Abstract
Fish species tolerance used as a component of fish-index of biological integrity (F-IBI) can be problematic as it is usually classified using the historical data, data from literature or expert judgments. In this study, fish assemblages, water quality parameters and physical habitat factors from 206 sampling sites in the Huntai River Basin were analyzed to develop tolerance indicator values (TIVs) of fish based on a (Fb-TIVs) and the weighted averaging (WA) method (FW-TIVs). The two quantitative methods for fish tolerance were then compared. The FW-TIVs and Fb-TIVs of fish species were calculated separately using a WA inference model based on ten water quality parameters (WT, pH, DO, SC, TDS, NH3, NO2−, NO3−, TP, Cl−, and SO42−), and six biological traits (lithophilic spawning, benthic invertivores, cold water species, equilibrium or periodic life history strategies, families of Cottidae, and species distribution range). Fish species were then classified into biological traits approach three categories (tolerant species, moderately tolerant species, and sensitive species). The results indicated that only 30.3% fish species have the same classification based on FW-TIVs and Fb-TIVs. However, the proportion of tolerant species based on two methods had a similar response to environmental stress, and these tolerant species were correlated with PCA axes 1 site scores obtained by (FW-TIVs, p < 0.05, R2 = 0.434; Fb-TIVs, p < 0.05, R2 = 0.334) and not correlated with PCA axis 2 site scores (FW-TIVs, p > 0.05, R2 = 0.001; Fb-TIVs, p > 0.05, R2 = 0.012) and PCA axis 3 site scores (FW-TIVs, p > 0.05, R2 = 0.000; Fb-TIVs, p > 0.05, R2 = 0.013). The results of linear regression analyses indicated that Fb-TIVs can be used for the study of fish tolerance. Fish tolerance assessments based on FW-TIVs requires long-term monitoring of fish assemblages and water quality parameters to provide sufficient data for quantitative studies. The Fb-TIV method relies on the accurate identification of fish traits by an ichthyologist. The two methods used in this study can provide methodological references for quantitative studies of fish tolerance in other regions, and are of great significance for the development of biological assessment tools.
1. Introduction
Aquatic organisms are commonly used as biological indicators of river ecosystem health [1] and are used to assess the state of a system relative to its reference conditions. This provides comprehensive information that could be missed or ignored during routine monitoring based on physicochemical parameters [2]. The Index of Biological Integrity (IBI) established by Karr [3] is a very useful fish community indicator that has been applied to other aquatic life such as algae, macro-benthos and macrophytes [4,5,6]. Fish IBI (F-IBI) is a multi-indices system, which describes five biological characteristics [3]. One of these characteristics is species tolerance. Tolerance values are generally obtained from historical records, publications or expert judgments from freshwater F-IBI research around the world, including Victoria Lake, Kenya [7], Chybaish marsh, Iraq [8], and the Guem River, Korea [9].
The sensitivity or tolerance of species is an appropriate indicator of aquatic environmental quality [10], since species survive at locations characterized by high oxygen, clean water, good habitat quality, and low levels of human disturbance. On the contrary, these species are poorly distributed at locations with low oxygen, turbid water, high concentrations of pollutants, and intensive human activities. Using fish species tolerance as a measure of F-IBI is problematic, as there is a lack of data for many countries, including China [11]. In most cases, the characteristics of fish tolerance available from the literature and historical data are only available for a small number of species. Thus, if a species tolerance is unknown, it is often ignored when calculating F-IBI, or else classified according to the existing classification for other species in the same genus or expert judgments. However, the degree of sensitivity and capacity of a species to adapt to changing environmental conditions is often species specific [12]. This subjective classification ignores the interspecific differences of tolerance within the same genus. Carlisle et al. reported that two fish species in the same genus (Etheostoma flabellare and E. olmstedi) sampled from Appalachian streams exhibited interspecific differences [13]. E. flabellare was an intolerant species, whereas E. olmstedi was a tolerant species. Although fish species may be in the same genus, a temporal small-scale disturbance can separate their spatial niches and change their living environment [14]. This indicates that fish species within a genus may have different variability or environmental tolerance under the same circumstances.
The collection of water quality parameters and biological data during routine water quality monitoring addresses the relationship between fish species and general environmental disturbances [15]. For example, the application of the weighted averaging (WA) method based on available datasets [16,17,18] can be used to quantify fish tolerance. The wide range of applications of qualitative classification of fish (FW-TIVs) in the United States provides a reliable framework for the development of fish species tolerance indicator values in other regions. Fish tolerance assessment based on FW-TIVs requires a large amount of data on individual fish species and environmental stress factors, which requires long-term aquatic ecology monitoring. However, this data is not currently available for many areas.
Species traits also reflect tolerance to environmental conditions [19]. For example, fish that are lithophilic spawners and benthic invertivores are intolerant to habitat alteration [20], while cold-adapted species are sensitive to increases in water temperature [21], and species that have equilibrium or periodic life history strategies are sensitive to changes in flow regimes [22,23]. Therefore it is important to understand the interaction between various environmental threats and fish reactions, as specific traits are likely to determine fish responses to different environmental threats [24,25,26]. A combination of species traits and environmental stress provides a new horizon for assessing fish species tolerance, without the need for complicated indoor water chemistry analysis of water quality parameters, and errors of water quality test values associated with seasonal variation (i.e., the water volume increases in the wet season and decreases in the dry season).
The current study aims to quantify fish tolerance using the biological traits approach (Fb-TIVs) and WA method (FW-TIVs), and to compare the application of the two methods for fish tolerance assessment in aquatic ecological monitoring. The quantitative results of fish tolerance arising from this study will be of great use in the development of fish diversity tools.
