Abstract
The efficiency of natural zeolite to remove ammonium from artificial wastewater (ammonium aqueous solutions) and to treat second cheese whey was examined, aiming to recover nitrogen nutrients that can be used for further applications, such as slow-release fertilizers. Sorption experiments were performed using artificial wastewater and zeolite of different granulometries (i.e., 0.71–1.0, 1.8–2.0, 2.0–2.8, 2.8–4.0, and 4.0–5.0 mm). The granulometry of the zeolite had no significant effect on its ability to absorb ammonium. Nevertheless, smaller particles (0.71–1.0 mm) exhibited quicker NH4+-N adsorption rates of up to 93.0% in the first 10 min. Maximum ammonium removal efficiency by the zeolite was achieved at ammonium concentrations ranging from 10 to 80 mg/L. Kinetic experiments revealed that chemisorption is the mechanism behind the adsorption process of ammonium on zeolite, while the Freundlich isotherm model fitted the experimental data well. Column sorption experiments under batch operating mode were performed using artificial wastewater and second cheese whey. Column experiments with artificial wastewater showed high NH4+-N removal rates (over 96% in the first 120 min) for all granulometries and initial NH4+-N concentrations tested (200 and 5000 mg/L). Column experiments with second cheese whey revealed that natural zeolite can remove significant organic loads (up to 40%, 14.53 mg COD/g of zeolite) and NH4+-N (about 99%). For PO43−-P, the zeolite appeared to saturate after day 1 of the experiments at a removal capacity of 0.15 mg P/g of zeolite. Desorption experiments with water resulted in low NH4+-N and PO43−-P desorption rates indicating that the zeolite could be used as a substrate for slow nitrogen release in soils.
1. Introduction
Agro-industrial development is considered to be major contributor of environmental pollution [1]. Agro-industries (e.g., dairies, olive mills, wineries) produce significant quantities of wastewaters characterized by high organic loads and nutrients [2]. Therefore, treatment of these wastewaters is necessary before their discharge.
Wastewaters originating from cheese manufacturing industries, such as cheese whey (CW), second cheese whey (SCW), and washing waters, are of high-strength as they contain significant amounts of ammonium nitrogen (0.06–0.270 g/L), total phosphorus (0.006–0.5 g/L chemical oxygen demand (COD) and biological oxygen demand (BOD) (in the range of 0.8–102.0 and 0.6–60.0 g/L, respectively) [3,4]. According to Jasko et al. [5], 45 million tons of CW are produced per year in Europe, while worldwide annual CW production is 160 million tons [6,7].
Various technologies and processes have been examined for the treatment of agro-industrial wastewaters such as cheese manufacturing industry wastewaters. These processes include physicochemical or biological systems, electrochemical methods, constructed wetlands, advanced oxidation processes, or hybrid systems including two or more of the above-mentioned methods [3,8,9]. Coagulation/flocculation is the simplest physicochemical treatment technology. However, according to Carvalho et al. [3] the treated supernatant still contains significant COD concentrations (COD removal of just 32%–50% from raw CW). Some researchers have claimed that anaerobic biological treatment is the most viable method for the treatment of CW (COD removal 81%–99% for raw CW (0.78–31.0 g COD/(L day)) [3]. However, in most anaerobic processes the residual COD concentration remains above the legal limit of 125 mg/L for industrial effluents [10]. Biological trickling filters and constructed wetlands (CWs) have proved relatively successful in treating SCW, as they can achieve high dissolved COD (d-COD) degradation rates of up to 26.3 g d-COD/(L day) for the biological trickling filters (100% dissolved COD (d-COD) removal, [8,11] and up to 685.49 g/(m2 d) for CWs [12]. Nevertheless, contemporary wastewater treatment methods should be combined with simultaneous energy and/or resource recovery. Thus, novel SCW treatment technologies should also include recovery of its naturally high nutrient content for re-use as alternative fertilizers, etc.
In recent years, wastewater treatment using zeolite as a low-cost absorbent has been examined by many researchers. Zeolites, either natural or synthetic, can improve water quality and wastewater treatment by removing substances such as heavy metals, ammonium, phosphorus, COD, dissolved organic matter, cations, and radioactive elements (Table 1). However, only a few works have focused on agro-industrial wastewater treatment using natural products such as natural zeolite. Aly et al. [13,14] proposed the treatment of olive mill wastewater by filtering it through three successive columns of gravel, fine sand and a mixture of cotton and zeolite (nanoparticles and normal zeolite particles). They found that most contaminants (K, nitrates, P, total phenols, etc.) were removed in the clinoptilolite column (third column) with nanoparticles (99.96%, 92.79%, 92.64%, 98.23%, respectively). Removal of ammonium from swine wastewaters was studied by Huang et al. [15] who revealed that parameters such as adsorbent dosage, contact time, competitive ions (i.e., Na+, K+), and pH significantly influence ammonium removal (40%–95%). The treatment of dairy wastewaters using zeolite was examined by Schierano et al. [16], Kolakovic et al. [17] and Samkutty and Gough [18]. Schierano et al. [16] studied the effect of different substrates, including natural.

Table 1.
Studies of zeolite use in wastewater treatment.
Zeolite, on the performance of a horizontal flow constructed wetland. High phosphorus and ammonium removal levels were achieved, of between 86%–99% and 88%–99%, respectively. Filtration treatment of dairy wastewater using natural zeolite was studied by Samkutty and Gough [18]. Their results showed that zeolite was the least effective medium (presenting only 30% COD removal) compared to crushed coral, charcoal, sand with crushed coral, and sand with glass beads (78%, 83%, 93%, 93%, respectively). Kolakovic et al. [17] examined dairy wastewater treatment using organo-zeolites. These organo-zeolites lead to COD, ammonium, nitrate and phosphorus removals between 29.55%–52.28%, 6.78%–20.34%, 49.68%–69.43% and 6.28%–19.37%, respectively. Zeolite has been proven to be a rather good alternative for ammonium removal, as it has a stable performance on NH4+-N, which is influenced by pH alterations (optimum pH is around 6) [23], SCW pH values are in this optimum range, therefore there was no need to examine pH effect on zeolite performance in this study.
This research investigates the treatment of SCW using natural zeolite. To the best of our knowledge, this is the first study to examine the efficiency of natural zeolites to reduce basic pollutants (i.e., COD, ammonium and phosphorus) from SCW. Initially, a series of experiments was performed using synthetic wastewater (ammonium aqueous solutions) and different granulometries of zeolite (i.e., 0.71–1.0, 1.8–2.0, 2.0–2.8, 2.8–4.0, and 4.0–5.0 mm) to study the effect of ammonium load, particle size, and contact time on ammonium removal capacity of the specific zeolite under batch operating mode. Adsorption kinetics and sorption isotherms were also proposed. A series of experiments was carried out in columns using both synthetic wastewaters and SCW at different dilution rates (100%, 75%, and 50%) to determine the feasibility of the process real conditions. Lastly, desorption experiments were carried out applying both wastewater type SCW and synthetic wastewater.
2. Materials and Methods
2.1. Artificial Wastewater and Secondary Cheese Whey
Artificial wastewater (AWW) was prepared by drying commercially available ammonium chloride at 104 °C for 24 h and then diluting it with deionized water (DW) to concentrations ranging from 1 to 5000 mg/L. The secondary cheese whey (SCW) used in this study was obtained from the factory of Papathanasiou S.A. (Agrinio, Greece), transported to the laboratory in plastic bottles and then stored at −20 °C until use at −20 °C to prevent any change in its composition. The COD concentration of the SCW was 28.500 mg/L, total Kjeldahl nitrogen (TKN) was 243 mg/L, NH4+ was 45.27 mg/L, PO43− was 196.69 mg/L, and its pH was recorded as 4.2.
