Abstract
The removal of bromate (BrO3−) as a byproduct of ozonation in subsequent managed aquifer recharge (MAR) systems has so far gained little attention. This preliminary study with anoxic batch experiments was executed to explore the feasibility of chemical BrO3− reduction in Fe-reducing zones of MAR systems and to estimate potential inhibition by NO3−. Results show that the reaction rate was affected by initial Fe2+/BrO3− ratios and by pH. The pH dropped significantly due to the hydrolysis of Fe3+ to hydrous ferric oxide (HFO) flocs. These HFO flocs were found to adsorb Fe2+, especially at high Fe2+/BrO3− ratios, whereas at low Fe2+/BrO3− ratios, the mass sum loss of BrO3− and Br− indicated intermediate species formation. Under MAR conditions with relatively low BrO3− and Fe2+ concentrations, BrO3− can be reduced by naturally occurring Fe2+, as the extensive retention time in MAR systems will compensate for the slow reaction kinetics of low BrO3− and Fe2+ concentrations. Under specific flow conditions, Fe2+ and NO3− may co-occur during MAR, but NO3− hardly competes with BrO3−, since Fe2+ prefers BrO3− over NO3−. However, it was found that when NO3− concentration exceeds BrO3− concentration by multiple orders of magnitude, NO3− may slightly inhibit BrO3− reduction by Fe2+.
1. Introduction
Managed aquifer recharge (MAR) has proven to be an effective barrier for many organic micropollutants (OMPs) present in surface water during drinking water production [1,2,3]. However, some highly persistent OMPs can still be detected in MAR filtrate [4] and may reach the drinking water supply [5]. Ozone-based advanced oxidation processes (AOPs) are increasingly being considered as effective alternatives for the removal of OMPs during drinking water treatment [6,7,8]. The combination of MAR with ozonation as a pretreatment has been suggested as a comprehensive multibarrier treatment system to effectively remove various OMPs during drinking water production [9,10,11]. However, bromate (BrO3−) is formed during ozone-based treatment when applied to bromide-containing water [12,13,14]. It has been reported that the BrO3− concentration in drinking water after ozone-based AOPs typically ranges from 0 to 127 μg/L (1 μM) [15]. BrO3− is classified as Group 2B, or possible human carcinogen, by the International Agency for Research on Cancer based on its major toxic effects [16,17,18]. The standard of BrO3− in drinking water regulated by the World Health Organization, the US Environmental Protection Agency, and the European Union is 10 μg/L [19,20,21], demanding water companies to control the BrO3− concentration in drinking water.
A number of physical, chemical, electrochemical, and biological techniques for BrO3− removal have already been proposed. With respect to physical techniques, various advanced sorption materials, e.g., ion-exchange resins [22], nanocrystalline akaganeite (β-FeOOH)–coated quartz sand [23], and layered double hydroxides [24,25], have shown the ability to adsorb BrO3− from aqueous solutions, but so far these have not been applied in drinking water treatment. The use of granular activated carbon (GAC) as a conventional physical sorption technique can successfully reduce BrO3− [26], but the regenerated GAC loses effectiveness for BrO3− removal after a certain running time [15]. BrO3− can be removed by reverse osmosis [27], but this is an expensive process, since membrane fluxes are low and high operating pressures are needed. Electrodialysis reversal (EDR) has been studied in an integrated membrane system for drinking water treatment [28], which showed only limited BrO3− removal: 64% in a two-stage EDR system and 78% in a three-stage EDR system. BrO3− removal with catalysts, including zero-valent iron (Fe) [29] and Pd/Al2O3 [30], has been found to be limited in the presence of coexisting anions. Different reducing agents, such as ferrous iron (FeSO4), react with dissolved oxygen (DO), and therefore their practical application during water treatment is quite difficult [31]. UV irradiation successfully reduces BrO3− but has a high energy demand [15], just like electrochemical methods [32,33]. With respect to biological techniques, biological activated carbon (BAC) filters are capable of reducing BrO3− effectively, but competitive DO remains a critical factor [34], because it is a challenge to construct a BAC filter with restricted oxygen transfer within the biofilm [35]. Hijnen et al. [36] showed that BrO3− was removed in a denitrifying bioreactor fed with methanol. However, they demonstrated that this did not seem to be a realistic option for drinking water treatment due to the long contact times required for BrO3− removal and extensive posttreatment necessary to remove excess methanol and released biomass. Altogether, there are few effective options to remove highly soluble and stable BrO3− in practice.
In this study, a new approach is being proposed, namely to utilize Fe-reducing zones of MAR as a barrier for BrO3− after ozonation. This sequence of AOP–MAR has been proposed to effectively remove various OMPs during drinking water production [9,10,11]. It is hypothesized that not only will the removal of OMPs improve with this sequence, but the produced BrO3− will be removed by MAR. Recently, it was found that BrO3− is partially biodegraded in NO3−-reducing zones of MAR [37,38]. However, the potential reduction of BrO3− to Br− in deeper Fe-reducing zones during soil passage has not yet been investigated.
The reduction of BrO3− by Fe2+ [31,39] and the hydrolysis of its product Fe3+ under near-neutral pH proceed as follows [40,41]:
BrO3− + 6 Fe2+ + 6 H+ → Br− + 6 Fe3+ + 3 H2O
Fe3+ + 3H2O → Fe(OH)3 (s) + 3H+
BrO3− + 6 Fe2+ + 15 H2O → Br− + 6 Fe(OH)3 (s) + 12 H+
The reduction rate of BrO3− by Fe2+ is dependent on Fe2+ concentration, contact time, pH, and DO [31,42]. In MAR systems, water flows from infiltration ponds through an oxic zone, via an NO3−-reducing anoxic zone and an Mn-reducing anoxic zone, to the Fe-reducing anoxic zone. So, depending on the local geochemical situation of MAR, Fe2+ may be released into the groundwater, leading to natural BrO3− reduction by Fe2+ in the Fe-reducing anoxic zone of MAR.