2. Methods
2.1. Study Area
The Huntai River Basin (40.45°~40.30° N, 122.00°~125.30° E) is located in Liaoning Province, China, and covers an area of 2.73 × 104 km2. The Hun and Taizi rivers rise in the Changbai Mountain Range and converge near the Daliao River, before flowing into the Bohai Sea at Yingkou City, Liaoning Province. The Hun River is 415 km long and Taizi River is 413 km long. The study area has a temperate monsoon climate. The Huntai River Basin is comprised of forested land (46.26%), dry land (22.54%), paddy fields (10.84%), and built urban land (15.45%). The upper and middle reaches of the river basin are mainly covered by deciduous broad-leaved forests, while the lower plain is characterized by intensive human activity and urbanization.
2.2. Fish Sampling
Sampling was carried out at a total of 206 sites in August, 2009 and July, 2010 (Figure 1). Fish were collected using electrofishing equipment and gill nets. Two technical operators with professional experience in backpack electrofishing (LR-24; Smith-Root Inc., Vancouver, WA, USA) collected fish along a zig-zag path within a 200-m reach over a 30-min period. Two gill nets with mesh sizes of 3 × 3 cm and 6 × 6 cm, respectively, were set in the water for 2 h as supplementary fish collection when a reach was not accessible by wading (deeper than 1.5 m). Fish were counted, identified and recorded immediately after collection. All fish were then released, with the exception of that that could not be identified in the field. Unidentified fish were stored in 10% formalin solution and brought back to the laboratory for identification.
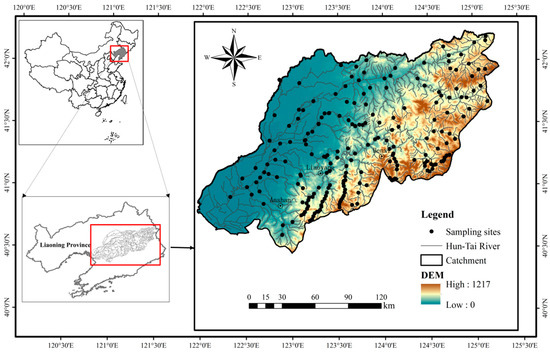
Figure 1.
Location of 206 sampling sites in the Huntai River Basin. Sampling was carried out at these sites in August 2009 and July 2010.
2.3. Water Quality Parameters
Physical and chemical water parameters including water temperature (WT, °C), pH, dissolved oxygen (DO, mg/L), specific conductivity (SC, μS/cm), total dissolved solids (TDS, mg/L), ammonia (NH3, mg/L), nitrites (NO2−, mg/L), nitrates (NO3−, mg/L), total phosphorus (TP, mg/L), chloride ions (Cl−, mg/L), and sulfate (SO42−, mg/L) were recorded. Water quality sampling at each site was carried out 500 m upstream of the fish sampling area to avoid any interference of fish sampling activity on water quality results. Water quality parameters including WT, pH, DO, SC, and TDS were measured with a portable Water Test Kit (YSI Pro 2030, Yellow Springs, OH, USA) in the field. Three water samples (one from the left bank, one from the middle channel and one from the right channel) were collected mixed into a 2 L bottle which was stored in the freezer. These water samples were transported to the laboratory within 48 h of collection to measure NH3, NO2−, NO3−, TP, Cl−, and SO42−. Water quality parameters were determined following the methods outlined in the environmental quality standards for surface water [27].
2.4. Physical Habitat Factors
The quality of physical habitats within the river were determined using the habitat evaluation index system. This system includes ten habitat factors: substrate, habitat complexity, velocity–depth combination, intensity of human activities, riverside land use, bank stability, channel alteration, stream flow conditions, vegetation diversity, and water quality conditions [28,29]. Each parameter has four scoring categories: optimal (score of 16–20), suboptimal (score of 11–15), marginal (score of 6–10), poor (score of 0–5). The score for each parameter at each sampling site was determined by expert visual evaluation in the field (Table A1).
2.5. Data Analysis
2.5.1. Calculation of FW-TIVs
FW-TIVs of fish species were calculated using a WA [16,30] inference model based on ten water quality parameters (TW, pH, DO, SC, TDS, NH3, TP, Cl−, SO42−, and NOx (NOx = NO2− + NO3−)) [17]. To increase the confidence of the estimates, species with less than thirty individuals recorded across all sites were removed before calculations [10]. The WA for each species under each water quality parameter was calculated using the following formula:
where the weighted average for each species j is expressed by WAj; X is one of the water quality parameters measured from site 1, 2, …, n; Y is the individual number of species j from site 1, 2, …, n. For example, in this study the WA of Abbottina liaoningensis for TW was obtained by multiplying the TW value for each site by the number of A. liaoningensis recorded at that site. The sum of this value for each site was then divided by the total number of A. liaoningensis recorded at all sampling sites.
The WA for each species under each water quality parameter was calculated separately to get a WA data set. The WA for each species was transformed into an ordinal rank using a 10-point ordinal classification [30,31] based on the 10% quantile of WAs. A species FW-TIV was equal to the mean of the ordinal ranks of ten WA, and fish species were classified into three categories according to FW-TIV: tolerant species (FW-TIV = 1–4), moderately tolerant species (FW-TIV = >4–<7), and sensitive species (FW-TIV = 7–10) [17].
