Experimental Study on the Palatability Impacts of Potable Water as a Hydronic Medium
Abstract
:1. Introduction
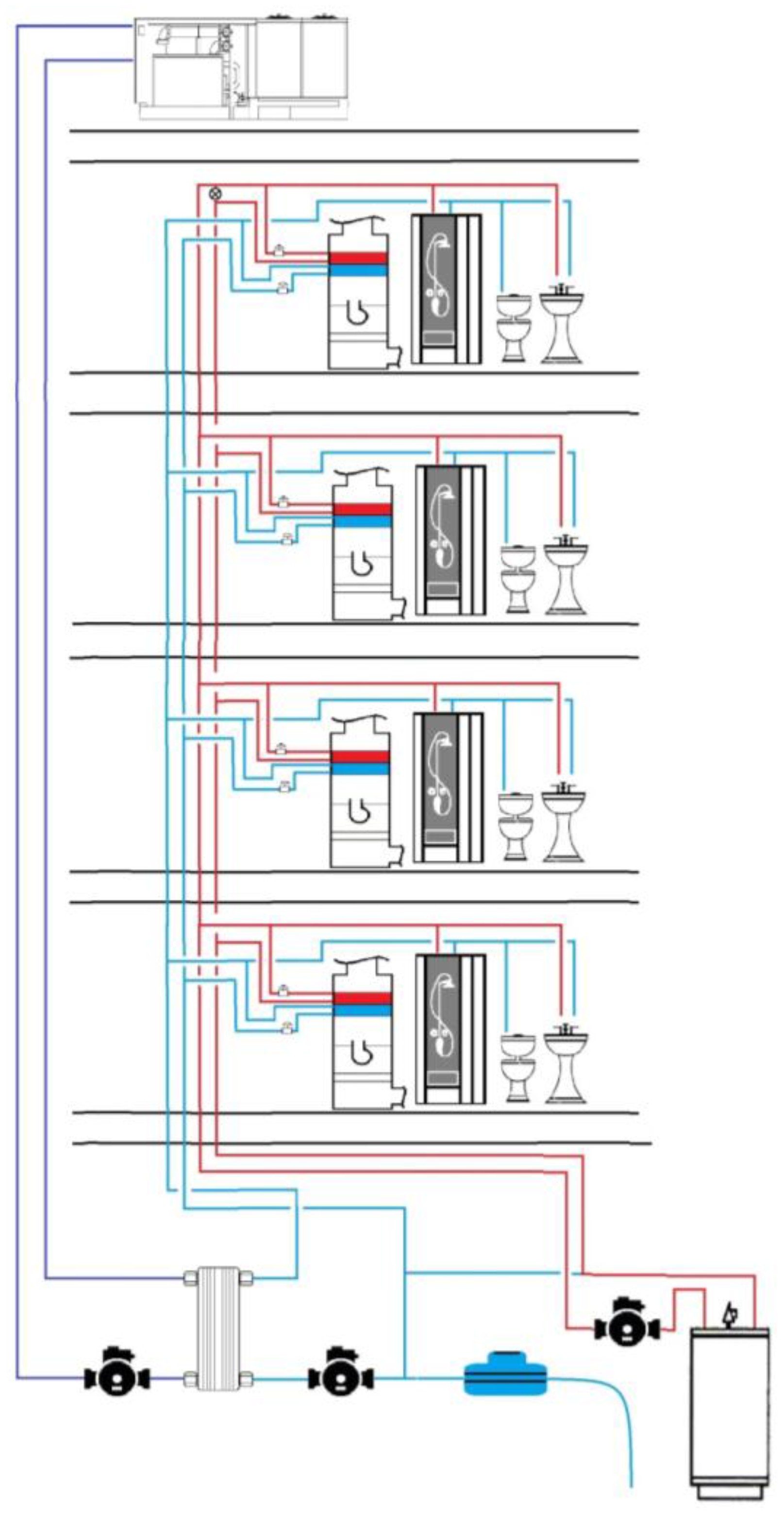
2. Materials and Methods
2.1. Experimental Design
2.2. Water Palatability Test Protocol
- Apparatus water, hot service water samples from the building system at the point of use by the simulated occupants.
