Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System
Abstract
1. Introduction
2. Methods and Materials
2.1. Substrate and Inoculum
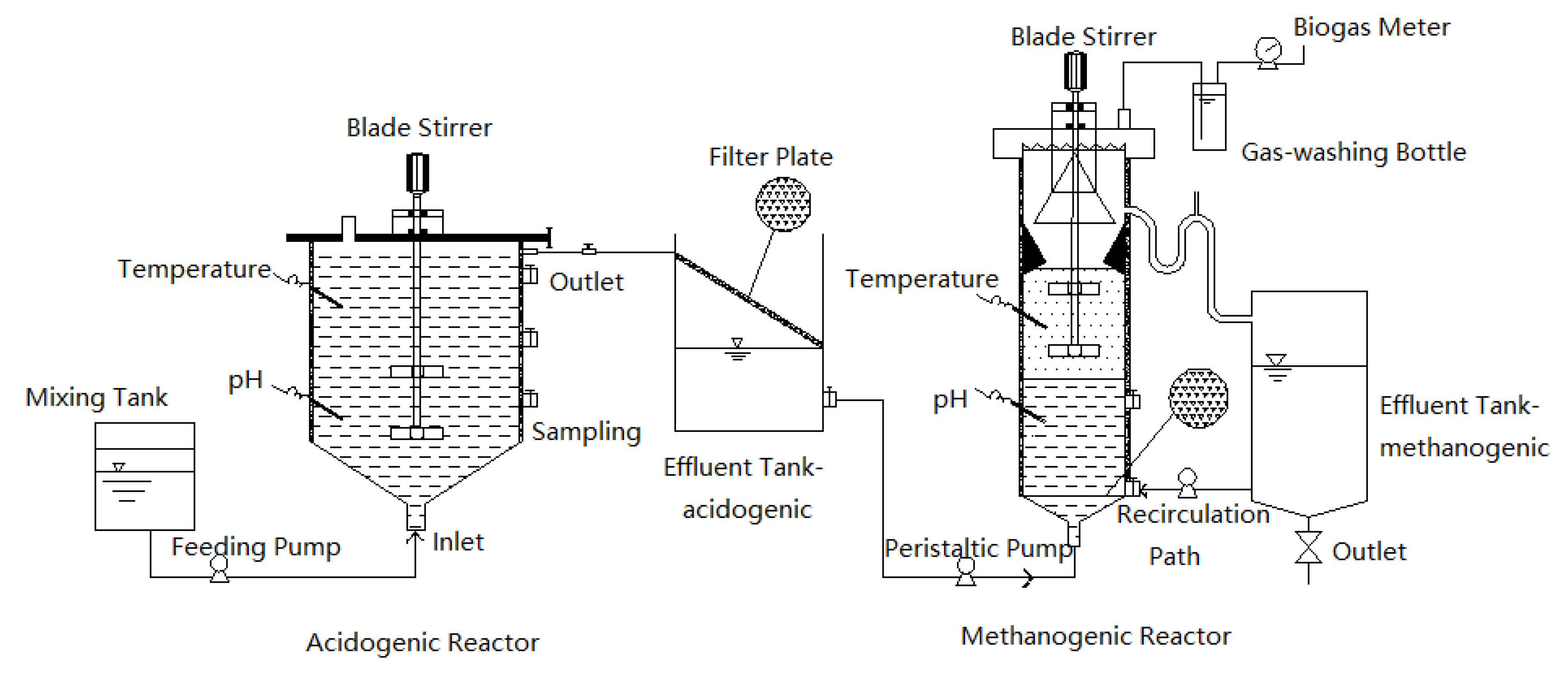
2.2. Two-Phase Anaerobic Digestion Reactor Configuration and Operation
2.3. Microbial Community Analysis
2.4. Analytical Methods
3. Results and Discussion
3.1. Performance of the Two-Phase Digestion Reactor under Different Temperatures
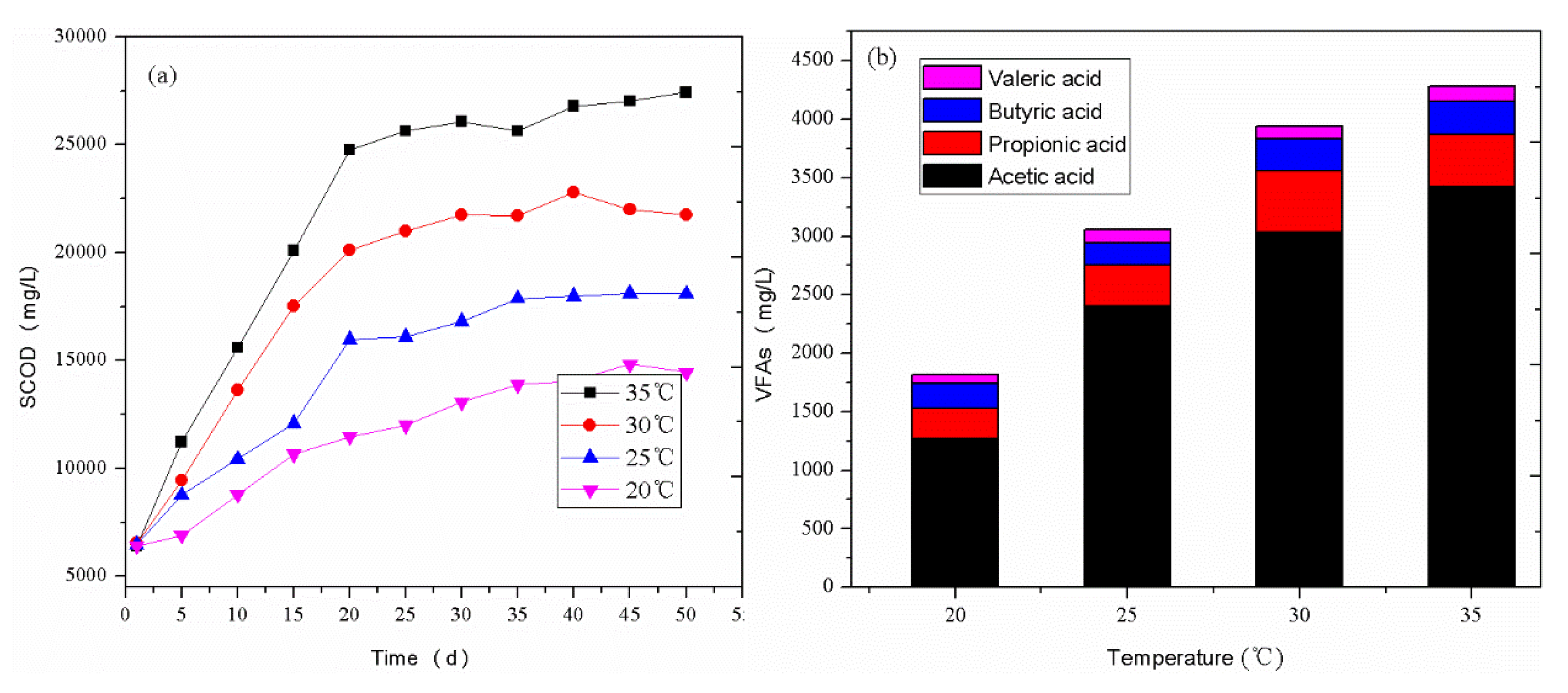
3.1.1. SCOD and VFAs Contents in the Acidogenic Phase
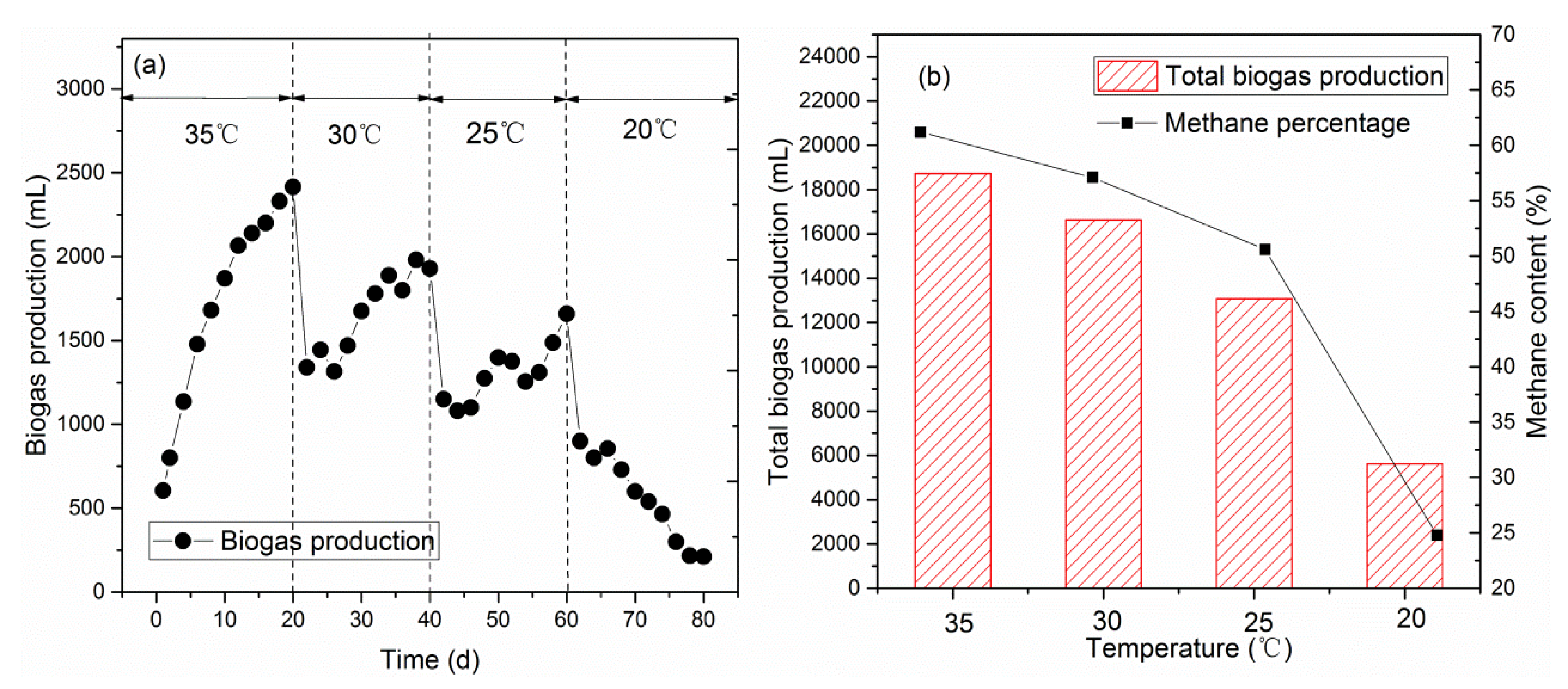
3.1.2. Biogas Production Efficiency in the Methanogenic Phase
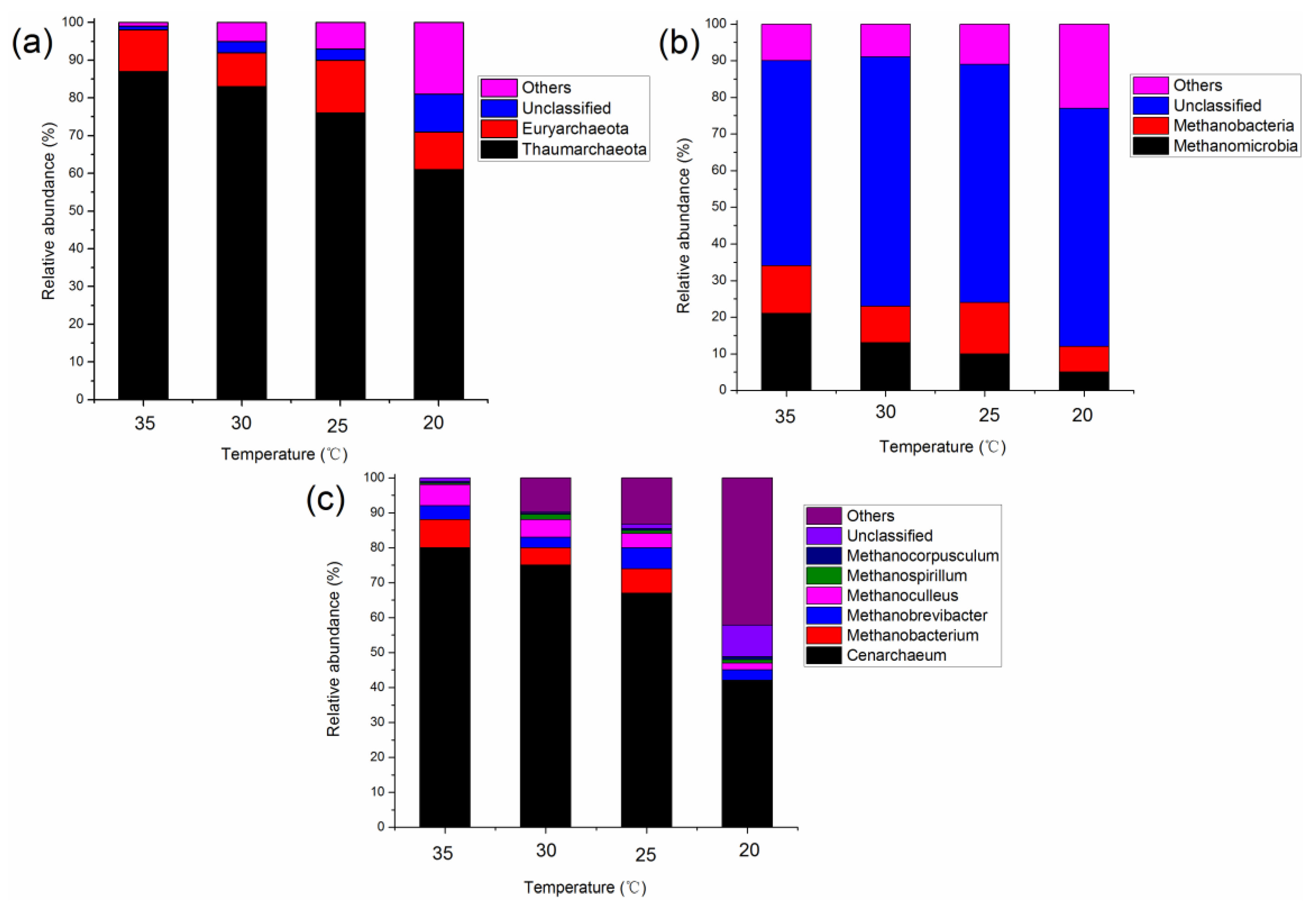
3.2. Characteristics of the Microbial Community at Different Temperatures
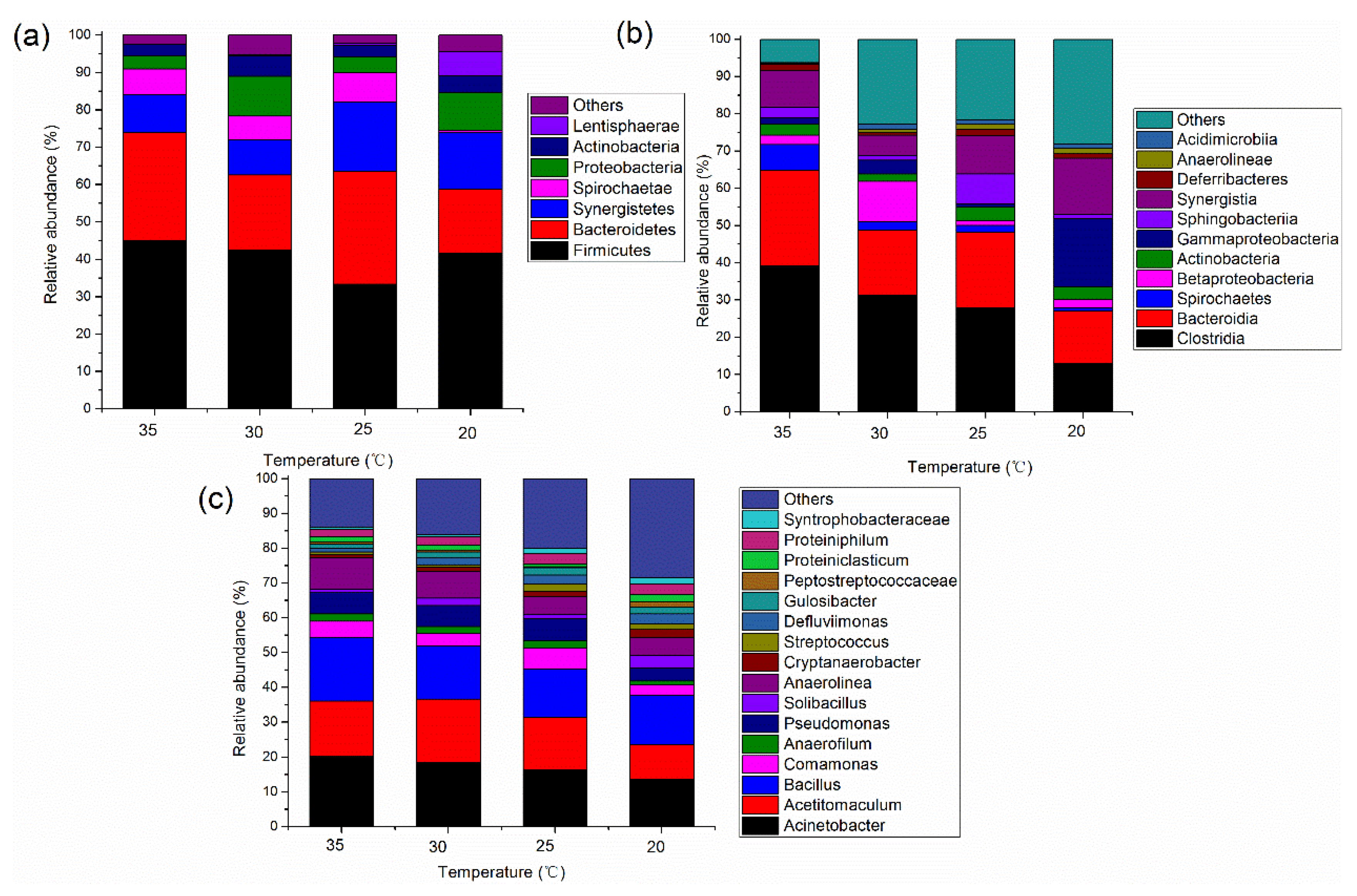
3.2.1. Microbial Community Structure in the Acidogenic Phase
3.2.2. Microbial Community Structure in the Methanogenic Phase
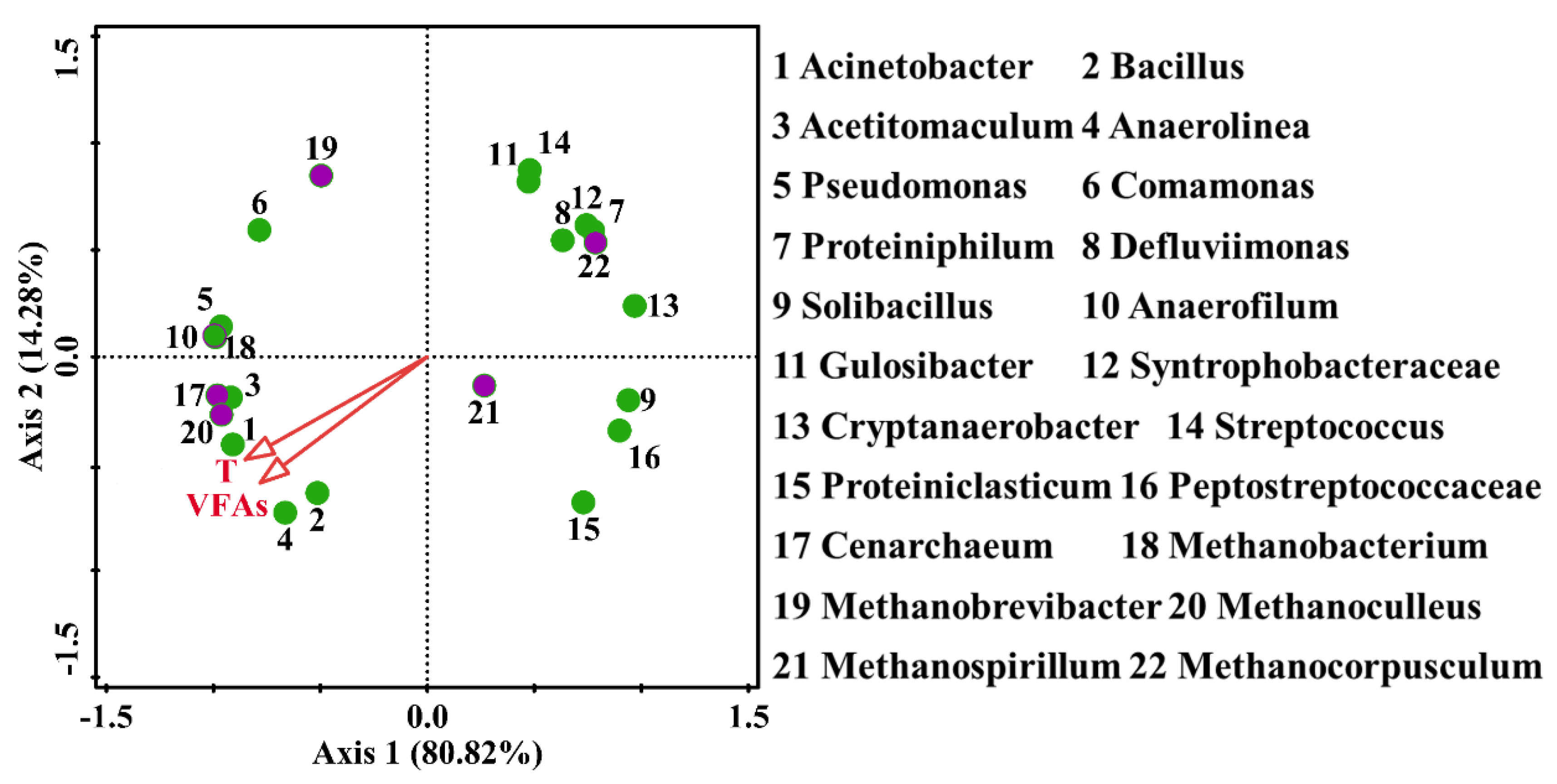
3.3. Correlation of Community Microorganisms with Environmental Variables (VFAs and Temperature)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbasi, T.; Tauseef, S.M.; Abbasi, S.A. Anaerobic digestion for global warming control and energy generation—An overview. Renew. Sustain. Energy Rev. 2012, 16, 3228–3242. [Google Scholar] [CrossRef]
- Albertson, M.L.; Pruden, A.; Oliver, R.T. Enhanced anaerobic digestion of biomass waste for optimized production of renewable energy and solids for compost. Int. Congr. Sci. 2006, 1293, 221–229. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, Y.; Xie, D. Insights into the production potential and trends of China’s rural biogas. Int. J. Energy Res. 2015, 39, 227–237. [Google Scholar] [CrossRef]