2.5.2. Calculation of Fb-TIVs
Both tolerance and sensitivity indicate the extent to which a species is physically or behaviorally affected by environmental conditions. Based on fish species traits and the sensitivity association approach [19,20], six biological traits (lithophilic spawning, benthic invertivores, cold water species, equilibrium or periodic life history strategies, families of Cottidae, and species distribution range) were selected to describe fish species tolerance. Species were also classified according to key biological traits [32], and each trait except for species range distribution was assigned a score of 0 (species do not have this trait) or 1 (species have this trait). The score for fish range distribution was calculated as the frequency of occurrence subtracted from one. The scores were scaled from 0 for species found in all sites to 1 for species found in a single site, with the exception of the minimum and maximum score. The frequency of occurrence for each species was given a score within this scale (e.g., a species found in 35% of sites would be given a score of 0.65, while one found at 75% of sites would be given a score of 0.25). The above scoring rules followed those described by Sievert et al. [20] and the Fb-TIVs were the sum of all six traits. For comparison with FW-TIV values, Fb-TIV values were also divided into three groups, i.e., tolerant species (Fb-TIV = 0–2), moderately tolerant species (Fb-TIV = >2–<4), sensitive species (Fb-TIV = 4–6).
2.5.3. Statistical Analysis
In order to compare the response of tolerant species to environmental disturbances based on the two methods, major environmental pressure gradients were established. The Shapiro–Wilk test was used to determine whether the data were normally distributed, and Spearman’s correlation coefficient was then used to remove the redundant parameters (|Pearson’s coefficients| > 0.7) [30]. Principal component analysis (PCA) was applied to identify the major environmental pressure gradients [33]. The number of PCA axes were determined using Kaiser’s rule requiring eigenvalues >1. Parameters were considered as major stressors, when the absolute value of loading for axes was ≥0.5. One-way analysis of variance (ANOVA) was used to analyze the differences in fish tolerance classifications based on two methods. To assess the concordance of tolerant species based on Fw-TIV values and Fb-TIV values to environment stress, linear regression was used. Data were transformed (lg(x+1)) before analysis. Correlation analysis and PCA were performed using CANOCO 4.5. One-way ANOVA was performed using SPSS 19.0 and linear regression was conducted using Sigmaplot 12.5.
3. Results
3.1. The Relationship between the FW-TIV Method and the Fb-TIV Method for Species Tolerance Classification
A total of 33,102 fish representing 42 species were collected from 206 sampling sites. Prior FW-TIV and Fb-TIV calculations, nine species that had a low frequency of occurrence were excluded from the dataset. Therefore, calculations were carried out using data relating to 33,013 individuals representing 33 species.
FW-TIV results found that seven species (21.2%) were tolerant, 18 species (54.6%) were moderately tolerant and eight species (24.2%) were sensitive. Cottus poecilopus (FW-TIV = 10.0) and Hypomesus olidus (FW-TIV = 9.1) were the most sensitive species. The two most tolerant species were Oryzias latipes (FW-TIV = 1.9) and Hemiculter leucisculus (FW-TIV = 2.0). However, fish tolerance classification based on Fb-TIV values found that 26 species (78.8%) were tolerant, six species (18.2%) were moderately tolerant and a single species (3%) was sensitive. C. poecilopus (Fb-TIV = 4) was the most sensitive species, while Cobitis granoei (Fb-TIV = 0.6) and Misgurnus anguillicaudatus (Fb-TIV = 0.6) were the most tolerant species (Table A2). One-way ANOVA revealed that there was a significant different between fish tolerance classification based on the two methods (F = 28.5, P = 0).
3.2. The Relationship between Fw-TIV, Fb-TIV and Environmental Pressure Gradients
Pearson’s correlation coefficients showed high redundancy between SC and TDS (r = 0.794, p < 0.01), SC and Cl− (r = 0.822, p < 0.01), SC and SO42− (r = 0.828, p < 0.01), Cl− with SO42− (r = 0.724, p < 0.01), Cl− with NH3 (r = 0.789, p < 0.01), TDS with Cl− (r = 0.705, p < 0.01), and TDS with SO42− (r = 0.907, p < 0.01). In this study, TDS, SC and Cl− were removed, whereas SO42− was retained for further analysis as sulfate is a dominant ion reflecting background characteristics of ion composition in the mountainous river.
Four significant PCA axes were derived, which explained 67.8% of the variation among these environmental parameters. Axis 1 was most heavily influenced by five physical habitat factors (substrate, habitat complexity, velocity-depth combination, water quality conditions, intensity of human activities) and four water quality parameters (WT, DO, SO42−, NH3), representing 39.9% of the variation. Axis 2 was most strongly associated with vegetation diversity which accounted for 10.3% of the variations. Axis 3 explained 10% of the variation and included NOx. Axis 4 only explained 7.6% of the variation and included intensity of human activities and riverside land use (Table 1). As PCA axes 1, 2, and 3 explained most of the data variability, they were used as stressor gradients for subsequent analysis.

Table 1.
Eigenvector matrix for principal component analysis (bold values are considered high ≥ |0.5|). WT: water temperature; DO: dissolved oxygen; TP: total phosphorus.
Proportion of tolerant species obtained by FW-TIV correlated with PCA axis 1 site scores (p < 0.05, R2 = 0.434; Figure 2a), but had no correlation with PCA axis 2 site scores (p > 0.05, R2 = 0.001; Figure 2c) and PCA axis 3 site scores (p > 0.05, R2 = 0.000; Figure 2e). Proportion of tolerant species obtained by Fb-TIV correlated with PCA axis 1 site scores (p < 0.05, R2 = 0.334; Figure 2b), although there was no correlation with PCA axis 2 site scores (p > 0.05, R2 = 0.012; Figure 2d) and PCA axis 3 site scores (p > 0.05, R2 = 0.013; Figure 2f).
