Abstract
A field study was carried out to investigate the feasibility of a riverbank filtration site using two vertical wells on the Nakdong River, South Korea. The riverbank filtration site was designed to have eleven horizontal collector wells in order to supply 280,000 m3/day. This field study provided more insight into the fate of the dissolved organic matter’s characteristics during soil passage. The vertical production wells (PWs) were located in different aquifer materials (PW-Sand and PW-Gravel) in order to determine the depth of the laterals for the horizontal collector wells. The turbidity of the riverbank filtrates from the PW-Sand (0.9 NTU) and PW-Gravel (0.7 NTU) was less than 1 NTU, which was the target turbidity of the riverbank filtrate in this study. The iron concentrations were 18.1 ± 0.8 and 25.9 ± 1.3 mg/L for PW-Sand and PW-Gravel respectively, and were higher than those of the land-side groundwater. The biodegradable organic matter-determined biochemical oxygen demand in the river water was reduced by more than 40% during soil passage, indicating that less microbial growth in the riverbank filtrate could be possible. Moreover, the influence of the pumping rates of the vertical wells on the removal of dissolved organic matter and the turbidity was not significant.
1. Introduction
Climate change influences both water availability and water quality through floods and droughts [1]. In Korea, the characteristics of water sources and their availability have been affected by economic growth, insufficient water management, and uncertainties due to climate change [2]. Therefore, it will become more difficult to secure clean water during extreme meteorological events. Korea is also included among the world’s water-stressed nations between 2000 and 2025 [3]. South Korea is heavily dependent on its surface water for sources of drinking water, and approximately more than 90% of its drinking water comes from a river or man-made reservoir. The rainfall from June to September provides nearly 70% of the regional drinking water supply [4]. The mean annual rainfall is 1274 mm, and heavy rainfall that occurs during the summer leads to water shortages during the dry season (spring). Environmental accidents, including the contamination of tap water sources in the 1990s, raised many concerns and caused people in Korea to be reluctant to use tap water as drinking water [2]. Therefore, there is a need to find other water resources that are safe and to improve the public view of tap water quality.
When surface water’s characteristics change due to extreme weather events, conventional water treatments have difficulty securing high quality water resources. To secure high quality water resources, alternative water resources such as managed aquifer recharge (MAR) systems were investigated [5]. MAR systems use natural water treatments and are effective at removing biodegradable organic matter. MAR systems such as riverbank filtration (RBF) are emerging in Korea as an alternative solution [2]. RBF is a water treatment process that uses the physical, chemical and biological degradation processes of aquifers [6,7], and it is a nature-friendly water treatment process that removes pollutants without using chemicals [8]. RBF is also effective at alleviating the production of disinfection by-products and reducing trace organic contaminants [9,10,11]. In addition, it is also suitable for water safety and management [8,12]. In the early 2000s, a RBF system was first introduced to improve the water quality of drinking water resources in South Korea, especially in the regions where there was poor water quality for a decade due to the wastewater effluents discharged from local industries. A number of chemical spills had occurred in the river, which caused people to lose confidence in tap water quality [5].
A number of cities located downstream of the Nakdong River (Busan, Korea) are vulnerable to various water pollution sources and the seasonal water quality changes. Therefore, it is necessary to improve their water treatment system by improving the water source’s quality. Currently, there are three drinking water treatment plants that are currently providing water via RBF using the Nakdong River (Table 1). The city of Changwon, which is on the Nakdong River in Korea, has been providing 80,000 m3/day of drinking water since 2006 using RBF systems with vertical and horizontal collector wells. This system was the first RBF site installed to supply drinking water in South Korea. The city of Gimhae, South Korea, is currently providing 127,000 m3/day via RBF (designed capacity: 180,000 m3/day). Moreover, the Korea Water Resources Corporation (K-Water, Dajeon) in South Korea is currently investigating potential RBF sites that can supply 680,000 m3/day to cities including Busan that are located near the lower part of the Nakdong River. This field study of two vertical wells will be used for the design of the horizontal collector wells that contribute part of the water supply to the city of Busan.

Table 1.
Riverbank filtration sites located along the Nakdong River, South Korea.