- Potable cold supply water from the municipal supply feeding the apparatus.
- Potable hot water from an adjacent, traditional source. This source was supplied with the same cold supply water as the apparatus, but the water was not used for HVAC purposes.
- Commercial bottled water (Nestle Pure Life purified water).
2.3. Apparatus Samples
3. Results and Discussion
3.1. Palatability Results
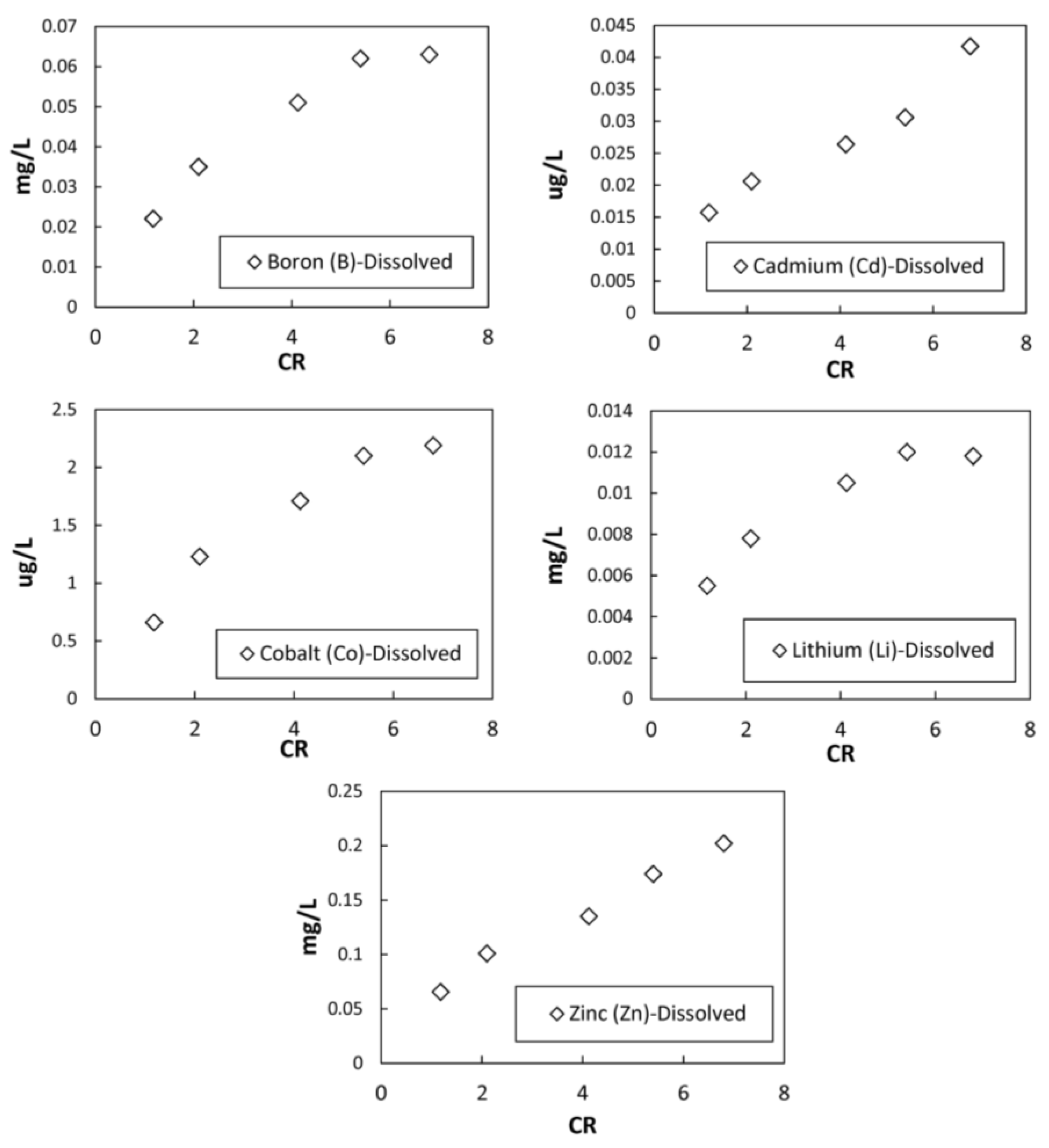
3.2. Chemical Analysis Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Butcher, T. Performance of Integrated Hydronic Heating Systems; Brookhaven Science Associates: Upton, NY, USA, 2007. [Google Scholar]
- ASHRAE. ASHRAE Handbook—Applications; ASHRAE: Atlanta, GA, USA, 2011; Chapter 50. [Google Scholar]
- Caron, R.; Wilson, R. Water-Heating Efficiency of Integrated Systems Designed for Space and Water Heating. ASHRAE Trans. 1983, 89, 18–29. [Google Scholar]
- Subherwal, B. Combination Water-Heating/SPace Heating Appliance Performance. ASHRAE Trans. 1986, 92, 415–432. [Google Scholar]
- MacNevin, L. Mixing hydronic heating water. In Modern Hydronics; Spring: Toronto, ON, Canada, 2016. [Google Scholar]
- Canadian Institute of Plumbing and Heating. Use of Water Heaters in Hydronic Applications; Canadian Institute of Plumbing and Heating: Toronto, ON, Canada, 2008. [Google Scholar]
- Whelton, A.; Dietrich, A.; Gallagher, D. Contaminant Diffusion, Solubility, and Material Property Differences between HDPE and PEX Potable Water Pipes. J. Environ. Eng. 2010, 136. [Google Scholar] [CrossRef]
- Kelley, K.; Stenson, A.; Dey, R.; Whelton, A. Release of Drinking Water Contaminants and Odor Impacts Caused by Green Building Cross-linked Polyethylene (PEX) Plumbing Systems. Water Res. 2014, 67, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Venere, E. Drinking Water Odors, Chemicals above Health Standards Caused by ‘Green Building’ Plumbing; Purdue University: West Lafayette, IN, USA, 2014. [Google Scholar]
- Causes of Copper Corrosion in Plumbing Systems; Foundation for Water Research: Marlow, UK, 2017.