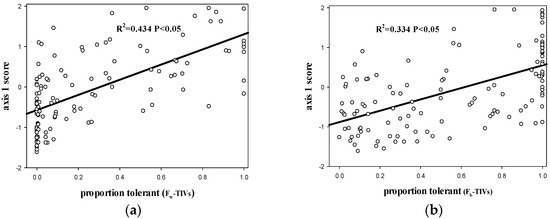
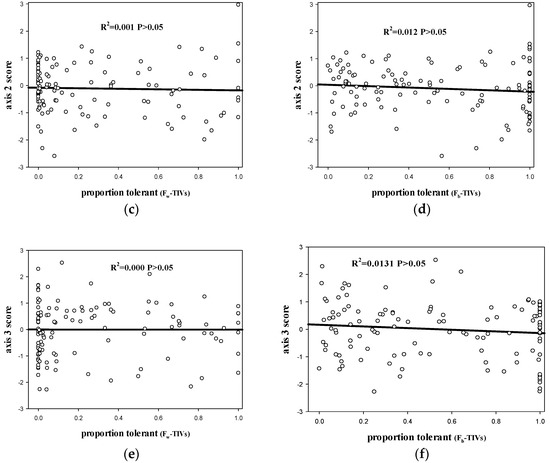
Figure 2.
(a) scatterplot of PC1 site scores versus the proportion of tolerant fish at each site based on FW-TIVs; (b) scatterplot of PC1 site scores versus the proportion of tolerant fish at each site based on Fb-TIVs; (c) scatterplot of PC2 site scores versus the proportion of tolerant fish at each site based on FW-TIVs; (d) scatterplot of PC2 site scores versus the proportion of tolerant fish at each site based on Fb-TIVs; (e) scatterplot of PC3 site scores versus the proportion of tolerant fish at each site based on FW-TIVs; (f) scatterplot of PC3 site scores versus the proportion of tolerant fish at each site based on Fb-TIVs. Line is best fit. (Fb-TIVs: tolerance indicator values (TIVs) of fish based on biological traits approach. FW-TIVs: tolerance indicator values (TIVs) of fish based on weighted averaging (WA) method).
4. Discussion
Determining fish tolerance based on quantitative methods (Fw-TIV and Fb-TIV) is more advantageous than using qualitative methods (e.g., expert judgments) in the application and development of biological assessment tools. Quantitative methods are better able to identify fish tolerance characteristics on environmental stress. Segurado et al. [10] suggested that experts tend to classify species which occur less frequently as moderately tolerant species. Species that were recorded to occur less frequently in the current study, such as Pseudogobio vaillanti (present at 5 of 206 sites) and Oryzias latipes sinensis (present at 17 of 206 sites), were considered moderately tolerant species by experts [34]. Both methods used in the current study found that these species were tolerant. Tolerance as defined by Bressler et al., is the ability of a living organism to survive and reproduce under environmental stress [35]. Tolerance classifications based on expert opinions are vague and may result in an underestimation of the tolerance of some fish species. For example, Phoxinus lagowskii and Opsariichthys bidens were classified as sensitive species by experts due to their distribution [34], however both methods used in the current study demonstrated that they were moderately tolerant species. Species distribution may result from historical factors rather than species sensitivity to environmental stressors. Quantitative methods may be more suitable for the development of a new biological assessment index. For example, the development of Biotic Indices (BI) and the Biological Monitoring Working Party (BMWP) are based on quantitative tolerance values of benthic macro-invertebrates [36].
The fish tolerance assessment system developed in the current study is based on the fact that traits are the result of environmental adaptation [37]. For example, Carassius auratus and M. anguillicaudatus are classified as tolerant species based on Fb-TIV values. Both of these species can be found in many types of water bodies in China, especially in urban reaches with low oxygen and high pollution levels. Their strong dispersal ability helps them adept to environmental changes, as they can choose favorable habitats for survival [38]. Furthermore, they are omnivores, which means they can consume a wide variety of prey, aiding their survival [39,40] when inhabiting highly disturbed environments. The relationship between fish biological traits and sensitivity also has been recognized by other researchers. Hermoso et al. [41] found the widely distributed species Luciobarbus comizo showed a higher tolerance than species that are only found upstream in Guadiana River basin. Species with low sensitivity have a relatively high potential for species distribution under environmental changes (i.e., pH, salinity, temperature) [42]. The biological traits approach helps to explain the adaptation mechanisms of fish in specific environmental conditions [43] and examines fish traits in relation to environmental pressure gradients as a novel approach in assessing fish tolerance.
It must be noted, however, that fish tolerance classification based on Fb-TIV showed a significant difference when compared with Fw-TIV. These discrepancies may be due to the number of available traits. In order to improve the accuracy of the assessment results, more traits would need to be included. It is well known that the fish traits such as morphology and body size vary in the process of environmental adaptation [44,45]. Generally, fish with spindle-shaped bodies display strong swimming ability to evade the impact of environmental pressures, and larger-bodied species can more readily populate new regions to avoid substrate changes due to the positive correlation of body size with home range size [46]. In addition, the reproductive period of fish is considered to be sensitive to changes in river flow [47]. These theories suggest that it is important to provide multifarious traits to improve the efficacy of the biological traits approach. Secondly, fish traits were assigned 0 or 1 scores in this study, which may limit the results of fish tolerance classification. If more grade divisions are allocated for each trait, the classification of a fish species tolerance may change.
