Microcosm Experiment to Assess the Capacity of a Poplar Clone to Grow in a PCB-Contaminated Soil
Abstract
:1. Introduction
2. Material and Methods
2.1. Soil Collection from the Historically Contaminated Area and Characterization
2.2. Microcosm Experimental Design
- -
- Microbiologically active soil (MA): Historically polluted soil where a poplar cutting was planted.
- -
- Pre-sterilized soil (Pre-sterilized): Historically polluted soil previously sterilized by autoclaving it (at 121 °C, 20 min), where a poplar cutting was subsequently planted;
- -
- Microbiologically active soil under hypoxic conditions (Hypoxic): Historically polluted soil where a poplar cutting was planted; then each pot was submerged in water for all the experimental period. This treatment was intended to limit the oxygen concentration in the soil in order to reproduce a hypoxic environment for promoting the transformation of higher-chlorinated PCBs.
2.3. Sampling of Soil and Plant for Various Analysis
2.3.1. PCB Markers in Soil and Roots
2.3.2. Microbial Abundance and Dehydrogenase Activity
2.4. Analysis for Growth Monitoring, Plant Physiology and Plant Antioxidants
2.4.1. Growth Monitoring Measurements
2.4.2. Plant Physiology Measurements
2.4.3. Plant Antioxidants
2.5. Statistical Analysis
3. Results
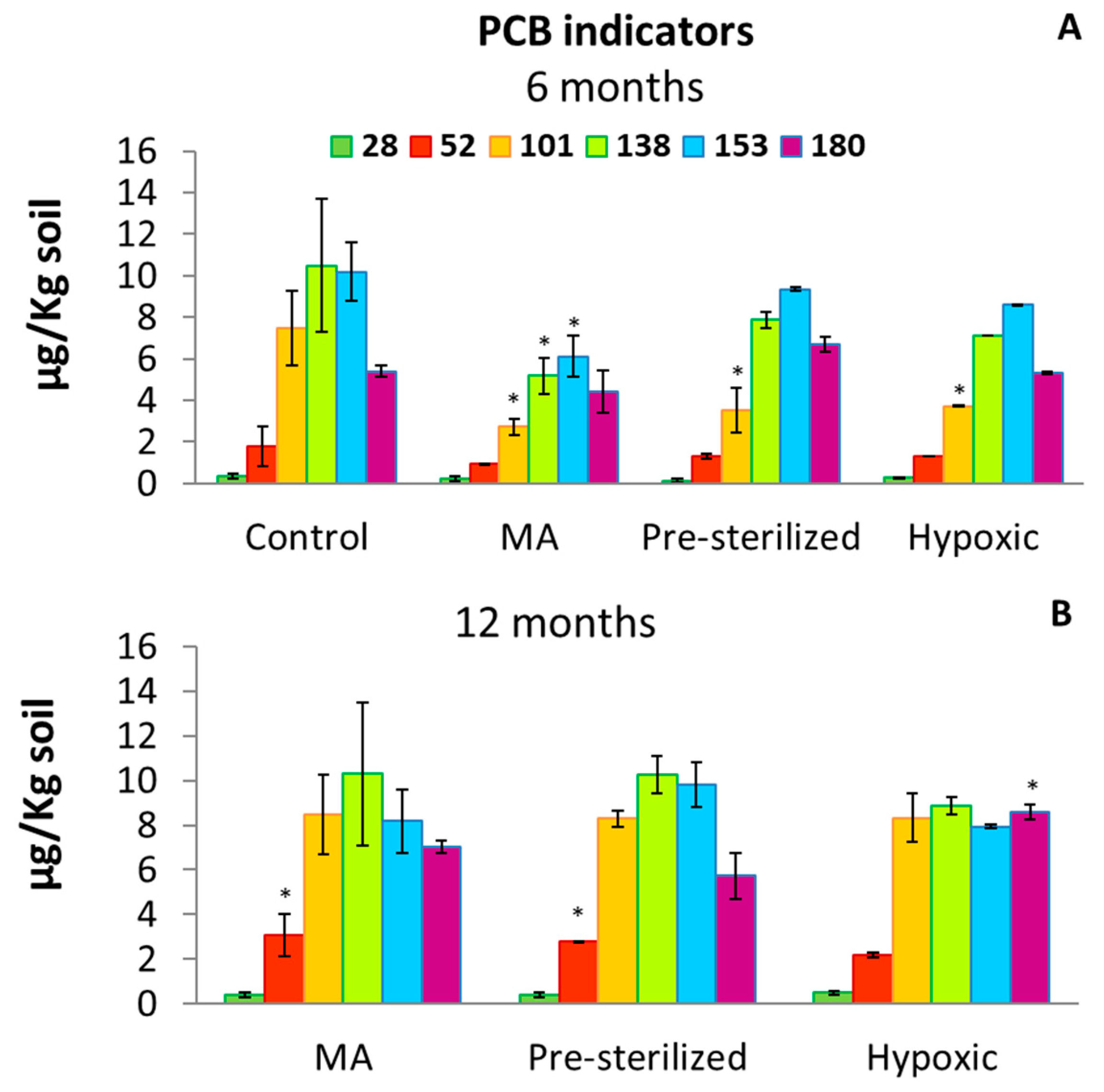
3.1. Soil PCB Concentration
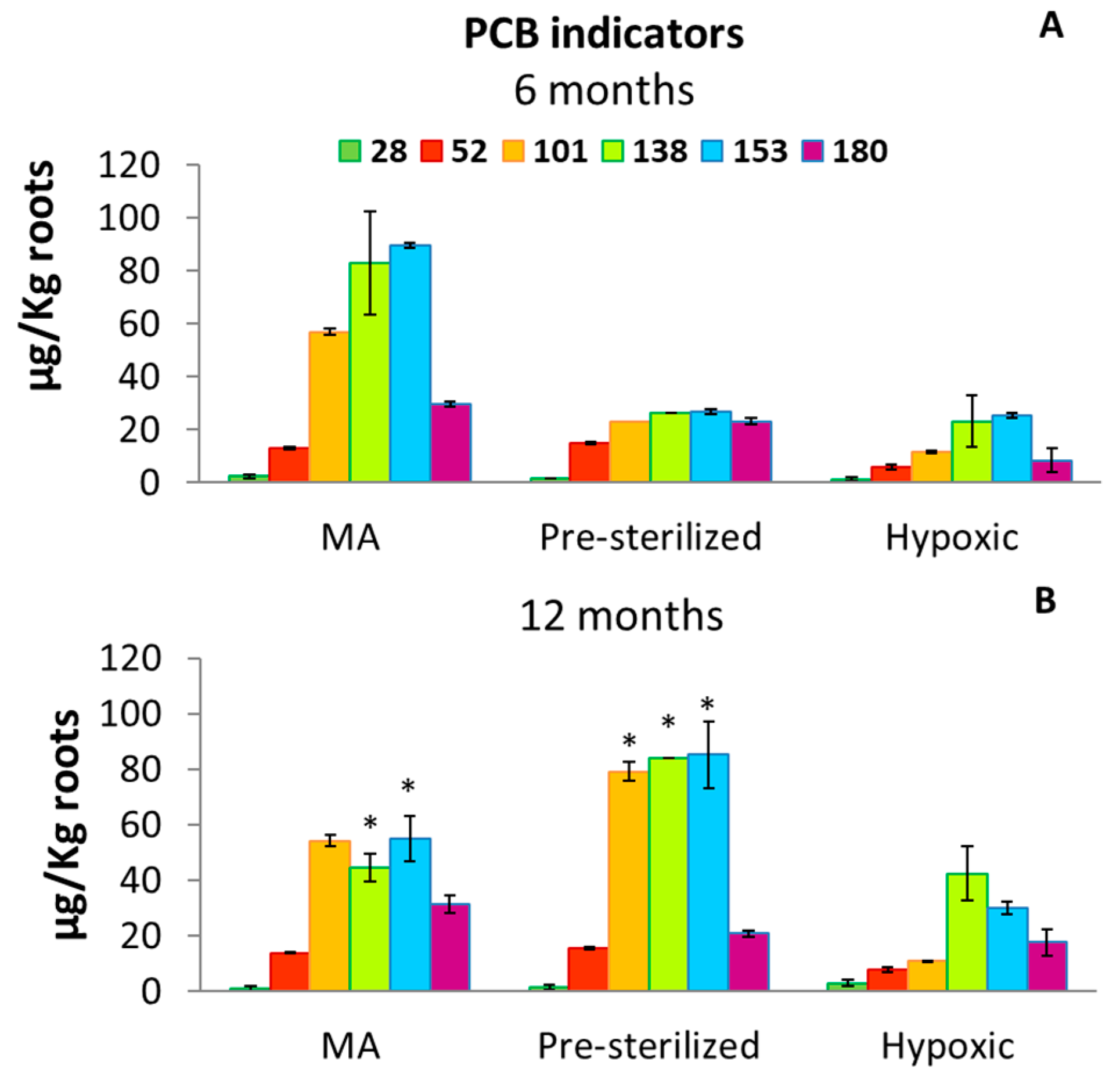
3.2. PCB Concentrations in Roots
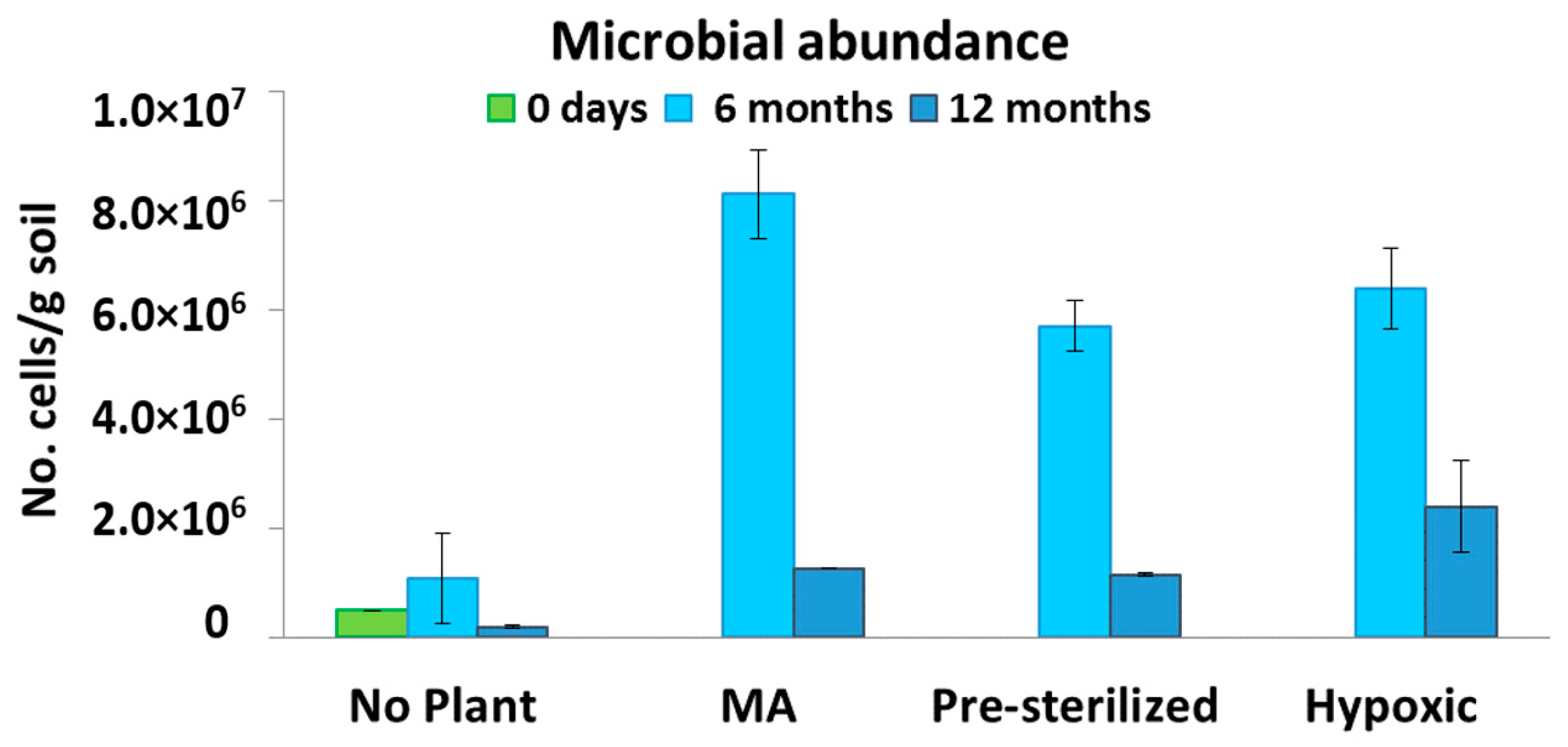
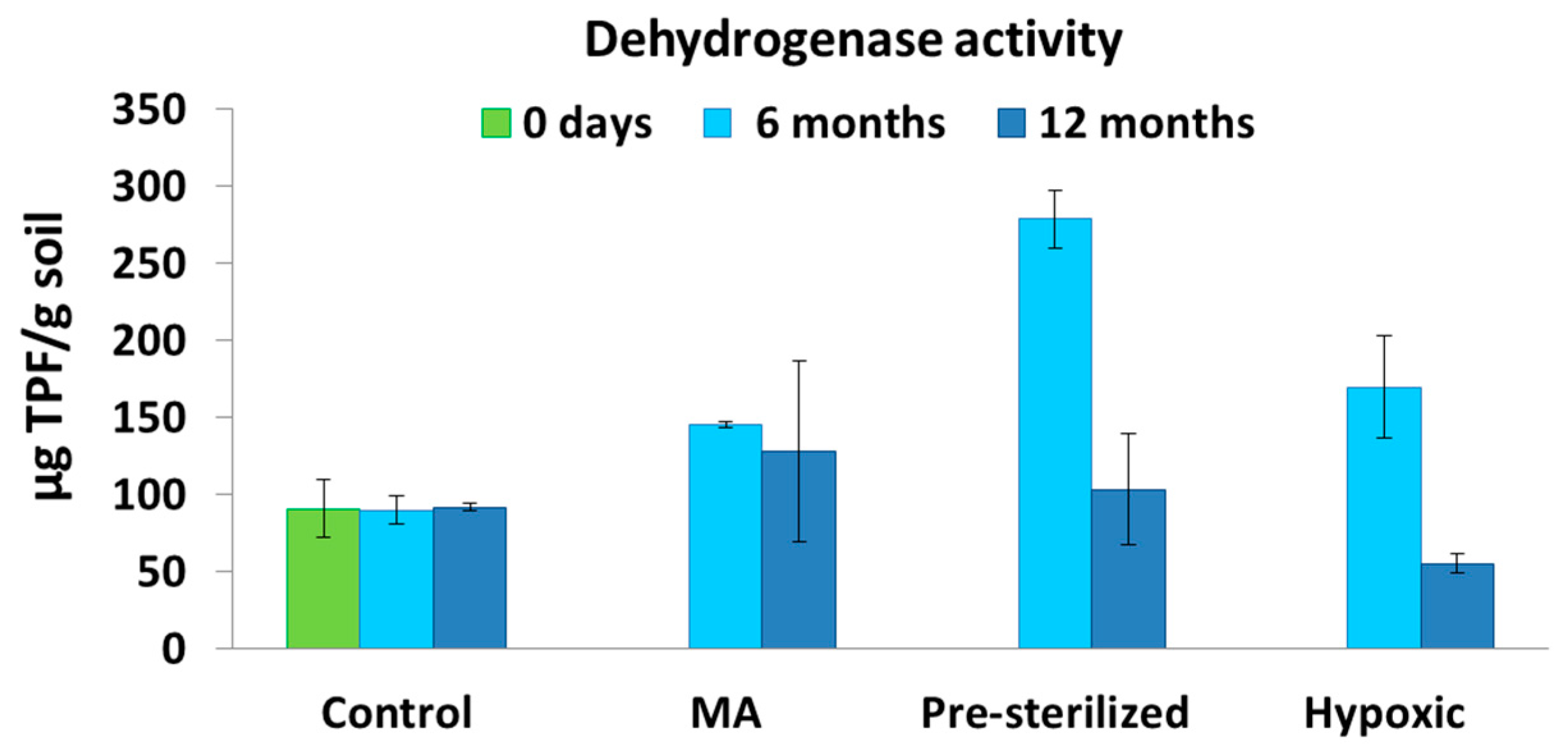
3.3. Microbiological Analysis
3.4. Plant Growth and Physiology
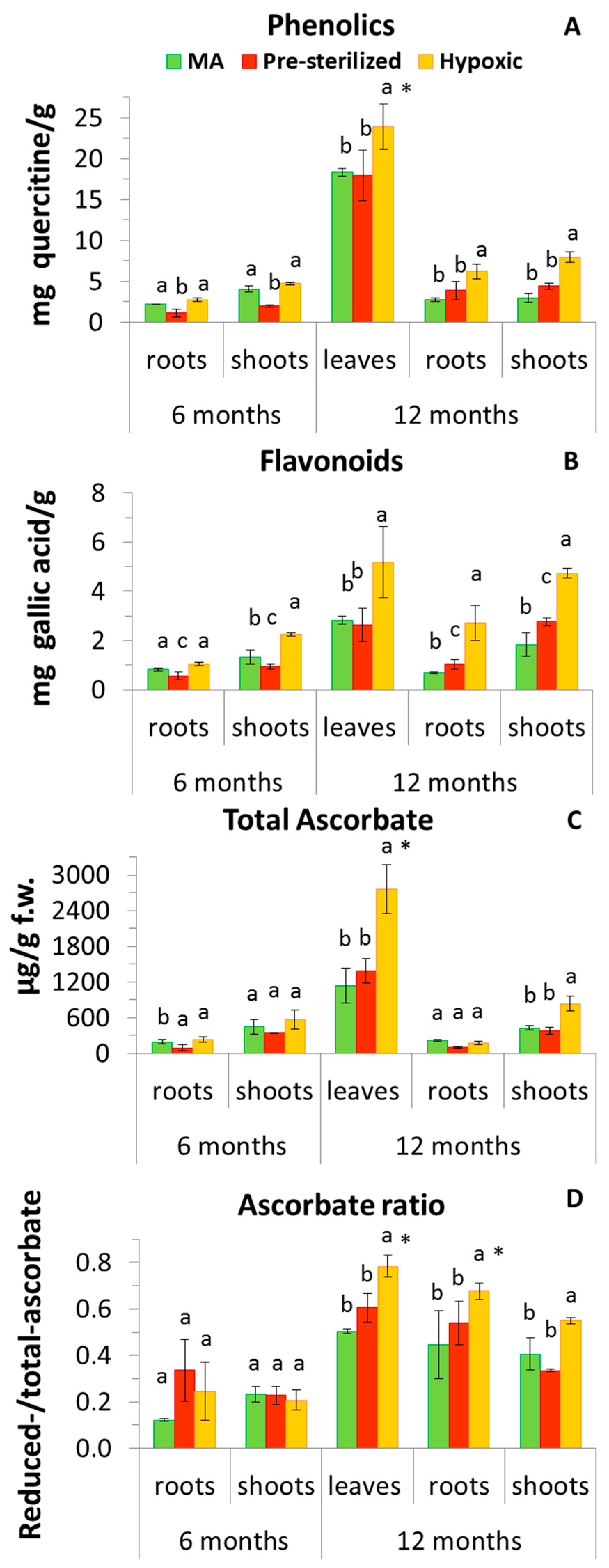