A study by Siddiqui et al. [31] with oxic water (0.22 mM DO) found that an initial BrO3− concentration of 0.4 µM was lowered to 0.08 µM within 30 minutes following a dose of 0.27 mM Fe2+. Dong et al. [42] worked with 0.2 µM BrO3−, a 0.54 mM Fe2+ dosage, and 0.07 mM DO, reaching a BrO3− reduction of 65%. In these studies, the Fe2+ dosage was extremely high compared to expected Fe2+ concentrations during MAR, when Fe concentrations below 0.03 mM are expected (e.g., the MAR site of Dunea, the Netherlands, shows concentrations ranging from 0.0015 to 0.029 mM Fe). However, little is known about the reaction of BrO3− and Fe2+ in such low concentrations. Additionally, to what extent BrO3− reduction is possible at such low concentrations of Fe2+ is not known, although the extensive residence times in the subsurface do not require fast kinetics for this technology to be effective. Also, competition of BrO3− with DO is not a problem in these anoxic zones. Fe2+ can be formed only when NO3− as an electron acceptor is exhausted in anaerobic zones of MAR systems [43,44]. However, water containing NO3− and water containing Fe2+ from different pathways have been found to mix in specific zones of MAR [45], so NO3− and Fe2+ can be present simultaneously in anaerobic zones of MAR systems. This was confirmed by Dunea measurements, where NO3− and dissolved Fe have been detected simultaneously in the effluent of MAR sites (Scheveningen and Monster, the Netherlands). Therefore, NO3− may compete with BrO3− for reduction by Fe2+ [46,47,48] during MAR. The investigation of BrO3− reduction by Fe2+ in the presence of NO3− may be an important reference for the feasibility of BrO3− removal in Fe-reducing zones of MAR systems. Examples of stoichiometric equations for the reaction of NO3− and Fe2+ in which the stable endpoint is nitrogen gas are given below, but less complete reactions may have endpoints anywhere along the reduction pathway [49]:
10Fe2+ + 2NO3− + 14H2O → 10FeOOH + N2 + 18H+
15Fe2+ + NO3− + 13H2O → 5Fe3O4 + N2 + 28H+
The focus of this preliminary study was to investigate the mechanism of chemical BrO3− reduction by Fe2+ and the feasibility of BrO3− reduction by naturally occurring Fe2+ in the Fe-reducing anoxic zones of MAR systems, with an emphasis on the potential competition with or inhibition by NO3−. Microbiological reactions and biochemical reactions were not included in this study.
2. Materials and Methods
2.1. Experimental Design
The research was designed with 2 sets of anoxic batch reactor experiments: (A) high Fe2+ and BrO3− concentrations to investigate reduction mechanisms, and (B) environmentally relevant concentrations of Fe2+ and BrO3− to simulate the concentrations during MAR. As the focus in all experiments was on chemical BrO3− reduction by Fe2+, no soil or sediment was added in the batch reactors. Both sets of experiments were executed in the absence and presence of NO3−. An overview of all experiments is provided in Figure 1.
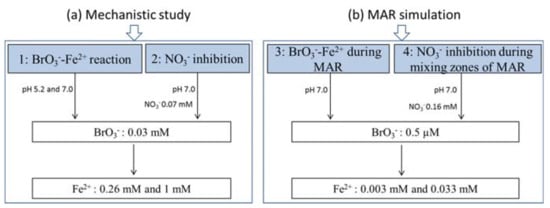
Figure 1.
Experimental overview of anoxic batch reactors. T = 11.5 ± 0.5 °C (n = 2). MAR: managed aquifer recharge.
For the experiments with high Fe2+ and BrO3− concentrations, anoxic batch experiments were performed with 0.03 mM BrO3− and 0.26 or 1 mM Fe2+. The concentration of 0.26 mM Fe2+ is close to the required concentration to reduce 0.03 mM BrO3− according to the stoichiometry of Equation (1). The experiments were executed under 2 pH conditions: pH 7.0, which is realistic for MAR water, and pH 5.2 to slow down the reaction in order to identify potential intermediate species.
To investigate the competition between NO3− and BrO3−, the same order of magnitude of NO3− (0.07 mM) and BrO3− (0.03 mM) was added to anoxic batch reactors, together with the Fe2+ (0.26 mM and 1 mM).
To simulate BrO3− reduction by Fe2+ at concentrations similar to MAR, the concentrations were lowered to 0.5 µM for BrO3− and 0.003–0.033 mM for Fe2+. The concentration of 0.003 mM Fe2+ was close to the stoichiometric amount to reduce 0.5 µM BrO3− (Equation (1)). These experiments were conducted at an initial pH of 7.0. The influence of NO3− was investigated by dosing with 0.16 mM NO3−, which was 3 orders of magnitude greater than the concentration of BrO3−. Our previous study showed that the NO3− concentration was in the range of 10.7 ± 6 mg/L in MAR influent water [37], so in this study 10 mg/L (0.16 mM) was chosen as a relevant concentration. BrO3− formation at concentrations ranging from <2–293 µg/L has been reported during ozonation of natural water under normal drinking water treatment conditions [28,50,51,52], but in 100 investigated drinking water utilities, BrO3− concentration was within the range of <2–60 µg/L after ozonation of water containing 2–429 µg/L Br− [53,54]. For this study, it was decided to investigate the upper value of this range, so 60 μg/L BrO3− (0.5 μM) was dosed. All experiments were performed in duplicate.
2.2. Anoxic Batch Reactors
Four series of laboratory-scale batch experiments using 250 mL (A experiments) and 1 L (B experiments) glass bottles were carried out under anoxic conditions at a controlled temperature (11.5 ± 0.5 °C). Anoxic conditions were reached by flushing nitrogen gas until a DO concentration below 0.3 µM (0.01 mg/L) was achieved in the batch reactors. The mouths of the batch reactors were sealed with rubber stoppers to prevent DO intrusion. On the rubber stoppers, there were 2 needles with valves, used as a sampling point and a reagent dosing point.
Water samples were collected 8–10 times within 120 h contact time to determine the concentrations of BrO3−, Br−, NO3−, and Fe2+. In the 0.03 mM BrO3− experiments (A) and 0.5 µM BrO3− experiments (B), 3 mL and 50 mL per sample were collected, respectively. After sample collection, several drops of diluted ethylenediamine (EDA) solution (11%) was added to the samples to prevent reactions of residual chemicals [55].
To test the stability of the anoxic system, Fe2+ concentrations were monitored in the batch reactors after dosing with 0.033, 0.003, 0.26, or 1 mM Fe2+. The Fe2+ concentrations remained stable during the 120 h experiment (Figure 2), indicating that the system was well sealed, and therefore no Fe2+ oxidation by DO was observed.
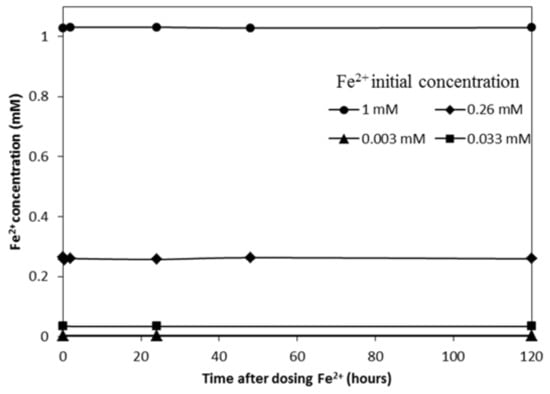
Figure 2.
Fe2+ concentrations over 120 h contact time in reactors with Fe2+ alone.
