Assessing Fish Species Tolerance in the Huntai River Basin, China: Biological Traits versus Weighted Averaging Approaches
Abstract
1. Introduction
2. Methods
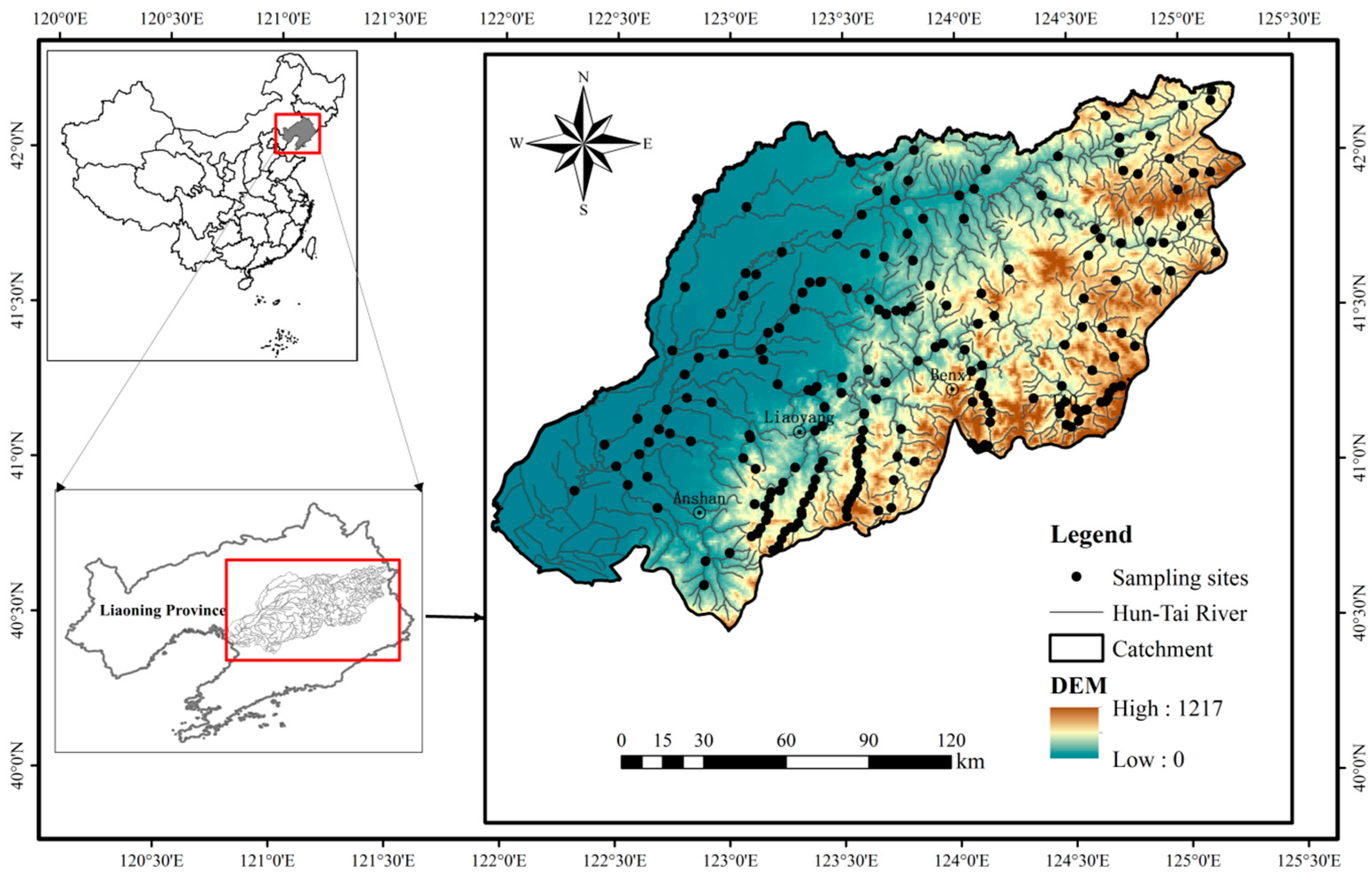
2.1. Study Area
2.2. Fish Sampling
2.3. Water Quality Parameters
2.4. Physical Habitat Factors
2.5. Data Analysis
2.5.1. Calculation of FW-TIVs
2.5.2. Calculation of Fb-TIVs
2.5.3. Statistical Analysis
3. Results
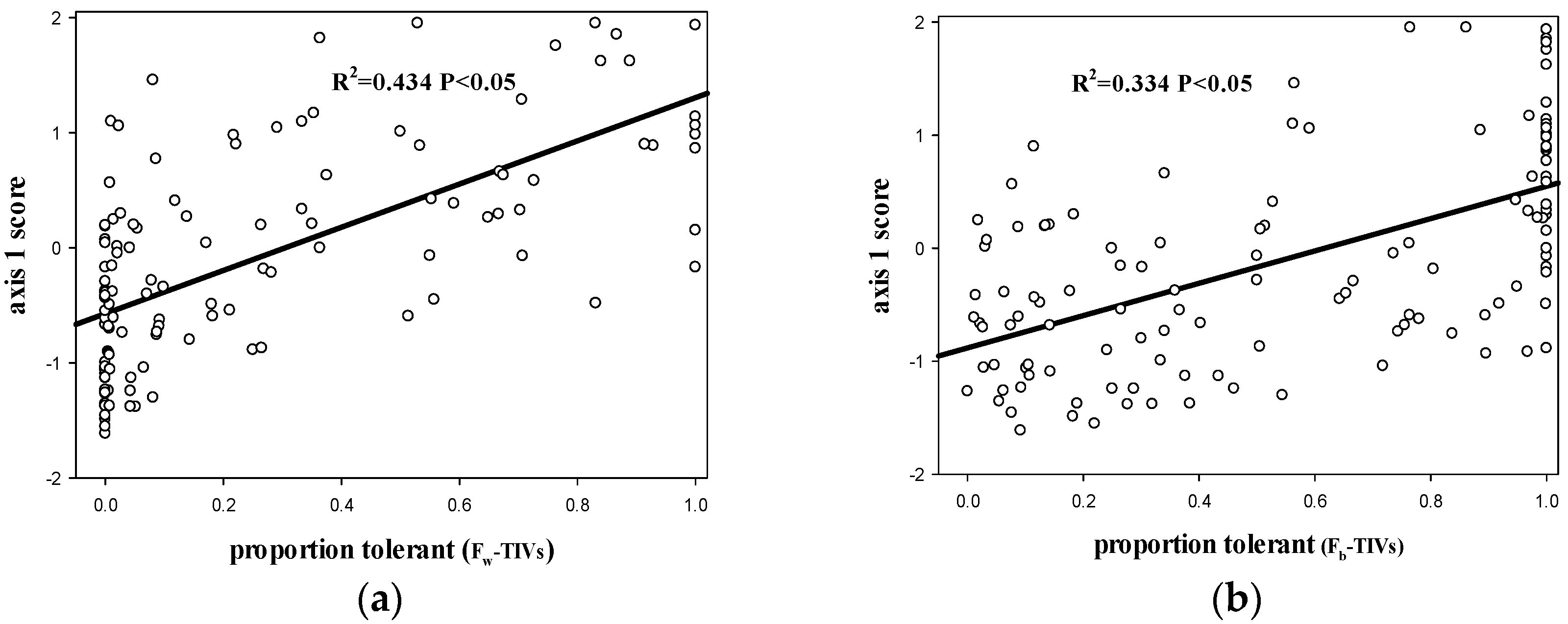
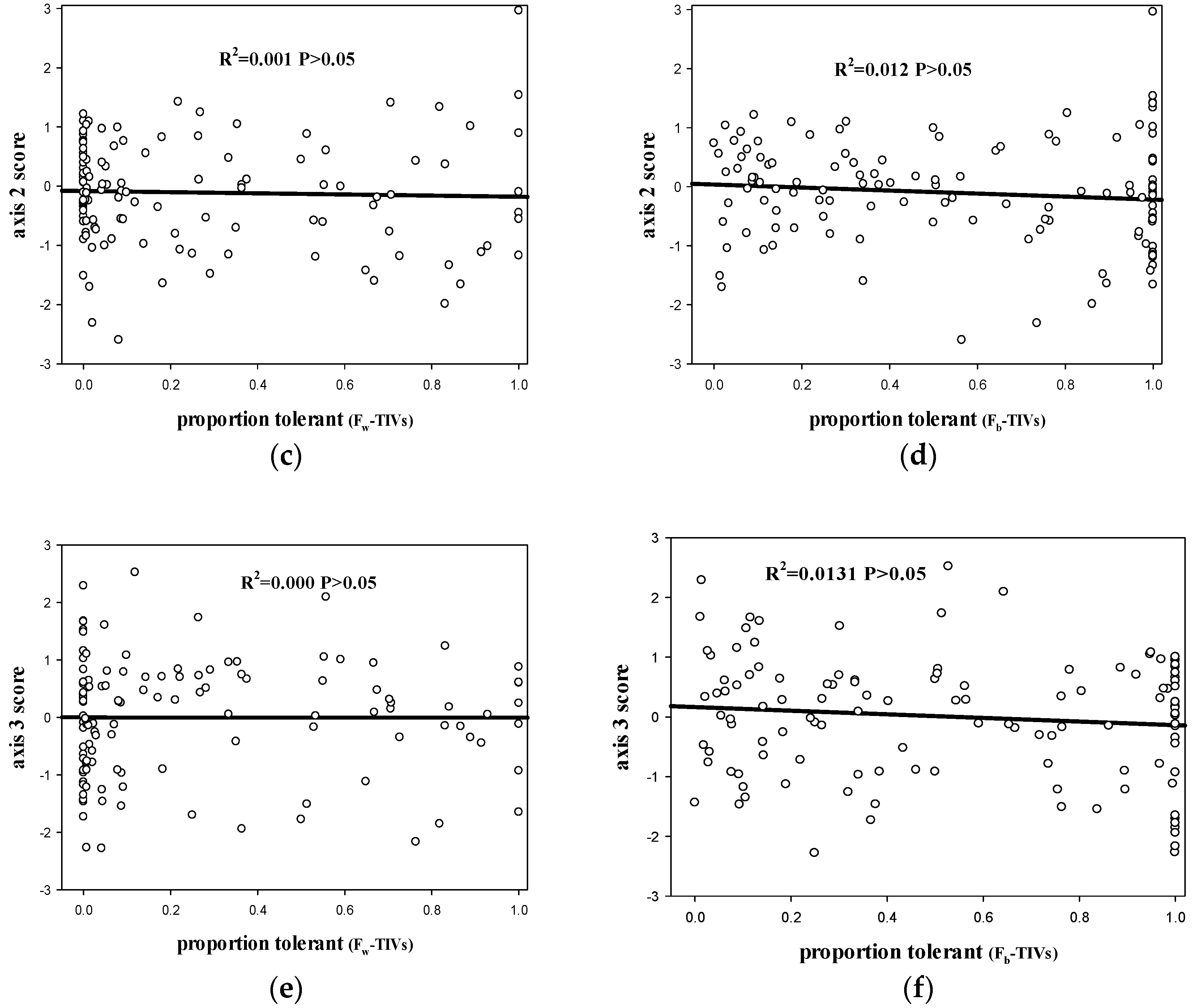
3.1. The Relationship between the FW-TIV Method and the Fb-TIV Method for Species Tolerance Classification
3.2. The Relationship between Fw-TIV, Fb-TIV and Environmental Pressure Gradients
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Factor | Optimal | Suboptimal | Marginal | Poor |
|---|---|---|---|---|
| Substrate | More than 75% composition of gravel, cobbles and big stones | 50%–75% composition of gravel, cobbles and big stones | 25%–50% composition of gravel, cobbles and big stones | Less than 25% composition of gravel, cobbles and big stones |
| Habitat complexity | Composition of aquatic vegetation, litter, fallen wood, concave banks and boulders, etc. | Composition of aquatic vegetation, litter, fallen wood, and concave banks, etc. | Domination by one or two kinds of microhabitat | Domination by one kind of microhabitat and the substrate mainly composed by silt or fine sand |
| Velocity-depth combination | Slow (<0.3 m/s)-deep (>0.5 m); slow-shallow (<0.5 m); fast (>0.3 m/s)-deep; fast-shallow three types of habitats | Only three types of habitat (fast-shallow type got the highest value) | Only two types of habitat (in the absence of fast-shallow type and slow-shallow type) | Predominated by one velocity–depth type (usually pools) |
| Intensity of human activities | Hardly any human disturbance | Minimal human disturbance by few walkers or bikes | Less human disturbance by vehicles | Serious human disturbance by motor vehicles |