- Wei, S.Z.; Zhang, H.F.; Cai, X.B.; Xu, J.; Fang, J.P.; Liu, H.M. Psychrophilic anaerobic co-digestion of highland barley straw with two animal manures at high altitude for enhancing biogas production. Energy Convers. Manag. 2014, 88, 40–48. [Google Scholar] [CrossRef]
- Nasir, I.M.; Mohd Ghazi, T.I.M.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Zheng, M.; Fu, G.; Lar, J.S. Anaerobic co-digestion of cattle manure with corn stover pretreated by Sodium Hydroxide for efficient biogas production. Energy Fuels 2009, 23, 4635–4639. [Google Scholar] [CrossRef]
- Barbanti, L.; Di Girolamo, G.; Grigatti, M.; Bertin, L.; Ciavatta, C. Anaerobic digestion of annual and multi-annual biomass crops. Ind. Crops Prod. 2014, 56, 137–144. [Google Scholar] [CrossRef]
- Dahiya, S.; Joseph, J. High rate biomethanation technology for solid waste management and rapid biogas production: An emphasis on reactor design parameters. Bioresour. Technol. 2015, 188, 73–78. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.J.; Rincón, B.; Fermoso, F.G.; Jiménez, A.M.; Borja, R. Assessment of two-phase olive mill solid waste and microalgae co-digestion to improve methane production and process kinetics. Bioresour. Technol. 2014, 157, 263–269. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Ghosh, S.; Klass, D.L. Two-phase anaerobic digestion. In Clean Fuels from BiomassClean Fuels from Biomass & Wastes; Institute of Gas Technology: Chicago, IL, USA, 1977. [Google Scholar]