2.2. Zeolite Characterization
The natural zeolite used in this research originated from Imerys Minerals Bulgaria AD (Kardzhali, Bulgaria) and was imported into Greece by ZeolifeTMCompany (Orestiada, Greece). Specifically, it is a hydrated aluminosilicate mineral of volcanic origin, with a clinoptilolite content of 85%, a moisture content of 10%–12%, while the remaining 3%–5% comprises impurities of feldspar, micas, and clays free of fibers and quartz. The chemical composition of the dry clinoptilolite is presented in Table 2. According to the technical data sheet provided, the minimum cation exchange capacity value of this specific zeolite is 150 meq/100 g. Prior to all experiments the zeolite was sieved to obtain different granulometries (i.e., 0.71–1.0, 1.8–2.0, 2.0–2.8, 2.8–4.0, and 4.0–5.0 mm), washed to remove dust, and finally dried.

Table 2.
Chemical composition of the zeolite used as analyzed by Imerys Minerals Bulgaria AD and reported in the technical data sheet provided online by ZeolifeTM Company. (https://zeolife.gr/products/zeolithos-apo-2-5-eos-5-chiliosta/).
2.3. Zeolite Characterization
Batch tests were carried out in 250 mL borosilicate bottles filled with 200 mL of AWW at low (5 mg/L) and high (200 mg/L) concentration, and 10 g of zeolite of different particle sizes (i.e., 0.71–1.0, 1.8–2.0, 2.0–2.8, 2.8–4.0, and 4.0–5.0 mm). Bottles were stirred at 200 rpm and samples were taken at regular intervals until equilibrium was achieved. In order to generate isotherm models (see Section 2.4) various concentrations of AWW were tested, at a range of 1 to 200 mg/LNH4+-N for the above mentioned granulometries. To determine the maximum amount of ammonium adsorbed per mass of zeolite at equilibrium (qe), additional batch adsorption experiments were conducted for zeolite of grain size 4–5 mm, using higher ammonium feed concentrations (i.e., 500, 1000, 2000, 3000, 4000, and 5000 mg/L).
Desorption was tested by placing the used zeolite sample (approximately 10 g) into a volumetric flask containing 200 mL of deionized water. The flasks were stirred at 200 rpm and samples were taken at regular intervals until equilibrium was reached [49].
2.4. Ammonium Adsorption Kinetics and Isotherms
2.4.1. Ammonium Adsorption Kinetics
The kinetics of ammonium adsorption by the zeolite was mathematically analyzed by applying pseudo-first order [50] and pseudo-second order [51] models according to the following non-linearized equations:
where qe (mg/g) and qt (mg/g) denote the amount of ammonium adsorbed per mass of zeolite at equilibrium and at any time t (min), respectively, and k1 (1/min) and k2 (g/mg min) are the rate constants for the pseudo-first order and pseudo-second order models.
2.4.2. Ammonium Isothermal Curves
The relationship between the ammonium concentration in solution and the various zeolite granulometries was studied using two adsorption isotherm models. The experimental equilibrium data were analyzed by applying isotherms with two parameter equations, Freundlich and Langmuir, that are frequently used in the literature [52,53].
The Freundlich isotherm describes heterogeneous adsorption systems [54] and the non-linearized form of this isotherm is expressed by the following equation:
where qe (mg/g) is the amount of ammonium adsorbed on the zeolite at equilibrium, Ce (mg/L) is the ammonium concentration at equilibrium, kF (mg/g)/(mg/L)n is the Freundlich constant, and n (dimensionless) is the Freundlich intensity parameter which indicates the magnitude of the adsorption driving force or the surface heterogeneity.
The Langmuir isotherm has been commonly used for the removal of a wide variety of compounds from waters using different adsorbents. It assumes monolayer coverage of the adsorbate over a homogeneous adsorbent surface [55]. The non-linearized model of Langmuir is described as:
where qe (mg/g) is the amount of ammonium adsorbed on the zeolite at equilibrium, qm (mg/g) is the maximum monolayer adsorption capacity of the zeolite, Ce (mg/L) denotes the equilibrium concentrations of ammonium, and kL (L/mg) is a constant related to the affinity between the adsorbent and adsorbate. The linearized model of Langmuir is described by the following equation:
The Langmuir isotherm model expressed extensively in terms of the separation factor or equilibrium parameter (RL) [56] can be given as:
where C0 (mg/L) is the initial maximum concentration of the analyzed ammonium.
The nature of the adsorption can be estimated through the isotherm profile according to the values of RL and the Freundlich exponent (n), being irreversible (RL = 0), favorable (0 < RL < 1), linear (RL > 1), or unfavorable (RL > 1) [57]. The Langmuir theory can be applied to homogeneous adsorption where each adsorbed species involves the same sorption activation energy.
Fitting of the kinetic and isotherm equations to the experimental data was evaluated using the coefficient of determination (R2).
2.5. Adsorption and Desorption Tests in Laboratory-Scale Columns Using AWW and SCW
Adsorption experiments were carried out in laboratory-scale columns. The columns used were Plexiglas tubes, 40.0 cm high and 4.0 cm internal diameter, equipped with four sample valves placed at different heights. The columns were first operated in batch mode and were filled with 500 g zeolite of various granulometries (as described in Section 2.2) and fed with 380 mL of either AWW at concentrations of 200 and 5000 mg/L or SCW diluted with tap water to different dilution ratios (i.e., 100%, 75%, and 50%). Samples were collected at regular intervals from the lower sample valve.
To achieve complete zeolite saturation, the columns were fed with an aqueous solution of ammonium chloride of concentration 5000 mg/L. Subsequently, the columns were fed with 380 mL of deionized water to study the desorption capacity of the zeolite [49]. Samples were taken at regular time intervals until equilibrium was achieved.
2.6. Analytical Methods
Total ammonium concentration was measured using the salicylate test as initially described by Verdouw et al. [58] with some modifications. In particular, filtered samples (0.45 µm, Whatman) reacted with sodium hypochloride solution 6% and salicylate/catalyst solution (sodium salicylate 10%, sodium nitroferricyanide 0.04%, and sodium hydroxide 0.5%). After color development, ammonium concentration was determined colorimetrically at 625 nm using a spectrophotometer (Hach Lange DR 500, Hach Lange, Loveland, CO, USA).
Ammonium and orthophosphoric ions were determined in filtered samples (0.2 µm, Whatman) by ion exchange chromatography (Thermo Dionex ICS-5000DC, Thermo Fischer Scientific, Wien, Austria) equipped with an AERS 500 (4 mm) conductivity detector. For anion determination, an IonPac AS23 (4 mm × 250 mm) column was used, eluted with 4.5 mM Na2CO3/0.8 mM NaHCO3 at flow rate 1.0 mL/min and T = 30 °C. For cation determination, an IonPac CS12A (2 mm × 250 mm) column was used, eluted with 20 mM H2SO4 at flow rate 1.0 mL/min and at room temperature.
Chemical oxygen demand (COD) was determined in filtered samples (0.45 µm, Whatman) using the “closed reflux” method following Standard Methods [59]. A HANNA Reactor C98000 was applied to measure digestion and absorbance was recorded using a Multiparameter Bench Photometer (HANNA C99, Hanna Instruments, Woonsocket, RI, USA). Total Kjeldahl nitrogen (TKN) was measured using the titrimetric method according to Standard Methods [59]. Finally, pH and conductivity were measured with a multiparameter meter (HANNA HI5521, Hanna Instruments, Woonsocket, RI, USA). All the reagents used in this study were of analytical grade.
