Progress Towards Bioelectrochemical Remediation of Hexavalent Chromium
Abstract
1. Introduction
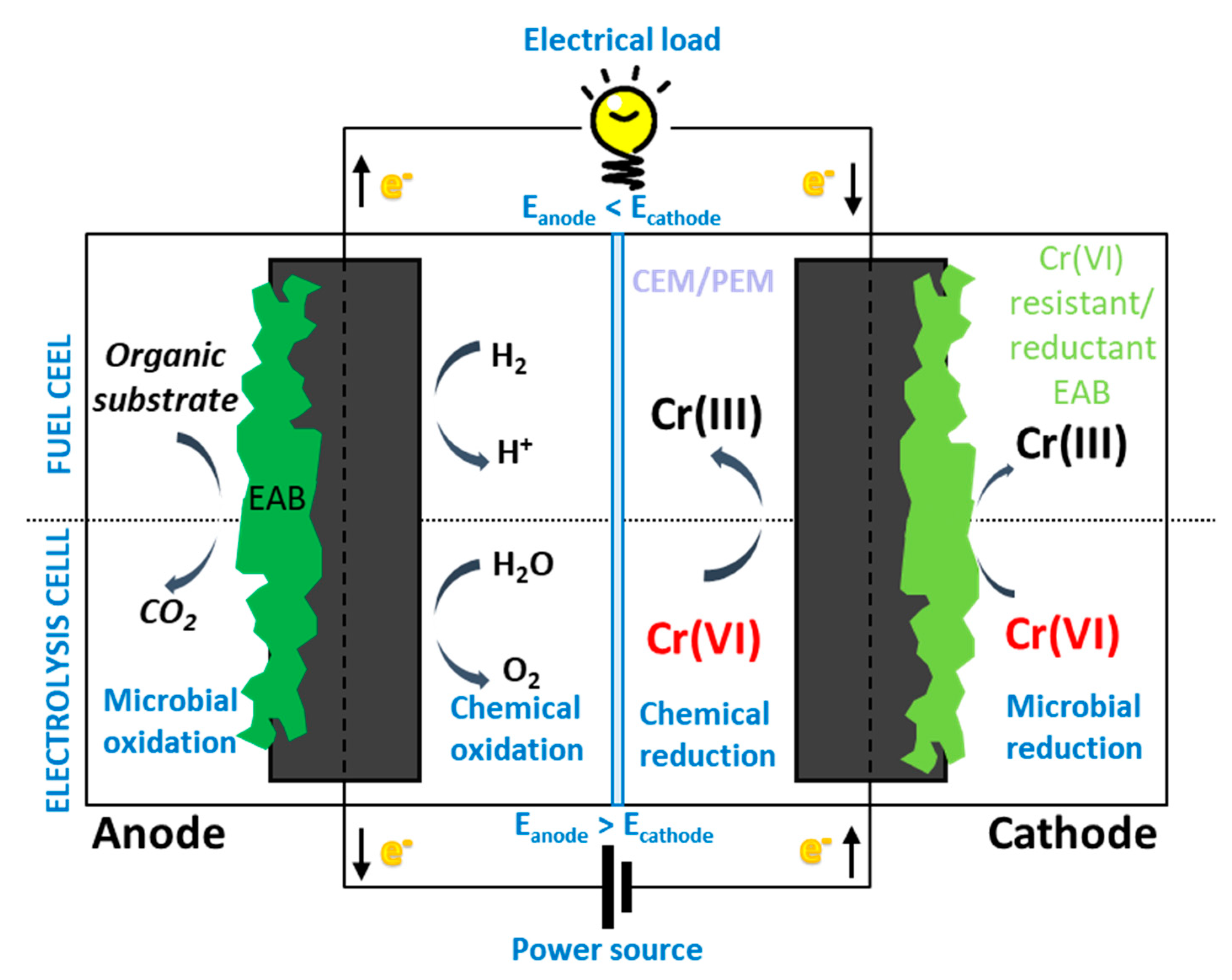
2. Principles of Cr(VI) Reduction in Bio-Electrochemical Systems
2.1. Electrochemical Reduction of Cr(VI)
2.2. Microbiological Mechanisms of Cr(VI) Reduction
3. Cr(VI) Biocathodic Reduction
3.1. Effects of pH and Cr(VI) Concentration
3.2. Effects of Cathode Potential
3.3. Effects of Materials, Reactor Design, and Other Operational Parameters
3.4. Simultaneous Reduction of Cr(VI) and Other Metals
4. Cr(VI) Reduction at Bioanode
5. Cr(VI) Bio-Electrochemical Remediation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Humphries, A.C.; Nott, K.P.; Hall, L.D.; Macaskie, L.E. Reduction of Cr(VI) by immobilized cells of desulfovibrio vulgaris NCIMB 8303 and Microbacterium sp. NCIMB 13776. Biotechnol. Bioeng. 2005, 90, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Marsh, T.L.; McInerney, M.J. Relationship of Hydrogen Bioavailability to Chromate Reduction in Aquifer Sediments. Appl. Environ. Microbiol. 2001, 67, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.D.; Puls, R.W. Natural Attenuation of Hexavalent Chromium in Ground Water and Soils; EPA/540/S-94/505; U.S. Environmental Protection Agency Ground Water Issue; Superfund Technology Support Center for Ground Water—Robert S. Kerr Environmental Research Laboratory: Ada, OK, USA, 1994; pp. 1–13. [Google Scholar]
- Rai, D.; Eary, L.E.; Zachara, J.M. Environmental chemistry of chromium. Sci. Total Environ. 1989, 86, 15–23. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised Pourbaix diagrams for Chromium at 25–300 °C. Corros. Sci. 1997, 39, 43–57. [Google Scholar] [CrossRef]
- Zayed, A.M.; Terry, N. Chromium in the environment: Factors affecting biological remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Chirwa, E.M.; Molokwane, P.P.P. Biological Cr(VI) reduction: Microbial diversity, kinetics and biotechnological solutions to pollution. In Biodiversity; Sofo, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 75–100. [Google Scholar]
- Jadhav, U.U.; Hocheng, H. A review of recovery of metals from industrial waste. J. Achiev. Mater. Manuf. Eng. 2012, 54, 159–167. [Google Scholar]
- Independent Environmental Technical Evaluation Group. Chromium (VI) Handbook; Guertin, J., Jacobs, J.A., Avakian, C.P., Eds.; CRC Press: New York, NY, USA, 2005. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on the risks to public health related to the presence of chromium in food and drinking water. EFSA J. 2014, 12, 3595. [Google Scholar]
- Malaviya, P.; Singh, A. Physicochemical Technologies for Remediation of Chromium-Containing Waters and Wastewaters. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1111–1172. [Google Scholar] [CrossRef]
- Jin, W.; Du, H.; Zheng, S.; Zhang, Y. Electrochemical processes for the environmental remediation of toxic Cr(VI): A review. Electrochim. Acta 2015, 191, 1044–1055. [Google Scholar] [CrossRef]
- Brungesh, K.; Nagabhushana, B.; Raveendra, R.; Krishna, K.; Prashantha, P.; Nagabhushana, H. Adsorption of Cr(VI) from Aqueous Solution onto a Mesoporous Carbonaceous Material Prepared from Naturally Occurring Pongamia pinnata Seeds. Environ. Anal. Toxicol. 2015, 5, 1. [Google Scholar]
- Caputo, D.; De Gennaro, B.; Pansini, M.; Colella, C. Chromium removal from water by ion exchange using zeolites and solidification of the resulting sludge in a cement matrix. In Studies in Surface Science and Catalysis; Kiricsi, I., Nagy, J.B., Karge, H.G., Palyi, G., Eds.; Elsevier Science Publisher B.V.: Amsterdam, the Netherlands, 1999; Volume 125, pp. 723–730. [Google Scholar]
- Rajeswari, V.; Janaki, V.; Shanthi, K.; Kamala-Kannan, S. Adsorption and subsequent detoxification of hexavalent chromium in aqueous solution using polypyrrole-bacterial extracellular polysaccharide nanocomposite. Environ. Prog. Sustain. Energy 2016, 35, 1293–1297. [Google Scholar] [CrossRef]