- Kvesitadze, G.; Sadunishvili, T.; Dudauri, T.; Zakariashvili, N.; Partskhaladze, G.; Ugrekhelidze, V.; Tsiklauri, G.; Metreveli, B.; Jobava, M. Two-stage anaerobic process for bio-hydrogen and bio-methane combined production from biodegradable solid wastes. Energy 2011, 37, 94–102. [Google Scholar] [CrossRef]
- Ren, J.; Yuan, X.; Li, J.; Ma, X.; Zhao, Y.; Zhu, W.; Wang, X.; Cui, Z. Performance and microbial community dynamics in a two-phase anaerobic co-digestion system using cassava dregs and pig manure. Bioresour. Technol. 2014, 155, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Mao, C.; Zhai, N.; Wang, X.; Yang, G. Influence of initial pH on thermophilic anaerobic co-digestion of swine manure and maize stalk. Waste Manag. 2015, 35, 119–126. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic Co-Digestion of dairy manure, chicken manure and rice straw: Focusing on ammonia inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, L.; Duan, Q.; Hu, G.; Zhang, G. Semi-continuous anaerobic co-digestion of dairy manure with three crop residues for biogas production. Bioresour. Technol. 2014, 156, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Lidén, G. The effect of temperature variation on biomethanation at high altitude. Bioresour. Technol. 2008, 99, 7278–7284. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zeeman, G.; van Loon, W.K.; Bot, G.P.; Lettinga, G. Effect of temperature and temperature fluctuation on thermophilic anaerobic digestion of cattle manure. Bioresour. Technol. 2004, 95, 191–201. [Google Scholar] [CrossRef]
- Xiong, H.; Chen, J.; Wang, H.; Shi, H. Influences of volatile solid concentration, temperature and solid retention time for the hydrolysis of waste activated sludge to recover volatile fatty acids. Bioresour. Technol. 2012, 119, 285–292. [Google Scholar] [CrossRef]
- Rademacher, A.; Nolte, C.; Schonberg, M.; Klocke, M. Temperature increases from 55 to 75 degrees C in a two-phase biogas reactor result in fundamental alterations within the bacterial and archaeal community structure. Appl. Microbiol. Biotechnol. 2012, 96, 565–576. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Cheng, H.; Wang, W.; Dong, R. Two-phase anaerobic digestion of municipal solid wastes enhanced by hydrothermal pretreatment: Viability, performance and microbial community evaluation. Appl. Energy 2017, 189, 613–622. [Google Scholar] [CrossRef]
- Meng, X.; Yuan, X.; Ren, J.; Wang, X.; Zhu, W.; Cui, Z. Methane production and characteristics of the microbial community in a two-stage fixed-bed anaerobic reactor using molasses. Bioresour. Technol. 