Application of a Rapid and Simple UV-Spectrophotometric Method for the Study of Desorption of Esterquat Collectors in Tailings–Seawater Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Calculations
2.2.1. Instruments and Apparatus
2.2.2. Stock and Standard Solution Preparation and Sample Analysis
2.2.3. Adsorption Tests
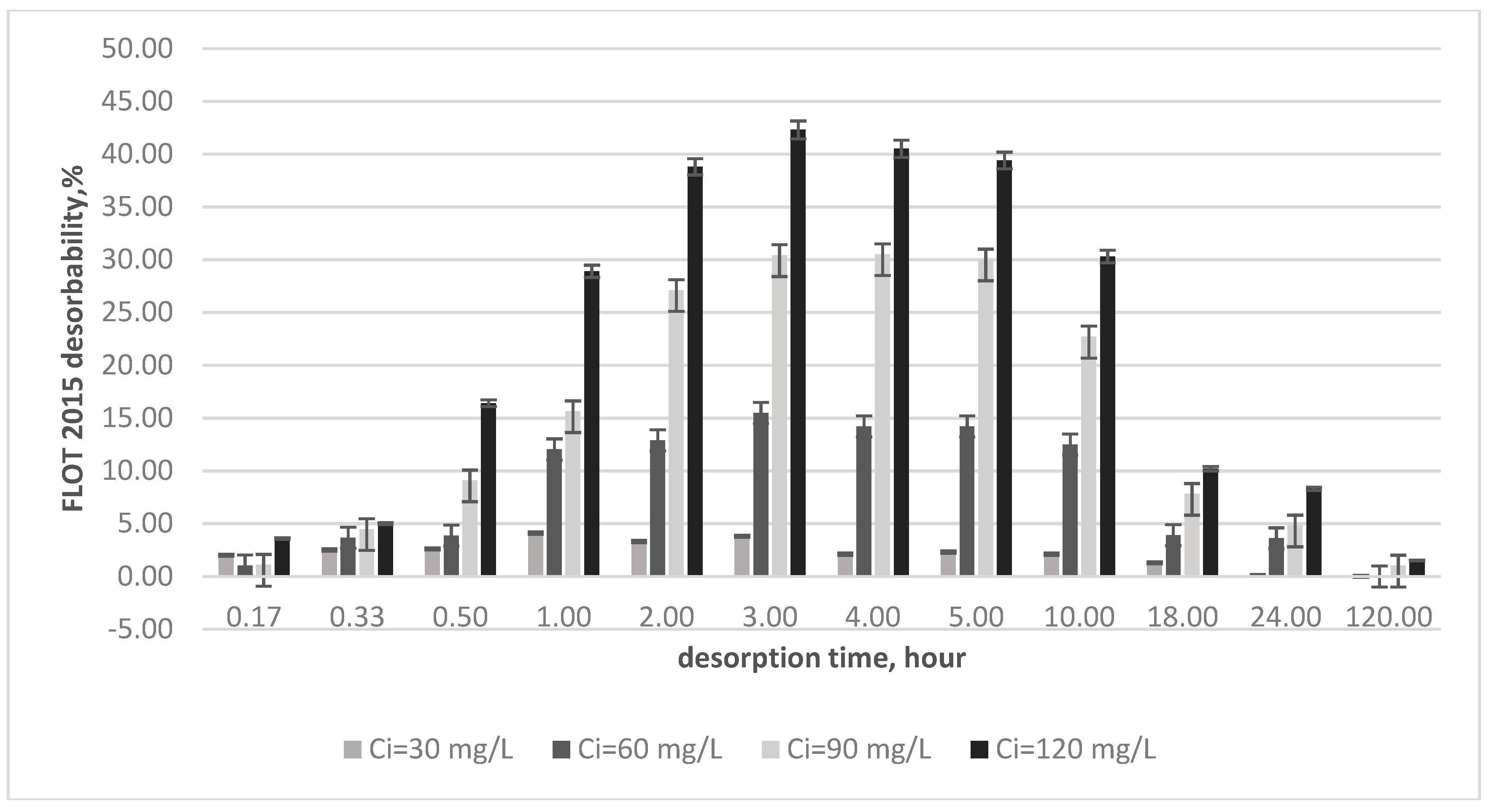
2.2.4. Desorption Tests
2.2.5. Degradation Tests
2.2.6. Calculations
3. Results and Discussion
3.1. Validation of the Proposed UV-Spectrophotometric Method
3.2. Adsorption Kinetics Tests
3.3. Desorption Kinetics Tests
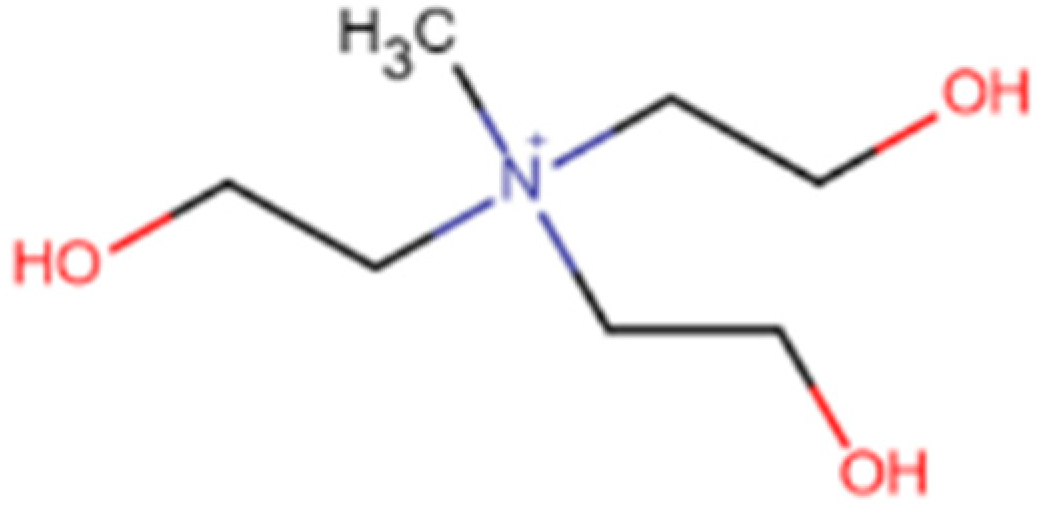
3.4. Study on Tris(2-hydroxyethyl)(methyl)azanium Methylsulfate (3HEMA MS)
3HEMA MS-BCP Complex Formation
3.5. Degradation Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Contributions of Ions | Concentration, % |
|---|---|
| Cl− | 19.353 |
| Na+ | 10.764 |
| SO42− | 2.700 |
| Mg2+ | 1.297 |
| Ca2+ | 0.408 |
| K+ | 0.387 |
| HCO3− | 0.142 |
| Br− | 0.066 |
| Sr2+ | 0.014 |
| H3BO3 | 0.026 |
| F− | 0.001 |
| Total | 35.158 |
| Salts | Concentration, g/kg of the Solution |
|---|---|
| NaCl | 23.926 |
| Na2SO4 | 4.008 |
| KCl | 0.877 |
| NaHCO3 | 0.196 |
| KBr | 0.098 |
| H3BO3 | 0.026 |
| Salts | Concentration, mol/kg of the Solution |
|---|---|
| MgCl2·6H2O | 0.05330 |
| CaCl2·2H2O | 0.01030 |
