Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies
Abstract
1. Introduction
2. Occurrence in Aquatic Systems
2.1. Wastewater Treatment Plant Effluent
2.2. Surface Water
2.3. Groundwater
3. Toxicological Effects
4. Regulations
5. Biological Treatment Technologies for CBZ Removal
5.1. Activated Sludge Based Processes
5.2. White-Rot Fungi and Their Extracellular Enzymes
6. CBZ Removal by Advanced Physicochemical Treatment Technologies
6.1. Performance of Nanofiltration and Reverse Osmosis Membranes
6.2. Adsorption of CBZ by Activated Carbon
6.3. CBZ Degradation by Advanced Oxidation Processes
6.4. Combined/Hybrid Treatment Systems
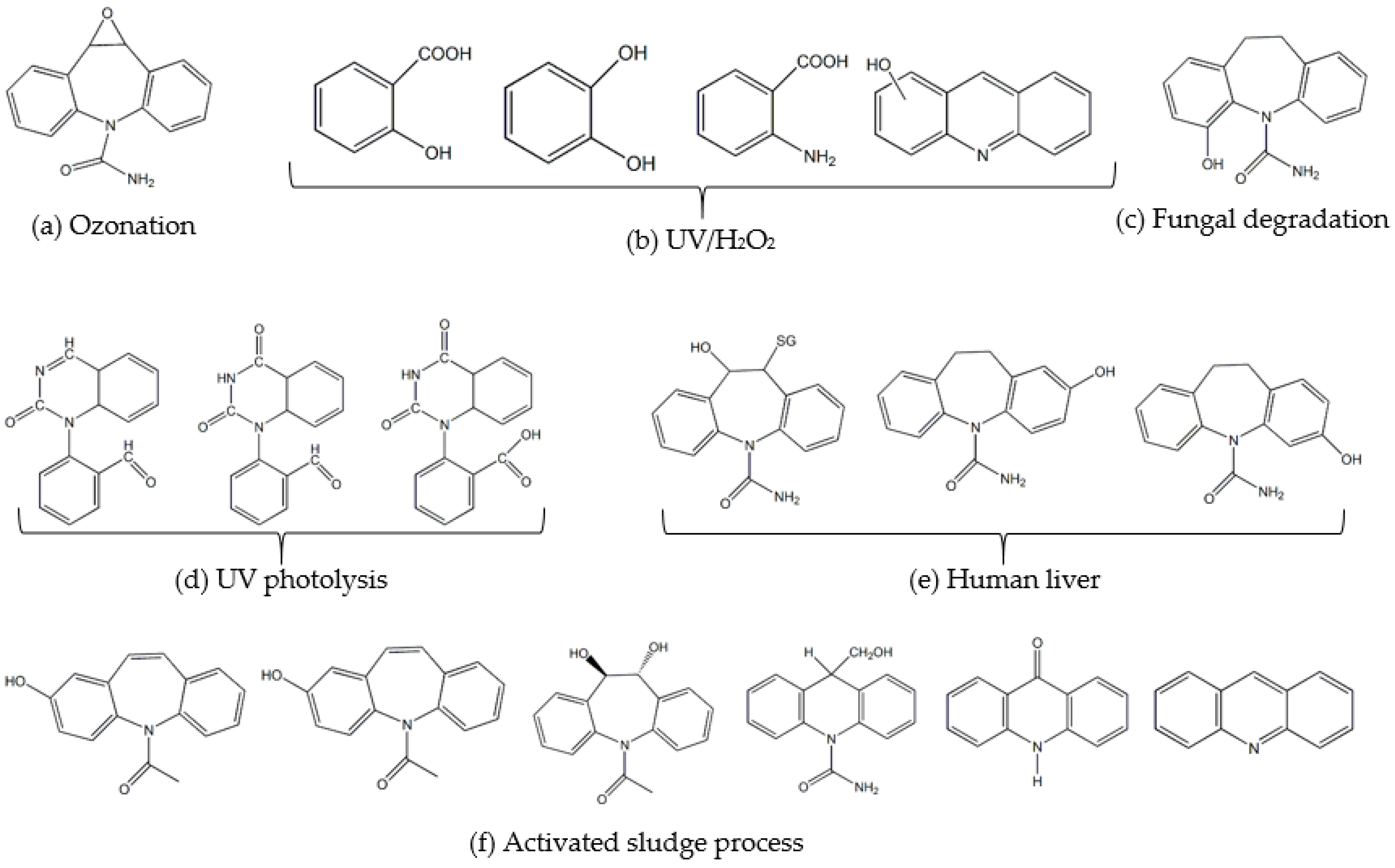
7. Fate and Metabolites of CBZ
8. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hughes, S.R.; Kay, P.; Brown, L.E. Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ. Sci. Technol. 2012, 47, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Nghiem, L.D.; Khan, S.J.; Price, W.E.; Yamamoto, K. Wastewater reuse: Removal of emerging trace organic contaminants. In Membrane biological Reactors: Theory, Modeling, Design, Management and Applications to Wastewater Reuse; Hai, F.I., Yamamoto, K., Lee, C., Eds.; IWA publishing: London, UK, 2014; pp. 165–205. ISBN 9781780400655. [Google Scholar]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Lapworth, D.; Baran, N.; Stuart, M.; Ward, R. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of pharmaceuticals in sewage treatment plants in finland. Water Res. 2007, 41, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Brack, W.; Dulio, V.; Ågerstrand, M.; Allan, I.; Altenburger, R.; Brinkmann, M.; Bunke, D.; Burgess, R.M.; Cousins, I.; Escher, B.I. Towards the review of the european union water framework directive: Recommendations for more efficient assessment and management of chemical contamination in european surface water resources. Sci. Total Environ. 2017, 576, 720–737. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Batley, G.E.; Nidumolu, B.; Hutchinson, T.H. Derivation of water quality guidelines for priority pharmaceuticals. Environ. Toxicol. Chem. 2016, 35, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.V.; Reis, S.; Lima, J.L.; Segundo, M.A. Analytical features of diclofenac evaluation in water as a potential marker of anthropogenic pollution. Curr. Pharm. Anal. 2017, 13, 39–47. [Google Scholar] [CrossRef]
- Arye, G.; Dror, I.; Berkowitz, B. Fate and transport of carbamazepine in soil aquifer treatment (sat) infiltration basin soils. Chemosphere 2011, 82, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Thurman, E.M. Analysis of 100 pharmaceuticals and their degradates in water samples by liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2012, 1259, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Fink, G.; Joss, A.; Siegrist, H.; Ternes, T.A. Fate of beta blockers and psycho-active drugs in conventional wastewater treatment. Water Res. 2009, 43, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. Understanding the removal mechanisms of ppcps and the influence of main technological parameters in anaerobic uasb and aerobic cas reactors. J. Hazard. Mater. 2014, 278, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, H.; Joss, A. Review on the fate of organic micropollutants in wastewater treatment and water reuse with membranes. Water Sci. Technol. 2012, 66, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xia, Y.; Li, T.; Yao, T.; Shi, Z.; Zhu, S.; Gao, N. Degradation of carbamazepine by uv/chlorine advanced oxidation process and formation of disinfection by-products. Environ. Sci. Pollut. Res. 2016, 23, 16448–16455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Peldszus, S.; Huck, P.M. Adsorption characteristics of selected pharmaceuticals and an endocrine disrupting compound-naproxen, carbamazepine and nonylphenol-on activated carbon. Water Res. 2008, 42, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Bolong, N.; Ismail, A.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.T.; Hai, F.I.; Al-aboud, T.M. Chemical coagulation-based processes for trace organic contaminant removal: Current state and future potential. J. Environ. Manag. 2012, 111, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Illicit drugs and pharmaceuticals in the environment—Forensic applications of environmental data. Part 1: Estimation of the usage of drugs in local communities. Environ. Pollut. 2009, 157, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.; Jaus, S.; Hanke, I.; Lück, A.; Hollender, J.; Alder, A.C. Determination of biocides and pesticides by on-line solid phase extraction coupled with mass spectrometry and their behaviour in wastewater and surface water. Environ. Pollut. 2010, 158, 3054–3064. [Google Scholar] [CrossRef] [PubMed]
- Dvory, N.Z.; Kuznetsov, M.; Livshitz, Y.; Gasser, G.; Pankratov, I.; Lev, O.; Adar, E.; Yakirevich, A. Modeling sewage leakage and transport in carbonate aquifer using carbamazepine as an indicator. Water Res. 2018, 128, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.-G.; Kookana, R.S.; Kolpin, D.W. Occurrence and removal of pharmaceutically active compounds in sewage treatment plants with different technologies. J. Environ. Monit. 2009, 11, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, K.C.; Hai, F.I.; Kang, J.; Price, W.E.; Guo, W.; Ngo, H.H.; Nghiem, L.D. The fate of pharmaceuticals, steroid hormones, phytoestrogens, uv-filters and pesticides during mbr treatment. Bioresour. Technol. 2013, 144, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Krasner, S.W. Occurence of primidone, carbamazepine, caffeine and precursors for n-nitrosodimethylamine in drinding water sources impacted by wastewater. J. Am. Water Resour. Assoc. 2009, 45, 58–67. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Miao, X.-S.; Koenig, B.G.; Struger, J. Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower great lakes, canada. Environ. Toxicol. Chem. 2003, 22, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.-S.; Metcalfe, C.D. Determination of carbamazepine and its metabolites in aqueous samples using liquid chromatography−electrospray tandem mass spectrometry. Anal. Chem. 2003, 75, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.-S.; Yang, J.-J.; Metcalfe, C.D. Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant. Environ. Sci. Technol. 2005, 39, 7469–7475. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; Blaise, C.; André, C. Occurrence of pharmaceutical products in a municipal effluent and toxicity to rainbow trout (Oncorhynchus mykiss) hepatocytes. Ecotoxicol. Environ. Saf. 2006, 64, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Peldszus, S.; Huck, P.M. Optimizing gas chromatographic–mass spectrometric analysis of selected pharmaceuticals and endocrine-disrupting substances in water using factorial experimental design. J. Chromatogr. A 2007, 1148, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, N.; Topp, E.; Metcalfe, C.; Edwards, M.; Payne, M.; Kleywegt, S.; Russell, P.; Lapen, D.R. Pharmaceutical and personal care products in groundwater, subsurface drainage, soil, and wheat grain, following a high single application of municipal biosolids to a field. Chemosphere 2012, 87, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Topp, E.; Metcalfe, C.D.; Li, H.; Gottschall, N.; Bolton, P.; Curnoe, W.; Payne, M.; Beck, A.; Kleywegt, S.; et al. Pharmaceutical and personal care products in tile drainage following surface spreading and injection of dewatered municipal biosolids to an agricultural field. Sci. Total Environ. 2009, 407, 4220–4230. [Google Scholar] [CrossRef] [PubMed]
