To What Degree Can the Specifics of Occurrence of Glacial Relic Betula humilis Schrank Be an Indicator of Habitat Conditions of Moderate Climate Peatlands?
Abstract
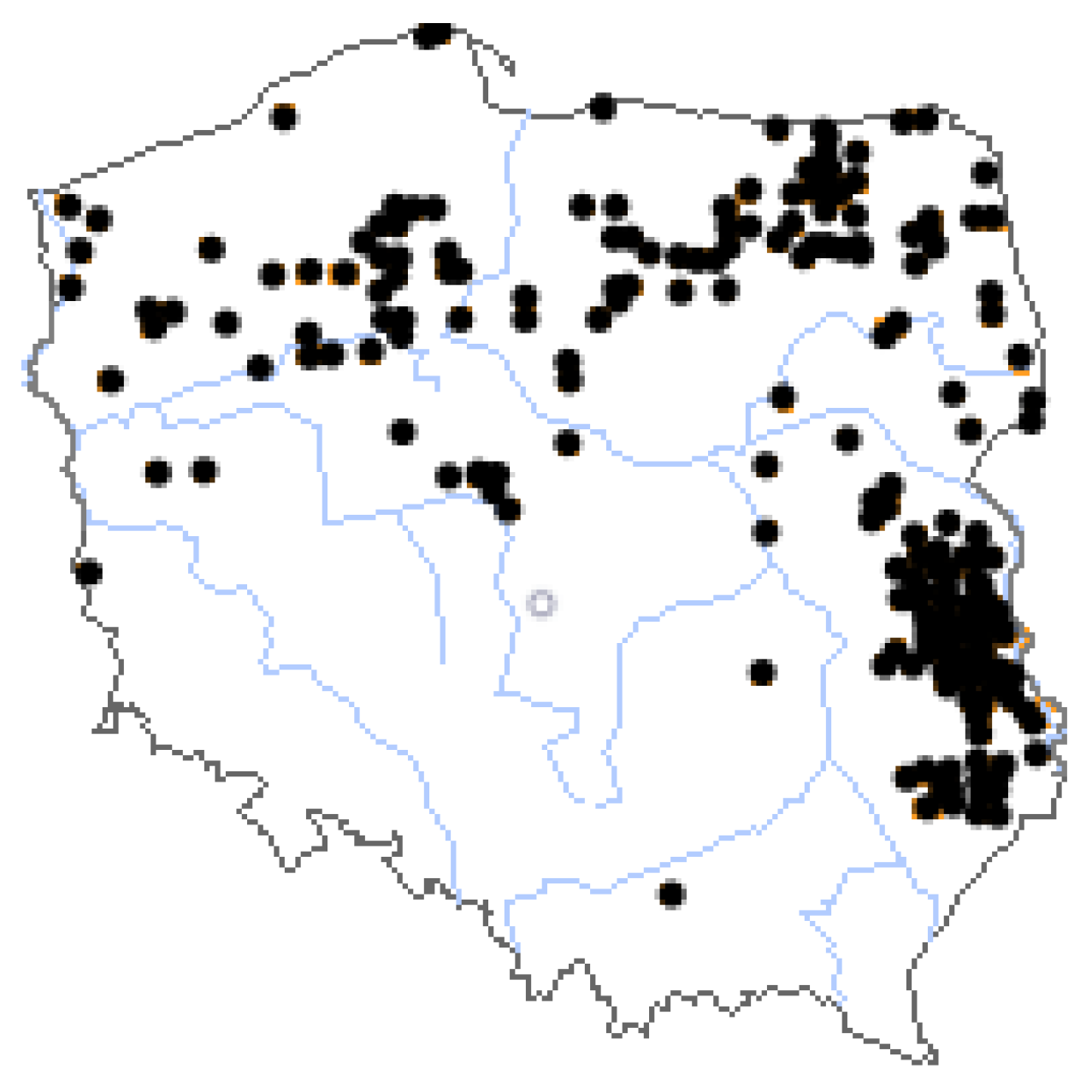
1. Introduction
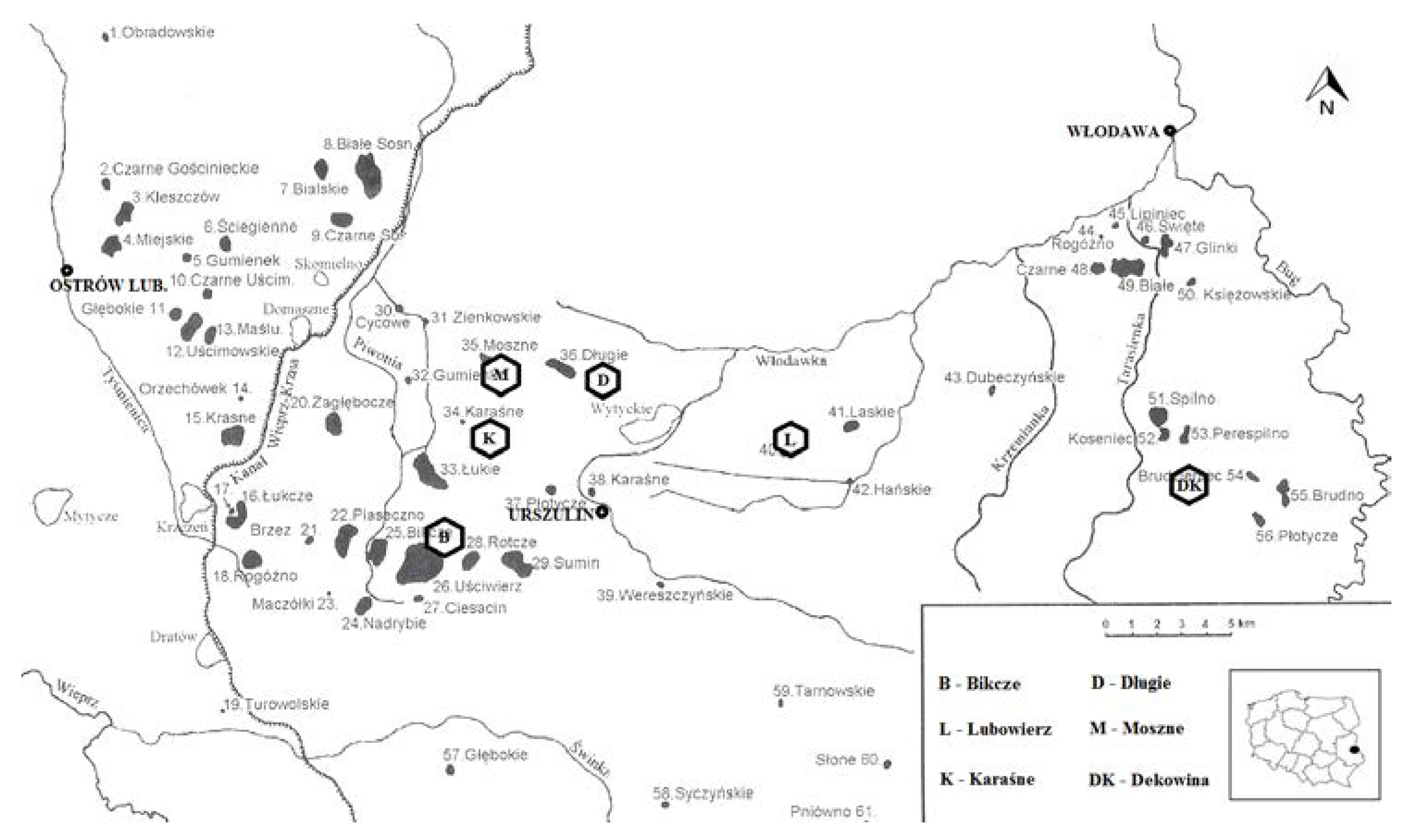
2. Materials and Methods
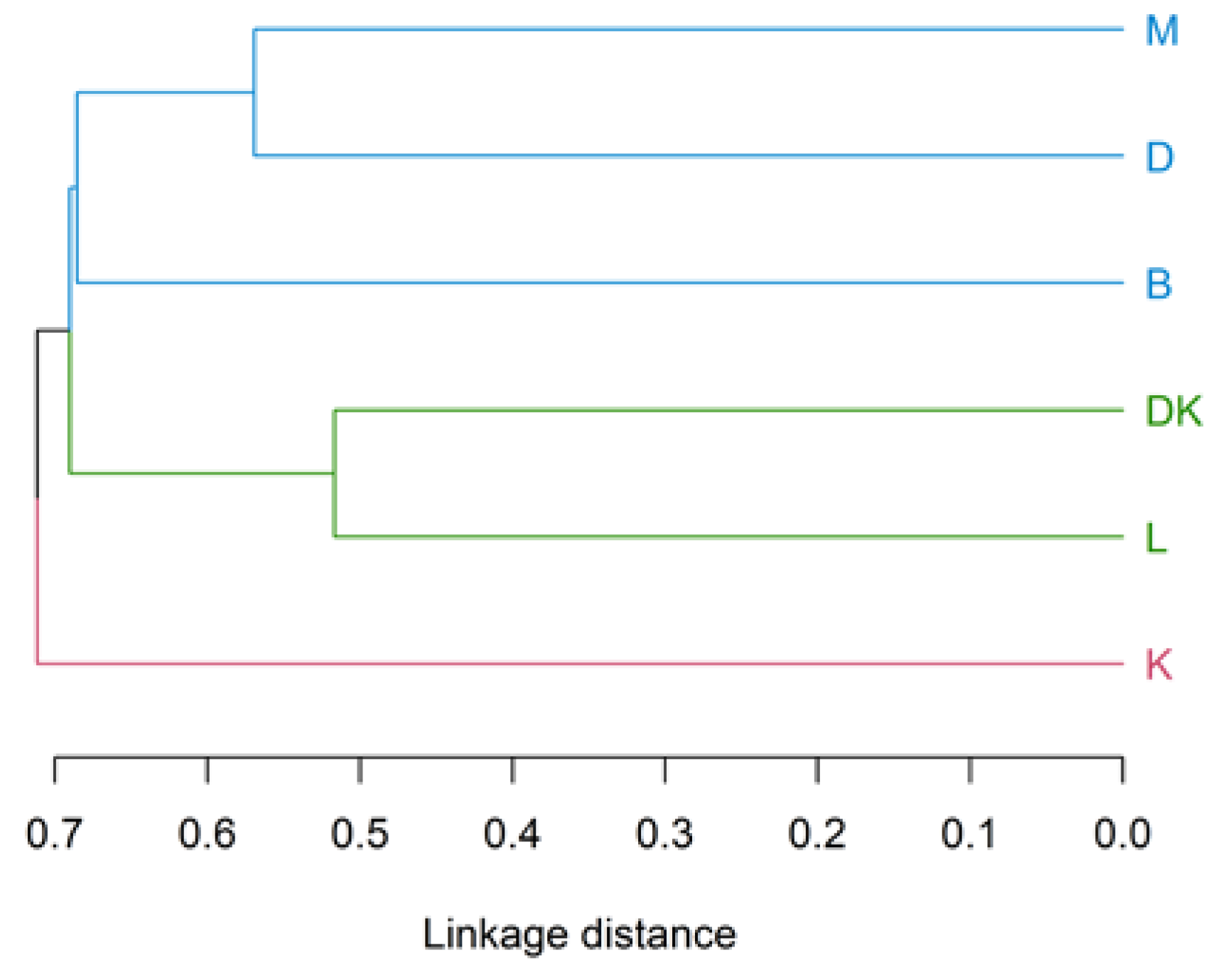
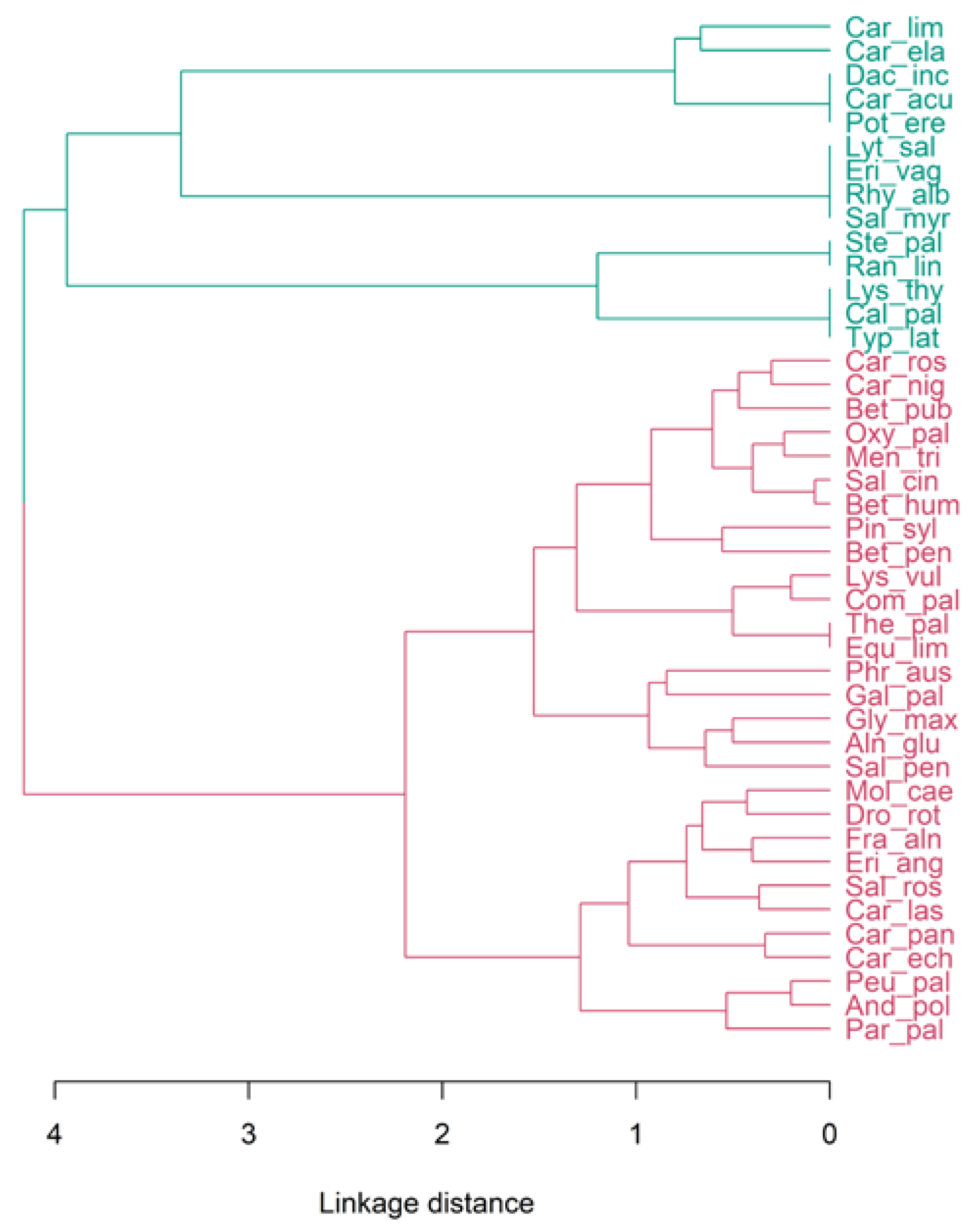
3. Results
4. Discussion
4.1. Anthropogenic Impacts in the Region
4.2. Botanical Analysis
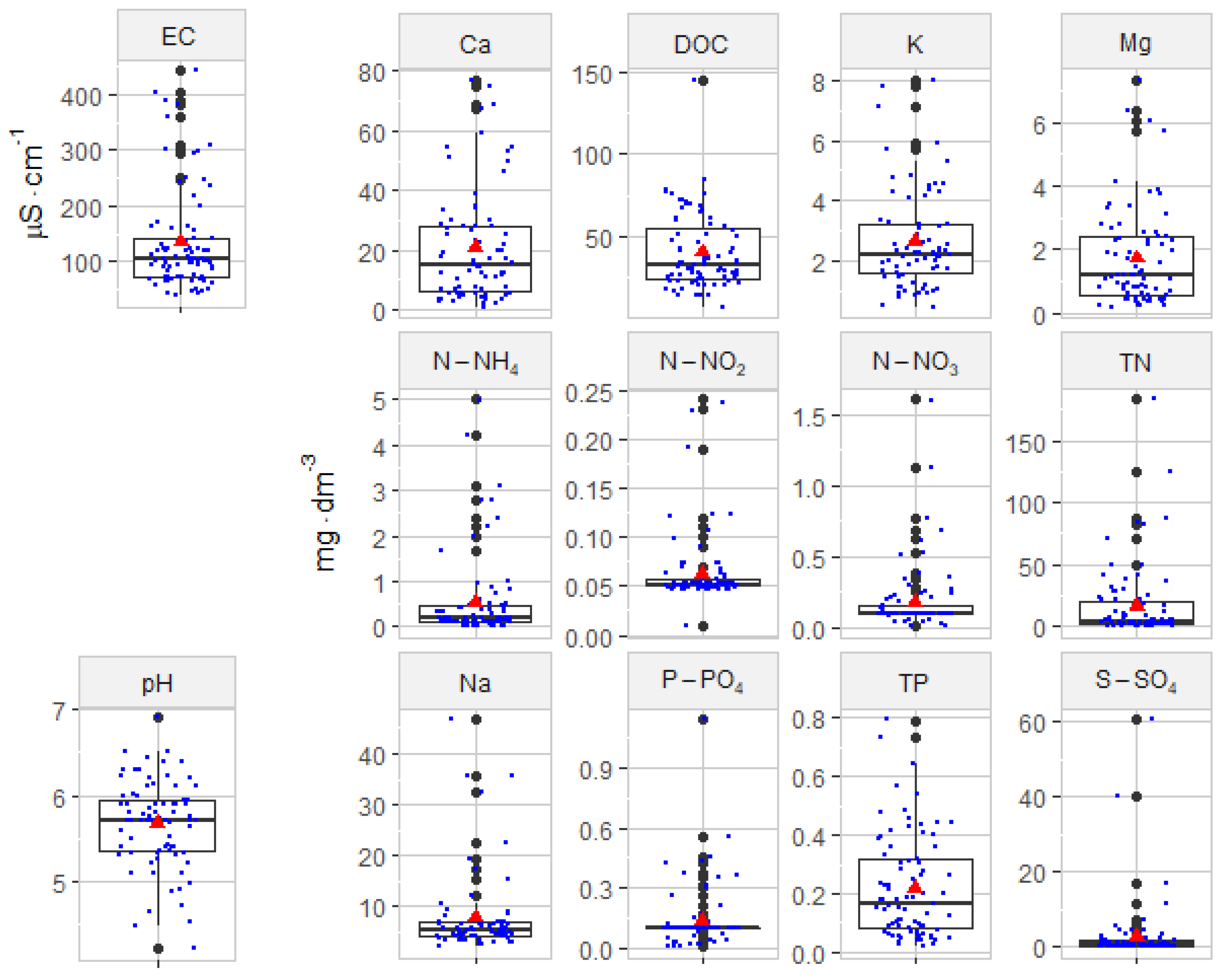
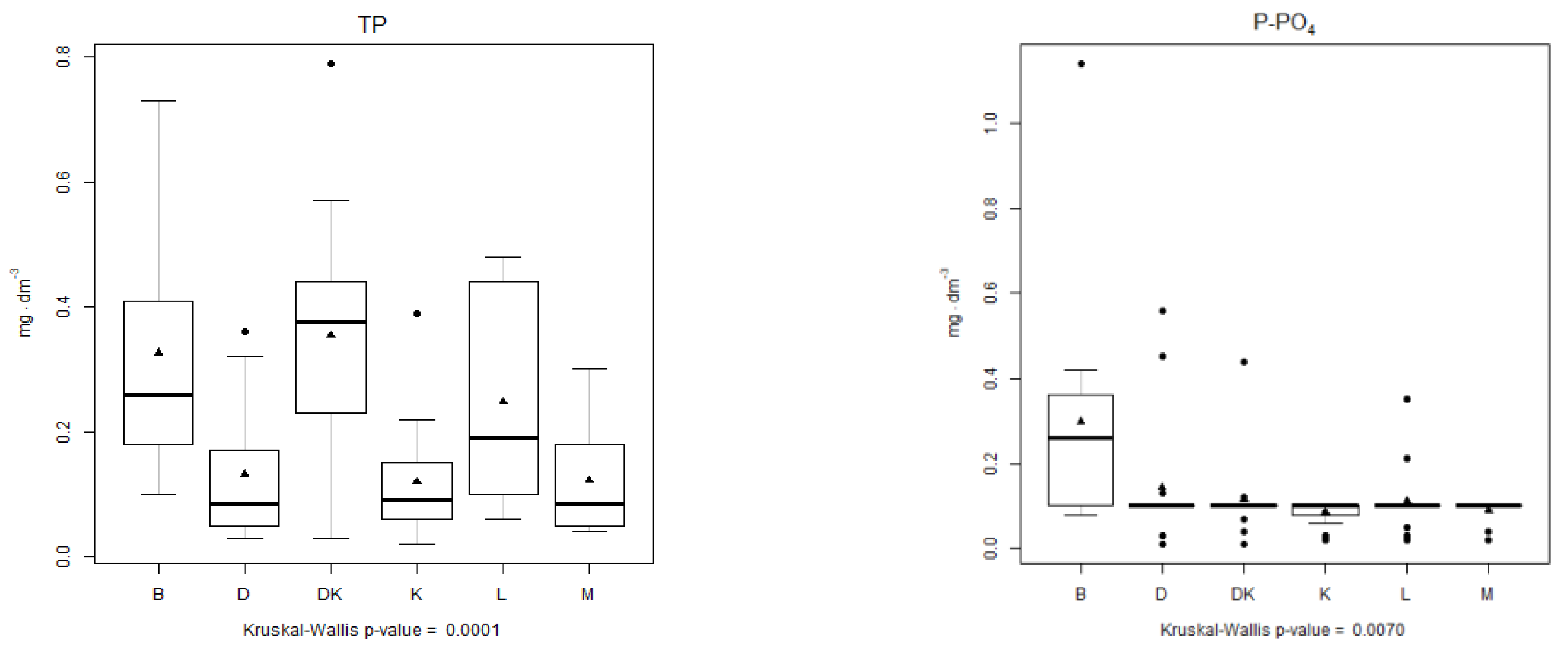
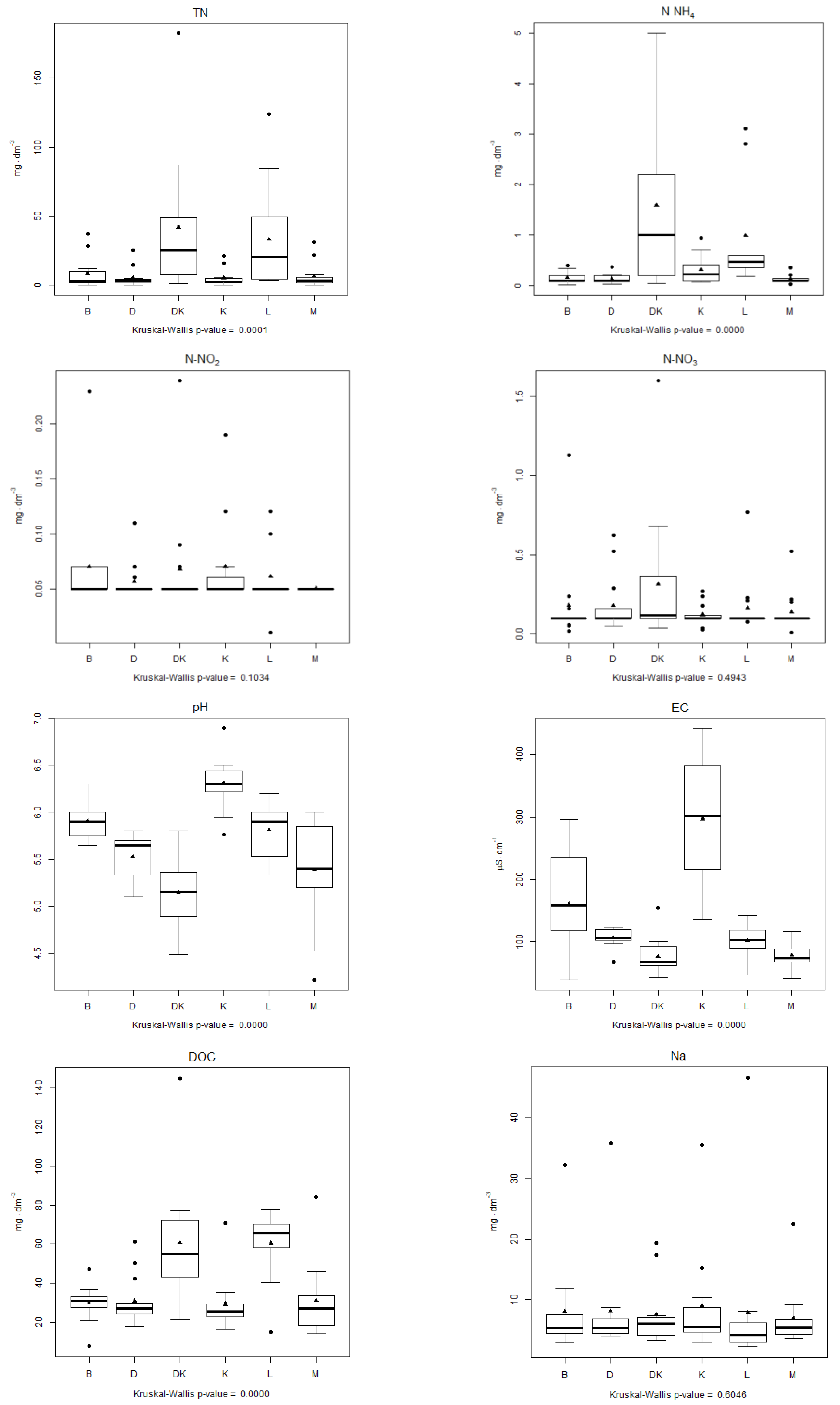
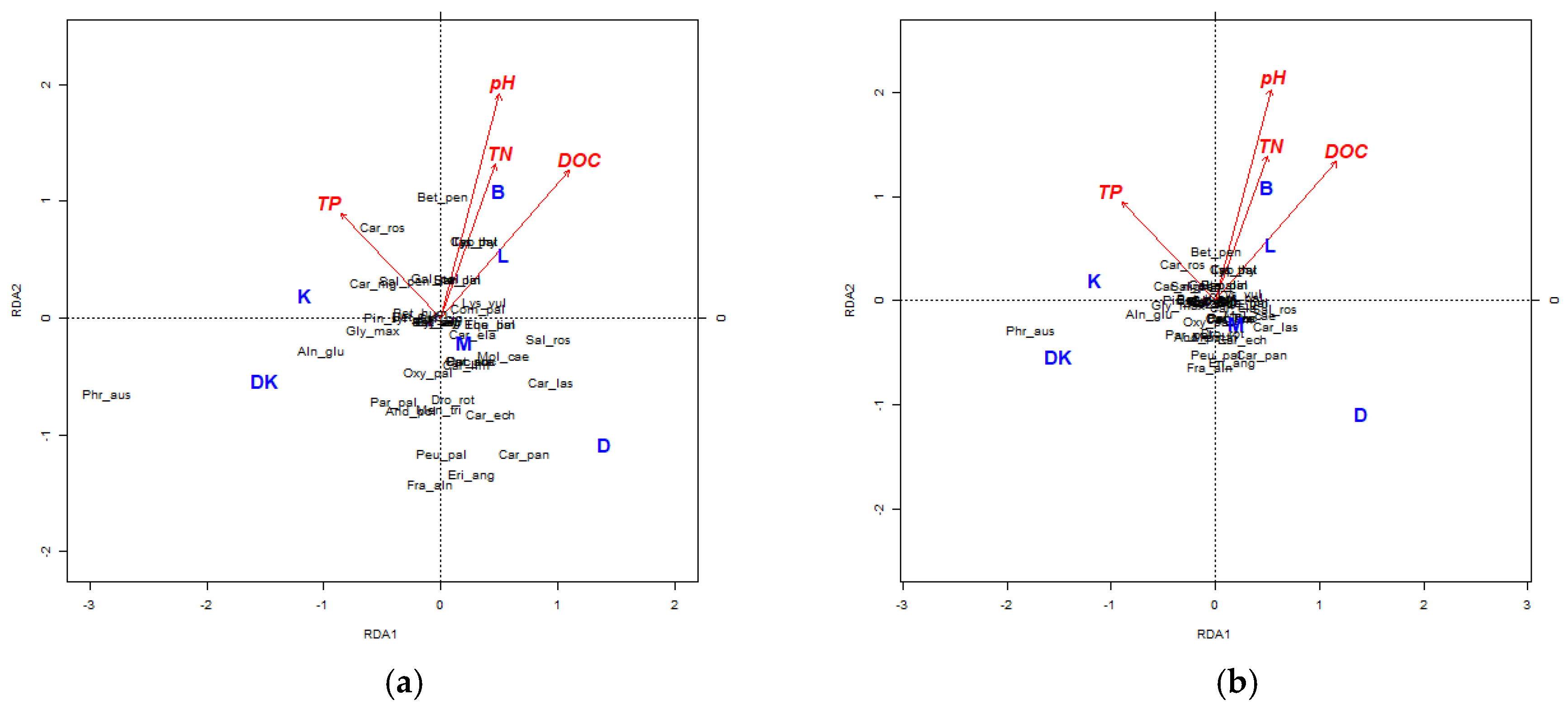
4.3. Analysis of the Physical–Chemical Parameters
4.4. Problems of the Active Protection of the Species
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wojciechowski, I. Funkcjonowanie Ekosystemów Torfowiskowych; Akademia Rolnicza w Lublinie—Katedra Ekologii Ogólnej: Lublin, Poland, 1997. [Google Scholar]
