.E., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 147–163. [Google Scholar] [CrossRef]
- Sridhar Reddy, M.; Parthasarathy, N. Liana diversity and distribution on host plants in four tropical dry evergreen forests of peninsular India. Trop. Ecol. 2005, 47, 109–123. [Google Scholar]
- Gianoli, E. The behavioural ecology of climbing plants. AoB Plants 2015, 7, plv013. [Google Scholar] [CrossRef]
- Gianoli, E.; Carrasco-Urra, F. Leaf mimicry in a climbing plant protects against herbivory. Curr. Biol. 2014, 24, 984–987. [Google Scholar] [CrossRef]
- Bagi, I.; Böszörményi, A. Wild cucumber (Echinocystis lobata Torr. et., Gray). In The Most Important Invasive Plants in Hungary; Botta-Dukát, Z., Balogh, L., Eds.; Institute of Ecology and Botany Hungarian Academy of Sciences: Vácrátót, Hungary, 2008; pp. 103–114. [Google Scholar]
- Parthenocissus quinquefolia (L.) Planch. Available online: https://neobiota.lu/parthenocissus-quinquefolia/ (accessed on 11 October 2019).
- Mooney, H.A.; Ehleringer, J.; Björkman, O. The energy balance of leaves of the evergreen desert shrub Atriplex hymenelytra. Oecologia 1977, 29, 301–310. [Google Scholar] [CrossRef]
- Klančnik, K.; Mlinar, M.; Gaberščik, A. Heterophylly results in a variety of “spectral signatures” in aquatic plant species. Aquat. Bot. 2012, 98, 20–26. [Google Scholar] [CrossRef]
- Carter, G.A.; Teramura, A.H. Vine photosynthesis and relationships to climbing mechanics in a forest understory. Am. J. Bot. 1988, 75, 1011–1018. [Google Scholar] [CrossRef]
- Végh, B.; Schmidt, G.; Diószegi, M. Characteristics of invasive taxa of Parthenocissus in the Buda arboretum, Hungary. Sci. Pap. Ser. B Hortic. 2015, 59, 427–434. [Google Scholar]
- Wraber, T. 2. Salix L.–vrba. In Mala Flora Slovenije: Ključ za Določanje Praprotnic in Semenk, 3rd ed.; Martinčič, A., Wraber, T., Jogan, N., Ravnik, V., Podobnik, A., Turk, B., Vreš, B., Eds.; Tehniška založba Slovenije: Ljubljana, Slovenia, 1999; pp. 396–400. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of photosynthetic tissues: Chlorophylls and carotenoids. Curr. Protocol. Food Anal. Chem. 2001, 1, 165–170. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protocol. Food Anal. Chem. 2001, 1, 171–178. [Google Scholar] [CrossRef]
- Drumm, H.; Mohr, H. The mode of interaction between blue (UV) light photoreceptor and phytochrome in anthocyanin formation of the Sorghum seedling. Photochem. Photobiol. 1978, 27, 241–248. [Google Scholar] [CrossRef]