2017, 241, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Rojas, P.; Morato, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Microbial communities of biomethanization digesters fed with raw and heat pre-treated microalgae biomasses. Chemosphere 2017, 168, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Song, Z.; Li, D.; Yuan, Y.; Liu, X.; Zheng, T. The effects of initial substrate concentration, C/N ratio, and temperature on solid-state anaerobic digestion from composting rice straw. Bioresour. Technol. 2015, 177, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Strauber, H.; Lucas, R.; Kleinsteuber, S. Metabolic and microbial community dynamics during the anaerobic digestion of maize silage in a two-phase process. Appl. Microbiol. Biotechnol. 2016, 100, 479–491. [Google Scholar] [CrossRef]
- Cai, M.; Liu, J.; Wei, Y. Enhanced biohydrogen production from sewage sludge with alkaline pretreatment. Environ. Sci. Technol. 2004, 38, 3195–3202. [Google Scholar] [CrossRef]
- Cho, S.K.; Im, W.T.; Kim, D.H.; Kim, M.H.; Shin, H.S.; Oh, S.E. Dry anaerobic digestion of food waste under mesophilic conditions: Performance and methanogenic community analysis. Bioresour Technol. 2013, 131, 210–217. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef]
- American Public Health Association Standard. Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Lai, B.; Zhou, Y.; Yang, P.; Yang, J.; Wang, J. Degradation of 3,3′-iminobispropanenitrile in aqueous solution by Fe(0)/GAC micro-electrolysis system. Chemosphere 2013, 90, 1470–1477. [Google Scholar] [CrossRef]
