Comparative Study of the Oxidative Degradation of Different 4-Aminobenzene Sulfonamides in Aqueous Solution by Sulfite Activation in the Presence of Fe(0), Fe(II), Fe(III) or Fe(VI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Iron-Based/Sulfite Systems
2.4. Analytical Methods
2.4.1. Fe (VI) Determination in Aqueous Solution
2.4.2. Determination of Sulfonamides in Aqueous Solution
2.4.3. Determination of Byproducts Degradation
2.4.4. Determination of Byproducts Cytotoxicity
2.4.5. Collection and Characterization of Natural Waters
3. Results and Discussion
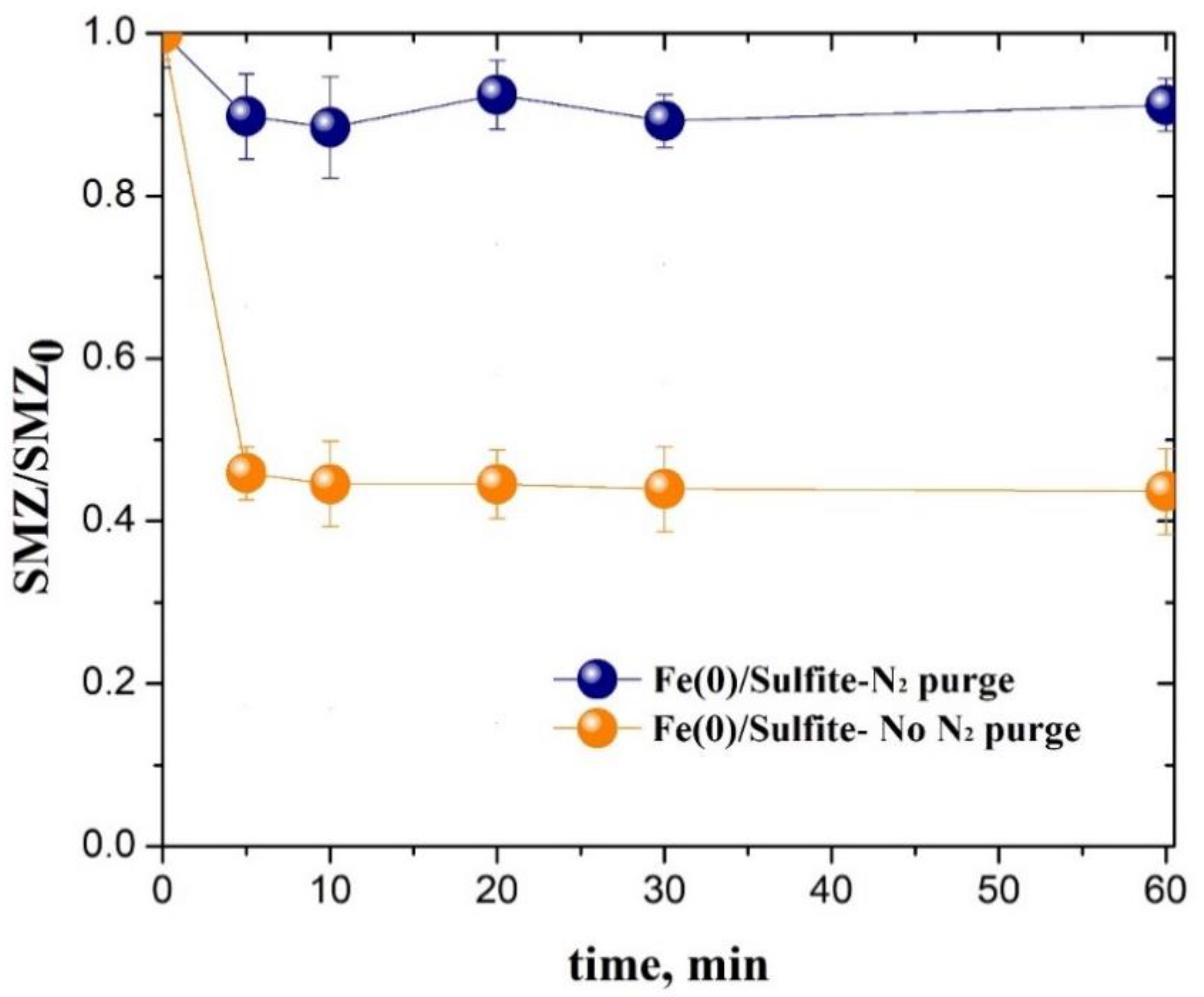
3.1. Sulfonamide Degradation by the Fe(0)/Sulfite System
3.2. Sulfonamide Degradation by the Fe(II)/Sulfite System
3.3. Sulfonamides Degradation by the Fe(III)/Sulfite System
3.4. Sulfonamides Degradation by the Fe(VI)/Sulfite System
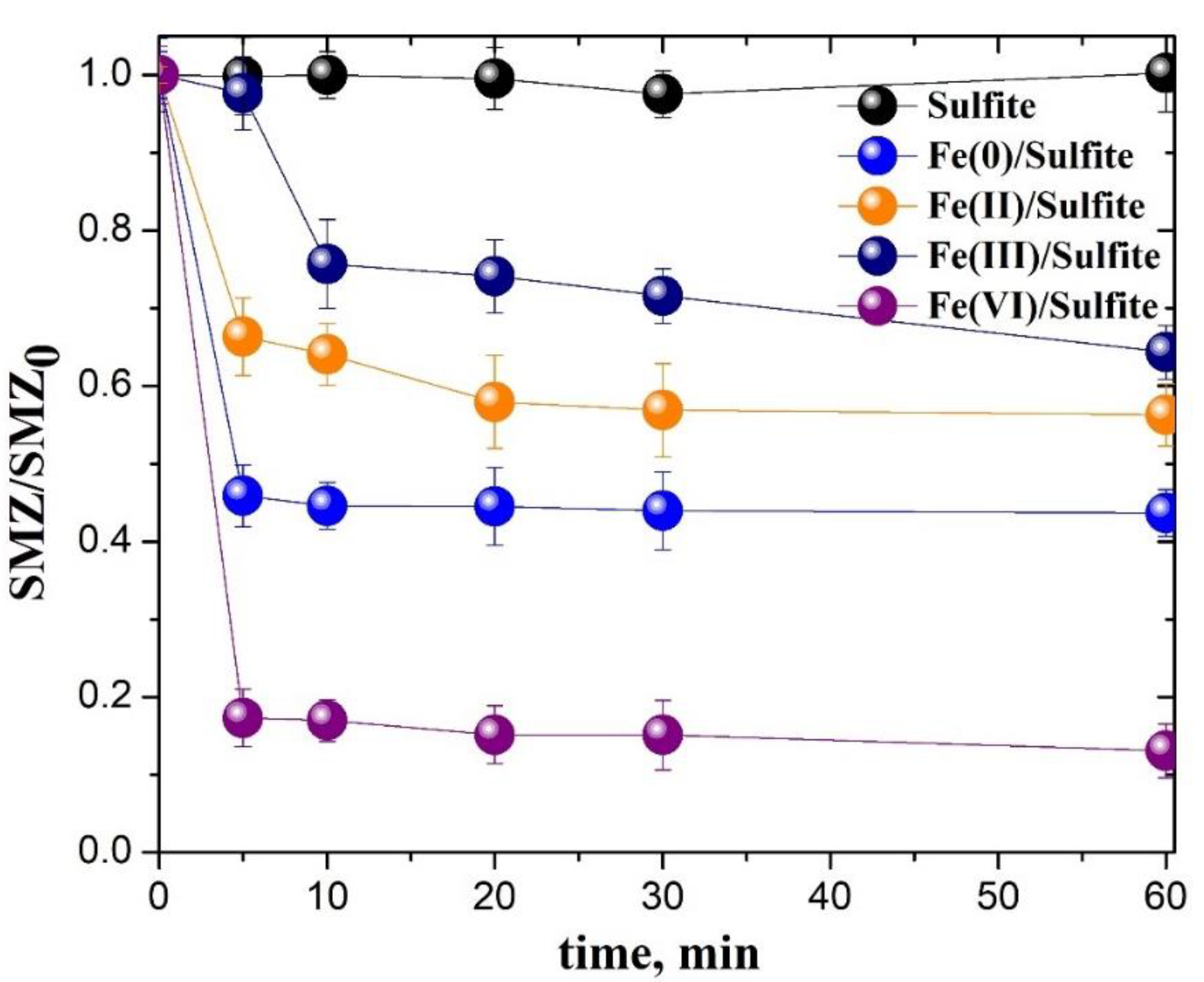
3.5. Comparison of Different Iron-Based/Sulfite Systems on the SAs Removal
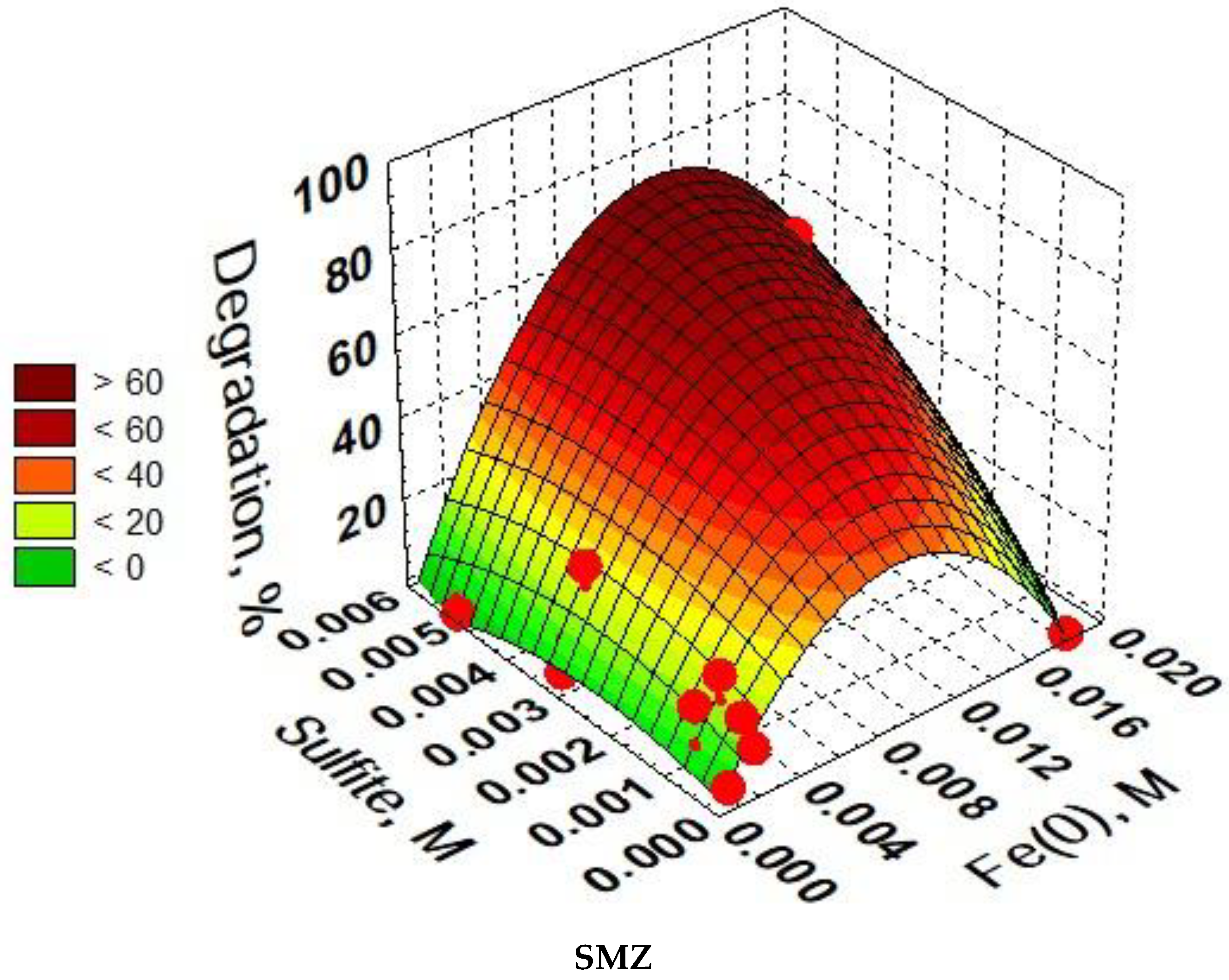
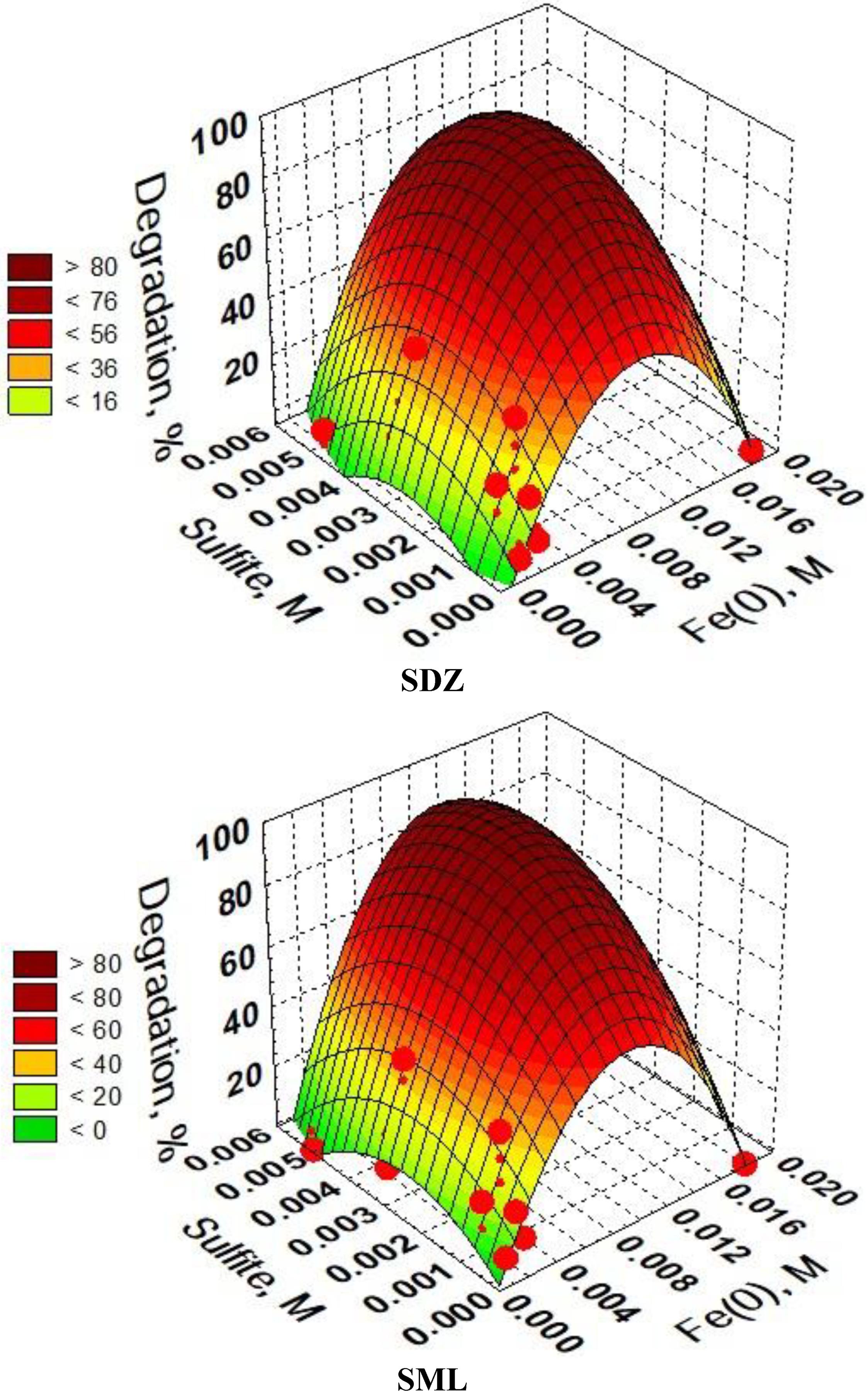
3.6. Optimization of SAs Degradation Process Conditions
3.7. Influence of the Water Matrix on Iron-Based/Sulfite Systems
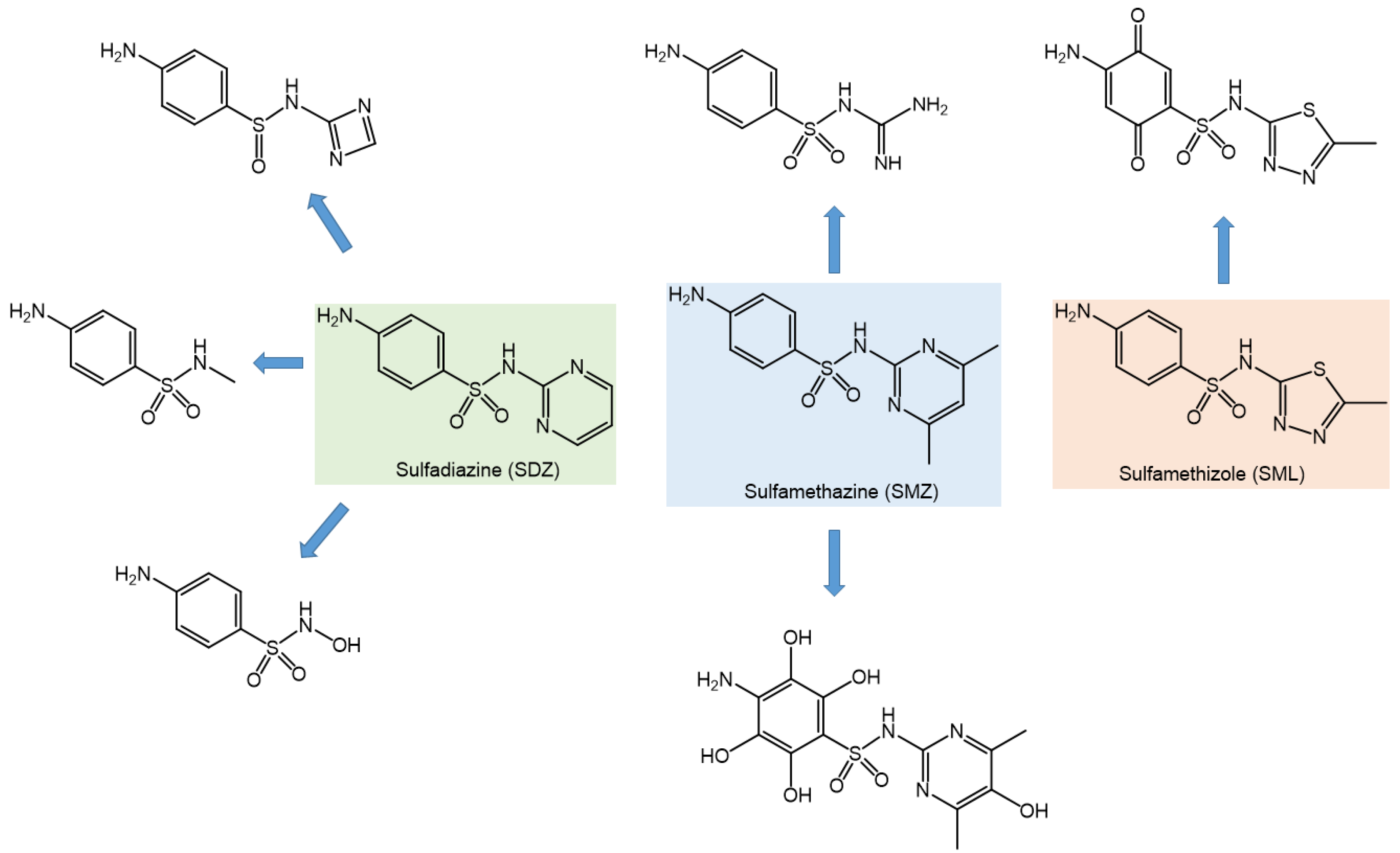
3.8. Degradation Byproducts and Pathways
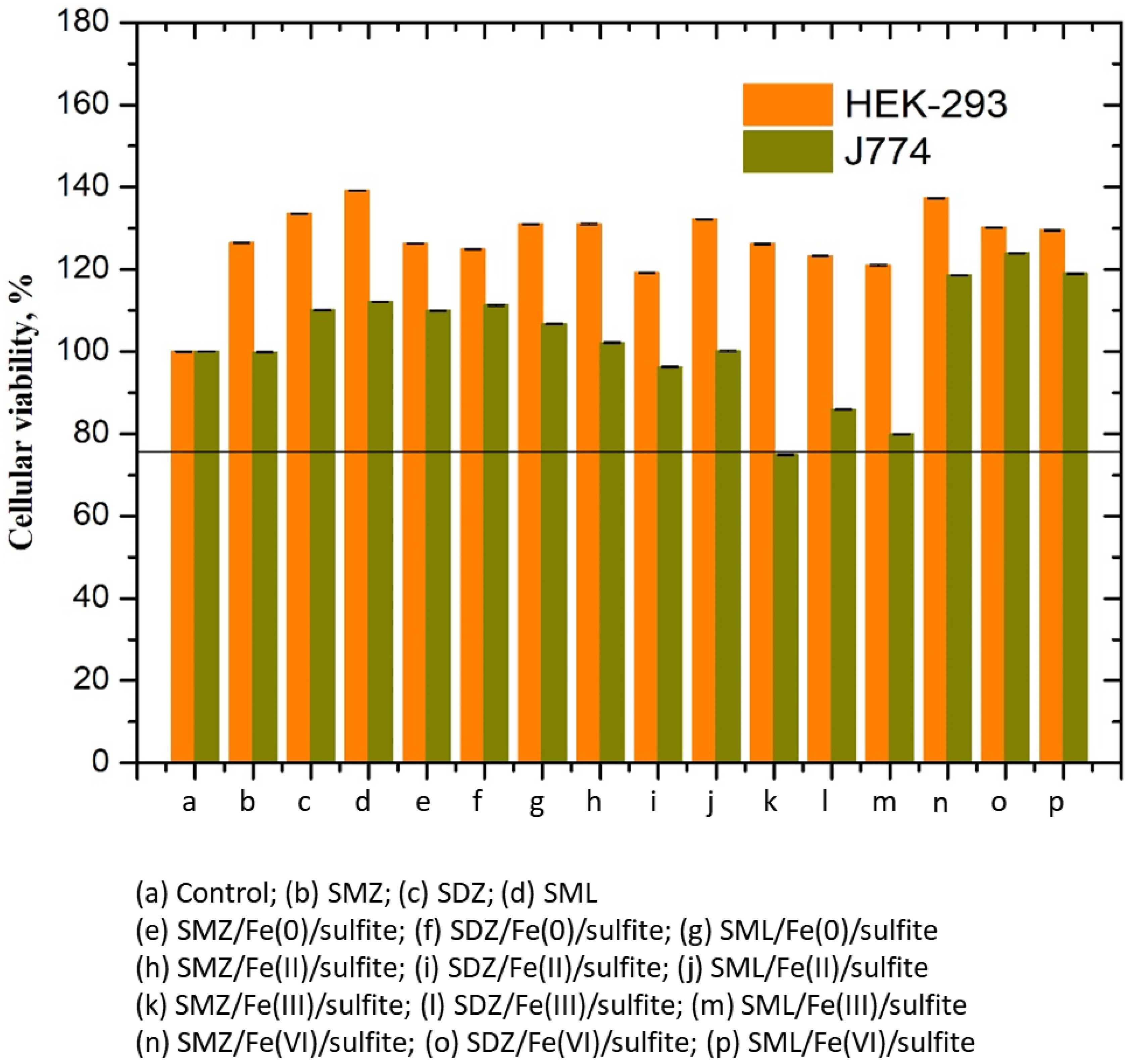
3.9. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.J.; Wang, Y.; Geng, Y.K.; Liu, R.D.; Pan, X.R.; Li, W.W.; Sheng, G.P. Application of membrane bioreactor for sulfamethazine-contained wastewater treatment. Chemosphere 2018, 193, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Duan, X.; Wang, C.; Sun, H.; Tan, X.; Tade, M.O.; Wang, S. Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability. Chem. Eng. J. 2018, 332, 398–408. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Sánchez-Polo, M.; Polo, A.M.S.; Rivera-Utrilla, J.; Berber-Mendoza, M.S. Tinidazole degradation assisted by solar radiation and iron-doped silica xerogels. Chem. Eng. J. 2018, 344, 21–33. [Google Scholar] [CrossRef]