Before the installation of eleven horizontal collector wells, which provide 280,000 m3/day, the field study was carried out using two vertical wells to investigate the quality of RBF. To improve the post-treatment requirements after the RBF, there is a need to investigate the water quality of RBF filtrates, including the dissolved organic matter’s characteristics. Previously, Lee et al. [5] investigated the performance of RBF filtrates by comparing river water quality. However, there were no studies conducted on the fate of dissolved organic matter characteristics during RBF from a field study in South Korea. Moreover, there has been no report on using vertical wells to determine the depths of the laterals for horizontal collector wells.
The objective of this study was to conduct a detailed investigation of the characteristics of dissolved organic matter during soil passage using two vertical wells along the Nakdong River, South Korea (January to June, 2011). This study also investigated the removal efficiency of dissolved organic matter and the turbidity of two vertical wells whose screens are located at different depths (i.e., sand and gravel layers). The performances of the vertical wells at different pumping rates were also investigated. The water quality characteristics from two different vertical wells helped to determine the depth of the laterals for horizontal collector wells, even when there were other factors that needed to be considered (such as quantity).
2. Materials and Methods
2.1. Field Site
Figure 1 shows the schematic diagram of a vertical well site, which consisted of two production wells (PWs) and land-side groundwater monitoring wells (GMWs). The pilot RBF site consisted of two PWs and two GMWs at the sand and gravel layers. The two PWs were located at 30 m from the river, and the well screens were 6 m long. The well depths for the PW-Sand and PW-Gravel were 18 and 27 m below the land surface, respectively. Horizontal collector wells were planned to be installed at the site; therefore, two vertical wells were located at two different depths/aquifer layers that consisted of different materials (e.g., sand or gravel) to investigate the water quality characteristics. Two GMWs at the same depths as the PWs screens were located 380 m from the PWs (PW-Sand and PW-Gravel) in order to compare the riverbank filtrates. A bank filtration (BF) simulator, developed as a part of the NASRI project (Germany), was used to estimate the shortest (i.e., minimum) travel time at different pumping rates (Table 2) [13]. It was reported that there was a small discrepancy, below 5%, when the numerically computed shortest travel time from the bank filtration simulator was compared to that of the MODFLOW model [14]. The shortest travel times estimated in the bank filtration simulator were used to determine the shortest travel time of the flow paths from the surface water to the well. Further information on the mathematical algorithms behind the BF simulator is given elsewhere [15].
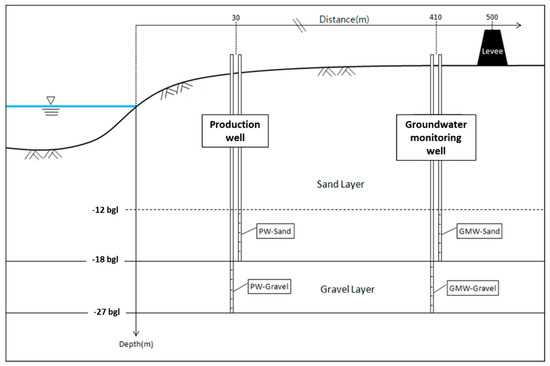
Figure 1.
Schematic diagram of the vertical wells installed at different depths and the groundwater monitoring wells.

Table 2.
Estimation of the shortest travel times (days) and the shares of riverbank filtrate and groundwater estimated by the bank filtration (BF) simulator.
2.2. Analytical Methods
The dissolved organic carbon (DOC) concentrations were analyzed using a total organic carbon (TOC) analyzer (TOC-V CPN, Shimadzu, Kyoto, Japan). For the DOC analysis, the samples were filtered using a 0.45-μm membrane filter (Whatman, Clifton, NJ, USA) and then analyzed using the TOC analyzer.