- Jeyasingh, J.; Philip, L. Bioremediation of chromium contaminated soil: Optimization of operating parameters under laboratory conditions. J. Hazard. Mater. 2005, 118, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.D.; Wittbrodt, P.R. Processes affecting the remediation of chromium-contaminated sites. Environ. Health Perspect. 1991, 92, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.K.; Bielefeldt, A.R. Low-Temperature Chromium(VI) Biotransformation in Soil with Varying Electron Acceptors. J. Environ. Qual. 2002, 31, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Turick, C.E.; Graves, C.; Apel, W.A. Bioremediation potential of Cr(VI)-contaminated soil using indigenous microorganisms. Bioremediat. J. 1998, 2, 1–6. [Google Scholar] [CrossRef]
- Pous, N.; Balaguer, M.D.; Colprim, J.; Puig, S. Opportunities for groundwater microbial electro-remediation. Microb. Biotechnol. 2018, 11, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Brodie, E.L.; Joyner, D.C.; Faybishenko, B.; Conrad, M.E.; Rios-Velazquez, C.; Malave, J.; Martinez, R.; Mork, B.; Willett, A.; Koenigsberg, S.; et al. Microbial community response to addition of polylactate compounds to stimulate hexavalent chromium reduction in groundwater. Chemosphere 2011, 85, 660–665. [Google Scholar] [CrossRef]
- Somenahally, A.C.; Mosher, J.J.; Yuan, T.; Podar, M.; Phelps, T.J.; Brown, S.D.; Yang, Z.K.; Hazen, T.C.; Arkin, A.P.; Palumbo, A.V.; et al. Hexavalent chromium reduction under fermentative conditions with lactate stimulated native microbial communities. PLoS ONE 2013, 8, e83909. [Google Scholar] [CrossRef]
- Deng, B.; Stone, A.T. Surface-Catalyzed Chromium(VI) Reduction: The TiO2−CrVI−Mandelic Acid System. Environ. Sci. Technol. 1996, 30, 463–472. [Google Scholar] [CrossRef]
- Beretta, G.; Mastorgio, A.F.; Pedrali, L.; Saponaro, S.; Sezenna, E. Support tool for identifying in situ remediation technology for sites contaminated by hexavalent chromium. Water 2018, 10, 1344. [Google Scholar] [CrossRef]
- Elangovan, R.; Philip, L.; Chandraraj, K. Hexavalent chromium reduction by free and immobilized cell-free extract of arthrobacter rhombi-RE. Appl. Biochem. Biotechnol. 2010, 160, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, M.; Wang, G. Removal of toxic chromate using free and immobilized Cr(VI)-reducing bacterial cells of intrasporangium sp. Q5-1. World J. Microbiol. Biotechnol. 2009, 25, 1579–1587. [Google Scholar] [CrossRef]
- Liu, H.; Guo, L.; Liao, S.; Wang, G. Reutilization of immobilized fungus Rhizopus sp. LG04 to reduce toxic chromate. J. Appl. Microbiol. 2012, 112, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.C.; Chen, L.; Hansel, C.M.; Krumholz, L.R.; Elwood Madden, A.S.; Lan, Y.; Madden, A.S.E.; Lan, Y. Biological versus mineralogical chromium reduction: Potential for reoxidation by manganese oxide. Environ. Sci. Process. Impacts 2015, 17, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Varadharajan, C.; Beller, H.R.; Bill, M.; Brodie, E.L.; Conrad, M.E.; Han, R.; Irwin, C.; Larsen, J.T.; Lim, H.C.; Molins, S.; et al. Reoxidation of Chromium(III) Products Formed under Different Biogeochemical Regimes. Environ. Sci. Technol. 2017, 51, 4918–4927. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microb. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Rabaey, K.; Angenent, L.T.T.; Schröder, U.; Keller, J. Bioelectrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application; IWA Publishing: London, UK, 2009. [Google Scholar]
- Kokabian, B.; Gude, V.G. Role of membranes in bioelectrochemical systems. Membr. Water Treat. 2015, 6, 53–75. [Google Scholar] [CrossRef]
- Gude, V.G.; Kokabian, B.; Gadhamshetty, V.G. Beneficial Bioelectrochemical Systems for Energy, Water, and Biomass Production. J. Microb. Biochem. Technol. 2013, S6:005, 1–14. [Google Scholar]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Singh, A.; Van Bogaert, G.; Irving Olsen, S.; Singh Nigam, P.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Li, X.; Abu-Reesh, I.; He, Z. Development of Bioelectrochemical Systems to Promote Sustainable Agriculture. Agriculture 2015, 5, 367–388. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N.L. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2011, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, H.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015, 33, 317–334. [Google Scholar] [CrossRef]