| Riverside land use | No agricultural land on either side of the river bank | Agricultural land present on one side of the river bank | Agricultural land present on both sides of the river bank | Weathered soils after fallow conditions present on both sides of the river bank |
| Bank stability | No erosion on the river banks. Less than 5% of the bank is damaged in the visual range (100 m) | 5%–30% erosion of the bank in the visual range (100 m) | 30%–60% erosion of the bank in the visual range (100 m) | More than 60% erosion of the bank in the visual range (100 m) |
| Channel alteration | No channelization of the river | Less channelization around the pier | Embankments or bridge pillars on both sides of the strait and more extensive channelization of the river | River bank fixed by wire and cement |
| Stream flow conditions | Large water volume, only a few exposed areas of the bank visible | Relatively large volume and 75% of the river bank is covered | Relatively high volume covering 25%–75% of the river bank | Small water volume and dry river course |
| Vegetation diversity | Over 50% coverage of vegetation | 25%–50% vegetation coverage | Less than 25% vegetation coverage | Hardly any vegetation coverage |
| Water quality conditions | Low turbidity, no sedimentation, no odor from the river water | Low turbidity, a small amount of odor from the river water | Water with a high turbidity and odorous water | High turbidity and foul smelling water |
| score | 15–20 | 10–15 | 5–10 | 0–5 |
| Species | Fw-TIVs | Fb-TIVs |
|---|---|---|
| Abbottina liaoningensis | 6.3 (M) | 1.8 (T) |
| Abbottina rivularis | 7.1 (S) | 1.6 (T) |
| Cobitis granoei | 6.7 (M) | 0.6 (T) |
| Barbatula barbatula nuda | 4.3 (M) | 2.4 (M) |
| Rhodeus lighti | 4.2 (M) | 0.9 (T) |
| Rhodeus sinensis | 4.6 (M) | 1.0 (T) |
| Squalidus chankaensis | 5.6 (M) | 2.0 (T) |
| Squalidus wolterstorffi | 7.6 (S) | 1.0 (T) |
| Leuciscus waleckii | 8.3 (S) | 2.9 (M) |
| Carassius auratus | 4.1 (M) | 0.6 (T) |
| Zacco platypus | 6.7 (M) | 1.6 (T) |
| Rostrogobio liaohensis | 5.9 (M) | 1.0 (T) |
| Gobio lingyuanensis | 3.4 (T) | 2.0 (T) |