The ultraviolet absorbance at the 254 nm wavelength (UVA254) is a useful indicator for predicting the trihalomethane (THM) formation potential [16] and characterizing the aromaticity of natural organic matter [17]. UVA254 was measured using a spectrophotometer (DR5000, Hach, Loveland, CO, USA). The biochemical oxygen demand (BOD5) was analyzed according to the standard methods [18]. The fluorescence excitation-emission matrix (EEM) spectra and liquid chromatography-organic carbon detection (LC-OCD) were used to determine the dissolved organic matter’s characteristics. For the fluorescence EEM spectra, the sample was filled with a quartz cuvette (Hellma, Plainview, NY, USA) and the fluorescence intensity was measured using a fluorescence spectrophotometer (LS45, Perkin Elmer, Waltham, MA, USA). The spectrophotometer scanned emission wavelengths between 280 nm and 600 nm with excitation wavelengths between 200 nm and 400 nm at 10-nm intervals. The four selected peak regions that were found in this study were aromatic protein-like substances T1 (excitation 220–240 nm and emission 330–360 nm), tryptophan protein-like substances T2 (excitation 270–280 nm and emission 330–360 nm), fulvic-like substances A (excitation 230–260 nm and emission 400–450 nm), and humic-like substances C (excitation 300–340 nm and emission 400–450 nm) [19]. LC-OCD (DOC-LABOR, Karlsruhe, Germany), which consists of a size-exclusion chromatography column (HW-55S, GROM Analytik + HPLC GmbH, Herrenberg, Germany) with ultraviolet and organic carbon detectors, was used to classify the dissolved organic matter into five different organic matter fractions (biopolymers, humic substances, building blocks, low molecular weight neutrals, and low molecular weight acids) according to their molecular weights. Further details of the fractions that were determined via LC-OCD are reported elsewhere [20].
3. Results and Discussion
3.1. Turbidity
The turbidity of river water significantly varied during the time of the study, but the turbidity of the riverbank filtrates from PW-Sand and PW-Gravel was fairly stable with levels less than 1.0 NTU (Figure 2, one-way variance (ANOVA) p < 0.05). The high turbidity removal has been proven to be effective when using RBF [21]. Even when the turbidity of the river was higher than 60 NTU (data are not shown) in this study, the turbidity of the riverbank filtrate was below 1 NTU, which was the target turbidity of the riverbank filtrate in this study, indicating fairly stable riverbank filtrates.
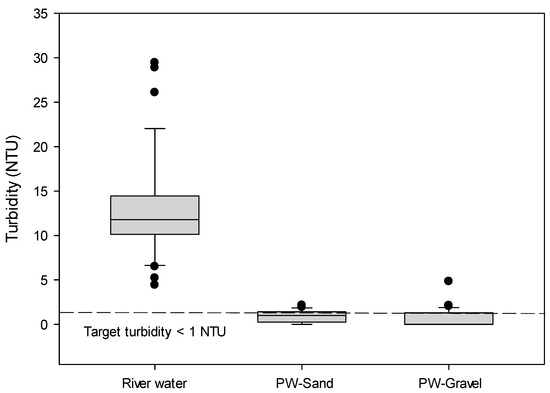
Figure 2.
The turbidity of the vertical wells installed at different depths (production well (PW)-Sand and PW-Gravel).
3.2. Iron and Manganese
Iron and manganese were investigated during RBF and compared to the river water and groundwater monitoring wells. The iron concentration in the river water was 1.2 ± 0.9 mg/L, which was lower than the samples collected from the PW-Sand (18.1 ± 0.8 mg/L) and the PW-Gravel (25.9 ± 1.3 mg/L) during the time of the study (Figure 3). The iron concentrations between the two production wells were different because the PW-Sand and the PW-Gravel were located at different layers (e.g., sand or gravel). This study showed that the gravel layer had higher iron concentrations compared to those of the sand layer. The iron concentrations from production wells (PW-Sand and PW-Gravel) were higher than those of the groundwater (GMW-Sand: 14.1 ± 1.1 mg/L and GMW-Gravel: 10.3 ± 0.9 mg/L). Previous studies reported the occurrence of high iron concentrations in MAR [22,23]. The amount of manganese in the river water was 0.2 mg/L, but it was higher in the RBF filtrate samples (PW-Sand (1.5 ± 0.1 mg/L) and PW-Gravel (2.6 ± 0.8 mg/L)). Oxide-forming metals such as iron and manganese are easily mobilized as result of the reduced zone occurring during soil passage. We observed that dissolved oxygen (DO) dropped from 12 mg/L (Nakdong River) to below 0.5 mg/L (PW-Sand and PW-Gravel) during RBF (Figure 4). The high concentrations of iron and manganese that were observed in the RBF filtrates were due to the reduced conditions that occurred during soil passage. It was necessary to treat the riverbank filtrates that have very high levels of iron and manganese, and the riverbank filtrate needs to be treated at below 0.3 mg/L for iron in order to meet South Korean Guidelines for drinking water. The high concentrations of iron and manganese in the riverbank filtrates must be reduced by existing drinking water treatment plants, even though they were not designed to treat iron. The most common method in the ex situ removal of iron is oxidation followed by filtration, and the removal can be simply carried out by aeration followed by rapid sand filtration.