3. Results and Discussion
3.1. Adsorption Batch Tests
3.1.1. Effect of Particle Size
Initially, the effect of zeolite particle size on its adsorbent capacity was tested for AWW of concentration 5 mg NH4+-N mg/L. NH4+-N is mainly removed with zeolite through ion exchange activity, which follows the order NH4+ > Na+ > Ca2+ [40]. According to the results, no great differences were recorded between the granulometries tested (Figure 1). For each grain size tested, ammonium removal by zeolite occurred rapidly within the first 60 min of contact time (removals ranged from 85.6%–98.8% depending on the granulometry). The removal rate of ammonium was initially fast then gradually decreased until equilibrium state which was achieved at 1440 min. Nevertheless, the finest zeolite particles (0.71–1.0 mm) demonstrated quicker NH4+-N adsorption, especially during the first 60 min (98.8%). This is attributed to their higher specific surface area which increases external surface adsorption, as also reported in other relative studies [29,32,60,61,62]. According to Wang et al. [62], grain size affects the amount of seawater NH4+-N adsorbed on zeolite, with smaller sized granules giving better results. However, Leyva-Ramos et al. [63] reported that decreasing the grain size does not significantly increase the adsorption capacity of zeolite. Zeolite is a porous material and interacts with the solution with both external and internal (pores and channels) surface area. By reducing particle size the external area increases considerably but the same does not apply for the internal area of the zeolite. Moreover, its exchange capacity is dependent on the cationic sites which are located inside the pores of the zeolite and the channels and cavities of the zeolite crystalline structure and not on its external area [64,65].
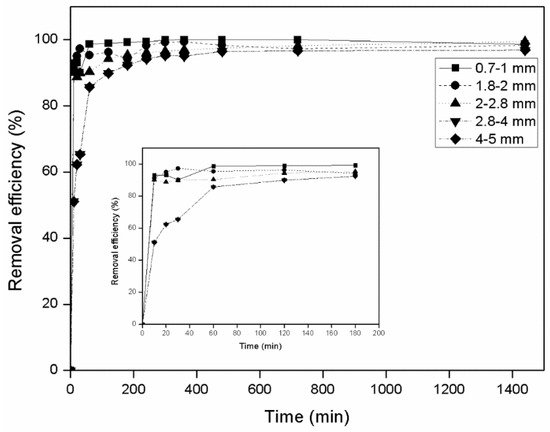
Figure 1.
Effect of zeolite particle size on ammonium removal (5 mg/L NH4+-N, T = 25 °C, 200 rpm, 50 g zeolite/L). Error bars indicate standard deviation of triplicate measurements.
Taking into consideration the above and that the present study aimed to apply the zeolite in adsorption columns, a grain size of 4–5 mm was selected for later experiments. Zeolite grains smaller than 4 mm may cause clogging when used as substrate in columns, trickling filters and constructed wetlands.
3.1.2. Effect of Contact Time
Adsorption of ammonium on zeolite was studied at various time intervals for low (5 mg/L) and high (200 mg/L) initial concentrations of AWW, and the results obtained are presented in Figure 2. The amount of ammonium retained by the zeolite increased gradually until adsorbent saturation while equilibrium was reached within 180 min for the low initial AWW concentration and within 300 min for the high initial AWW concentration. The removal efficiency of ammonium ions by the zeolite was initially fast, with up to 85% being achieved within the first 60 min for AWW of 5 mg/L. The rate then slowed considerably with increased contact time and remained almost constant before reaching equilibrium. After 2880 min ammonium removal had reached up to 99% for both initial AWW concentrations tested. These results are similar to those recorded in other relevant studies [66,67]. Using batch experiments, Martins et al. [66] reported that ammonium removal by natural zeolite (originating from Cuba) occurred within the first 180 min of contact time. Removal rates were initially fast then gradually decreased with increased contact time. Alshameri et al. [67] observed that natural zeolite from China (Hulaodu) exceeded 78% removal rate at 300 min but then plateaued. This behavior may be attributed to the rapid utilization of the zeolite’s available adsorbing sites followed by fast diffusion into its pores and channels until equilibrium is achieved [29,68].
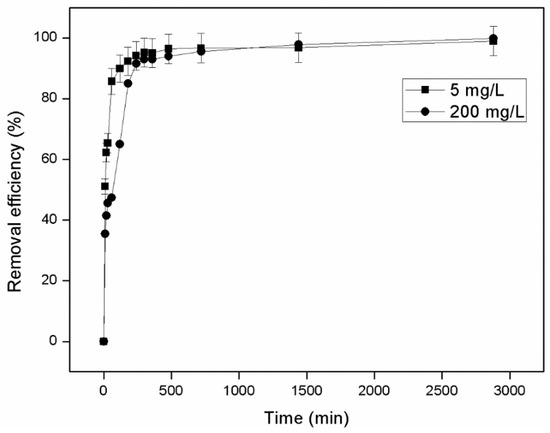
Figure 2.
Effect of contact time on ammonium removal (5 and 200 mg/L NH4+-N, T = 25 °C, 200 rpm, 50 g zeolite/L, 4–5mm). Error bars indicate standard deviation of triplicate measurements.
3.1.3. Effect of Initial Concentration
The effect of initial ammonium concentration on zeolite’s removal efficiency was studied and the results are shown in Figure 3. The experimental data indicated that the maximum removal efficiency of the zeolite was achieved with ammonium concentrations ranging from 10 to 80 mg/L, while increasing the initial ammonium concentration decreased its removal percentage (Figure 3). Similar results were observed by Sarioglu [69] who reported that maximum removal efficiency was achieved at concentrations of 8.8 to 40 mg/L, leading to the conclusion that initial ammonium concentration plays a key role in the adsorption mechanism of ammonium on zeolite. Huang et al. [29] also observed that the efficiency of a zeolite (Chende zeolite from China) to remove ammonium (initial NH4+ concentration was 80 mg/L) decreased when the concentration of ammonium in solution was increased. Alshameri et al. [67] also reported that maximum ammonium removal was achieved at initial concentrations ranging from 10 to 50 mg/L. These results can be expected for porous materials such as zeolite. Increasing the initial ammonium concentration leads to an increase of the mass transfer driving force and consequently the rate at which ammonium ions pass from the bulk solution to the surface of the zeolite. Above a critical concentration, where the driving force is high, the ammonium ions migrate from the zeolite’s surface to its core (pores and channels) and when all the ion exchange processes are completed equilibrium state is achieved [70,71].
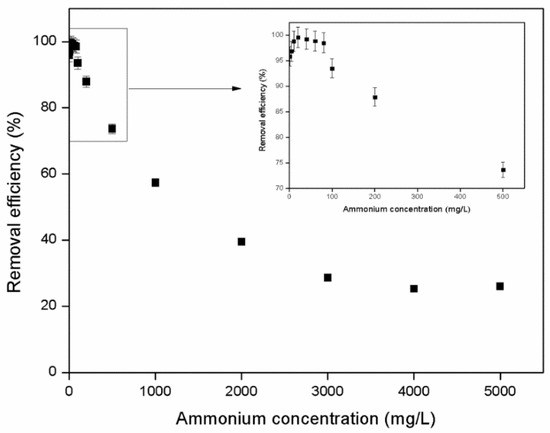
Figure 3.
Effect of initial ammonium concentration on the removal of NH4+-N by the zeolite (T = 25 °C, 200 rpm, 50 g zeolite/L, 4–5mm). Error bars indicate standard deviation of triplicate measurements.