| Gobio rivuloides | 2.1 (T) | 1.0 (T) |
| Gobio cynocephalus | 4.1 (M) | 2.9 (M) |
| Phoxinus lagowskii | 6.2 (M) | 2.4 (M) |
| Opsariichthys bidens | 4.7 (M) | 2.0 (T) |
| Pseudorasbora parva | 5.6 (M) | 1.6 (T) |
| Hemiculter leucisculus | 2.0 (T) | 0.9 (T) |
| Huigobio chinssuensis | 8.2 (S) | 0.9 (T) |
| Pseudogobio vaillanti | 2.3 (T) | 2.0 (T) |
| Acheilognathus chankaensis | 2.4 (T) | 0.9 (T) |
| Misgurnus anguillicaudatus | 5.0 (M) | 0.6 (T) |
| Lefua costata | 4.5 (M) | 0.8 (T) |
| Hypomesus olidus | 9.1 (S) | 3.0 (M) |
| Lampetra mori | 6.6 (M) | 2.0 (T) |
| Perccottus glenni | 5.1 (M) | 1.0 (T) |
| Ctenogolius brunneus | 4.0 (T) | 2.8 (M) |
| Cottus poecilopus | 10.0 (S) | 4.0 (S) |
| Hypseleotris swinhonis | 4.6 (M) | 0.9 (T) |
| Odontobutis yaluensis | 7.8 (S) | 1.8 (T) |
| Oryzias latipes sinensis | 1.9 (T) | 0.9 (T) |
| Pungitius pungitius | 9.0 (S) | 0.9 (T) |
References
- O’Brien, A.; Townsend, K.; Hale, R.; Sharley, D.; Pettigrove, V. How is ecosystem health defined and measured? A critical review of freshwater and estuarine studies. Ecol. Indic. 2016, 69, 722–729. [Google Scholar] [CrossRef]
- Ogren, S.A. Using Indicators of Biotic Integrity for Assessment of Stream Condition. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2014. [Google Scholar]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Zalack, J.T.; Smucker, N.J.; Vis, M.L. Development of a diatom index of biotic integrity for acid mine drainage impacted streams. Ecol. Indic. 2010, 10, 287–295. [Google Scholar] [CrossRef]
- Beck, M.W.; Vondracek, B.; Hatch, L.K. Environmental clustering of lakes to evaluate performance of a macrophyte index of biotic integrity. Aquat. Bot. 2013, 108, 16–25. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, J.F.; Cai, Y.J.; Yin, H.B.; Gao, Y.N.; Zhao, J.H.; Liu, L.Z.; Huang, J.C. Development and application of benthic macroinvertebrate-based multimetric indices for the assessment of streams and rivers in the Taihu Basin, China. Ecol. Indic. 2015, 48, 649–659. [Google Scholar] [CrossRef]