2.3. Water and Chemicals
The water used in batch experiments was prepared using chemical reagents and deionized water from a Millipore Milli-Q system. Sodium bromate (NaBrO3), sodium nitrate (NaNO3), ferrous sulfate (FeSO4·7H2O), sodium bicarbonate (NaHCO3), and EDA were purchased from Sigma-Aldrich (St. Louis, MO, USA). The amount of 2 mM NaHCO3 was prepared for use as a pH buffer, and 0.2 M NaOH was prepared to further adjust the pH. To prevent Fe2+ oxidation in FeSO4 solutions, the solutions were always prepared immediately before the experiments and concentrated acid (HCl) was used to acidify the FeSO4 solutions to pH 2 [55]. All chemicals were of analytical grade.
2.4. Analytical Methods
DO and temperature were measured with an FDO® 925 optical oxygen sensor (WTW) (Xylem Inc., Weilheim, Germany) and pH was measured with a SenTix® 940 (WTW) electrode (Xylem Inc., Weiheim, Germany), both using the WTW Multi 3420 meter (Xylem Inc., Weiheim, Germany).
Fe2+ was measured by photometry using the Spectroquant® iron test (Merck, Kenilworth, NJ, USA) with a detection range of 0.0002–0.09 mM. Dilution factors of 4 and 16 were needed to measure the Fe2+ in the experiments with dosages of 0.26 and 1 mM, respectively. For the dosages of 0.003 and 0.033 mM Fe2+, no dilution was required.
The NO3− concentration in all experiments was determined by an ion chromatograph (Metrohm 881 Compact IC pro-Anion, Metrohm AG, Herisau, Switzerland) with an A Supp 16-150/4.0 anion column (Metrohm AG, Herisau, Switzerland). For experiments using 0.03 mM BrO3− (A), BrO3− and Br− were measured by the same equipment as for NO3−. The detection limits of BrO3−, Br−, and NO3− were 0.008 mM, 0.001 mM, and 0.002 mM, respectively. For experiments using 0.5 µM BrO3− (B), water samples were analyzed at Het Waterlaboratorium (Haarlem, the Netherlands), where an ion chromatograph (Dionex ICS-300, Thermo Fisher Scientific Inc., Waltham, MA, USA) with IonPac AS9SC column (250 mm × 4 mm ID, Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to measure BrO3−. An ion chromatograph (Dionex ICS-1100, Thermo Fisher Scientific Inc., Waltham, MA, USA) with IonPac AG22 column (4 × 50 mm, Thermo Fisher Scientific Inc., Waltham, MA, USA) and IonPac AS22SC column (4 × 250 mm, Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to measure Br−. The detection limits for BrO3− and Br− were 0.004 µM and 0.125 µM, respectively.
3. Results
3.1. BrO3− Reduction Rate and Mass Balance
Figure 3 presents the kinetics of BrO3− reduction and Br− formation within 120 h after the addition of 0.26 and 1 mM Fe2+. The experiments were executed at pH 5.2 and 7.0, the latter being most representative of MAR water. For BrO3− (0.03 mM), the 0.26 mM Fe2+ dosage was close to the stoichiometric ratio (1 mol BrO3−:6 mol Fe2+) according to Equation (1). For this particular setting, >90% of initial BrO3− reduced into Br− within 120 h (Figure 3a,c). The BrO3− reduction fit second-order reaction kinetics well. Moreover, for pH 5.2 and 7.0, the kinetic constant was 2.5 and 3.3, respectively, and the BrO3− reduction rate was 0.00024 μM/min and 0.00026 μM/min, respectively, indicating that pH 7 promoted BrO3− reduction compared to pH 5.2. In the case of the 1 mM Fe2+ dosage, BrO3− reduction was accelerated, with almost 100% BrO3− reduction to Br− within 1 h at pH 5.2 (Figure 3b) and pH 7.0 (Figure 3d). These results indicate that the higher the Fe2+ dosage, the higher the BrO3− reduction rate, which is in line with existing literature [31,42].
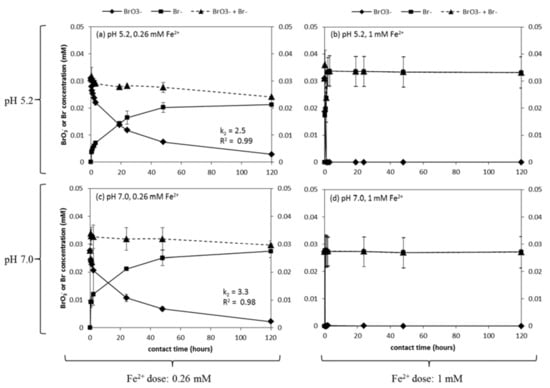
Figure 3.
BrO3− reduction after dosing with (a,c) 0.26 mM or (b,d) 1 mM Fe2+. Initial BrO3− concentration was 0.03 mM and initial pH levels were 5.2 and 7.0. The calculated BrO3− reduction rates were 0.00024 μM/min and 0.00026 μM/min for a and c.
Figure 4 shows the consumed Fe2+/BrO3− ratios after 24, 48, and 120 h. In the case of the 1 mM Fe2+ dosage (corresponding to an initial ratio of Fe2+/BrO3− = 33), consumed Fe2+/BrO3− ratios were higher than the theoretical ratio of 6 according to Equation (1), which assumes a total reduction of BrO3− to Br−. After 24 h, the ratios were 8.0 and 9.2 for pH 5.2 and pH 7.0, respectively, with BrO3− reduced to below the detection limit. Between 24 and 120 h, Fe2+ continued to be consumed, and correspondingly, Fe2+/BrO3− ratios increased. This may be explained by Fe2+ adsorption onto hydrolyzed Fe3+ flocs of hydrous ferric oxide (HFO). Interestingly, there was a consumed Fe2+/BrO3− ratio below the stoichiometric ratio of 6 in the case of the 0.26 mM Fe2+ dosage (corresponding initial Fe2+/BrO3− = 8). The ratio below 6 could indicate the production of intermediate Br species during the reduction of BrO3−, requiring less Fe2+ compared to the total reduction of BrO3− to Br− as in Equation (1). Additionally, the molar mass sum of BrO3− and Br− slightly decreased during the experiment by 10–20%, indicating that intermediate products may have formed.
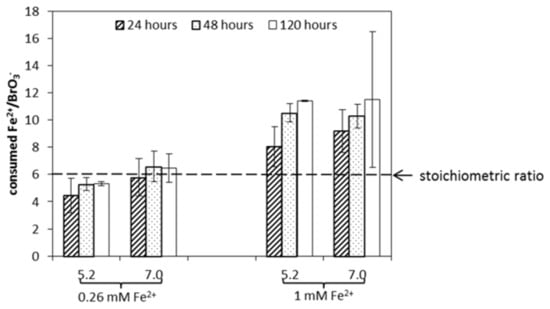
Figure 4.