3.1.4. Kinetic Models
To comprehend the mechanism involved during the adsorption of ammonium on zeolite and the potential controlling steps, two kinetic models were applied and evaluated, namely, pseudo-first order and pseudo-second order. The best fit model was based on both the linear regression correlation coefficient (R2) and the calculated qe values. According to the results obtained (Table 3), low R2 values and notable variances between the experimental and theoretical uptakes reveal clearly the poor fitting of the pseudo-first order model. On the contrary, the pseudo-second model shows better fitting, for all the initial tested ammonium concentration, which is in agreement with previous research [29,72,73]. A fitting to the pseudo-second order reveals that chemisorptions dominate the adsorption process of ammonium on zeolite, and that this process involves three stages: (i) diffusion from the liquid state to the liquid–solid interface, (ii) movement of ammonium ions toward the solid surface, and (iii) movement of ammonium ions to the pores and channels of the zeolite [74].

Table 3.
Fitting kinetics parameters of adsorption of ammonium onto zeolite according to pseudo-first order and pseudo-second order models at various N-NH4+ concentrations (zeolite granulometry: 4.0–5.0 mm).
3.1.5. Equilibrium Isotherms
The two most popular isothermal models, Langmuir and Freundlich (see Section 2.4.2) were employed to describe the equilibrium adsorption isotherms. The estimated parameters and error function values for the applied adsorption isotherms are shown in Table 4. The Freundlich isotherm model shows higher relative R2 values, thus indicating that the Freundlich model fits the experimental data better than the Langmuir model. A 1/n value of less than 1 indicates that NH4+-N adsorbance on zeolite is favorable. However, according to the literature, the optimal adsorption isotherm for NH4+ adsorption on zeolite with different characteristics can vary. Many studies suggest that the Freundlich isothermal model fits better in describing NH4+-N adsorption on zeolite (natural or modified) [15,23,24,29,48,75], while others propose that the Langmuir model has the better fitting [24,30,37,76].

Table 4.
Isotherm constants for ammonium exchange.
3.2. Desorption Batch Tests
Natural zeolite is particularly useful for controlled agricultural soil fertilization as it provides the slow release of nutrients, such as N and P, which help ensure high crop yields. For this reason, desorption experiments were performed to test its suitability for direct use as a soil additive. The desorption tests were conducted using deionized water and the results showed that ammonium release was remarkably slow and final desorption equilibrium was achieved after 7200 min, at which time only 1.59% of the absorbed ammonium had been released. These results may be explained by the characteristics of the aqueous solution used in the desorption experiments [41] since the solution lacks any ion exchange capacity to release and replace ammonium anions. According to the literature, a strong ion exchange solution is needed to regenerate used zeolite, such as sodium hydroxide that can increase NH4+-N desorption rates by up to 99% [42].
3.3. Adsorption and Desorption Experiments on Laboratory Scale Column Using AWW
Following the batch adsorption experiments, column experiments were carried out to treat AWW. Previous column adsorption experiments (using zeolitic adsorbents) have shown that fixed-bed columns are more efficient than fluidized bed columns [22,77]. Therefore, fixed-bed column experiments were performed here to study the removal of ammonium from AWW and then treat SCW. Figure 4 presents the results of NH4+-N adsorption obtained in column kinetic experiments (under batch operation) for an initial ammonium concentration of 200 mg/L using natural zeolite of various granulometries. Each experimental column contained 500 g of zeolite and was filled with 380 mL of NH4+-N aqueous solution. For all granulometries tested the solution covered the zeolite entirely. It was observed that ammonium ion concentrations decreased more sharply in the experiments with smaller-sized fractions. Τhis result was expected because total surface area increases as particle size decreases. Specifically, the times required to meet the discharge standard of 5 mg/L (discharge limit for total nitrogen) were: <5 min for granulometries of 0.71–1.0 mm, 20 min for 1.8–2.0 mm, 30 min for 2.0–2.8 mm, and >60 min for 2.8–4.0 mm and 4.0–5.0 mm. For all the particle sizes tested, the zeolite was able to absorb almost all available NH4+-N (above 99%) in the first 120 min, while 1440 min were required to reach equilibrium (99.7% of the NH4+-N was absorbed). However, similar results were not observed when the initial concentration of NH4+-N was 5000 mg/L (Figure 5). At this concentration, only zeolite with 0.71–1.0 mm sized particles was able to remove over 99% of the NH4+-N (99.9%, 3.8 mg/g) in 120 min. All the other granulometries established equilibrium much later, at 1440 min, with 96%–97% NH4+-N removal. According to the literature, 3.8 mg/g falls within the range of the recorded ammonium capacity for natural zeolites (2.7–22.9 mg/g) [60].
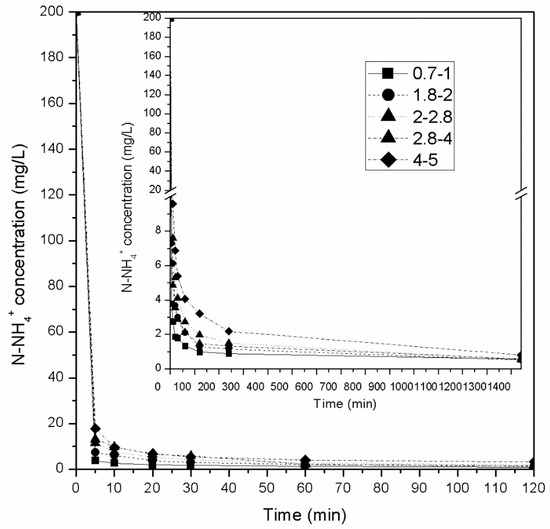
Figure 4.
Effect of contact time on ammonium removal by natural zeolite of different particle sizes in column experiments and with initial NH4+-N concentration of 200 mg/L.
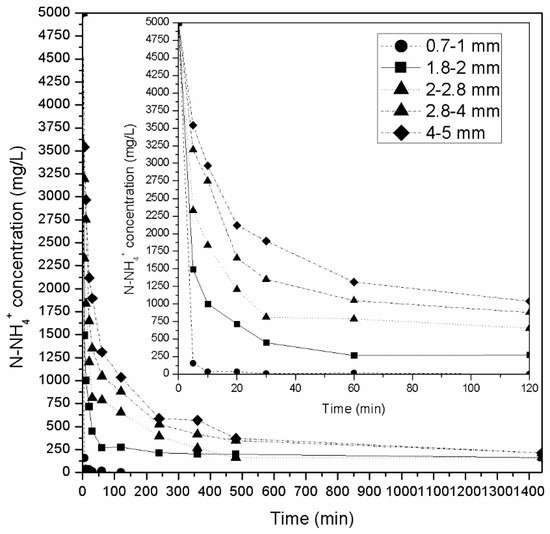
Figure 5.
Effect of contact time on ammonium removal by natural zeolite of different-sized particles in column experiments with an initial NH4+-N concentration of 5000 mg/L.