- Raburu, P.O.; Masese, F.O. Development of a fish-based index of biotic integrity (FIBI) for monitoring riverine ecosystems in the Lake Victoria drainage Basin, Kenya. River Res. Appl. 2012, 28, 23–38. [Google Scholar] [CrossRef]
- Mohamed, A.R.M. A fish index of biotic integrity for evaluation of fish assemblage environment in restored Chybaish marsh, Iraq. Glob. J. Biol. Agric. Health Sci. 2014, 3, 32–37. [Google Scholar]
- Kim, J.; Koh, D.K.; Cho, S. An assessment of habitat conditions for fish species in the Guem River, Korea. Ecol. Environ. 2011, 167, 309–320. [Google Scholar]
- Segurado, P.; Santos, J.M.; Pont, D.; Melcher, A.H.; Jalon, D.G.; Hughes, R.M.; Ferreira, M.T. Estimating species tolerance to human perturbation: Expert judgment versus empirical approaches. Ecol. Indic. 2011, 11, 1623–1635. [Google Scholar] [CrossRef]
- Wu, W.; Xu, Z.; Zhan, C.; Yin, X.; Yu, S. A new framework to evaluate ecosystem health: A case study in the Wei River basin, China. Environ. Monit. Assess. 2015, 187, 460. [Google Scholar] [CrossRef] [PubMed]
- Chevin, L.M.; Lande, R.; Mace, G.M. Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biol. 2010, 8, e1000357. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, D.M.; Hawkins, C.P.; Meador, M.R.; Potapova, M.; Falcone, J. Biological assessments of Appalachian streams based on predictive models for fish, macroinvertebrate, and diatom assemblages. J. N. Am. Benthol. Soc. 2008, 27, 16–37. [Google Scholar] [CrossRef]
- Brenner, M.; Buck, B.; Cordes, S.; Dietrich, L.; Jacob, U.; Mintenbeck, K.; Schröder, A.; Brey, T.; Knust, R.; Arntz, W. The role of iceberg sours in niche separation within the Antarctic fish genus Trematomus. Polar Biol. 2001, 24, 502–507. [Google Scholar]
- Benejam, L.; Aparicio, E.; Vargas, M.; Vila-Gispert, A.; García-Berthou, E. Assessing fish metrics and biotic indices in a Mediterranean stream: Effects of uncertain native status of fish. Hydrobiologia 2008, 603, 197–210. [Google Scholar] [CrossRef]