- Palma, E.; Daghio, M.; Franzetti, A.; Petrangeli Papini, M.; Aulenta, F. The bioelectric well: A novel approach for in situ treatment of hydrocarbon-contaminated groundwater. Microb. Biotechnol. 2018, 11, 112–118. [Google Scholar] [CrossRef]
- Daghio, M.; Aulenta, F.; Vaiopoulou, E.; Franzetti, A.; Arends, J.B.A.; Sherry, A.; Suárez-Suárez, A.; Head, I.M.; Bestetti, G.; Rabaey, K. Electrobioremediation of oil spills. Water Res. 2017, 114, 351–370. [Google Scholar] [CrossRef]
- Zhang, T.; Gannon, S.M.; Nevin, K.P.; Franks, A.E.; Lovley, D.R. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ. Microbiol. 2010, 12, 1011–1020. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Park, Y.; Yu, J.; Lee, T. Simultaneous arsenite oxidation and nitrate reduction at the electrodes of bioelectrochemical systems. Environ. Sci. Pollut. Res. 2016, 23, 19978–19988. [Google Scholar] [CrossRef]
- Lai, A.; Aulenta, F.; Mingazzini, M.; Palumbo, M.T.; Papini, M.P.; Verdini, R.; Majone, M. Bioelectrochemical approach for reductive and oxidative dechlorination of chlorinated aliphatic hydrocarbons (CAHs). Chemosphere 2017, 169, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Leitão, P.; Nouws, H.; Danko, A.S.; Aulenta, F. Bioelectrochemical Dechlorination of 1,2-DCA with an AQDS-Functionalized Cathode Serving as Electron Donor. Fuel Cells 2017, 17, 612–617. [Google Scholar] [CrossRef]
- Cecconet, D.; Devecseri, M.; Callegari, A.; Capodaglio, A.G. Effects of process operating conditions on the autotrophic denitrification of nitrate-contaminated groundwater using bioelectrochemical systems. Sci. Total Environ. 2018, 613, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.B.; Lovley, D.R. Remediation and Recovery of Uranium from Contaminated Subsurface Environments with Electrodes. Environ. Sci. Technol. 2005, 39, 8943–8947. [Google Scholar] [CrossRef]
- Harnisch, F.; Aulenta, F.; Schröder, U. Microbial Fuel Cells and Bioelectrochemical Systems: Industrial and Environmental Biotechnologies Based on Extracellular Electron Transfer. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, NJ, USA, 2011; Volume 6, pp. 643–659. [Google Scholar]
- Wang, H.; Ren, J.; Ren, Z.J.; Ren, J.; Ren, Z.J. Bioelectrochemical metal recovery from wastewater: A review. Water Res. 2014, 66, 219–232. [Google Scholar] [CrossRef]
- Wu, Y.; Qi, H.; Gao, Y.; Wang, L. Heavy Metal Sensor Research Based on Microbial Fuel Cell. Int. J. Environ. Monit. Anal. 2018, 6, 53–64. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, P.; Niu, Y.; Chen, Z.; Khan, A.; Zhang, P.; Li, X. A Novel Early Warning System Based on a Sediment Microbial Fuel Cell for In Situ and Real Time Hexavalent Chromium Detection in Industrial Wastewater. Sensors 2018, 18, 642. [Google Scholar] [CrossRef]
- Zhou, T.; Han, H.; Liu, P.; Xiong, J.; Tian, F.; Li, X. Microbial fuels cell-based biosensor for toxicity detection: A review. Sensors 2017, 17, 2230. [Google Scholar] [CrossRef]
- Williams, K.H.; Nevin, K.P.; Franks, A.; Englert, A.; Long, P.E.; Lovley, D.R. Electrode-based approach for monitoring in situ microbial activity during subsurface bioremediation. Environ. Sci. Technol. 2010, 44, 47–54. [Google Scholar] [CrossRef]
- Sophia, A.C.; Saikant, S. Reduction of chromium(VI) with energy recovery using microbial fuel cell technology. J. Water Process Eng. 2016, 11, 39–45. [Google Scholar] [CrossRef]
- Yeon, R.E.; Kim, M.; Lee, S.J. Characterization of microbial fuel cells enriched using Cr(VI)-containing sludge. J. Microbiol. Biotechnol. 2011, 21, 187–191. [Google Scholar]
- Clauwaert, P.; Aelterman, P.; Pham, T.H.; De Schamphelaire, L.; Carballa, M.; Rabaey, K.; Verstraete, W. Minimizing losses in bio-electrochemical systems: The road to applications. Appl. Microbiol. Biotechnol. 2008, 79, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, L.; Zhang, Y. Cathodic reduction of hexavalent chromium [Cr(VI)] coupled with electricity generation in microbial fuel cells. Biotechnol. Lett. 2008, 30, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.C.; Bourg, A.C.M. Aqueous geochemistry of chromium: A review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- Sophia, A.C.; Sai, S. Modified microbial fuel cell for Cr(VI) reduction and simultaneous bio-electricity production. J. Environ. Chem. Eng. 2016, 4, 2402–2409. [Google Scholar] [CrossRef]
