Support Tool for Identifying In Situ Remediation Technology for Sites Contaminated by Hexavalent Chromium
Abstract
:1. Introduction
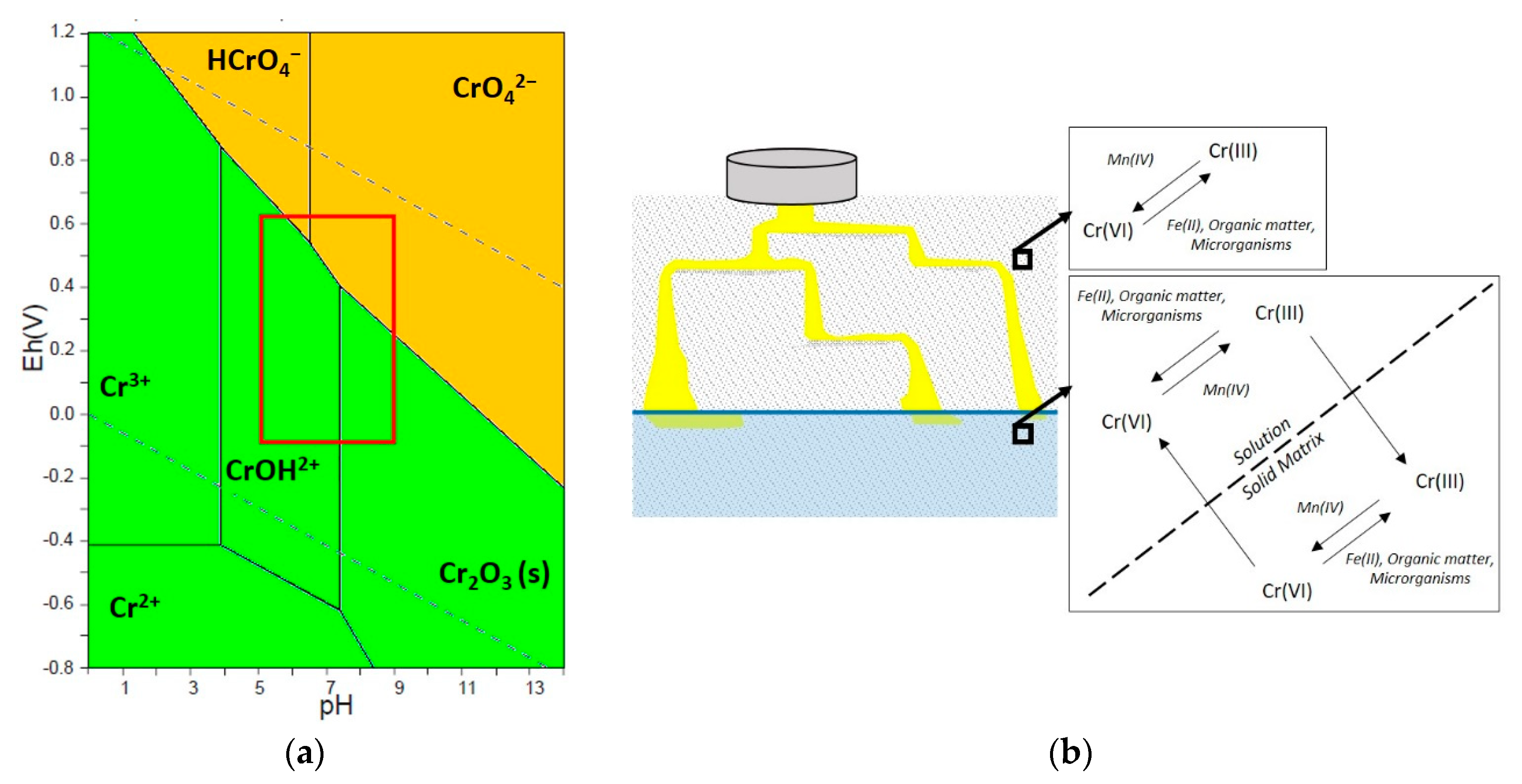
2. Behaviour of Chromium in Soil and Groundwater
3. Technologies
3.1. Innovative Technologies for Cr (VI) Remediation
3.1.1. Chemical Process
3.1.2. Biological Process
3.1.3. Chemical-Physical Process
3.2. Full Scale Implementation
4. Scenarios and Decision Support Tool
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guertin, J.; Jacobs, J.A.; Avakian, C.P. Chromium (VI) Handbook; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Panagiotakis, I.; Dermatas, D.; Vatseris, C.; Chrysochoou, M.; Papassiopi, N.; Xenidis, A.; Vaxevanidou, K. Forensic investigation of a Chromium (VI) groundwater plume in Thiva, Greece. J. Hazard. Mater. 2015, 281, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lilli, M.A.; Moraetis, D.; Nikolaidis, N.P.; Karatzas, G.P.; Kalogerakis, N. Characterization and mobility of geogenic chromium in soils and riverbed sediments of asopos basin. J. Hazard. Mater. 2015, 281, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Chrysochoou, M.; Theologou, E.; Bompoti, N.; Dermatas, D.; Panagiotakis, I. Occurrence, origin and transformation processes of geogenic chromium in soils and sediments. Curr. Pollut. Rep. 2016, 2, 224–235. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. In Situ Treatment of Soil and Groundwater Contaminated with Chromium–Technical Resource Guide; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- United States Environmental Protection Agency. Introduction to In Situ Bioremediation of Groundwater–Technical Resource Guide; United States Environmental Protection Agency, Office of Solid Waste and Emergency Response: Cincinnati, OH, USA, 2013.
- Bekele, T.C.; Bucklin, K.; Burger, S.; Martinez, L.; Parnass, B.; Renzi, E.; Rodriguez, M.; Wong, P. Investigation and Remediation of Plating Facilities–Technical Resource Guide; United States Environmental Protection Agency, Department of Toxic Substances Control: Sacramento, CA, USA, 2011.
- California Environmental Protection Agency. Proven Technologies and Remedies Guidance–Remediation of Metals in Soil; California Environmental Protection Agency, Department of Toxic Substances Control: Sacramento, CA, USA, 2008.