- Kaszewski, B.M. Warunki klimatyczne Poleskiego Parku Narodowego. In Poleski Park Narodowy Monografia Przyrodnicza; Radwan, S., Ed.; Morpol: Lublin, Poland, 2002; pp. 19–28. [Google Scholar]
- Fijałkowski, D. Zespoły Roślinne Lubelszczyzny; UMCS: Lublin, Poland, 1991. [Google Scholar]
- Kruszelnicki, J.; Gostyńska-Jakuszewska, M.; Rutkowski, L. Salix lapponum L. (wierzba lapońska). In Polska Czerwona Księga Roślin. Paprotniki i Rośliny Kwiatowe; Kaźmierczakowa, R., Zarzycki, K., Eds.; Instytut Botaniki PAN: Kraków, Poland, 2001; pp. 71–73. [Google Scholar]
- Churski, M.; Danielewicz, W. Salix myrtilloides in the north central Poland. Distribution, threats and conservation. Dendrobiology 2008, 60, 3–9. [Google Scholar]
- Pogorzelec, M. Influence of chosen environmental abiotic factors on Salix lapponum L. populations in Polesie Lubelskie Region. Pol. J. Environ. Stud. 2008, 17, 581–586. [Google Scholar]
- Pogorzelec, M. Downy willow (Salix lapponum L.) as a component of different phytocoenoses in Polesie National Park. Acta Agrobot. 2009, 62, 107–116. [Google Scholar] [CrossRef][Green Version]
- Serafin, A.; Pogorzelec, M.; Banach, B.; Mielniczuk, J. Habitat conditions of the endangered species Salix myrtilloides in Eastern Poland. Dendrobiology 2015, 73, 55–64. [Google Scholar] [CrossRef]
- Serafin, A.; Pogorzelec, M.; Banach, B.; Szczurowska, A.; Mielniczuk, J. Physico-chemical groundwater conditions at Salix lapponum stands in Eastern Poland. Dendrobiology 2015, 73, 65–74. [Google Scholar] [CrossRef]
- Kłosowski, G.; Kłosowski, S. Flora Polski. Rośliny Wodne i Bagienne; Multico: Warszawa, Poland, 2001. [Google Scholar]
