Selection of Coagulants for the Removal of Chosen PAH from Drinking Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Jar Test Procedure
2.3. Analytical Procedure
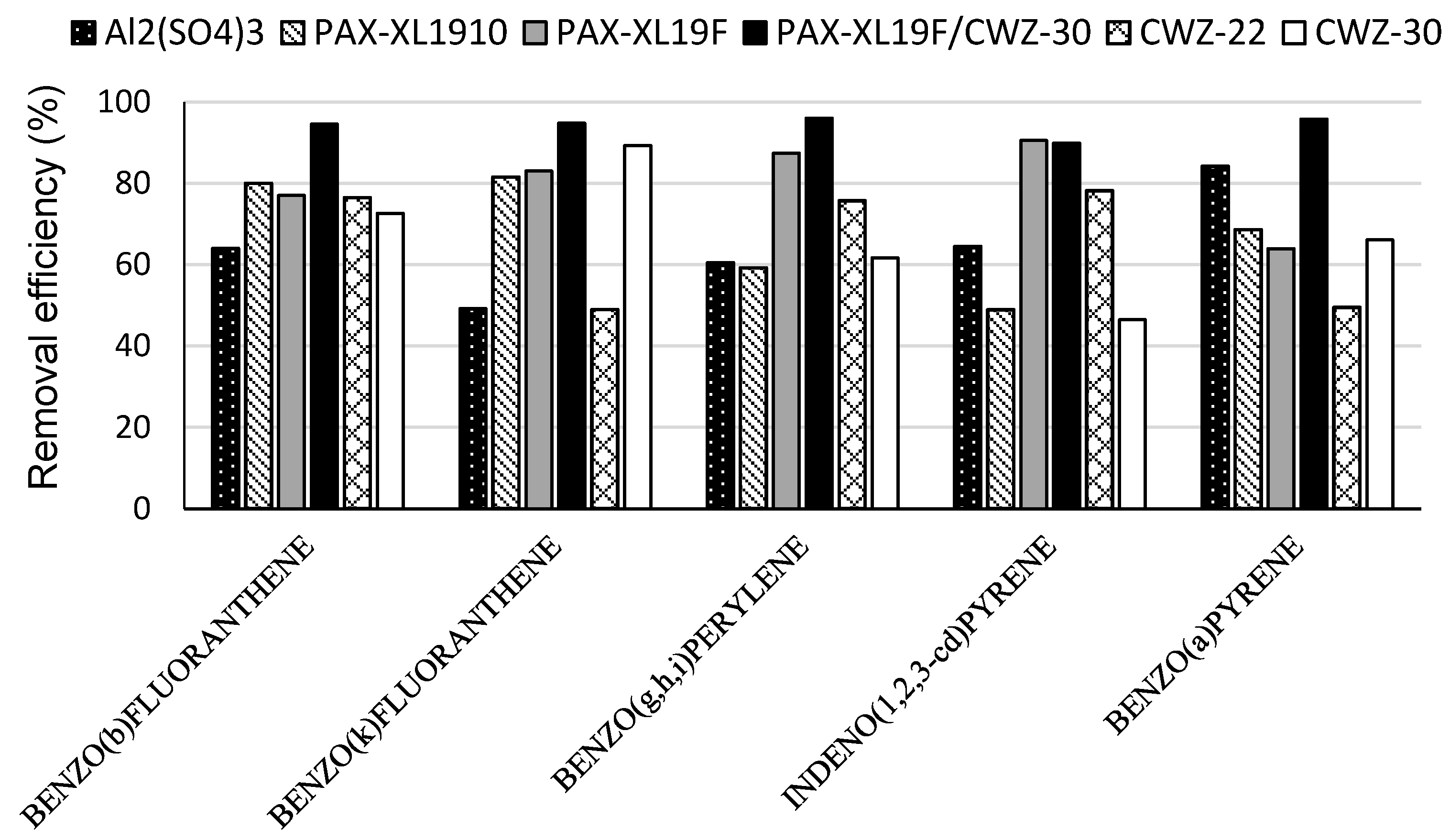
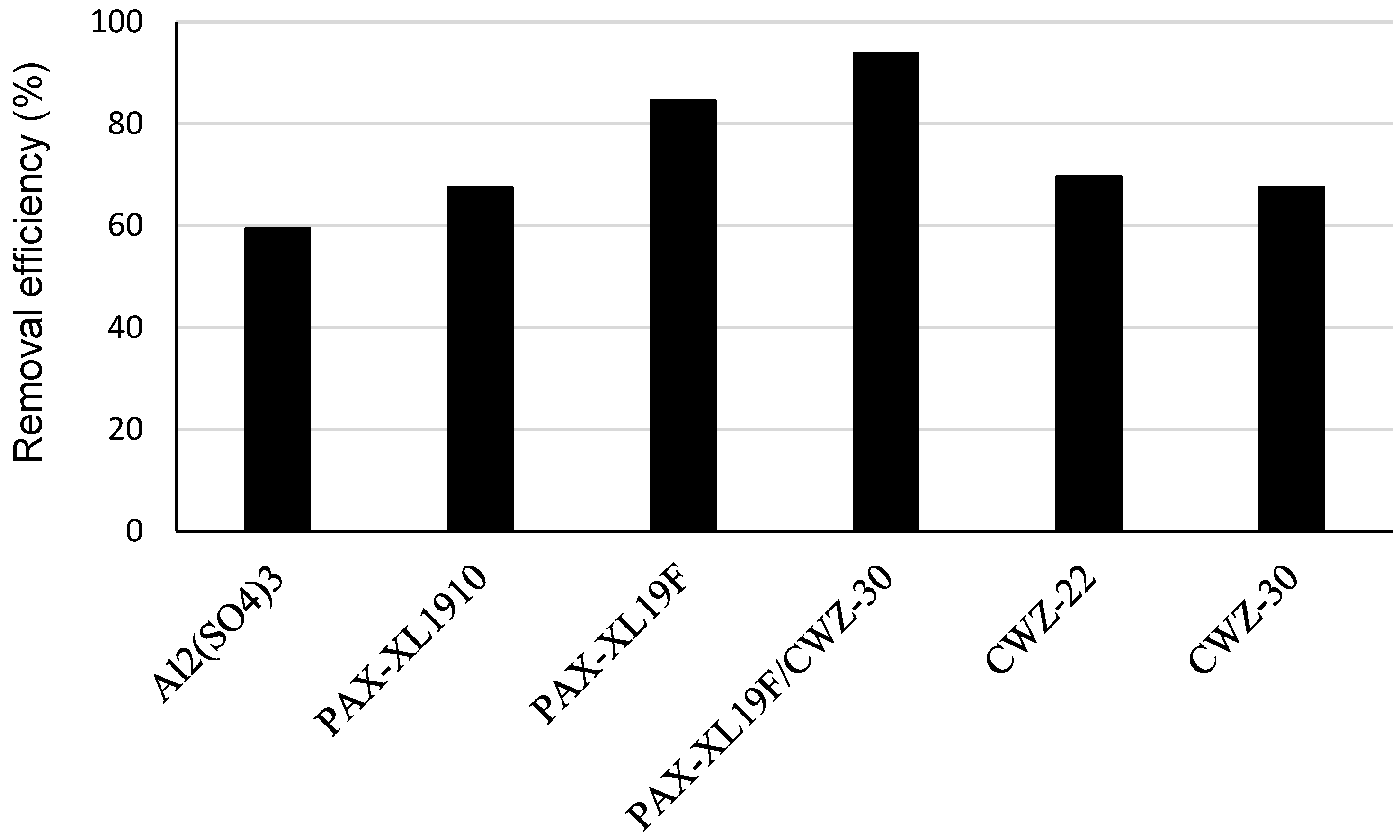
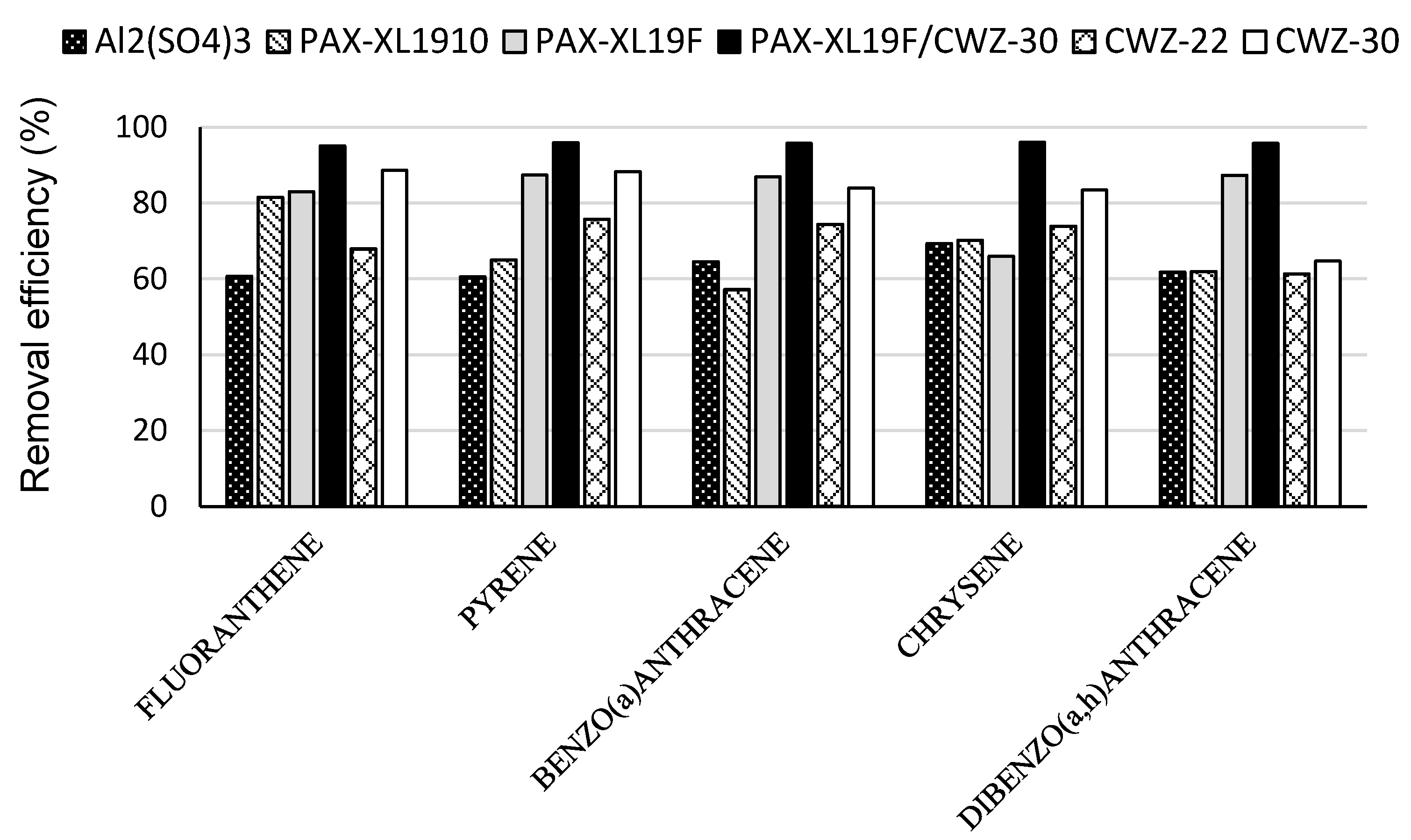
3. Results and Discussion
- -
- standardized in the Council Directive 98/83/EC on the quality of water intended for human consumption: benzo(a)pyrene and the total of: benzo(b)fluoranthene, benzo(k)fluoranthene; benzo(g,h,i)perylene, indeno(1,2,3-cd)pyrene (compounds classified as 5- and 6-ring PAH);
- -
- 2-ring PAH (naphthalene);
- -
- 3-ring PAH: acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene;
- -
- 4-ring PAH: fluoranthene, pyrene, benzo(a)anthracene, chrysene;
- -
- 6-ring PAH: dibenzo(a,h)anthracene.
