Decomposition of Contaminants of Emerging Concern in Advanced Oxidation Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Water Samples
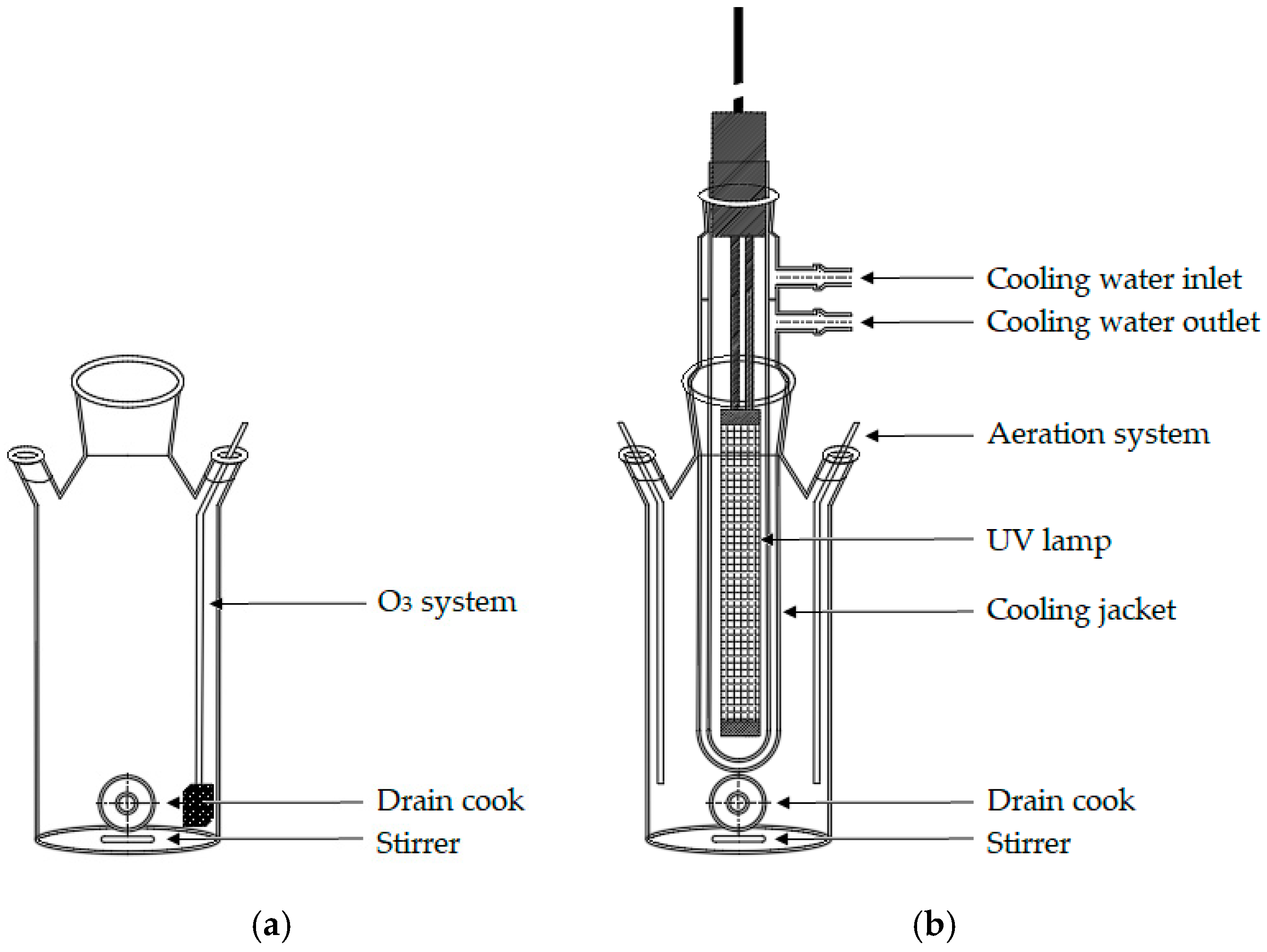
2.3. Advanced Oxidation Processes
2.4. Analytical Procedure and Toxicity Assestment
2.5. Toxicity Assestment