- Ilnicki, P. Torfowiska i Torf; Wyd. Akademii Rolniczej im. Augusta Cieszkowskiego: Poznań, Poland, 2002. [Google Scholar]
- Tobolski, K. Torfowiska na Przykładzie Ziemi Świeckiej; Wyd. Towarzystwo Przyjaciół Dolnej Wisły: Toruń, Poland, 2003. [Google Scholar]
- Geertsema, W.; Opdam, P.; Kropff, M.J. Plant strategies and agricultural landscapes: Survival in spatially and temporally fragmented habitat. Landsc. Ecol. 2002, 17, 263–279. [Google Scholar] [CrossRef]
- Soomers, H.; Karssenberg, D.; Verhoeven, J.T.A.; Verweij, P.A.; Wassen, M.J. The effect of habitat fragmentation and abiotic factors on fen plant occurrence. Biodivers. Conserv. 2013, 22, 405–424. [Google Scholar] [CrossRef]
- Staszkiewicz, J.; Białobrzeska, M.; Truchanowicz, J.; Wójcicki, J.J. Variability of Betula humilis (Betulaceae) in Poland. 2. Variability of the generative organs. Fragm. Flor. Geobot. 1991, 36, 375–401. [Google Scholar]
- Jadwiszczak, K.A.; Banaszek, A.; Jabłońska, E.; Sozinov, O.V. Chloroplast DNA variation of Betula Humilis Schrk. in Poland and Belarus. Tree Genet. Genomes 2012, 8, 1017–1030. [Google Scholar] [CrossRef]
- Kaźmierczakowa, R.; Zarzycki, K.; Mirek, Z.; Adamowski, W.; Babczyńska-Sendek, B. Polska Czerwona Księga Roślin. Paprotniki i Rośliny Kwiatowe; Instytut Ochrony Przyrody. Polska Akademia Nauk: Kraków, Poland, 2014; ISBN 978-83-61191-72-8. [Google Scholar]
- Hultén, E.; Fries, M. Atlas of North European Vascular Plants; Koeltz Scientific Books: Königstein, Germany, 1986. [Google Scholar]
- Jabłońska, E. Brzoza Niska Betula Humilis Schrank w Polsce-Status Fitocenotyczny, Warunki Siedliskowe, Zagrożenia i Ochrona. Ph.D. Thesis, Uniwersytet Warszawski, Warszawa, Poland, 2009. Available online: https://www.researchgate.net/publication/270283403 (accessed on 4 August 2018).
- Jabłońska, E. Vegetation with Betula humilis in Central Europe. Phytocoenologia 2012, 42, 259–277. [Google Scholar] [CrossRef]
- Zając, A.; Zając, M. Atlas Rozmieszczenia Roślin Naczyniowych w Polsce; Pracownia Chorologii Komputerowej Instytutu Botaniki UJ: Kraków, Poland, 2001. [Google Scholar]
- Zarzycki, K.; Trzcińska-Tacik, H.; Różański, W.; Szaląg, Z.; Wołek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plants of Vascular Plants of Poland; W. Szafer Institute of Botany, PAN: Kraków, Poland, 2002. [Google Scholar]
- Rozporządzenie Ministra Leśnictwa i Przemysłu Drzewnego z Dnia 30 Kwietnia 1983 r. w Sprawie Wprowadzenia Gatunkowej Ochrony Roślin. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU19830270134 (accessed on 4 August 2018).