- Carlisle, D.M.; Meador, M.R.; Li, S.R.M.; Ruhl, P.M. Estimation and application of indicator values for common macroinvertebrate genera and families of the United States. Ecol. Indic. 2007, 7, 22–33. [Google Scholar] [CrossRef]
- Meador, M.R.; Carlisle, D.M. Quantifying tolerance indicator values for common stream fish species of the United States. Ecol. Indic. 2007, 7, 329–338. [Google Scholar] [CrossRef]
- Minnesota Pollution Control Agency (MPCA). Development of a Fish-Based Index of Biological Integrity for Minnesota’s Rivers and Streams; Number wq-bsm2-03; Minnesota Pollution Control Agency, Environmental Analysis and Outcomes Division: St. Paul, MN, USA, 2014. [Google Scholar]
- Poff, L.R.; Olden, J.D.; Strayer, D.L. Climate Change and Freshwater Fauna Extinction Risk; Island Press-Center for Resource Economics: Washington, DC, USA, 2012. [Google Scholar]
- Sievert, N.A.; Paukert, C.P.; Tsang, Y.P.; Infante, D. Development and assessment of indices to determine stream fish vulnerability to climate change and habitat alteration. Ecol. Indic. 2016, 67, 403–416. [Google Scholar] [CrossRef]
- Morgan, I.J.; McDonald, D.G.; Wood, C.M. The cost of living for freshwater fish in a warmer, more polluted world. Glob. Chang. Biol. 2001, 7, 345–355. [Google Scholar] [CrossRef]
- Mims, M.; Olden, J. Life history theory predicts fish assemblage response to hydrologic regimes. Ecology 2012, 93, 35–45. [Google Scholar] [CrossRef]
- Mims, M.C.; Olden, J.D. Fish assemblages respond to altered flow regimes via ecological filtering of life history strategies. Freshw. Biol. 2013, 58, 50–62. [Google Scholar] [CrossRef]
- Murray, K.A.; Rosauer, D.; McCallum, H.; Skerratt, L.F. Integrating species traits with extrinsic threats: Closing the gap between predicting and preventing species declines. Proc. R. Soc. B Biol. Sci. 2011, 278, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, M.; Gomez, A.; Revilla, E. Which intrinsic traits predict vulnerability to extinction dependson the actual threatening processes. Ecosphere 2013, 4, art76. [Google Scholar] [CrossRef]