- Savannah River National Laboratory. The Scenarios Approach to Attenuation-Based Remedies for Inorganic and Radionuclide Contaminants; Savannah River National Laboratory: Aiken, SC, USA, 2011.
- Unceta, N.; Séby, F.; Malherbe, J.; Donard, O.F.X. Chromium speciation in solid matrices and regulation: A review. Anal. Bioanal. Chem. 2010, 397, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250, 272–291. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Toxicological Review of Hexavalent Chromium; United States Environmental Protection Agency: Washington, DC, USA, 1998.
- US Department of Labor Occupational Safety and Health Administration. Hexavalent Chromium; US Department of Labor Occupational Safety and Health Administration: Washington, DC, USA, 2009.
- Canadian Council of Ministers of the Environment. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health—Chromium; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999. [Google Scholar]
- United States Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. Toxicological Profile for Chromium. 2012. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp7.pdf (accessed on 15 July 2018).
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- US Department of Agriculture. Soil Science Division Staff, 2017. In Soil Survey Manual—Handbook No. 18; US Department of Agriculture: Washington, DC, USA, 2017. [Google Scholar]
- Ohio Environmental Protection Agency. Reduction-Oxidation (Redox) Control in Ohio’s Ground Water Quality; Ohio Environmental Protection Agency: Columbus, OH, USA, 2014.
- Palmer, C.D.; Wittbrodt, P.R. Processes affecting the remediation of chromium contaminated sites. Environ. Health Perspect. 1991, 92, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Hug, S.J. Kinetics and pH dependence of Chromium (VI) reduction by Iron (II). Environ. Sci. Technol. 1997, 3, 1–7. [Google Scholar] [CrossRef]
- He, Y. Chromate Reduction and Immobilization under High pH and High Ionic Strength Conditions. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2003. [Google Scholar]
- Palmer, C.D.; Puls, R.W. Natural Attenuation of Hexavalent Chromium in Groundwater and Soils; USEPA, Office of Emergency and Remedial Response: Washington, DC, USA, 1994.
