Source Apportionment of PM2.5 during Haze and Non-Haze Episodes in Wuxi, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
2.2. Chemical Analysis
2.3. Reconstruction of Oxidized Species
2.4. CMB Model
3. Results
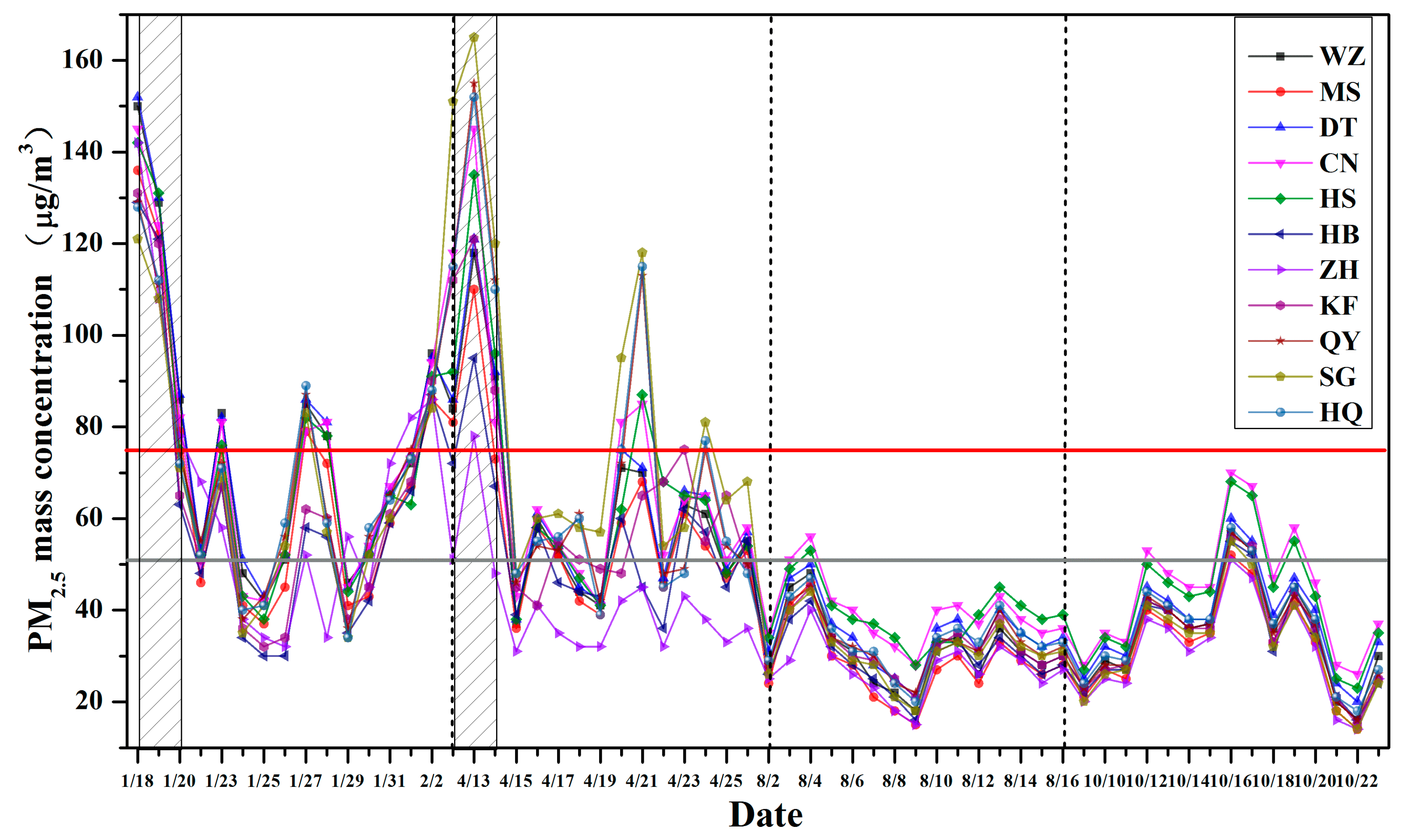
3.1. PM2.5 Mass Concentration
3.2. PM2.5 Chemical Compositions
3.2.1. Water-Soluble Ions
3.2.2. Organic and Elemental Carbon
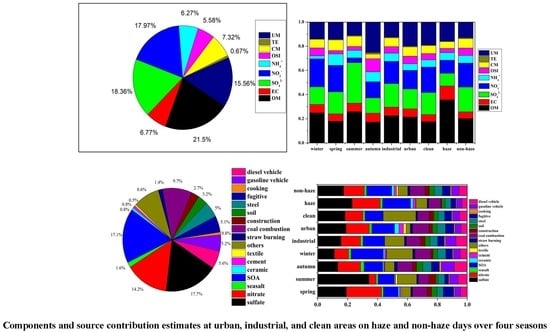
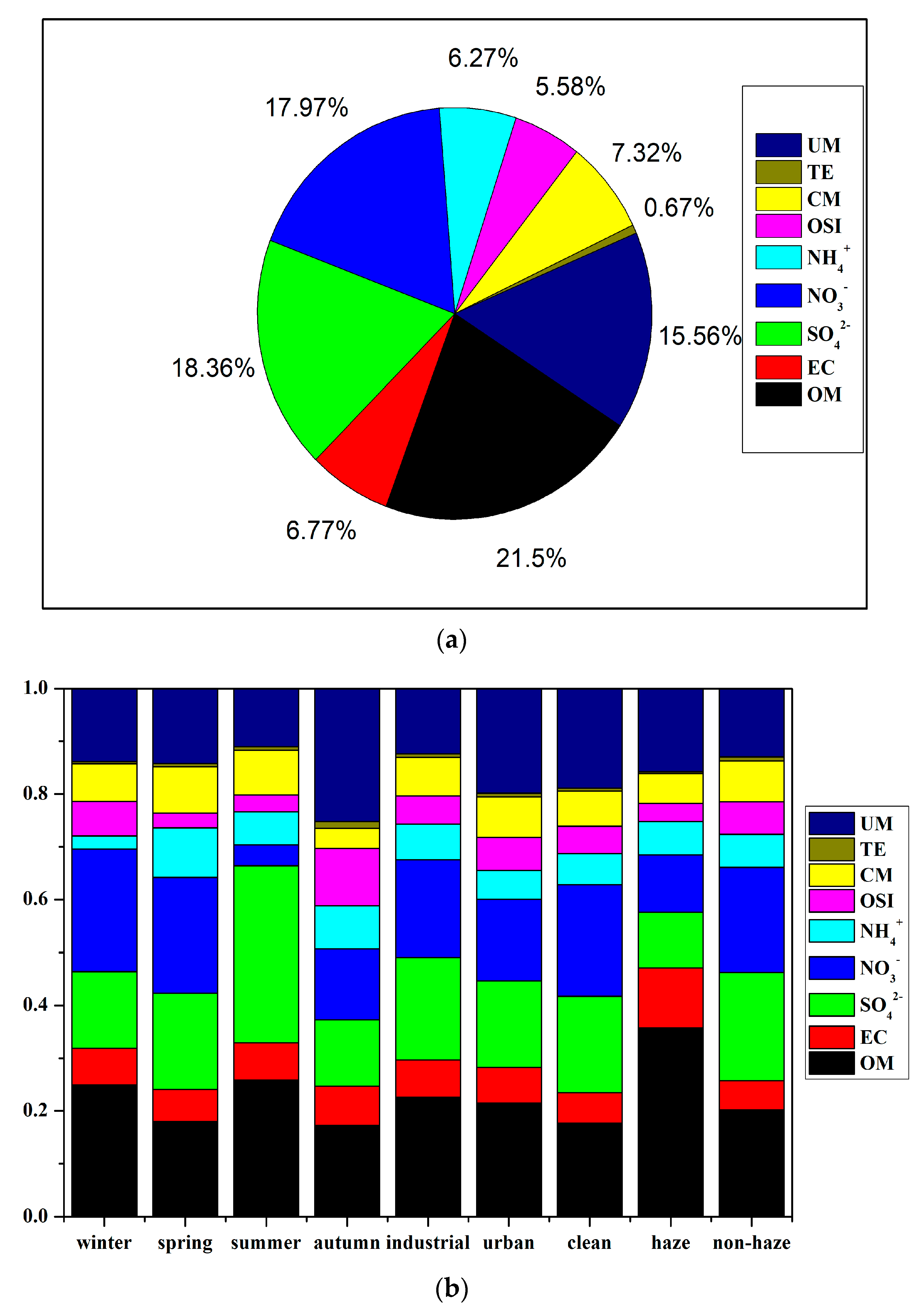
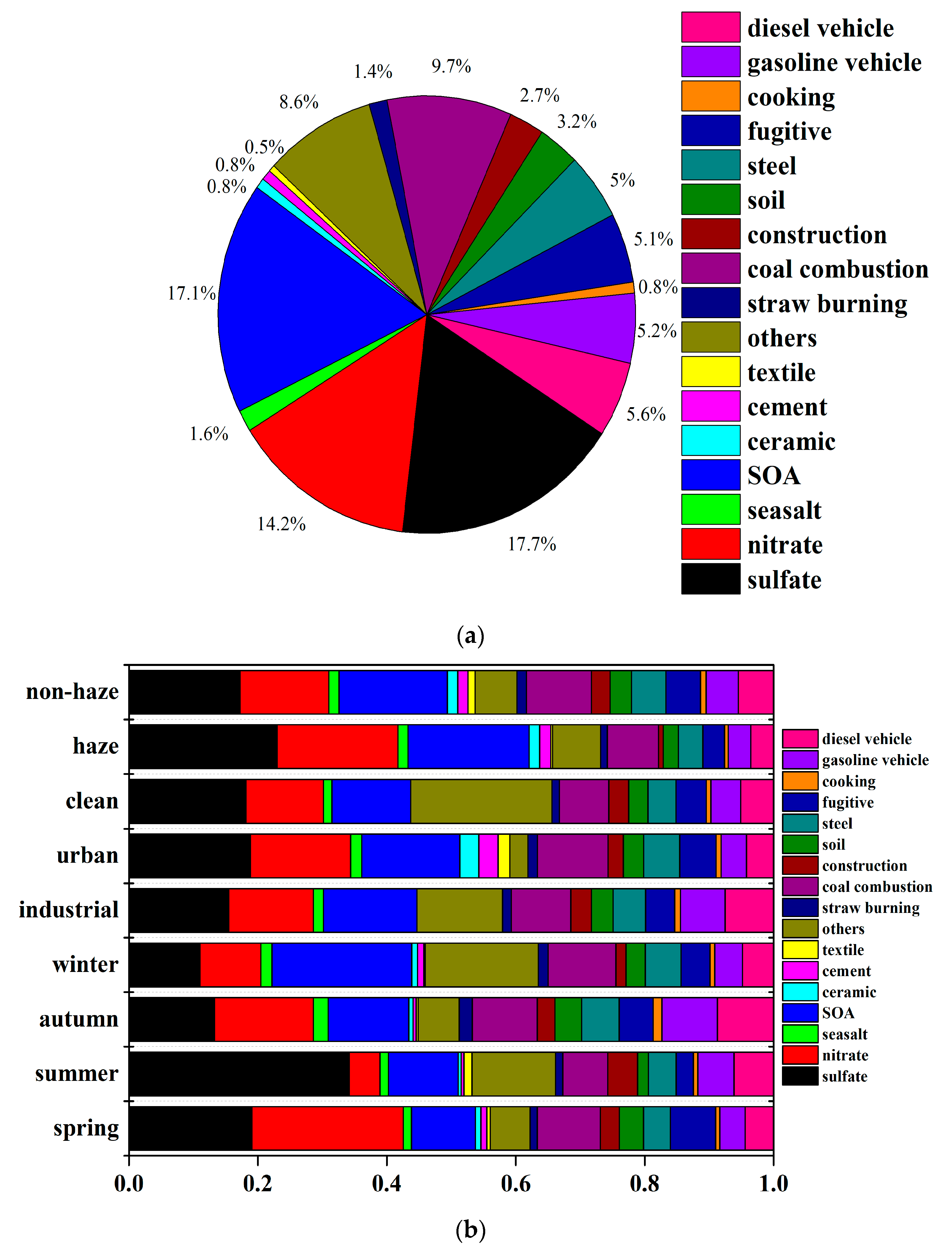
3.3. Source Apportionment by CMB Model
3.3.1. Variation in Seasons and Sites
3.3.2. Variation between Haze and Non-Haze Days
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dockerey, D.; Pope, A. Epidemiology of acute health effects: Summary of time-series studies. In Particles in Air: Concentration and Health Effects; Wilson, R., Spengler, J.D., Eds.; University Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistryand Physics: From Air Pollutionto Climate Change; John Wiley & Sons, Inc.: New York, NY, USA, 2006. [Google Scholar]
- IARC (International Agency for Research on Cancer). IARC: Outdoor Air Pollution a Leading Environmental Cause of Cancer Deaths (Press Release N 221); IARC: Lyon, France, 2013. [Google Scholar]
- Remer, L.A.; Chin, M.; DeCoal, P.; Fein-gold, G.; Halthore, R.; Kahn, R.A. Aerosols and Their Climate Effects, 1–2. In Atmospheric Aerosol Properties and Climate Impacts; U.S. Climate Change Science Program Synthesis and Assessment Product 2.3; 2009. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20090032661.pdf (accessed on 20 May 2018).