- Molokwane, P.E.; Meli, K.C.; Nkhalambayausi-Chirwa, E.M. Chromium (VI) reduction in activated sludge bacteria exposed to high chromium loading: Brits culture (South Africa). Water Res. 2008, 42, 4538–4548. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Xu, Y.; Liu, Z.; Ma, F.; Zhi, L.; Zhou, X. Simultaneous Cr(VI) reduction and bioelectricity generation in a dual chamber microbial fuel cell. Chem. Eng. J. 2018, 334, 1621–1629. [Google Scholar] [CrossRef]
- Huang, L.; Chai, X.; Chen, G.; Logan, B.E. Effect of set potential on hexavalent chromium reduction and electricity generation from biocathode microbial fuel cells. Environ. Sci. Technol. 2011, 45, 5025–5031. [Google Scholar] [CrossRef]
- Huang, L.; Chai, X.; Cheng, S.; Chen, G. Evaluation of carbon-based materials in tubular biocathode microbial fuel cells in terms of hexavalent chromium reduction and electricity generation. Chem. Eng. J. 2011, 166, 652–661. [Google Scholar] [CrossRef]
- Thatoi, H.; Das, S.; Mishra, J.; Rath, B.P.; Das, N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review. J. Environ. Manag. 2014, 146, 383–399. [Google Scholar] [CrossRef]
- Viti, C.; Marchi, E.; Decorosi, F.; Giovannetti, L. Molecular mechanisms of Cr(VI) resistance in bacteria and fungi. FEMS Microbiol. Rev. 2014, 38, 633–659. [Google Scholar] [CrossRef] [PubMed]
- Horitsu, H.; Futo, S.; Miyazawa, Y.; Ogai, S.; Kawai, K. Enzymatic reduction of hexavalent chromium by hexavalent chromium tolerant Pseudomonas ambigua G-1. Agric. Biol. Chem. 1987, 51, 2417–2420. [Google Scholar] [CrossRef]
- Ackerley, D.F.; Gonzalez, C.F.; Park, C.H.; Ii, R.B.; Matin, A.; Keyhan, M. Chromate-Reducing Properties of Soluble Flavoproteins from Pseudomonas putida and Escherichia coli. Am. Soc. Microbiol. 2004, 70, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.H.; Gu, J.D. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review. Int. Biodeterior. Biodegrad. 2007, 59, 8–15. [Google Scholar] [CrossRef]
- Camargo, F.A.O.; Bento, F.M.; Okeke, B.C.; Frankenberger, W.T. Hexavalent chromium reduction by an actinomycete, Arthrobacter crystallopoietes ES 32. Biol. Trace Elem. Res. 2004, 97, 183–194. [Google Scholar] [CrossRef]
- Tahri Joutey, N.; Bahafid, W.; Sayel, H.; Ananou, S.; El Ghachtouli, N. Hexavalent chromium removal by a novel Serratia proteamaculans isolated from the bank of Sebou River (Morocco). Environ. Sci. Pollut. Res. 2014, 21, 3060–3072. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Cervantes, C.; Silver, S. Chromium reduction in Pseudomonas putida. Appl. Environ. Microbiol. 1990, 56, 2268–2270. [Google Scholar]
- Mohapatra, R.K.; Parhi, P.K.; Thatoi, H.; Panda, C.R. Bioreduction of hexavalent chromium by Exiguobacterium indicum strain MW1 isolated from marine water of Paradip Port, Odisha, India. Chem. Ecol. 2017, 33, 114–130. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Reduction of Chromate by Desulfovibrio-Vulgaris and Its c3 Cytochrome. Appl. Environ. Microbiol. 1994, 60, 726–728. [Google Scholar]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223, 1–12. [Google Scholar] [CrossRef]
- Wang, P.C.; Mori, T.; Komori, K.; Sasatsu, M.; Toda, K.; Ohtake, H. Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl. Environ. Microbiol. 1989, 55, 1665–1669. [Google Scholar]
- Bencheikh-Latmani, R. Global transcriptional profiling of Shewanella oneidensis MR-1 during Cr (VI) and U (VI) reduction. Appl. Environ. Microbiol. 2005, 71, 7453–7460. [Google Scholar] [CrossRef]
- Belchik, S.M.; Kennedy, D.W.; Dohnalkova, A.C.; Wang, Y.; Sevinc, P.C.; Wu, H.; Lin, Y.; Lu, H.P.; Fredrickson, J.K.; Shi, L. Extracellular reduction of hexavalent chromium by cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2011, 77, 4035–4041. [Google Scholar] [CrossRef]
- Němeček, J.; Pokorný, P.; Lhotský, O.; Knytl, V.; Najmanová, P.; Steinová, J.; Černík, M.; Filipová, A.; Filip, J.; Cajthaml, T. Combined nano-biotechnology for in-situ remediation of mixed contamination of groundwater by hexavalent chromium and chlorinated solvents. Sci. Total Environ. 2016, 563, 822–834. [Google Scholar] [CrossRef]