3.5. Plant Antioxidants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Terzaghi, E.; Zanardini, E.; Morosini, C.; Raspa, G.; Borin, S.; Mapelli, F.; Vergani, L.; Di Guardo, A. Rhizoremediation half-lives of PCBs: Role of congener composition, organic carbon forms, bioavailability, microbial activity, plant species and soil conditions, on the prediction of fate and persistence in soil. Sci. Total Environ. 2018, 612, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Meli, P.; Rey Benayas, J.M.; Balvanera, P.; Martínez Ramos, M. Restoration enhances wetland biodiversity and ecosystem service supply, but results are context-dependent: a meta-analysis. PLoS ONE 2014, 9, e93507. [Google Scholar] [CrossRef] [PubMed]
- Keesstra, S.; Nunes, J.; Novara, A.; Finger, D.; Avelar, D.; Kalantari, Z.; Cerdà, A. The superior effect of nature based solutions in land management for enhancing ecosystem services. Sci. Total Environ. 2018, 610, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W. Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 2009, 321, 385–408. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Abrantes, I.; Barra Caracciolo, A.; Bevivino, A.; Ciancio, A.; Grenni, P.; Hrynkiewicz, K.; Kredics, L.; Proença, D.N. Belowground Microbiota and the Health of Tree Crops. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef] [Green Version]
- Field, J.A.; Sierra-Alvarez, R. Microbial transformation and degradation of polychlorinated biphenyls. Environ. Pollut. 2008, 155, 1–12. [Google Scholar] [CrossRef]
- Singer, A.C.; Crowley, D.E.; Thompson, I.P. Secondary plant metabolites in phytoremediation and biotransformation. Trends Biotechnol. 2003, 21, 123–130. [Google Scholar] [CrossRef]
- Chekol, T.; Vough, L.R.; Chaney, R.L. Phytoremediation of polychlorinated biphenyl-contaminated soils: The rhizosphere effect. Environ. Int. 2004, 30, 799–804. [Google Scholar] [CrossRef]
