Enhancing the Adsorption of Lead (II) by Bentonite Enriched with pH-Adjusted Meranti Sawdust
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical and Chemical Properties of the Adsorbents
2.1.1. Na-Bentonite
2.1.2. Sawdust
2.1.3. Alkali and Acidic Treatment of Sawdust
2.2. Sample Preparation and Experimental Methods
3. Results and Discussion
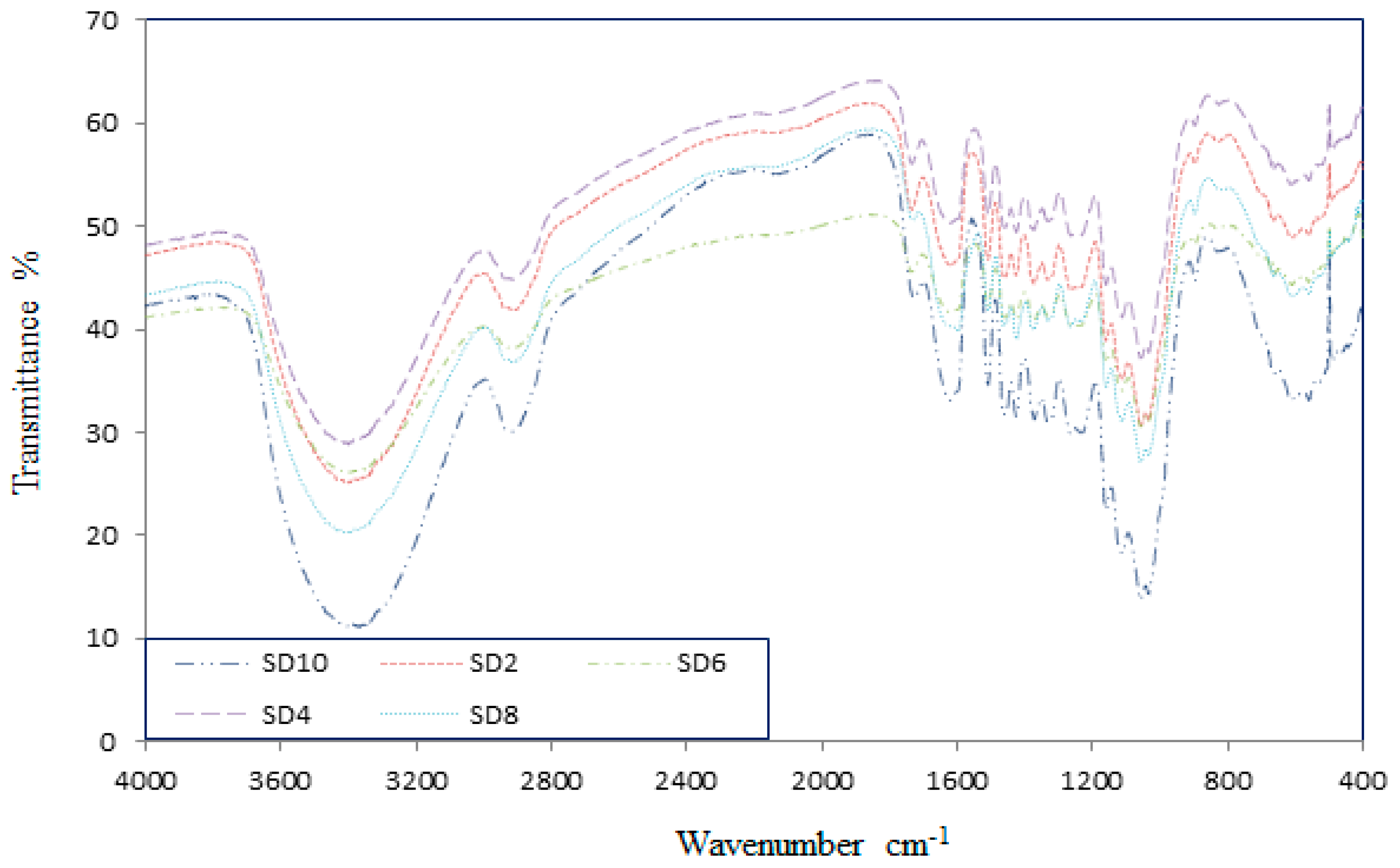
3.1. Component Analysis of the Sawdust