- Jørgensen, B.B. Mineralization of organic matter in the sea bed—The role of sulphate reduction. Nature 1982, 296, 643–645. [Google Scholar] [CrossRef]
- Inglett, K.S.; Bae, H.S.; Aldrich, H.C.; Hatfield, K.; Ogram, A.V. Clostridium chromiireducens sp. nov., isolated from Cr(VI)-contaminated soil. Int. J. Syst. Evol. Microbiol. 2011, 61, 2626–2631. [Google Scholar] [CrossRef]
- Battaglia-Brunet, F.; Foucher, S.; Denamur, A.; Ignatiadis, I.; Michel, C.; Morin, D. Reduction of chromate by fixed films of sulfate-reducing bacteria using hydrogen as an electron source. J. Ind. Microbiol. Biotechnol. 2002, 28, 154–159. [Google Scholar] [CrossRef]
- Chung, J.; Nerenberg, R.; Rittmann, B.E. Bio-reduction of soluble chromate using a hydrogen-based membrane biofilm reactor. Water Res. 2006, 40, 1634–1642. [Google Scholar] [CrossRef]
- McLean, J.S.; Beveridge, T.J.; Phipps, D. Isolation and characterization of a chromium-reducing bacterium from a chromated copper arsenate-contaminated site. Environ. Microbiol. 2000, 2, 611–619. [Google Scholar] [CrossRef]
- Mclean, J.; Beveridge, T.J. Chromate Reduction by a Pseudomonad Isolated from a Site Contaminated with Chromated Copper Arsenate. Appl. Environ. Microbiol. 2001, 67, 1076–1084. [Google Scholar] [CrossRef]
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial Electron Transport and Energy Conservation—The Foundation for Optimizing Bioelectrochemical Systems. Front. Microbiol. 2015, 6, 575. [Google Scholar] [CrossRef]
- Cordas, C.M.; Guerra, L.T.; Xavier, C.; Moura, J.J.G. Electroactive biofilms of sulphate reducing bacteria. Electrochim. Acta 2008, 54, 29–34. [Google Scholar] [CrossRef]
- Tandukar, M.; Huber, S.J.; Onodera, T.; Pavlostathis, S.G. Biological chromium(VI) reduction in the cathode of a microbial fuel cell. Environ. Sci. Technol. 2009, 43, 8159–8165. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Quan, X.; Yang, F. Enhancement of hexavalent chromium reduction and electricity production from a biocathode microbial fuel cell. Bioprocess Biosyst. Eng. 2010, 33, 937–945. [Google Scholar] [CrossRef]
- Hsu, L.; Masuda, S.A.; Nealson, K.H.; Pirbazari, M. Evaluation of microbial fuel cell Shewanella biocathodes for treatment of chromate contamination. RSC Adv. 2012, 2, 5844–5855. [Google Scholar] [CrossRef]
- Xafenias, N.; Zhang, Y.; Banks, C.J. Enhanced performance of hexavalent chromium reducing cathodes in the presence of Shewanella oneidensis MR-1 and lactate. Environ. Sci. Technol. 2013, 47, 4512–4520. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Puranik, S.; Lei, Y.; Vadas, T.; Li, B. Metals as electron acceptors in single-chamber microbial fuel cells. J. Power Sources 2014, 269, 430–439. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Q.; Jiang, L.; Zhou, P.; Quan, X.; Logan, B.E. Adaptively Evolving Bacterial Communities for Complete and Selective Reduction of Cr(VI), Cu(II), and Cd(II) in Biocathode Bioelectrochemical Systems. Environ. Sci. Technol. 2015, 49, 9914–9924. [Google Scholar] [CrossRef]
- Xafenias, N.; Zhang, Y.; Banks, C.J. Evaluating hexavalent chromium reduction and electricity production in microbial fuel cells with alkaline cathodes. Int. J. Environ. Sci. Technol. 2015, 12, 2435–2446. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, X.; Song, T.; Zhang, L.; Jia, H.; Wei, P. Effect of acclimatization on hexavalent chromium reduction in a biocathode microbial fuel cell. Bioresour. Technol. 2015, 180, 185–191. [Google Scholar] [CrossRef]
- Chen, D.; Wang, H.; Yang, K. Effective biodegradation of nitrate, Cr(VI) and p-fluoronitrobenzene by a novel three dimensional bioelectrochemical system. Bioresour. Technol. 2016, 203, 370–373. [Google Scholar] [CrossRef]
- Wu, X.; Tong, F.; Yong, X.; Zhou, J.; Zhang, L.; Jia, H.; Wei, P. Effect of NaX zeolite-modified graphite felts on hexavalent chromium removal in biocathode microbial fuel cells. J. Hazard. Mater. 2016, 308, 303–311. [Google Scholar] [CrossRef]