The consumed Fe2+/BrO3− ratios after dosing with 0.26 mM or 1 mM Fe2+ to a solution containing 0.03 mM BrO3− at two initial pH levels, 5.2 and 7.0.
3.2. NO3−, A Competing Electron Acceptor?
NO3− is known to act as a competitive electron acceptor in the reaction with Fe2+ [48]. Figure 5 depicts BrO3− reduction by Fe2+ in the presence of NO3− at a concentration at the same order of magnitude as BrO3− (0.07 mM). The rate of BrO3− reduction in the presence of NO3− was slightly lower compared to the absence of NO3− (Figure 3c). NO3− concentrations in these experiments were steady during the 120 h for both Fe2+ dosages (Figure 5a,b), indicating that Fe2+ did not reduce NO3− when BrO3− and NO3− were present simultaneously.
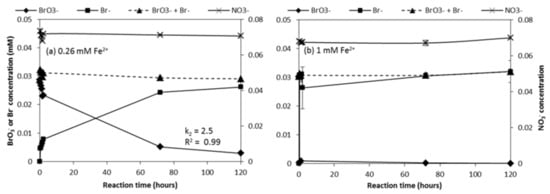
Figure 5.
BrO3− reduction after dosing with (a) 0.26 mM or (b) 1 mM Fe2+ in the presence of NO3−. Initial BrO3− and NO3− concentrations were 0.03 mM and 0.07 mM, respectively, and initial pH was 7.0.
Figure 6 shows BrO3− and Fe2+ consumption in the presence and absence of 0.07 mM NO3−. BrO3− removal in the presence and absence of NO3− was the same for both Fe2+ dosages, while the presence of NO3− led to higher Fe2+ consumption with the 0.26 mM Fe2+ dosage. The additional Fe2+ removal (62%→71%), 0.02 mM, might have reacted with NO3−, but the change would have remained undetected, given that it would have resulted in a calculated reduction of <0.005 mM NO3− (NO3−/Fe2+ ratio, Equations (4) and (5)). This would not have been noted by our NO3− analytical methods. Nevertheless, based on the above results, it can be concluded that BrO3− reduction was hardly affected by NO3− presence and that Fe2+ preferred BrO3− to NO3− as an electron acceptor. This was also observed by Westerhoff [56], who suggested that the difference in structure (atomic radii and O-bonds) makes it relatively easier to remove an O atom from a BrO3− ion than a NO3− ion. In addition, the preference of Fe2+ for BrO3− reduction vs. NO3− reduction can also be attributed to the higher ΔΕ [57,58,59] of oxidation-reduction reactions:
Fe2+ → Fe3+ + e, E1 = −0.771 V
2BrO3− + 12H+ + 10e → Br2 + 6H2O, E2 = 1.52 V
Br2 + 2e → 2 Br−, E3 = 1.087 V
NO3− +3H+ + 2e → HNO2 + H2O, E4 = 0.94 V
HNO2 + H+ + e → NO + H2O, E5 = 1.00 V
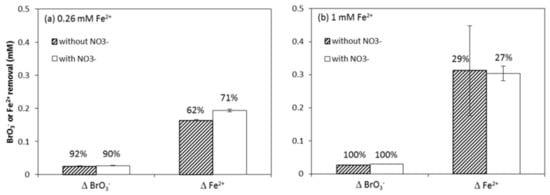
Figure 6.
The effect of 0.07 mM NO3− on the reduction of 0.03 mM BrO3− 120 h after dosing with (a) 0.26 mM or (b) 1 mM Fe2+ at initial pH 7.0 corresponds to the removal percentages.
Reactions 6 and 7 are preferable over 6 and 9.
3.3. pH Change and Fe3+ Hydrolysis
Although the BrO3− reduction in Equation (1) shows a pH increase, reduction of BrO3− by Fe2+ consequently means that Fe2+ is oxidized to Fe3+, and subsequently Fe3+ will hydrolyze to form flocs of hydrous ferric oxide (HFO) [40]. Therefore, the pH will drop based on Equation (3), the combined BrO3− reduction with Fe3+ hydrolysis. The pH drop was observed in all of the 0.03 mM BrO3− experiments: 1.5–1.6 drop and 2.6–3.0 drop with initial pH 5.2 and 7.0, respectively. The pH drop was an indicator of Fe3+ hydrolysis. Moreover, the observed yellow flocs in the batch reactors also provide evidence of HFO formation. The above two phenomena (pH decrease and visible flocs) are a strong indication that HFO flocs were formed in the reactors. The adsorption of Br− or BrO3− onto HFO flocs was not expected to have occurred, as BrO3− and Br− have no affinity for HFO [60]. However, Fe2+ adsorption onto the flocs has been frequently reported [31,61,62], which may explain the observed Fe2+/BrO3− removal ratios beyond the stoichiometric ratio of 6 (in Figure 4).
3.4. BrO3− Reduction under Concentrations Similar to MAR
To investigate the rate of BrO3− reduction by Fe2+ in concentrations similar to MAR, the reduction kinetics were monitored for 0.5 µM BrO3− after dosing with 0.003 and 0.033 mM Fe2+. Figure 7a,b show the BrO3− and Br− kinetics in the absence of NO3−, while Figure 7c,d show the kinetics of BrO3− and Br− in the presence of 0.16 mM NO3−. As in the previous experiments with high BrO3− concentrations, the reduction rate of 0.5 µM BrO3− also depends on the Fe2+ concentration, with a higher rate at a higher concentration. Figure 7a,c show that after 120 h contact time, there was limited BrO3− reduction (7% in the absence of NO3− and 12% in the presence of NO3−) at 0.003 mM Fe2+, while Figure 7b,d show considerable BrO3− reduction at 0.033 mM Fe2+ (74% in the absence and 58% in the presence of NO3−). Assuming second-order reaction kinetics, it was calculated that the rate constant of BrO3− reduction at a higher Fe2+ dosage (0.033 mM) was 0.049 and 0.023 in the absence and presence of NO3−, respectively. Although the NO3− concentration was three orders of magnitude higher than the BrO3− concentration, the NO3− concentration was steady (Figure 7c,d). It is noteworthy that during these experiments the molar mass sum of BrO3− and Br− also slightly decreased from 0.50 µM to 0.48 µM and 0.46 µM for 0.003 mM and 0.033 mM Fe2+ dosages, respectively, indicating the formation of Br intermediate species.
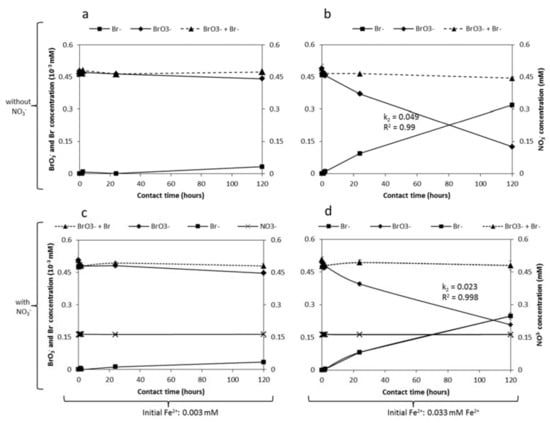
Figure 7.