- Velo-Gala, I.; Pirán-Montaño, J.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Mota, A.J. Advanced oxidation processes based on the use of UVC and simulated solar radiation to remove the antibiotic tinidazole from water. Chem. Eng. J. 2017, 323, 605–617. [Google Scholar] [CrossRef]
- Niu, H.; Zheng, Y.; Wang, S.; Zhao, L.; Yang, S.; Cai, Y. Continuous generation of hydroxyl radicals for highly efficient elimination of chlorophenols and phenols catalyzed by heterogeneous Fenton-like catalysts yolk/shell Pd@Fe3O4@metal organic frameworks. J. Hazard. Mater. 2018, 346, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Dmitrienko, S.G.; Kochuk, E.V.; Apyari, V.V.; Tolmacheva, V.V.; Zolotov, Y.A. Recent advances in sample preparation techniques and methods of sulfonamides detection–A review. Anal. Chim. Acta 2014, 850, 6–25. [Google Scholar] [CrossRef]
- Ait Lahcen, A.; Amine, A. Mini-review: Recent advances in electrochemical determination of sulfonamides. Anal. Lett. 2018, 51, 424–441. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Enhanced peroxymonosulfate activation for sulfamethazine degradation by ultrasound irradiation: Performances and mechanisms. Chem. Eng. J. 2018, 335, 145–153. [Google Scholar] [CrossRef]
- Sabri, N.A.; Schmitt, H.; Van der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng. 2018, 6, 898–905. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Munir, M.; Xagoraraki, I. Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci. Total Environ. 2012, 421, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Wang, Y.; Loftin, K.; Meyer, M. Removal of antibiotics from surface and distilled water in conventional water treatment processes. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Wallace, J.S.; Garner, E.; Pruden, A.; Aga, D.S. Occurrence and transformation of veterinary antibiotics and antibiotic resistance genes in dairy manure treated by advanced anaerobic digestion and conventional treatment methods. Environ. Pollut. 