- Shan, L.; Yu, Y.; Zhu, Z.; Zhao, W.; Wang, H.; Ambuchi, J.J.; Feng, Y. Microbial community analysis in a combined anaerobic and aerobic digestion system for treatment of cellulosic ethanol production wastewater. Environ. Sci. Pollut. Res. 2015, 22, 17789–17798. [Google Scholar] [CrossRef]
- Hao, J.; Wang, H. Volatile fatty acids productions by mesophilic and thermophilic sludge fermentation: Biological responses to fermentation temperature. Bioresour. Technol. 2015, 175, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Koropatkin, N.M.; Smith, T.J.; Gordon, J.I. Complex glycan catabolism by the human gut microbiota: The Bacteroidetes Sus-like paradigm. J. Biol. Chem. 2009, 284, 24673–24677. [Google Scholar] [CrossRef] [PubMed]
- Tsapekos, P.; Kougias, P.G.; Treu, L.; Campanaro, S.; Angelidaki, I. Process performance and comparative metagenomic analysis during co-digestion of manure and lignocellulosic biomass for biogas production. Appl. Energy 2017, 185, 126–135. [Google Scholar] [CrossRef]
- Shima, S.; Warkentin, E.; Thauer, R.K.; Ermler, U. Structure and function of enzymes involved in the methanogenic pathway utilizing carbon dioxide and molecular hydrogen. J. Biosci. Bioeng. 2002, 93, 519–530. [Google Scholar] [CrossRef]
- Griffin, M.E.; Mcmahon, K.D.; Mackie, R.I.; Raskin, L. Methanogenic population dynamics during start-up of anaerobic digesters treating municipal solid waste and biosolids. Biotechnol. Bioeng. 1998, 57, 342–355. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, Y.; Dong, X. Methanol as the primary methanogenic and acetogenic precursor in the cold Zoige wetland at Tibetan plateau. Microb. Ecol. 2010, 60, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Tang, Y.; Urakami, T.; Morimura, S.; Kida, K. Digestion performance and microbial community in full-scale methane fermentation of stillage from sweet potato-shochu production. J. Environ. Sci. 2014, 26, 423–431. [Google Scholar] [CrossRef]
- O’Reilly, J.; Lee, C.; Chinalia, F.; Collins, G.; Mahony, T.; O’Flaherty, V. Microbial community dynamics associated with biomass granulation in low-temperature (15 degrees C) anaerobic wastewater treatment bioreactors. Bioresour. Technol. 2010, 101, 6336–6344. [Google Scholar] [CrossRef]
- Galagan, J.E.; Nusbaum, C.; Roy, A.; Endrizzi, M.G.; Macdonald, P.; Fitzhugh, W.; Calvo, S.; Engels, R.; Smirnov, S.; Atnoor, D.; et al. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 2002, 12, 532–542. [Google Scholar] [CrossRef]
- Siggins, A.; Enright, A.M.; O’Flaherty, V. Methanogenic community development in anaerobic granular bioreactors treating trichloroethylene (TCE)-contaminated wastewater at 37 degrees C and 15 degrees C. Water Res. 2011, 45, 2452–2462. [Google Scholar] [CrossRef]
| Parameters | CS | CS (5% NaOH) | CM | BS |
|---|---|---|---|---|
| pH | 7.18 | - | 7.12 | 7.02 |
| Total solid (TS, %) | 86.3 | 30.8 | 17.2 | 5.4 |
| Volatile solid (VS, %) | 61.4 | 24.6 | 12.45 | 4.2 |
| Total carbon (TC, %) | 36.6 | 26.3 | 35.4 | 22.5 |
| Total nitrogen (TN, %) | 0.89 | 0.47 | 2.02 | 1.28 |
| Carbon to nitrogen ratio (C/N) | 41.1 | 56.2 | 17.6 | 17.3 |