| SrCl2·2H2O | 0.00009 |
Appendix B


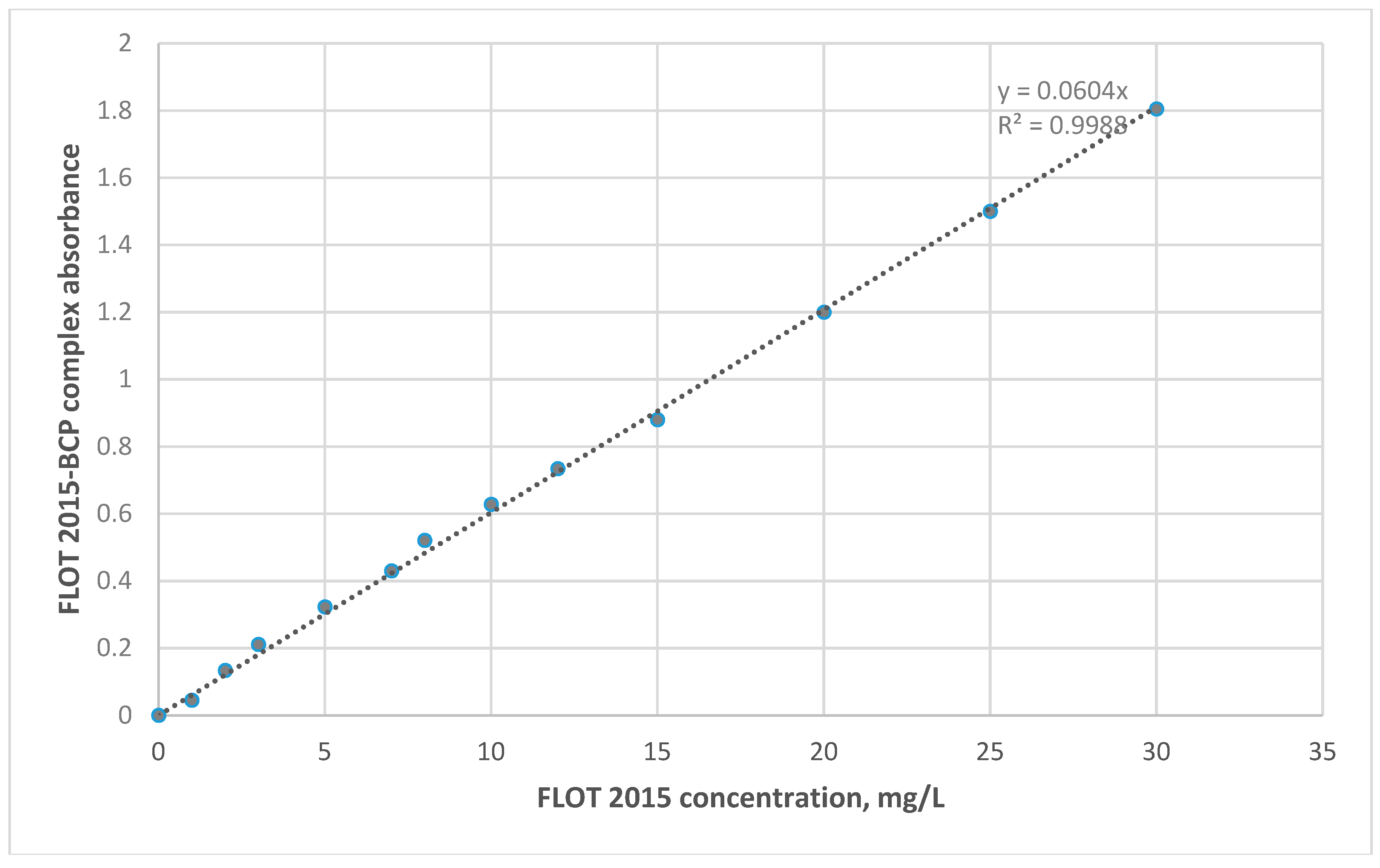
| Parameter | Result |
|---|---|
| Absorption maximum (nm) | 380 |
| Linearity range (mg/L) | 0.5–30 |
| Standard regression equation | y = 0.0604x |
| Correlation coefficient R2 | 0.9988 |
| Standard deviation (SD) | 0.00515 |
| Relative standard deviation (RSD) | 0.5789 |
| Intraday precision (RSD%) | 2.08 |
| Interday precision (RSD%) | 2.8 |
| LOD (mg/L) | 1 |
| LOQ (mg/L) | 2 |
References
- Orecchio, S.; Bianchini, F.; Bonsignore, R.; Blandino, P.; Barreca, S.; Amorello, D. Profiles and Sources of PAHs in Sediments from an Open-Pit Mining Area in the Peruvian Andes. Polycycl. Aromat. Compd. 2016, 36, 429–451. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Bastida, E. Sustainable investment in the minerals sector: Re-examining the paradigm. International Environmental Agreements. Polit. Law Econ. 2006, 6, 401–406. [Google Scholar]
- Vare, L.L.; Baker, M.C.; Howe, J.A.; Levin, L.A.; Neira, C.; Ramirez-Llodra, E.Z.; Reichelt-Brushett, A.; Rowden, A.A.; Shimmield, T.M.; Simpson, S.L.; et al. Scientific Considerations for the Assessment and Management of Mine Tailings Disposal in the Deep Sea. Front. Mar. Sci. 2018, 5, 1–14. [Google Scholar] [CrossRef]