- Song, T.; Jin, Y.; Bao, J.; Kang, D.; Xie, J. Graphene/biofilm composites for enhancement of hexavalent chromium reduction and electricity production in a biocathode microbial fuel cell. J. Hazard. Mater. 2016, 317, 73–80. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, P.; Quan, X.; Logan, B.E. Removal of binary Cr(VI) and Cd(II) from the catholyte of MFCs and determining their fate in EAB using fluorescence probes. Bioelectrochemistry 2018, 122, 61–68. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Wang, J.; Song, Q.; Zhang, W.; He, Q.; Song, J.; Ma, F. Comparison of performance and microbial communities in a bioelectrochemical system for simultaneous denitrification and chromium removal: Effects of pH. Process Biochem. 2018, 73, 154–161. [Google Scholar] [CrossRef]
- Wu, X.; Ren, X.; Owens, G.; Brunetti, G.; Zhou, J.; Yong, X.; Wei, P.; Jia, H. A Facultative Electroactive Chromium(VI)-Reducing Bacterium Aerobically Isolated From a Biocathode Microbial Fuel Cell. Front. Microbiol. 2018, 9, 2883. [Google Scholar] [CrossRef]
- Sagar, S.; Dwivedi, A.; Yadav, S.; Tripathi, M.; Kaistha, S.D. Hexavalent chromium reduction and plant growth promotion by Staphylococcus arlettae Strain Cr11. Chemosphere 2012, 86, 847–852. [Google Scholar] [CrossRef]
- Jung, R.K.; Cheng, S.; Oh, S.E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar]
- Malaviya, P.; Singh, A. Bioremediation of chromium solutions and chromium containing wastewaters. Crit. Rev. Microbiol. 2016, 42, 607–633. [Google Scholar] [CrossRef]
- Clauwaert, P.; Desloover, J.; Shea, C.; Nerenberg, R.; Boon, N.; Verstraete, W. Enhanced nitrogen removal in bio-electrochemical systems by pH control. Biotechnol. Lett. 2009, 31, 1537–1543. [Google Scholar] [CrossRef]
- Liang, P.; Fan, M.; Cao, X.; Huang, X. Evaluation of applied cathode potential to enhance biocathode in microbial fuel cells. J. Chem. Technol. Biotechnol. 2009, 84, 794–799. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Xiao, C. Focus on factors affecting pH, flow of Cr and transformation between Cr(VI) and Cr(III) in the soil with different electrolytes. Electrochim. Acta 2016, 211, 652–662. [Google Scholar] [CrossRef]
- Chai, L.; Huang, S.; Yang, Z.; Peng, B.; Huang, Y.; Chen, Y. Cr(VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag. J. Hazard. Mater. 2009, 167, 516–522. [Google Scholar] [CrossRef]
- Kavita, B.; Keharia, H. Reduction of hexavalent chromium by Ochrobactrum intermedium BCR400 isolated from a chromium-contaminated soil. 3 Biotech 2012, 2, 79–87. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.; Adholeya, A. Hexavalent chromium reduction in tannery effluent by bacterial species isolated from tannery effluent contaminated soil. J. Environ. Sci. Technol. 2012, 5, 142–154. [Google Scholar] [CrossRef]
- Squillace, P.J.; Scott, J.C.; Moran, M.J.; Nolan, B.T.; Kolpin, D.W. VOCs, pesticides, nitrate, and their mixtures in groundwater used for drinking water in the United States. Environ. Sci. Technol. 2002, 36, 1923–1930. [Google Scholar] [CrossRef]
- Santini, M.; Marzorati, S.; Fest-Santini, S.; Trasatti, S.; Cristiani, P. Carbonate scale deactivating the biocathode in a microbial fuel cell. J. Power Sources 2017, 356, 400–407. [Google Scholar] [CrossRef]
- Duca, M.; Koper, M.T.M. Powering denitrification: The perspectives of electrocatalytic nitrate reduction. Energy Environ. Sci. 2012, 5, 9726. [Google Scholar] [CrossRef]
- Coma, M.; Puig, S.; Pous, N.; Balaguer, M.D.; Colprim, J. Biocatalysed sulphate removal in a BES cathode. Bioresour. Technol. 2013, 130, 218–223. [Google Scholar] [CrossRef]
- Bardiya, N.; Bae, J.H. Dissimilatory perchlorate reduction: A review. Microbiol. Res. 2011, 166, 237–254. [Google Scholar] [CrossRef]
- Daghio, M.; Vaiopoulou, E.; Patil, S.A.; Suárez-Suárez, A.; Head, I.M.; Franzetti, A.; Rabaey, K. Anodes stimulate anaerobic toluene degradation via sulfur cycling in marine sediments. Appl. Environ. Microbiol. 2016, 82, 297–307. [Google Scholar] [CrossRef]
- Zhang, T.; Bain, T.S.; Barlett, M.A.; Dar, S.A.; Snoeyenbos-West, O.L.; Nevin, K.P.; Lovley, D.R. Sulfur oxidation to sulfate coupled with electron transfer to electrodes by Desulfuromonas strain TZ1. Microbiology 2014, 160, 123–129. [Google Scholar] [CrossRef]