4. Conclusions
- -
- the application of coagulants Al2(SO4)3, PAX-XL1910, PAX-XL19F and powdered activated carbons CWZ-22 and CWZ-30 was effective in PAH removal in the range of 32.9 (naphthalene) up to 90.5% (indeno(1,2,3-cd)pyrene);
- -
- in the coagulation process, high efficiency of reduction of the sum of the four standardized PAH, i.e., benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(g,h,i)perylene, indeno(1,2,3-cd)pyrene, was obtained using PAX-19F coagulant (84.5%); the usage of Al2(SO4)3 or PAX-XL1910 obtained an efficiency of removal of these compounds of 59.4% and 67.4%, respectively;
- -
- a good efficiency of removal of benzo(a)pyrene (84.2%) was obtained during coagulation using Al2(SO4)3;
- -
- among the applied coagulants, good removal for most PAHs was obtained in the coagulation process with polyaluminum chloride PAX-XL19F;
- -
- the application of powdered activated carbons CWZ-22 and CWZ-30 reduced the concentrations of benzo(a)pyrene by 49.5% and 66.1%, respectively, while the total concentration of four PAH decreased by 69.7% and 67.6%, respectively;
- -
- better removal efficiency for micropollutants in the process of coagulation and adsorption was demonstrated for PAH containing 4–6 rings;
- -
- the adsorption on powdered activated carbon reduced the concentrations of analyzed PAH max. up to 89.3%; the best results were obtained using activated carbon CWZ-30, which was characterized by a higher specific surface area;
- -
- the combination of the coagulation and the adsorption increased the efficiency of removal of PAH from contaminated water; the best results of removal of analyzed PAH were obtained using polyaluminum chloride PAX-XL19F and powdered activated carbon CWZ-30; a decrease in benzo(a)pyrene concentration and total concentration of four PAH in water by 95.8% and 93.8%, respectively, was obtained.
Author Contributions
Funding
Conflicts of Interest
References
- Di-Toro, D.M.; McGrath, J.A.; Hansen, D.J. Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. I. Water and tissue. Environ. Toxicol. Chem. 2000, 19, 1951–1970. [Google Scholar] [CrossRef]
- Boström, C.-E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators and guidelines for polycyclic aromatic hydrocarbons in ambient air. Environ. Health Perspect. 2002, 110, 451–489. [Google Scholar] [CrossRef] [PubMed]
- Arey, J.; Atkinson, R. Photochemical Reactions of PAH in the Atmosphere. PAHs: An Ecotoxicological Perspective; Douben, P.E.J., Ed.; John Wiley and Sons Ltd.: New York, NY, USA, 2003; ISBN 978-0-471-56024-1. [Google Scholar]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Masih, A.; Lal, J.K. Concentrations and carcinogenic profiles of polycyclic aromatic hydrocarbons (PAHs) in groundwater of an urban site at a terai belt of North India. IJAER 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Vigneswaran, S.; Kandasamy, J.; Slee, D.; Stevenson, G.; Naidu, R. Polycyclic aromatic hydrocarbons in road-deposited sediments, water sediments, and soils in Sydney, Australia: Comparisons of concentration distribution, sources and potential toxicity. Ecotoxicol. Environ. Saf. 2014, 104, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T. In silico investigation of polycyclic aromatic hydrocarbons against bacterial 1-2 dioxygenase. J. Chem. Pharm. Res. 2014, 6, 873–877. [Google Scholar]
- Neff, J.M.; Stout, S.A.; Gunster, D.G. Ecological risk assessment of polycyclic aromatic hydrocarbons in sediments: Identifying sources and ecological hazard. Integr. Environ. Assess. Manag. 2005, 1, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.G.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: A review and meta-analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef]
- Bach, P.B.; Kelley, M.J.; Tate, R.C.; McCrory, D.C. Screening for lung cancer: A review of the current literature. Chest 2003, 123, 72S–82S. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.S.; Syage, J.A.; Hanold, K.A.; Balogh, M.P. Ultra performance liquid chromatography-atmospheric pressure photoionization-tandem mass spectrometry for high-sensitivity and high-throughput analysis of US Environmental Protection Agency 16 priority pollutants polynuclear aromatic hydrocarbons. Anal. Chem. 2009, 81, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.H. The source of US EPA’s sixteen PAH priority pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31998L0083 (accessed on 29 June 2018).