- Xu, H.M.; Cao, J.J.; Chow, J.C.; Huang, R.; Shen, Z.; Chen, L.; Ho, K.; Watson, J.G. Inter-annual variability of wintertime PM2.5 chemical composition in Xi’an, China: Evidences of changing source emissions. Sci. Total Environ. 2016, 545, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Wang, S.; He, K.; Zheng, M. Review of receptor-based source apportionment research of fine particulate matter and its challenges in China. Sci. Total Environ. 2017, 586, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G. Review of PM2.5 and PM10 apportionment for fossil fuel combustion and other sources by the chemical mass balance receptor model. Energy Fuels 2002, 16, 222–260. [Google Scholar] [CrossRef]
- Antony Chen, L.; Watson, J.G.; Chow, J.C.; DuBois, D.W.; Herschberger, L. Chemical mass balance source apportionment for combined PM2.5 measurements from U.S. Non-urban and Urban Long-term networks. Atmos. Environ. 2010, 44, 4908–4918. [Google Scholar] [CrossRef]
- Deshmukh, D.K.; Deb, M.K.; Tsai, Y.I.; Mkoma, S.L. Water soluble ions in PM2.5 and PM2.1 aerosols in Durg City, Chhattisgarh, India. Aerosol Air Qual. Res. 2011, 11, 696–708. [Google Scholar]
- Chan, C.K.; Yao, X. Air pollution in mega cities in China. Atmos. Environ. 2008, 42, 1–42. [Google Scholar] [CrossRef]
- Belis, C.A.; Karagulian, F.; Amato, F.; Almeida, M.; Artaxo, P.; Beddows, D.C.S.; Bernardoni, V.; Bove, M.C.; Carbone, S.; Cesari, D. A new methodology to assess the performance and uncertainty of source apportionment models II: The results of teo European intercomparison exercises. Atmos. Environ. 2015, 123, 240–250. [Google Scholar] [CrossRef]
- Cesari, D.; Donateo, A.; Conte, M.; Contini, D. Inter-comparison of source apportionment of PM10 using PMF and CMB in three sites nearby an industrial area in central Italy. Atmos. Res. 2016, 182, 282–293. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhuang, Y.H.; Wang, Y.; Sun, Y.L.; Yuan, H.; Zhuang, G.S.; Hao, Z. Long-term monitoring and source apportionment of PM2.5/PM10 in Beijing, China. J. Environ. Sci. 2008, 20, 1323–1327. [Google Scholar] [CrossRef]
- Xie, S.D.; Liu, Z.; Chen, T.; Hua, L. Spatiotemporal variations of ambient PM10 source contributions in Beijing in 2004 using positive matrix factorization. Atmos. Chem. Phys. 2008, 8, 2701–2716. [Google Scholar] [CrossRef]
- Deng, J.J.; Wang, T.J.; Jiang, Z.Q.; Xie, M.; Zhang, R.J.; Huang, X.X.; Zhu, J.L. Characterization of visibility and its affecting factors over Nanjing, China. Atmos. Res. 2011, 101, 681–691. [Google Scholar] [CrossRef]
- Xu, H.M.; Cao, J.J.; Ho, K.F.; Ding, H.; Han, Y.M.; Wang, G.H.; Chow, J.C.; Watson, J.G.; Khol, S.D.; Qiang, J.; et al. Lead concentrations in fine particulate matter after the phasing out of leaded gasoline in Xi’an, China. Atmos. Environ. 2012, 46, 217–224. [Google Scholar] [CrossRef]
- Zhu, C.S.; Cao, J.J.; Shen, Z.X.; Liu, S.X.; Zhang, T.; Zhao, Z.Z.; Xu, H.; Zhang, E. Indoor and outdoor chemical components of PM2.5 in the rural areas of Northwestern China. Aerosol Air Qual. Res. 2012, 12, 1157–1165. [Google Scholar] [CrossRef]