Following the same procedure as the batch sorption tests, experiments were then performed to investigate the degree of desorption of sorbed ammonium from the zeolite using deionized water. These experiments aimed to study the slow release of nitrogen [49] and were performed for all granulometries and an initial NH4+-N concentration of 5000 mg/L. Specifically, 380 mL of deionized water was added into each column. The results are presented in Table 5 and are in line with those of the adsorption experiments (Table 4), as finer-grained zeolite (i.e., 0.70–1.0 mm and 1.8–2.0 mm) desorbed higher amounts of NH4+-N (over 4% of the absorbed NH4+-N) than coarser zeolite (i.e., 2.0–2.8, 2.8–4.0, and 4.0–5.0 mm) (between 2.77% and 3.8% of the absorbed NH4+-N). Finally, the NH4+-N desorption equilibrium was reached after 11,520 min (192 h). The rates of NH4+-N desorption recorded in the column experiments were also low compared with those of previous related studies in which tap or deionized water was used for desorption [49,78]. Specifically, Cyrus and Reddy [49] showed that after 250 h of desorption with water, a significant amount of nitrogen remained in the column (>20%) and was available for desorption using only 0.1 N HCl, thus confirming that zeolite could be a suitable medium for slow nitrogen release in soils. Hedstrom and Amofah [78] also studied NH4+-N desorption and found that only 23% of NH4+-N was desorbed by tap water, while desorption was more pronounced in saturated conditions.

Table 5.
Results of the column desorption experiments.
3.4. Sorption Column Studies Using SCW
Column sorption experiments under batch operation were also performed using pre-treated SCW. The pre-treatment step included a biological trickling filter and the COD concentration of the SCW used was 29,000 mg/L. A series of experiments was carried out in columns using SCW at different dilution ratios (100%, 75%, and 50%) to determine the feasibility of the adsorption process using the specific zeolite. The three different columns used for this experiment were filled with zeolite of the coarser grain (4.0–5.0 mm) to prevent pore clogging. The first column was fed with undiluted SCW, the second was fed with a solution of 75% SCW and 25% tap water, and the third was fed with a solution of 50% SCW and 50% tap water. The columns were fed with fresh wastewater every day (380 mL on day one, 320 mL on day two, and 310 mL on day three) as the water was absorbed by the zeolite, and samples were taken at specific time intervals to determine the kinetics of COD and NH4+-N removal. The total duration of the experiments was three days.
Figure 6 presents the time series data for COD removal in the three columns. A short, three-day, experimental duration was chosen to avoid the formation of biofilm that could interfere with the zeolite’s adsorption ability. All three columns presented the same pattern concerning COD removal, as maximum removal was observed in day one of the experiments (i.e., 36.89 for 100% SCW (7.7 mg COD/g absorbed); 24.33% for 75% SCW (3.78 mg COD/g absorbed); 23.14% for 50% SCW (2.52 mg COD/g absorbed). COD removal rates were observed to decrease in the following two days (Day two: 19.29% for 100% SCW (3.52 mg COD/g absorbed); 20% for 75% SCW (2.88 mg COD/g absorbed); 19.31% for 50% SCW (1.79 mg COD/g absorbed); Day three: 17.63 for 100% SCW (3.31mg COD/g absorbed); 17.11% for 75% SCW (2.38 mg COD/g absorbed); 15.86% for 50% SCW (1.43 mg COD/g absorbed)). The total adsorption capacity of the zeolite recorded in these experiments was 14.53, 9.04, and 5.74 mg COD/g of zeolite for 100%, 75%, and 50% SCW, respectively. Although COD removal decreased with time, the zeolite was probably not fully saturated as it continued to remove organic matter. Nevertheless, the decrease in COD removal does indicate partial zeolite saturation.
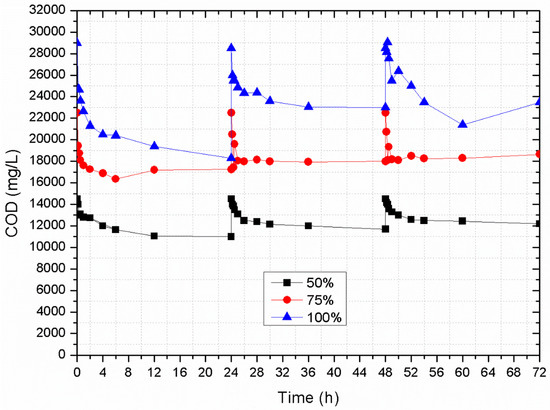
Figure 6.
Effect of contact time on chemical oxygen demand (COD) removal by natural zeolite, granulometry 4.0–5.0 mm in column experiments using second cheese whey (SCW) at different dilution rates (100%, 75%, and 50%).
The ability of zeolites to absorb organic matter has been previously reported in various studies [17,18,39]. Kolakovic et al. [17] reported that COD reduction from dairy wastewater using organo-zeolite and a filter column ranged from 30% to 50% when a mean initial COD concentration of 2676 mg/L was applied. Nevertheless, the exact mechanism of COD removal was not examined by Kolakovic et al. [17] and was attributed to the zeolite’s ability to absorb both organic and inorganic substances. Samkutty and Gough [18] reported higher COD removal (76%) when applying dairy processing wastewater with low initial COD concentrations (between 713 and 1410 mg/L), however provided no data on the zeolite used. High COD removal (71.8%) was also recorded by Guo et al. [48], however this research studied the treatment of swine wastewater using a composite of MgO-modified zeolite and a bioflocculant rather than a natural zeolite. Lakdawala and Patel [44] studied the use of natural zeolite for the removal of both COD and BOD from sugar industry wastewater and reported COD removal rates of up to 26.6%. These values are similar to those presented in this study. However, direct comparison of the above results with previous studies is not currently possible, since almost all adsorption experiments with zeolite were performed in batch systems (flasks) or in columns under continuous operation and not in columns under batch operation.
To investigate the possible removal of COD by biodegradation, a void column was loaded only with SCW and was operated under the same experimental conditions as the other three columns. At the end of day one COD concentration had decreased by about 8% (due to the indigenous microorganisms existing in the SCW), thus confirming the hypothesis that COD in the other three columns was removed mainly through adsorption by the zeolite.
Table 6 presents the daily initial and final concentrations of nitrogen and phosphorus recorded in the three columns. The zeolite filters managed to remove most of the TKN and NH4+-N as approximately 80% of TKN and 99% of NH4+-N was removed daily. The fact that NO2−-N was not recorded and that NO3−-N concentrations were slightly increased during the experiments, indicates that biological removal of nitrogen is limited and can be neglected. Therefore, nitrogen removal can be attributed to adsorption on zeolite particles. From Table 6 it can be seen that in the experiments with raw SCW (100%) (containing the highest initial NH4+-N concentration: 45.3 mg/L), NH4+-N was not completely removed even after 24 h (final NH4+-N concentration: 0.41 mg/L). It should be mentioned that in the experiment on AWW with an initial NH4+-N concentration of 200 mg/L and zeolite particles of 4–5 mm, NH4+-N removal reached 99.7% after 1440 min (1 day), while when using SCW with lower initial ammonium concentration (45.3 mg/L) 99% removal was recorded. As reported by Huang et al. [15], NH4+-N adsorption on zeolite is affected by the presence of competitive cations (i.e., Na+, K+, Ca2+, and Mg2+) that occupy the available ion-exchange sites on the zeolites. The SCW used in this study contained significant concentrations of the above-mentioned cations (initial concentrations in the raw SCW were 1188 mg/L, 783.8 mg/L, 312.2 mg/L, and 73.1 mg/L, of Na+, K+, Ca2+, and Mg2+, respectively), therefore, NH4+-N removal was reduced compared to experiments where aqueous NH4+-N solutions were used.

Table 6.
Initial and final concentrations of nitrogen and phosphorus in the columns treating secondary cheese whey (SCW).