3. Results and Discussion
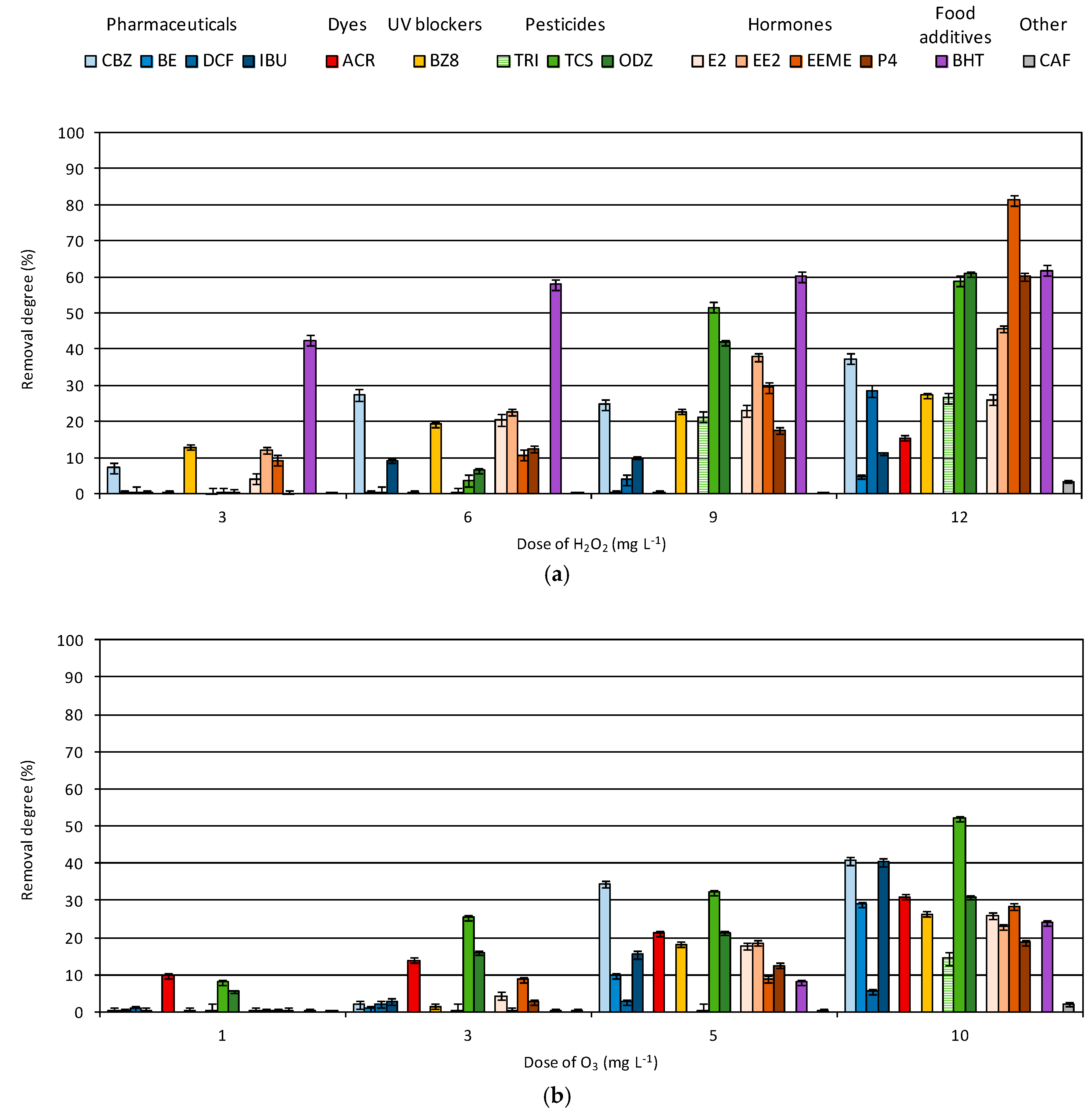
3.1. Degradation of Micropollutants in Single AOP
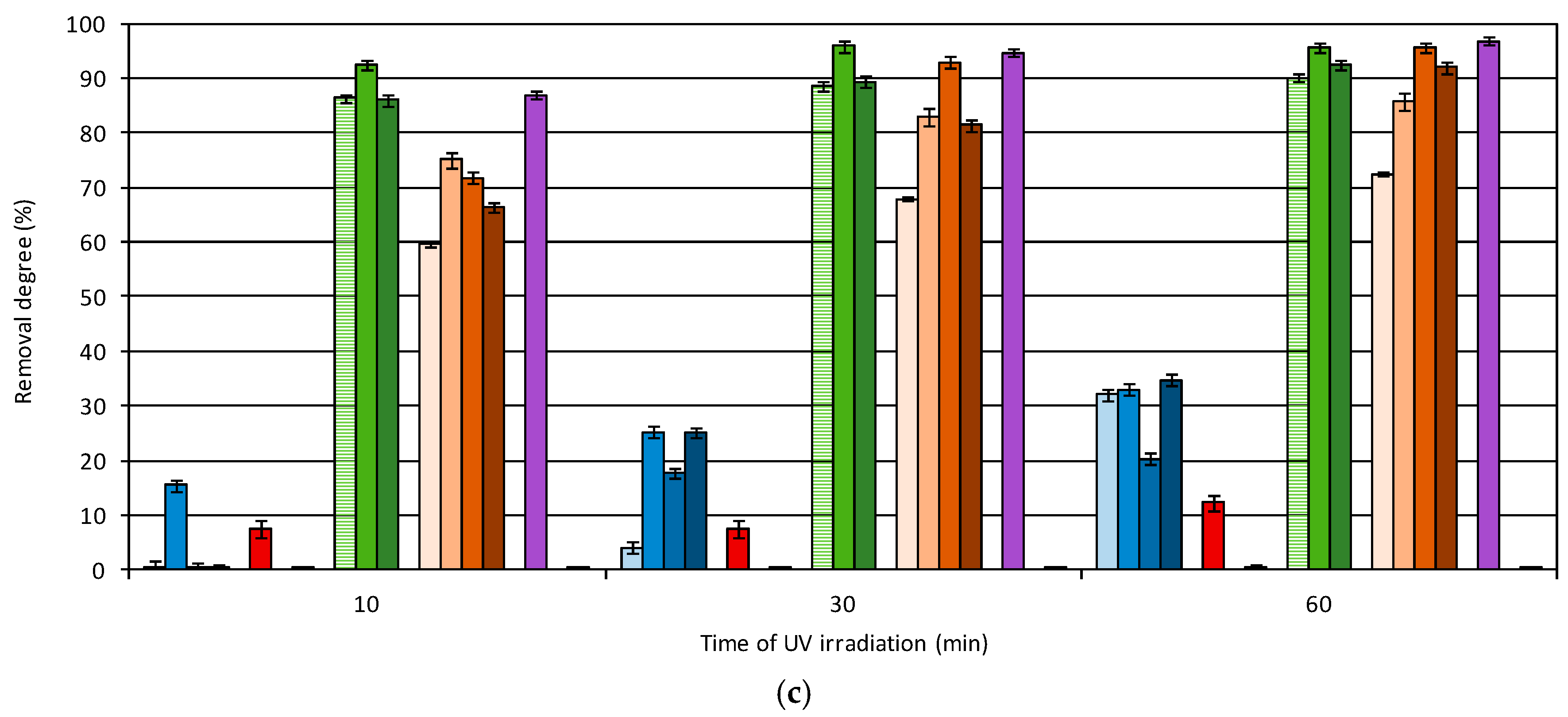
3.2. Degradation of Micropollutants in UV-Based AOP
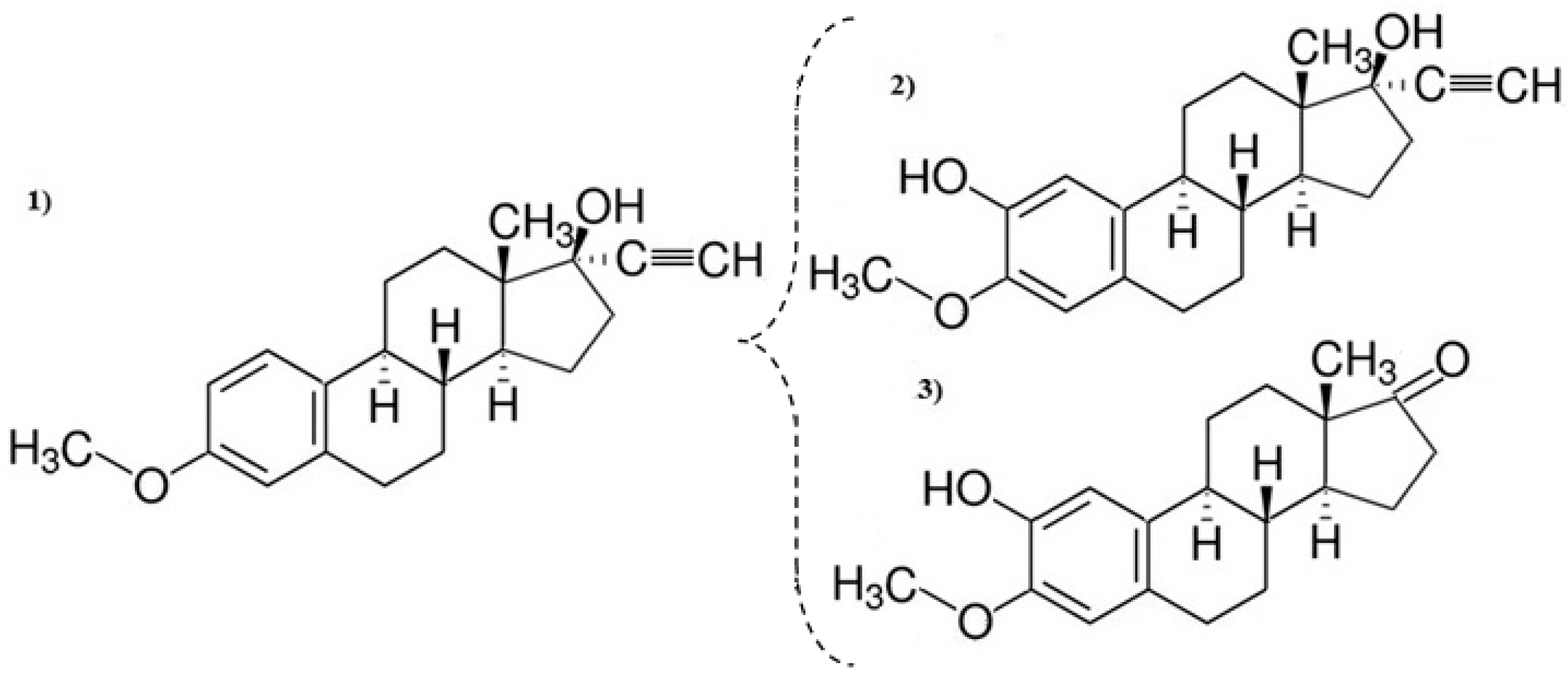
3.3. Decomposition By-Product Identification
3.4. Toxicological Assessment of Post-Processed Water Solution
4. Conclusions
- The highest removal rate of pharmaceutical compounds was observed during the UV/TiO2 process. Only acridine and benzocaine were more effectively oxidized by the UV/O3 process.