- Dold, B. Submarine tailings disposal (STD)—A review. Minerals 2014, 4, 642–666. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Trannum, H.C.; Evenset, A.; Levin, L.A.; Andersson, M.; Finne, T.E.; Hilario, A.; Flem, B.; Christensen, G.; Schaanning, M.; et al. Submarine and deep-see mine tailing placements: A review of current practices, environmental issues, natural analogs and knowledge gaps in Norway and internationally. Mar. Pollut. Bull. 2015, 97, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Rubingh, D.N.; Holland, P.M. Cationic Surfactants: Physical Chemistry, Surfactant Science Series; Marcell Dekker: New York, NY, USA, 1992; Volume 37, p. 449. [Google Scholar]
- Hellberga, P.E.; Bergstroma, K.; Holmbergb, K. Cleavable Surfactants. J. Surfactants Deterg. 2000, 3, 81–91. [Google Scholar] [CrossRef]
- Overkempe, C.; Annerling, A.; Gingel, C.; Tomas, P.C.; Boltersdort, D.; Speelman, J. Esterquats. Novel Surfactants: Preparation, Application and Biodegradability; Marcell Dekker: New York, NY, USA, 2005; Volume 37, p. 449. [Google Scholar]
- Ginkel, C.G. Biodegradation of Cationic Surfactants: An Environmental perspective. In Handbook of Detergents. Part B: Environmental Impact; Marcel Dekker: New York, NY, USA, 2004; pp. 523–555. [Google Scholar]