Adsorption of PO43−-P was observed to be less than that of NH4+-N, as the zeolite appears to become saturated after day one (day one: 77%–79%, day two: 20%–30%, and day three: 7%–17% for all three SCW dilution ratios), this phenomenon was expected as zeolite ability on adsorbing PO43−P is rather limited compared to its ability adsorbing NH4+-N [17,34]. PO43−-P is mainly adsorbed on zeolite through an inner-sphere complexation [28]. The maximum adsorption capacity of the zeolite for PO43−-P (days one to three) was 0.15 mg P/g zeolite, and was observed in experiments with raw SCW (100%). Zeolite’s low adsorption capacity for PO43−-P was also reported by Drizo et al. [79], who recorded a maximum capacity of 0.46 mg P/g. Lin et al. [33] stated that zeolite’s ability for PO43−-P adsorption increases when pH values exceed the value of 9, while for neutral pH values adsorption capacity is limited to 0.30 mg P/g of zeolite. Adsorption capacity for PO43−-P could be increased to 8.2 mg P/g when the zeolite is impregnated with hydrated metal oxides [26], or to 17.2 mg P/g when zeolite is modified with lanthanum oxide [28]. Although modified zeolites show up to 400% improved adsorption capacity for PO43−-P, this value is still low (1.58 mg P/g of zeolite) compared to that for NH4+-N [34]. The relatively limited ability of zeolite for PO43−-P removal was also reported by Kolakovic et al. [17], who examined its use in dairy wastewater treatment and reported extremely low phosphorus removal (approximately 20%), even though the zeolite was modified with stearin-dimethyl-benzyl ammonium chloride.
To determine the COD desorption rate, the experimental columns were filled with deionized water (DI) which was replaced every 24 h, while COD was measured at regular intervals. After two days, almost all the absorbed COD was recovered, indicating that organic substances are not firmly bound onto zeolite grains. In contrast to COD, zeolite desorbed part of the absorbed NH4+-N (1%) and PO43−-P (23.2%). Therefore, the use of an appropriate quantity of zeolite in soils may promote the slow release of N from zeolite without the desorbed COD affecting plant growth.
3.5. Effect of Temperature and Biofilm Growth
During the three-day column experiments, the zeolite did not become saturated, as it was observed to remove most of the nitrogen present. Therefore, additional prolonged column experiments were conducted using initial COD concentrations of 2600 ± 200 mg/L. The experimental set-up was identical to that described above, extending only the experimental duration to six days. Furthermore, three additional columns were operated inside a refrigerator (4 °C) to prevent biofilm formation and inhibit any biological processes. Table 7 presents the mean values and standard deviations of all pollutant concentrations at the start and end of each experimental day. Concerning COD, final concentrations from day 1 to day 3 did not present great differences (COD removal 16%–23%), from those observed during the previous three-day experiments, thus verifying that no significant biofilm growth occurred in the columns in the first three days. However, from day four to six, the columns operating at ambient temperature (approximately 22 °C) achieved higher COD removal rates (28%–36%) than the refrigerated columns (16%–22%), indicating that biodegradation of organic matter occurs from day four onward.

Table 7.
Initial and final concentration of nitrogen and phosphorus in the columns, concerning the experiment for temperature effect.
TKN and PO43−-P adsorption by zeolite do not seem to be greatly affected either by temperature or by biofilm formation, as final concentrations of both pollutants did not differ significantly between the refrigerated columns and those at ambient temperature throughout the experiment. It is possible however that the concentrations of these pollutants were so low that any decrease in them could not be detected.
4. Conclusions
In this study, natural Bulgarian zeolite was investigated for its effectiveness to remove ammonium from synthetic wastewater and to treat second cheese whey. Batch experiments were performed in both flasks and fixed-bed columns to examine the effect of various operating conditions on zeolite performance. The conclusions reached from this study are:
- Batch experiments revealed that zeolite granulometry had no significant effect on its ability to absorb ammonium, while maximum removal efficiency was achieved at ammonium concentrations ranging from 10 to 80 mg/L.
- The pseudo-second order model fitted the experimental data thus revealing that chemisorption is the mechanism for the adsorption process of ammonium on zeolite. The Freundlich isotherm model best fit the experimental data.
- Experiments in columns using synthetic wastewater with an initial NH4+-N concentration of 200 mg/L revealed that for all granulometries tested, the zeolite was able to absorb almost all available NH4+-N (over 99%) in the first 120 min. The same was not observed for initial concentrations of 5000 mg/L where only zeolite with grain sizes of 0.71–1.0 mm managed to remove over 99% NH4+-N (99.9%) in 120 min. All other zeolite granulometries reached equilibrium much later (24 h, 96%–97% removal of NH4+-N).
- Natural zeolite may be used as an alternative substrate for second cheese whey treatment, as significant removal of organic load (up to 40%, 14.53 mg COD/g of zeolite) and NH4+-N (about 99%) can be achieved. Concerning PO43−-P, the zeolite appeared to saturate after day one of the experiments at a removal capacity of 0.15 mg P/g of zeolite. Desorption experiments with deionized water in batch and columns presented low desorption rates for NH4+-N and PO43−-P, thus indicating that this zeolite could be used as substrate for slow nitrogen release in soils.
- Prolonged use of zeolite in SCW treatment (after three days) led to the formation of biomass by increasing the percentage of organic load removal.
Author Contributions
Conceptualization, C.S.A. and D.V.V.; Methodology, C.S.A, A.K., D.A., T.I.T., A.G.T., and D.V.V.; Validation, C.S.A, A.K., D.A., T.I.T., and A.G.T.; Formal analysis, C.S.A, I.E.T., T.I.T., A.G.T., and D.V.V.; investigation, A.K., D.A., and T.I.T.; Writing—original draft preparation, C.S.A, I.E.T., and A.G.T.; Writing—review and editing, C.S.A, A.G.T., and D.V.V.; supervision, C.S.A, A.G.T., and D.V.V.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank ZeolifeTM and Chrysovallantis Chatzigeorgiou for providing the zeolite used in this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alayu, E.; Yirgu, Z. Advanced technologies for the treatment of wastewaters from agro-processing industries and cogeneration of by-products: A case of slaughterhouse, dairy and beverage industries. Int. J. Environ. Sci. Technol. 2018, 15, 1581–1596. [Google Scholar] [CrossRef]
- Abdallh, M.N.; Abdelhalim, W.S.; Abdelhalim, H.S. Industrial Wastewater Treatment of Food Industry Using Best Techniques. Int. J. Eng. Sci. Invent. 2016, 5, 15–28. [Google Scholar]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A. Treatment of Cheese Processing Wastewater by Physicochemical and Biological Methods. Int. J. Microbiol. Res. 2013, 4, 321–332. [Google Scholar]
- Jasko, J.; Skripts, E.; Dubrovskis, V.; Zabarovskis, E.; Kotelenecs, V. Biogas production from cheese whey in two phase anaerobic digestion. Eng. Rural Dev. 2011, 373–376. [Google Scholar]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- OECD-FAO, Agricultural Outlook 2014. Highlights. Paris. Available online: http://www.oecd-ilibrary.org/agriculture-and-food/oecdfao-agricultural-outlook_19991142 (accessed on 10 November 2014).
- Tatoulis, T.I.; Tekerlekopoulou, A.G.; Akratos, C.S.; Pavlou, S.; Vayenas, D.V. Aerobic biological treatment of second cheese whey in suspended and attached growth reactors. J. Chem. Technol. Biotechnol. 2015, 90, 2040–2049. [Google Scholar] [CrossRef]
- Chatzipaschali, A.A.; Stamatis, S.A. Biotechnological utilization with a focus on anaerobic treatment of cheese whey: Current status and prospects. Energies 2012, 5, 3492–3525. [Google Scholar] [CrossRef]
- Gazette of the Greek Government (GR) 2011/354B of 8 March 2011 on the “Establishment of Measures, Conditions and Procedures for the Reuse of Treated Wastewater and Other Provisions”. Available online: www.et.gr (accessed on 11 January 2019).