| Properties | Data |
|---|---|
| TS (%) | 12.8 |
| VS (% TS) | 9.1 |
| Acetic Acid (mg/L) | 3295 |
| Propionic Acid (mg/L) | 371 |
| Butyric Acid (mg/L) | 265 |
| SCOD (mg/L) | 26039 |
| pH | 6.3 |
| Temperature (°C) | OTU | ACE | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|
| 35 | 1078 | 1210 | 1214 | 6.23 | 0.012 |
| 30 | 1009 | 1145 | 1132 | 6.46 | 0.023 |
| 25 | 1032 | 1021 | 1025 | 5.61 | 0.025 |
| 20 | 890 | 954 | 932 | 4.51 | 0.031 |
| Temperature (°C) | OTU | ACE | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|
| 35 | 980 | 854 | 867 | 5.01 | 0.017 |
| 30 | 976 | 856 | 894 | 5.09 | 0.021 |
| 25 | 982 | 845 | 800 | 4.47 | 0.025 |
| 20 | 789 | 765 | 696 | 4.03 | 0.038 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ma, F.; Ma, W.; Wang, P.; Zhao, G.; Lu, X. Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System. Water 2019, 11, 133. https://doi.org/10.3390/w11010133
Wang S, Ma F, Ma W, Wang P, Zhao G, Lu X. Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System. Water. 2019; 11(1):133. https://doi.org/10.3390/w11010133
Chicago/Turabian StyleWang, Shiwei, Fang Ma, Weiwei Ma, Ping Wang, Guang Zhao, and Xiaofei Lu. 2019. "Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System" Water 11, no. 1: 133. https://doi.org/10.3390/w11010133
APA StyleWang, S., Ma, F., Ma, W., Wang, P., Zhao, G., & Lu, X. (2019). Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System. Water, 11(1), 133. https://doi.org/10.3390/w11010133