- Hummel, D.; Löffler, D.; Fink, G.; Ternes, T.A. Simultaneous determination of psychoactive drugs and their metabolites in aqueous matrices by liquid chromatography mass spectrometry. Environ. Sci. Technol. 2006, 40, 7321–7328. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Stüber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in german sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Wiegel, S.; Aulinger, A.; Brockmeyer, R.; Harms, H.; Löffler, J.; Reincke, H.; Schmidt, R.; Stachel, B.; von Tümpling, W.; Wanke, A. Pharmaceuticals in the river elbe and its tributaries. Chemosphere 2004, 57, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Musolff, A.; Leschik, S.; Möder, M.; Strauch, G.; Reinstorf, F.; Schirmer, M. Temporal and spatial patterns of micropollutants in urban receiving waters. Environ. Pollut. 2009, 157, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Osenbrück, K.; Gläser, H.-R.; Knöller, K.; Weise, S.M.; Möder, M.; Wennrich, R.; Schirmer, M.; Reinstorf, F.; Busch, W.; Strauch, G. Sources and transport of selected organic micropollutants in urban groundwater underlying the city of halle (saale), Germany. Water Res. 2007, 41, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Kobayashi, Y.; Nagao, R.; Yamashita, N.; Tanaka, H.; Tanaka, S.; Fujii, S.; Konishi, C.; Houwa, I. Removal efficiency of 66 pharmaceuticals during wastewater treatment process in Japan. Water Sci. Technol. 2008, 57, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Nakada, N.; Komori, K.; Suzuki, Y.; Konishi, C.; Houwa, I.; Tanaka, H. Occurrence of 70 pharmaceutical and personal care products in tone river basin in Japan. Water Sci. Technol. 2007, 56, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Nakada, N.; Kiri, K.; Shinohara, H.; Harada, A.; Kuroda, K.; Takizawa, S.; Takada, H. Evaluation of pharmaceuticals and personal care products as water-soluble molecular markers of sewage. Environ. Sci. Technol. 2008, 42, 6347–6353. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Murakami, M.; Oguma, K.; Muramatsu, Y.; Takada, H.; Takizawa, S. Assessment of groundwater pollution in tokyo using ppcps as sewage markers. Environ. Sci. Technol. 2011, 46, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, Y.; Park, J.; Park, C.K.; Kim, M.; Kim, H.S.; Kim, P. Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea. Sci. Total Environ. 2008, 405, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Kim, H.W.; Oh, J.-E.; Park, H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Jang, H.-S.; Kim, J.-G.; Ishibashi, H.; Hirano, M.; Nasu, K.; Ichikawa, N.; Takao, Y.; Shinohara, R.; Arizono, K. Occurrence of pharmaceutical and personal care products (ppcps) in surface water from Mankyung River, South Korea. J. Health Sci. 2009, 55, 249–258. [Google Scholar] [CrossRef]
- Yoon, Y.; Ryu, J.; Oh, J.; Choi, B.-G.; Snyder, S.A. Occurrence of endocrine disrupting compounds, pharmaceuticals, and personal care products in the Han river (Seoul, South Korea). Sci. Total Environ. 2010, 408, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Wang, P.-L.; Ding, W.-H. Using liquid chromatography–ion trap mass spectrometry to determine pharmaceutical residues in taiwanese rivers and wastewaters. Chemosphere 2008, 72, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Chen, H.-C.; Ding, W.-H. Determination of pharmaceutical residues in waters by solid-phase extraction and large-volume on-line derivatization with gas chromatography–mass spectrometry. J. Chromatogr. A 2005, 1065, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Zhou, J.L. Simultaneous determination of various pharmaceutical compounds in water by solid-phase extraction–liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2007, 1154, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Zhang, Z.L.; Banks, E.; Grover, D.; Jiang, J.Q. Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J. Hazard. Mater. 2009, 166, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in south wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multi-residue method for the determination of basic/neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra performance liquid chromatography–positive electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.E.; Manamsa, K.; Talbot, J.C.; Crane, E.J. Emerging Contaminants in Groundwater, 2011. Available online: https://nora.nerc.ac.uk/14557/ (accessed on 15 October 2017).
- Lapworth, D.; Stuart, M.; Hart, A.; Crane, E.; Baran, N. Emerging Contaminants in Groundwater; Groundwater Forum: London, UK, 2011; Available online: http://nora.nerc.ac.uk/14093/ (accessed on 15 October 2017).
- Spongberg, A.L.; Witter, J.D. Pharmaceutical compounds in the wastewater process stream in Northwest Ohio. Sci. Total Environ. 2008, 397, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Vanderford, B.J.; Snyder, S.A. Analysis of pharmaceuticals in water by isotope dilution liquid chromatography/tandem mass spectrometry. Environ. Sci. Technol. 2006, 40, 7312–7320. [Google Scholar] [CrossRef] [PubMed]
- Glassmeyer, S.T.; Furlong, E.T.; Kolpin, D.W.; Cahill, J.D.; Zaugg, S.D.; Werner, S.L.; Meyer, M.T.; Kryak, D.D. Transport of chemical and microbial compounds from known wastewater discharges: Potential for use as indicators of human fecal contamination. Environ. Sci. Technol. 2005, 39, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Skopec, M.; Meyer, M.T.; Furlong, E.T.; Zaugg, S.D. Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci. Total Environ. 2004, 328, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Conley, J.M.; Symes, S.J.; Kindelberger, S.A.; Richards, S.M. Rapid liquid chromatography–tandem mass spectrometry method for the determination of a broad mixture of pharmaceuticals in surface water. J. Chromatogr. A 2008, 1185, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.G.; Griffin, D.W.; Davis, J.H. Groundwater quality impacts from the land application of treated municipal wastewater in a large karstic spring basin: Chemical and microbiological indicators. Sci. Total Environ. 2009, 407, 2872–2886. [Google Scholar] [CrossRef] [PubMed]
- Fram, M.S.; Belitz, K. Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in california. Sci. Total Environ. 2011, 409, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Jelić, A.; Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Occurrence and elimination of pharmaceuticals during conventional wastewater treatment. In Emerging and Priority Pollutants in Rivers; Guasch, H., Ginebreda, A., Geiszinger, A., Eds.; Springer: Berlin, Germany, 2012; pp. 1–23. ISBN 9783642257223. [Google Scholar]
- Petrovic, M.; de Alda, M.J.L.; Diaz-Cruz, S.; Postigo, C.; Radjenovic, J.; Gros, M.; Barcelo, D. Fate and removal of pharmaceuticals and illicit drugs in conventional and membrane bioreactor wastewater treatment plants and by riverbank filtration. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 3979–4003. [Google Scholar] [CrossRef] [PubMed]
- Al-Rifai, J.H.; Gabelish, C.L.; Schäfer, A.I. Occurrence of pharmaceutically active and non-steroidal estrogenic compounds in three different wastewater recycling schemes in Australia. Chemosphere 2007, 69, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Strenn, B.; Kreuzinger, N. Carbamazepine as a possible anthropogenic marker in the aquatic environment: Investigations on the behaviour of carbamazepine in wastewater treatment and during groundwater infiltration. Water Res. 2004, 38, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Analysis of neutral and basic pharmaceuticals in sewage treatment plants and in recipient rivers using solid phase extraction and liquid chromatography–tandem mass spectrometry detection. J. Chromatogr. A 2006, 1134, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Vochezer, K. Modelling of Carbamazepine and Diclofenac in a River Network—Photolytic Degradation in Swiss Rivers; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2010. [Google Scholar]
- Modick, H.; Weiss, T.; Dierkes, G.; Brüning, T.; Koch, H.M. Ubiquitous presence of paracetamol in human urine: Sources and implications. Reproduction 2014, 147, R105–R117. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Muñoz, D.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Concentration evolution of pharmaceutically active compounds in raw urban and industrial wastewater. Chemosphere 2014, 111, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Alemán, R.M.; Aceña, J.; Barceló, D.; de Alda, M.L. Study of pharmaceuticals in surface and wastewater from cuernavaca, morelos, mexico: Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, L.; Chang, A.C. Seasonal variation of endocrine disrupting compounds, pharmaceuticals and personal care products in wastewater treatment plants. Sci. Total Environ. 2013, 442, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two wwtp effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.-H.; Lin, A.Y.-C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Gioia, R.; Dachs, J. The riverine input–output paradox for organic pollutants. Front. Ecol. Environ. 2012, 10, 405–406. [Google Scholar] [CrossRef]