- Caldwell, M.M. Solar ultraviolet radiation as an ecological factor for alpine plants. Ecol. Monogr. 1968, 38, 243–268. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Fierke, M.K.; Kauffman, J.B. Invasive species influence riparian plant diversity along a successional gradient, Willamette River, Oregon. Nat. Areas J. 2006, 26, 376–382. [Google Scholar] [CrossRef]
- Vitousek, P.M. Biological invasions and ecosystem processes: Towards an integration of population biology and ecosystem studies. Oikos 1990, 57, 7–13. [Google Scholar] [CrossRef]
- Braatne, J.H.; Sullivan, S.M.P.; Chamberlain, E. Leaf decomposition and stream macroinvertebrate colonisation of Japanese knotweed, an invasive plant species. Int. Rev. Hydrobiol. 2007, 92, 656–665. [Google Scholar] [CrossRef]
- McNeish, R.E.; Benbow, M.E.; McEwan, R.W. Removal of the invasive shrub, Lonicera maackii (Amur honeysuckle), from a headwater stream riparian zone shifts taxonomic and functional composition of the aquatic biota. Invasive Plant Sci. Manag. 2017, 10, 232–246. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Mendez-Alonzo, R.; Ewers, F.W.; Sack, L. Ecological variation in leaf biomechanics and its scaling with tissue structure across three mediterranean-climate plant communities. Funct. Ecol. 2013, 27, 544–554. [Google Scholar] [CrossRef]
- Gaberščik, A.; Vončina, M.; Trošt, T.; Germ, M.; Björn, L.O. Growth and production of buckwheat (Fagopyrum esculentum) treated with reduced, ambient, and enhanced UV-B radiation. J. Photochem. Photobiol. B 2002, 66, 30–36. [Google Scholar] [CrossRef]
- Rozema, J.; Björn, L.O.; Bornman, J.F.; Gaberščik, A.; Hader, D.P.; Trošt, T.; Germ, M.; Klisch, M.; Gröniger, A.; Sinha, R.P.; et al. The role of UV-B radiation in aquatic and terrestrial ecosystems—an experimental and functional analysis of the evolution of UV-absorbing compounds. J. Photochem. Photobiol. B 2002, 66, 2–12. [Google Scholar] [CrossRef]
- Kuiters, A.T. Role of phenolic substances from decomposing forest litter in plant–soil interactions. Acta Bot. Neerl. 1990, 39, 329–348. [Google Scholar] [CrossRef]
- Züst, T.; Joseph, B.; Shimizu, K.K.; Kliebenstein, D.J.; Turnbull, L.A. Using knockout mutants to reveal the growth costs of defensive traits. Proc. R. Soc. B 2011, 278, 2598–2603. [Google Scholar] [CrossRef]