The stressors affecting fish tolerance in the Fw-TIV method have been the focus of previous research. Fedorenkova et al. [48] stated that physical parameters should be considered in species tolerance studies. With the degraded gradient of riparian vegetation, sensitive fish species are gradually disappearing and being replaced by tolerant species [49]. Casatti et al. [50] found that the abundance of fish species changed with physical habitat index, and some species showed optimal distribution corresponding to the degree of habitat conservation. The PCA analysis results from the current study also indicate that physical habitat factors are important environmental stress factors. However, measured physical habitat factors were not used to calculate Fw-TIV values in the current study. There are many environmental factors affecting fish, including natural variables such as elevation, slopes and longitude, anthropogenic variables such as hydro-morphology, land use, and substrate. However, water quality is the most direct factor affecting fish. Therefore, the Fw-TIV method used in this study is a reliable means of identifying fish tolerance. In order to improve the accuracy of the Fw-TIV method, more environmental factors will be included in future studies.
Geographical scales should also be recognized as a common problem between the two methods used. Many researchers have found that the tolerance range of individual species varies between different regions [30,51]. From the individual level of fish, the geographical distribution of river basins determines the broader environmental gradient that ultimately selects suitable species traits [52]. Lourenco et al. [53] found that the maturation time of Piabina argentea differed between basins. Fish living in different eco-regions are subject to different levels of environmental stresses. Some researchers have indicated that fish reproduction can be promoted or inhibited by different levels of environmental stress [54,55]. The fish tolerance values obtained in the current study are for an eco-region with similar aquatic ecosystems. For example, Whittier and Hughes [56] divided their study area into three eco-regions to calculate the tolerance values of fish and amphibians. The United States Environmental Protection Agency have developed five regional systems for TIVs of macro-invertebrates [29] in order to reduce estimation errors caused by regional differences in environment.
Both methods used in the current study can be used for quantitative studies of fish tolerance and both have advantages in application. For the Fw-TIV method, data from a high number of individuals and environmental stress factors from long-term aquatic ecology monitoring are required. However, the development of emerging technologies and informatization makes processing Fw-TIV values simple. If there is a large amount of biological monitoring available data for an eco-region, it is more convenient to use the Fw-TIV method for identifying fish tolerance. For example, fish communities and water quality are monitored in U.S. for the long-term National Water-Quality Assessment Program and the collected data can be used directly by managers for Fw-TIV analysis. In the Fb-TIV method, less survey effort is required, but professional identification of fish traits by an ichthyologist is needed. When aquatic ecology data is not available in some eco-regions, the Fb-TIV method is acceptable for the quantitative study of fish tolerance in these areas. The two methods used in the current study provide methodological references for quantitative studies of fish tolerance in other regions and are of great significance for the development of biological assessment tools.
Author Contributions
All authors listed have contributed to this study. X.-N.W., S.D. and G.-H.X. had a substantial involvement in the conception, guidance, and revising of the manuscript. The data acquisition and analysis were done by H.-Y.D., H.D. and Y.Z. The manuscript was written by X-N.W. and S.D.
Funding
This study was supported by grants from the National Natural Science Foundation of China (41401066), the National Natural Science Foundation of China (41571050), and the Fundamental Research Funds for the Central Universities (2662015QD004).
Acknowledgments
All authors listed have contributed to this study. Xianwei Huang supplied excellent field assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Habitat assessment scoring descriptions.
Table A1.
Habitat assessment scoring descriptions.
| Factor | Optimal | Suboptimal | Marginal | Poor |
|---|---|---|---|---|
| Substrate | More than 75% composition of gravel, cobbles and big stones | 50%–75% composition of gravel, cobbles and big stones | 25%–50% composition of gravel, cobbles and big stones | Less than 25% composition of gravel, cobbles and big stones |
| Habitat complexity | Composition of aquatic vegetation, litter, fallen wood, concave banks and boulders, etc. | Composition of aquatic vegetation, litter, fallen wood, and concave banks, etc. | Domination by one or two kinds of microhabitat | Domination by one kind of microhabitat and the substrate mainly composed by silt or fine sand |
| Velocity-depth combination | Slow (<0.3 m/s)-deep (>0.5 m); slow-shallow (<0.5 m); fast (>0.3 m/s)-deep; fast-shallow three types of habitats | Only three types of habitat (fast-shallow type got the highest value) | Only two types of habitat (in the absence of fast-shallow type and slow-shallow type) | Predominated by one velocity–depth type (usually pools) |
| Intensity of human activities | Hardly any human disturbance | Minimal human disturbance by few walkers or bikes | Less human disturbance by vehicles | Serious human disturbance by motor vehicles |
| Riverside land use | No agricultural land on either side of the river bank | Agricultural land present on one side of the river bank | Agricultural land present on both sides of the river bank | Weathered soils after fallow conditions present on both sides of the river bank |
| Bank stability | No erosion on the river banks. Less than 5% of the bank is damaged in the visual range (100 m) | 5%–30% erosion of the bank in the visual range (100 m) | 30%–60% erosion of the bank in the visual range (100 m) | More than 60% erosion of the bank in the visual range (100 m) |
| Channel alteration | No channelization of the river | Less channelization around the pier | Embankments or bridge pillars on both sides of the strait and more extensive channelization of the river | River bank fixed by wire and cement |
| Stream flow conditions | Large water volume, only a few exposed areas of the bank visible | Relatively large volume and 75% of the river bank is covered | Relatively high volume covering 25%–75% of the river bank | Small water volume and dry river course |
| Vegetation diversity | Over 50% coverage of vegetation | 25%–50% vegetation coverage | Less than 25% vegetation coverage | Hardly any vegetation coverage |
| Water quality conditions | Low turbidity, no sedimentation, no odor from the river water | Low turbidity, a small amount of odor from the river water | Water with a high turbidity and odorous water | High turbidity and foul smelling water |
| score | 15–20 | 10–15 | 5–10 | 0–5 |
Note: reference as [28].