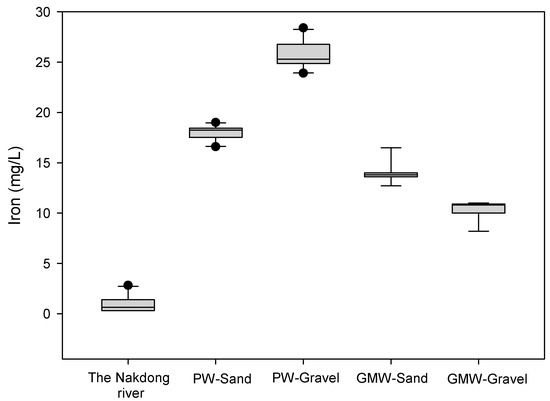
Figure 3.
Iron concentrations of the vertical wells (PW-Sand and PW-Gravel).
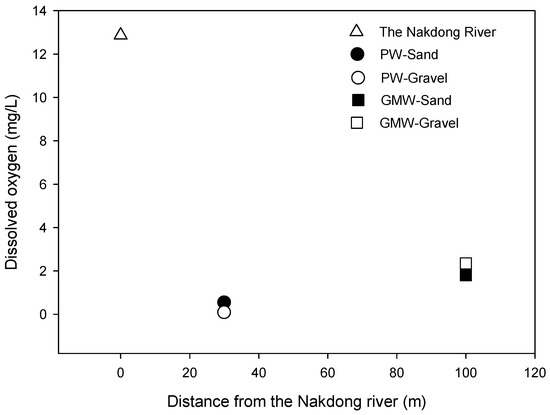
Figure 4.
Changes in the dissolved oxygen during riverbank filtration.
3.3. Bulk Organic Matter
3.3.1. Biochemical Oxygen Demand (BOD5)
The dissolved organic matter consisted of non-biodegradable and biodegradable organic matter fractions. In this study, BOD5 was used to investigate the changes in the biodegradable organic matter during RBF. BOD5 decreased during soil passage and was lower than that of the river. The removal efficiencies of BOD5 were 64 and 40% for PW-Sand and PW-Gravel, respectively. The significant reduction of DO during soil passage reflected the biodegradation of bulk organic matter during soil passage. The DO reduction as a result of biodegradation was also reported during soil passage [24]. Biodegradable organic matter determined by BOD5 was effectively removed during RBF which can reduce the occurrence of bio-regrowth and disinfection byproducts using less chlorine in distribution systems. There are limited treatments available (e.g., biological activated carbon, slow sand filter, etc.) to remove biodegradable organic matter during drinking water treatment processes. Therefore, RBF is an efficient treatment technology suitable for providing biostable water.
3.3.2. Dissolved Organic Carbon and UVA254
The DOC was used to investigate the fate of dissolved organic matter during RBF. As shown in Table 3, DOC was effectively reduced. The reduction was not different from BOD and DO. The DOC is known to be the fraction that is most often removed by biodegradation during soil passage and it corresponds to the DO reduction [24]. The removal of DOC during RBF was 50 and 57% for PW-Sand and PW-Gravel, respectively. It is clear that the removal of DOC was effective during RBF. UVA254 is a useful tool to characterize the dissolved organic matter characteristics and can be used as an indicator of its aromaticity and hydrophobicity. The reduction of UVA254 was clearly observed during RBF, thus indicating the attenuation of the aromaticity in dissolved organic matter. The UVA254 levels were significantly high at 0.36 and 0.23 cm−1 for GMW-Sand and GMW-Gravel, respectively, indicating the high amount of aromatic compounds in the organic matter. Allochthonous dissolved organic matter, which consists of more humic-like compounds including polycyclic aromatics, is strongly associated with the formation of THMs [25]. Therefore, the mixing effects from groundwater that contained high concentrations of humic substances should be minimized at this site in order to reduce the THM formation potential during treatment of the riverbank filtrate. In this study, it is important to investigate the effect of the portion of groundwater in the pumped water, which is a mixture of riverbank filtrate and land-side groundwater.