2018, 236, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ding, F.; Weng, C.H.; Hwang, C.C.; Lin, Y.T. Effective degradation of primary color direct azo dyes using Fe0 aggregates-activated persulfate process. J. Environ. Manag. 2018, 206, 565–576. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, F.; Luo, F.; Li, C. Degradation of sulfonamides and formation of trihalomethanes by chlorination after pre-oxidation with Fe(VI). J. Environ. Sci. 2018, 73, 89–95. [Google Scholar] [CrossRef]
- Pagano, M.; Ciannarella, R.; Locaputo, V.; Mascolo, G.; Volpe, A. Oxidation of azo and anthraquinonic dyes by peroxymonosulphate activated by UV light. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2018, 53, 393–404. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Gao, N.Y.; Yin, D.Q.; Tian, F.X.; Zheng, Q.F. Oxidation of the β-blocker propranolol by UV/persulfate: Effect, mechanism and toxicity investigation. Chemosphere 2018, 201, 50–58. [Google Scholar] [CrossRef]
- Watts, R.J.; Ahmad, M.; Hohner, A.K.; Teel, A.L. Persulfate activation by glucose for in situ chemical oxidation. Water Res. 2018, 133, 247–254. [Google Scholar] [CrossRef]
- Bao, Y.; Lim, T.T.; Wang, R.; Webster, R.D.; Hu, X. Urea-assisted one-step synthesis of cobalt ferrite impregnated ceramic membrane for sulfamethoxazole degradation via peroxymonosulfate activation. Chem. Eng. J. 2018, 343, 737–747. [Google Scholar] [CrossRef]
- Xie, P.; Guo, Y.; Chen, Y.; Wang, Z.; Shang, R.; Wang, S.; Ding, J.; Wan, Y.; Jiang, W.; Ma, J. Application of a novel advanced oxidation process using sulfite and zero-valent iron in treatment of organic pollutants. Chem. Eng. J. 2017, 314, 240–248. [Google Scholar] [CrossRef]