Table A2.
Summary of Fw-TIVs and Fb-TIVs for fish species and tolerance classifications.
Table A2.
Summary of Fw-TIVs and Fb-TIVs for fish species and tolerance classifications.
| Species | Fw-TIVs | Fb-TIVs |
|---|---|---|
| Abbottina liaoningensis | 6.3 (M) | 1.8 (T) |
| Abbottina rivularis | 7.1 (S) | 1.6 (T) |
| Cobitis granoei | 6.7 (M) | 0.6 (T) |
| Barbatula barbatula nuda | 4.3 (M) | 2.4 (M) |
| Rhodeus lighti | 4.2 (M) | 0.9 (T) |
| Rhodeus sinensis | 4.6 (M) | 1.0 (T) |
| Squalidus chankaensis | 5.6 (M) | 2.0 (T) |
| Squalidus wolterstorffi | 7.6 (S) | 1.0 (T) |
| Leuciscus waleckii | 8.3 (S) | 2.9 (M) |
| Carassius auratus | 4.1 (M) | 0.6 (T) |
| Zacco platypus | 6.7 (M) | 1.6 (T) |
| Rostrogobio liaohensis | 5.9 (M) | 1.0 (T) |
| Gobio lingyuanensis | 3.4 (T) | 2.0 (T) |
| Gobio rivuloides | 2.1 (T) | 1.0 (T) |
| Gobio cynocephalus | 4.1 (M) | 2.9 (M) |
| Phoxinus lagowskii | 6.2 (M) | 2.4 (M) |
| Opsariichthys bidens | 4.7 (M) | 2.0 (T) |
| Pseudorasbora parva | 5.6 (M) | 1.6 (T) |
| Hemiculter leucisculus | 2.0 (T) | 0.9 (T) |
| Huigobio chinssuensis | 8.2 (S) | 0.9 (T) |
| Pseudogobio vaillanti | 2.3 (T) | 2.0 (T) |
| Acheilognathus chankaensis | 2.4 (T) | 0.9 (T) |
| Misgurnus anguillicaudatus | 5.0 (M) | 0.6 (T) |
| Lefua costata | 4.5 (M) | 0.8 (T) |
| Hypomesus olidus | 9.1 (S) | 3.0 (M) |
| Lampetra mori | 6.6 (M) | 2.0 (T) |
| Perccottus glenni | 5.1 (M) | 1.0 (T) |
| Ctenogolius brunneus | 4.0 (T) | 2.8 (M) |
| Cottus poecilopus | 10.0 (S) | 4.0 (S) |
| Hypseleotris swinhonis | 4.6 (M) | 0.9 (T) |
| Odontobutis yaluensis | 7.8 (S) | 1.8 (T) |
| Oryzias latipes sinensis | 1.9 (T) | 0.9 (T) |
| Pungitius pungitius | 9.0 (S) | 0.9 (T) |
Note: T represents tolerant species, M represents moderate species, S represents sensitive species.
References
- O’Brien, A.; Townsend, K.; Hale, R.; Sharley, D.; Pettigrove, V. How is ecosystem health defined and measured? A critical review of freshwater and estuarine studies. Ecol. Indic. 2016, 69, 722–729. [Google Scholar] [CrossRef]
- Ogren, S.A. Using Indicators of Biotic Integrity for Assessment of Stream Condition. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2014. [Google Scholar]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Zalack, J.T.; Smucker, N.J.; Vis, M.L. Development of a diatom index of biotic integrity for acid mine drainage impacted streams. Ecol. Indic. 2010, 10, 287–295. [Google Scholar] [CrossRef]
- Beck, M.W.; Vondracek, B.; Hatch, L.K. Environmental clustering of lakes to evaluate performance of a macrophyte index of biotic integrity. Aquat. Bot. 2013, 108, 16–25. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, J.F.; Cai, Y.J.; Yin, H.B.; Gao, Y.N.; Zhao, J.H.; Liu, L.Z.; Huang, J.C. Development and application of benthic macroinvertebrate-based multimetric indices for the assessment of streams and rivers in the Taihu Basin, China. Ecol. Indic. 2015, 48, 649–659. [Google Scholar] [CrossRef]
- Raburu, P.O.; Masese, F.O. Development of a fish-based index of biotic integrity (FIBI) for monitoring riverine ecosystems in the Lake Victoria drainage Basin, Kenya. River Res. Appl. 2012, 28, 23–38. [Google Scholar] [CrossRef]
- Mohamed, A.R.M. A fish index of biotic integrity for evaluation of fish assemblage environment in restored Chybaish marsh, Iraq. Glob. J. Biol. Agric. Health Sci. 2014, 3, 32–37. [Google Scholar]
- Kim, J.; Koh, D.K.; Cho, S. An assessment of habitat conditions for fish species in the Guem River, Korea. Ecol. Environ. 2011, 167, 309–320. [Google Scholar]
- Segurado, P.; Santos, J.M.; Pont, D.; Melcher, A.H.; Jalon, D.G.; Hughes, R.M.; Ferreira, M.T. Estimating species tolerance to human perturbation: Expert judgment versus empirical approaches. Ecol. Indic. 2011, 11, 1623–1635. [Google Scholar] [CrossRef]
- Wu, W.; Xu, Z.; Zhan, C.; Yin, X.; Yu, S. A new framework to evaluate ecosystem health: A case study in the Wei River basin, China. Environ. Monit. Assess. 2015, 187, 460. [Google Scholar] [CrossRef] [PubMed]
- Chevin, L.M.; Lande, R.; Mace, G.M. Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biol. 2010, 8, e1000357. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, D.M.; Hawkins, C.P.; Meador, M.R.; Potapova, M.; Falcone, J. Biological assessments of Appalachian streams based on predictive models for fish, macroinvertebrate, and diatom assemblages. J. N. Am. Benthol. Soc. 2008, 27, 16–37. [Google Scholar] [CrossRef]