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More plant growth but less plant defence? First global gene expression data for plants grown in soil amended with biochar. GCB Bioenergy 2014, 7, 658–672. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Canhoto, C. Leaf litter processing in low order streams. Limnetica 2006, 25, 1–10. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Javelle, M.; Vernoud, V.; Rogowsky, P.M.; Ingram, G.C. Epidermis: The formation and functions of a fundamental plant tissue. New Phytol. 2011, 189, 17–39. [Google Scholar] [CrossRef]
- Hladyz, S.; Åbjörnsson, K.; Giller, P.S.; Woodward, G. Impacts of an aggressive riparian invader on community structure and ecosystem functioning in stream food webs. J. Appl. Ecol. 2011, 48, 443–452. [Google Scholar] [CrossRef]
- Quist, C.W.; Vervoort, M.T.W.; van Megen, H.H.B.; Gort, G.; Bakker, J.; van der Putten, W.H.; Helder, J. Selective alteration of soil food web components by invasive Giant goldenrod (Solidago gigantea) in two distinct habitat types. Oikos 2014, 123, 837–845. [Google Scholar] [CrossRef]
- Seeney, A.; Eastwood, S.; Pattison, Z.; Willby, N.J.; Bull, C.D. All change at the water’s edge: Invasion by non-native riparian plants negatively impacts terrestrial invertebrates. Biol. Invasions 2019, 21, 1933–1946. [Google Scholar] [CrossRef]
- Friberg, N. Impacts and indicators of change in lotic ecosystems. Wiley Interdiscip. Rev. Water 2014, 1, 513–531. [Google Scholar] [CrossRef]
- Robertson, D.J.; Coll, M. Effects of riparian invasive nonindigenous plants on freshwater quantity and ecological functioning in mesic temperate landscapes. Nat. Areas J. 2019, 39, 22–32. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Roelofsen, H.D.; van Bodegom, P.M.; Kooistra, L.; Witte, J.-P.M. Predicting leaf traits of herbaceous species from their spectral characteristics. Ecol. Evol. 2014, 4, 706–719. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Asner, G.P. Applications of remote sensing to alien invasive plant studies. Sensors 2009, 9, 4869–4889. [Google Scholar] [CrossRef]

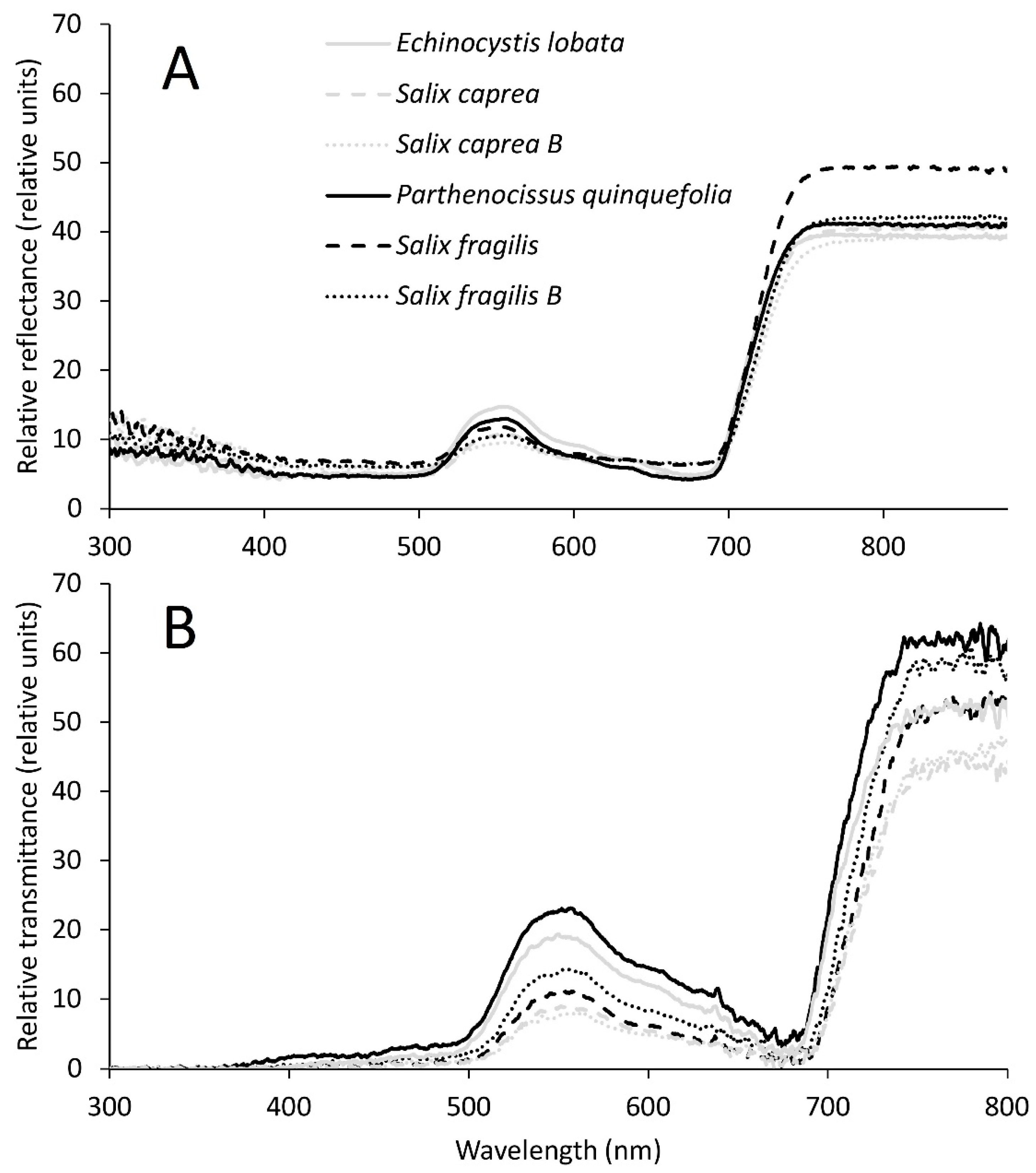

| Parameter | Units | Stand Location | Salix caprea | Salix fragilis | ||
|---|---|---|---|---|---|---|
| Without | Overgrown a | Without | Overgrown b | |||
| Air temperature (ns) | °C | Outside | 30.8 ± 1.4 | 30.5 ± 1.4 | 30.9 ± 1.7 | 30.9 ± 1.6 |
| Above | 28.1 ± 1.6 | 28.9 ± 1.5 | 29.4 ± 1.5 | 27.9 ± 0.7 | ||
| Within | 25.6 ± 1.2 | 25.6 ± 1.0 | 26.4 ± 1.4 | 25.0 ± 1.3 | ||
| Relative air humidity (ns) | % | Outside | 41.4 ± 2.3 | 41.3 ± 3.9 | 44.8 ± 6.4 | 44.5 ± 2.7 |
| Above | 45.6 ± 3.2 | 45.2 ± 5.4 | 48.5 ± 4.5 | 52.0 ± 2.6 | ||