BrO3− reduction after dosing with (a,c) 0.003 mM or (b,d) 0.033 mM Fe2+, simulating MAR concentrations, in the (a,b) presence and (c,d) absence of NO3−. (c,d) Initial BrO3− and NO3− concentrations were 0.5 µM and 0.16 mM, respectively. Initial pH was 7.0.
Figure 8 shows reduction of BrO3− and consumption of Fe2+ in the presence and absence of 0.16 mM NO3−. In the case of the 0.003 mM Fe2+ dosage, it appears that the presence of NO3− did not influence BrO3− reduction and Fe2+ oxidation (Figure 8a). In the case of the 0.033 mM Fe2+ dosage, the presence of NO3− led to lower BrO3− reduction and lower Fe2+ oxidation (Figure 8b). Combining the results in Figure 7 and Figure 8 indicates that Fe2+ preferred BrO3− to NO3− as an electron acceptor, but it did inhibit BrO3− reduction to some extent. This could possibly have been set off by considerably higher NO3− concentrations compared to BrO3−, in combination with the stoichiometric excess of Fe2+. One potential reason to explain the inhibition by NO3− is the hypothesized formation of NO from NO3− complexed with Fe2+ [63], slowing down the reduction of BrO3−.
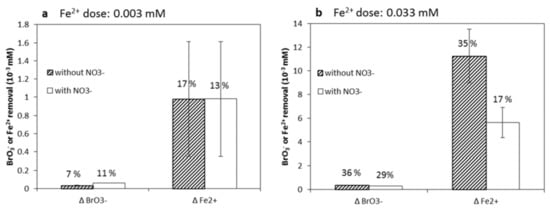
Figure 8.
Consumed BrO3− and Fe2+ 120 h after dosing with (a) 0.003 mM or (b) 0.033 mM Fe2+ in the presence and absence of NO3− at initial pH 7.0. Initial BrO3− and NO3− concentrations were 0.5 µM and 0.16 mM, respectively. % corresponds to the removal percentages.
4. Discussion
4.1. BrO3− Reduction Mechanism
Figure 9a shows a summary of the mole sum of BrO3− and Br− in all of the experiments with an initial dosage of 0.03 mM BrO3−. No Br mass loss was observed in the case of a sufficiently high initial Fe2+/BrO3− ratio (33), while the mole sum of BrO3− and Br− was 78–90% of the initial Br in the case of lower stoichiometric Fe2+/BrO3− ratios (8). It is possible that Br intermediate species formed during the reduction of BrO3−. Equations (11)–(13) show the reduction pathways of BrO3− to intermediate species requiring less Fe2+, as reported by Shen et al. [60] and Siddiqui et al. [31]:
BrO3− + 2Fe2+ + 2H+ → 2Fe3+ + BrO2− + H2O
BrO2− + 2Fe2+ + 2H+ → 2Fe3+ + BrO− + H2O
BrO− + 2Fe2+ + 2H+ → 2Fe3+ + Br− + H2O
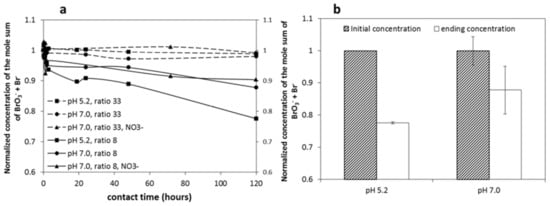
Figure 9.
(a) Mole mass sum of BrO3− and Br− in all experiments with initial BrO3− concentration of 0.03 mM and (b) comparison of mole mass loss of BrO3− and Br− in 120 h between pH 5.2 and pH 7.0. Ratio means the initial ratio of Fe2+/BrO3− (n = 2).
The most frequently reported intermediate species is hypobromous acid (HOBr/BrO−) [31,64]. Furthermore, the study of Shen et al. [60] showed that the sum of BrO3−, HBrO/BrO−, and Br− was 98–101% of the initial Br (as BrO3−) concentration, and therefore almost no other intermediate species except for HOBr/BrO− existed. Taken together, BrO3− was reduced into the end product Br− most likely via the intermediate species HBrO/BrO during the reaction of BrO3− and Fe2+ in this study.
Figure 9b shows the mole sum change of BrO3− and Br− in the case of an initial Fe2+/BrO3− ratio of 8 at pH 5.2 and 7.0. More Br loss was observed at pH 5.2 (22%) than at pH 7.0 (12%). Based on the total reaction (Equation (3)), pH 5.2 would slow down the BrO3− reduction, providing the intermediate species with a longer lifetime and thus a better chance to be detected in this experiment. Moreover, the intermediate species formation requires less Fe2+, as shown in Equations (11)–(13). This may be one possible reason for the observation in Figure 4: a relatively low consumed Fe2+/BrO3− ratio at pH 5.2 compared to pH 7.0. However, the lower ratio for pH 5.2 can also be partially explained by the decreased sorption of Fe2+ onto precipitating HFO in this experiment, while the higher Fe2+/BrO3− ratio for pH 7.0 correlates with promoted Fe2+ sorption at higher pH [61]. The lower Fe2+/BrO3− ratio at pH 5.2 compared to pH 7.0 in Figure 4 may also be related to the surface charge density. It has been reported that the lower the pH of FeOOH formation, the higher the positive surface charge density [65]. So, at pH 5.2, the formed FeOOH presents high positive charge density, potentially promoting BrO3− adsorption and Fe2+ rejection, which in turn may explain the lower Fe2+/BrO3− ratio. In contrast, at pH 7, the neutral to positively charged surface of FeOOH will favor Fe2+ adsorption and BrO3− rejection.
4.2. Feasibility of BrO3− Reduction by Fe2+ during MAR
Based on the results in Section 3.1 and Section 3.4, a preliminary conclusion can be drawn that under anoxic conditions and at a sufficiently high Fe2+/BrO3− ratio, chemical BrO3− reduction can be achieved. In MAR systems, Fe2+ concentrations tend to be 10−3 to 10−2 mM. Fortunately, the same is the case for BrO3− production after ozone-based AOPs, where concentrations are generally limited to 10−5 to 10−4 mM [16,66]. Fe2+ concentrations detected in Dunea MAR effluent range from 0.0015–0.029 mM, so the Fe2+/BrO3− ratios in MAR systems are sufficiently high (15–2900). From a drinking water production perspective, the extremely slow BrO3− reduction shown in Section 3.4 might seem to be a very inefficient process, since treatment technologies most often have contact times of minutes. However, MAR residence times in the subsurface are weeks to months [67,68], making this process a very viable BrO3− removal pathway. Assuming that Fe2+ and BrO3− concentrations in Fe-reducing anoxic zones and BrO3− reduction follow second-order kinetics, as in Figure 7b (k2 = 0.049), the required time to reduce BrO3− below the drinking water guideline of 10 μg/L (0.08 µM) is on the order of 10–20 days.