3.2. Charge Characteristic
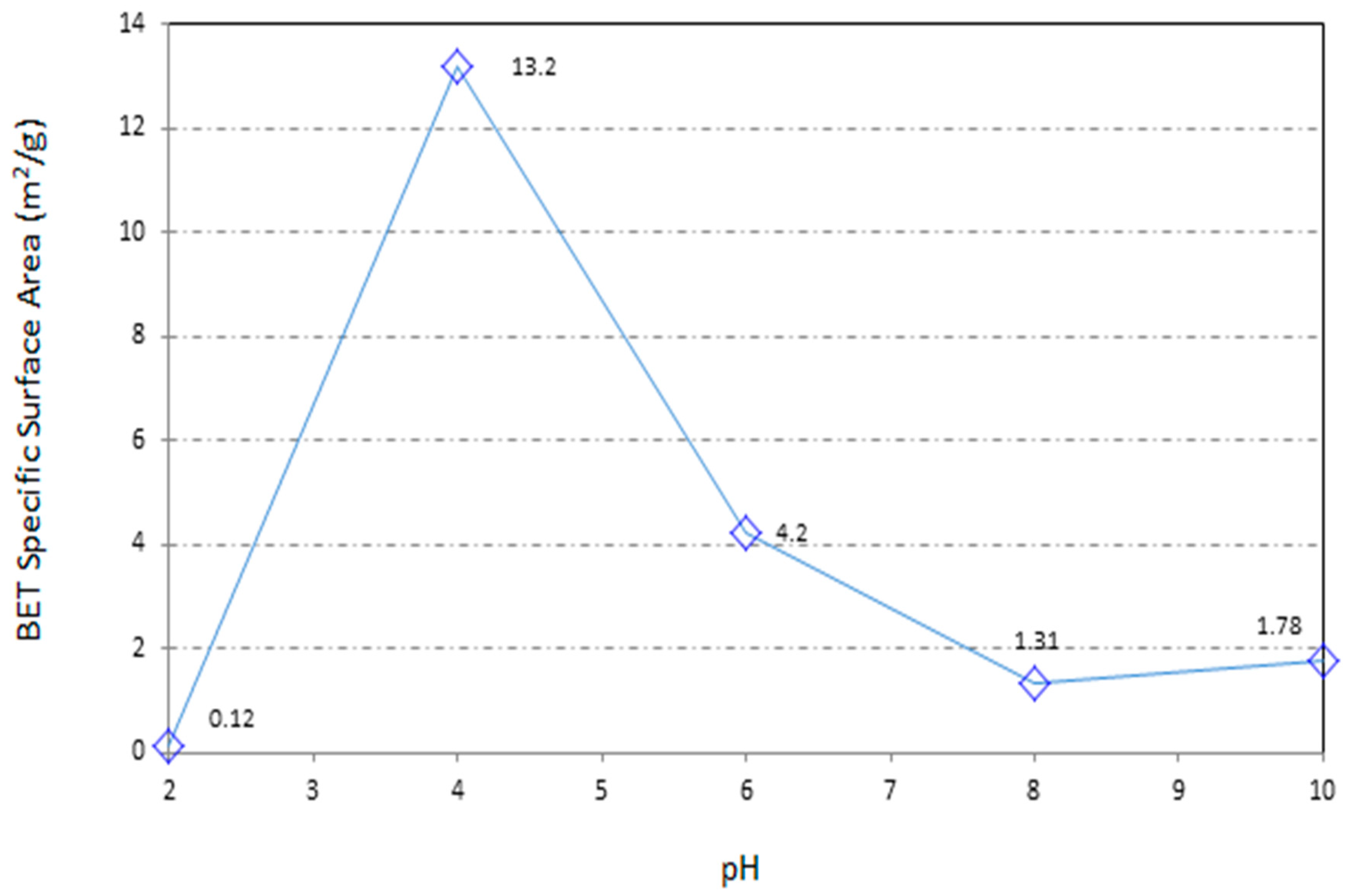
3.3. Brunauer-Emmett-Teller (BET) Surface Area and Particle Size
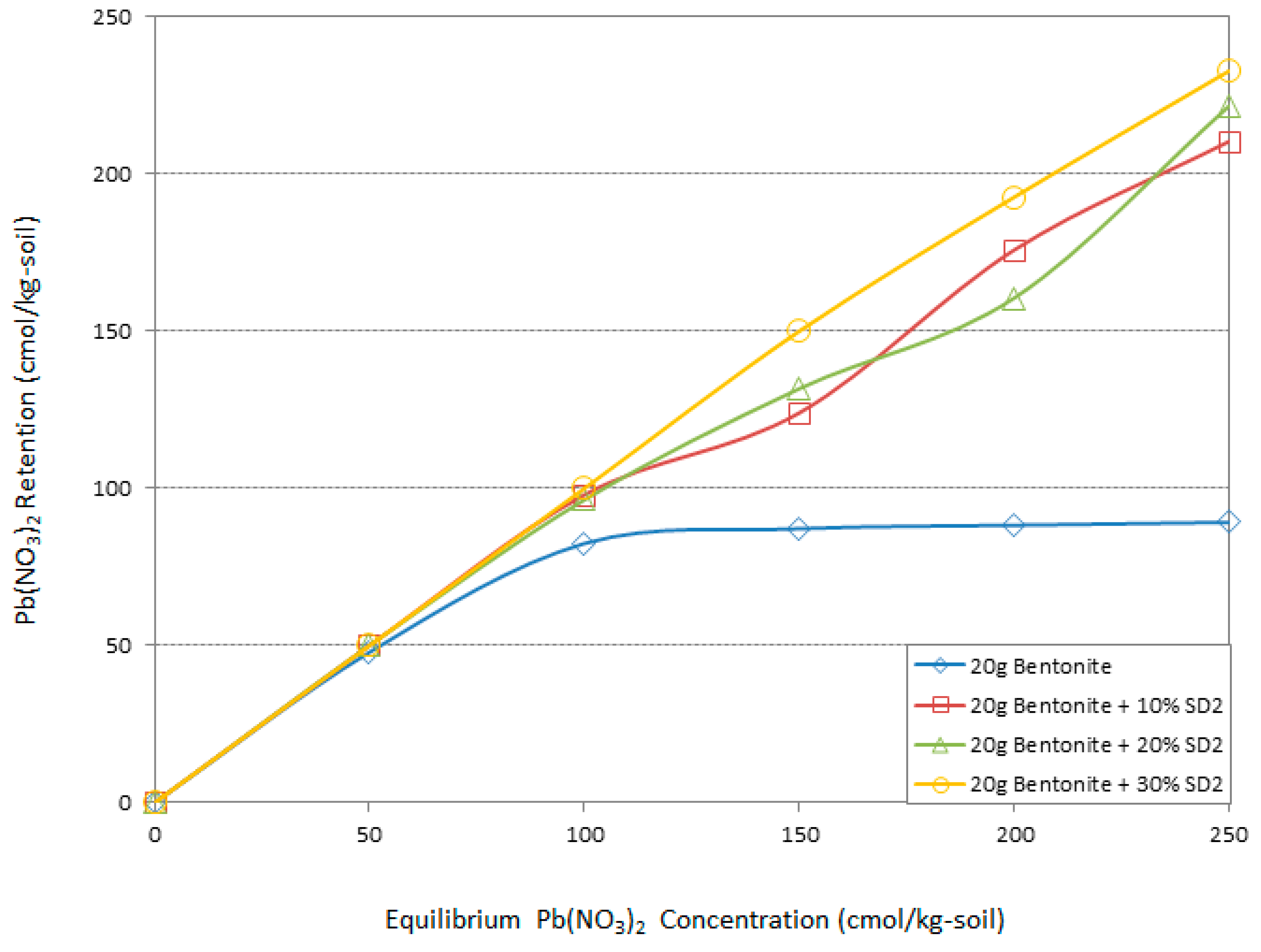
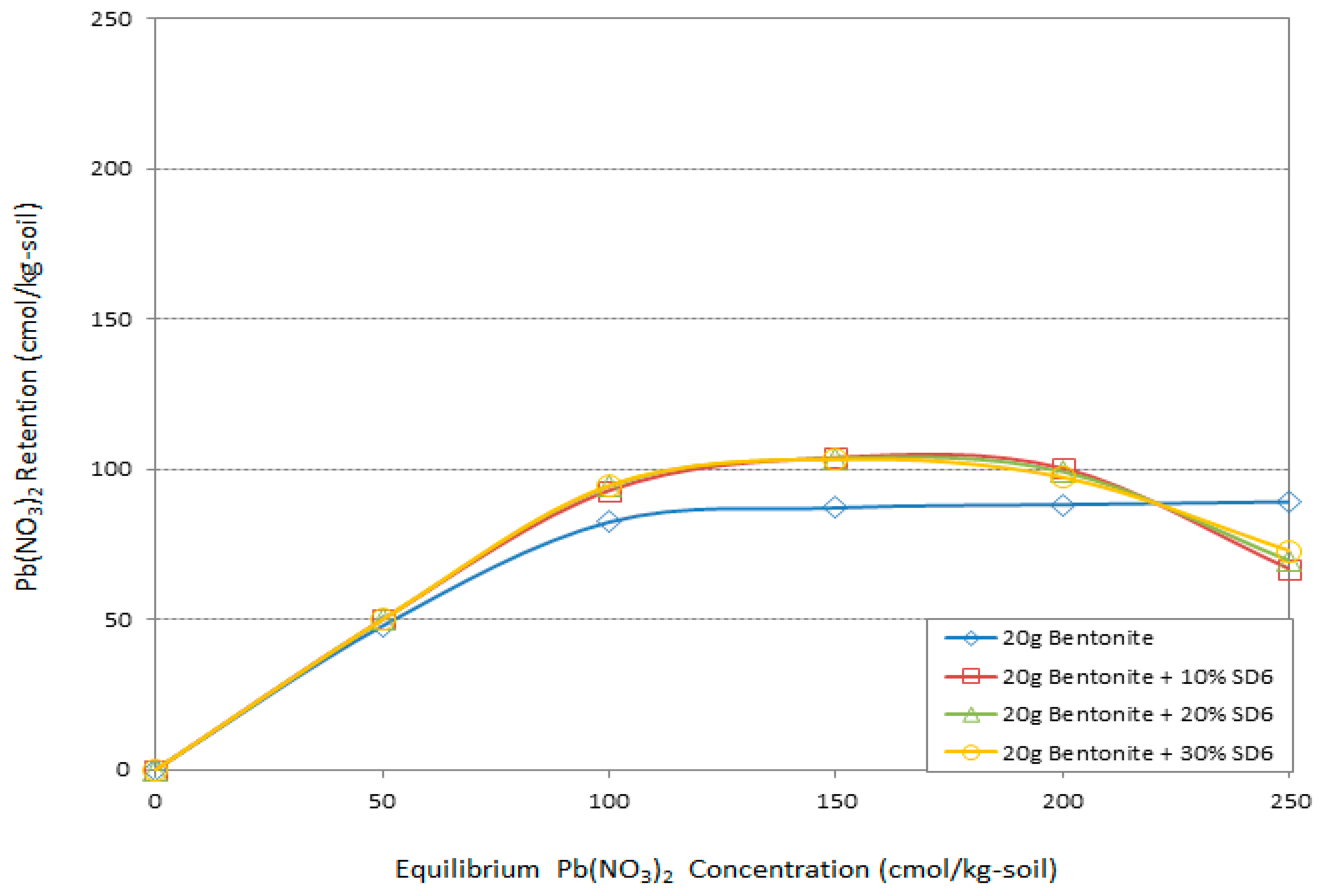
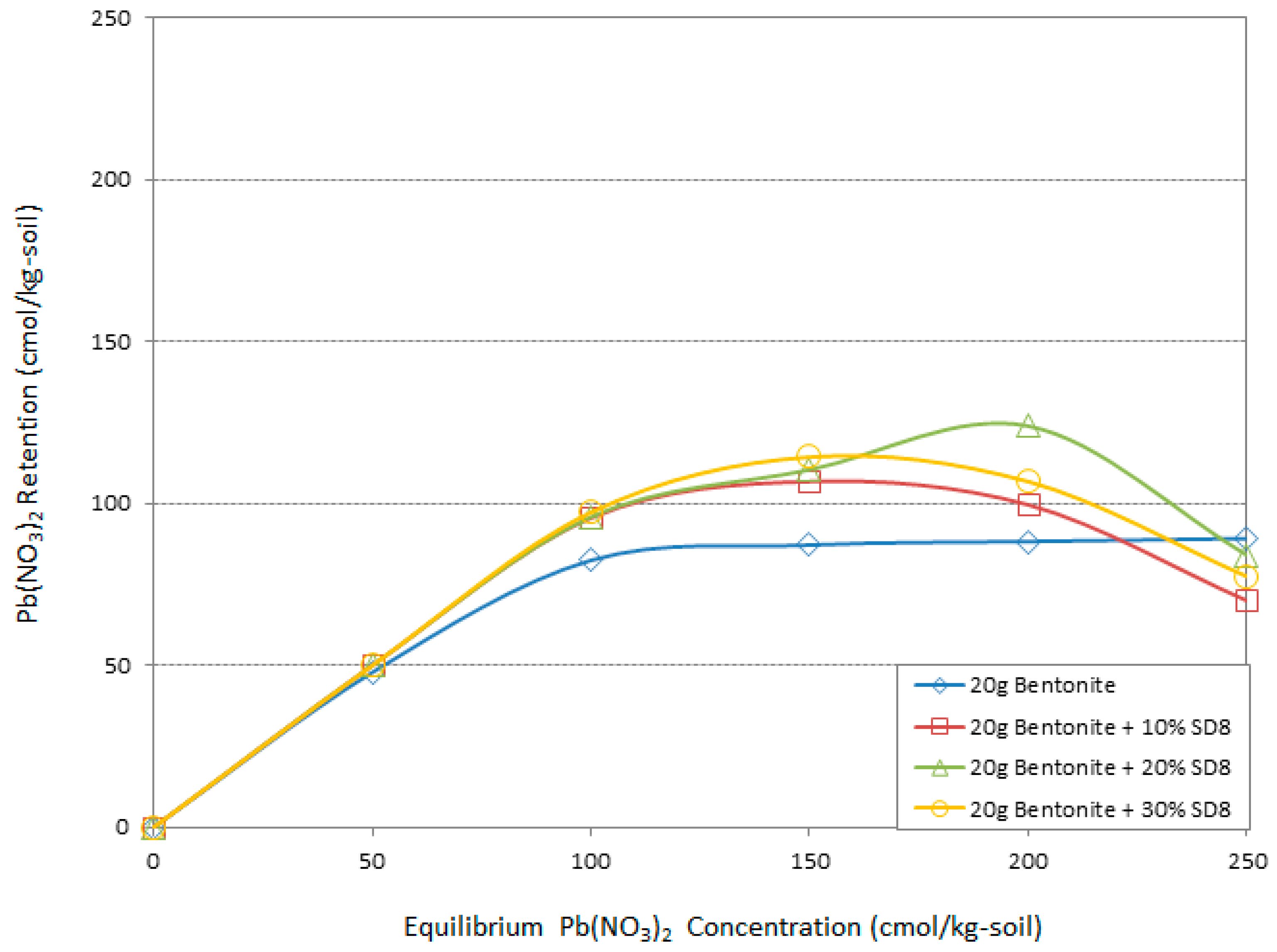
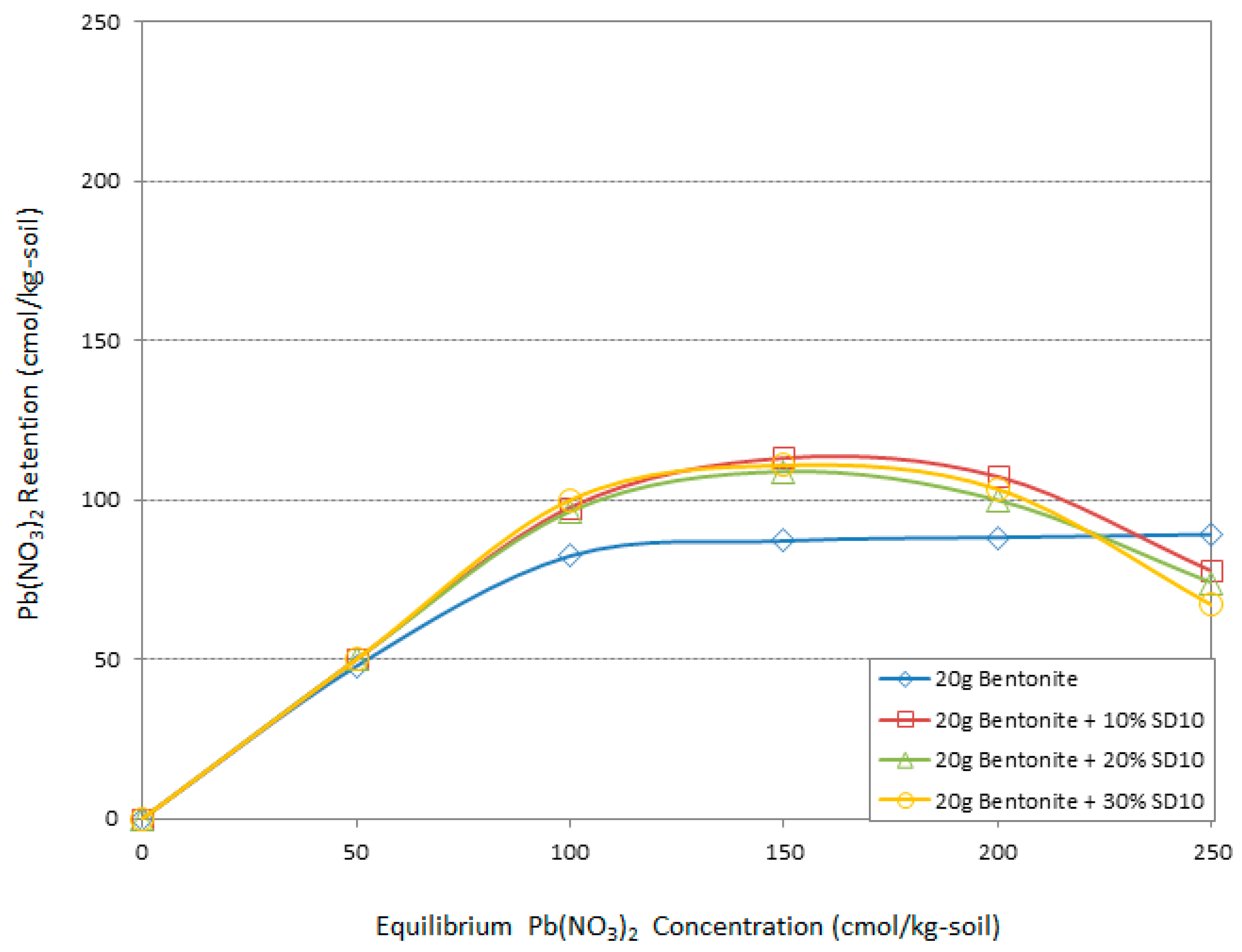
3.4. Atomic Adsorption Spectrometry (AAS) “Adsorption Behavior” Results
3.5. Adsorption Mechanisms
3.5.1. Na-Bentonite Adsorption Mechanism