- Wang, P.; Cao, J.J.; Shen, Z.X.; Han, Y.M.; Lee, S.C.; Huang, Y.; Zhu, C.S.; Wang, Q.Y.; Xu, H.M.; Huang, R.J. Spatial and seasonal variations of PM2.5 mass and species during 2010 in Xi’an, China. Sci. Total Environ. 2015, 508, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.L.; Wang, T.J.; Hu, X. Chemical Mass Balance Source Apportionment of Size-Fractionated Particulate Matter in Nanjing, China. Aerosol Air Qual. Res. 2015, 15, 1855–1867. [Google Scholar] [CrossRef]
- Deng, J.J.; Zhang, Y.R.; Hong, Y.W.; Xu, L.L.; Chen, Y.T.; Du, W.J.; Chen, J.S. Optical properties of PM2.5 and the impacts of chemical compositions in the coastal city Xiamen in China. Sci. Total Environ. 2016, 557, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, T.; Lu, X.; Yu, Y.; Kasoar, M.; Xie, M.; Zhuang, B. Source apportionment of size-fractionated particles during the 2013 Asian Youth Games and the 2014 Youth Olympic Games in Nanjing, China. Sci. Total Environ. 2017, 579, 860–870. [Google Scholar] [CrossRef] [PubMed]
- He, K.B.; Huo, H.; Zhang, Q. Urban air pollution in China: Current status, characterizes and progress. Annu. Rev. Energy Environ. 2002, 27, 397–431. [Google Scholar] [CrossRef]
- Tie, X.; Cao, J. Aerosol pollution in China: Present and future impact on environment. Particuology 2009, 7, 426–431. [Google Scholar] [CrossRef]
- Gao, M.; Guttikunda, S.K.; Carmichael, G.R.; Wang, Y.; Liu, Z.; Stanier, C.O. Health impacts and economic losses assessment of the 2013 severe haze event in Beijing area. Sci. Total Environ. 2015, 511, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Bi, X.H.; Xue, Y.; Wu, J.; Zhu, T.; Zhang, B.; Ding, J.; Du, Y. Source apportionment of ambient PM10 in urban areas of Wuxi, China. Front. Environ. Sci. Eng. 2011, 5, 552–563. [Google Scholar] [CrossRef]
- Wang, T.J.; Jiang, F.; Deng, J.J.; Shen, Y.; Fu, Q.Y.; Wang, Q.; Fu, Y.; Xu, J.H.; Zhang, D.N. Urban air quality and regional haze weather forecast for Yangtze River Delta region. Atmos. Environ. 2012, 58, 70–83. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.C.; Wang, G.; Cao, J.; Lee, C.; Zhu, L.; Chen, Z.; Zhao, Y.; et al. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. 2013, 13, 7053–7074. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Xu, H.; Tian, Y.Z.; Shia, G.L.; Zeng, F.; Wu, J.H.; Zhang, X.Y.; Li, X.; Zhu, T.; Feng, Y.C. The Study on Vertical Variability of PM10 and the Possible Sources on a 220 m Tower in Tianjin China. Atmos. Environ. 2011, 45, 6133–6140. [Google Scholar] [CrossRef]
- Fu, Q.; Zhuang, G.; Wang, J.; Xu, C.; Huang, K.; Li, J.; Hou, B.; Lu, T.; Streets, D.G. Mechanism of formation of the heaviest pollution episode ever recorded in the Yangtze River Delta, China. Atmos. Environ. 2008, 42, 2023–2036. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Zhang, X.; Zhang, X.; Huang, K.; Xu, C.; Tang, A.; Chen, J.; An, Z. The ion chemistry, seasonal cycle, and sources of PM2.5 and TSP aerosol in Shanghai. Atmos. Environ. 2006, 40, 2935–2952. [Google Scholar] [CrossRef]
- Ambient Air Quality Standards in China; GB3095-2012; Ministry of Environmental Protection and General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2012.