- Vergani, L.; Mapelli, F.; Zanardini, E.; Terzaghi, E.; Di Guardo, A.; Morosini, C.; Raspa, G.; Borin, S. Phyto-rhizoremediation of polychlorinated biphenyl contaminated soils: An outlook on plant-microbe beneficial interactions. Sci. Total Environ. 2017, 575, 1395–1406. [Google Scholar] [CrossRef]
- Sharma, J.K.; Gautam, R.K.; Nanekar, S.V.; Weber, R.; Singh, B.K.; Singh, S.K.; Juwarkar, A.A. Advances and perspective in bioremediation of polychlorinated biphenyl-contaminated soils. Environ. Sci. Pollut. Res. 2018, 25, 16355–16375. [Google Scholar] [CrossRef]
- Meggo, R.E.; Schnoor, J.L. Rhizospere redox cycling and implications for rhizosphere biotransformation of selected polychlorinated biphenyl (PCB) congeners. Ecol. Eng. 2013, 57, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germaine, K.J.; McGuinness, M.; Dowling, D.N. Improving Phytoremediation through Plant-Associated Bacteria. In Molecular Microbial Ecology of the Rhizosphere; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 961–973. [Google Scholar]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Uhlik, O.; Musilova, L.; Ridl, J.; Hroudova, M.; Vlcek, C.; Koubek, J.; Holeckova, M.; Mackova, M.; Macek, T. Plant secondary metabolite-induced shifts in bacterial community structure and degradative ability in contaminated soil. Appl. Microbiol. Biotechnol. 2013, 97, 9245–9256. [Google Scholar] [CrossRef] [PubMed]
- Slater, H.; Gouin, T.; Leigh, M.B. Assessing the potential for rhizoremediation of PCB contaminated soils in northern regions using native tree species. Chemosphere 2011, 84, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Segura, A.; Rodríguez-Conde, S.; Ramos, C.; Ramos, J.L. Bacterial responses and interactions with plants during rhizoremediation. Microb. Biotechnol. 2009, 2, 452–464. [Google Scholar] [CrossRef]