- Brenner, M.; Buck, B.; Cordes, S.; Dietrich, L.; Jacob, U.; Mintenbeck, K.; Schröder, A.; Brey, T.; Knust, R.; Arntz, W. The role of iceberg sours in niche separation within the Antarctic fish genus Trematomus. Polar Biol. 2001, 24, 502–507. [Google Scholar]
- Benejam, L.; Aparicio, E.; Vargas, M.; Vila-Gispert, A.; García-Berthou, E. Assessing fish metrics and biotic indices in a Mediterranean stream: Effects of uncertain native status of fish. Hydrobiologia 2008, 603, 197–210. [Google Scholar] [CrossRef]
- Carlisle, D.M.; Meador, M.R.; Li, S.R.M.; Ruhl, P.M. Estimation and application of indicator values for common macroinvertebrate genera and families of the United States. Ecol. Indic. 2007, 7, 22–33. [Google Scholar] [CrossRef]
- Meador, M.R.; Carlisle, D.M. Quantifying tolerance indicator values for common stream fish species of the United States. Ecol. Indic. 2007, 7, 329–338. [Google Scholar] [CrossRef]
- Minnesota Pollution Control Agency (MPCA). Development of a Fish-Based Index of Biological Integrity for Minnesota’s Rivers and Streams; Number wq-bsm2-03; Minnesota Pollution Control Agency, Environmental Analysis and Outcomes Division: St. Paul, MN, USA, 2014. [Google Scholar]
- Poff, L.R.; Olden, J.D.; Strayer, D.L. Climate Change and Freshwater Fauna Extinction Risk; Island Press-Center for Resource Economics: Washington, DC, USA, 2012. [Google Scholar]
- Sievert, N.A.; Paukert, C.P.; Tsang, Y.P.; Infante, D. Development and assessment of indices to determine stream fish vulnerability to climate change and habitat alteration. Ecol. Indic. 2016, 67, 403–416. [Google Scholar] [CrossRef]
- Morgan, I.J.; McDonald, D.G.; Wood, C.M. The cost of living for freshwater fish in a warmer, more polluted world. Glob. Chang. Biol. 2001, 7, 345–355. [Google Scholar] [CrossRef]
- Mims, M.; Olden, J. Life history theory predicts fish assemblage response to hydrologic regimes. Ecology 2012, 93, 35–45. [Google Scholar] [CrossRef]
- Mims, M.C.; Olden, J.D. Fish assemblages respond to altered flow regimes via ecological filtering of life history strategies. Freshw. Biol. 2013, 58, 50–62. [Google Scholar] [CrossRef]
- Murray, K.A.; Rosauer, D.; McCallum, H.; Skerratt, L.F. Integrating species traits with extrinsic threats: Closing the gap between predicting and preventing species declines. Proc. R. Soc. B Biol. Sci. 2011, 278, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, M.; Gomez, A.; Revilla, E. Which intrinsic traits predict vulnerability to extinction dependson the actual threatening processes. Ecosphere 2013, 4, art76. [Google Scholar] [CrossRef]
- Garcia, R.A.; Araújo, M.B.; Burgess, N.D.; Foden, W.B.; Gutsche, A.; Rahbek, C.; Cabeza, M. Matching species traits to projected threats and opportunities from climate change. J. Biogeogr. 2014, 41, 724–735. [Google Scholar] [CrossRef] [PubMed]
- CSEPB (Chinese State Environment Protection Bureau). Water and Wastewater Monitoring and Analysis Methods, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002; ISBN 9787801634009. [Google Scholar]
- Zheng, B.H.; Zhang, Y.; Li, Y.B. Study of indicators and methods for river habitat assessment of Liao River Basin. Acta Sci. Circumst. 2007, 27, 928–936. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wade Able Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999.
- Macedaveiga, A.; Sostoa, A.D. Observational evidence of the sensitivity of some fish species to environmental stressors in Mediterranean rivers. Ecol. Indic. 2011, 11, 311–317. [Google Scholar] [CrossRef]
- Stevens, C.E.; Council, T.; Sullivan, M.G. Influences of human stressors on fish-based metrics for assessing river condition in Central Alberta. Water Qual. Res. J. Can. 2010, 45, 35–46. [Google Scholar] [CrossRef]
- Xie, Y.H. Freshwater Fishes in Northeast Region of China; Liaoning Science and Technology Press: Shenyang, China, 2007; ISBN 978-7-5381-5259-3. (In Chinese) [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier Science: Amsterdam, The Netherland, 1998. [Google Scholar]
- Zhang, H.; Ding, S.; Zhang, Y.; Jia, X.B.; Meng, W.; Guo, B. Assessment of the fish index of biotic integrity and its relationship with environmental factors in the Xiliao River Basin. J. Lake Sci. 2015, 27, 829–839. (In Chinese) [Google Scholar]
- Bressler, D.W.; Stribling, J.B.; Paul, M.J.; Hicks, M.B. Stressor tolerance values for benthic macroinvertebrates in Mississippi. Hydrobiologia 2006, 573, 155–172. [Google Scholar] [CrossRef]
- Mandaville, S.M. Benthic Macroinvertebrates in Freshwaters Taxa Tolerance Values, Metrics, and Protocols; New York State Department of Environmental Conservation: Albany, NY, USA, 2002.