- Tatoulis, T.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V.; Stefanakis, A.I. A novel horizontal subsurface flow constructed wetland: Reducing area requirements and clogging risk. Chemosphere 2017, 186, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.-Y.; Mourti, C.; Tatoulis, T.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Effect of hydraulic retention time, temperature, and organic load on a horizontal subsurface flow constructed wetland treating cheese whey wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 726–732. [Google Scholar] [CrossRef]
- Aly, A.A.; Hasan, N.Y.Y.; Al-Farraj, S.A. Olive mill wastewater treatment using a simple zeolite-based low cost method. J. Environ. Manag. 2014, 145, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Alashgar, N.S.K.; Al-Farraj, S.A.; Ibrahim, M.H. Contaminants and salinity removal of olive mill wastewater using zeolite nanoparticles. Sep. Sci. Technol. 2018, 53, 1638–1653. [Google Scholar] [CrossRef]
- Huang, H.; Yang, L.; Xue, Q.; Liu, J.; Hou, L.; Ding, L. Removal of ammonium from swine wastewater by zeolite combined with chlorination for regeneration. J. Environ. Manag. 2015, 160, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Schierano, M.C.; Maine, M.A.; Panigatti, M.C. Dairy farm wastewater treatment using horizontal subsurface flow wetlands with Typhadomingensis and different substrates. Environ. Technol. 2017, 38, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kolakovic, S.; Stefanovic, D.; Milicevic, D.; Trajkovic, S.; Milenkovic, S.; Kolakovic, S.; Andjelkovic, L. Effects of reactive filters based on modified zeolite in dairy industry wastewater treatment process. Chem. Ind. Chem. Eng. Q. 2013, 19, 583–592. [Google Scholar] [CrossRef]
- Samkutty, J.P.; Gough, H.R. Filtration treatment of dairy processing wastewater. J. Environ. Sci. Health Part A 2002, 37, 195–199. [Google Scholar] [CrossRef]
- An, S.-W.; Jeong, Y.-C.; Cho, H.-H.; Park, J.-W. Adsorption of NH4+-N and E. coli onto Mg2+-modified zeolites. Environ. Earth Sci. 2016, 75, 437. [Google Scholar] [CrossRef]
- Beebe, D.A.; Castle, J.W.; Rodgers, J.H., Jr. Treatment of ammonia in pilot-scale constructed wetland systems with clinoptilolite. J. Environ. Chem. Eng. 2013, 1, 1159–1165. [Google Scholar] [CrossRef]
- Das, P.; Prasad, B.; Singh, K.K.K. Applicability of zeolite based systems for ammonia removal and recovery from wastewater. Water Environ. Res. 2017, 89, 840. [Google Scholar] [CrossRef]
- Delkash, M.; Bakhshayesh, E.B.; Kazemian, H. Using zeolitic absorbents to clean up special wastewater streams: A review. Microporous Mesoporous Mater. 2015, 214, 224–241. [Google Scholar] [CrossRef]
- Ding, Y.; Sartaj, M. Statistical analysis and optimization of ammonia removal from aqueous solution by zeolite using factorial design and response surface methodology. J. Environ. Chem. Eng. 2015, 3, 807–814. [Google Scholar] [CrossRef]
- Dong, Y.-B.; Lin, H. Ammonia nitrogen removal from aqueous solution usingzeolite modified by microwave-sodium acetate. J. Cent. South Univ. 2016, 23, 1345–1352. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Modification of a natural zeolite with Fe(III) for simultaneous phosphate and ammonium removal from aqueous solutions. J. Chem. Technol. Biotechnol. 2016, 91, 1737–1746. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Liu, J. Ion-exchange capability for ammonium removal using zeolite modified by potassium permanganate. Chem. Eng. Trans. 2016, 55, 163–168. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Dong, Y.; Wang, L. Preferable adsorption of phosphate using lanthanum-incorporated porous zeolite: Characteristics and mechanism. Appl. Surf. Sci. 2017, 426, 995–1004. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Yan, B.; Yang, L. Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. J. Hazard. Mater. 2010, 175, 247–252. [Google Scholar] [CrossRef]
- Huo, H.; Lin, H.; Dong, Y.; Cheng, H.; Wang, H.; Cao, L. Ammonia-nitrogen and phosphates sorption from simulated reclaimed waters bymodified clinoptilolite. J. Hazard. Mater. 2012, 229–230, 292–297. [Google Scholar] [CrossRef]
- Khosravi, A.; Esmhosseini, M.; Khezri, S. Removal of ammonium ion from aqueous solutions using natural zeolite: Kinetic, equilibrium and thermodynamic studies. Res. Chem. Intermed. 2014, 40, 2905–2917. [Google Scholar] [CrossRef]
- Langwaldt, J. Ammonium removal from water by eight natural zeolites: A comparative study. Sep. Sci. Technol. 2008, 43, 2166–2182. [Google Scholar] [CrossRef]
- Lin, L.; Wan, C.; Lee, D.; Lei, Z.; Liu, X. Ammonium assists orthophosphate removal from high-strength wastewaters by natural zeolite. Sep. Purif. Technol. 2014, 133, 351–356. [Google Scholar] [CrossRef]
- Moharami, S.; Jalali, M. Use of modified clays for removal of phosphorus from aqueous solutions. Environ. Monit. Assess. 2015, 187, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Araújo, A.; Azevedo, G.; Pereira, M.F.R.; Neves, I.C.; Machado, A.V. Kinetic and equilibrium studies of phosphorous adsorption: Effect of physical and chemical properties of adsorption agent. Ecol. Eng. 2015, 82, 527–530. [Google Scholar] [CrossRef]
- Pizzaro, C.; Rubio, M.A.; Escudey, M.; Albornoz, M.F.; Muñoz, D.; Denardin, J.; Fabris, J.D. Nanomagnetite-zeolite composites in the removal of arsenate from aqueous systems. J. Braz. Chem. Soc. 2015, 26, 1887–1896. [Google Scholar] [CrossRef]
- Sancho, I.; Licon, E.; Valderrama, C.; de Arespacochaga, N.; López-Palau, S.; Cortina, J.L. Recovery of ammonia from domestic wastewater effluents as liquidfertilizers by integration of natural zeolites and hollow fibre membrane contactors. Sci. Total Environ. 2017, 584–585, 244–251. [Google Scholar] [CrossRef]
- Song, W.; Shi, T.; Yang, D.; Ye, J.; Zhou, Y.; Feng, Y. Pretreatment effects on the sorption of Cr(VI) onto surfactantmodified zeolite: Mechanism analysis. J. Environ. Manag. 2015, 162, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, A.I.; Tsihrintzis, V.A. Use of zeolite and bauxite as filter media treating the effluent of Vertical Flow Constructed Wetlands. Microporpous Mesoporous Mater. 2012, 155, 106–116. [Google Scholar] [CrossRef]
- Wan, C.; Ding, S.; Zhang, C.; Tan, X.; Zou, W.; Liu, X.; Yang, X. Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: Mechanism and application. Sep. Purif. Technol. 2017, 180, 1–12. [Google Scholar] [CrossRef]
- Yapsakli, K.; Aktan, C.K.; Mertoglu, B. Anammox-zeolite system acting as buffer to achieves table effluent nitrogen values. Biodegradation 2017, 28, 69–79. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Valderrama, C.; Queroland, J.L.C.X. Recovery of Ammonium by Powder Synthetic Zeolites from Wastewater Effluents: Optimization of the Regeneration Step. Water Air Soil Pollut. 2017, 228, 396. [Google Scholar] [CrossRef]