- Garcia, R.A.; Araújo, M.B.; Burgess, N.D.; Foden, W.B.; Gutsche, A.; Rahbek, C.; Cabeza, M. Matching species traits to projected threats and opportunities from climate change. J. Biogeogr. 2014, 41, 724–735. [Google Scholar] [CrossRef] [PubMed]
- CSEPB (Chinese State Environment Protection Bureau). Water and Wastewater Monitoring and Analysis Methods, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002; ISBN 9787801634009. [Google Scholar]
- Zheng, B.H.; Zhang, Y.; Li, Y.B. Study of indicators and methods for river habitat assessment of Liao River Basin. Acta Sci. Circumst. 2007, 27, 928–936. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wade Able Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999.
- Macedaveiga, A.; Sostoa, A.D. Observational evidence of the sensitivity of some fish species to environmental stressors in Mediterranean rivers. Ecol. Indic. 2011, 11, 311–317. [Google Scholar] [CrossRef]
- Stevens, C.E.; Council, T.; Sullivan, M.G. Influences of human stressors on fish-based metrics for assessing river condition in Central Alberta. Water Qual. Res. J. Can. 2010, 45, 35–46. [Google Scholar] [CrossRef]
- Xie, Y.H. Freshwater Fishes in Northeast Region of China; Liaoning Science and Technology Press: Shenyang, China, 2007; ISBN 978-7-5381-5259-3. (In Chinese) [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier Science: Amsterdam, The Netherland, 1998. [Google Scholar]
- Zhang, H.; Ding, S.; Zhang, Y.; Jia, X.B.; Meng, W.; Guo, B. Assessment of the fish index of biotic integrity and its relationship with environmental factors in the Xiliao River Basin. J. Lake Sci. 2015, 27, 829–839. (In Chinese) [Google Scholar]
- Bressler, D.W.; Stribling, J.B.; Paul, M.J.; Hicks, M.B. Stressor tolerance values for benthic macroinvertebrates in Mississippi. Hydrobiologia 2006, 573, 155–172. [Google Scholar] [CrossRef]
- Mandaville, S.M. Benthic Macroinvertebrates in Freshwaters Taxa Tolerance Values, Metrics, and Protocols; New York State Department of Environmental Conservation: Albany, NY, USA, 2002.