- Macek, T.; Macková, M.; Káš, J. Exploitation of plants for the removal of organics in environmental remediation. Biotechnol. Adv. 2000, 18, 23–34. [Google Scholar] [CrossRef]
- Gomes, H.I.; Dias-Ferreira, C.; Ribeiro, A.B. Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Sci. Total Environ. 2013, 445, 237–260. [Google Scholar] [CrossRef]
- Di Baccio, D.; Tognetti, R.; Sebastiani, L.; Vitagliano, C. Responses of Populus deltoides x Populus nigra (Populus x euramericana) clone I-214 to high zinc concentrations. New Phytol. 2003, 159, 443–452. [Google Scholar] [CrossRef]
- Sebastiani, L.; Scebba, F.; Tognetti, R. Heavy metal accumulation and growth responses in poplar clones Eridano (Populus deltoides × maximowiczii) and I-214 (P. × euramericana) exposed to industrial waste. Environ. Exp. Bot. 2004, 52, 79–88. [Google Scholar] [CrossRef]
- Meggo, R.E.; Schnoor, J.L.; Hu, D. Dechlorination of PCBs in the rhizosphere of switchgrass and poplar. Environ. Pollut. 2013, 178, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Schnoor, J.L. Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in whole hybrid poplar plants after hydroponic exposure. Chemosphere 2008, 73, 1608–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, G.; Hu, D.; Lehmler, H.-J.; Schnoor, J.L. Enantioselective biotransformation of chiral PCBs in whole poplar plants. Environ. Sci. Technol. 2011, 45, 2308–2316. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Wright, S.J.; Sardans, J.; Pérez-Trujillo, M.; Oravec, M.; Večeřová, K.; Urban, O.; Fernández-Martínez, M.; Parella, T.; Peñuelas, J. Long-term fertilization determines different metabolomic profiles and responses in saplings of three rainforest tree species with different adult canopy position. PLoS ONE 2017, 12, e0177030. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.; Schaffer, B.; Davies, F.S. Flooding, root temperature, physiology and growth of two Annona species. Tree Physiol. 2004, 24, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.D. Changes in photosynthesis and fluorescence in response to flooding in emerged and submerged leaves of Pouteria orinocoensis. Photosynthetica 2006, 44, 32–38. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant. Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef]
- Jaeger, C.; Gessler, A.; Biller, S.; Rennenberg, H.; Kreuzwieser, J. Differences in C metabolism of ash species and provenances as a consequence of root oxygen deprivation by waterlogging. J. Exp. Bot. 2009, 60, 4335–4345. [Google Scholar] [CrossRef] [Green Version]
- Parent, C.; Crèvecoeur, M.; Capelli, N.; Dat, J.F. Contrasting growth and adaptive responses of two oak species to flooding stress: Role of non-symbiotic haemoglobin. Plant. Cell Environ. 2011, 34, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of stomatal closure in plants exposed to drought and cold stress. In Survival Strategies in Extreme Cold and Desiccation; Springer: Singapore, 2018; pp. 215–232. [Google Scholar]
- Wang, F.; Wu, N.; Zhang, L.; Ahammed, G.J.; Chen, X.; Xiang, X.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; et al. Light signaling-dependent regulation of photoinhibition and photoprotection in Tomato. Plant Physiol. 2018, 176, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Banti, V.; Giuntoli, B.; Gonzali, S.; Loreti, E.; Magneschi, L.; Novi, G.; Paparelli, E.; Parlanti, S.; Pucciariello, C.; Santaniello, A.; et al. Low oxygen response mechanisms in green organisms. Int. J. Mol. Sci. 2013, 14, 4734–4761. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Dietz, K.-J. Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J. Exp. Bot. 2015, 66, 2401–2414. [Google Scholar] [CrossRef] [Green Version]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Mitra, T.; Singha, B.; Bar, N.; Das, S.K. Removal of Pb(II) ions from aqueous solution using water hyacinth root by fixed-bed column and ANN modeling. J. Hazard. Mater. 2014, 273, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant. Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Bianconi, D.; De Paolis, M.R.; Agnello, M.C.; Lippi, D.; Pietrini, F.; Zacchini, M.; Polcaro, C.; Donati, E.; Paris, P.; Spina, S.; et al. Field-scale rhyzoremediation of a contaminated soil with hexachlorocyclohexane (HCH) isomers: The potential of poplars for environmental restoration and economical sustainability. In Handbook of Phytoremediation; Nova Science Publishers: New York, NY, USA, 2011; pp. 783–794. ISBN 978-161728753-4. [Google Scholar]
- Italian Ministry of the Environment Legislative Decree no. 152, 2006, Rules in environmental field. Ital. Off. J. 2006, 88, 1–425.
- Di Lenola, M.; Barra Caracciolo, A.; Grenni, P.; Ancona, V.; Rauseo, J.; Laudicina, V.A.; Uricchio, V.F.; Massacci, A. Effects of apirolio addition and Alfalfa and compost treatments on the natural microbial community of a historically PCB-contaminated soil. Water Air Soil Pollut. 2018, 229, 143. [Google Scholar] [CrossRef]
- EFSA. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the presence of non dioxin-like polychlorinated biphenyls (PCB) in feed and food. EFSA J. 2005, 284, 1–137. [Google Scholar]
- US-EPA. Method 1668, Revision A Chlorinated Biphenyl Congeners in Water, Soil, Sediment, Biosolids, and Tissue by HRGC/HRMS; U.S. Environmental Protection Agency, Office of Water, Office of Science and Technology, Engineering and Analysis Division (4303T), 1200 Pennsylvania Avenue, NW: Washington, DC, USA, 2003; p. 20460.