- Walas, J. Atlas Roślin Chronionych; Liga Ochrony Przyrody: Warszawa, Poland, 1973. [Google Scholar]
- Kucharczyk, M.; Szukałowicz, I. Rzadkie i zagrożone gatunki roślin Polesia Zachodniego. Kosmos 2003, 52, 321–330. [Google Scholar]
- Zarzycki, K.; Szeląg, Z. Red list of the vascular plants in Poland. In Red List of Plants and Fungi in Poland; Mirek, Z., Zarzycki, K., Wojewoda, W., Szeląg, Z., Eds.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2006; pp. 11–20. [Google Scholar]
- Harasimiuk, M.; Michalczyk, Z.; Turczyński, M. Jeziora Łęczyńsko—Włodawskie; Biblioteka Monitoringu Środowiska: Lublin, Poland, 1998. [Google Scholar]
- Piernik, A. Metody Numeryczne w Ekologii; Wydawnictwo Naukowe Uniwersytetu Mikołaja Kopernika: Toruń, Poland, 2008. [Google Scholar]
- Chmiel, J. Flora Roślin Naczyniowych Wschodniej Części Pojezierza Gnieźnieńskiego i Jej Antropogeniczne Przeobrażenia w Wieku XIX i XX. Cz. 1; Sorus: Poznań, Poland, 1993. [Google Scholar]
- Chmiel, J. Flora Roślin Naczyniowych Wschodniej Części Pojezierza Gnieźnieńskiego i Jej Antropogeniczne Przeobrażenia w Wieku XIX i XX. Cz. 2, Atlas Rozmieszczenia Roślin; Sorus: Poznań, Poland, 1993. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 4 August 2018).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5–2. 2018. Available online: http://CRAN.R-project.org/package=vegan (accessed on 4 August 2018).
- Matuszkiewicz, W. Przewodnik Do Oznaczania Zbiorowisk Roślinnych Polski; Wydawn. Naukowe PWN: Warszawa, Poland, 2001. [Google Scholar]
- Fijałkowski, D. Vascular Flora of Lublin Region. I; Lubelskie Towarzystwo Naukowe: Lublin, Poland, 1994. [Google Scholar]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7976-6. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 0-444-89250-8. [Google Scholar]
- Juśkiewicz-Swaczyna, B.; Choszcz, D. Effect of habitat quality on the structure of populations of Pulsatilla patens (L.) Mill. (Ranunculaceae)—Rare and endangered species of European flora. Pol. J. Ecol. 2012, 60, 567–576. [Google Scholar]
- Skrajna, T.; Kubicka, H.; Rzymowska, Z. Illecebrum verticillatum L.—Endangered species in agrocenoses of eastern Poland: Assessment of ecological and genetic indicators for protection goals. Pol. J. Ecol. 2012, 60, 577–589. [Google Scholar]
- Kostrakiewicz-Gieralt, K. The effect of vegetation character on abundance and structure of subpopulations of rare herb species Gentiana pneumonanthe L. Pol. J. Ecol. 2014, 61, 35–46. [Google Scholar]
- Tilaki, G.A.D.; Kamarei, R.; Vafakhah, M. Determining the relation between soil properties and spatial variability of Nitraria schoberi Linn. Using geostatistical analysis: A case study in Meighan Playa in Iran. Pol. J. Ecol. 2013, 61, 93–104. [Google Scholar]
- Matthies, D.; Bräuer, I.; Maibom, W.; Tscharntke, T. Population size and the risk of local extinction: Empirical evidence from rare plants. Oikos 2004, 105, 481–488. [Google Scholar] [CrossRef]
- Lyons, K.G.; Brigham, C.A.; Traut, B.H.; Schwartz, M.W. Rare species and ecosystem functioning. Conserv. Biol. 2005, 19, 1019–1024. [Google Scholar] [CrossRef]
- Wilgat, T. Jeziora Łęczyńsko-Włodawskie. Ann. Univ. Mariae Curie-Skłodowska Sect. B Biol. 1954, 8, 37–121. [Google Scholar]
- Wilgat, T. Zagadnienia Ochrony środowiska w Lubelskim Zagłębiu Węglowym. Sylwan 1975, 12, 25–34. [Google Scholar]
- Wilgat, T. Wstęp, Czynniki terenowe kształtujące stosunki wodne, morfometria jezior, zakończenie. In Jeziora Łęczyńsko-Włodawskie; Studia Ośrodka Dokumentacji Fizjograficznej: Kraków, Poland, 1991; Volume 19. [Google Scholar]
- Guz, T. Ekologiczna rola mokradeł w środowisku przyrodniczym Lubelszczyzny. Ekoinżynieria 1996, 1, 21–25. [Google Scholar]
- Fijałkowski, D.; Urban, D. Szata roślinna obiektu wodno-torfowiskowego “Uściwierzek” i jej przekształcenia. Ann. Univ. Mariae Curie-Skłodowska Sect. C 1997, 52, 119–143. [Google Scholar]
- Janiec, B. Naturalna i antropogeniczna ewolucja właściwości wód jezior w zachodniej części Pojezierza Łęczyńsko-Włodawskiego. In Przewodnik Ogólnopolskiego Zjazdu; PTG: Lublin, Poland, 1984. [Google Scholar]
- Michalczyk, Z. Aktualny Stan Środowiska w Rejonie Kanału Wieprz-Krzna i Propozycje Ekologicznego Zagospodarowania Tego Terenu; Wydawnictwo Hydrotrust: Warszawa, Poland, 1992. [Google Scholar]
- Michalczyk, Z. Zmiany sieci hydrograficznej w rejonie oddziaływania Kanału Wieprz-Krzna. In Środowisko Przyrodnicze w Strefie Oddziaływania Kanału Wieprz-Krzna; Radwan, S., Ed.; Wydawnictwo TWWP: Lublin, Poland, 1994; pp. 43–46. [Google Scholar]
- Michalczyk, Z.; Zarębski, K. Wymiana wód podziemnych w południowo-zachodniej części Pojezierza Łęczyńsko-Włodawskiego w rejonie KWK “Bogdanka”. In Współczesne Problemy Hydrogeologii. T. 7 cz. 2; Wydawnictwo AGH: Kraków, Poland, 1995; pp. 119–126. [Google Scholar]
- Jasnowski, M. Rozmiary i kierunki przekształceń szaty roślinnej torfowisk. Phytocoenosis 1972, 1, 193–209. [Google Scholar]