3.5.2. Sawdust Adsorption Mechanism
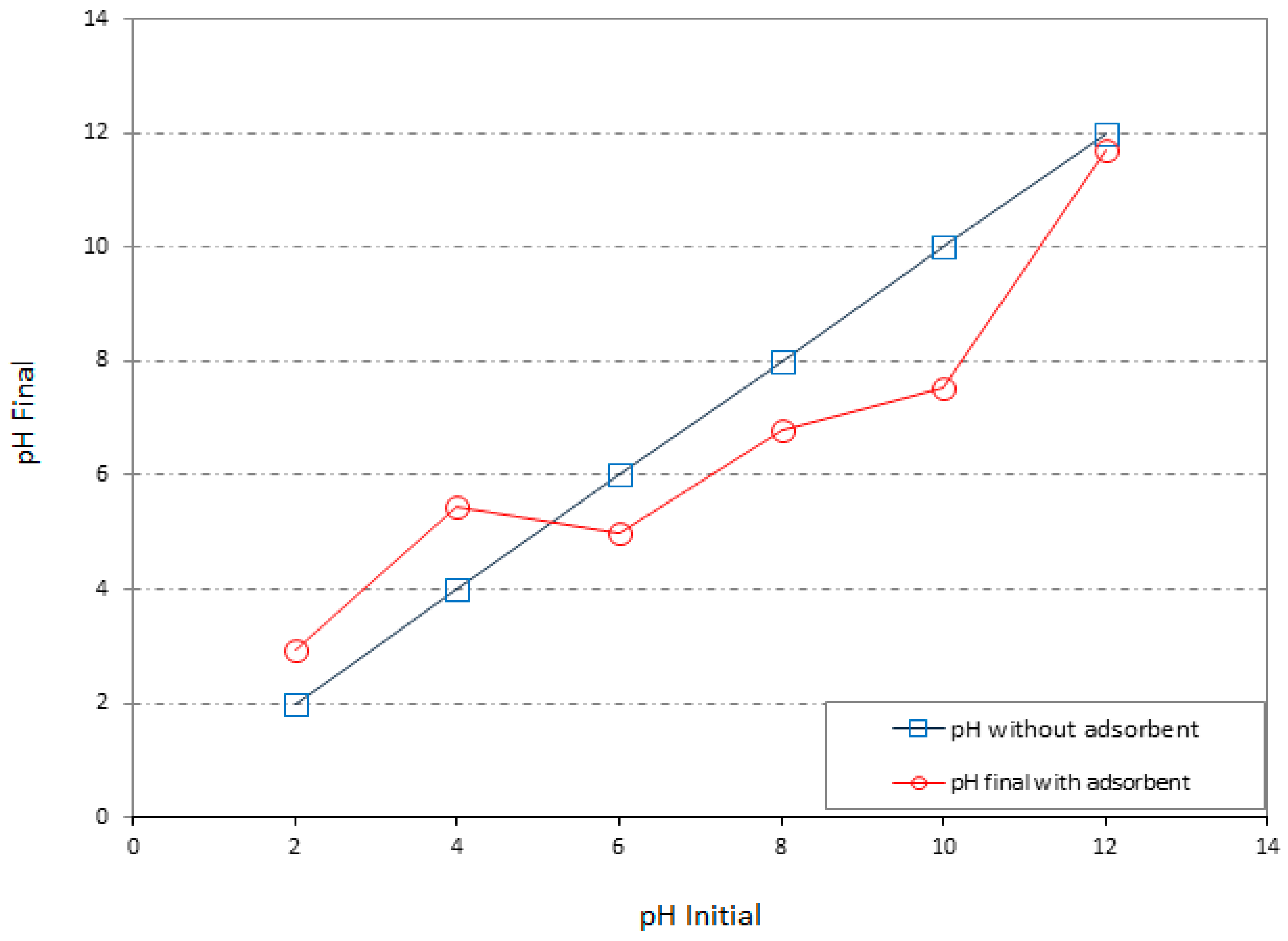
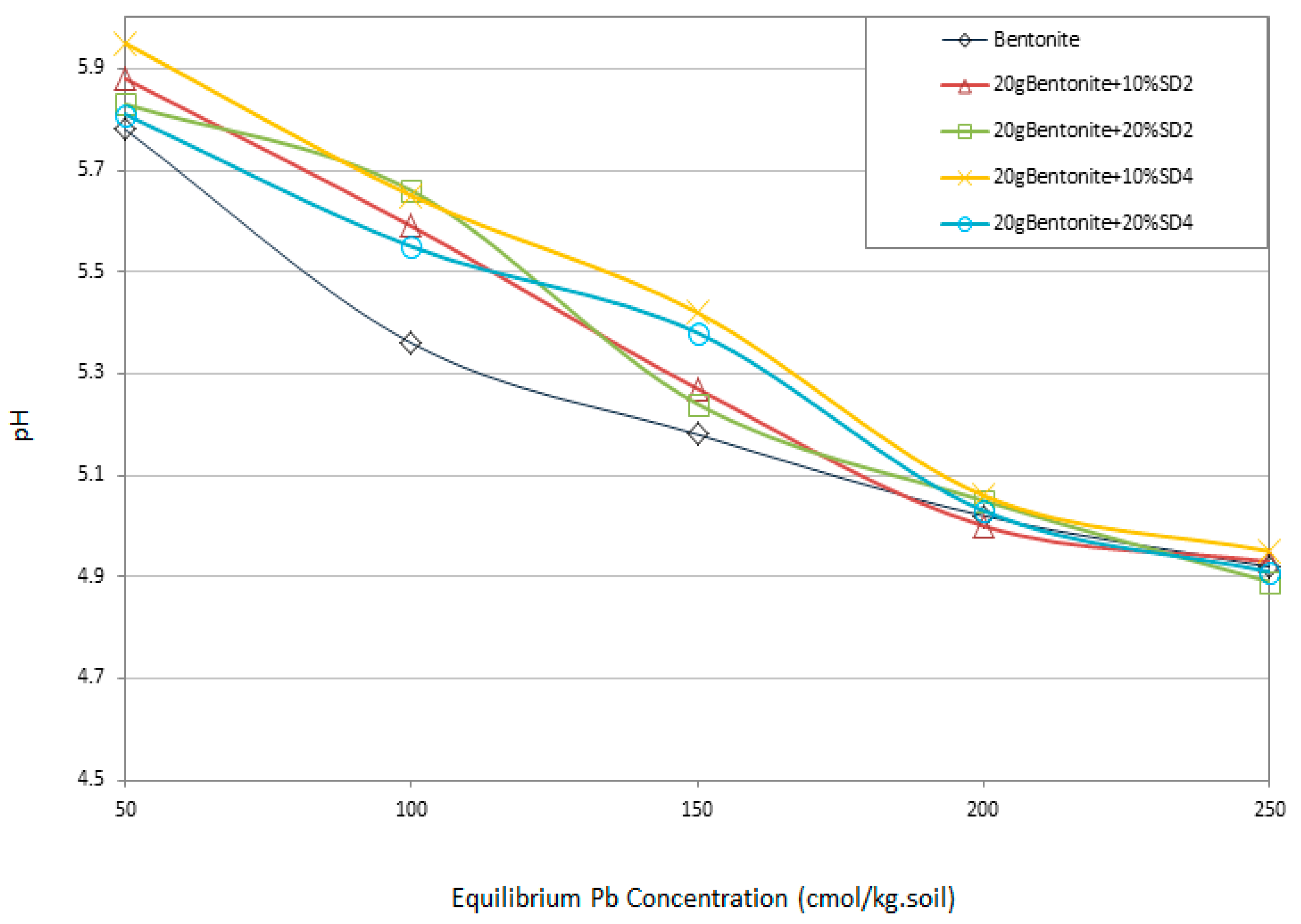
3.6. pH Variation of the System
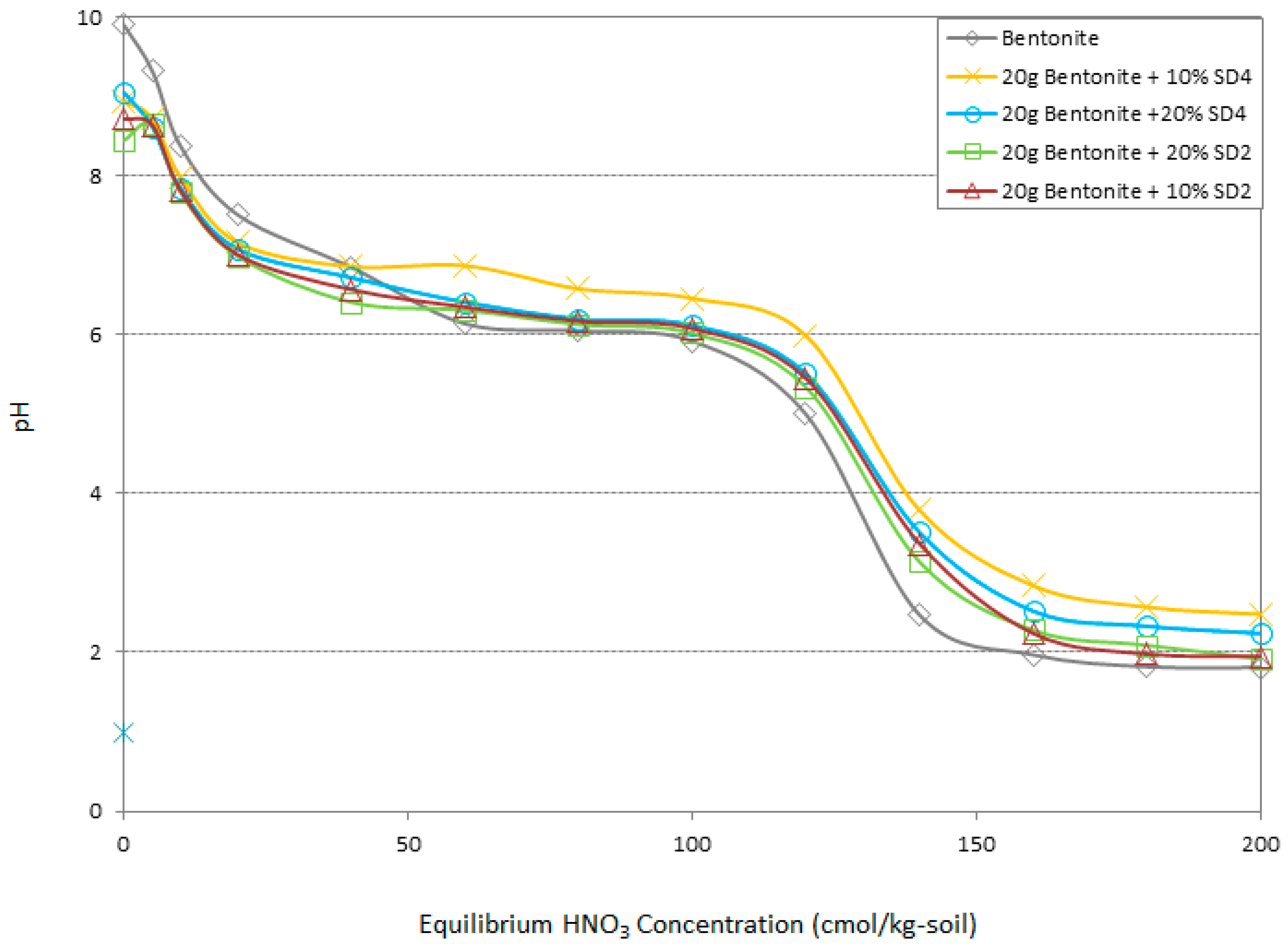
3.7. Buffering Capacity
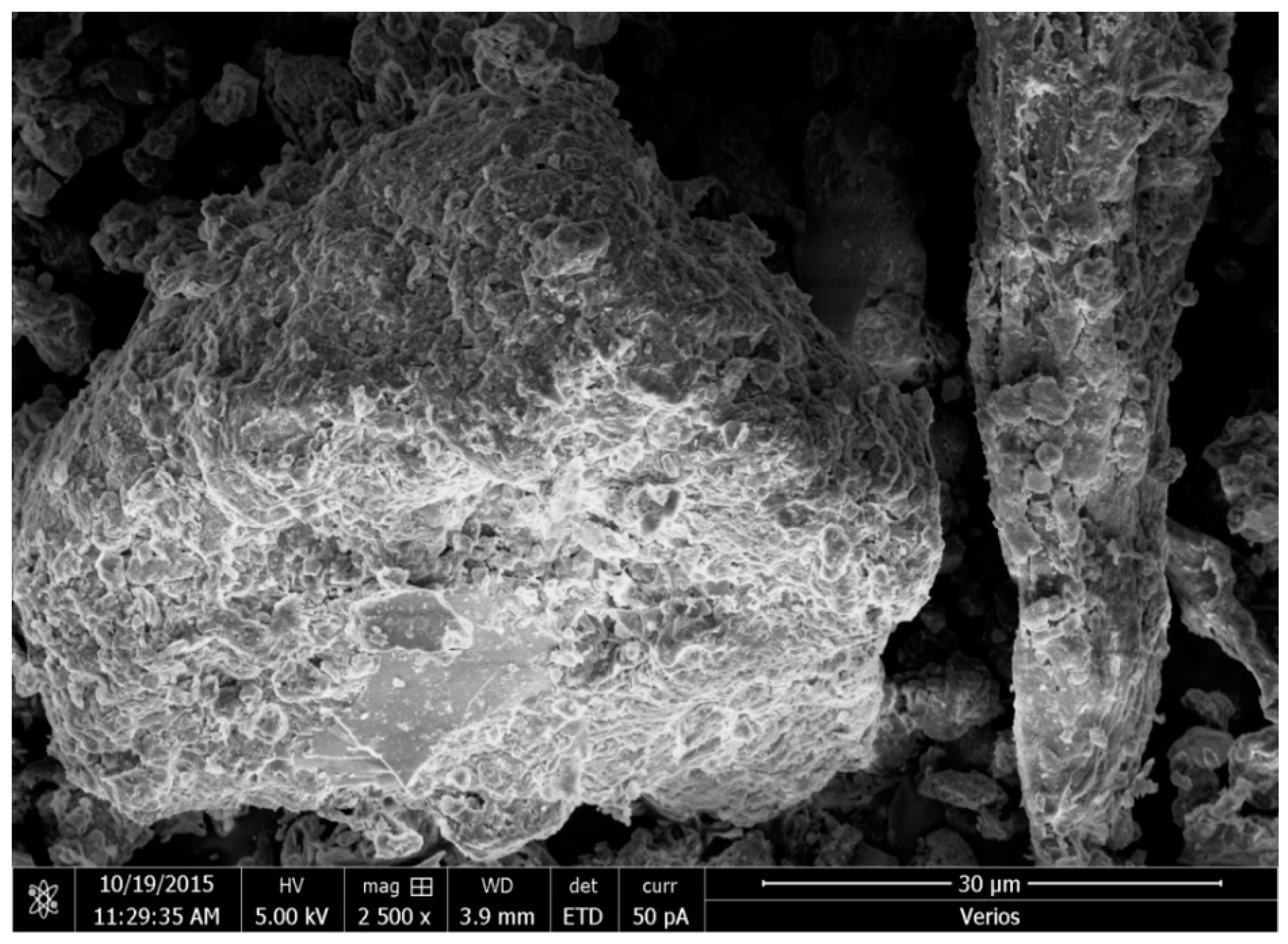
3.8. Scanning Electron Microscope (SEM)
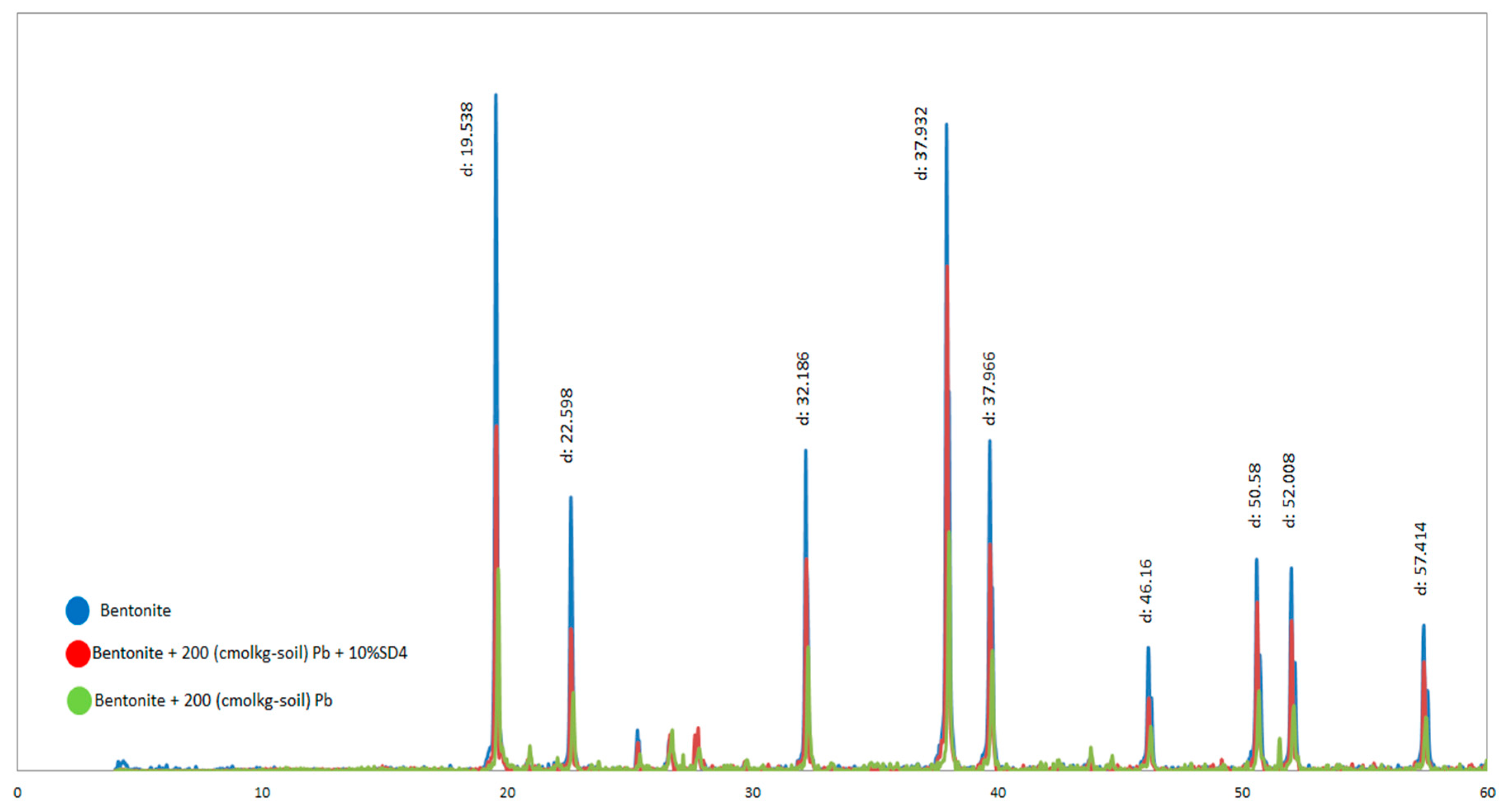
3.9. XRD Analysis
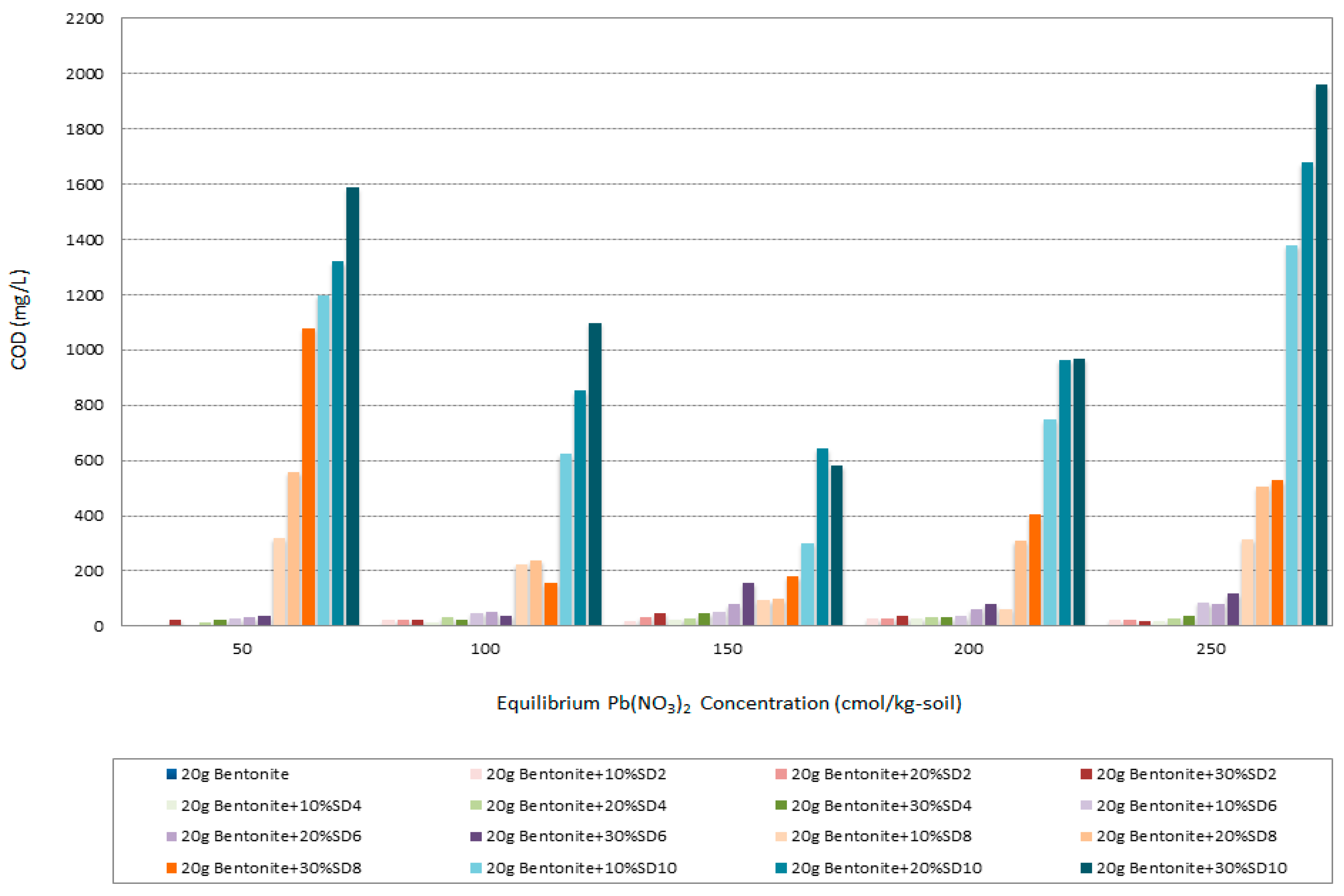
3.10. Chemical Oxygen Demand (COD)
4. Conclusions