- Goldstein, R.M.; Meador, M.R. Multilevel assessment of fish species traits to evaluate habitat degradation in streams of the upper Midwest. N. Am. J. Fish Manag. 2005, 25, 180–194. [Google Scholar] [CrossRef]
- Bonada, N.; Doledec, S.; Statzner, B. Taxonomic and biological trait differences of stream macroinvertebrate communities between Mediterranean and temperate regions: Implications for future climatic scenarios. Glob. Chang. Biol. 2007, 13, 1658–1671. [Google Scholar] [CrossRef]
- Chessman, B.C. Identifying species at risk from climate change: Traits predict the drought vulnerability of freshwater fishes. Biol. Conserv. 2013, 160, 40–49. [Google Scholar] [CrossRef]
- Lima, A.C.; Wrona, F.J.; Soares, A.M.V.M. Fish traits as an alternative tool for the assessment of impacted rivers. Rev. Fish Biol. Fish. 2017, 27, 31–42. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M.; Blanco-Garrido, F.; Prenda, J. Assessing freshwater fish sensitivity to different sources of perturbation in a Mediterranean basin. Ecol. Freshw. Fish. 2010, 18, 269–281. [Google Scholar] [CrossRef]
- Hare, J.A.; Morrison, W.E.; Nelson, M.W.; Stachura, M.M.; Teeters, E.J.; Griffis, R.B.; Alexander, M.A.; Scott, J.D.; Alade, L.; Bell, R.J.; Chute, A.S.; et al. A vulnerability assessment of fish and invertebrates to climate change on the Northeast U.S. Continental Shelf. PLoS ONE 2016, 11, e0146756. [Google Scholar] [CrossRef]
- Lamouroux, N.; Poff, N.L.; Angermeier, P.L. Intercontinental convergence of stream fish community traits in France and Virginia (USA) streams along hydraulic and geomorphic gradients. Ecology 2002, 83, 1792–1807. [Google Scholar] [CrossRef]
- Blanck, A.; Lamouroux, N. Large-scale intraspecific variation in life history traits of 44 freshwater fish. J. Biogeogr. 2007, 34, 862–875. [Google Scholar] [CrossRef]
- Lee, H.J.; Valentin, H.; Axel, M. Genetic and environmental effects on the morphological asymmetry in the scale-eating cichlid fish, Perissodus microlepis. Ecol. Evolut. 2015, 5, 4277–4286. [Google Scholar] [CrossRef]
- Kramer, D.L.; Chapman, M.R. Implications of fish home range size and relocation for marine reserve function. Environ. Biol. Fishes 1999, 55, 65–79. [Google Scholar] [CrossRef]
- Vinyoles, D.; De-Sostoa, A.; Franch, C.; Maceda-Veiga, A.; Casals, F.; Caiola, N. Life-history traits of the stone loach Barbatula barbatula. J. Fish Biol. 2010, 77, 20–32. [Google Scholar] [CrossRef]
- Fedorenkova, A.; Vonk, J.A.; Breure, A.M.; Hendriks, A.J.; Leuven, R.S.E.W. Tolerance of native and non-native fish species to chemical stress: A case study for the River Rhine. Aquat. Invasions. 2013, 8, 231–241. [Google Scholar] [CrossRef]
- Casatti, L.; Teresa, F.B.; Gonçalves-Souza, T.; Bessa, E.; Manzotti, A.R.; Gonçalves, C.S.; Zeni, J.O. From forests to cattail: How does the riparian zone influence stream fish. Neotrop. Ichthyol. 2012, 10, 205–214. [Google Scholar] [CrossRef]