- The TiO2-supported process also allows 96% removal of hormones. Triallat and the food additive BHT were most effectively oxidized by the UV process and their removal degrees exceeded 90%. Triclosan was reduced by 98% during the UV/TiO2 process and oxadiazon reached the highest removal degree during the UV/H2O2 process.
- Dioxybenzone was mainly reduced by the process of adsorption on the surface of the TiO2 catalyst—70% removal was achieved.
- The lowest removal degree in all examined processes was observed in the case of caffeine. The removal of this compound requires the implementation of different types of treatment processes, such as membrane technologies.
Funding
Conflicts of Interest
References
- Qiu, L.; Dong, Z.; Sun, H.; Li, H.; Chang, C.C. Emerging pollutants—Part I: Occurrence, fate and transport. Water Environ. Res. 2016, 1855–1875. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Kudlek, E. Toxicological analysis of water mixtures of organic micropollutants subjected to UV irradiation. E3S Web Conf. 2018, 44. [Google Scholar] [CrossRef]
- Fekete-Kertész, I.; Kunglné-Nagy, Z.; Gruiz, K.; Magyar, Á.; Farkas, É.; Molnár, M. Assessing Toxicity of Organic Aquatic Micropollutants Based on the Total Chlorophyll Content of Lemna minor as a Sensitive Endpoint. Period. Polytech. Chem. Eng. 2015, 59, 262–271. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, D.; Roy, R.; Azzouz, A. Advances in catalytic oxidation of organic pollutants—Prospects for thorough mineralization by natural clay catalysts. Appl. Catal. B Environ. 2015, 174–175, 277–292. [Google Scholar] [CrossRef]
- Mahmoud, W.M.M.; Rastogi, T.; Kümmerer, K. Application of titanium dioxide nanoparticles as a photocatalyst for the removal of micropollutants such as pharmaceuticals from water. Curr. Opin. Green Sustain. Chem. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Kosera, V.S.; Cruz, T.M.; Chaves, E.S.; Tiburtius, E.R.L. Triclosan degradation by heterogeneous photocatalysis using ZnO immobilized in biopolymer as catalyst. J. Photochem. Photobiol. A 2017, 344, 184–191. [Google Scholar] [CrossRef]