- Zendehdel, M.; Zendehnam, A.; Hoseini, F.; Azarkish, M. Investigation of removal of chemical oxygen demand (COD) wastewater and antibacterial activity of nanosilver incorporated in poly (acrylamide-coacrylic acid)/NaY zeolite nanocomposite. Polym. Bull. 2015, 72, 1281–1300. [Google Scholar] [CrossRef]
- Lakdawala, M.M.; Patel, Y.S. Studies on Adsorption Capacity of Zeolite for Removal of Chemical and Bio-Chemical Oxygen Demands. Chem. J. 2015, 1, 139–143. [Google Scholar]
- Zhou, L.; Boyd, C.E. Total ammonia nitrogen removal from aqueous solutions by the natural zeolite, mordenite: A laboratory test and experimental study. Aquaculture 2014, 432, 252–257. [Google Scholar] [CrossRef]
- Vocciante, M.; De Folly D’Auris, A.; Finocchi, A.; Tagliabue, M.; Bellettato, M.; Ferrucci, A.; Reverberi, A.P.; Ferro, S. Adsorption of ammonium on clinoptilolite in presence of competing cations: Investigation on groundwater remediation. J. Clean. Prod. 2018, 198, 480–487. [Google Scholar] [CrossRef]
- Yin, H.; Yang, C.; Jia, Y.; Chen, H.; Gu, X. Dual removal of phosphate and ammonium from high concentrations of aquaculture wastewaters using an efficient two-stage infiltration system. Sci. Total Environ. 2018, 635, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Du, J.; Chen, P.; Huang, X.; Chen, O. Enhanced efficiency of swine wastewater treatment by the composite of modified zeolite and a bioflocculant enriched from biological sludge. Environ. Technol. 2018, 39, 3096–3103. [Google Scholar] [CrossRef] [PubMed]
- Cyrus, S.J.; Reddy, B.G. Sorption and Desorption of Ammonium by Zeolite: Batch and Column Studies. J. Environ. Sci. Health Part A 2011, 46, 408–414. [Google Scholar] [CrossRef]
- Lagergren, S.Y. Zur Theorie der sogenannten adsorption gelosterstoffe. Handlingar 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Poreand solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling; De Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Verdouw, H.; Van Echteld, C.J.A.; Dekkers, E.M.J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- (American Public Health Association) APHA. Standard Methods for Examination of Water and Wastewater, 23rd ed.; American Public Health Association: New York, NY, USA, 2017. [Google Scholar]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, Z.; Zhang, J.; Wen, D.; Tang, X. Adsorption characteristics of ammonium exchange by zeolite and the optimal application in the tertiary treatment of coking wastewater using response surface methology. Water Sci. Technol. 2013, 67, 619–627. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.; Zhang, X.; Cheng, Y.; Wang, J.; Zhang, Y.; Li, L. Adsorption properties of ammonium nitrogen in seawater by natural zeolite. Chin. J. Environ. Eng. 2015, 9, 4281–4286. [Google Scholar]
- Leyva-Ramos, R.; Aguilar-Armenta, G.; Gonzalez-Gutierrez, L.V.; Guerrero-Coronado, R.M.; Mendoza-Barron, J. Ammonia exchange on clinoptilolite from mineral deposits located in Mexico. J. Chem. Technol. Biotechnol. 2004, 79, 651–657. [Google Scholar] [CrossRef]
- Ören, A.H.; Kaya, A. Factors affecting adsorption characteristics of Zn2+ on two natural zeolites. J. Hazard. Mater. 2006, 131, 59–65. [Google Scholar] [CrossRef]
- Ziyath, A.M.; Mahbub, P.; Goonetilleke, A.; Adebajo, M.O.; Kokot, S.; Oloyede, A. Influence of physical and chemical parameters on the treatment of heavy metals in polluted stormwater using zeolite: A review. J. Water Resour. Prot. 2011, 3, 758–767. [Google Scholar] [CrossRef]
- Martins, T.H.; Souza, T.S.O.; Foresti, E. Ammonium removal from landfill leachate by Clinoptilolite adsorption followed by bioregeneration. J. Environ. Chem. Eng. 2017, 5, 63–68. [Google Scholar] [CrossRef]
- Alshameri, A.; Chunjie, Y.; Al-Ani, Y.; Dawood, A.S.; Ibrahim, A.; Zhou, C.; Wang, H. An investigation into the adsorption removal of ammonium by salt activated Chinese (Hulaodu) natural zeolite: Kinetics, isotherms, and thermodynamics. J. Taiwan Inst. Chem. Eng. 2014, 45, 554–564. [Google Scholar] [CrossRef]
- Karadag, D.; Koc, Y.; Turan, M.; Armagan, B. Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite. J. Hazard. Mater. 2006, 136, 604–609. [Google Scholar] [CrossRef]
- Sarioglu, M. Removal of ammonium from municipal wastewater using natural Turkish (Dogantepe) zeolite. Sep. Purif. Technol. 2005, 41, 1–11. [Google Scholar] [CrossRef]
- Townsend, R.P.; Loizidou, M. Ion exchange properties of natural clinoptilolite, ferrierite and mordenite: 1. Sodium—Ammonium equilibria. Zeolites 1984, 4, 191–195. [Google Scholar] [CrossRef]
- Thomas, J.M.; Thomas, W.J. Principles and Practice of Heterogeneous Catalysis; John Wiley &Sons: Hoboken, NJ, USA, 2014; p. 1560. [Google Scholar]
- Wen, D.; Ho, Y.S.; Tang, X. Comparative sorption kinetic studies of ammonium onto zeolite. J. Hazard. Mater. 2006, 133, 252–256. [Google Scholar] [CrossRef]
- Taddeo, R.; Prajapati, S.; Lepistö, R. Optimizing ammonium adsorption on natural zeolite for wastewaters with high loads of ammonium and solids. J. Porous Mater. 2017, 24, 1545–1554. [Google Scholar] [CrossRef]
- Shaban, M.; AbuKhadra, M.R.; Nasief, F.M.; El-Salam, H.A. Removal of ammonia from aqueous solutions, ground water, and wastewater using mechanically activated clinoptilolite and synthetic zeolite-a: Kinetic and equilibrium studies. Water Air Soil Pollut. 2017, 228, 450. [Google Scholar] [CrossRef]
- Cheng, Z.; Ding, W. Ammonium removal from water by naturaland modified zeolite: Kinetic, equilibrium, andthermodynamic studies. Desalin. Water Treat. 2015, 55, 978–985. [Google Scholar] [CrossRef]
- Sánchez-Hernández, R.; Padilla, I.; López-Andrés, S.; López-Delgado, A. Al-Waste-Based Zeolite Adsorbent Used for the Removal of Ammonium from Aqueous Solutions. Int. J. Chem. Eng. 2018, 2018, 1256197. [Google Scholar] [CrossRef]
- Celik, S.M.; Ozdemir, H.B.; Turan, E.Y.; Koyuncu, I.; Atesok, G.; Sarikaya, H.Z. Removal of ammonia by natural clay minerals using fixed and fluidised bed column reactors. Water Sci. Technol. 2001, 1, 81–88. [Google Scholar] [CrossRef]
- Hedström, A.; Amofah, L.R. Adsorption and desorption of ammonium by clinoptilolite adsorbent in municipal wastewater treatment systems. J. Environ. Eng. Sci. 2008, 7, 53–61. [Google Scholar] [CrossRef]
- Drizo, A.; Frost, C.A.; Grace, J.; Smith, K.A. Physico-chemical screening of phosphate-removing substrates for use in constructed wetland systems. Water Res. 1999, 33, 3595–3602. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).