- Casatti, L.; Langeani, F.; Silva, A.M.; Castro, R.M. Stream fish, water and habitat quality in a pasture dominated basin, southeastern Brazil. Braz. J. Biol. 2006, 66, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Kennard, M.J.; Arthington, A.H.; Pusey, B.J.; Harch, B.D. Are alien fish a reliable indicator of river health? Freshw. Biol. 2005, 50, 174–193. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Leibold, M.A.; Dray, S. Assessing the effects of spatial contingency and environmental filtering on metacommunity phylogenetics. Ecology 2012, 93, S14–S30. [Google Scholar] [CrossRef]
- Lourenco, L.S.; Souza, U.P.; Fernandes, I.M.; Petrerejr, M. Spatiotemporal variation in life history traits of three small fishes in streams of south-eastern Brazil. Fisher. Manag. Ecol. 2015, 22, 143–151. [Google Scholar] [CrossRef]
- Toft, G.; Baatrup, E.; Guillette, L.J., Jr. Altered social behavior and sexual characteristics in mosquito fish (Gambusia holbrooki) living downstream of a paper mill. Aquat. Toxicol. 2004, 70, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef]
- Whittier, T.R.; Hughes, R.M. Fish and Amphibian Tolerance Values and an Assemblage Tolerance Index for Streams and Rivers in the Western USA. Trans. Am. Fish Soc. 2007, 136, 254–271. [Google Scholar] [CrossRef]
| Items | Axi1 | Axi2 | Axi3 | Axi4 |
|---|---|---|---|---|
| Substrate | −0.8569 | 0.0562 | 0.0997 | 0.2706 |
| Habitat complexity | −0.7431 | 0.2754 | 0.1846 | 0.2906 |
| Velocity-depth combination | −0.7194 | 0.2810 | 0.0990 | 0.2664 |
| Bank stability | −0.4523 | 0.4871 | −0.0140 | −0.0551 |
| Channel alteration | −0.3885 | 0.2352 | 0.0617 | −0.2449 |
| Stream flow conditions | −0.3481 | −0.0686 | −0.4399 | −0.2449 |
| Vegetation diversity | −0.2241 | 0.6394 | 0.2703 | −0.3494 |
| Water quality conditions | −0.7110 | −0.1939 | 0.3191 | −0.2219 |
| Intensity of human activities | −0.5963 | −0.0299 | 0.1136 | −0.5523 |
| Riverside land use | −0.2955 | 0.2746 | 0.2010 | −0.6377 |
| WT | 0.6404 | 0.0177 | 0.3955 | −0.0094 |
| pH | −0.3131 | −0.3660 | −0.2698 | −0.0087 |
| DO | −0.6137 | −0.4973 | −0.1304 | −0.1228 |
| TP | 0.4629 | 0.3555 | −0.3085 | −0.0092 |
| SO42− | 0.7642 | 0.2781 | 0.4459 | 0.1492 |
| NOX | 0.2364 | −0.4163 | 0.7136 | −0.0329 |
| NH3 | 0.6945 | 0.3964 | −0.2868 | 0.0299 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-N.; Ding, H.-Y.; He, X.-G.; Dai, Y.; Zhang, Y.; Ding, S. Assessing Fish Species Tolerance in the Huntai River Basin, China: Biological Traits versus Weighted Averaging Approaches. Water 2018, 10, 1843. https://doi.org/10.3390/w10121843
Wang X-N, Ding H-Y, He X-G, Dai Y, Zhang Y, Ding S. Assessing Fish Species Tolerance in the Huntai River Basin, China: Biological Traits versus Weighted Averaging Approaches. Water. 2018; 10(12):1843. https://doi.org/10.3390/w10121843
Chicago/Turabian StyleWang, Xiao-Ning, Hai-Yu Ding, Xu-Gang He, Yang Dai, Yuan Zhang, and Sen Ding. 2018. "Assessing Fish Species Tolerance in the Huntai River Basin, China: Biological Traits versus Weighted Averaging Approaches" Water 10, no. 12: 1843. https://doi.org/10.3390/w10121843
APA StyleWang, X.-N., Ding, H.-Y., He, X.-G., Dai, Y., Zhang, Y., & Ding, S. (2018). Assessing Fish Species Tolerance in the Huntai River Basin, China: Biological Traits versus Weighted Averaging Approaches. Water, 10(12), 1843. https://doi.org/10.3390/w10121843