| Within | 50.1 ± 4.6 | 52.8 ± 3.6 | 51.3 ± 3.9 | 54.9 ± 3.6 | ||
| Soil temperature (ns) | °C | Outside | 31.2 ± 2.9 | 32.3 ± 3.4 | 27.3 ± 3.3 | 24.9 ± 1.5 |
| Within | 22.2 ± 0.8 | 21.9 ± 1.7 | 24.0 ± 1.4 | 23.2 ± 0.5 | ||
| Soil humidity (ns) | % | Outside | 18.7 ± 6.3 | 23.0 ± 3.8 | 24.0 ± 7.4 | 21.1 ± 4.8 |
| Within | 19.7 ± 7.0 | 21.6 ± 4.4 | 23.1 ± 4.6 | 21.6 ± 7.6 | ||
| Leaf Traits | Units | Echinocystis lobata | Salix caprea | Parthenocissus quinquefolia | Salix fragilis | ||
|---|---|---|---|---|---|---|---|
| Without | Overgrown # | Without | Overgrown § | ||||
| Biochemical | |||||||
| Chlorophyll a | mg/cm2 | 0.025 ± 0.004 a | 0.035 ± 0.004 c | 0.046 ± 0.006 b | 0.019 ± 0.0048 a | 0.044 ± 0.0051 b | 0.035 ± 0.004 c |
| Chlorophyll b | mg/cm2 | 0.022 ± 0.006 c | 0.028 ± 0.006 ac | 0.036 ± 0.005 b | 0.019 ± 0.005 ac | 0.040 ± 0.008 b | 0.022 ± 0.005 ac |
| Carotenoids | mg/cm2 | 0.006 ± 0.001 b | 0.008 ± 0.001 a | 0.011 ± 0.002 c | 0.003 ± 0.001 d | 0.001 ± 0.000 e | 0.008 ± 0.001 a |
| Anthocyanins | RU/cm2 | 0.65 ± 0.17 b | 1.32 ± 0.44 acd | 1.90 ± 0.12 ac | 0.99 ± 0.53 abc | 0.97 ± 0.25 bc | 1.57 ± 0.14 d |
| UVB–AS | RU/cm2 | 1.88 ± 0.38 b | 3.86 ± 0.87 a | 5.18 ± 0.65 a | 0.95 ± 0.29 c | 4.27 ± 0.80 a | 4.45 ± 1.22 a |
| UVA–AS | RU/cm2 | 2.94 ± 0.56 d | 7.94 ± 1.34 a | 6.15 ± 1.13 ab | 1.18 ± 0.23 d | 4.31 ± 0.72 c | 5.14 ± 0.97 bc |
| Morphological | |||||||
| Specific leaf area | cm²/mg | 0.44 ± 0.07 a | 0.14 ± 0.02 bc | 0.13 ± 0.02 b | 0.55 ± 0.11 a | 0.15 ± 0.02 b | 0.18 ± 0.04 c |
| Upper cuticle | µm | 4.4 ± 1.1 ab | 4.5 ± 1.0 ab | 1.5 ± 0.2 c | 8.7 ± 1.6 d | 5.8 ± 1.4 a | 3.6 ± 0.3 b |
| Upper epidermis | µm | 14.0 ± 2.6 a | 15.4 ± 4.2 ab | 8.3 ± 1.0 c | 15.5 ± 3.7 b | 12.0 ± 2.8 ab | 14.8 ± 0.9 ab |
| Lower cuticle | µm | 3.6 ± 1.3 ac | 4.3 ± 1.0 a | 1.2 ± 0.2 d | 6.3 ± 1.0 b | 4.7 ± 1.4 ab | 2.2 ± 0.1 a |
| Lower epidermis | µm | 8.7 ± 2.8 a | 11.2 ± 2.6 ab | 9.1 ± 1.1 a | 16.7 ± 3.6 b | 8.2 ± 2.7 a | 12.6 ± 1.8 b |
| Leaf thickness | µm | 116.3 ± 14.7 a | 136.7 ± 14.9 ab | 124.6 ± 17.0 a | 237.9 ± 26.5 c | 141.3 ± 15.6 b | 156.1 ± 14.9 b |
| Leaf tissue density | mg/cm3 | 203 ± 39 b | 537 ± 110 d | 618 ± 99 d | 80 ± 15 a | 511 ± 163 d | 358 ± 72 c |
| Reflectance | % | ||||||
| UV-B | 8.39 ± 1.21 a | 11.10 ± 1.12 c | 12.70 ± 1.69 b | 9.23 ± 0.81 a | 13.20 ± 0.52 b | 10.55 ± 0.44 c | |
| UV-A | 7.17 ± 1.06 c | 9.22 ± 0.99 a | 9.78 ± 1.04 a | 8.12 ± 0.81 b | 9.54 ± 0.37 a | 8.43 ± 0.21 b | |