- Abdennouri, M.; Baâlala, M.; Galadi, A.; El Makhfouk, M.; Bensitel, M.; Nohair, K.; Sadiq, M.; Boussaoud, A.; Barka, N. Photocatalytic degradation of pesticides by titanium dioxide and titanium pillared purified clays. Arab. J. Chem. 2016, 9, S313–S318. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Arshad, T.; Khan, S.A.; Faisal, M.; Shah, Z.; Akhtar, K.; Asiri, A.M.; Ismail, A.A.; Alhogbi, B.G.; Khan, S.B. Cerium based photocatalysts for the degradation of acridine orange in visible light. J. Mol. Liq. 2017, 241, 20–26. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Kudlek, E.; Dudziak, M. Influence of the catalyst type (TiO2 and ZnO) on the photocatalytic oxidation of pharmaceuticals in the aquatic environment. Desalin. Water Treat. 2016, 57, 1552–1563. [Google Scholar] [CrossRef]
- Shaykhi Mehrabadi, Z. Performance of advanced oxidation process (UV/O3/H2O2) degrading amoxicillin wastewater: A comparative study. J. Appl. Res. Water Wastewater 2016, 3, 222–231. [Google Scholar]
- Fodi, T.; Didaskalou, C.; Kupai, J.; Balogh, G.T.; Huszthy, P.; Szekely, G. Nanofiltration-Enabled In Situ Solvent and Reagent Recycle for Sustainable Continuous-Flow Synthesis. ChemSusChem 2017, 10, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Schaepertoens, M.; Didaskalou, C.; Kim, J.F.; Livingston, A.G.; Szekely, G. Solvent recycle with imperfect membranes: A semi-continuous workaround for diafiltration. J. Membr. Sci. 2016, 514, 646–658. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Chanakya, H.N.; Ramachandra, T.V. Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J. Appl. Phycol. 2013, 25, 855–865. [Google Scholar] [CrossRef]
- Li, W.-W.; Yu, H.-Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Kudlek, E.; Dudziak, M.; Bohdziewicz, J. Influence of inorganic ions and organic substances on the degradation of pharmaceutical compound in water matrix. Water 2016, 8, 532. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A Novel Nanohybrid Nanofibrous Adsorbent for Water Purification from Dye Pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Menz, J.; Schneider, M.; Kümmerer, K. Toxicity testing with luminescent bacteria—Characterization of an automated method for the combined assessment of acute and chronic effects. Chemosphere 2013, 93, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Mahugo Santana, C.; Sosa Ferrera, Z.; Torres Padron, M.E.; Santana Rodríguez, J.J. Methodologies for the extraction of phenolic compounds from environmental samples: New Approaches. Molecules 2009, 14, 298–320. [Google Scholar] [CrossRef] [PubMed]
- Werle, S.; Dudziak, M. Evaluation of toxicity of sewage sludge and gasification waste-products. Przem. Chem. 2013, 92, 1350–1353. [Google Scholar]
- AlAani, H.; Hashem, S.; Karabet, F. Photocatalytic (UV-A/TiO2) and photolytic (UV-A) degradation of steroid hormones: Ethinyl Estradiol, Levonorgestrel, and Progesterone. Int. J. ChemTech Res. 2017, 10, 1061–1070. [Google Scholar]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamagiwa, S.; Araki, S.; Yamamoto, H. Effects of Advanced Oxidation Processes on the Decomposition Properties of Organic Compounds with Different Molecular Structures in Water. JWARP 2016, 8, 823–834. [Google Scholar] [CrossRef]
- Xue, J.; Chen, L.; Wang, H. Degradation mechanism of Alizarin Red in hybrid gas–liquid phase dielectric barrier discharge plasmas: Experimental and theoretical examination. Chem. Eng. J. 2008, 138, 120–127. [Google Scholar] [CrossRef]
- Luo, Y.R. Handbook of Bond Dissociation Energies in Organic Compounds; Science Press: Beijing, China, 2005. [Google Scholar]
- Da Silva, J.C.; Teodoro, J.A.; Afonso, R.J.; Aquino, S.F.; Augusti, R. Photolysis and photocatalysis of ibuprofen in aqueous medium: Characterization of by-products via liquid chromatography coupled to high-resolution mass spectrometry and assessment of their toxicities against Artemia Salina. J. Mass Spectrom. 2014, 49, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Son, H.S.; Ko, G.; Zoh, K.D. Kinetics and mechanism of photolysis and TiO2 photocatalysis of triclosan. J. Hazard Mater. 2009, 166, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Deborde, M.; Rabouan, S.; Mazellier, P.; Legube, B. Kinetic and mechanistic investigations of progesterone reaction with ozone. Water Res. 2006, 40, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Svanfelt, J.; Kronberg, L. A photochemical study of diclofenac and its major transformation products. Photochem. Photobiol. 