- Chen, L.; Peng, X.; Liu, J.; Li, J.; Wu, F. Decolorization of orange II in aqueous solution by an Fe (II)/sulfite system: Replacement of persulfate. Ind. Eng. Chem. Res. 2012, 51, 13632–13638. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Xiao, M.; Zhang, L.; Wu, F.; Ge, L. Enhanced decolorization of orange II solutions by the Fe (II)–sulfite system under xenon lamp irradiation. Ind. Eng. Chem. Res. 2013, 52, 10089–10094. [Google Scholar] [CrossRef]
- Xu, J.; Ding, W.; Wu, F.; Mailhot, G.; Zhou, D.; Hanna, K. Rapid catalytic oxidation of arsenite to arsenate in an iron (III)/sulfite system under visible light. Appl. Catal. B Environ. 2016, 186, 56–61. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, Y.; Yang, S.; Gao, H.; Chen, L. Roles of oxysulfur radicals in the oxidation of acid orange 7 in the Fe (III)–sulfite system. J. Sulfur Chem. 2015, 36, 373–384. [Google Scholar] [CrossRef]
- Jegatheesan, V.; Pramanik, B.K.; Chen, J.; Navaratna, D.; Chang, C.Y.; Shu, L. Treatment of textile wastewater with membrane bioreactor: A critical review. Bioresour. Technol. 2016, 204, 202–212. [Google Scholar] [CrossRef]
- Sun, S.; Pang, S.; Jiang, J.; Ma, J.; Huang, Z.; Zhang, J.; Liu, Y.; Xu, C.; Liu, Q.; Yuan, Y. The combination of ferrate (VI) and sulfite as a novel advanced oxidation process for enhanced degradation of organic contaminants. Chem. Eng. J. 2018, 333, 11–19. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Shi, Z.; Gao, Y. Rapid removal of organic pollutants by activation sulfite with ferrate. Chemosphere 2017, 186, 576–579. [Google Scholar] [CrossRef]
- Guo, Y.; Lou, X.; Fang, C.; Xiao, D.; Wang, Z.; Liu, J. Novel photo-sulfite system: Toward simultaneous transformations of inorganic and organic pollutants. Environ. Sci. Technol. 2013, 47, 11174–11181. [Google Scholar] [CrossRef]
- Du, J.; Guo, W.; Wang, H.; Yin, R.; Zheng, H.; Feng, X.; Che, D.; Ren, N. Hydroxyl radical dominated degradation of aquatic sulfamethoxazole by Fe0/bisulfite/O2: Kinetics, mechanisms, and pathways. Water Res. 2018, 138, 323–332. [Google Scholar] [CrossRef]