- Regulation of the Minister of Health of 7 December 2017 on the Quality of Water Intended for Human Consumption. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 29 June 2018).
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Skurlatov, Y.I.; Vichutinskaya, E.V.; Zaitsevaa, N.I.; Shtammb, E.V.; Shvydkiib, V.O.; Semenyakc, L.V.; Baikovad, I.S. Forms and pathways of migration and transformation of hazardous chemicals in the environment. Russ. J. Phys. Chem. B 2017, 11, 576–586. [Google Scholar] [CrossRef]
- Latimer, J.; Zheng, J. The Sources, Transport, and Fate of PAH in the Marine Environment. PAHs: An Ecotoxicological Perspective; Douben, P.E.J., Ed.; John Wiley and Sons Ltd.: New York, NY, USA, 2003; ISBN 978-0-471-56024-1. [Google Scholar]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cog. Environ. Sci. 2017, 3, 1–86. [Google Scholar] [CrossRef]
- Broyde, S.; Wang, L.Y.; Cai, Y.; Jia, L.; Shapiro, R.; Patel, D.J.; Geacintov, N.E. Covalent polycyclic aromatic hydrocarbon-DNA adducts: Carcinogenicity, structure, and function. Curr. Cancer Res. 2011, 6, 181–207. [Google Scholar] [CrossRef]
- Klein, S.J. Acenaphthene. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 17–19. [Google Scholar]
- Sun, C.; Zhang, J.; Ma, Q.; Chen, Y.; Ju, H. Polycyclic aromatic hydrocarbons (PAHs) in water and sediment from a river basin: Sediment–water partitioning, source identification and environmental health risk assessment Environ. Geochem. Health 2017, 39, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; He, W.; Kong, X.-Z.; Liu, W.-X.; He, Q.-S.; Yang, B.; Ouyang, H.-L.; Wang, Q.-M.; Xu, F.-L. Ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the water from a large Chinese lake based on multiple indicators. Ecol. Indic. 2013, 24, 599–608. [Google Scholar] [CrossRef]
- Valderrama, C.; Cortina, J.L.; Farran, A.; Gamisans, X.; Lao, C. Kinetics of sorption of polyaromatic hydrocarbons onto granular activated carbon and Macronet hyper-cross-linked polymers (MN200). J. Colloid Interface Sci. 2007, 310, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zia-Manhood, S.; Deepak, P. Adsorption of polyaromatic pollutants from water system using carbon/ZnFe2O4 nanocomposite: Equilibrium, kinetic and thermodynamic mechanism. J. Mol. Liq. 2017, 240, 361–371. [Google Scholar] [CrossRef]
- Alexander, J.T.; Hai, F.I.; Al-aboud, T.M. Chemical coagulation-based processes for trace organic contaminant removal: Current state and future potential. J. Environ. Manag. 2012, 111, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.Y.; Wu, T.Y. The potential use of natural coagulants and flocculants in the treatment of urban waters. Chem. Eng. Trans. 2014, 39, 1603–1608. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Sperczyńska, E.; Dąbrowska, L.; Wiśniowska, E. Removal of turbidity, colour and organic matter from surface water by coagulation with polyaluminium chlorides and with activated carbon as coagulant aid. Desalin. Water Treat. 2016, 57, 1139–1144. [Google Scholar] [CrossRef]