- Para, G.; Łuczyński, J.; Palus, J.; Jarek, E.; Wilk, K.A.; Warszyński, P. Hydrolysis driven surface activity of esterquats surfactants. J. Colloid Interface Sci. 2016, 465, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Giolando, S.T.; Rapaport, R.A.; Larson, R.A.; Federle, T.W.; Stalmans, M.; Masscheleyn, P. Environmental Safety Assessment of DEEDMAC: A new rapidly biodegradable cationic surfactant. Chemosphere 1995, 30, 1067–1083. [Google Scholar] [CrossRef]
- Waters, J.; Kupfer, W. Cationic surfactants in the presence of anionic surfactant in biodegradation test liquors. Anal. Chim. Acta 1976, 85, 241–251. [Google Scholar] [CrossRef]
- Simms, J.R.; Woods, D.A.; Walley, D.R.; Keough, T.; Schwab, B.S.; Larson, R.J. Integrated approach to surfactant environmental safety assessment: Fast bombardment mass spectrometry and liquid scintillation counting to determine the mechanism and kinetics of surfactant biodegradation. Anal. Chem. 1992, 64, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Puchta, P.; Krings, P.; Sandkiihler, P. A new generation of softeners. Tenside Surfactants Deterg. 1993, 30, 186–192. [Google Scholar]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surface Chemistry of Surfactants and Polymers; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2014; p. 496. Available online: http://site.ebrary.com.libproxy.tut.fi/lib/ttyk/reader.action?docID=10937139&ppg=395 (accessed on 14 May 2018).