- Tan, J.H.; Duan, J.C.; Chen, D.H.; Wang, X.H.; Guo, S.J.; Bi, X.H.; Sheng, G.Y.; He, K.B.; Fu, J.M. Chemical characteristics of haze during summer and winter in Guangzhou. Atmos. Res. 2009, 94, 238–245. [Google Scholar] [CrossRef]
- Sun, Y.; Zhuang, G.; Tang, A.; Wang, Y.; An, Z. Chemical characteristics of PM2.5 and PM10 in haze-fog episodes in Beijing. Environ. Sci. Technol. 2006, 40, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Wang, Y.; He, H.; Liu, J.; Wang, X.; Zhu, T.; Ge, M.; Zhou, J.; Tang, G.; Ma, J. Haze insights and mitigation in China: An overview. J. Environ. Sci. 2014, 26, 2–12. [Google Scholar] [CrossRef]
- Chen, P.; Wang, T.; Dong, M.; Kasoar, M.; Han, Y.; Xie, M.; Li, S.; Zhuang, B.; Li, M.; Huang, T. Characterization of major natural and anthropogenic source profiles for size-fractionated PM in Yangtze River Delta. Sci. Total Environ. 2017, 598, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Pritchett, L.C.; Pierson, W.R.; Frazier, C.A.; Purcell, R.G. The dri thermal/optical reflectance carbon analysis system: Description, evaluation and applications in U.S. air quality studies. Atmos. Environ. 1993, 27, 1185–1201. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Chen, L.W.A.; Arnott, W.P.; Moosmuller, H. Equivalence of elemental carbon by thermal/optical reflectance and transmittance with different temperature protocols. Environ. Sci. Technol. 2004, 38, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Chen, L.W.A.; Paredes-Mir, G.; Chang, M.C.O.; Trimble, D. Atmospheric chemistry and physics refining temperature measures in thermal/optical carbon analysis. Atmos. Chem. Phys. 2005, 5, 2961–2972. [Google Scholar] [CrossRef]
- Tian, S.L.; Pan, Y.P.; Wang, Y.S. Size-resolved source apportionment of particulate matter in urban Beijing during haze and non-haze episodes. Atmos. Chem. Phys. 2016, 16, 1–19. [Google Scholar] [CrossRef]
- Tan, J.; Duan, J.; Zhen, N.; He, K.; Hao, J. Chemical characteristics and source of size-fractionated atmospheric particle in haze episode in Beijing. Atmos. Res. 2016, 167, 24–33. [Google Scholar] [CrossRef]
- Kunwar, B.; Kawamura, K. One-year observations of carbonaceous and nitrogenous components and major ions in the aerosols from subtropical Okinawa Island, an outflow region of Asian dusts. Atmos. Chem. Phys. 2014, 14, 1819–1836. [Google Scholar] [CrossRef]
- Yao, L.; Yang, L.X.; Yuan, Q.; Yan, C.; Dong, C.; Meng, C. Source apportionment of PM2.5 in a background site in the North China Plain. Sci. Total Environ. 2016, 541, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.W.; Huang, L.; Leaitch, W.R.; Sharma, S.; Brook, J.R.; Slowik, J.G. Observations of OM/OC and specific attenuation coefficients (SAC) in ambient fine PM at a rural site ini central Ontario, Canada. Atmos. Chem. Phys. 2010, 10, 2393–2411. [Google Scholar] [CrossRef]
- Thurston, G.D.; Spengler, J.D. A quantitative assessment of source contributions to inhalable particulate matter pollution in metropolitan Boston. Atmos. Environ. 1985, 19, 9–25. [Google Scholar] [CrossRef]
- Watson, J.G.; Cooper, J.A.; Huntzicker, J.J. The effective variance weighting for least squares calculations applied to the mass balance receptor model. Atmos. Environ. 1984, 18, 1347–1355. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Kong, S.; Han, B.; Bai, Z.; Chen, L.; Shi, J.; Xu, Z. Receptor Modeling of PM2.5, PM10 and TSP in Different Seasons and Long-range Transport Analysis at A coastal Site of Tianjin, China. Sci. Total Environ. 2010, 408, 4681–4694. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Y.; Han, S.; Wu, J.; Bi, X.; Shi, G.; Wang, J.; Yao, Q.; Cai, Z.; Liu, J.; et al. Vertical characteristics of PM2.5 during the heating season in Tianjin, China. Sci. Total Environ. 2015, 523, 152–160. [Google Scholar] [CrossRef] [PubMed]
- US EPA. EPA-CMB8.2 User’s Manual; EPA-452/R-04-011; US EPA: Research Triangle Park, NC, USA, 2004.