Table 3.
UV254 and dissolved organic carbon (n = 10).
3.3.3. Dissolved Organic Matter’s Characteristics
The characteristics of the dissolved organic matter between surface water (Nakdong River), riverbank filtrates, and land-side groundwater were different. The fluorescence EEM spectra of the Nakdong River, PW-Sand and PW-Gravel were used to investigate dissolved organic matter’s characteristics during soil passage. As previously mentioned, there are peaks at known wavelengths that represent protein-like substances and humic-like substances. The protein-like substances were also reported to indicate the presence of biodegradable organic matter [11]. The protein-like substances that were contained in the river water were attenuated in the RBF filtrate. Figure 5 demonstrated that the Fluorescence EEM intensities of the protein-like substances (T1 and T2) in the river water were reduced by more than 50% for the PW-Sand and PW-Gravel during soil passage. In addition, the reductions in the intensities of protein-like substances were similar to those of the DOC removals that are shown in Table 3 (PW-Sand: 63% and PW-Gravel: 68%). The fulvic-like and humic-like substances in groundwater were relatively high compared to those of PW-Sand and PW-Gravel. It was found that the humic-like substances in the Nakdong River were relatively low. In the case of land-side groundwater, it was confirmed that humic-like substances were dominant. The origins of aquatic humic-like substances are different from one another, and dissimilar properties could also result in high DOC [26]. The high fulvic-like and humic-like substances that were detected in PW-Gravel may be due to the influence of land-side groundwater, which contained high amounts of humic-like substances.
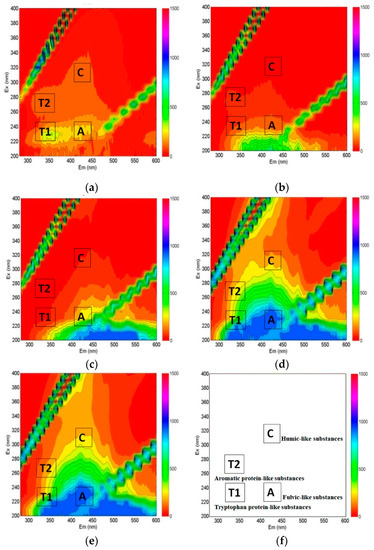
Figure 5.
Fluorescence excitation-emission matrix (EEM) spectra: (a) river water, (b) PW-Sand, (c) PW-Gravel, (d) Groundwater (GW)-Sand, (e) GW-Gravel, and (f) the location of the EEM peaks for four groups of substances.
LC-OCD was used to investigate the changes in the dissolved organic matter’s characteristics in the RBF filtrates according to their molecular weights. The biopolymers in the river water were significantly degraded compared to other DOM fractions (Figure 6). A previous study reported that biodegradation was found to be an important mechanism for removing biodegradable organic matter including biopolymers during soil passage [10]. As shown in Figure 6, biopolymers were relatively high in the Nakdong River. In the RBF filtrates taken from PW-Sand and PW-Gravel, the dissolved organic matter fractions with relatively high molecular weights, such as biopolymers and humics, were effectively removed during RBF. In particular, the biopolymers can be easily used as a carbon source for microorganisms; therefore, the biodegradation during soil passage is an important mechanism for supplying biologically stable water. It is important to understand the dissolved organic matter characteristics in riverbank filtrates using advanced organic matter characterization tools such as fluorescence EEM and LC-OCD. The humic-like substances were relatively high in the riverbank filtrate; therefore, post-treatments should focus on the removal of humics and biological filtration may not be necessary since most of the biodegradable organic matter was effectively removed. Based on the fluorescence EEM and LC-OCD results, RBF could effectively remove biodegradable organic matter, such as biopolymers, including protein-like substances.