| Violet | 4.87 ± 0.57 a | 4.84 ± 0.37 a | 6.03 ± 0.50 b | 4.83 ± 0.35 a | 7.08 ± 0.22 b | 6.38 ± 0.11 b | |
| Blue | 5.06 ± 0.50 a | 4.65 ± 0.33 b | 5.91 ± 0.48 c | 4.67 ± 0.36 ab | 6.70 ± 0.23 d | 6.10 ± 0.14 c | |
| Green | 11.45 ± 1.34 d | 8.54 ± 0.86 ab | 8.21 ± 0.72 a | 10.06 ± 1.49 bc | 9.82 ± 1.01 bc | 8.90 ± 0.52 abc | |
| Yellow | 9.11 ± 1.24 a | 7.08 ± 0.98 b | 7.47 ± 0.58 b | 7.52 ± 1.13 b | 8.08 ± 0.74 ab | 7.65 ± 0.48 b | |
| Red | 6.27 ± 0.65 c | 5.37 ± 0.58 a | 6.92 ± 0.51 b | 5.25 ± 0.56 a | 6.88 ± 0.32 b | 6.88 ± 0.30 b | |
| Near infrared | 34.61 ± 1.79 a | 33.80 ± 1.84 a | 35.46 ± 4.40 bc | 35.31 ± 1.35 ab | 44.60 ± 1.60 d | 38.23 ± 2.07 c | |
| Transmittance | % | ||||||
| UV-B | 0.02 ± 0.08 a | -0.08 ± 0.13 a | 0.14 ± 0.14 a | 0.04 ± 0.11 a | -0.10 ± 0.10 a | 0.13 ± 0.10 a | |
| UV-A | -0.07 ± 0.07 a | -0.08 ± 0.09 a | 0.12 ± 0.30 a | -0.04 ± 0.10 a | -0.16 ± 0.12 a | 0.13 ± 0.10 a | |
| Violet | 0.91 ± 0.54 acd | 0.19 ± 0.18 b | 0.52 ± 0.89 abc | 1.99 ± 1.16 d | 0.29 ± 0.22 ab | 0.86 ± 0.36 cd | |
| Blue | 2.02 ± 1.07 bc | 0.72 ± 0.39 a | 0.94 ± 1.27 ac | 3.38 ± 1.62 b | 0.92 ± 0.45 ac | 1.71 ± 0.54 bc | |
| Green | 14.35 ± 1.96 c | 6.34 ± 1.02 a | 5.70 ± 1.76 a | 17.59 ± 4.02 d | 7.91 ± 1.65 ab | 10.40 ± 1.30 b | |
| Yellow | 12.28 ± 2.08 a | 5.36 ± 0.92 b | 4.90 ± 2.18 b | 14.54 ± 3.75 a | 6.04 ± 1.49 bc | 8.36 ± 1.23 c | |
| Red | 6.20 ± 1.70 ab | 2.56 ± 0.73 c | 3.14 ± 2.39 c | 8.37 ± 2.68 a | 2.84 ± 1.03 c | 4.57 ± 0.86 bc | |
| Near infrared | 45.47 ± 3.07 ab | 36.10 ± 3.44 a | 42.32 ± 4.89 a | 54.60 ± 3.75 c | 43.14 ± 2.35 ab | 53.94 ± 1.91 c | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grašič, M.; Piberčnik, M.; Zelnik, I.; Abram, D.; Gaberščik, A. Invasive Alien Vines Affect Leaf Traits of Riparian Woody Vegetation. Water 2019, 11, 2395. https://doi.org/10.3390/w11112395
Grašič M, Piberčnik M, Zelnik I, Abram D, Gaberščik A. Invasive Alien Vines Affect Leaf Traits of Riparian Woody Vegetation. Water. 2019; 11(11):2395. https://doi.org/10.3390/w11112395
Chicago/Turabian StyleGrašič, Mateja, Mateja Piberčnik, Igor Zelnik, Dragan Abram, and Alenka Gaberščik. 2019. "Invasive Alien Vines Affect Leaf Traits of Riparian Woody Vegetation" Water 11, no. 11: 2395. https://doi.org/10.3390/w11112395
APA StyleGrašič, M., Piberčnik, M., Zelnik, I., Abram, D., & Gaberščik, A. (2019). Invasive Alien Vines Affect Leaf Traits of Riparian Woody Vegetation. Water, 11(11), 2395. https://doi.org/10.3390/w11112395