- Muir, D.; Sverko, E. Analytical methods for PCBs and organochlorine pesticides in environmental monitoring and surveillance: A critical appraisal. Anal. Bioanal. Chem. 2006, 386, 769–789. [Google Scholar] [CrossRef]
- Ribani, M.; Collins, C.H.; Bottoli, C.B.G. Validation of chromatographic methods: Evaluation of detection and quantification limits in the determination of impurities in omeprazole. J. Chromatogr. A 2007, 1156, 201–205. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Grenni, P.; Cupo, C.; Rossetti, S. In situ analysis of native microbial communities in complex samples with high particulate loads. FEMS Microbiol. Lett. 2005, 253, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Barra Caracciolo, A.; Giuliano, G.; Grenni, P.; Guzzella, L.; Pozzoni, F.; Bottoni, P.; Fava, L.; Crobe, A.; Orrù, M.; Funari, E. Degradation and leaching of the herbicides metolachlor and diuron: A case study in an area of Northern Italy. Environ. Pollut. 2005, 134, 525–534. [Google Scholar] [CrossRef]
- Grenni, P.; Barra Caracciolo, A.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Changes in the microbial activity in a soil amended with oak and pine residues and treated with linuron herbicide. Appl. Soil Ecol. 2009, 41, 2–7. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Bustamante, M.A.; Nogues, I.; Di Lenola, M.; Luprano, M.L.; Grenni, P. Changes in microbial community structure and functioning of a semiarid soil due to the use of anaerobic digestate derived composts and rosemary plants. Geoderma 2015, 245–246, 89–97. [Google Scholar] [CrossRef]
- Pietrini, F.; Di Baccio, D.; Iori, V.; Veliksar, S.; Lemanova, N.; Juškaitė, L.; Maruška, A.; Zacchini, M. Investigation on metal tolerance and phytoremoval activity in the poplar hybrid clone “Monviso” under Cu-spiked water: Potential use for wastewater treatment. Sci. Total Environ. 2017, 592, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Passatore, L.; Patti, V.; Francocci, F.; Giovannozzi, A.; Zacchini, M. Morpho-Physiological and Metal Accumulation Responses of Hemp Plants (Cannabis Sativa L.) Grown on Soil from an Agro-Industrial Contaminated Area. Water 2019, 11, 808. [Google Scholar] [CrossRef]
- Ugulin, T.; Bakonyi, T.; Berčič, R.; Urbanek Krajnc, A. Variations in leaf total protein, phenolic and thiol contents amongst old varieties of mulberry from the Gorizia region. Agricultura 2015, 12, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Hernandez, M.; Fernandez-Garcia, N.; Diaz-Vivancos, P.; Olmos, E. A different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J. Exp. Bot. 2010, 61, 521–535. [Google Scholar] [CrossRef]
- Pietrini, F.; Zacchini, M.; Iori, V.; Pietrosanti, L.; Bianconi, D.; Massacci, A. Screening of poplar clones for cadmium phytoremediation using photosynthesis, biomass and cadmium content analyses. Int. J. Phytoremediation 2009, 12, 105–120. [Google Scholar] [CrossRef]
- Marmiroli, M.; Pietrini, F.; Maestri, E.; Zacchini, M.; Marmiroli, N.; Massacci, A. Growth, physiological and molecular traits in Salicaceae trees investigated for phytoremediation of heavy metals and organics. Tree Physiol. 2011, 31, 1319–1334. [Google Scholar] [CrossRef] [Green Version]
- Pajević, S.; Borišev, M.; Nikolić, N.; Arsenov, D.D.; Orlović, S.; Župunski, M. phytoextraction of heavy metals by fast-growing trees: A review. In Phytoremediation; Springer International Publishing: Cham, Vietnam, 2016; pp. 29–64. ISBN 9783319401485. [Google Scholar]
- Sylvestre, M. Prospects for using combined engineered bacterial enzymes and plant systems to rhizoremediate polychlorinated biphenyls. Environ. Microbiol. 2013, 15, 907–915. [Google Scholar] [CrossRef]
- Meggo, R.E.; Schnoor, J.L. Cleaning polychlorinated biphenyl (PCB) contaminated garden soil by phytoremediation. Environ. Sci. 2013, 1, 33–52. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Brookes, P.C.; Xu, J. Cucurbita spp. and Cucumis sativus enhance the dissipation of polychlorinated biphenyl congeners by stimulating soil microbial community development. Environ. Pollut. 2014, 184, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Ancona, V.; Barra Caracciolo, A.; Grenni, P.; Di Lenola, M.; Campanale, C.; Calabrese, A.; Uricchio, V.F.; Mascolo, G.; Massacci, A. Plant-assisted bioremediation of a historically PCB and heavy metal-contaminated area in Southern Italy. N. Biotechnol. 2017, 38, 65–73. [Google Scholar] [CrossRef]
- Campanella, B.F.; Bock, C.; Schröder, P. Phytoremediation to increase the degradation of PCBs and PCDD/Fs. Environ. Sci. Pollut. Res. 2002, 9, 73–85. [Google Scholar] [CrossRef]