- Sedlazeck, K.P.; Hollen, D.; Muller, P.; Mischitz, R.; Giere, R. Mineralogical and geochemical characterization of a chromium contamination in an aquifer—A combined analytical and modeling approach. Appl. Geochem. 2017, 87, 44–56. [Google Scholar] [CrossRef]
- Network for Industrially Contaminated Land in Europe. How to Implement Sustainable Remediation in a Contaminated Land Management Project? 2012. Available online: http://www.nicole.org/uploadedfiles/wg-sustainableremediation-finalreport.pdf (accessed on 15 July 2018).
- Department of Toxic Substances Control, California Environmental Protection Agency. Interim Advisory for Green Remediation; Department of Toxic Substances Control: Sacramento, CA, USA, 2009. [Google Scholar]
- Beretta, G.P.; Pellegrini, R. Linee Guida per la Verifica del Trattamento Chimico In Situ dei Terreni e Delle Acque Sotterranee; Technical Report: Provincia di Milano, Italy, 2006. (In Italian) [Google Scholar]
- Ludwig, R.D.; Su, C.; Lee, T.R.; Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Keeley, A. In situ chemical reduction of Cr (VI) in groundwater using a combination of ferrous sulfate and sodium dithionite: A field investigation. Environ. Sci. Technol. 2007, 41, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Němeček, J.; Lhotský, O.; Cajthaml, T. Nanoscale zero-valent iron application for in situ reduction of hexavalent chromium and its effects on indigenous microorganism populations. Sci. Total. Environ. 2014, 485, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lei, L.; Yang, C.; Zhang, W. Reduction of Cr (VI) by nanoscale zero valent Iron (nZVI): The reaction kinetics. In Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering (IWBBIO 2016), Granada, Spain, 20–22 April 2016. [Google Scholar]
- Yoon, I.H.; Kim, K.W. Effect of pH and Dissolved Oxygen on the Cr (VI) Removal by Zero-Valent Iron. Available online: https://www.researchgate.net/publication/242201190_Effect_of_pH_and_Dissolved_Oxygen_on_the_CrVI_Removal_by_Zero-Valent_Iron (accessed on 15 July 2018).
- Storch, P.; Messer, A.; Palmer, D.; Pyrih, R. Pilot test for in situ geochemical fixation of chromium (VI) using calcium polysulfide. In Proceedings of the Third International Conference on Remediation of Chlorinated and Recalcitrant Compounds: pts 1a–2b; Battelle Press: San Diego, CA, USA, 2002. [Google Scholar]
- Chrysochoou, M.; Ferreira, D.R.; Johnston, C.P. Calcium polysulfide treatment of Cr (VI)-contaminated soil. J. Hazard. Mater. 2010, 179, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Chrysochoou, M.; Ting, A. A kinetic study of Cr (VI) reduction by calcium polysulfide. Sci. Total. Environ. 2011, 409, 4072–4077. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cwiertny, D.M. Use of dithionite to extend the reactive lifetime of nanoscale zero-valent iron treatment systems. Environ. Sci. Technol. 2010, 44, 8649–8655. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Plus, R.W. In situ abiotic detoxification and immobilization of hexavalent chromium. Ground Water Monit. Remediat. 2003, 23, 77–84. [Google Scholar] [CrossRef]
- Beukes, J.P; Pienaar, J.; J; Lachmann, G; Giesekke, E.W. The reduction of hexavalent chromium by sulphite in wastewater. Water 1999, 25, 363. Available online: http://www.wrc.org.za (accessed on 15 July 2018).