- Yu, Y.; Li, S.; Peng, X.; Yang, S.; Zhu, Y.; Chen, L.; Wu, F.; Mailhot, G. Efficient oxidation of bisphenol A with oxysulfur radicals generated by iron-catalyzed autoxidation of sulfite at circumneutral pH under UV irradiation. Environ. Chem. Lett. 2016, 14, 527–532. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Li, Y.; Xiang, G.J. Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese loess plateau: A case study of Tongchuan, Northwest China. Expo. Health 2019, 11, 95–107. [Google Scholar] [CrossRef]
- Feng, M.; Jinadatha, C.; McDonald, T.J.; Sharma, V.K. Accelerated oxidation of organic contaminants by ferrate (VI): The overlooked role of reducing additives. Environ. Sci. Technol. 2018, 52, 11319–11327. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, Y.; Shang, R.; Fang, Z.; Wu, F.; Wang, Z. A triple system of Fe (III)/sulfite/persulfate: Decolorization and mineralization of reactive Brilliant Red X-3B in aqueous solution at near-neutral pH values. J. Taiwan Inst. Chem. Eng. 2016, 68, 162–168. [Google Scholar] [CrossRef]
- Kim, C.; Ahn, J.Y.; Kim, T.Y.; Shin, W.S.; Hwang, I. Activation of persulfate by nanosized zero-valent iron (NZVI): Mechanisms and transformation products of NZVI. Environ. Sci. Technol. 2018, 52, 3625–3633. [Google Scholar] [CrossRef]
- Velo-Gala, I.; López-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Comparative study of oxidative degradation of sodium diatrizoate in aqueous solution by H2O2/Fe2+, H2O2/Fe3+, Fe (VI) and UV, H2O2/UV, K2S2O8/UV. Chem. Eng. J. 2014, 241, 504–512. [Google Scholar] [CrossRef]
- Wang, F.; Gao, F.; Lan, M.; Yuan, H.; Huang, Y.; Liu, J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol. Vitr. 2009, 23, 808–815. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Z.; Zhang, T.; Gao, N.; Ma, Y. Degradation effect of sulfa antibiotics by potassium ferrate combined with ultrasound (Fe (VI)-US). BioMed Res. Int. 2015, 2015, 169215. [Google Scholar] [CrossRef]