- The acid treatment (also called acidification) of the sawdust has led to the improvement in its adsorption capacity through the activation of particle surfaces and the creation of the honeycomb structures.
- The acid treatment has caused an increase in the specific surface area of the sawdust particles. Thus, due to the improved pore sizes in the process, beside the chemically adsorption mechanisms the physical adsorption mechanisms have also participated in retaining the Pb contaminant.
- The reduction in the particle size can lead to increased specific surface area (SSA) and the subsequent augmentation in the adsorption opportunity and adsorption capacity of the outer sawdust surface.
- The high specific surface area (SSA) and high cation exchange capacity (CEC) of the Na-bentonite clay with high plasticity characteristics has increased the capability for high Pb retention through different adsorption mechanisms out of a solution with 100 (cmol/kg-soil) of Pb concentration, without using any sawdust additive.
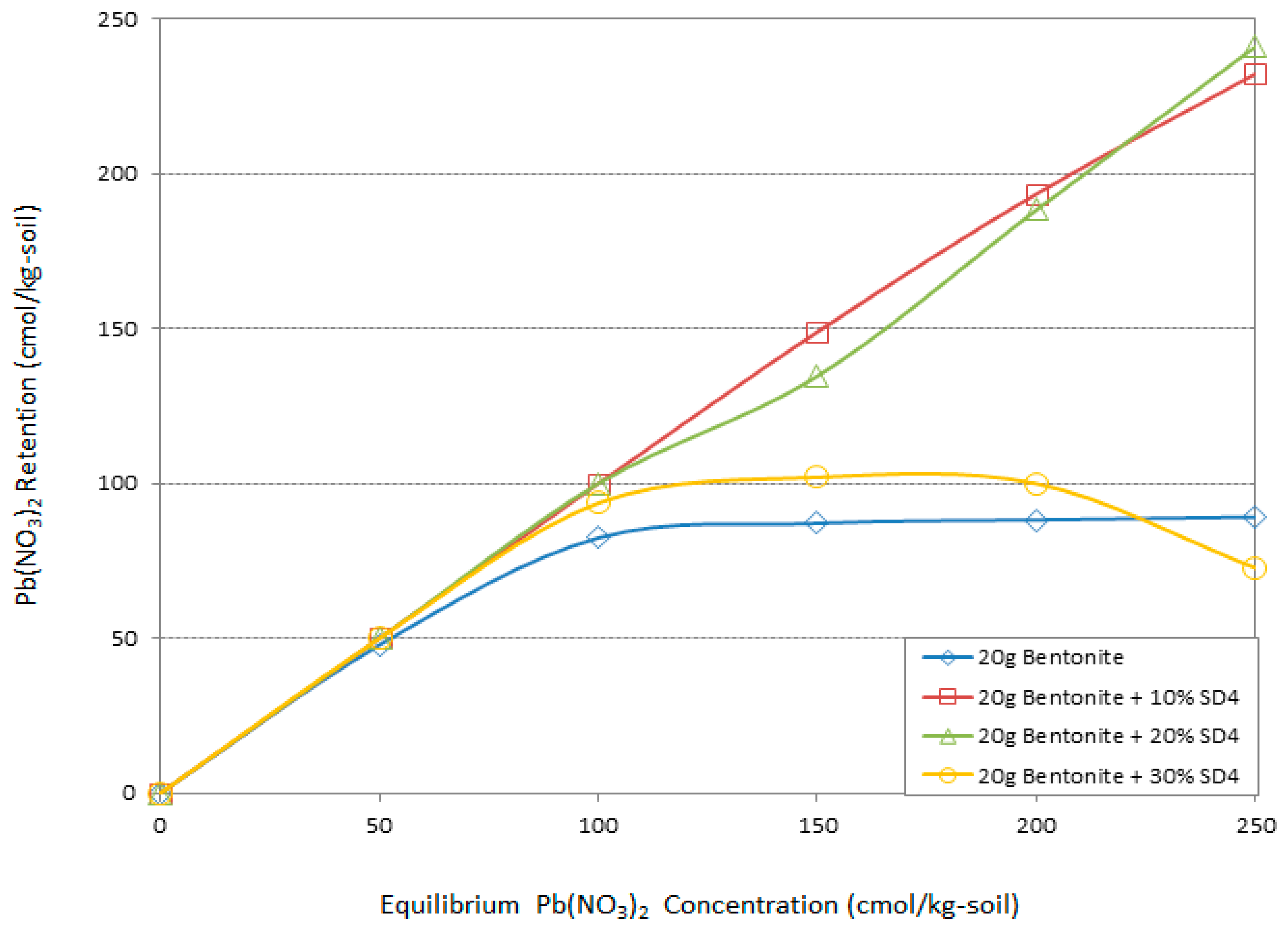
- The 10% SD4 and the 30% SD2 designs had substantial effects on the Pb adsorption capacity of the Na-Bentonite and therefore were the optimum mix designs. These are the 10% sawdust additive of pH4 and the 30% sawdust additive of pH2.