As stated previously, the theoretical sequence of MAR infiltration zones follows the sequence of oxic-NO3−-reducing-Mn-reducing-Fe-reducing-SO42−-reducing [69], but the possible practical cross of different flowlines may result in the joint presence of NO3− and Fe2+. The results in Figure 7 indicate a small negative effect of NO3− as an inhibitor for BrO3− reduction by Fe2+, though at sufficiently high Fe2+ concentrations, bromate reduction is still not inhibited. Although NO3− reduction by Fe2+ is thermodynamically not feasible, in the presence of catalysts this reaction may occur [70]. A previous study reported that the presence of Ni2+, Cu2+, and Ag2+ promoted the reaction of Fe2+ with NO3− [48]. Given the presence of these elements in nature (for example, the concentration of Cu2+ at Dunea’s MAR site is 10−2 mM), these may well set off NO3− reduction by Fe2+. Moreover, previous studies [71,72,73] reported NO3−-dependent Fe2+ oxidation mediated by anaerobic ammonium oxidation bacteria, Escherichia coli, and NO3−-reducing bacteria. Therefore, a microbial mediated kinetic reaction of Fe2+ and NO3− could also occur, leading to competition for BrO3− reduction in these mixing flow paths in MAR systems.
Altogether, this study has shown that chemical BrO3− reduction by Fe2+ is expected to occur in Fe-reducing anoxic zones during MAR and that NO3− on its own is not a strong inhibitor or competitor; nevertheless, the complexity of subsurface processes may still set off conditions where NO3− reduction is favored over BrO3−. Therefore, a subsequent study to investigate BrO3− reduction in simulated Fe-reducing zones, such as a column study, is highly recommended, also to include microbiological and biochemical processes that take place during MAR.
5. Conclusions
Based on anoxic batch experiments, it is concluded that BrO3− is readily reduced by Fe2+. The reaction rate was influenced by the initial Fe2+/BrO3− ratio and by the initial pH, i.e., a higher Fe2+ concentration and higher pH accelerated the reaction. The pH dropped considerably during the experiments, set off by the hydrolysis of Fe3+ to HFO flocs. These HFO flocs were found to adsorb Fe2+, particularly at high Fe2+/BrO3− ratios, whereas at low Fe2+/BrO3− ratios the incomplete BrO3−–Br− mass balance indicated formation of intermediate species. Overall, it can be concluded that the chemical reduction of BrO3− by naturally occurring Fe2+ during MAR can occur, as extensive retention times in the subsurface will compensate for the slow reaction kinetics of low BrO3− and Fe2+ concentrations. In the specific case that Fe2+-containing and NO3−-containing water crosses flow paths during MAR, the presence of NO3− will not compete with BrO3−, as Fe2+ is preferred over NO3− as an electron acceptor. However, it was found that the presence of NO3− may somewhat inhibit BrO3− reduction when NO3− concentrations are far higher than BrO3− concentrations. The findings in this study show that application of MAR following ozone-based AOPs broadens the application of ozone-based AOPs, as MAR removes the byproduct BrO3−.
Acknowledgments
The research was funded by the Dunea drinking water company and by the Top Sector TKI Water Technology Program of the Dutch Ministry of Economic Affairs (No. 2013TUD001). The authors thank Katie Friedman for English editing and the China Scholarship Council for supporting our work (201206140009).
Author Contributions
Doris van Halem, Jan Peter van der Hoek, Feifei Wang, and Vanida Salgado conceived and designed the experiments; Vanida Salgado and Feifei Wang performed the experiments; Feifei Wang and Vanida Salgado analyzed the data; Vanida Salgado and Feifei Wang contributed reagents/materials/analysis tools; Feifei Wang, Vanida Salgado, Jan Peter van der Hoek, and Doris van Halem wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Laws, B.V.; Dickenson, E.R.V.; Johnson, T.A.; Snyder, S.A.; Drewes, J.E. Attenuation of contaminants of emerging concern during surface-spreading aquifer recharge. Sci. Total Environ. 2011, 409, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Noh, J.H.; Chae, S.R.; Choi, J.; Lee, Y.; Maeng, S.K. A multi-parametric approach assessing microbial viability and organic matter characteristics during managed aquifer recharge. Sci. Total Environ. 2015, 524–525, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Postigo, C.; Barceló, D. Synthetic organic compounds and their transformation products in groundwater: Occurrence, fate and mitigation. Sci. Total Environ. 2015, 503–504, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Drewes, J.E.; Heberer, T.; Rauch, T.; Reddersen, K. Fate of pharmaceuticals during ground water recharge. Ground Water Monit. Remediat. 2003, 23, 64–72. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; McDowell, D.; Sacher, F.; Brauch, H.J.; Haist-Gulde, B.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of pharmaceuticals during drinking water treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Hübner, U.; Miehe, U.; Jekel, M. Optimized removal of dissolved organic carbon and trace organic contaminants during combined ozonation and artificial groundwater recharge. Water Res. 2012, 46, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef] [PubMed]
- Scheideler, J.; Lekkerkerker-Teunissen, K.; Knol, T.; Ried, A.; Verberk, J.; van Dijk, H. Combination of O3/H2O2 and uv for multiple barrier micropollutant treatment and bromate formation control—An economic attractive option. Water Pract. Technol. 2011, 6. [Google Scholar] [CrossRef]
- Lekkerkerker, K.; Scheideler, J.; Maeng, S.K.; Ried, A.; Verberk, J.Q.J.C.; Knol, A.H.; Amy, G.; van Dijk, J.C. Advanced oxidation and artificial recharge: A synergistic hybrid system for removal of organic micropollutants. Water Sci. Technol. Water Supply 2009, 9, 643–651. [Google Scholar] [CrossRef]
- Lekkerkerker-Teunissen, K.; Chekol, E.T.; Maeng, S.K.; Ghebremichael, K.; Houtman, C.J.; Verliefde, A.R.D.; Verberk, J.Q.J.C.; Amy, G.L.; van Dijk, J.C. Pharmaceutical removal during managed aquifer recharge with pretreatment by advanced oxidation. Water Sci. Technol. Water Supply 2012, 12, 755–767. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Maekawa, A.; Takahashi, M.; Hayashi, Y. Toxicity and carcinogenicity of potassium bromate—A new renal carcinogen. Environ. Health Perspect. 1990, 87, 309–335. [Google Scholar] [PubMed]
- Haag, W.R.; Holgne, J. Ozonation of bromide-containing waters: Kinetics of formation of hypobromous acid and bromate. Environ. Sci. Technol. 1983, 17, 261–267. [Google Scholar] [CrossRef]
- Assuncao, A.; Martins, M.; Silva, G.; Lucas, H.; Coelho, M.R.; Costa, M.C. Bromate removal by anaerobic bacterial community: Mechanism and phylogenetic characterization. J. Hazard. Mater. 2011, 197, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shang, C. A review on bromate occurrence and removal strategies in water supply. Water Sci. Technol. Water Supply 2006, 6, 131–136. [Google Scholar] [CrossRef]
- Xiao, Q.; Yu, S.; Li, L.; Wang, T.; Liao, X.; Ye, Y. An overview of advanced reduction processes for bromate removal from drinking water: Reducing agents, activation methods, applications and mechanisms. J. Hazard. Mater. 2017, 324, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Aoki, S.; Matsushima, Y.; Takamura, N.; Imazawa, T.; Hayashi, Y. Dose-response studies on the carcinogenicity of potassium bromate in F344 rats after long-term oral administration. J. Natl. Cancer Inst. 1986, 77, 977–982. [Google Scholar] [PubMed]
- Crofton, K.M. Bromate: Concern for developmental neurotoxicity? Toxicology 2006, 221, 212–216. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 216, pp. 303–304. [Google Scholar]
- U.S. EPA. Guidelines for Carcinogen Risk Assessment; Risk Assessment Forum: Washington, DC, USA, 2005.