- Kabziński, A.K.M.; Cyran, J.; Juszczak, R. Determination of polycyclic aromatic hydro-carbons in water (including drinking water) of Łódź. Pol. J. Environ. Stud. 2002, 11, 695–706. [Google Scholar]
- Olivella, M.A. Isolation and analysis of polycyclic aromatic hydrocarbons from natural water using accelerated solvent extraction followed by gas chromatography–mass spectrometry. Talanta 2006, 69, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Cao, W.; Liu, B.; Zhao, X.; Qu, J. Simultaneous detection of chlorinated polycyclic aromatic hydrocarbons with polycyclic aromatic hydrocarbons by gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 3465–3473. [Google Scholar] [CrossRef] [PubMed]
- Kinga, A.J.; Readman, J.W.; Zhoua, J.L. Determination of polycyclic aromatic hydrocarbons in water by solid-phase microextraction–gas chromatography–mass spectrometry. Anal. Chim. Acta 2004, 523, 259–267. [Google Scholar] [CrossRef]
- Kraleva, E.; Karamfilov, V.; Hibaum, G. Determination of PAH in the black sea water by GC/MS following preconcentration with solid-phase extraction. Ecol. Chem. Eng. S 2012, 19, 393–403. [Google Scholar] [CrossRef]
- Anderson, K.A.; Szelewski, M.J.; Wilson, G.; Quimby, B.D.; Hoffman, P.D. Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses. J. Chromatogr. A 2015, 1419, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Corrotea, Y.; Sánchez, K.; Rubio, M.A.; Richter, P. Extraction of polycyclic aromatic hydrocarbons from water samples into a rotating-disk microextractor and the subsequent determination by gas chromatography-mass spectrometry. J. Chil. Chem. Soc. 2014, 59, 2477–2480. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Ni, J.; Qu, J.; Chow, C.W.K.; Liu, H. Mechanism of natural organic matter removal by polyaluminum chloride: Effect of coagulant particle size and hydrolysis kinetics. Water Res. 2008, 42, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Lin, L., Jr.; Huang, C.; Dempsey, B.; Hu, J.-H. Fate of hydrolyzed Al species in humic acid coagulation. Water Res. 2014, 56, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Brändli, R.C.; Hartnik, T.; Henriksen, T.; Cornelissen, G. Sorption of native polyaromatic hydrocarbons (PAH) to black carbon and amended activated carbon in soil. Chemosphere 2008, 73, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Clemente, A.; Torres-Palma, R.A.; Peñuela, G.A. Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: A review. Sci. Total Environ. 2014, 478, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Zietzschmann, F.; Geiling, E.-L.; Ruhl, A.S.; Sperlich, A.; Jekel, M. Impacts of coagulation on the adsorption of organic micropollutants onto powdered activated carbon in treated domestic wastewater. Chemosphere 2015, 125, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Subramonian, W.; Wu, T.Y.; Chai, S.-P. An application of response surface methodology for optimizing coagulation process of raw industrial effluent using Cassia obtusifolia seed gum together with alum. Ind. Crops Prod. 2015, 70, 107–115. [Google Scholar] [CrossRef]