- Loganathan, G.; Lee, J.; Bosch, D.; Dwyer, S.; Willis-Walton, S.; Kleczyk, E. Preference Trade-Offs in Choosing Domestic Plumbing Meterials; Pipelines: Reston, VA, USA, 2006. [Google Scholar]
- Kelly, M.; Pomfret, J. Tastes and odours in potable water: Perception versus reality. In Microbiological Quality of Water; Freshwater Biological Association: Ambleside, UK, 1997; pp. 71–80. [Google Scholar]
- American Water Works Association (AWWA). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA, AWWA, and WEF: Washington, DC, USA, 2012. [Google Scholar]
- Canadian Standards Association. CSA B214-12, Installation Code for Hydronic Heating Systems; Canadian Standards Association: Mississauga, ON, Canada, 2012. [Google Scholar]
- Heim, T.; Dietrich, A. Sensory aspects and water quality impacts of chlorinated and chloraminated drinking water in contact with HDPE and cPVC pipe. Water Res. 2007, 41, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Heim, T. Impact of Polymeric Plumbing Materials on Drinking Water Quality and Aesthetics; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2006. [Google Scholar]
- Wiesenthal, K.; McGuire, M.; Suffet, I. Characteristics of salt taste and free chlorine or chloramine in drinking water. Water Sci. Technol. 2007, 55, 293–300. [Google Scholar] [CrossRef] [PubMed]
- American Water Works Association (AWWA). Effects of Water Age on Distribution System Water Quality; U.S. Environmental Protection Agency: Washington, DC, USA, 2002.
- Teillet, E.; Urbano, C.; Cordelle, S.; Schlich, P. Consumer Perception and Preference of Bottled and Tap Water. J. Sens. Stud. 2010, 3, 463–480. [Google Scholar] [CrossRef]
- Liu, B.; Reckhow, D. Impact of Water heaters on the Formation of Disinfection By-Products. J. Am. Water Works Assoc. 2015, 107, E328–E338. [Google Scholar] [CrossRef]
- Rushing, J.; Edwards, M. The role of termperature gradients in residential copper pipe corrosion. Corros. Sci. 2004, 46, 1883–1894. [Google Scholar] [CrossRef]
- Federal-State Toxicology Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines; FSTRAC: Durham, NC, USA, 1995.