- Xu, X.; Li, H.; Li, X.; Gu, J. Reduction of hexavalent chromium by ascorbic acid in aqueous solutions. Chemosphere 2004, 57, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Bianco Prevot, A.; Ginepro, M.; Peracaciolo, E.; Zelano, V.; De Luca, D.A. Chemical vs bio-mediated reduction of hexavalent chromium. An in-vitro study for soil and deep waters remediation. Geoderma 2018, 312, 17–23. [Google Scholar] [CrossRef]
- Thornton, E.C.; Gilmore, T.J.; Olsen, K.B.; Giblin, J.T.; Phelan, J.M. Treatment of a chromate-contaminated soil site by in situ gaseous reduction. Ground Water Monit. Remediat. 2007, 27, 56–64. [Google Scholar] [CrossRef]
- Kim, C.; Zhou, Q.; Deng, B.; Thorton, E.C.; Xu, H. Chromium (VI) reduction by hydrogen sulfide in aqueous media: stoichiometry and kinetics. Environ. Sci. Technol. 2001, 35, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Deng, B. Influences of water vapor on Cr (VI) reduction by gaseous hydrogen sulfide. Environ. Sci. Technol. 2003, 37, 4771–4777. [Google Scholar] [CrossRef] [PubMed]
- Ibbini, J.; Santharam, S.; Davis, L.C.; Erickson, L.E. Laboratory and Field Scale Bioremediation of Tetrachloroethene (PCE) Contaminated Groundwater. Jordan J. Mech. Ind. Eng. 2010, 4, 35–44. [Google Scholar]
- Somenahally, A.C.; Mosher, J.J.; Yuan, T.; Podar, M.; Phelps, T.J.; Brown, S.D.; Yang, Z.K.; Hazen, T.C.; Arkin, A.P.; Palumbo, A.V.; et al. Hexavalent Chromium Reduction under Fermentative Conditions with Lactate Stimulated Native Microbial Communities. 2013. Available online: www.plosone.org (accessed on 15 July 2018).
- Liao, Y.; Min, X.; Yang, Z.; Chai, L.; Zhang, S.; Wang, Y. Physicochemical and biological quality of soil in hexavalent chromium-contaminated soils as affected by chemical and microbial remediation. Environ. Sci. Pollut. Res. 2014, 21, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Viti, C.; Mini, A.; Ranalli, G.; Lustrato, G.; Giovannetti, L. Response of microbial communities to different doses of chromate in soil microcosms. Appl. Soil Ecol. 2006, 34, 125–139. [Google Scholar] [CrossRef]
- Liu, J.; Duan, C.; Zhang, X.; Zhu, Y.; Lu, X. Potential of leersia hexandra swartz for phytoextraction of Cr from soil. J. Hazard. Mater. 2011, 188, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Panda, S.S.; Basu, A.; Dhal, N.K. Enhancement of toxic Cr (VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides L. Int. J. Phytoremediat. 2018, 20, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M. Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017, 198, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; Petros, G. Effects of plants for reduction and removal of hexavalent chromium from a contaminated soil. Water Air Soil Pollut. 2014, 225. [Google Scholar] [CrossRef]
- Chai, L.; Huang, S.; Yang, Z.; Peng, B.; Huang, Y.; Chen, Y. Cr (VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag. J. Hazard. Mater. 2009, 167, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Höhener, P.; Ponsin, V. In situ vadose zone bioremediation. Curr. Opin. Biotechnol. 2014, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thatoi, H.; Das, S.; Mishra, J.; Rath, B.P.; Das, N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review. J. Environ. Manag. 2014, 146, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Chen, J.; Huang, Q.; Chen, J.; Xu, Y.; Luo, J.; Wang, K.; Guo, W.; Zheng, Y. Bioremediation of hexavalent chromium contaminated soil by a bioleaching system with weak magnetic fields. Int. Biodeterior. Biodegrad. 2018, 128, 41–47. [Google Scholar] [CrossRef]