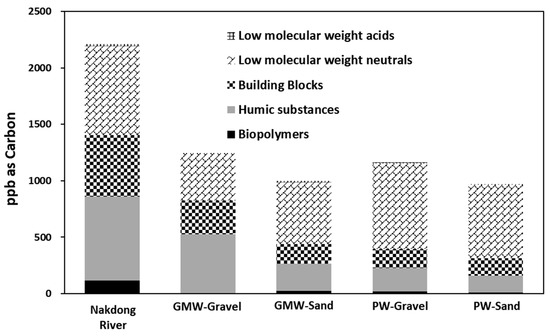
Figure 6.
The changes of the organic matter fractions (biopolymers, humic substances, building blocks, neutrals, and acids) determined using liquid chromatography-organic carbon detection (LC-OCD) for river water, PW-Sand, PW-Gravel, GW-Sand, and GW-Gravel.
3.4. Effect of Different Pumping Rates
The pumping rates of the two vertical wells were varied for two weeks in order to investigate the impact of the shortest travel times on the removal of dissolved organic matter. The BF simulator, part of the NASRI Project (Germany), was used to estimate the shortest travel times at different pumping rates (Table 2) [13,14,15]. The different pumping rates (700, 1000, 1300 and 2000 m3/day) that were tested in this study showed that there were no significant changes in the removal of turbidity, ammonia (data not shown), and DOC (Figure 7).
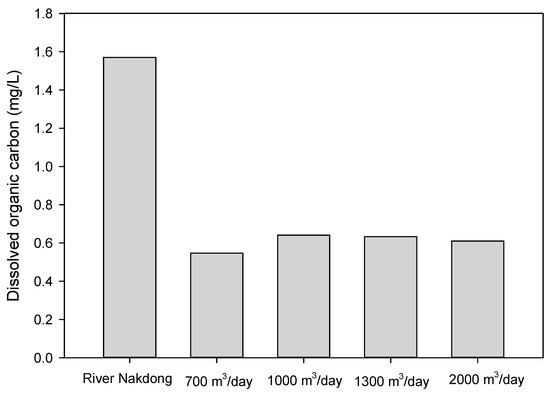
Figure 7.
Changes in the dissolved organic carbon (PW-Sand) at different pumping rates (700, 1000, 1300 and 2000 m3/day, n = 2).
The removal of ammonia and DOC are associated with biodegradation, which occurs predominantly within a few meters of infiltration [27]. Therefore, longer travel times may enhance the removal of slowly biodegradable organic matter that requires a longer time to degrade. However, there were no significant changes in the removal of dissolved organic matter when the estimated travel time increased from 1 to 3 d.
The iron concentrations with different pumping rates were also investigated. The iron concentrations were 18.1 ± 0.8, 17.2± 0.5, 14.7 ± 0.9, and 14.5 ± 0.6 mg/L at 2000, 1300, 1000 and 700 m3/day, respectively. As the pumping rates decrease, there is more land-side groundwater in the pumped water, containing relatively lower iron concentrations (Table 2), indicating the higher fraction of land-side groundwater in the riverbank filtrate. As the pumping rates were changed, the water quality in the riverbank filtrates could also be influenced by the groundwater portion.
4. Conclusions
RBF is a robust water treatment method, which provides a degree of bulk organic matter removal and protection against water contamination. This study was carried out to investigate the feasibility of horizontal collector wells, and two vertical PWs were used to assess the water quality of filtrates during soil passage and the influence of land-side groundwater. Based on the field studies described in this paper, the following conclusions can be drawn:
- -
- The turbidity was effectively removed via RBF, although there were significant turbidity increases in the river water.
- -
- The iron concentrations from the RBF wells (i.e., PW-Sand and PW-Gravel) were higher than those of the groundwater monitoring wells (GMW-Sand and GMW-Gravel) and were 18.1 ± 0.8 and 25.9 ± 1.3 mg/L for the PW-Sand and PW-Gravel, respectively, during the study. The occurrence of a high iron concentration was due to the biodegradation of dissolved organic matter, which led to the reduced redox potential during soil passage. The reduced zone occurred between the river and RBF wells, which enhanced the mobilization of iron under more reducing conditions compared to that of land-side groundwater.