- Federal-State Toxicology Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines; FSTRAC: Durham, NC, USA, 1999.
- World Health Organization. Cobalt and Inorganic Cobalt Compounds; WHO: Geneva, Switzerland, 2006. [Google Scholar]

| Detectable Limit | Units | Source Water Average Chemistry | Min | Max | Apparatus Water Average Chemistry | Min | Max | |
|---|---|---|---|---|---|---|---|---|
| Chloramines | ||||||||
| Chlorine, Free | 0.1 | mg/L | 0.468 | 0.1 | 0.74 | 0 | 0 | 0 |
| Chlorine, Total | 0.1 | mg/L | 1.116 | 0.13 | 1.84 | 0 | 0 | 0 |
| Total Chlorine minus Free Chlorine | 0.2 | mg/L | 0.805 | 0.38 | 1.54 | 0 | 0 | 0 |
| Ammonia, Total (as N) | 0.05 | mg/L | 0.3296 | 0.308 | 0.349 | 0.4362 | 0.416 | 0.453 |
| Chloride (Cl) | 0.5 | mg/L | 5.066 | 4.78 | 5.58 | 5.888 | 5.59 | 6.26 |
| Fluoride (F) | 0.02 | mg/L | 0.6712 | 0.655 | 0.693 | 0.759 | 0.743 | 0.796 |
| Ion Balance | % | 98.28 | 96.3 | 99.2 | 98.22 | 96.4 | 99.7 | |
| TDS (Calculated) | mg/L | 212.8 | 205 | 220 | 208 | 200 | 220 | |
| Hardness (as CaCO3) | mg/L | 168 | 162 | 172 | 162.6 | 157 | 173 | |
| Nitrate (as N) | 0.02 | mg/L | 0.0498 | 0.022 | 0.074 | 0.0572 | 0.03 | 0.079 |
| Nitrate and Nitrite (as N) | 0.022 | mg/L | 0.05675 | 0.045 | 0.074 | 0.0572 | 0.03 | 0.079 |
| Nitrite (as N) | 0.01 | mg/L | 0 | 0 | 0 | 0 | 0 | 0 |
| Sulfate (SO4) | 0.3 | mg/L | 68.32 | 64.3 | 72.1 | 66.76 | 64.8 | 68.9 |
| pH | 0.1 | pH | 8.134 | 8.01 | 8.2 | 8.112 | 8.08 | 8.16 |
| Conductivity (EC) | 0.2 | uS/cm | 396.2 | 384 | 410 | 389.2 | 375 | 405 |
| Bicarbonate (HCO3) | 5 | mg/L | 140 | 131 | 147 | 135 | 128 | 145 |
| Carbonate (CO3) | 5 | mg/L | 0 | 0 | 0 | 0 | 0 | 0 |
| Hydroxide (OH) | 5 | mg/L | 0 | 0 | 0 | 0 | 0 | 0 |
| Alkalinity, Total (as CaCO3) | 2 | mg/L | 115 | 108 | 121 | 110.6 | 105 | 119 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prybysh, R.; Al-Hussein, M.; Fleck, B.; Sadrzadeh, M.; Osolu, J. Experimental Study on the Palatability Impacts of Potable Water as a Hydronic Medium. Water 2018, 10, 218. https://doi.org/10.3390/w10020218
Prybysh R, Al-Hussein M, Fleck B, Sadrzadeh M, Osolu J. Experimental Study on the Palatability Impacts of Potable Water as a Hydronic Medium. Water. 2018; 10(2):218. https://doi.org/10.3390/w10020218
Chicago/Turabian StylePrybysh, Robert, Mohamed Al-Hussein, Brian Fleck, Mohtada Sadrzadeh, and Jeremiah Osolu. 2018. "Experimental Study on the Palatability Impacts of Potable Water as a Hydronic Medium" Water 10, no. 2: 218. https://doi.org/10.3390/w10020218
APA StylePrybysh, R., Al-Hussein, M., Fleck, B., Sadrzadeh, M., & Osolu, J. (2018). Experimental Study on the Palatability Impacts of Potable Water as a Hydronic Medium. Water, 10(2), 218. https://doi.org/10.3390/w10020218