- Kunkel, U.; Radke, M. Fate of pharmaceuticals in rivers: Deriving a benchmark dataset at favorable attenuation conditions. Water Res. 2012, 46, 5551–5565. [Google Scholar] [CrossRef] [PubMed]
- Acuña, V.; von Schiller, D.; García-Galán, M.J.; Rodríguez-Mozaz, S.; Corominas, L.; Petrovic, M.; Poch, M.; Barceló, D.; Sabater, S. Occurrence and in-stream attenuation of wastewater-derived pharmaceuticals in iberian rivers. Sci. Total Environ. 2015, 503, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Writer, J.H.; Antweiler, R.C.; Ferrer, I.; Ryan, J.N.; Thurman, E.M. In-stream attenuation of neuro-active pharmaceuticals and their metabolites. Environ. Sci. Technol. 2013, 47, 9781–9790. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.J.; Herrera, S.; Solé, D.; García-Calvo, E.; Fernández-Alba, A.R. Spatio-temporal evaluation of organic contaminants and their transformation products along a river basin affected by urban, agricultural and industrial pollution. Sci. Total Environ. 2012, 420, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, H.; Adams, C.D.; Gamagedara, S.; Stayton, I.; Timmons, T.; Ma, Y. Investigation of pharmaceuticals in missouri natural and drinking water using high performance liquid chromatography-tandem mass spectrometry. Water Res. 2011, 45, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, E.; Brunsch, A.; Wunderlich-Pfeiffer, J.; Mertens, F.M. Monitoring micropollutants in the swist river basin. Water Sci. Technol. 2016, 74, 2280–2296. [Google Scholar] [CrossRef] [PubMed]
- Kickham, P.; Otton, S.; Moore, M.M.; Ikonomou, M.G.; Gobas, F.A. Relationship between biodegradation and sorption of phthalate esters and their metabolites in natural sediments. Environ. Toxicol. Chem. 2012, 31, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Riml, J.; Wörman, A.; Kunkel, U.; Radke, M. Evaluating the fate of six common pharmaceuticals using a reactive transport model: Insights from a stream tracer test. Sci. Total Environ. 2013, 458, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Nakamura, Y.; Moriguchi, S.; Nakamura, Y.; Honda, Y.; Tamura, I.; Hirata, Y.; Hayashi, A.; Sekizawa, J. Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: Laboratory photolysis, biodegradation, and sorption experiments. Water Res. 2009, 43, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Weigel, S.; Berger, U.; Jensen, E.; Kallenborn, R.; Thoresen, H.; Hühnerfuss, H. Determination of selected pharmaceuticals and caffeine in sewage and seawater from tromsø/norway with emphasis on ibuprofen and its metabolites. Chemosphere 2004, 56, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Weigel, S.; Bester, K.; Hühnerfuss, H. New method for rapid solid-phase extraction of large-volume water samples and its application to non-target screening of north sea water for organic contaminants by gas chromatography–mass spectrometry. J. Chromatogr. A 2001, 912, 151–161. [Google Scholar] [CrossRef]
- Kinney, C.A.; Furlong, E.T.; Werner, S.L.; Cahill, J.D. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ. Toxicol. Chem. 2006, 25, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, G. Pharmaceuticals in the environment. In Encyclopedia of Quantitative Risk Analysis and Assessment; Melnick, E.L., Everitt, B.S., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; Volume 3, ISBN 9780470035498. [Google Scholar]
- Thacker, P. Pharmaceutical data elude researchers. Environ. Sci. Technol. 2005, 39, 193A. [Google Scholar] [PubMed]
- Postigo, C.; Barceló, D. Synthetic organic compounds and their transformation products in groundwater: Occurrence, fate and mitigation. Sci. Total Environ. 2015, 503, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Chefetz, B.; Mualem, T.; Ben-Ari, J. Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere 2008, 73, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes—A review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.T.; Paxéus, N.; Giudice, R.L.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370. [Google Scholar] [CrossRef]
- Han, G.H.; Hur, H.G.; Kim, S.D. Ecotoxicological risk of pharmaceuticals from wastewater treatment plants in Korea: Occurrence and toxicity to daphnia magna. Environ. Toxicol. Chem. 2006, 25, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Dussault, E.B.; Balakrishnan, V.K.; Sverko, E.; Solomon, K.R.; Sibley, P.K. Toxicity of human pharmaceuticals and personal care products to benthic invertebrates. Environ. Toxicol. Chem. 2008, 27, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Jos, A.; Repetto, G.; Rios, J.C.; Hazen, M.J.; Molero, M.L.; del Peso, A.; Salguero, M.; Fernández-Freire, P.; Pérez-Martín, J.M.; Cameán, A. Ecotoxicological evaluation of carbamazepine using six different model systems with eighteen endpoints. Toxicol. In Vitro 2003, 17, 525–532. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Li, Z.-H.; Zlabek, V.; Velisek, J.; Grabic, R.; Machovaa, J.; Randaka, T. Physiological condition status and musclebased biomarkers in rainbow trout (Oncorhynchus mykiss), after long-term exposure to carbamazepine. J. Appl. Toxicol. 2009, 30, 197–203. [Google Scholar]
- Van den Brandhof, E.-J.; Montforts, M. Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol. Environ. Saf. 2010, 73, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.G.; Antunes, P.; Sibley, P.K.; Klironomos, J.N.; Solomon, K.R. Structural responses of daucus carota root-organ cultures and the arbuscular mycorrhizal fungus, glomus intraradices, to 12 pharmaceuticals. Chemosphere 2008, 73, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Gagné, F.; Blaise, C. An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian, hydra attenuata. Sci. Total Environ. 2008, 389, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.P.; Kime, D.E.; Van der Ven, L.T.; Wester, P.W.; Brion, F.; Maack, G.; Stahlschmidt-Allner, P.; Tyler, C.R. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ. Health Perspect. 2004, 112, 1725. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Vanderford, B.J.; Drewes, J.; Dickenson, E.; Snyder, E.M.; Bruce, G.M.; Pleus, R.C. State of Knowledge of Endocrine Disruptors and Pharmaceuticals in Drinking Water; IWA Publishing: London, UK, 2008; p. 264. ISBN 9781843392415. [Google Scholar]
- Chen, P.; Lin, J.-J.; Lu, C.-S.; Ong, C.-T.; Hsieh, P.F.; Yang, C.-C.; Tai, C.-T.; Wu, S.-L.; Lu, C.-H.; Hsu, Y.-C.; et al. Carbamazepine-induced toxic effects and HLA-B* 1502 screening in taiwan. N. Engl. J. Med. 2011, 364, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.A.; Mudgil, A.V.; Rosmarin, D.M. Toxic epidermal necrolysis. J. Am. Acad. Dermatol. 2007, 56, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Jentink, J.; Dolk, H.; Loane, A.M.; Morris, K.J.; Wellesley, D.; Garne, E.; de Jong-van den Berg, L. Intrauterine exposure to carbamazepine and specific congenital malformations: Systematic review and case-control study. Br. Med. J. 2010, 341, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.; Stewart, M.; Stevenson, M.; Morrow, J.; Nelson, J. Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch. Dis. Child. 2011, 96, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, E.D.; Brice-Bennett, S.; D’Souza, W.S. Antiepileptic medication during pregnancy: Does fetal genotype affect outcome? Pediatr. Res. 2007, 62, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Virkutyte, J.; Varma, R.S.; Jegatheesan, V. Treatment of Micropollutants in Water and Wastewater; IWA Publishing: London, UK, 2010; p. 520. ISBN 9781843393160. [Google Scholar]
- Lofgren, H.; Boer, R.D. Pharmaceuticals in Australia: Developments in regulation and governance. Soc. Sci. Med. 2004, 58, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- MDH. Carbamazepine in Drinking Water; Health Risk Assessment Unit, Environmental Health Division: St. Paul, MN, USA, 2011.