- Lee, C.; Keenan, C.R.; Sedlak, D.L. Polyoxometalate-enhanced oxidation of organic compounds by nanoparticulate zero-valent iron and ferrous ion in the presence of oxygen. Environ. Sci. Technol. 2008, 42, 4921–4926. [Google Scholar] [CrossRef]
- Reddy, K.B.; Van Eldik, R. Kinetics and mechanism of the sulfite-induced autoxidation of Fe (II) in acidic aqueous solution. Atmos. Environ. Part A 1992, 26, 661–665. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Trimethoprim degradation by Fenton and Fe (II)-activated persulfate processes. Chemosphere 2018, 191, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Qiang, Z.; Liu, S.; Li, J.; Yu, J.; Qu, J. Oxidation of iopamidol with ferrate (Fe (VI)): Kinetics and formation of toxic iodinated disinfection by-products. Water Res. 2018, 130, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Dubrawski, K.; Cataldo, M.; Dubrawski, Z.; Mazumder, A.; Wilkinson, D.; Mohseni, M. In-situ electrochemical Fe (VI) for removal of microcystin-LR from drinking water: Comparing dosing of the ferrate ion by electrochemical and chemical means. Water Health 2018, 16, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Panditi, V.R.; Gardinali, P.R.; Varma, R.S.; Kim, H.; Sharma, V.K. Ferrate promoted oxidative cleavage of sulfonamides: Kinetics and product formation under acidic conditions. Chem. Eng. J. 2015, 279, 307–316. [Google Scholar] [CrossRef]
- Sharma, V.K. Oxidation of inorganic compounds by ferrate (VI) and ferrate (V): One-electron and two-electron transfer steps. Environ. Sci. Technol. 2010, 44, 5148–5152. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Giannakis, S.; Liu, S.; Carratalà, A.; Rtimi, S.; Bensimon, M.; Pulgarin, C. Effect of Fe(II)/Fe(III) species, pH, irradiance and bacterial presence on viral inactivation in wastewater by the photo-Fenton process: Kinetic modeling and mechanistic interpretation. Appl. Catal. B 2017, 204, 156–166. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of sulfate radical and hydroxyl radical by reaction of ozone with peroxymonosulfate: A novel advanced oxidation process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Wang, J. Fenton-like degradation of sulfamethazine using Fe3O4/Mn3O4 nanocomposite catalyst: Kinetics and catalytic mechanism. Environ. Sci. Pollut. Res. 2017, 24, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C.; Yau, M.; Bolton, J.R.; Qiang, Z. Sulfamethazine degradation in water by the VUV/UV process: Kinetics, mechanism and antibacterial activity determination based on a mini-fluidic VUV/UV photoreaction system. Water Res. 2017, 108, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.S.; Pires, F.C.C.; Teixeira, A.C.S.C. The role of reactive oxygen species in sulfamethazine degradation using UV-based technologies and products identification. J. Photochem. Photobiol. A Chem. 2014, 290, 77–85. [Google Scholar] [CrossRef]
- Pomati, F.; Castiglioni, S.; Zuccato, E.; Fanelli, R.; Vigetti, D.; Rossetti, C.; Calamari, D. Effects of a complex mixture of therapeutic drugs at environmental levels on human embryonic cells. Environ. Sci. Technol. 2006, 40, 2442–2447. [Google Scholar] [CrossRef]
- Cizmas, L.; Sharma, V.K.; Gray, C.M.; McDonald, T.J. Pharmaceuticals and personal care products in waters: Occurrence, toxicity, and risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef]
- Weaver, K.D.; Kim, H.J.; Sun, J.; MacFarlane, D.R.; Elliott, G.D. Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications. Green Chem. 2010, 12, 507–513. [Google Scholar] [CrossRef]
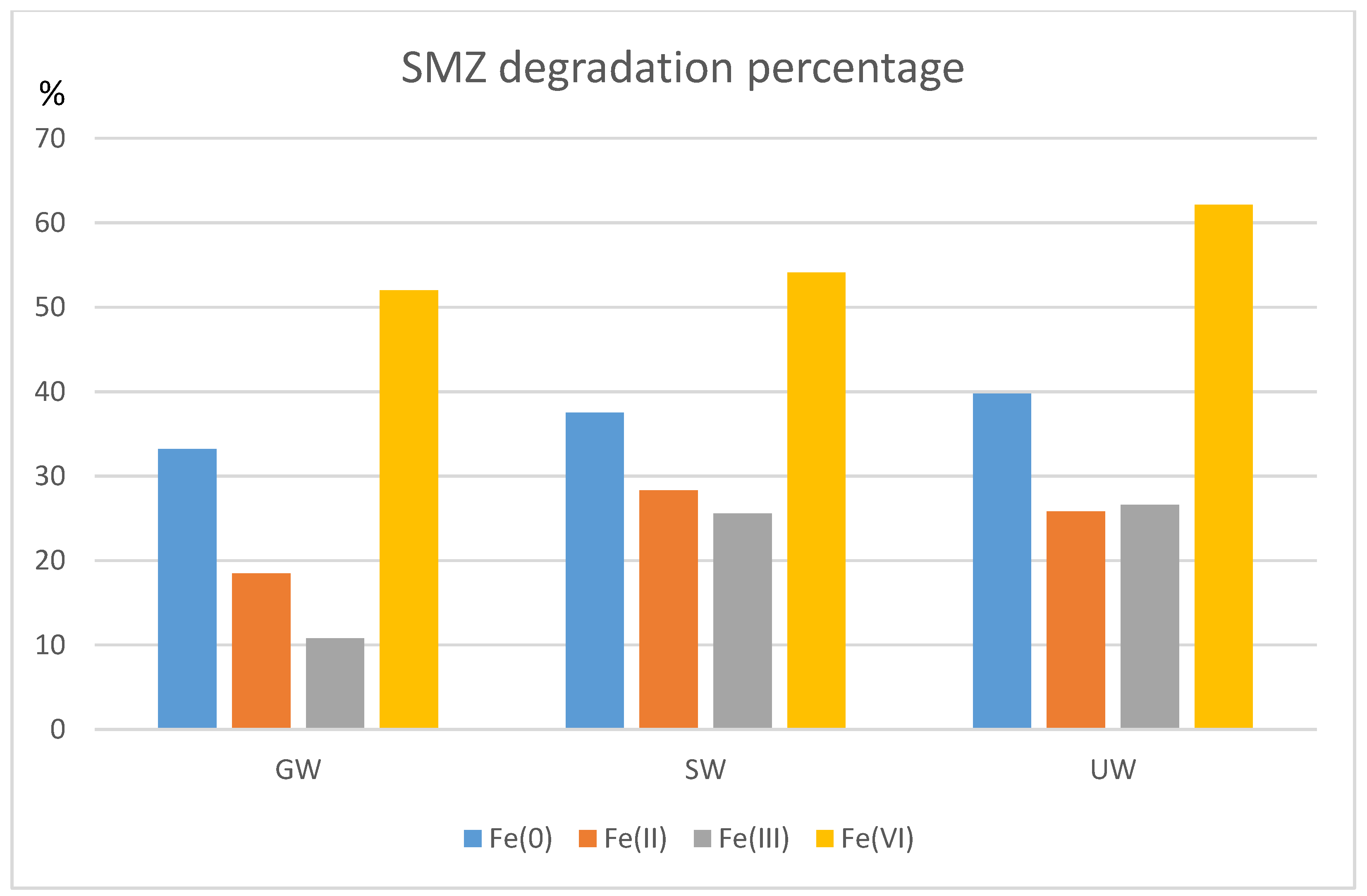
| Sulfonamide | Molecular Structure (3D) | Molar Mass (g/mol) | LogKow a | Solubility b (mg/L) | pKa |
|---|---|---|---|---|---|
| Sulfamethazine C12H14N4O2S (SMZ) |  | 278.33 | 0.19 | 1500 (29 °C) | pKa1: 2.00 pKa2: 6.99 |