- Dutta, P.K.; Keller, J.; Yuan, Z.; Rozendal, R.A.; Rabaey, K. Role of sulfur during acetate oxidation in biological anodes. Environ. Sci. Technol. 2009, 43, 3839–3845. [Google Scholar] [CrossRef]
- Daghio, M.; Espinoza Tofalos, A.; Leoni, B.; Cristiani, P.; Papacchini, M.; Jalilnejad, E.; Bestetti, G.; Franzetti, A. Bioelectrochemical BTEX removal at different voltages: Assessment of the degradation and characterization of the microbial communities. J. Hazard. Mater. 2018, 341, 120–127. [Google Scholar] [CrossRef]
- Wang, C.; Deng, H.; Zhao, F. The Remediation of Chromium (VI)-Contaminated Soils Using Microbial Fuel Cells. Soil Sediment Contam. 2016, 25, 1–12. [Google Scholar] [CrossRef]
- Habibul, N.; Hu, Y.; Wang, Y.K.; Chen, W.; Yu, H.Q.; Sheng, G.P. Bioelectrochemical Chromium(VI) Removal in Plant-Microbial Fuel Cells. Environ. Sci. Technol. 2016, 50, 3882–3889. [Google Scholar] [CrossRef]
- Guan, C.Y.; Hu, A.; Yu, C.P. Stratified chemical and microbial characteristics between anode and cathode after long-term operation of plant microbial fuel cells for remediation of metal contaminated soils. Sci. Total Environ. 2019, 670, 585–594. [Google Scholar] [CrossRef]
| Reaction | Eh0 (V vs. SHE) | Eh’ at pH 7 (V vs. SHE) |
|---|---|---|
| Cr2O72− + 14H+ + 6e− → 2Cr3+ +7H2O | 1.33 | 0.33 |
| HCrO4− + 7H+ + 3e− → Cr3+ + 4H2O | 1.35 | 0.35 |
| HCrO4− + 4H+ + 3e− → Cr(OH)2+ + 3H2O | 1.31 | 0.76 |
| CrO42− + 4H2O + 3e− → Cr(OH)3(s, hydrated) + 5OH− | −0.13 | 0.21 |
| O2 + 4H+ + 4e− → H2O | 1.23 | 0.805 (pO2 = 0.2 bar) |
| NO3− + 6H+ + 5e− → 1/2 N2 + 3H2O | 1.24 | 0.71 (pN2 = 0.8 bar) |
| Fe3+ + e− → Fe2+ | 0.77 | 0.77 |
| SO42− + 10H+ + 8e− → H2S + 4H2O | 0.30 | −0.21 |
| HCO3− + 9H+ + 8e− → CH4(g) + 3H2O | 0.20 | −0.26 |
| 2HCO3− + 9H+ + 8e− → CH3COO− + 4H2O | 0.19 | −0.29 |
| 2H+ + 2e− → H2(g) | 0 | −0.41 (pH2 = 1 bar) |
| MnO2(s) + 4H+ + 2e− → Mn2+ + 2H2O | 1.23 | 0.402 |
| BES Type 1 | Anode Material | Anodic Inoculum/Mediator 2 | Cathode Material | Cathodic Inoculum/Mediator | Cathode Potential (V vs. SHE) | Initial Cr(VI) (mg/L) | Initial pH | Test Period (h) 3 | CrVI Removal (%) | Rate (mgCrVI/L/h) (Specific Rate (mgCrVI/ gVSS /h)) 4 | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2CMFC-PEM, H(b) | graphite plate | anaerobic sludge. ED: acetate | graphite plate | Cr(VI) enriched denitrifying and anaerobic mixed culture | NA | 22 | 7.2–7.6 | 552 | 100 | 0.14 (0.18) | [89] |
| 31 | (0.22) | ||||||||||
| 40 | (0.36) | ||||||||||
| 63 | 0.45 (0.46) | ||||||||||
| 2CMFC, PEM, H(b) | graphite plate | MFC effluent. ED: acetate | graphite plate and granules | Anaerobic mixed culture from contaminated soil | NA | 13 | ≈7 | 7 | 100 | 3.8 (2) | [90] |
| NA | 39 | 5.3 (2.4) | |||||||||
| 2CMFC, CEM, TR(b) | graphite fiber | effluent and acclimated anode from MFC | graphite fiber (C/A = 3) | MFC anaerobic effluent | 0.013 | 20 | 5 | 3 | 98.5 | 6.57 | [65] |
| 0.03 | 8 | 61 | 4.07 | ||||||||
| NA | 40 | 7 | 3.5 | 75 | 8.57 | ||||||
| −0.05 | 50 | 3 | NA | (5.2) | |||||||
| NA | 20 | 5 | 100 | 4.08 (12.4) | |||||||
| graphite fiber (C/A = 10) | 4 | 5.12 (15.5) | |||||||||
| graphite fiber (C/A = 20) | 3 | 6.8 (20.6) | |||||||||
| graphite fiber (C/A = 3) | 5 | 90.25 | 3.61 ± 0.1 (11.3 ± 2.2) | ||||||||
| graphite felt (C/A = 3) | 76.75 | 3.07 ± 0.12 (9.5 ± 1) | |||||||||
| graphite granules (C/A = 3) | 86.5 | 3.46 ± 0.09 (5.6 ± 0.6) | |||||||||
| 2CMFC, CEM, TR(b) | graphite brush | anaerobic wastewater ED: acetate | graphite granules | WWTP primary clarifier effluent | NA | 20 | 7 | 24 | 70 | 0.6 | [64] |
| 2CBES (poised cathode), CEM, TR(b) | graphite brush | graphite granules | −0.45 | 55 | 0.43 | ||||||
| −0.3 | 100 | 0.83 | |||||||||
| −0.15 | 100 | 0.83 | |||||||||
| 0.2 | 43 | 0.36 | |||||||||
| 2CMFC, CEM, H(sb) | reticulated vitreous carbon | Shewanella oneidensis MR-1. ED: lactate | reticulated vitreous carbon | Shewanella oneidensis MR1 | NA | 2.5 (3 cycles) | 7 | 72 (each cycle) | 60–100 | 0.02–0.04 | [91] |
| Shewanella putrefaciens | 92–100 | 0.03–0.04 | |||||||||
| Shewanella amazonensis | 72–100 | 0.025–0.04 | |||||||||