- Cunliffe, D. Australian Guidelines for Water Recycling: Augmentation of Drinking Water Supplies; Environment Protection and Heritage Council: Canberra, Australia; National Health and Medical Research Council: Canberra, Australia; Natural Resource Management Ministerial Council: Canberra, Australia, 2008. [Google Scholar]
- Garcia-Rodríguez, A.; Matamoros, V.; Fontàs, C.; Salvadó, V. The ability of biologically based wastewater treatment systems to remove emerging organic contaminants—A review. Environ. Sci. Pollut. Res. 2014, 21, 11708–11728. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, E.; EDDY, M. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Professional: New York, NY, USA, 2013; p. 2048. ISBN 9780073401188. [Google Scholar]
- Habib, R.; Asif, M.B.; Iftekhar, S.; Khan, Z.; Gurung, K.; Srivastava, V.; Sillanpää, M. Influence of relaxation modes on membrane fouling in submerged membrane bioreactor for domestic wastewater treatment. Chemosphere 2017, 181, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Urase, T.; Ngo, H.H.; Hu, J.; Ong, S.L. Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresour. Technol. 2013, 146, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fontaina, E.; Carballa, M.; Omil, F.; Lema, J. Modelling cometabolic biotransformation of organic micropollutants in nitrifying reactors. Water Res. 2014, 65, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (cas) and advanced membrane bioreactor (mbr) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Different fouling modes of submerged hollow-fiber and flat-sheet membranes induced by high strength wastewater with concurrent biofouling. Desalination 2005, 180, 89–97. [Google Scholar] [CrossRef]
- Radjenovic, J.; Petrovic, M.; Barceló, D. Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal. Bioanal. Chem. 2007, 387, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Kreuzinger, N.; Strenn, B.; Gans, O.; Kroiss, H. The solids retention time—A suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Gao, X.; Chen, Y.-P.; Peng, X.-Y.; Zhang, Y.-X.; Gan, X.-M.; Zi, C.-F.; Guo, J.-S. Occurrence, fate and ecotoxicological assessment of pharmaceutically active compounds in wastewater and sludge from wastewater treatment plants in chongqing, the three gorges reservoir area. Sci. Total Environ. 2014, 470, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Kannan, K. Occurrence and fate of select psychoactive pharmaceuticals and antihypertensives in two wastewater treatment plants in new york state, USA. Sci. Total Environ. 2015, 514, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Li, X.; Price, W.E.; Nghiem, L.D. Removal of carbamazepine and sulfamethoxazole by MBR under anoxic and aerobic conditions. Bioresour. Technol. 2011, 102, 10386–10390. [Google Scholar] [CrossRef] [PubMed]
- Tadkaew, N.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Nghiem, L.D. Removal of trace organics by mbr treatment: The role of molecular properties. Water Res. 2011, 45, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Habib, R.; Iftekhar, S.; Khan, Z.; Majeed, N. Optimization of the operational parameters in a submerged membrane bioreactor using box behnken response surface methodology: Membrane fouling control and effluent quality. Desalination 2017, 82, 26–38. [Google Scholar] [CrossRef]
- Jumat, M.R.; Hasan, N.A.; Subramanian, P.; Heberling, C.; Colwell, R.R.; Hong, P.-Y. Membrane bioreactor-based wastewater treatment plant in saudi arabia: Reduction of viral diversity, load, and infectious capacity. Water 2017, 9, 534. [Google Scholar] [CrossRef]
- Kimura, K.; Hara, H.; Watanabe, Y. Elimination of selected acidic pharmaceuticals from municipal wastewater by an activated sludge system and membrane bioreactors. Environ. Sci. Technol. 2007, 41, 3708–3714. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Barceló, D. First evidence for occurrence of hydroxylated human metabolites of diclofenac and aceclofenac in wastewater using QqLIT-MS and QqTOF-MS. Anal. Chem. 2008, 80, 8135–8145. [Google Scholar] [CrossRef] [PubMed]
- Cirja, M.; Ivashechkin, P.; Schäffer, A.; Corvini, P. Factors affecting the removal of organic micropollutants from wastewater in conventional treatment plants (CTP) and membrane bioreactors (MBR). Rev. Environ. Sci. Biotechnol. 2008, 7, 61–78. [Google Scholar] [CrossRef]
- Luo, W.; Hai, F.I.; Kang, J.; Price, W.E.; Guo, W.; Ngo, H.H.; Yamamoto, K.; Nghiem, L.D. Effects of salinity build-up on biomass characteristics and trace organic chemical removal: Implications on the development of high retention membrane bioreactors. Bioresour. Technol. 2015, 177, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Price, W.E.; Zhang, J.; Liang, S.; Deng, L.; Guo, W. Effect of hydraulic retention time on the performance of a hybrid moving bed biofilm reactor-membrane bioreactor system for micropollutants removal from municipal wastewater. Bioresour. Technol. 2018, 247, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Gurung, K.; Ncibi, M.C.; Sillanpää, M. Assessing membrane fouling and the performance of pilot-scale membrane bioreactor (mbr) to treat real municipal wastewater during winter season in nordic regions. Sci. Total Environ. 2017, 579, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hara, H.; Watanabe, Y. Removal of pharmaceutical compounds by submerged membrane bioreactors (mbrs). Desalination 2005, 178, 135–140. [Google Scholar] [CrossRef]
- Abegglen, C.; Joss, A.; McArdell, C.S.; Fink, G.; Schlüsener, M.P.; Ternes, T.A.; Siegrist, H. The fate of selected micropollutants in a single-house mbr. Water Res. 2009, 43, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Andersen, H.; Ternes, T.; Richle, P.R.; Siegrist, H. Removal of estrogens in municipal wastewater treatment under aerobic and anaerobic conditions: Consequences for plant optimization. Environ. Sci. Technol. 2004, 38, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.V.; Hai, F.I.; Kang, J.; Dam, H.K.; Zhang, R.; Price, W.E.; Broeckmann, A.; Nghiem, L.D. Simultaneous nitrification/denitrification and trace organic contaminant (troc) removal by an anoxic–aerobic membrane bioreactor (mbr). Bioresour. Technol. 2014, 165, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.; Lema, J.M.; Omil, F. Removal of pharmaceutical and personal care products (ppcps) under nitrifying and denitrifying conditions. Water Res. 2010, 44, 3214–3224. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hai, F.I.; Nghiem, L.D.; Price, W.E.; Roddick, F.; Moreira, M.T.; Magram, S.F. Understanding the factors controlling the removal of trace organic contaminants by white-rot fungi and their lignin modifying enzymes: A critical review. Bioresour. Technol. 2013, 141, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Hai, F.I.; Hou, J.; Price, W.E.; Nghiem, L.D. Impact of wastewater derived dissolved interfering compounds on growth, enzymatic activity and trace organic contaminant removal of white rot fungi—A critical review. J. Environ. Manag. 2017, 201, 89–109. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Development of a submerged membrane fungi reactor for textile wastewater treatment. Desalination 2006, 192, 315–322. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Hybrid treatment systems for dye wastewater. Crit. Rev. Environ. Sci. Technol. 2007, 37, 315–377. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Fukushi, K.; Nakajima, F. Fouling resistant compact hollow-fiber module with spacer for submerged membrane bioreactor treating high strength industrial wastewater. J. Membr. Sci. 2008, 317, 34–42. [Google Scholar] [CrossRef]
- Margot, J.; Maillard, J.; Rossi, L.; Barry, D.A.; Holliger, C. Influence of treatment conditions on the oxidation of micropollutants by trametes versicolor laccase. New Biotechnol. 2013, 30, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Hai, F.I.; Singh, L.; Price, W.E.; Nghiem, L.D. Degradation of pharmaceuticals and personal care products by white-rot fungi—A critical review. Curr. Pollut. Rep. 2017, 3, 88–103. [Google Scholar] [CrossRef]
- Golan-Rozen, N.; Chefetz, B.; Ben-Ari, J.; Geva, J.; Hadar, Y. Transformation of the recalcitrant pharmaceutical compound carbamazepine by pleurotus ostreatus: Role of cytochrome p450 monooxygenase and manganese peroxidase. Environ. Sci. Technol. 2011, 45, 6800–6805. [Google Scholar] [CrossRef] [PubMed]
- Rodarte-Morales, A.; Feijoo, G.; Moreira, M.; Lema, J. Degradation of selected pharmaceutical and personal care products (ppcps) by white-rot fungi. World J. Microbiol. Biotechnol. 2011, 27, 1839–1846. [Google Scholar] [CrossRef]