- Němeček, J.; Pokorný, P.; Lhotský, O.; Knytl, V.; Najmanová, P.; Steinová, J.; Černík, M.; Filipová, A.; Filip, J.; Cajthaml, T. Combined nano-biotechnology for in-situ remediation of mixed contamination of groundwater by hexavalent chromium and chlorinated solvents. Sci. Total Environ. 2016, 563, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jin, C.; Zhao, Z.; Tian, G. 2D crossed electric field for electrokinetic remediation of chromium contaminated soil. J. Hazard. Mater. 2010, 177, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Guo, S.; Wu, B.; Li, F.; Li, G. Effects of reducing agent and approaching anodes on chromium removal in electrokinetic soil remediation. Front. Environ. Sci. Eng. 2016, 10, 253–261. [Google Scholar] [CrossRef]
- Yan, Y.; Xue, F.; Muhammad, F.; Yu, L.; Xu, F.; Jiao, B.; Li, D. Application of iron-loaded activated carbon electrodes for electrokinetic remediation of chromium-contaminated soil in a three-dimensional electrode system. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Contaminated Land: Applications in Real Environments. Electrokinetic Ferric Ion Remediation and Stabilisation (FIRS) of Hexavalent Chromium Contaminated Soils: An Ex Situ Field Scale Demonstration. Available online: http://www.claire.co.uk (accessed on 15 July 2018).
- Vocciante, M.; Caretta, A.; Bua, L.; Bagatin, R.; Ferro, S. Enhancements in ElectroKinetic Remediation Technology: Environmental assessment in comparison with other configurations and consolidated solutions. Chem. Eng. J. 2016, 289, 123–134. [Google Scholar] [CrossRef]
- Tang, S.; Yin, K.; Lo, I.M. Column study of Cr (VI) removal by cationic hydrogel for in-situ remediation of contaminated groundwater and soil. J. Contam. Hydrol. 2011, 125, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.Y.; Lo, I.M. Pyrophosphate coupling with chelant-enhanced soil flushing of field contaminated soils for heavy metal extraction. J. Hazard. Mater. 2012, 199, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Yaqiao, S.; Lei, D. The influence mechanism of ash-flushing water from power plant on groundwater environment. In Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering (IWBBIO 2016), Granada, Spain; 2016. [Google Scholar]
- Fruchter, J. In situ treatment of chromium-contaminated groundwater. Environ. Sci. Technol. 2002, 36, 464A–472A. [Google Scholar] [CrossRef] [PubMed]
- Jeyasingh, J.; Somasundaram, V.; Philip, L.; Bhallamudi, S.M. Pilot scale studies on the remediation of chromium contaminated aquifer using bio-barrier and reactive zone technologies. Chem. Eng. J. 2011, 167, 206–214. [Google Scholar] [CrossRef]
- Ohio Environmental Protection Agency. Use of direct push technologies for soil and ground water sampling. In Technical Resource Guide for Ground Water Investigations; Ohio Environmental Protection Agency: Columbus, OH, USA, 2005. [Google Scholar]
- ASTM International. Standard Guide for Direct-Push Groundwater Sampling for Environmental Site Characterization; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- United States Environmental Protection Agency. Injection Wells: An Introduction to Their Use, Operation, and Regulation; United States Environmental Protection Agency, Office of Drinking Water: Washington, DC, USA, 1989.
- ASTM International. Standard Guide for Development of Groundwater Monitoring Wells in Granular Aquifers; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- United States Environmental Protection Agency. In Situ Permeable Reactive Barrier for Contaminated Groundwater at the U.S. Coast Guard Support Center Elisabeth City, North Carolina; United States Environmental Protection Agency: Washington, DC, USA, 1988.