| Shewanella sp ANA3 | 32–100 | 0.01–0.04 | |||||||||
| Shewanella loihica | 44–100 | 0.015–0.04 | |||||||||
| Shewanella sp MR-4 | 20–100 | 0.007–0.04 | |||||||||
| 2CMFC, PEM, H(b) | graphite felt | S.oneidensis MR-1. ED: lactate | graphite felt | Shewanella oneidensis MR-1 | NA | 10 (6 cycles) | 7 | 300 | 90 | 0.1–1.1 | [92] |
| 2CBES (poised cathode), PEM, H(b) | graphite felt | Shewanella oneidensis MR-1. ED: lactate | graphite felt | Shewanella oneidensis MR1 | 10 | 0.5 | |||||
| Shewanella oneidensis MR1; MED: riboflavin | 13 | 0.65 | |||||||||
| Shewanella oneidensis MR1; ED: lactate | −0.3 | 20 | 7 | 4 | 45 | 2.25 | |||||
| SCMFC (b) | carbon brush | municipal wastewater. ED: acetate | carbon cloth with Pt | municipal wastewater; ED: acetate | 2.93 | 1 | 6.5 | 120 | 89 | 0.01 | [93] |
| 3.03 | 3 | 95.7 | 0.02 | ||||||||
| 3.13 | 10 | 98.8 | 0.08 | ||||||||
| 2CMFC, CEM, TR(b) | graphite brush | MFC anodic effluent. ED: acetate | graphite felt | WWTP primary clarifier effluent acclimated to Cr(VI) | −0.074 | 5 | 5.8 | 4 | 100 | 1.21 | [94] |
| NA | 5 (with Cu(II), Cd(II)) | 39 | 0.49 | ||||||||
| WWTP primary clarifier effluent, acclimated to Cr(VI), Cu(II) and Cd(II) | 100 | 1.24 | |||||||||
| 2CMFC, PEM, H(b) | graphite felt | Shewanella oneidensis | graphite felt | Shewanella oneidensis | NA | 10 (8 cycles) | 8 | 840 | 90–100 | 0.9–1.95 | [95] |
| 2CMFC, PEM, C(b) | graphite felt | anaerobic sludge. ED: glucose | graphite felt | mixed culture from MFC anode (ex situ) | NA | 20 | 7 | 24 | 79.3 | 0.66 | [96] |
| anaerobic digester sludge enriched in presence of Cr(VI) (in situ) | 20.2 | 0.17 | |||||||||
| SCMEC, Cyl(c) | carbon rod | anaerobic sludge | graphite felt | anaerobic sludge | NA | 100 | 16 HRT | 43.12–96.68 | 2.69–6.04 | [97] | |
| 100 (200 NO3−, 100 p-FNB) | 41.38 | 2.59 | |||||||||
| 100 (200 NO3−, 150 p-FNB) | 49.14 | 3.07 | |||||||||
| 100 (200 NO3−, 200 p-FNB) | 55.21 | 3.45 | |||||||||
| 100 (200 NO3−, 300 p-FNB) | 58.93 | 3.68 | |||||||||
| 2CMFC-PEM, C(b) | graphite felt | anaerobic sludge. ED: glucose | graphite felt | acclimated MFC anode | NA | 20 | 7 | 5 | 28.3 | 1.13 ± 0.01 | [98] |
| graphite felt /NaX | NA | 69 | 2.76 ± 0.09 | ||||||||
| graphite felt /NaX-HNO3 | NA | 3 | 100 | 10.39 ± 0.28 | |||||||
| 2CMFC-PEM, H(b) | graphite felt | sewage sludge. ED: glucose | graphite felt | acclimated MFC | NA | 40 | 7 | 48 | 58.3 | 0.49 | [99] |
| graphene-modified felt | 100 | 0.83 | |||||||||
| 2CMFC, CEM, Cyl(b) | graphite felt | MFC anodic effluent. ED: acetate | graphite felt | Stenotrophomonas sp., S. maltophilia, Serratia marcescens, Achromobacter xylosoxidans | −0.04 | 20 | 5.8 | 5 | 74–83 | 2.96–3.32 | [100] |
| −0.05 | 20 (20 mg/L Cd(II)) | 63–71 | 2.52–2.84 | ||||||||
| SCMEC, Cyl(c) | graphite rod | activated sludge | carbon felt | activated sludge | NA | 30 (20 mg/L NO3−) | 6 | 20 HRT | 58.96 | 0.88 | [101] |
| 7 | 72.65 | 1.09 | |||||||||
| 8 | 65.08 | 0.98 | |||||||||
| 2CMFC-CEM, H(b) | graphite felt | anaerobic sludge | graphite felt | Bacillus cereus | NA | 27 | 7 | 25 | 100 | 2.56 | [102] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beretta, G.; Daghio, M.; Espinoza Tofalos, A.; Franzetti, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Progress Towards Bioelectrochemical Remediation of Hexavalent Chromium. Water 2019, 11, 2336. https://doi.org/10.3390/w11112336
Beretta G, Daghio M, Espinoza Tofalos A, Franzetti A, Mastorgio AF, Saponaro S, Sezenna E. Progress Towards Bioelectrochemical Remediation of Hexavalent Chromium. Water. 2019; 11(11):2336. https://doi.org/10.3390/w11112336
Chicago/Turabian StyleBeretta, Gabriele, Matteo Daghio, Anna Espinoza Tofalos, Andrea Franzetti, Andrea Filippo Mastorgio, Sabrina Saponaro, and Elena Sezenna. 2019. "Progress Towards Bioelectrochemical Remediation of Hexavalent Chromium" Water 11, no. 11: 2336. https://doi.org/10.3390/w11112336
APA StyleBeretta, G., Daghio, M., Espinoza Tofalos, A., Franzetti, A., Mastorgio, A. F., Saponaro, S., & Sezenna, E. (2019). Progress Towards Bioelectrochemical Remediation of Hexavalent Chromium. Water, 11(11), 2336. https://doi.org/10.3390/w11112336