- Tran, N.H.; Urase, T.; Kusakabe, O. Biodegradation characteristics of pharmaceutical substances by whole fungal culture trametes versicolor and its laccase. J. Water Environ. Technol. 2010, 8, 125–140. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Yang, S.; Kang, J.; Leusch, F.D.L.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of pharmaceuticals, steroid hormones, phytoestrogens, uv-filters, industrial chemicals and pesticides by trametes versicolor: Role of biosorption and biodegradation. Int. Biodeterior. Biodegrad. 2014, 88, 169–175. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.; Feijoo, G.; Moreira, M.; Lema, J. Operation of stirred tank reactors (strs) and fixed-bed reactors (fbrs) with free and immobilized phanerochaete chrysosporium for the continuous removal of pharmaceutical compounds. Biochem. Eng. J. 2012, 66, 38–45. [Google Scholar] [CrossRef]
- Jelic, A.; Cruz-Morató, C.; Marco-Urrea, E.; Sarrà, M.; Perez, S.; Vicent, T.; Petrović, M.; Barcelo, D. Degradation of carbamazepine by trametes versicolor in an air pulsed fluidized bed bioreactor and identification of intermediates. Water Res. 2012, 46, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Hai, F.I.; Yang, S.; Kang, J.; Leusch, F.D.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of trace organic contaminants by an mbr comprising a mixed culture of bacteria and white-rot fungi. Bioresour. Technol. 2013, 148, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Kang, J.; Leusch, F.D.; Roddick, F.; van de Merwe, J.P.; Magram, S.F.; Nghiem, L.D. Degradation of a broad spectrum of trace organic contaminants by an enzymatic membrane reactor: Complementary role of membrane retention and enzymatic degradation. Int. Biodeterior. Biodegrad. 2015, 99, 115–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U. In vitro degradation of carbamazepine and diclofenac by crude lignin peroxidase. J. Hazard. Mater. 2010, 176, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; van de Merwe, J.P.; Hai, F.I.; Leusch, F.D.; Kang, J.; Price, W.E.; Roddick, F.; Magram, S.F.; Nghiem, L.D. Laccase–syringaldehyde-mediated degradation of trace organic contaminants in an enzymatic membrane reactor: Removal efficiency and effluent toxicity. Bioresour. Technol. 2016, 200, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Rodarte-Morales, A.; Feijoo, G.; Moreira, M.; Lema, J. Biotransformation of three pharmaceutical active compounds by the fungus phanerochaete chrysosporium in a fed batch stirred reactor under air and oxygen supply. Biodegradation 2012, 23, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hai, F.I.; Nghiem, L.D.; Roddick, F.; Price, W.E. Removal of trace organic contaminants by nitrifying activated sludge and whole-cell and crude enzyme extract of trametes versicolor. Water Sci. Technol. 2013, 67, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Ortiz, E.J.; Rene, E.R.; Pakshirajan, K.; van Hullebusch, E.D.; Lens, P.N. Fungal pelleted reactors in wastewater treatment: Applications and perspectives. Chem. Eng. J. 2016, 283, 553–571. [Google Scholar] [CrossRef]
- Mir-Tutusaus, J.; Sarrà, M.; Caminal, G. Continuous treatment of non-sterile hospital wastewater by trametes versicolor: How to increase fungal viability by means of operational strategies and pretreatments. J. Hazard. Mater. 2016, 318, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Mir-Tutusaus, J.A.; Parladé, E.; Llorca, M.; Villagrasa, M.; Barceló, D.; Rodriguez-Mozaz, S.; Martinez-Alonso, M.; Gaju, N.; Caminal, G.; Sarrà, M. Pharmaceuticals removal and microbial community assessment in a continuous fungal treatment of non-sterile real hospital wastewater after a coagulation-flocculation pretreatment. Water Res. 2017, 116, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Badia-Fabregat, M.; Lucas, D.; Pereira, M.A.; Alves, M.; Pennanen, T.; Fritze, H.; Rodríguez-Mozaz, S.; Barceló, D.; Vicent, T.; Caminal, G. Continuous fungal treatment of non-sterile veterinary hospital effluent: Pharmaceuticals removal and microbial community assessment. Appl. Microbiol. Biotechnol. 2016, 100, 2401–2415. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Morató, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barceló, D.; Vicent, T.; Sarrà, M.; Marco-Urrea, E. Hospital wastewater treatment by fungal bioreactor: Removal efficiency for pharmaceuticals and endocrine disruptor compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Goyal, P. Novel Biomaterials: Decontamination of Toxic Metals from Wasterwater; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.; Zhang, T.; Valero, J. Membrane processes for removal of pharmaceutically active compounds (phacs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Racar, M.; Dolar, D.; Špehar, A.; Košutić, K. Application of uf/nf/ro membranes for treatment and reuse of rendering plant wastewater. Process Saf. Environ. Prot. 2017, 105, 386–392. [Google Scholar] [CrossRef]
- Schulte-Herbrüggen, H.M.; Semião, A.J.; Chaurand, P.; Graham, M.C. Effect of ph and pressure on uranium removal from drinking water using nf/ro membranes. Environ. Sci. Technol. 2016, 50, 5817–5824. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Bellona, C.; Drewes, J.E.; Oelker, G.; Luna, J.; Filteau, G.; Amy, G. Comparing nanofiltration and reverse osmosis for drinking water augmentation. Am. Water Work. Assoc. J. 2008, 100, 102–116. [Google Scholar]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Hao, C. The rejection of endocrine disrupting and pharmaceutically active compounds by nf and ro membranes as a function of compound and water matrix properties. J. Membr. Sci. 2008, 313, 323–335. [Google Scholar] [CrossRef]
- Röhricht, M.; Krisam, J.; Weise, U.; Kraus, U.R.; Düring, R.-A. Elimination of carbamazepine, diclofenac and naproxen from treated wastewater by nanofiltration. CLEAN Soil Air Water 2009, 37, 638–641. [Google Scholar] [CrossRef]
- Gur-Reznik, S.; Koren-Menashe, I.; Heller-Grossman, L.; Rufel, O.; Dosoretz, C.G. Influence of seasonal and operating conditions on the rejection of pharmaceutical active compounds by ro and nf membranes. Desalination 2011, 277, 250–256. [Google Scholar] [CrossRef]
- Beier, S.; Köster, S.; Veltmann, K.; Schröder, H.; Pinnekamp, J. Treatment of hospital wastewater effluent by nanofiltration and reverse osmosis. Water Sci. Technol. 2010, 61, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Coleman, P.J.; Espendiller, C. Mechanisms underlying the effects of membrane fouling on the nanofiltration of trace organic contaminants. Desalination 2010, 250, 682–687. [Google Scholar] [CrossRef]
- Vogel, D.; Simon, A.; Alturki, A.A.; Bilitewski, B.; Price, W.E.; Nghiem, L.D. Effects of fouling and scaling on the retention of trace organic contaminants by a nanofiltration membrane: The role of cake-enhanced concentration polarisation. Sep. Purif. Technol. 2010, 73, 256–263. [Google Scholar] [CrossRef]
- Simon, A.; Price, W.E.; Nghiem, L.D. Effects of chemical cleaning on the nanofiltration of pharmaceutically active compounds (phacs). Sep. Purif. Technol. 2012, 88, 208–215. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Hawkes, S. Effects of membrane fouling on the nanofiltration of pharmaceutically active compounds (phacs): Mechanisms and role of membrane pore size. Sep. Purif. Technol. 2007, 57, 176–184. [Google Scholar] [CrossRef]
- Hajibabania, S.; Verliefde, A.; Drewes, J.E.; Nghiem, L.D.; McDonald, J.; Khan, S.; Le-Clech, P. Effect of fouling on removal of trace organic compounds by nanofiltration. Drink. Water Eng. Sci. 2011, 4, 117–149. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Andreozzi, R.; Napolitano, A.; D’Ischia, M. Kinetic and chemical assessment of the uv/h2o2 treatment of antiepileptic drug carbamazepine. Chemosphere 2004, 54, 497–505. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M. The influence of natural organic matter and cations on the rejection of endocrine disrupting and pharmaceutically active compounds by nanofiltration. Water Res. 2009, 43, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Kårelid, V.; Larsson, G.; Björlenius, B. Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manag. 2017, 193, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Skouteris, G.; Saroj, D.; Melidis, P.; Hai, F.I.; Ouki, S. The effect of activated carbon addition on membrane bioreactor processes for wastewater treatment and reclamation—A critical review. Bioresour. Technol. 2015, 185, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Real, F.J.; Benitez, F.J.; Acero, J.L.; Casas, F. Adsorption of selected emerging contaminants onto pac and gac: Equilibrium isotherms, kinetics, and effect of the water matrix. J. Environ. Sci. Health Part A 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kovalova, L.; Siegrist, H.; Von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-J.; Kim, S.-G.; Kim, S.-H. Removal of antibiotics by coagulation and granular activated carbon filtration. J. Hazard. Mater. 2008, 151, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Rossner, A.; Snyder, S.A.; Knappe, D.R. Removal of emerging contaminants of concern by alternative adsorbents. Water Res. 2009, 43, 3787–3796. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Meisenheimer, M.; McDowell, D.; Sacher, F.; Brauch, H.