- Nowacka, A.; Włodarczyk-Makuła, M. The coagulant type influence on removal efficiency of 5- and 6-ring PAHs during water coagulation process. CEER 2014, 13, 63–73. [Google Scholar] [CrossRef]
- Li, X.; Peng, P.; Zhanga, S.; Man, R.; Sheng, G.; Fu, J. Removal of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans by three coagulants in simulated coagulation processes for drinking water treatment. J. Hazard. Mater. 2009, 162, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Tadkaew, N.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Nghiem, L.D. Removal of trace organics by MBR treatment: The role of molecular properties. Water Res. 2011, 45, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Deshayes, S.; Zedek, S.; Cren-Oliv, C.; Cartiser, N.; Eudes, V.; Bressy, A.; Caupos, E.; et al. Study of a large scale powdered activated carbon pilot: Removals of a wide range of emerging and priority micropollutants from wastewater treatment plant effluents. Water Res. 2015, 72, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Amstaetter, K.; Eek, E.; Cornelissen, G. Sorption of PAHs and PCBs to activated carbon: Coal versus biomass-based quality. Chemosphere 2012, 87, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Anita, C.O.; Cabal, B.; Pevida, C.; Arenillas, A.; Parra, J.B.; Rubiera, F.; Pis, J. Removal of naphthalene from aqueous solution on chemically modified activated carbons. Water Res. 2007, 41, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhao, Y.; Sun, W.; Lou, Z. Hydrophobic organic chemicals (HOCs) removal from biologically treated landfill leachate by powder-activated carbon (PAC), granular-activated carbon (GAC) and biomimetic fat cell (BFC). J. Hazard. Mater. 2009, 163, 1084–1089. [Google Scholar] [CrossRef]
- Guo, Y.; Kaplanb, S.; Karanfilb, T. The significance of physical factors on the adsorption of polyaromatic compounds by activated carbons. Carbon 2008, 46, 1885–1891. [Google Scholar] [CrossRef]

| Properties | Unit | Powdered Activated Carbon | |
|---|---|---|---|
| CWZ-22 | CWZ-30 | ||
| Specific surface area | m2 g−1 | 960 | 1134 |
| Iodine number | mg g−1 | 1032 | 1190 |
| Methylene number | cm3 | 29 | 30 |
| Granulation < 0.06 mm | % | 93 | 90 |
| PAH | Molecular Formula | Retention Times | The Strongest Ions |
|---|---|---|---|
| Naphthalene | C10H8 | 5.40 | 129 |
| 128 | |||
| 127 | |||
| Acenaphtylene | C12H8 | 10.09 | 153 |
| 152 | |||
| 151 | |||
| Acenaphtene | C10H10 | 10.76 | 154 |
| 153 | |||
| 152 | |||
| Fluorene | C13H10 | 12.96 | 167 |
| 166 | |||
| 165 | |||
| Phenanthrene | C14H10 | 17.48 | 179 |
| 178 | |||
| 176 | |||
| Anthracene | C14H10 | 17.73 | 179 |
| 178 | |||
| 176 | |||
| Fluoranthene | C16H10 | 23.60 | 203 |
| 202 | |||
| 101 | |||
| Pyrene | C16H10 | 24.77 | 203 |
| 202 | |||
| 101 | |||
| Benzo(a)anthracene | C18H12 | 31.95 | 229 |
| 228 | |||
| 226 | |||
| Chrysene | C18H12 | 32.95 | 229 |
| 228 | |||
| 226 | |||
| Benzo(b)fluoranthene | C20H12 | 36.17 | 252 |
| 250 | |||
| 126 | |||
| Benzo(k)fluoranthene | C20H12 | 36.17 | 252 |