- -
- As a result of the dissolved organic matter characteristics via LC-OCD and fluorescence EEM, the biopolymers contained in the river were effectively removed while passing through the aquifer. It was also confirmed that most of the humic components, which are difficult to reduce biologically and were detected from the land-side groundwater, could influence the quality of the RBF filtrate.
- -
- The RBF wells (PW-Sand and PW-Gravel) in this study did not show any changes with respect to turbidity and DOC at different pumping rates (700, 1000, 1300 and 2000 m3/day).
- -
- Vertical wells at different layers (sand and gravel layers in the aquifer) were tested in this study in order to determine the depth of the laterals for the horizontal wells. This study used vertical wells in order to investigate the RBF site before the construction of the horizontal collectors, which usually cost much more than vertical wells.
Author Contributions
S.K.M. and K.-H.L. prepared the article. S.K.M. was responsible for the water quality data collection.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which was funded by the Ministry of Science, ICT & Future Planning [Grant number: 2017R1A1A1A05001231].
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Delpla, I.; Jung, A.-V.; Baures, E.; Clement, M.; Thomas, O. Impacts of climate change on surface water quality in relation to drinking water production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-C.; Shin, H.-J.; Nguyen, T.T.; Tenhunen, J. Water policy reforms in South Korea: A historical review and ongoing challenges for sustainable water governance and management. Water 2017, 9, 717. [Google Scholar] [CrossRef]
- Pryor, F.L. Water stress and water wars. Econ. Peace Secur. J. 2007, 2, 7–18. [Google Scholar] [CrossRef]
- Kim, J.-S.; Jain, S. Precipitation trends over the Korean peninsula: Typhoon-induced changes and a typology for characterizing climate-related risk. Environ. Res. Lett. 2011, 6, 034033. [Google Scholar] [CrossRef]
- Lee, J.-H.; Hamm, S.-Y.; Cheong, J.-Y.; Kim, H.-S.; Ko, E.-J.; Lee, K.-S.; Lee, S.-I. Characterizing riverbank-filtered water and river water qualities at a site in the lower Nakdong River basin, Republic of Korea. J. Hydrol. 2009, 376, 209–220. [Google Scholar] [CrossRef]
- Greskowiak, J.; Prommer, H.; Massmann, G.; Johnston, C.D.; Nützmann, G.; Pekdeger, A. The impact of variably saturated conditions on hydrogeochemical changes during artificial recharge of groundwater. Appl. Geochem. 2005, 20, 1409–1426. [Google Scholar] [CrossRef]
- Henzler, A.F.; Greskowiak, J.; Massmann, G. Modeling the fate of organic micropollutants during river bank filtration (Berlin, Germany). J. Contam. Hydrol. 2014, 56, 78–92. [Google Scholar] [CrossRef]
- Kim, H.-C.; Lee, W.M.; Lee, S.; Choi, J.; Maeng, S.K. Characterization of organic precursors in DBP formation and AOC in urban surface water and their fate during managed aquifer recharge. Water Res. 2017, 123 (Suppl. C), 75–85. [Google Scholar] [CrossRef]
- Bertelkamp, C.; Reungoat, J.; Cornelissen, E.R.; Singhal, N.; Reynisson, J.; Cabo, A.J.; van der Hoek, J.P.; Verliefde, A.R.D. Sorption and biodegradation of organic micropollutants during river bank filtration: A laboratory column study. Water Res. 2014, 52, 231–241. [Google Scholar] [CrossRef]
- Maeng, S.K.; Sharma, S.K.; Abel, C.D.T.; Magic-Knezev, A.; Amy, G.L. Role of biodegradation in the removal of pharmaceutically active compounds with different bulk organic matter characteristics through managed aquifer recharge: Batch and column studies. Water Res. 2011, 45, 4722–4736. [Google Scholar] [CrossRef]
- Maeng, S.K.; Sharma, S.K.; Abel, C.D.T.; Magic-Knezev, A.; Song, K.-G.; Amy, G.L. Effects of effluent organic matter characteristics on the removal of bulk organic matter and selected pharmaceutically active compounds during managed aquifer recharge: Column study. J. Contam. Hydrol. 2012, 140–141, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P. Future management of aquifer recharge. Hydrogeol. J. 2005, 13, 313–316. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chaweza, D.; Bosuben, N.; Holzbecher, E.; Amy, G. Framework for feasibility assessment and performance analysis of riverbank filtration systems for water treatment. J. Water Supply Res. Technol. 2012, 61, 73–81. [Google Scholar] [CrossRef]
- Rustler, M.; Grützmacher, G. Bank Filtration Simulator-Manual, TECHNEAU Executive Summary. 2009. Available online: https://www.kompetenz-wasser.de/wp-content/uploads/2017/05/d5-2-5.pdf (accessed on 4 April 2018).