- The presence of 10% SD4 or 30% SD2 could improve the adsorption capacity of the system as it was capable of adsorbing 100% of the Pb for concentrations up to 150 (cmol/kg-soil).
- For higher Pb concentrations, i.e., between 150 (cmol/kg-soil) and 250 (cmol/kg-soil), the given optimum mix designs of 10% SD4 and the 30% SD2 were capable of 58% adsorption of the Pb.
- The ion exchange and the hydrogen bonding were the main mechanisms introduced by the chemical adsorption capability of the sawdust. Also, the surface complexes and the functional groups have affected the cation adsorption capacity of the sawdust.
- The buffering capacity of the system has been improved by the addition of 10% SD4. Thus the change in pH and the resulting improved buffering capacity of a clay linear system in contact with heavy metals were the most significant geo-environmental behaviors that have aided the adsorption.
- The maximum COD was found with specimens of the highest alkali degree, i.e., involving the SD10 samples, while the minimum COD was found with specimens of the most acidic degree, i.e., involving the SD2 samples. Therefore, the acidification of the sawdust could solve the problem associated with the COD creation, which also corresponds to the maximum adsorption capacity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bradbury, M.H.; Baeyens, B. Sorption modelling on illite. Part II: Actinide sorption and linear free energy relationships. Geochim. Cosmochim. Acta 2009, 73, 1004–1013. [Google Scholar] [CrossRef]
- Cruz-Guzmán, M.; Celis, R.; Hermosín, M.C.; Koskinen, W.C.; Nater, E.A.; Cornejo, J. Heavy Metal Adsorption by Montmorillonites Modified with Natural Organic Cations. Soil Sci. Soc. Am. J. 2006, 70, 215. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, D.; Zhang, H.; Lu, S.; Chen, L.; Yu, X. Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions. J. Hazard. Mater. 2010, 183, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Ozdes, D.; Gundogdu, A.; Kemer, B.; Duran, C.; Senturk, H.B.; Soylak, M. Removal of Pb(II) ions from aqueous solution by a waste mud from copper mine industry: Equilibrium, kinetic and thermodynamic study. J. Hazard. Mater. 2009, 166, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Ho, S.H.; Wang, D.; Wei, Z.S.; Chang, J.S.; Ren, N.Q. Lead removal by a magnetic biochar derived from persulfate-ZVI treated sludge together with one-pot pyrolysis. Bioresour. Technol. 2018, 247, 463–470. [Google Scholar]
- Wei, Z.; Luo, S.; Xiao, R.; Khalfin, R.; Semiat, R. Characterization and quantification of chromate adsorption by layered porous iron oxyhydroxide: An experimental and theoretical study. J. Hazard. Mater. 2017, 338, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Semiat, R. Applying a modified Donnan model to describe the surface complexation of chromate to iron oxyhydroxide agglomerates with heteromorphous pores. J. Colloid Interface Sci. 2017, 506, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wei, Z.; Dionysiou, D.D.; Spinney, R.; Hu, W.P.; Chai, L.; Yang, Z.; Ye, T.; Xiao, R. Mechanistic insight into reactivity of sulfate radical with aromatic contaminants through single-electron transfer pathway. Chem. Eng. J. 2017, 327, 1056–1065. [Google Scholar] [CrossRef]
- Ferrero, F.; Gaglia Prati, M.P. Coal fly ash and alginate for the removal of heavy metals from aqueous solutions. Ann. Chim. 1996, 86, 125–132. [Google Scholar]
- Mathur, A.; Khare, S.K.; Rupainwar, D.C. Removal of heavy metals from main sewer-water of Varanasi city by adsorption on fly ash and blast furnace slag. J. Ind. Pollut. Control 1989, 5, 52–57. [Google Scholar]
- Ouki, S.; Kavannagh, M. Treatment of metals-contaminated wastewaters by use of natural zeolites. Water Sci. Technol. 1999, 39, 115–122. [Google Scholar] [CrossRef]
- Kumar, P.; Dara, S.S. Binding heavy metal ions with polymerized onion skin. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 397–402. [Google Scholar] [CrossRef]
- Khalid, N.; Ahmad, S.; Kiani, S.N.; Ahmed, J. Removal of Mercury from Aqueous Solutions by Adsorption to Rice Husks. Sep. Sci. Technol. 1999, 34, 3139–3153. [Google Scholar] [CrossRef]
- Wei, Z.; Seo, Y. Trichloroethylene (TCE) adsorption using sustainable organic mulch. J. Hazard. Mater. 2010, 181, 147–153. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing and Materials (ASTM). Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 1992. [Google Scholar]
- Hendershot, W.; Duquette, M. A simple barium chloride method for determining cation exchange capacity and exchangeable cations. Soil Sci. Soc. Am. J. 1986, 50, 605–608. [Google Scholar] [CrossRef]
- Moore, D.; Reynolds, R. X-ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Hesse, P. A Textbook of Soil Chemical Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Fagerlund, G. Determination of specific surface by the BET method. Matériaux Constr. 1973, 6, 239–245. [Google Scholar] [CrossRef]
- ASTM D4749. StandardTest Method for Performing the Sieve Analysis of Coal and Designating Coal Size, USA; American Society for Testing and Materials: Philadelphia, PA, USA, 1994. [Google Scholar]
- Brown, P.; Jefcoat, I.; Parrish, D. Evaluation of the adsorptive capacity of peanut hull pellets for heavy metals in solution. Adv. Environ. Res. 2000, 4, 19–29. [Google Scholar] [CrossRef]
- EPA. Process Design Manual, Land Application of Municipal Sludge; EPA-625/1-83-016; Municipal Environmental Research Laboratory: Washington, DC, USA, 1983.