-J.; Haist-Gulde, B.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of pharmaceuticals during drinking water treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Pojana, G.; Fantinati, A.; Marcomini, A. Occurrence of environmentally relevant pharmaceuticals in italian drinking water treatment plants. Int. J. Environ. Anal. Chem. 2011, 91, 537–552. [Google Scholar] [CrossRef]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Grover, D.P.; Zhou, J.L.; Frickers, P.E.; Readman, J.W. Improved removal of estrogenic and pharmaceutical compounds in sewage effluent by full scale granular activated carbon: Impact on receiving river water. J. Hazard. Mater. 2011, 185, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.; Lema, J.M.; Omil, F. Influence of the employment of adsorption and coprecipitation agents for the removal of ppcps in conventional activated sludge (cas) systems. Water Sci. Technol. 2010, 62, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Meinel, F.; Ruhl, A.; Sperlich, A.; Zietzschmann, F.; Jekel, M. Pilot-scale investigation of micropollutant removal with granular and powdered activated carbon. Water Air Soil Pollut. 2015, 226, 2260. [Google Scholar] [CrossRef]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Lipp, P.; Groß, H.-J.; Tiehm, A. Improved elimination of organic micropollutants by a process combination of membrane bioreactor (mbr) and powdered activated carbon (pac). Desalin. Water Treat. 2012, 42, 65–72. [Google Scholar] [CrossRef]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J. Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Nevskaia, D.; Guerrero-Ruiz, A. Comparative study of the adsorption from aqueous solutions and the desorption of phenol and nonylphenol substrates on activated carbons. J. Colloid Interface Sci. 2001, 234, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Nghiem, L.D.; Price, W.E.; Guo, W.; Ngo, H.H.; Tung, K.-L. Comparison between sequential and simultaneous application of activated carbon with membrane bioreactor for trace organic contaminant removal. Bioresour. Technol. 2013, 130, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hai, F.I.; Nghiem, L.D. Simultaneous activated carbon adsorption within a membrane bioreactor for an enhanced micropollutant removal. Bioresour. Technol. 2011, 102, 5319–5324. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Leal, L.; Temmink, H.; Zeeman, G.; Buisman, C. Removal of micropollutants from aerobically treated grey water via ozone and activated carbon. Water Res. 2011, 45, 2887–2896. [Google Scholar] [CrossRef] [PubMed]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.K.; Price, W.E. Degradation and fate of pharmaceutically active contaminants by advanced oxidation processes. Curr. Pollut. Rep. 2017, 3, 268–280. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Pinto, G.; Pollio, A. Carbamazepine in water: Persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res. 2002, 36, 2869–2877. [Google Scholar] [CrossRef]
- Hua, W.; Bennett, E.R.; Letcher, R.J. Ozone treatment and the depletion of detectable pharmaceuticals and atrazine herbicide in drinking water sourced from the upper detroit river, ontario, canada. Water Res. 2006, 40, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Rosario-Ortiz, F.L.; Snyder, S.A. Effect of ozone exposure on the oxidation of trace organic contaminants in wastewater. Water Res. 2009, 43, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Buffle, M.-O.; Schumacher, J.; Salhi, E.; Jekel, M.; von Gunten, U. Measurement of the initial phase of ozone decomposition in water and wastewater by means of a continuous quench-flow system: Application to disinfection and pharmaceutical oxidation. Water Res. 2006, 40, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.K.; Kang, J.; Nghiem, L.D.; van de Merwe, J.P.; Leusch, F.D.; Price, W.E. Photolysis and UV/H2O2 of diclofenac, sulfamethoxazole, carbamazepine, and trimethoprim: Identification of their major degradation products by ESI–LC–MS and assessment of the toxicity of reaction mixtures. Process Saf. Environ. Prot. 2017, 112, 222–234. [Google Scholar] [CrossRef]
- Im, J.-K.; Cho, I.-H.; Kim, S.-K.; Zoh, K.-D. Optimization of carbamazepine removal in O3/UV/H2O2 system using a response surface methodology with central composite design. Desalination 2012, 285, 306–314. [Google Scholar] [CrossRef]
- Monteagudo, J.; Durán, A.; González, R.; Expósito, A. In situ chemical oxidation of carbamazepine solutions using persulfate simultaneously activated by heat energy, UV light, Fe2+ ions, and H2O2. Appl. Catal. B Environ. 2015, 176, 120–129. [Google Scholar] [CrossRef]
- Shirazi, E.; Torabian, A.; Nabi-Bidhendi, G. Carbamazepine removal from groundwater: Effectiveness of the Tio2/UV, nanoparticulate zero-valent iron, and fenton (NZVI/H2O2) processes. CLEAN Soil Air Water 2013, 41, 1062–1072. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Fu, W.; Shang, C.; Li, Y.; Chen, Y.; Gan, W.; Fang, J. Ppcp degradation by uv/chlorine treatment and its impact on dbp formation potential in real waters. Water Res. 2016, 98, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Ortiz, F.L.; Wert, E.C.; Snyder, S.A. Evaluation of UV/H2O2 treatment for the oxidation of pharmaceuticals in wastewater. Water Res. 2010, 44, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Sichel, C.; Garcia, C.; Andre, K. Feasibility studies: UV/chlorine advanced oxidation treatment for the removal of emerging contaminants. Water Res. 2011, 45, 6371–6380. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.J.; Linden, K.G.; Weinberg, H.S. Evaluation of uv irradiation for photolytic and oxidative degradation of pharmaceutical compounds in water. Water Res. 2007, 41, 4413–4423. [Google Scholar] [CrossRef] [PubMed]
- Doll, T.E.; Frimmel, F.H. Photocatalytic degradation of carbamazepine, clofibric acid and iomeprol with p25 and hombikat UV100 in the presence of natural organic matter (nom) and other organic water constituents. Water Res. 2005, 39, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, A.; Palacios, S.; Vicente, R.; Vercher, R.F.; Malato, S.; Arques, A.; Amat, A.M. Solar photo-fenton at mild conditions to treat a mixture of six emerging pollutants. Chem. Eng. J. 2012, 198–199, 65–72. [Google Scholar] [CrossRef]
- Klamerth, N.; Rizzo, L.; Malato, S.; Maldonado, M.I.; Agüera, A.; Fernández-Alba, A.R. Degradation of fifteen emerging contaminants at μg/L initial concentrations by mild solar photo-fenton in mwtp effluents. Water Res. 2010, 44, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zheng, M.; Sijak, S.; Tang, L.; Xu, G.; Wu, M. Aquatic photolysis of carbamazepine by UV/H2O2 and UV/Fe(ii) processes. Res. Chem. Interm. 2015, 41, 7015–7028. [Google Scholar] [CrossRef]
- Dai, C.-m.; Zhou, X.-F.; Zhang, Y.-L.; Duan, Y.-P.; Qiang, Z.-M.; Zhang, T.C. Comparative study of the degradation of carbamazepine in water by advanced oxidation processes. Environ. Technol. 2011, 33, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef] [PubMed]
- Reungoat, J.; Macova, M.; Escher, B.; Carswell, S.; Mueller, J.; Keller, J. Removal of micropollutants and reduction of biological activity in a full scale reclamation plant using ozonation and activated carbon filtration. Water Res. 2010, 44, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar]
- Hai, F.I.; Nguyen, L.N.; Nghiem, L.D.; Liao, B.-Q.; Koyuncu, I.; Price, W.E. Trace organic contaminants removal by combined processes for wastewater reuse. In Advanced Treatment Technologies for Urban Wastewater Reuse; Fatta-Kassinos, D., Dionysiou, D.D., Kümmerer, K., Eds.; Springer: Cham, Switzerland, 2014; pp. 39–77. ISBN 9783319238869. [Google Scholar]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Removal of emerging trace organic contaminants by mbr-based hybrid treatment processes. Int. Biodeterior. Biodegrad. 2013, 85, 474–482. [Google Scholar] [CrossRef]
- Laera, G.; Chong, M.N.; Jin, B.; Lopez, A. An integrated MBR–Tio2 photocatalysis process for the removal of carbamazepine from simulated pharmaceutical industrial effluent. Bioresour. Technol. 2011, 102, 7012–7015. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J. Carbamazepine degradation by gamma irradiation coupled to biological treatment. J. Hazard. Mater. 2017, 321, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Ivancev-Tumbas, I.; Hobby, R. Removal of organic xenobiotics by combined out/in ultrafiltration and powdered activated carbon adsorption. Desalination 2010, 255, 124–128. [Google Scholar] [CrossRef]