- Wang, L.T.; Wei, Z.; Yang, J.; Zhang, Y.; Zhang, F.; Su, J.; Meng, C.C.; Zhang, Q. The 2013 severe haze over southern Hebei, China: Model evaluation, source apportionment, and policy implications. Atmos. Chem. Phys. 2014, 14, 3151–3173. [Google Scholar] [CrossRef]
- Lu, Z.; Streets, D.G.; Zhang, Q.; Wang, S.; Carmichael, G.R.; Cheng, Y.F. Sulfur dioxide emissions in China and sulfur trends in East Asia since 2000. Atmos. Chem. Phys. 2010, 10, 6311–6331. [Google Scholar] [CrossRef]
- Turpin, B.J.; Cary, R.A.; Huntzicker, J.J. An in-situ, time-resolved analyzer for aerosol organic and elemental carbon. Aerosol Sci. Technol. 1990, 12, 161–171. [Google Scholar] [CrossRef]
- Yang, F.; Tan, J.; Zhao, Q.; Du, Z.; He, K.; Ma, W.; Duan, F.; Chen, G.; Zhao, Q. Characteristics of PM2.5 speciation in representative megacities and across China. Atmos. Chem. Phys. 2011, 11, 5207–5219. [Google Scholar] [CrossRef]
- Cesari, D.; Merico, E.; Dinoi, A.; Marinoni, A.; Bonasoni, P.; Contini, D. Seasonal variability of carbonaceous aerosols in an urban background area in Southern Italy. Atmos. Res. 2018, 200, 97–108. [Google Scholar] [CrossRef]
- Khan, M.B.; Masiol, M.; Formenton, G.; Di Gilio, A.; de Gennaro, G.; Agostinelli, C.; Pavoni, B. Carbonaceous PM2.5 and secondary organic aerosol across the Veneto region (NE Italy). Sci. Total Environ. 2016, 542, 172–181. [Google Scholar] [CrossRef] [PubMed]