- Holzbecher, E.; Grützmacher, G.; Amy, G.; Wiese, B.; Sharma, S.K. The Bank Filtration Simulator—A MATLAB GUI. In Environmental Informatics and Industrial Ecology, Proceedings of the 22nd International Conference on Informatics for Environmental Protection (Enviroinfo 2008), Lüneburg, Germany, 10–12 September 2008; Möller, A., Page, B., Schreiber, M., Eds.; Shaker: Aachen, Germany, 2008. [Google Scholar]
- Chow, A.T.; Dahlgren, R.A.; Zhang, Q.; Wong, P.K. Relationships between specific ultraviolet absorbance and trihalomethane precursors of different carbon sources. J. Water Supply Res. Technol. 2008, 57, 471–480. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y.; Quan, X.; Fan, X.; Zhao, H. Performing a microfiltration integrated with photocatalysis using an Ag-TiO2/HAP/Al2O3 composite membrane for water treatment: Evaluating effectiveness for humic acid removal and anti-fouling properties. Water Res. 2010, 44, 6104–6114. [Google Scholar] [CrossRef]
- Korean Ministry of Environment. Water Quality Standard and Test; Korean Ministry of Environment: Sejong City, Korea, 2011.
- Park, J.W.; Kim, H.C.; Meyer, A.S.; Kim, S.; Maeng, S.K. Influences of NOM composition and bacteriological characteristics on biological stability in a full-scale drinking water treatment plant. Chemosphere 2016, 160, 189–198. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography–organic carbon detection–organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Kumar, P.; Mehrotra, I.; Gupta, A.; Kumar, S. Riverbank Filtration: A Sustainable Process to Attenuate Contaminants during Drinking Water Production. J. Sustain. Dev. Energy Water Environ. Syst. 2018, 6, 150–161. [Google Scholar] [CrossRef]
- Vanderzalm, J.L.; Page, D.W.; Barry, K.E.; Dillon, P.J. A comparison of the geochemical response to different managed aquifer recharge operations for injection of urban storm water in a carbonate aquifer. Appl. Geochem. 2010, 25, 1350–1360. [Google Scholar] [CrossRef]
- Vanderzalm, J.L.; Page, D.W.; Barry, K.E.; Scheiderich, K.; Gonzalez, D.; Dillon, P.J. Probabilistic approach to evaluation of metal(loid) fate during stormwater aquifer storage and recovery. Clean Soil Air Water 2016, 44, 1672–1684. [Google Scholar] [CrossRef]
- Maeng, S.K.; Sharma, S.K.; Amy, G.; Magic-Knezev, A. Fate of effluent organic matter (EfOM) and natural organic matter (NOM) through riverbank filtration. Water Sci. Technol. 2008, 57, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Conrad, D.; Kothawala, D.N.; Baulch, H.M. Selective removal of dissolved organic matter affects the production and speciation of disinfection byproducts. Sci. Total Environ. 2019, 652, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Artinger, R.; Buckau, G.; Geyer, S.; Fritz, P.; Wolf, M.; Kim, J.I. Characterization of groundwater humic substances: Influence of sedimentary organic carbon. Appl. Geochem. 2000, 15, 97–116. [Google Scholar] [CrossRef]
- Schmidt, C.; Lange, F.; Brauch, H.; Kuhn, W. Experiences with riverbank filtration and infiltration in Germany. Proceeding of the International Symposium of Artificial Recharge of Groundwater, Daejon, Korea, 7–8 July 2003. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).