- Carney, M. European drinking water standards. J. Am. Water Works Assoc. 1991, 83, 48–55. [Google Scholar] [CrossRef]
- Chen, R.; Yang, Q.; Zhong, Y.; Li, X.; Liu, Y.; Li, X.M.; Du, W.X.; Zeng, G.M. Sorption of trace levels of bromate by macroporous strong base anion exchange resin: Influencing factors, equilibrium isotherms and thermodynamic studies. Desalination 2014, 344, 306–312. [Google Scholar] [CrossRef]
- Xu, C.; Shi, J.; Zhou, W.; Gao, B.; Yue, Q.; Wang, X. Bromate removal from aqueous solutions by nano crystalline akaganeite (β-FeOOH)-coated quartz sand (CACQS). Chem. Eng. J. 2012, 187, 63–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X. Preparation of Zn-Al CLDH to remove bromate from drinking water. J. Environ. Eng. (USA) 2014, 140. [Google Scholar] [CrossRef]
- Theiss, F.L.; Couperthwaite, S.J.; Ayoko, G.A.; Frost, R.L. A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides. J. Coll. Interface Sci. 2014, 417, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yu, S.; Tang, Y. Adsorptive characteristics of bromate from aqueous solutions by modified granular activated carbon. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2014, 34, 630–637. [Google Scholar]
- Gyparakis, S.; Diamadopoulos, E. Formation and reverse osmosis removal of bromate ions during ozonation of groundwater in coastal areas. Sep. Sci. Technol. 2007, 42, 1465–1476. [Google Scholar] [CrossRef]
- Van Der Hoek, J.P.; Rijnbende, D.O.; Lokin, C.J.A.; Bonné, P.A.C.; Loonen, M.T.; Hofman, J.A.M.H. Electrodialysis as an alternative for reverse osmosis in an integrated membrane system. Desalination 1998, 117, 159–172. [Google Scholar] [CrossRef]
- Wang, Q.; Snyder, S.; Kim, J.; Choi, H. Aqueous ethanol modified nanoscale zerovalent iron in Bromate reduction: Synthesis, characterization, and reactivity. Environ. Sci. Technol. 2009, 43, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, Z.; Wan, H.; Zheng, J.; Yin, D.; Zheng, S. Aqueous bromate reduction by catalytic hydrogenation over Pd/Al2O3 catalysts. Appl. Catal. B Environ. 2010, 96, 307–313. [Google Scholar] [CrossRef]
- Siddiqui, M.; Amy, G.; Ozekin, K.; Zhai, W.; Westerhoff, P. Alternative strategies for removing bromate. J. Am. Water Works Assoc. (USA) 1994, 86, 81–96. [Google Scholar] [CrossRef]
- Kishimoto, N.; Matsuda, N. Bromate ion removal by electrochemical reduction using an activated carbon felt electrode. Environ. Sci. Technol. 2009, 43, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Zhao, X.; Qu, J. Electrochemical Reduction of Bromate by a Pd Modified Carbon Fiber Electrode: Kinetics and Mechanism. Electrochim. Acta 2014, 132, 151–157. [Google Scholar] [CrossRef]
- Kirisits, M.J.; Snoeyink, V.L.; Inan, H.; Chee-sanford, J.C.; Raskin, L.; Brown, J.C. Water quality factors affecting bromate reduction in biologically active carbon filters. Water Res. 2001, 35, 891–900. [Google Scholar] [CrossRef]
- Liu, J.; Yu, J.; Li, D.; Zhang, Y.; Yang, M. Reduction of bromate in a biological activated carbon filter under high bulk dissolved oxygen conditions and characterization of bromate-reducing isolates. Biochem. Eng. J. 2012, 65, 44–50. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Jong, R.; van der Kooij, D. Bromate removal in a denitrifying bioreactor used in water treatment. Water Res. 1999, 33, 1049–1053. [Google Scholar] [CrossRef]
- Wang, F.; van Halem, D.; Ding, L.; Bai, Y.; Lekkerkerker-Teunissen, K.; van der Hoek, J.P. Effective removal of bromate in nitrate-reducing anoxic zones during managed aquifer recharge for drinking water treatment. Water Res. 2018, 130, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Hübner, U.; Kuhnt, S.; Jekel, M.; Drewes, J.E. Fate of bulk organic carbon and bromate during indirect water reuse involving ozone and subsequent aquifer recharge. J. Water Reuse Desal. 2016, 6, 413–420. [Google Scholar] [CrossRef]
- Xie, L.; Shang, C. The effects of operational parameters and common anions on the reactivity of zero-valent iron in bromate reduction. Chemosphere 2007, 66, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Stefánsson, A. Iron(III) hydrolysis and solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Dong, Z.J.; Dong, W.Y.; Zhang, X.M.; Yu, X.H.; Ou, Y.F.; Du, H. Removal of bromate by ferrous sulfate reduction in drinking water. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Beijing, China, 11–13 June 2009. [Google Scholar]
- Barbieri, M.; Carrera, J.; Sanchez-Vila, X.; Ayora, C.; Cama, J.; Köck-Schulmeyer, M.; de Alda, M.L.; Barceló, D.; Brunet, J.T.; García, M.H. Microcosm experiments to control anaerobic redox conditions when studying the fate of organic micropollutants in aquifer material. J. Contam. Hydrol. 2011, 126, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Kedziorek, M.A.M.; Geoffriau, S.; Bourg, A.C.M. Organic matter and modeling redox reactions during river bank filtration in an alluvial aquifer of the Lot River, France. Environ. Sci. Technol. 2008, 42, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Grischek, T.; Paufler, S. Prediction of iron release during riverbank filtration. Water 2017, 9, 317. [Google Scholar] [CrossRef]
- Song, X.; Wang, S.; Wang, Y.; Zhao, Z.; Yan, D. Addition of Fe2+ increase nitrate removal in vertical subsurface flow constructed wetlands. Ecol. Eng. 2016, 91, 487–494. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhang, T.C. Effects of low pH on nitrate reduction by iron powder. Water Res. 2004, 38, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Buresh, R.J.; Moraghan, J. Chemical reduction of nitrate by ferrous iron. J. Environ. Qual. 1976, 5, 320–325. [Google Scholar] [CrossRef]