2010, 86, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.; Weiss, S.; Schymanski, E.; Von der Ohe, P.C.; Schmitt-Jansen, M.; Altenburger, R.; Streck, G.; Brack, W. Identification of a phytotoxic photo-transformation product of diclofenac using effect-directed analysis. Environ. Pollut. 2010, 158, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, M.; Juretic Perisic, D.; Biosic, M.; Kusic, H.; Babic, S.; Loncaric Bozic, A. UV photolysis of diclofenac in water; kinetics, degradation pathway and environmental aspects. Environ. Sci. Pollut. Res. Int. 2016, 23, 14908–14917. [Google Scholar] [CrossRef] [PubMed]
- Calza, P.; Massolino, C.; Pelizzetti, E. Photo-induced transformation of hexaconazole and dimethomorph over TiO2 suspension. J. Photochem. Photobiol. A 2008, 200, 356–363. [Google Scholar] [CrossRef]
- Juretic, D.; Kusic, H.; Dionysiou, D.D.; Loncaric Bozic, A. Environmental aspects of photooxidative treatment of phenolic compounds. J. Hazard. Mater. 2013, 262, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Milovac, N.; Juretic, D.; Kusic, H.; Dermadi, J.; Loncaric Bozic, A. Photooxidative degradation of aromatic carboxylic acids in water; influence of hydroxyl substituents. Ind. Eng. Chem. Res. 2014, 53, 10590–10598. [Google Scholar] [CrossRef]

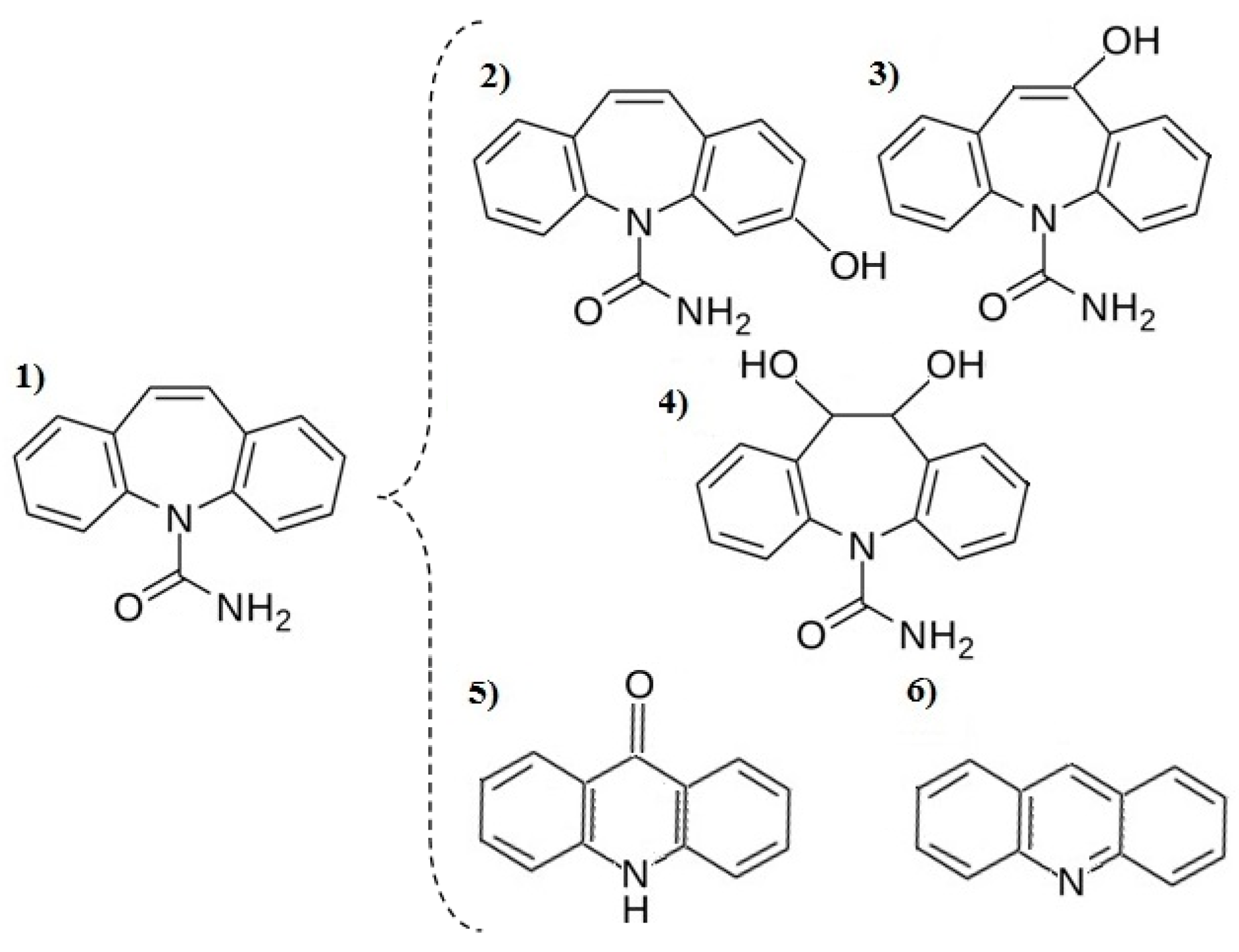
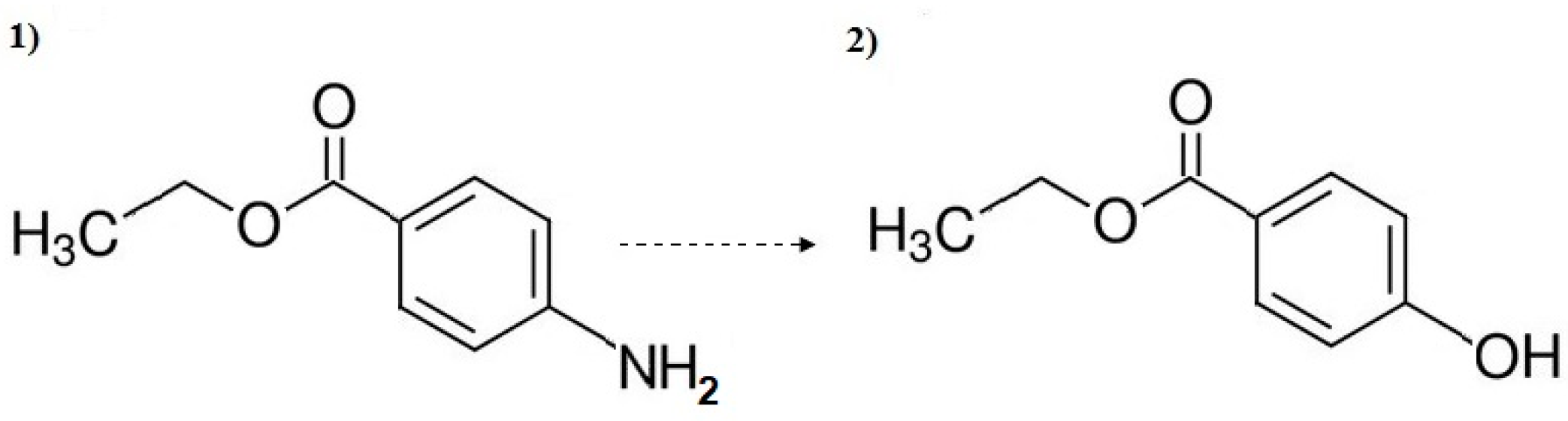



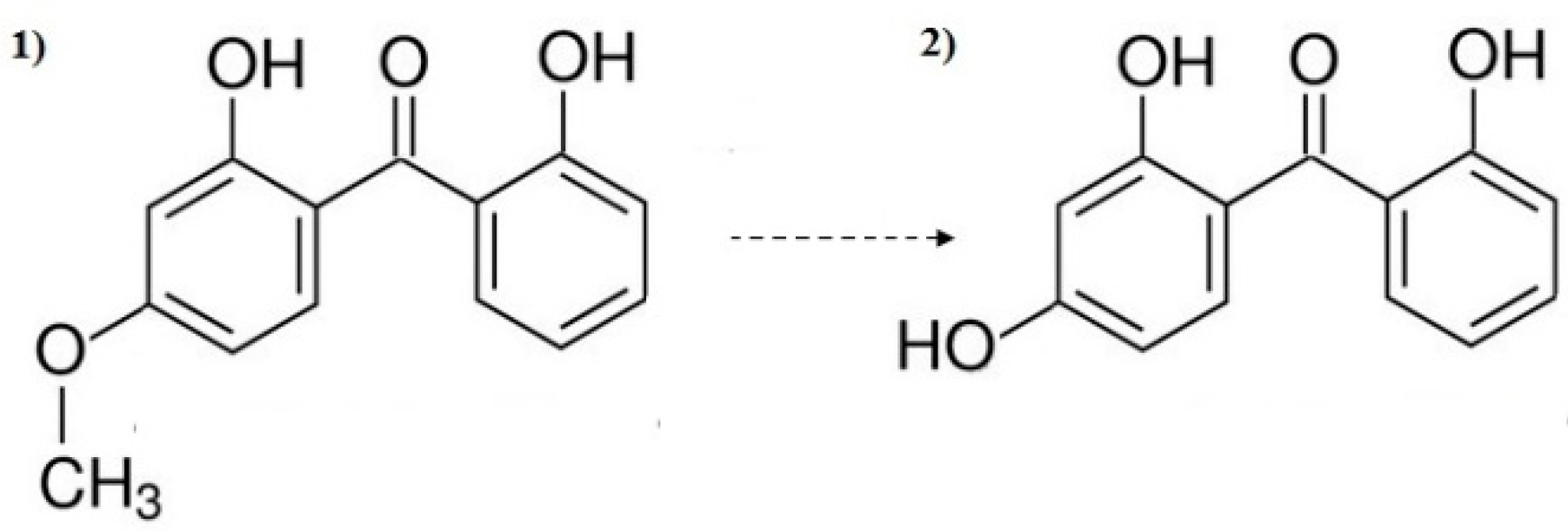
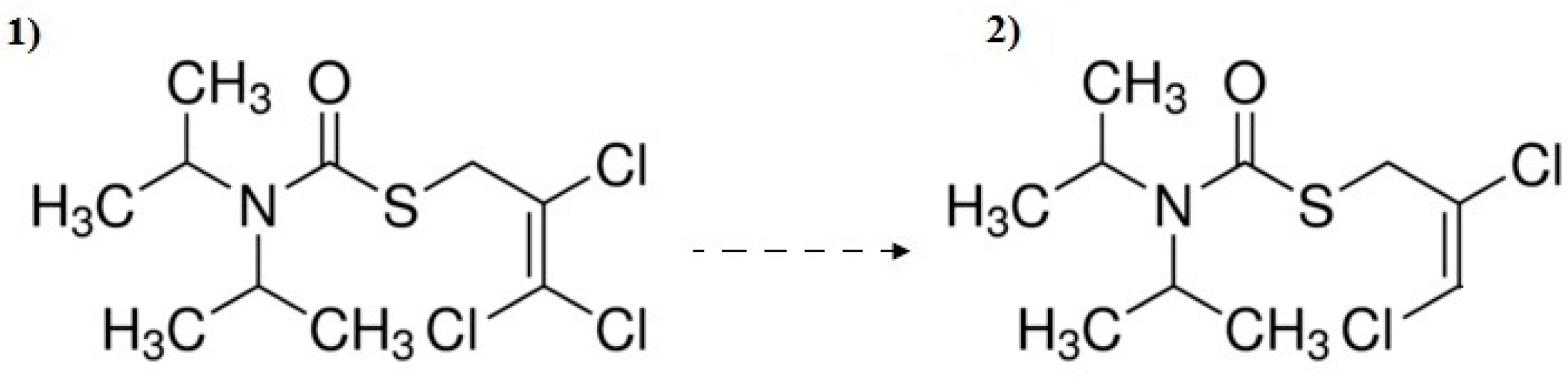








| Group | Name | Molecular Formula | Molecular Weight, g mol−1 | Solubility in Water, mg L−1 | pKa |
|---|---|---|---|---|---|
| Pharmaceuticals | Carbamazepine, CBZ | C16H12N2O | 236.30 | 17 | 2.30 |
| Benzocaine, BE | C9H11NO2 | 165.19 | 1310 | 2.51 | |
| Diclofenac sodium salt, DCF | C14H10Cl2NNaO2 | 318.13 | 50 | 4.15 | |
| Ibuprofen sodium salt, IBU | C13H17NaO2 | 228.26 | 100 | 4.91 | |
| Dyes | Acridine, ACR | C13H9N | 179.22 | 38.4 | 5.6 |
| UV blockers | Dioxybenzone, BZ8 | C14H12O4 | 244.24 | Insoluble | 6.99 |