| Sulfadiazine C10H10N4O2S (SDZ) |  | 250.278 | −0.09 | 77 (25 °C) | pKa1:2.01 pKa2: 6.99 |
| Sulfamethizole C9H10N4O2S2 (SML) |  | 270.33 | 0.54 | 1050 (37 °C) | pKa1: 1.95 pKa2: 6.71 |
| Water | pH | [HCO3−] (mg L−1) | [SO42−] (mg L−1) | [Cl−] (mg L−1) | [NO3−] (mg L−1) | TOC* (mg L−1) |
|---|---|---|---|---|---|---|
| Ultrapure water (UW) | 6.8 | <BDL | <BDL | <BDL | <DL | <BDL |
| Surface water (SW) | 8.61 | 143 | 35.8 | <10 | 2.50 | 3.5 |
| Ground water (GW) | 7.34 | 156 | 2404 | 121 | <0.30 | 2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Rangel, A.; Sánchez-Polo, M.; Rozalen, M.; Rivera-Utrilla, J.; Polo, A.M.S.; Mota, A.J. Comparative Study of the Oxidative Degradation of Different 4-Aminobenzene Sulfonamides in Aqueous Solution by Sulfite Activation in the Presence of Fe(0), Fe(II), Fe(III) or Fe(VI). Water 2019, 11, 2332. https://doi.org/10.3390/w11112332
Acosta-Rangel A, Sánchez-Polo M, Rozalen M, Rivera-Utrilla J, Polo AMS, Mota AJ. Comparative Study of the Oxidative Degradation of Different 4-Aminobenzene Sulfonamides in Aqueous Solution by Sulfite Activation in the Presence of Fe(0), Fe(II), Fe(III) or Fe(VI). Water. 2019; 11(11):2332. https://doi.org/10.3390/w11112332
Chicago/Turabian StyleAcosta-Rangel, A., M. Sánchez-Polo, M. Rozalen, J. Rivera-Utrilla, A.M.S. Polo, and A. J. Mota. 2019. "Comparative Study of the Oxidative Degradation of Different 4-Aminobenzene Sulfonamides in Aqueous Solution by Sulfite Activation in the Presence of Fe(0), Fe(II), Fe(III) or Fe(VI)" Water 11, no. 11: 2332. https://doi.org/10.3390/w11112332
APA StyleAcosta-Rangel, A., Sánchez-Polo, M., Rozalen, M., Rivera-Utrilla, J., Polo, A. M. S., & Mota, A. J. (2019). Comparative Study of the Oxidative Degradation of Different 4-Aminobenzene Sulfonamides in Aqueous Solution by Sulfite Activation in the Presence of Fe(0), Fe(II), Fe(III) or Fe(VI). Water, 11(11), 2332. https://doi.org/10.3390/w11112332