- Olaczek, R.; Kucharski, L.; Pisarek, W. Zanikanie obszarów podmokłych i jego skutki środowiskowe na przykładzie województwa piotrkowskiego (zlewni Pilicy i Warty). Stud. Ośrodka Dok. Fizjogr. 1990, 18, 141–199. [Google Scholar]
- Herbich, J. Zróżnicowanie i problemy ochrony roślinności torfowisk Pojezierza Kaszubskiego. Acta Bot. Cassubica 2001, 1, 59–69. [Google Scholar]
- Sołtys, M.; Różycki, A. Rzadkie i zagrożone gatunki flory naczyniowej w Poleskim Parku Narodowym. In Funkcjonowanie Ekosystemów Wodno-Błotnych w Obszarach Chronionych Polesia; Radwan, S., Ed.; UMCS: Lublin, Poland, 1996; pp. 89–95. [Google Scholar]
- Fijałkowski, D. Zmiany szaty roślinnej na Lubelszczyźnie w ostatnim dwudzietoleciu. Ann. Univ. Mariae Curie-Skłodowska Sect. C 1988, 43, 215–238. [Google Scholar]
- Boiński, M.; Boińska, U.; Ceynowa-Giełdon, M. Charakterystyka Fitosocjologiczna rezerwatu wodno-torfowiskowego “Mętno” na obszarze Borów Tucholskich. Acta Univ. Nicol. Copernic. Biol. 1975, 17, 89–113. [Google Scholar]
- Fijałkowski, D. Szata roślinna Jezior Łęczyńsko-Włodawskich i przylegających do nich torfowisk. Ann. Univ. Mariae Curie-Skłodowska Sect. B 1959, 14, 131–207. [Google Scholar]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin, Germany, 2003. [Google Scholar]
- Gawlik, J.; Dębek, W. Ekosystemy torfowiskowe Polesia (Rodzaje i Przemiany Strukturalne). Acta Agrophys. 2002, 66, 121–145. [Google Scholar]
- Schep, S.A.; Zeefat, R.; van Zuidam, J.P. The Relationship between Site Conditions, Nutrient Availability and Vegetation in Western-Siberian Mires-A Reference for Western-European Mires; Manuscript; Utrecht University: Utrecht, The Netherlands, 2003; p. 105. [Google Scholar]
- El Kahloun, M.; Gerard, M.; Meire, P. Phosphorus and nitrogen cycling in fen vegetation along different trophic conditions in the Biebrza valley, Poland. Ecohydrol. Hydrobiol. 2005, 5, 67–78. [Google Scholar]
- Wheeler, B.D.; Proctor, M.C.F. Ecological gradients, subdivisions and terminology of north-west European mires. J. Ecol. 2000, 88, 187–203. [Google Scholar] [CrossRef]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands—Background and Principles Including a Framework for Decision-Making; International Mire Conservation Group and International Peat Society: Saarijärvi, Finland, 2002. [Google Scholar]
- Kamiński, D.; Kamińska, A.M.; Załuski, T. Populacja brzozy niskiej Betula humilis Schrank na terenie projektowanego rezerwatu “Ostoje Koszelewskie” w Welskim Parku Krajobrazowym. Przegląd Przyr. 2000, 11, 125–131. [Google Scholar]
- Szańkowski, M. Zbiorowiska Brzozy Niskiej (Betula Humilis Schrank) w Białowieskim Parku Narodowym i Ich Przyszłość w Środowisku Uwolnionym Spod Presji; Wydawnictwo UW: Warszawa, Poland, 1991. [Google Scholar]
- Ellstrand, N.C.; Elam, D.R. Population genetic consequences of small population size: Implications for plant conservation. Annu. Rev. Ecol. Syst. 1993, 24, 217–242. [Google Scholar] [CrossRef]


| Analysed Parameter | Kruskal–Wallis Statistic | p-Value | Multiple Comparison Test, Kruskal–Wallis Test | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-D | B-DK | B-K | B-L | B-M | D-DK | D-K | D-L | D-M | DK-K | DK-L | DK-M | K-L | K-M | L-M | |||
| I | II | III | IV | ||||||||||||||
| TN | 26.543 | 6.999 × 10−5 | |||||||||||||||
| TP | 27.115 | 5.419 × 10−5 | |||||||||||||||
| N-NH4 | 31.095 | 8.969 × 10−6 | |||||||||||||||
| P-PO4 | 15.951 | 0.006984 | |||||||||||||||
| pH | 50.385 | 1.156 × 10−9 | |||||||||||||||
| DOC | 31.313 | 8.124 × 10−6 | |||||||||||||||
| EC | 47.465 | 4.566 × 10−9 | |||||||||||||||
| Mg | 25.025 | 0.0001378 | |||||||||||||||
| Ca | 33.986 | <0.001 | |||||||||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafin, A.; Urban, D.; Bronowicka-Mielniczuk, U.; Szczurowska, A. To What Degree Can the Specifics of Occurrence of Glacial Relic Betula humilis Schrank Be an Indicator of Habitat Conditions of Moderate Climate Peatlands? Water 2018, 10, 1062. https://doi.org/10.3390/w10081062
Serafin A, Urban D, Bronowicka-Mielniczuk U, Szczurowska A. To What Degree Can the Specifics of Occurrence of Glacial Relic Betula humilis Schrank Be an Indicator of Habitat Conditions of Moderate Climate Peatlands? Water. 2018; 10(8):1062. https://doi.org/10.3390/w10081062
Chicago/Turabian StyleSerafin, Artur, Danuta Urban, Urszula Bronowicka-Mielniczuk, and Agnieszka Szczurowska. 2018. "To What Degree Can the Specifics of Occurrence of Glacial Relic Betula humilis Schrank Be an Indicator of Habitat Conditions of Moderate Climate Peatlands?" Water 10, no. 8: 1062. https://doi.org/10.3390/w10081062
APA StyleSerafin, A., Urban, D., Bronowicka-Mielniczuk, U., & Szczurowska, A. (2018). To What Degree Can the Specifics of Occurrence of Glacial Relic Betula humilis Schrank Be an Indicator of Habitat Conditions of Moderate Climate Peatlands? Water, 10(8), 1062. https://doi.org/10.3390/w10081062