- Ottley, C.J.; Davison, W.; Edmunds, M.W. Chemical catalysis of nitrate reduction by iron (II). Geochim. Cosmochim. Acta 1997, 61, 1819–1828. [Google Scholar] [CrossRef]
- Krasner, S.W.; Glaze, W.H.; Weinberg, H.S.; Daniel, P.A.; Najm, I.N. Formation and control of bromate during ozonation of waters containing bromide. J. Am. Water Works Assoc. 1993, 85, 73–81. [Google Scholar] [CrossRef]
- Glaze, W.H.; Weinberg, H.S.; Cavanagh, J.E. Evaluating the formation of brominated DBPs during ozonation. J. Am. Water Works Assoc. 1993, 85, 96–103. [Google Scholar] [CrossRef]
- Amy, G.; Bull, R.; Craun, G.F.; Pegram, R.; Siddiqui, M. Disinfectants and Disinfectant by-Products; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Butler, R.; Godley, A.; Lytton, L.; Cartmell, E. Bromate environmental contamination: Review of impact and possible treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 193–217. [Google Scholar] [CrossRef]
- Kirisits, M.J.; Snoeyink, V.L. Reduction of bromate in a BAC filter. J. Am. Water Works Assoc. 1999, 91, 74–84. [Google Scholar] [CrossRef]
- Thomas, D.; Rohrer, J. Determination of Chlorite, Bromate, Bromide, and Chlorate in Drinking Water by Ion Chromatography with an On-Line-Generated Postcolumn Reagent for Sub-µg/L Bromate Analysis; Thermo Fisher Scientific: Sunnyvale, CA, USA, 2017. [Google Scholar]
- Westerhoff, P. Reduction of nitrate, bromate, and chlorate by zero valent iron (Fe0). J. Environ. Eng. 2003, 129, 10–16. [Google Scholar] [CrossRef]
- Lengyel, I.; Nagy, I.; Bazsa, G. Kinetic study of the autocatalytic nitric acid-bromide reaction and its reverse, the nitrous acid-bromine reaction. J.Phys. Chem. 1989, 93, 2801–2807. [Google Scholar] [CrossRef]
- Britton, H.; Britton, H.G. 746. A potentiometric study of the reduction by potassium iodide of potassium bromate in sulphuric and hydrochloric acid solutions. J. Chem. Soc. (Resumed) 1952, 3887–3892. [Google Scholar] [CrossRef]
- Aksut, A. Investigation of Fe3+/Fe2+ redox reaction by electrochemical methods in aqueous solution. Commun. Fac. Sci. Univ. Ank. Ser. B 1994, 40, 83–93. [Google Scholar]
- Shen, W.; Lin, F.; Jiang, X.; Li, H.; Ai, Z.; Zhang, L. Efficient removal of bromate with core-shell Fe@Fe2O3 nanowires. Chem. Eng. J. 2017, 308, 880–888. [Google Scholar] [CrossRef]
- Hiemstra, T.; van Riemsdijk, W.H. Adsorption and surface oxidation of Fe(II) on metal (hydr)oxides. Geochim. Cosmochim. Acta 2007, 71, 5913–5933. [Google Scholar] [CrossRef]
- Williams, A.G.B.; Scherer, M.M. Spectroscopic evidence for Fe(II)-Fe(III) electron transfer at the iron oxide-water interface. Environ. Sci. Technol. 2004, 38, 4782–4790. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.A.; van Weert, G. On the catalysis of ferrous sulphate oxidation in autoclaves by nitrates and nitrites. Hydrometallurgy 1996, 42, 209–219. [Google Scholar] [CrossRef]
- Ohura, H.; Imato, T.; Kameda, K.; Yamasaki, S. Potentiometric determination of bromate using an Fe(III)-Fe(II) potential buffer by circulatory flow-injection analysis. Anal. Sci. 2004, 20, 513–518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tresintsi, S.; Simeonidis, K.; Vourlias, G.; Stavropoulos, G.; Mitrakas, M. Kilogram-scale synthesis of iron oxy-hydroxides with improved arsenic removal capacity: Study of Fe (II) oxidation–precipitation parameters. Water Res. 2012, 46, 5255–5267. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; van Halem, D.; van der Hoek, J.P. The fate of H2O2 during managed aquifer recharge: A residual from advanced oxidation processes for drinking water production. Chemosphere 2016, 148, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; van Halem, D.; Liu, G.; Lekkerkerker-Teunissen, K.; van der Hoek, J.P. Effect of residual H2O2 from advanced oxidation processes on subsequent biological water treatment: A laboratory batch study. Chemosphere 2017, 185, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.K.; Sharma, S.K.; Lekkerkerker-Teunissen, K.; Amy, G.L. Occurrence and fate of bulk organic matter and pharmaceutically active compounds in managed aquifer recharge: A review. Water Res. 2011, 45, 3015–3033. [Google Scholar] [CrossRef] [PubMed]
- Stuyfzand, P.J. Hydrology and water quality aspects of Rhine bank groundwater in The Netherlands. J. Hydrol. 1989, 106, 341–363. [Google Scholar] [CrossRef]
- Eckert, P.; Appelo, C.A.J. Hydrogeochemical modeling of enhanced benzene, toluene, ethylbenzene, xylene (BTEX) remediation with nitrate. Water Resour. Res. 2002, 38. [Google Scholar] [CrossRef]
- Benz, M.; Brune, A.; Schink, B. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch. Microbiol. 1998, 169, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Oshiki, M.; Ishii, S.; Yoshida, K.; Fujii, N.; Ishiguro, M.; Satoh, H.; Okabe, S. Nitrate-dependent ferrous iron oxidation by anaerobic ammonium oxidation (anammox) bacteria. Appl. Environ. Microbiol. 2013, 79, 4087–4093. [Google Scholar] [CrossRef] [PubMed]
- Brons, H.J.; Hagen, W.R.; Zehnder, A.J.B. Ferrous iron dependent nitric oxide production in nitrate reducing cultures of Escherichia coli. Arch. Microbiol. 1991, 155, 341–347. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).