- Argun, M.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tabil, L.; Panigrahi, S. Chemical treatments of natural fiber for use in natural fiber-reinforced composites: A review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Gaballah, I.; Kilbertus, G. Recovery of heavy metal ions through decontamination of synthetic solutions and industrial effluents using modified barks. J. Geochem. Explor. 1998, 62, 241–286. [Google Scholar] [CrossRef]
- Mohajeri, P.; Selamat, M.R.B.; Aziz, H.A.; Vakili, A.H.; Farraji, H. Geo-environmental Behaviour of Pb Contaminated Clay with Sawdust. Environ. Geotech. 2018, 1–40. [Google Scholar] [CrossRef]
- Singh, J.; Mishra, N.; Banerjee, S.; Sharma, Y. Comparative studies of physical characteristics of raw and modified sawdust for their use as adsorbents for removal of acid dye. BioResources 2011, 6, 2732–2743. [Google Scholar]
- Sakurai, K.; Teshima, A.; Kyuma, K. Changes in Zero Point of Charge (ZPC), Specific Surface Area (SSA), and Cation Exchange Capacity (CEC) of kaolinite and montmorillonite, and strongly weathered soils caused by Fe and Al coatings. Soil Sci. Plant Nutr. 1990, 36, 73–81. [Google Scholar] [CrossRef]
- Bulut, Y. Removal of heavy metals from aqueous solution by sawdust adsorption. J. Environ. Sci. 2007, 19, 160–166. [Google Scholar] [CrossRef]
- Ajmal, M.; Khan, A.; Ahmad, S.; Ahmad, A. Role of sawdust in the removal of copper (II) from industrial wastes. Water Res. 1998, 32, 3085–3091. [Google Scholar] [CrossRef]
- Raji, C.; Anirudhan, T. Kinetics of Pb (II) adsorption by polyacrylamide grafted sawdust. Indian J. Chem. Technol. 1997, 4, 157–162. [Google Scholar]
- Yong, R. Geoenvironmental Engineering: Contaminated Soils, Pollutant Fate, and Mitigation; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Stewart, D.; Studds, P.; Cousens, T. The factors controlling the engineering properties of bentonite-enhanced sand. Appl. Clay Sci. 2003, 23, 97–110. [Google Scholar] [CrossRef]
- Kraepiel, A.; Keller, K.; Morel, F. A Model for Metal Adsorption on Montmorillonite. J. Colloid Interface Sci. 1999, 210, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Schindler, P.W.; Fürst, B.; Dick, R.; Wolf, P.U. Ligand properties of surface silanol groups. I. surface complex formation with Fe3+, Cu2+, Cd2+, and Pb2+. J. Colloid Interface Sci. 1976, 55, 469–475. [Google Scholar] [CrossRef]
- Suemitsu, R.; Uenishi, R.; Akashi, I.; Nakano, M. The use of dyestuff-treated rice hulls for removal of heavy metals from waste water. J. Appl. Polym. Sci. 1986, 31, 75–83. [Google Scholar] [CrossRef]
- Ibrahim, N. Animation of wood sawdust for removing anionic dyes from aqueous solutions. Polym. Plast. Technol. Eng. 1997, 36, 963–971. [Google Scholar] [CrossRef]
- Setyono, D.; Valiyaveettil, S. Chemically Modified Sawdust as Renewable Adsorbent for Arsenic Removal from Water. ACS Sustain. Chem. Eng. 2014, 2, 2722–2729. [Google Scholar] [CrossRef]
- Anirudhan, T.; Sreedhar, M. Adsorption thermodynamics of Co (II) on polysulphide treated sawdust. Indian J. Chem. Technol. 1998, 5, 41–47. [Google Scholar]
- Yong, R.; Warkentin, B. Buffer capacity and lead retention in some clay materials. Water Air Soil Pollut. 1990, 53, 53–67. [Google Scholar] [CrossRef]
- Abollino, O.; Aceto, M.; Malandrino, M.; Sarzanini, C.; Mentasti, E. Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res. 2003, 37, 1619–1627. [Google Scholar] [CrossRef]
- Ouhadi, V.; Yong, R. Impact of carbonate on the efficiency of heavy metal removal from kaolinite soil by the electrokinetic soil remediation method. J. Hazard. Mater. 2010, 173, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.; Yong, R.; Gibbs, B. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Mulligan, C.; Yong, R. Natural attenuation of contaminated soils. Environ. Int. 2004, 30, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.A.; Shimp, N.F.; Steele, J.D.; Ruch, R.R.; White, W.A.; Hughes, G.M. Attenuation of pollutants in municipal landfill leachate by passage through clay. Environ. Sci. Technol. 1976, 10, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Plassard, F.; Winiarski, T.; Petit-Ramel, M. Retention and distribution of three heavy metals in a carbonated soil: Comparison between batch and unsaturated column studies. J. Contam. Hydrol. 2000, 42, 99–111. [Google Scholar] [CrossRef]
- Sipos, P.; Németh, T.; Mohai, I.; Dódony, I. Effect of soil composition on adsorption of lead as reflected by a study on a natural forest soil profile. Geoderma 2005, 124, 363–374. [Google Scholar] [CrossRef]
- Yong, R.; Ouhadi, V.; Goodarzi, A. Effect of Cu2+ ions and buffering capacity on smectite microstructure and performance. J. Geotech. Geoenviron. Eng. 2009, 135, 1981–1985. [Google Scholar] [CrossRef]
- Sulkowski, M.; Hirner, A. Element fractionation by sequential extraction in a soil with high carbonate content. Appl. Geochem. 2006, 21, 16–28. [Google Scholar] [CrossRef]
- Buckman, H.O.; Brady, N.C. The Nature and Properties of Soils; Macmillan: New York, NY, USA, 1968. [Google Scholar]
- Ouhadi, V.; Yong, R.; Sedighi, M. Desorption response and degradation of buffering capability of bentonite, subjected to heavy metal contaminants. Eng. Geol. 2006, 85, 102–110. [Google Scholar] [CrossRef]
- Alvarez, P.; Blanco, C.; Santamarıa, R.; Granda, M. Improvement of the thermal stability of lignocellulosic materials by treatment with sulphuric acid and potassium hydroxide. J. Anal. Appl. Pyrolysis 2004, 72, 131–139. [Google Scholar] [CrossRef]
- Camacho, F.; Gonzalez-Tello, P.; Jurado, E.; Robles, A. Microcrystalline-cellulose hydrolysis with concentrated sulphuric acid. J. Chem. Technol. Biotechnol. 1996, 67, 350–356. [Google Scholar] [CrossRef]
- Rodrıguez, J.; Castrillon, L.; Maranon, E. Removal of non-biodegradable organic matter from landfill leachates by adsorption. Water Res. 2004, 38, 3297–3303. [Google Scholar] [CrossRef] [PubMed]

| Physical Characteristics | Quantity Measured |
|---|---|
| Clay (%) | 76 |
| Silt (%) | 23 |
| Sand (%) | 1 |
| Liquid limit (%) | 423 |
| Plastic limit (%) | 32 |
| Plasticity index (PI) (%) | 391 |
| Activity (%) | 3.73 |
| Soil classification (%) | CH |
| Water content (air-dried) (%) | 5.9 |
| Water content (oven-dried) (%) | 7.1 |
| Specific gravity (Gs) (%) | 2.45 |
| Chemical Characteristics | Quantity Measured |
|---|---|
| Mineral composition in decreasing abundance | Montmorilonite, Quartz, Calsit |
| Carbonite content (%) | 8 |
| Organic content (%) | 1.4 |
| Cation Exchange Capacity (CEC) (Cmol/kg soil) | 80 |
| Specific surface area (SSA) (10−3 m2/kg) | 425 |
| pH (1:10, Soil/Water ratio) | 9.9 |
| Constituent | SiO2 | Al2O3 | Fe2O3 | FeO | Na2O | K2O | CaO | MgO | MnO | TiO2 | Loss of Ignition |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage present | 69.17 | 14.43 | 3.12 | 0.02 | 1.95 | 0.83 | 1.29 | 3.31 | 0.04 | 0.13 | 5.40 |
| Surface Area Data | Pore Volume Data | ||||
|---|---|---|---|---|---|
| Single Point Surface Area at P/Po (m2/g) | BET Surface Area (m2/g) | Langmuir Surface Area (m2/g) | t-Plot External Surface Area (m2/g) | t-Plot Micro-Pore Volume (cm3/g) | |
| Sawdust pH2 | 0.0973 | 0.1237 | 0.2237 | 0.1907 | 0.000032 |
| Sawdust pH4 | 0.3869 | 13.2607 | 1.1502 | 13.7163 | 0.000468 |
| Sawdust pH6 | 1.8732 | 4.2070 | 1.29.21 | 4.5690 | 0.001185 |
| Sawdust pH8 | 1.1082 | 1.3198 | 2.3011 | 1.5219 | 0.000152 |
| Sawdust pH10 | 1.2048 | 1.7812 | 4.0343 | 1.8255 | 0.000183 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohajeri, P.; Smith, C.; Selamat, M.R.; Abdul Aziz, H. Enhancing the Adsorption of Lead (II) by Bentonite Enriched with pH-Adjusted Meranti Sawdust. Water 2018, 10, 1875. https://doi.org/10.3390/w10121875
Mohajeri P, Smith C, Selamat MR, Abdul Aziz H. Enhancing the Adsorption of Lead (II) by Bentonite Enriched with pH-Adjusted Meranti Sawdust. Water. 2018; 10(12):1875. https://doi.org/10.3390/w10121875
Chicago/Turabian StyleMohajeri, P., C. Smith, M. R. Selamat, and H. Abdul Aziz. 2018. "Enhancing the Adsorption of Lead (II) by Bentonite Enriched with pH-Adjusted Meranti Sawdust" Water 10, no. 12: 1875. https://doi.org/10.3390/w10121875
APA StyleMohajeri, P., Smith, C., Selamat, M. R., & Abdul Aziz, H. (2018). Enhancing the Adsorption of Lead (II) by Bentonite Enriched with pH-Adjusted Meranti Sawdust. Water, 10(12), 1875. https://doi.org/10.3390/w10121875