- Sethi, R.; Day, S.; Di Molfetta, A. Clamshell vs. Backhoe excavation of permeable reactive barriers. Am. J. Environ. Sci. 2011, 7, 463–467. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.; Rawaa, J.M. Performance of zero-valent iron barrier through the migration of lead-contaminated groundwater. Assoc. Arab Univ. J. Eng. Sci. 2018, 25, 132–144. [Google Scholar]
- Scherer, M.M.; Richter, S.; Valentine, R.L.; Alvarez, P.J. Chemistry and microbiology of permeable reactive barriers for In Situ groundwater clean up. Crit. Rev. Microbiol. 2000, 30, 363–411. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. A Citizen’s guide to Permeable Reactive Barriers. 2012. Available online: https://clu-in.org/download/Citizens/a_citizens_guide_to_permeable_reactive_barriers.pdf (accessed on 15 July 2018).
- Fuller, S.J.; Stewart, D.I; Ian, T.B. Chromate reduction in highly alkaline groundwater by zerovalent iron: implications for its use in a permeable reactive barrier. Ind. Eng. Chem. Res. 2013, 52, 4704–4714. [Google Scholar] [CrossRef]
- Liu, Y.; Mou, H.; Chen, L.; Mirza, Z.A.; Liu, L. Cr (VI)-contaminated groundwater remediation with simulated permeable reactive barrier (PRB) filled with natural pyrite as reactive material: Environmental factors and effectiveness. J. Hazard. Mater. 2015, 298, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Careghini, A.; Saponaro, S.; Sezenna, E. Biobarriers for groundwater treatment: A review. Water Sci. Technol. 2013, 67, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Christoph, W.; Eggenberger, U.; Kurz, D.; Zink, S.; Mäder, U. A chromate-contaminated site in southern Switzerland—Part 1: Site characterization and the use of Cr isotopes to delineate fate and transport. Appl. Geochem. 2012, 27, 644–654. [Google Scholar]
- Baldi, F.; Barbieri, P. Microbiologia Ambientale ed Elementi di Ecologia Microbica. 2008. Available online: https://iris.unive.it/handle/10278/22405?mode=full.156#.W62VsbgRVPY (accessed on 15 July 2018).
- Kamaludden, S.P.B.; Arunkumar, K.R.; Avudainayagam, S.; Ramasamy, K. Bioremediation of chromium contaminated environments. Indian J. Exp. Biol. 2003, 41, 972–985. [Google Scholar]
- Hassan, Z.; Ali, S.; Farid, M.; Rizwan, M.; Shahid, J. Effect of Chromium (Cr) on the Microbial and Enzymatic Activities in the Soil: A Review. 2017. Available online: https://www.researchgate.net/publication/317167651_Effect_of_Chromium_Cr_on_the_Microbial_and_Enzymatic_Activities_in_the_Soil_A_Review (accessed on 15 July 2018).
- Dresel, P.E.; Wellman, D.M.; Cantrell, K.J.; Truex, M.J. Review: Technical and policy challenges in deep vadose zone remediation of metals and radionuclides. Environ. Sci. Technol. 2011, 45, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, B.; Sundquist, J.; Schmitz, R.J. Removal of Cr (VI) from Cr-contaminated groundwater through electrochemical addition of Fe (II). J. Environ. Manag. 2007, 82, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Elkateb, T.; Chalaturnyk, R.; Robertson, P.K. An overview of soil heterogeneity: Quantification and implications on geotechnical field problems. Can. Geotech. J. 2003, 40, 1–15. [Google Scholar] [CrossRef]
- Uzielli, M.; Simonini, P.; Cola, S. Statistical identification of homogeneous soil units for Venice lagoon soils. In Proceedings of the 3rd International Conference on Site Characterization, Taipei, Taiwan, 1–4 April 2008. [Google Scholar]
- European Achievements in Soil Remediation and Brownfield Redevelopment. A Report of the European Information and Observation Network’s National Reference Centers for Soil. 2017. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC102681/kj0217891enn.pdf (accessed on 15 July 2018).