- Serrano, D.; Suárez, S.; Lema, J.M.; Omil, F. Removal of persistent pharmaceutical micropollutants from sewage by addition of pac in a sequential membrane bioreactor. Water Res. 2011, 45, 5323–5333. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Removal of trace organic contaminants by a membrane bioreactor–granular activated carbon (mbr–gac) system. Bioresour. Technol. 2012, 113, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Hübner, U.; Seiwert, B.; Reemtsma, T.; Jekel, M. Ozonation products of carbamazepine and their removal from secondary effluents by soil aquifer treatment–indications from column experiments. Water Res. 2014, 49, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Kleywegt, S.; Pileggi, V.; Yang, P.; Hao, C.; Zhao, X.; Rocks, C.; Thach, S.; Cheung, P.; Whitehead, B. Pharmaceuticals, hormones and bisphenol a in untreated source and finished drinking water in Ontario, Canada—Occurrence and treatment efficiency. Sci. Total Environ. 2011, 409, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Martin, H.M.; Arce-Bulted, O.; Sugihara, M.N.; Keating, K.A.; Strathmann, T.J. Oxidation of carbamazepine by Mn(vii) and Fe(vi): Reaction kinetics and mechanism. Environ. Sci. Technol. 2008, 43, 509–515. [Google Scholar] [CrossRef]
- Kosjek, T.; Andersen, H.R.; Kompare, B.; Ledin, A.; Heath, E. Fate of carbamazepine during water treatment. Environ. Sci. Technol. 2009, 43, 6256–6261. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.; Borea, L.; Cesaro, A.; Liu, H.; Naddeo, V.; Belgiorno, V.; Ballesteros, F. Removal of emerging contaminant and fouling control in membrane bioreactors by combined ozonation and sonolysis. Int. Biodeterior. Biodegrad. 2017, 119, 577–586. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Pre-treatment of hospital wastewater by coagulation-flocculation and flotation. Bioresour. Technol. 2009, 100, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Removal of pharmaceuticals in drinking water treatment: Effect of chemical coagulation. Environ. Technol. 2006, 27, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.M.; Härkki, H.; Tuhkanen, T.; Kronberg, L. Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environ. Sci. Technol. 2007, 41, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Gonzales, S.; Dong, M.M.; Rosario-Ortiz, F.L. Evaluation of enhanced coagulation pretreatment to improve ozone oxidation efficiency in wastewater. Water Res. 2011, 45, 5191–5199. [Google Scholar] [CrossRef] [PubMed]
- Ciardelli, G.; Ranieri, N. The treatment and reuse of wastewater in the textile industry by means of ozonation and electroflocculation. Water Res. 2001, 35, 567–572. [Google Scholar] [CrossRef]
- McDowell, D.C.; Huber, M.M.; Wagner, M.; von Gunten, U.; Ternes, T.A. Ozonation of carbamazepine in drinking water: Identification and kinetic study of major oxidation products. Environ. Sci. Technol. 2005, 39, 8014–8022. [Google Scholar] [CrossRef] [PubMed]
- Marco-Urrea, E.; Radjenović, J.; Caminal, G.; Petrović, M.; Vicent, T.; Barceló, D. Oxidation of atenolol, propranolol, carbamazepine and clofibric acid by a biological fenton-like system mediated by the white-rot fungus trametes versicolor. Water Res. 2010, 44, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.-Z.; Zhao, P.; Dalvie, D.K.; Pool, W.F. Identification of primary and sequential bioactivation pathways of carbamazepine in human liver microsomes using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, M.; Mathieu, O.; Gomez, E.; Casellas, C.; Fenet, H.; Hillaire-Buys, D. Presence and fate of carbamazepine, oxcarbazepine, and seven of their metabolites at wastewater treatment plants. Arch. Environ. Contam. Toxicol. 2009, 56, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Brar, S.K.; Tyagi, R.D.; Picard, P.; Surampalli, R.Y. A comparative study of ultrasonication, fenton’s oxidation and ferro-sonication treatment for degradation of carbamazepine from wastewater and toxicity test by yeast estrogen screen (yes) assay. Sci. Total Environ. 2013, 447, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Vom Eyser, C.; Börgers, A.; Richard, J.; Dopp, E.; Janzen, N.; Bester, K.; Tuerk, J. Chemical and toxicological evaluation of transformation products during advanced oxidation processes. Water Sci. Technol. 2013, 68, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fenet, H.; Gomez, E.; Chiron, S. Transformation of the antiepileptic drug oxcarbazepine upon different water disinfection processes. Water Res. 2011, 45, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
| Structure |  |
| Formula | C15H12N2O |
| CAS No. | 298-46-4 |
| Molecular Weight | 236.2686 g·mol−1 |
| Usage | Anticonvulsant/mood stabilizing drug |
| Water solubility | 17.7 mg/L (25 °C) |
| Log P (octanol-water partition coefficient) | 2.45 |
| Log D at pH = 7 a | 1.32 |
| Henry’s Law Constant | 1.09 × 10−5 Pa·m3·mol−1 (25 °C) |
| Half-life (t1/2) | 25–65 h |
| Excretion | 72% absorbed and metabolized in liver, 28% excreted in feces |
| Metabolites in urine | CBZ, CBZ-epoxide, CBZ-diol, CBZ-acridan, 2-OH-CBZ, 3-OH-CBZ |
| Dosage | 800–1200 mg/day |
| Other information | Autoinduction i.e., induces its own metabolism during continued intake |
| Country | W WWTP Effluent | Surface Water | Groundwater | |
|---|---|---|---|---|
| No. of WWTPs * | Concentration (ng/L) | Concentration (ng/L) | Concentration (ng/L) | |
| Canada | 7 | 33–426 [a] | 0.7–126 [b] | 10–49 [c] |
| Germany | 5 | 1075–6300 [d] | 81–1100 [e] | 1–100 [f] |
| Japan | 20 | 81–86 [g] | 0.1–34.7 [h] | 1.64–97 [i] |
| South Korea | 11 | 73–729 [j] | 6–61 [k] | NA |
| Taiwan | 4 | 290–960 [l] | 0.5–120 [m] | NA |
| UK | 3 | 152–4596 [n] | 9–327 [o] | 425–3600 [p] |
| USA | 16 | 33–270 [q] | 2–172 [r] | 1.5–42 [s] |
| Country | WWTP Effluent | Consumption Rate (tons/year) | ||
|---|---|---|---|---|
| No. of WWTPs* | Concentration (ng/L) | |||
| Winter | Summer | |||
| Australia | 3 | 1480 [70] | NA | 10 [16] |
| Austria | 11 | 952 [71] | 1337–1594 [3,71] | 6 [16] |
| 11 | 1000 [72] | 1500 [72] | ||
| Canada | 4 | 426 [32] | 300 [33] | 28 [16] |
| Finland | 12 | 500 [6] | NA | 4.8 [16] |
| 3 | 380–470 [73] | NA | ||
| Germany | 3 | 1900 [38] | 2100 [40] | 76 [16] |
| Korea | 4 | 103–195 [48] | 5–6 [48] | 9.2 [48] |
| Switzerland | 2 | 1000 [74] | 950 [74] | 4.1 [75] |
| 3 | 400–800 [26] | 200–600 [26] | ||
| UK | 3 (Winter) 2 (Summer) | 637–950 [56] | 2499 [54] | 40 [16] |
| Species | Critical Effects | Exposure Time | LC50 (mg/L) | EC50 (mg/L) | NOEC (mg/L) | LOEC (mg/L) | References |
|---|---|---|---|---|---|---|---|
| Water flea | |||||||
| Daphnia magna | Mortality | 2 days | 111 | - | - | - | [101] |
| Chironomus dilutus | Survival | 10 days | 47.3 | 10.2 | - | - | [102] |
| Hyalella azteca | Growth | 10 days | 1.5 | 9.5 | - | - | [102] |
| Brachionus calyciflorus | Reproduction inhibition | 2 days | - | - | 0.4 | 0.8 | [100] |
| Ceriodaphnia dubia | Reproduction inhibition | 7 days | - | - | 0.025 | 0.1 | [100] |
| Daphnia magna | Mobility inhibition | 2 days | - | 77.7 | - | - | [100] |
| Daphnia magna | Immobilization | 2 days | - | 97.8 | - | - | [103] |
| Bacteria | |||||||
| Aliivibrio fischeri | Bioluminescence | 30 min | - | 64.2 | - | - | [100] |
| Vibrio fischeri | Bioluminescence | 5 min | - | 87.4 | - | - | [103] |
| Algae | |||||||
| Chlorella vulgaris | Growth inhibition | 24 h | - | 110.9 | - | - | [103] |
| Desmodesmus subspicatus | Inhibition of average growth rate | 3 days | - | 74 | - | - | [104] |
| Raphidocelis subcapitata | Growth inhibition | 4 days | 89 | >100 | >100 | [100] | |
| Rainbow trout | |||||||
| Oncorhynchus mykiss | Condition factor | 42 days | - | - | - | 2 | [105] |
| Oncorhynchus mykiss | Antioxidant responses in muscle | 42 days | - | - | - | 0.001 | [105] |
| Oncorhynchus mykiss | Changes in RNA-DNA ratio | 42 days | - | - | - | 2 | [105] |
| Zebrafish | |||||||
| Danio rerio | Developmental effects | 3 days | >245 | 85.6 | 30.6 | - | [106] |
| Danio rerio | Embryos and larvae mortality | 10 days | - | - | 25 | 50 | [100] |
| Mycorrhizal fungus | |||||||
| Glomus intraradices | Spore production | 28 days | - | 0.1 | - | - | [107] |
| Duckweed | |||||||
| Lemna minor | Inhibition of average growth rate | 7 days | - | 25.5 | - | - | [104] |
| Cnidarian | |||||||
| Hydra attenuate | Morphological changes | 4 days | 29.4 | 15.5 | 1 | 5 | [108] |
| Process Type | Influent Concentration (ng/L) | SRT (days) | HRT (h) | Aerobic/Anoxic | Removal (%) | References |
|---|---|---|---|---|---|---|
| CAS | 240 | 3 | 12 | aerobic | negligible | [128] |
| 156 | 10 | 11.5 | both | negligible | [126] | |
| 350–1850 | 52–114 | 12.5–13.6 | both | negligible | [71] | |
| 350 | 2–20 | 1.5–20 | both | negligible | [6] | |
| 15–270 | 3.8–8.4 | 7.1–9.4 | aerobic | negligible–80 | [129] | |
| 1000 | 10 | 7.3 | both | negligible | [74] | |
| 670–704 | 19 | NA | aerobic | 0 | [130] | |
| 10–20 | 11–15 | 9–17 | both | negligible–25 | [131] | |
| 200–600 | 15–25 | 16–24 | aerobic | negligible | [132] | |
| MBR | 240 | infinite | 14 | aerobic | negligible | [128] |
| 156 | >60 | 15 | aerobic | negligible | [126] | |
| 156 | >60 | 7.2 | aerobic | negligible | [126] | |
| 704–1850 | 10–55 | 0.5–4 | both | negligible | [71] | |
| 1000 | 16 | 13 | both | 25 | [74] | |
| 704–1200 | 22 | NA | both | negligible | [130] | |
| 750,000 | infinite | 24 | near-anoxic | 68 | [133] | |