| Pesticides | Triallat, TRI | C10H16Cl3NOS | 304.66 | 4.1 | - 1 |
| Triclosan, TCS | C12H7Cl3O2 | 289.54 | 0.1 | 7.9 | |
| Oxadiazon, ODZ | C15H18Cl2N2O3 | 345.22 | 0.7 | - 2 | |
| Hormones | β-Estradiol, E2 | C18H24O2 | 272.38 | 3.6 | 10.33 |
| 17α-Ethinylestradiol, EE2 | C20H24O2 | 296.40 | 11.3 | 10.33 | |
| Mestranol, EEME | C21H26O2 | 310.43 | 1.13 | 17.59 | |
| Progesterone, P4 | C21H30O2 | 314.46 | 8.81 | 18.92 | |
| Food additives | Butylated hydroxytoluene, BHT | C15H24O | 220.35 | 0.6 | 12.23 |
| Other | Caffeine, CAF | C8H10N4O2 | 194.19 | 21600 | 14.0 |
| Compound Group | Pharmaceuticals; Food Additive | Dyes; UV Blocker; Pesticides; Other | Hormones |
|---|---|---|---|
| Cartridge type | Supelclean™ ENVI-8 | Supelclean™ ENVI-18 | |
| Cartridge bed | silica gel base material with C8 (octyl) bonding, polymerically bonded | silica gel base material with C18 (octadecyl) bonding, polymerically bonded | |
| Bed weight (mg) | 1000 | ||
| Bed pore size (Å) | 60 | ||
| Bed surface area (m2 g−1) | 475 | ||
| Material of cartridge filter | PE frit (20 μm porosity) | ||
| Conditioning | 5.0 mL of MeOH | 5.0 mL of ACN; 5.0 mL of MeOH | 3.0 mL of DCM; 3.0 mL of ACN; 3.0 mL of MeOH |
| Washing | 5.0 mL of deionized water | ||
| Sample flow (mL min−1) | 1.0 | ||
| Vacuum drying time (min) | 5.0 | ||
| Extract elution | 3.0 mL of MeOH | 1.5 mL of MeOH; 1.5 mL of ACN | 2.0 mL of DCM; 1.5 mL of ACN; 1.5 mL of MeOH |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudlek, E. Decomposition of Contaminants of Emerging Concern in Advanced Oxidation Processes. Water 2018, 10, 955. https://doi.org/10.3390/w10070955
Kudlek E. Decomposition of Contaminants of Emerging Concern in Advanced Oxidation Processes. Water. 2018; 10(7):955. https://doi.org/10.3390/w10070955
Chicago/Turabian StyleKudlek, Edyta. 2018. "Decomposition of Contaminants of Emerging Concern in Advanced Oxidation Processes" Water 10, no. 7: 955. https://doi.org/10.3390/w10070955
APA StyleKudlek, E. (2018). Decomposition of Contaminants of Emerging Concern in Advanced Oxidation Processes. Water, 10(7), 955. https://doi.org/10.3390/w10070955