| 250 | |||
| 126 | |||
| Benzo(a)pyrene | C20H12 | 41.28 | 252 |
| 250 | |||
| 126 | |||
| Indeno(1,2,3-cd)pyrene | C22H12 | 51.78 | 277 |
| 276 | |||
| 138 | |||
| Dibenzo(a,h)anthracene | C22H14 | 52.86 | 278 |
| 277 | |||
| 276 | |||
| Benzo(g,h,i)perylene | C22H12 | 54.48 | 277 |
| 276 | |||
| 138 |
| Parameter | Unit | Surface Water | Al2(SO4)3 | PAX-XL1910 | PAX-XL19F | PAX19F + CWZ-30 | CWZ-22 * | CWZ-30 * |
|---|---|---|---|---|---|---|---|---|
| pH | - | 7.28 | 6.92 | 7.08 | 7.12 | 7.16 | 7.23 | 7.26 |
| Color | mgPt L−1 | 40.0 | 20.0 | 15.0 | 10.0 | 5.0 | 15.0 | 10.0 |
| Turbidity | NTU | 7.5 | 2.1 | 1.6 | 0.8 | 1.3 | - | - |
| TOC | mgC L−1 | 9.1 | 6.9 | 6.1 | 5.6 | 5.1 | 7.4 | 7.0 |
| Aluminum | mgAl L−1 | 0.03 | 0.28 | 0.07 | 0.07 | 0 | 0 | 0 |
| PAH | Detection Limit (ng L−1) | Water after Modification | Al2(SO4)3 | PAX-XL1910 | PAX-XL19F | PAX19F + CWZ-30 | CWZ-22 | CWZ-30 |
|---|---|---|---|---|---|---|---|---|
| Benzo(a)pyrene | 0.01 | 50.85 | 8.06 | 15.96 | 18.33 | 2.15 | 25.70 | 17.22 |
| Benzo(b)fluoranthene | 0.01 | 49.76 | 18.03 | 10.01 | 11.50 | 2.74 | 11.78 | 13.71 |
| Benzo(k)fluoranthene | 0.01 | 51.99 | 26.44 | 9.62 | 8.83 | 2.74 | 26.55 | 5.58 |
| Benzo(g,h,i)perylene | 0.01 | 51.26 | 20.25 | 20.93 | 6.46 | 2.07 | 12.46 | 19.65 |
| Indeno(1,2,3-cd)pyrene | 0.01 | 50.85 | 18.06 | 25.97 | 4.83 | 5.16 | 11.10 | 27.23 |
| Σ | 203.86 | 82.78 | 66.53 | 31.62 | 12.71 | 61.89 | 66.17 | |
| Naphthalene | 0.05 | 58.58 | 35.80 | 39.33 | 36.69 | 20.10 | 33.20 | 35.40 |
| Acenaphthylene | 0.01 | 48.83 | 20.18 | 24.13 | 22.60 | 11.61 | 28.90 | 19.01 |
| Acenaphthene | 0.01 | 49.24 | 15.50 | 20.38 | 19.4 | 13.77 | 27.12 | 20.95 |
| Fluorene | 0.01 | 52.59 | 22.84 | 12.81 | 21.01 | 10.63 | 22.58 | 20.59 |
| Phenanthrene | 0.01 | 48.39 | 17.88 | 14.43 | 16.32 | 13.77 | 20.68 | 18.00 |
| Anthracene | 0.01 | 48.52 | 20.03 | 16.01 | 16.49 | 9.73 | 17.77 | 17.70 |
| Fluoranthene | 0.01 | 51.99 | 20.44 | 9.61 | 8.82 | 2.53 | 16.69 | 5.87 |
| Pyrene | 0.01 | 51.27 | 20.25 | 17.93 | 6.46 | 2.06 | 12.45 | 5.99 |
| Benzo(a)anthracene | 0.01 | 50.88 | 18.06 | 21.76 | 6.63 | 2.15 | 13.04 | 8.12 |
| Chrysene | 0.01 | 50.59 | 15.50 | 15.08 | 17.18 | 2.01 | 13.20 | 8.37 |
| Dibenzo(a,h)anthracene | 0.01 | 51.26 | 19.15 | 19.03 | 6.32 | 2.10 | 19.35 | 17.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosińska, A.; Dąbrowska, L. Selection of Coagulants for the Removal of Chosen PAH from Drinking Water. Water 2018, 10, 886. https://doi.org/10.3390/w10070886
Rosińska A, Dąbrowska L. Selection of Coagulants for the Removal of Chosen PAH from Drinking Water. Water. 2018; 10(7):886. https://doi.org/10.3390/w10070886
Chicago/Turabian StyleRosińska, Agata, and Lidia Dąbrowska. 2018. "Selection of Coagulants for the Removal of Chosen PAH from Drinking Water" Water 10, no. 7: 886. https://doi.org/10.3390/w10070886
APA StyleRosińska, A., & Dąbrowska, L. (2018). Selection of Coagulants for the Removal of Chosen PAH from Drinking Water. Water, 10(7), 886. https://doi.org/10.3390/w10070886