- Goldstein, R.M.; Meador, M.R. Multilevel assessment of fish species traits to evaluate habitat degradation in streams of the upper Midwest. N. Am. J. Fish Manag. 2005, 25, 180–194. [Google Scholar] [CrossRef]
- Bonada, N.; Doledec, S.; Statzner, B. Taxonomic and biological trait differences of stream macroinvertebrate communities between Mediterranean and temperate regions: Implications for future climatic scenarios. Glob. Chang. Biol. 2007, 13, 1658–1671. [Google Scholar] [CrossRef]
- Chessman, B.C. Identifying species at risk from climate change: Traits predict the drought vulnerability of freshwater fishes. Biol. Conserv. 2013, 160, 40–49. [Google Scholar] [CrossRef]
- Lima, A.C.; Wrona, F.J.; Soares, A.M.V.M. Fish traits as an alternative tool for the assessment of impacted rivers. Rev. Fish Biol. Fish. 2017, 27, 31–42. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M.; Blanco-Garrido, F.; Prenda, J. Assessing freshwater fish sensitivity to different sources of perturbation in a Mediterranean basin. Ecol. Freshw. Fish. 2010, 18, 269–281. [Google Scholar] [CrossRef]
- Hare, J.A.; Morrison, W.E.; Nelson, M.W.; Stachura, M.M.; Teeters, E.J.; Griffis, R.B.; Alexander, M.A.; Scott, J.D.; Alade, L.; Bell, R.J.; Chute, A.S.; et al. A vulnerability assessment of fish and invertebrates to climate change on the Northeast U.S. Continental Shelf. PLoS ONE 2016, 11, e0146756. [Google Scholar] [CrossRef]
- Lamouroux, N.; Poff, N.L.; Angermeier, P.L. Intercontinental convergence of stream fish community traits in France and Virginia (USA) streams along hydraulic and geomorphic gradients. Ecology 2002, 83, 1792–1807. [Google Scholar] [CrossRef]
- Blanck, A.; Lamouroux, N. Large-scale intraspecific variation in life history traits of 44 freshwater fish. J. Biogeogr. 2007, 34, 862–875. [Google Scholar] [CrossRef]
- Lee, H.J.; Valentin, H.; Axel, M. Genetic and environmental effects on the morphological asymmetry in the scale-eating cichlid fish, Perissodus microlepis. Ecol. Evolut. 2015, 5, 4277–4286. [Google Scholar] [CrossRef]
- Kramer, D.L.; Chapman, M.R. Implications of fish home range size and relocation for marine reserve function. Environ. Biol. Fishes 1999, 55, 65–79. [Google Scholar] [CrossRef]
- Vinyoles, D.; De-Sostoa, A.; Franch, C.; Maceda-Veiga, A.; Casals, F.; Caiola, N. Life-history traits of the stone loach Barbatula barbatula. J. Fish Biol. 2010, 77, 20–32. [Google Scholar] [CrossRef]
- Fedorenkova, A.; Vonk, J.A.; Breure, A.M.; Hendriks, A.J.; Leuven, R.S.E.W. Tolerance of native and non-native fish species to chemical stress: A case study for the River Rhine. Aquat. Invasions. 2013, 8, 231–241. [Google Scholar] [CrossRef]
- Casatti, L.; Teresa, F.B.; Gonçalves-Souza, T.; Bessa, E.; Manzotti, A.R.; Gonçalves, C.S.; Zeni, J.O. From forests to cattail: How does the riparian zone influence stream fish. Neotrop. Ichthyol. 2012, 10, 205–214. [Google Scholar] [CrossRef]
- Casatti, L.; Langeani, F.; Silva, A.M.; Castro, R.M. Stream fish, water and habitat quality in a pasture dominated basin, southeastern Brazil. Braz. J. Biol. 2006, 66, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Kennard, M.J.; Arthington, A.H.; Pusey, B.J.; Harch, B.D. Are alien fish a reliable indicator of river health? Freshw. Biol. 2005, 50, 174–193. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Leibold, M.A.; Dray, S. Assessing the effects of spatial contingency and environmental filtering on metacommunity phylogenetics. Ecology 2012, 93, S14–S30. [Google Scholar] [CrossRef]
- Lourenco, L.S.; Souza, U.P.; Fernandes, I.M.; Petrerejr, M. Spatiotemporal variation in life history traits of three small fishes in streams of south-eastern Brazil. Fisher. Manag. Ecol. 2015, 22, 143–151. [Google Scholar] [CrossRef]
- Toft, G.; Baatrup, E.; Guillette, L.J., Jr. Altered social behavior and sexual characteristics in mosquito fish (Gambusia holbrooki) living downstream of a paper mill. Aquat. Toxicol. 2004, 70, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef]
- Whittier, T.R.; Hughes, R.M. Fish and Amphibian Tolerance Values and an Assemblage Tolerance Index for Streams and Rivers in the Western USA. Trans. Am. Fish Soc. 2007, 136, 254–271. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).