- Pace, A.; Barreca, S. Environmental Organic Photochemistry: Advances and Perspectives. Curr. Organ. Chem. 2013, 17, 3032–3041. [Google Scholar] [CrossRef]
- Games, L.M.; King, J.E.; Larson, R.J. Fate and distribution of a quaternary ammonium surfactant, octadecyltrimethylarnmonium chloride (OTAC) in wastewater treatment. Environ. Sci. Technol. 1982, 16, 483–488. [Google Scholar] [CrossRef]
- Kortstee, G.J.J. The aerobic decomposition of choline by micro-organisms. The ability of aerobic organisms, particularly coryneform bacteria, to utilize choline as sole carbon and nitrogen source. Arch. Microbiol. 1970, 71, 235–244. [Google Scholar]
- Fendrich, E.; Hippe, H.; Gottschalk, G. Clostridium halophilium sp. nov. and a marine species degrading betaine in the Stickland reaction. Arch. Microbiol. 1990, 154, 127–132. [Google Scholar] [CrossRef]
- Yoshimura, K.; Machida, S.; Masuda, F. Biodegradation of long chain alkylamines. Am. Oil Chem. Soc. 1980, 57, 238–241. [Google Scholar] [CrossRef]
- Larson, R.J.; Vashon, R.D. Adsorption and biodegradation of cationic surfactants in laboratory and environmental systems. Dev. Ind. Microbiol. 1983, 24, 425–434. [Google Scholar]
- Vives-Rego, J.; Vaque, M.D.; Sanchez Leal, J.; Parra, J. Surfactants biodegradation in sea water. Tenside Surfactants Deterg. 1987, 24, 20–22. [Google Scholar]
- Wulf, V.; Wienand, N.; Wirtz, M.; Kling, H.W.; Gäb, S.; Schmitz, O.J. Analysis of special surfactants by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Bassarab, P.; Williams, D.; Dean, J.R.; Ludkin, E.; Perry, J.J. Determination of quaternary ammonium compounds in seawater samples by solid-phase extraction and liquid chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Wigman, L.S.; Thomson, M.L.; Wayne, R.S. Single column IC determination of anions in agriculturally useful quaternary compounds using indirect UV detection. J. Liq. Chromatogr. 1989, 12, 3219–3229. [Google Scholar]
- Yamamoto, K.; Motomizu, S. Spectrophotometric method for ionic surfactants by flow injection analysis with acidic dyes. Anal. Chim. Acta 1991, 246, 333–339. [Google Scholar] [CrossRef]
- Motomizu, S.; Oshima, M.; Hosoi, Y. Spectrophotometric determination of cationic and anionic surfactants with anionic dyes in the presence of nonionic surfactants. Part I: General aspect. Mikrochim. Acta 1992, 706, 57–66. [Google Scholar] [CrossRef]
- El-Khateeb, S.; Abdel-Moety, E.M. Determination of quaternary ammonium surfactants in pharmaceutical formulations by the hypochromic effect. Talanta 1988, 35, 813–815. [Google Scholar] [CrossRef]
- Akbaş, H.; Kartal, C. Spectrophotometric studies of anionic dye-cationic surfactant interactions in mixture of cationic and nonionic surfactants. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 961–966. [Google Scholar] [CrossRef] [PubMed]
- El-Didamony, A.M.; Moustafa, M.A. Spectrophotometric determination of diphenhydramine hydrochloride in pharmaceutical preparations and biological fluids via ion-pair formation. Arab. J. Chem. 2010, 3, 265–270. [Google Scholar] [CrossRef]
- Hylland, K.; Bratrud, T.; Storset, A.; Wisbech, C. Ecotoxicological Assessment of Flotation Chemicals; Omya Hustadmarmor AS: Elnesvågen, Norway, 2014. [Google Scholar]
- Kester, D.R.; Duedall, I.W.; Connors, D.N.; Pytkowicz, R.M. Preparation of Artificial Seawater. Limnol. Oceanogr. 1967, 12, 176–179. [Google Scholar] [CrossRef]
- Miller, J.N. Basic Statistical Methods for Analytical Chemistry. Part 2. Calibration and Regression methods. A Review. Analyst 1991, 116, 3–14. [Google Scholar] [CrossRef]