- Whitfieldaslund, M.; Zeeb, B.; Rutter, A.; Reimer, K. In situ phytoextraction of polychlorinated biphenyl—(PCB)contaminated soil. Sci. Total Environ. 2007, 374, 1–12. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Ademollo, N.; Cardoni, M.; Di Giulio, A.; Grenni, P.; Pescatore, T.; Rauseo, J.; Patrolecco, L. Assessment of biodegradation of the anionic surfactant sodium lauryl ether sulphate used in two foaming agents for mechanized tunnelling excavation. J. Hazard. Mater. 2019, 365, 538–545. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Grenni, P.; Ciccoli, R.; Di Landa, G.; Cremisini, C. Simazine biodegradation in soil: Analysis of bacterial community structure byin situ hybridization. Pest Manag. Sci. 2005, 61, 863–869. [Google Scholar] [CrossRef]
- Fagervold, S.K.; May, H.D.; Sowers, K.R. Microbial reductive dechlorination of Aroclor 1260 in Baltimore harbor sediment microcosms is catalyzed by three phylotypes within the phylum Chloroflexi. Appl. Environ. Microbiol. 2007, 73, 3009–3018. [Google Scholar] [CrossRef]
- Imamoglu, I.; Christensen, E.R. PCB sources, transformations, and contributions in recent Fox River, Wisconsin sediments determined from receptor modeling. Water Res. 2002, 36, 3449–3462. [Google Scholar] [CrossRef]
- Glick, B.R. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 2005, 251, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Low, J.E.; Whitfield Åslund, M.L.; Rutter, A.; Zeeb, B.A. Effect of plant age on PCB accumulation by Cucurbita pepo ssp. pepo. J. Environ. Qual. 2010, 39, 245. [Google Scholar] [CrossRef] [PubMed]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M.; Szopiński, M.; Małkowski, E. photosynthetic efficiency as bioindicator of environmental pressure in A. halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, A.; Krupa, J.; Lesak, I. Effect of hypoxia on photosynthetic activity and antioxidative response in gametophores of Mnium undulatum. Acta Physiol. Plant. 2005, 27, 205–212. [Google Scholar] [CrossRef]
- Horchani, F.; Aloui, A.; Brouquisse, R.; Aschi-Smiti, S. Physiological responses of tomato plants (Solanum lycopersicum) as affected by root hypoxia. J. Agron. Crop Sci. 2008, 194, 297–303. [Google Scholar] [CrossRef]
- Iori, V.; Pietrini, F.; Bianconi, D.; Mughini, G.; Massacci, A.; Zacchini, M. Analysis of biometric, physiological, and biochemical traits to evaluate the cadmium phytoremediation ability of eucalypt plants under hydroponics. iForest-Biogeosciences For. 2017, 10, 416–421. [Google Scholar] [CrossRef] [Green Version]

| PCB Congener (IUPAC Number) | LOQ (pg) |
|---|---|
| 28 (7012-37-5) | 43.73 |
| 52 (35693-99-3) | 25.46 |
| 101 (37680-73-2) | 11.00 |
| 138 (35065-28-2) | 29.65 |
| 153 (35065-27-1) | 26.59 |
| 180 (35065-29-3) | 27.97 |
| Conditions | Roots (g) | Leaves (g) | Branches (g) |
|---|---|---|---|
| 6 months | |||
| MA | 1.31 a ± 1.11 | --- | 5.42 a ± 1.95 |
| Pre-sterilized | 4.55 b ± 1.30 | --- | 11.98 b ± 1.84 |
| Hypoxic | 3.35 ab ± 2.46 | --- | 11.00 b ± 0.27 |
| 12 months | |||
| MA | 5.14 a ± 0.03 | 5.77 a ± 2.26 | 8.35 a ± 0.03 |
| Pre-sterilized | 7.64 b ± 2.06 | 8.28 a ± 1.25 | 8.02 a ± 0.37 |
| Hypoxic | 5.40 ab ± 1.34 | 7.53 a ± 1.00 | 10.34 a ± 1.61 |
| Treatment | Fv/Fm | Chlorophyll (µg/cm2) |
|---|---|---|
| 4 months | ||
| MA | 0.76 a ± 0.11 | 37.40 a ± 5.76 |
| Hypoxic | 0.80 a ± 0.04 | 28.93 b ± 2.98 |
| Pre-sterilized | 0.79 a ± 0.08 | 27.97 b ± 3.96 |
| 12 months | ||
| MA | 0.80 a ± 0.06 | 31.88 a ± 2.41 |
| Hypoxic | 0.82 a ± 0.05 | 32.90 a ± 4.70 |
| Pre-sterilized | 0.82 a ± 0.11 | 31.59 a ± 5.68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogues, I.; Grenni, P.; Di Lenola, M.; Passatore, L.; Guerriero, E.; Benedetti, P.; Massacci, A.; Rauseo, J.; Barra Caracciolo, A. Microcosm Experiment to Assess the Capacity of a Poplar Clone to Grow in a PCB-Contaminated Soil. Water 2019, 11, 2220. https://doi.org/10.3390/w11112220
Nogues I, Grenni P, Di Lenola M, Passatore L, Guerriero E, Benedetti P, Massacci A, Rauseo J, Barra Caracciolo A. Microcosm Experiment to Assess the Capacity of a Poplar Clone to Grow in a PCB-Contaminated Soil. Water. 2019; 11(11):2220. https://doi.org/10.3390/w11112220
Chicago/Turabian StyleNogues, Isabel, Paola Grenni, Martina Di Lenola, Laura Passatore, Ettore Guerriero, Paolo Benedetti, Angelo Massacci, Jasmin Rauseo, and Anna Barra Caracciolo. 2019. "Microcosm Experiment to Assess the Capacity of a Poplar Clone to Grow in a PCB-Contaminated Soil" Water 11, no. 11: 2220. https://doi.org/10.3390/w11112220
APA StyleNogues, I., Grenni, P., Di Lenola, M., Passatore, L., Guerriero, E., Benedetti, P., Massacci, A., Rauseo, J., & Barra Caracciolo, A. (2019). Microcosm Experiment to Assess the Capacity of a Poplar Clone to Grow in a PCB-Contaminated Soil. Water, 11(11), 2220. https://doi.org/10.3390/w11112220