| Technology | Unsaturated 0–1 m | Unsaturated 1–10 m | Unsaturated > 10 m | Saturated < 10 m | Saturated 10–25 m | Saturated > 25 m |
|---|---|---|---|---|---|---|
| Chemical process with solutions or slurry | - | - | - | x | x | x |
| Chemical process with gaseous reagent | - | x | x | - | - | - |
| Indirect biological process | - | - | - | x | x | x |
| Biological process-Phytoremediation | x | - | - | - | - | - |
| Chemical-physical process-Electrokinetics | x | x | - | x | - | - |
| Chemical-physical process-Flushing | x | x | - | - | - | - |
| Factor | Scenario | Value |
|---|---|---|
| Soil pH | Acid | 5 ÷ 7 |
| Alkaline | 7 ÷ 9 | |
| Cr (VI) concentration | Low | < 102 mg unsaturated soil; < 10 mg L−1 in aquifer |
| High | > 102 mg kg−1 unsaturated soil; > 10 mg L−1 in aquifer | |
| Fe concentration in soil | Low | < 1 g Fe kg−1 |
| High | > 1 g Fe kg−1 | |
| Soil homogeneity | Yes | Variation of hydraulic conductivity or intrinsic permeability within 2 orders of magnitude |
| No | Variation of hydraulic conductivity or intrinsic permeability more than 2 orders of magnitude |
| pH 1 | Cr (VI) Concentration 2 | Fe Concentration in Soil 3 | Soil Homogeneity | Fe (0), C6H8O6, H2S | Sodium Dithionite | Calcium Polysulphurs | Indirect Biological Process | Phytoremediation | Electrokinetics | Soil Flushing |
|---|---|---|---|---|---|---|---|---|---|---|
| A | L | L | Yes | - | X | X | X | - | X | X |
| A | L | L | No | X 4 | X | X | X | - | X | X |
| A | L | H | Yes | - | - | X | - | - | X | X |
| A | L | H | No | X 4 | X4 | X | X 4 | - | X | X |
| A | H | L | Yes | - | X | X | X | X | - | X |
| A | H | L | No | X 4 | X | X | X | X | - | X |
| A | H | H | Yes | - | - | X | X | X | - | X |
| A | H | H | No | X 4 | X 4 | X | X | X | - | X |
| B | L | L | Yes | X | X | - | X | - | X | - |
| B | L | L | No | X | X | X 4 | X | - | X | X |
| B | L | H | Yes | X | X | - | - | - | X | |
| B | L | H | No | X | X | X 4 | X 4 | - | X | X |
| B | H | L | Yes | X | X | - | X | X | - | - |
| B | H | L | No | X | X | X 4 | X | X | - | X |
| B | H | H | Yes | X | X | - | X | X | - | - |
| B | H | H | No | X | X | X 4 | X | X | - | X |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beretta, G.; Mastorgio, A.F.; Pedrali, L.; Saponaro, S.; Sezenna, E. Support Tool for Identifying In Situ Remediation Technology for Sites Contaminated by Hexavalent Chromium. Water 2018, 10, 1344. https://doi.org/10.3390/w10101344
Beretta G, Mastorgio AF, Pedrali L, Saponaro S, Sezenna E. Support Tool for Identifying In Situ Remediation Technology for Sites Contaminated by Hexavalent Chromium. Water. 2018; 10(10):1344. https://doi.org/10.3390/w10101344
Chicago/Turabian StyleBeretta, Gabriele, Andrea Filippo Mastorgio, Lisa Pedrali, Sabrina Saponaro, and Elena Sezenna. 2018. "Support Tool for Identifying In Situ Remediation Technology for Sites Contaminated by Hexavalent Chromium" Water 10, no. 10: 1344. https://doi.org/10.3390/w10101344
APA StyleBeretta, G., Mastorgio, A. F., Pedrali, L., Saponaro, S., & Sezenna, E. (2018). Support Tool for Identifying In Situ Remediation Technology for Sites Contaminated by Hexavalent Chromium. Water, 10(10), 1344. https://doi.org/10.3390/w10101344