| 5 | 88 | 26 | aerobic | 40 | [27] | |
| 1400 | 70 | 24 | aerobic | 10 | [134] |
| Bioreactor Type | WRF Species/Enzyme Type | HRT (h)/Incubation Time (days) | Initial Concentration (ng/L) | Removal Efficiency (%) | References |
|---|---|---|---|---|---|
| Removal by whole-cell WRF | |||||
| Stirred tank (batch) | Bjerkandera sp. R1 | 14 | 1,000,000 | 99 | [156] |
| Stirred tank (batch) | B. adusta (Laccase, LiP and MnP) | 14 | 1,000,000 | 99 | [156] |
| Stirred tank (batch) | T. versicolor (Laccase, LiP and MnP) | 2 | 10,000 | 75 | [157] |
| Stirred tank (batch) | T. versicolor (Laccase, LiP and MnP) | 1 | 100,000 | 2 | [158] |
| Stirred tank (batch) | P. ostreatus (Florida N001) (Laccase, MnP) | 32 | 1000 | 50 | [155] |
| Stirred tank (batch) | P. ostreatus (Florida F6) (Laccase, MnP) | 32 | 1000 | 60 | [155] |
| Stirred tank (batch) | P. ostreatus (PC9) (Laccase, MnP) | 32 | 1000 | 99 | [155] |
| Stirred tank a (continuous) | P. chrysosporium (MnP, LiP) | 24 | 500,000 | 25–60 | [159] |
| Fluidized bed a (continuous) | T. versicolor (Laccase, MnP, LiP) | 72 | 200,000 | 95.6 | [160] |
| Membrane bioreactor (continuous) a | T. versicolor (Laccase, MnP, LiP) | 48 | 5000 | 20 | [161] |
| Removal by extracellular enzymes | |||||
| Stirred tank (batch) | Laccase from A. oryzae | 1 | 100,000 | <5 | [162] |
| Stirred tank (batch) | Laccase from T. versicolor | 2 | 10,000 | 5 | [157] |
| Stirred tank (batch) | LiP from P. chrysosporium | 4 | NA | 10–15 | [163] |
| Membrane bioreactor (continuous) | Laccase from A. oryzae | 8 | 5000 | <5 | [162] |
| Membrane bioreactor (continuous) | Laccase from A. oryzae | 8 | 5000 | 7 | [164] |
| Membrane Type (Pore Size) | Configuration | Water Matrix | Initial CBZ Concentration (ng/L) | Applied Pressure (psi) | Removal (%) | References |
|---|---|---|---|---|---|---|
| NF (0.34 nm) | Flat-sheet | Groundwater | 84.5 | NA | >98 | [176] |
| NF (0.27 nm) | Flat-sheet (tight) | MBR permeate | 150 | 150 | 97.3 ± 0.6 | [178] |
| NF (0.42 nm) | Flat-sheet (loose) | MBR permeate | 150 | 75 | 71.2 ± 3.1 | [178] |
| NF | Flat-sheet | WWTP effluent | 500–850 | 4.35 | 6 | [179] |
| NF | Flat-sheet | WWTP effluent | 500–850 | 10 | 8 | [179] |
| NF (0.84 nm) | Flat-sheet | Primary effluent | 2000 | 72 | 74 | [180] |
| NF | Spiral-wound | Hospital wastewater | 1000 | 98 | 88 | [181] |
| NF (0.84 nm) | Flat-sheet | Synthetic wastewater | 750,000 | 261 | 80 a, 60 b | [182] |
| NF (0.68 nm) | Flat-sheet | Synthetic wastewater | 750,000 | 261 | 95 a, 90 b | [182] |
| NF (0.84 nm) | Flat-sheet | Synthetic wastewater | 750,000 | 986 | 70 a, 20 b | [183] |
| NF (0.84 nm) | Flat-sheet | Synthetic wastewater | 750,000 | 261 | 80 a, 90 b | [184] |
| RO (0.34 nm) | Flat-sheet | Groundwater | 84.5 | NA | >98 | [176] |
| RO | Flat-sheet | MBR permeate | 150 | 250 | 91 ± 8.4 | [178] |
| RO | Flat-sheet | MBR permeate | 150 | 150 | 97.9 ± 1.5 | [178] |
| RO | Flat-sheet | Primary effluent | 1000 | 72 | 100 | [180] |
| RO | Spiral-wound module | Hospital wastewater | 1000 | 196 | 99 | [181] |
| AC Type | Water Matrix | Initial CBZ concentration (ng/L) | Contact Time (min) | Removal (%) | References |
|---|---|---|---|---|---|
| GAC | Synthetic wastewater | 1000 | 1440 | 99–80 a | [197] |
| Ozonation effluent | 36 | – | 88 b | [198] | |
| Groundwater | 9 | 15 | >75 c | [199] | |
| Surface water | 25 | – | 99 | [200] | |
| Disinfected surface water | 600 | 1.5–3 | 79 d | [201] | |
| WWTP effluent | 67 | – | 30 e | [202] | |
| GAC added to an activated sludge based bioreactor | 22,000 | 1440 | 43 f | [203] | |
| WWTP effluent | 4000 | 100 | 80 g | [204] | |
| WWTP effluent | 30–100 | 130 | >99 | [190] | |
| PAC | Surface water | 78 | 300 | 95 h | [205] |
| Surface water | 78 | 300 | 36 i | [205] | |
| MBR permeate | 1000 | 30 | 99.4 j | [206] | |
| Surface water | 50 | 240 | 80 | [207] | |
| WWTP effluent | 30–100 | 20–40 | 95–100 | [190] |
| AOP Type | CBZ Initial Concentration (ng/L) | Operating Conditions | Removal (%) | References |
|---|---|---|---|---|
| Ozonation | 1000 | Dose = 0.5 mg/L Contact time = 20 min | >99 | [197] |
| 35 | Dose = 1-1.5 mg/L Contact time = 10 min | >97 | [197] | |
| 9 | Dose = 0.2 mg/L Contact time = 15 min | >99 | [199] | |
| 8 × 105 | Dose = 1 mg/L Contact time = 10 min | >99 | [215] | |
| 3.8 | Dose = 1.5–2 mg/L Contact time = 20 min | 80–99 | [216] | |
| 1.18 × 105 | Dose = 0.1–2 mg/L Contact time = 10 h | 80–99 | [217] | |
| 170 | Dose = 0.8 mg/L Contact time = 24 min | 100 | [218] | |
| 4.72 × 105 | Dose = 1.5–4 mg/L Contact time = 20 min | >99 | [219] | |
| UV alone | 5000 | UV Wavelength = 254 nm Energy output = 83 W Irradiation time = 60 min | 20 | [220] |
| NA | UV Wavelength = 200–280 nm Energy output = 120 W Irradiation time = NA | 7 | [221] | |
| 1.5 × 107 | UV Wavelength = 254 nm Energy output = 220 W Irradiation time = 2 h | 16 | [222] | |
| 5 × 106 | UV Wavelength = 254 nm Energy output = 400 W Irradiation time = 30 min | <5 | [223] | |
| 19–59 | UV Wavelength = 254 nm Energy output = 10 W Irradiation time = 3 min | <10 | [224] | |
| UV/H2O2 | 4.72 × 106 | UV Wavelength = 254 nm Energy output = 83 W H2O2 dose = 170 mg/L Irradiation time = 60 min | 90 | [187] |
| 210 | UV Wavelength = 254 nm Energy output = 30 W H2O2 dose = 2–20 mg/L Irradiation time = 20 min | 14–74 | [225] | |
| 1000 | UV Wavelength = 254 nm Energy output = 20 W H2O2 dose = 5 mg/L Irradiation time = 15 min | 60 | [226] | |
| 50,000 | UV Wavelength = 254 nm Energy output = 83 W H2O2 dose: 10–200 mg/L Irradiation time = 60 min | 90–99 | [220] | |
| 19–59 | UV Wavelength = 254 nm Energy output = 10 W H2O2 dose = 5 mg/L Irradiation time = 3 min | 20 | [224] | |
| 2.36 × 105 | UV Wavelength = 254 nm Energy output = 1000 W H2O2 dose = 5 mg/L Irradiation time = NA | 90 | [227] | |
| UV/Cl2 | 1000 | UV Wavelength = 254 nm Energy output = 80 W Cl2 dose = 1 mg/L Irradiation time = 20 min | 55 | [226] |
| 19–59 | UV Wavelength = 254 nm Energy output = 10 W Cl2 dose = 5 mg/L Irradiation time = 1.5 min | 30–60 | [224] | |
| 19–59 | UV Wavelength = 254 nm Energy output = 10 W Cl2 dose = 3 mg/L Irradiation time = 3 min | 50 | [224] | |
| UV/TiO2 | 4 × 106 | UV Wavelength = 200–296 nm Energy output = 1000 W TiO2 dose = 100 mg/L Irradiation time = 9 min | 99 | [228] |
| 5 × 106 | UV Wavelength = 254 nm Energy output = 250 W TiO2 dose = 20–500 mg/L Irradiation time = 30 min | 80–98 | [223] | |
| 5 × 106 | UV Wavelength = 254 nm Energy output = 400 W TiO2 dose = 20–500 mg/L Irradiation time = 30 min | 90–99 | [223] | |
| Photo-Fenton | 5 × 107 | UV Wavelength = 254 nm Energy output = 400 W Fe2+ dose = 5 mg/L Irradiation time = 15 min | >99 | [229] |
| 1 × 105 | UV Wavelength = 200–296 nm Energy output = 30 W Fe2+ dose = 5 mg/L Irradiation time = 1.5 h | 95 | [230] |
| Treatment Systems | CBZ Initial Concentration (ng/L) | Operating Conditions | Removal (%) | References |
|---|---|---|---|---|
| Integrated MBR-UV/TiO2 | 1 × 107 | SRT = 60 d HRT = 50 h UV wavelength = 360 nm TiO2 dose = NA | up to 95 | [238] |
| Integrated MBR-PAC | 5000 | SRT = infinite HRT = 24 h PAC dose = 0.1 g/L | 50 | [210] |
| Integrated MBR-PAC | 5000 | SRT = infinite HRT = 24 h PAC dose = 0.5 g/L | 90 | [210] |
| Integrated Gamma radiation-CAS (batch experiment) | 1.7 × 107 | Incubation time = 10 d Radiation dose = 800 Gy | >99 | [239] |
| Integrated PAC-UF | 3 × 105 | PAC dose = 5–10 mg/L Contact time = 1.5 h | 40 | [240] |
| Integrated MBR-PAC | 7.5 × 105 | SRT = infinite HRT = 24 h PAC dose = 0.1 and 1 g/L | 34 and 90 | [211] |
| Integrated MBR-PAC | 2 × 104 | SRT = infinite HRT = 24 h PAC dose = 1 g/L | >99 | [241] |
| Integrated MBR-PAC | 390–1800 | SRT = 20 d HRT = 24 h PAC dose = 1 g/L | 99.4 | [206] |
| Integrated CAS-GAC | 2 × 104 | SRT = NA HRT = NA GAC dose = 100–1000 mg/L | 10–50 | [203] |
| MBR followed by GAC | 5000 | SRT of MBR = infinite HRT of MBR = 24 h GAC contact time = 7 min | 98 | [242] |
| Ozonation followed by biological sand column | 2.06 | HRT of sand column = 5–6 d Ozone contact time = NA | 80 | [243] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.-Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107. https://doi.org/10.3390/w10020107
Hai FI, Yang S, Asif MB, Sencadas V, Shawkat S, Sanderson-Smith M, Gorman J, Xu Z-Q, Yamamoto K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water. 2018; 10(2):107. https://doi.org/10.3390/w10020107
Chicago/Turabian StyleHai, Faisal I., Shufan Yang, Muhammad B. Asif, Vitor Sencadas, Samia Shawkat, Martina Sanderson-Smith, Jody Gorman, Zhi-Qiang Xu, and Kazuo Yamamoto. 2018. "Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies" Water 10, no. 2: 107. https://doi.org/10.3390/w10020107
APA StyleHai, F. I., Yang, S., Asif, M. B., Sencadas, V., Shawkat, S., Sanderson-Smith, M., Gorman, J., Xu, Z.-Q., & Yamamoto, K. (2018). Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water, 10(2), 107. https://doi.org/10.3390/w10020107