- Patel, J.; Kevin, G.; Patel, A.; Raval, M.; Sheth, N. UV spectrophotometric method development of Olmesartan medoxomil in bulk drug and pharmaceutical formulation and stress degradation studies. Pharm. Methods 2011, 2, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Barreca, S.; Orecchio, S.; Pace, A. Photochemical sample treatment for extracts clean up in PCB analysis from sediments. Talanta 2012, 103, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Baer, M.D.; Mundy, C.J.; Schenter, G.K. Marcus Theory of Ion-Pairing. J. Chem. Theory Comput. 2017, 13, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.M. Analysis of Surfactants; Marcel Dekkert, Inc.: New York, NY, USA; Basel, Switzerland, 2001; 637p. [Google Scholar]
- Ibragimova, O.; Kleiv, R.A. Equilibria and Kinetics of Flotation Chemical Sorption Reactions in Tailings-Seawater Systems. In Proceedings of the 5th International Seminar on Tailings Management, Santiago, Chile, 11–13 July 2018; pp. 80–81. [Google Scholar]
- Aparicio, S.; Atilhan, M.; Khraisheh, M.; Alcalde, R. Study on Hydroxylammonium-Based Ionic Liquids. I. Characterization. J. Phys. Chem. B 2011, 115, 12473–12486. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, S.; Atilhan, M. Insights into tris-(2-hydroxylethyl)methylammonium methylsulfate aqueous solutions. ChemPhysChem 2012, 13, 3340–3349. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Escudero-Onate, C.; Gomes, T.; Ferrando-Climent, L. An integrative biological effect assessment of a mine discharge into a Norwegian fjord using field transplanted mussels. Sci. Total Environ. 2018, 644, 1056–1069. [Google Scholar] [CrossRef]
- Blaedel, W.J.; Meloche, V.W. Elementary Quantitative Analysis. Theory and Practice; Harper & Row: Evanston, IL, USA, 1957; 826p. [Google Scholar]



| Name and Structure | Molecular Formula |
|---|---|
tris (2-hydroxyethyl) (methyl)azanium (3HEMA MS) | C7H18NO3 |
bis(2-hydroxyethyl) (methyl){2-((15E)-octadec-15-enoyloxy) ethyl}azanium (monoester)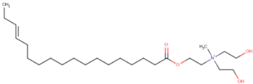 | C25H50NO4 |
(2-hydroxyethyl) (methyl)bis {2-((15E)-octadec-15-enoyloxy) ethyl}azanium (diester) | C43H82NO5 |
| Fe2O3 | TiO2 | CaO | K2O | P2O5 | SiO2 | Al2O3 | MgO | Na2O | MnO2 | LOI * |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.64 | 0.01 | 54.76 | 0.01 | 0.01 | 0.44 | 0.03 | 1.08 | 0.02 | 0.22 | 42.38 |
| Concentration of 3HEMA MS, mg/L | Absorbance | Concentration of 3HEMA MS Found after Addition, mg/L |
|---|---|---|
| 0 | 0.00 | 0.00 |
| 20 | 0.00 | 0.00 |
| 50 | 0.00 | 0.00 |
| 1000 | 0.00 | 0.00 |
| Concentration of FLOT 2015, mg/L | Concentration of 3HEMA MS, mg/L | Absorbance | Concentration of FLOT 2015 Found after Addition, mg/L | Total FLOT 2015 Recovered, % | Mean Recovery, % |
|---|---|---|---|---|---|
| 4.00 | 0 | 0.260 | 4.00 | 100.00 | 100.00 |
| 4.00 | 20 | 0.261 | 4.00 | 100.00 | |
| 4.00 | 50 | 0.260 | 4.00 | 100.00 | |
| 4.00 | 1000 | 0.260 | 4.00 | 100.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibragimova, O.; Kleiv, R.A. Application of a Rapid and Simple UV-Spectrophotometric Method for the Study of Desorption of Esterquat Collectors in Tailings–Seawater Systems. Water 2018, 10, 1544. https://doi.org/10.3390/w10111544
Ibragimova O, Kleiv RA. Application of a Rapid and Simple UV-Spectrophotometric Method for the Study of Desorption of Esterquat Collectors in Tailings–Seawater Systems. Water. 2018; 10(11):1544. https://doi.org/10.3390/w10111544
Chicago/Turabian StyleIbragimova, Olga, and Rolf Arne Kleiv. 2018. "Application of a Rapid and Simple UV-Spectrophotometric Method for the Study of Desorption of Esterquat Collectors in Tailings–Seawater Systems" Water 10, no. 11: 1544. https://doi.org/10.3390/w10111544
APA StyleIbragimova, O., & Kleiv, R. A. (2018). Application of a Rapid and Simple UV-Spectrophotometric Method for the Study of Desorption of Esterquat Collectors in Tailings–Seawater Systems. Water, 10(11), 1544. https://doi.org/10.3390/w10111544