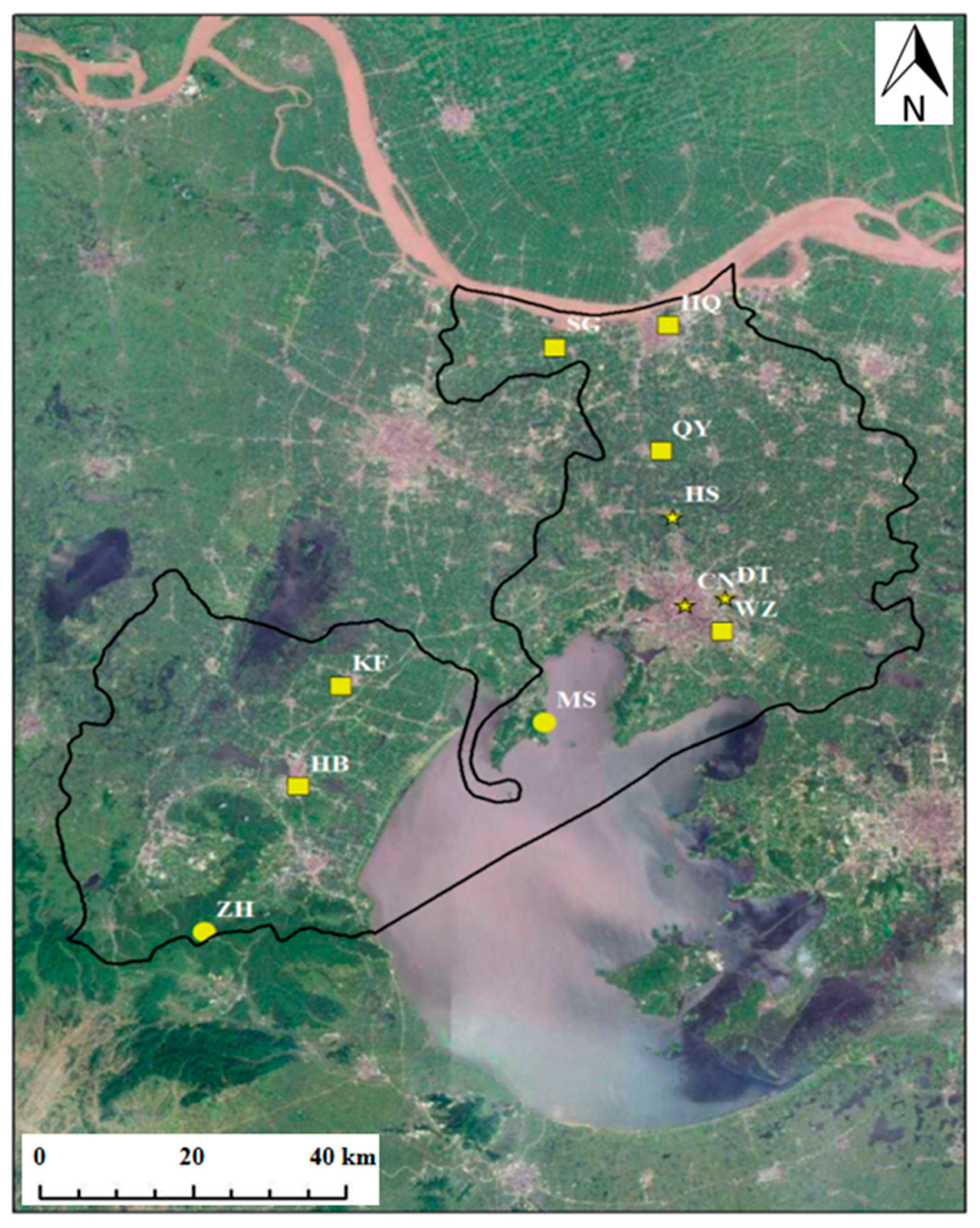

| Site | DT | CN | HS | HB | KF | QY | SG | HQ | WZ | MS | ZH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Urban | Industrial | Clean | ||||||||
| Mean a | 53.4 ± 26.5 b | 56.9 ± 26.3 | 55.2 ± 25.3 | 44.9 ± 22.3 | 49.0 ± 25.2 | 52.6 ± 28.7 | 53.4 ± 32.0 | 53.1 ± 28.3 | 51.2 ± 27.0 | 47.2 ± 25.0 | 41.2 ± 22.7 |
| Winter | 76.7 ± 30.3 | 74.1 ± 28.8 | 72.3 ± 29.5 | 61.7 ± 29.3 | 64.0 ± 28.6 | 69.6 ± 25.1 | 66.3 ± 24.1 | 69.3 ± 25.3 | 75.7 ± 30.2 | 68.5 ± 28.6 | 65.7 ± 29.6 |
| Spring | 64.6 ± 21.4 | 69.9 ± 27.9 | 67.3 ± 24.8 | 54.9 ± 14.8 | 65.9 ± 23.3 | 73.3 ± 33.0 | 83.9 ± 36.2 | 73.2 ± 32.7 | 62.7 ± 21.0 | 58.5 ± 18.3 | 41.1 ± 11.6 |
| Summer | 34.6 ± 7.3 | 39.3 ± 6.8 | 38.8 ± 6.3 | 29.2 ± 6.2 | 31.8 ± 5.8 | 32.7 ± 5.9 | 30.9 ± 6.3 | 33.7 ± 6.6 | 31.1 ± 7.7 | 28.0 ± 7.7 | 26.9 ± 5.7 |
| Autumn | 37.9 ± 10.8 | 44.4 ± 13.2 | 42.3 ± 13.1 | 33.9 ± 11.0 | 34.4 ± 11.3 | 34.8 ± 11.2 | 32.7 ± 11.1 | 36.1 ± 11.0 | 35.2 ± 11.1 | 32.7 ± 10.6 | 31.1 ± 10.3 |
| Haze | 111.3 ± 24.8 | 115.8 ± 26.3 | 111.8 ± 25.2 | 91.2 ± 26.1 | 106.2 ± 22.7 | 116.0 ± 24.1 | 122.7 ± 30.2 | 114.8 ± 23.9 | 109.7 ± 24.6 | 100.2 ± 23.8 | 84.2 ± 26.1 |
| Non-haze | 47.0 ± 17.2 | 50.4 ± 16.2 | 48.9 ± 15.7 | 39.8 ± 14.5 | 42.7 ± 15.6 | 45.5 ± 18.9 | 45.7 ± 21.0 | 46.2 ± 18.9 | 44.7 ± 18.0 | 41.2 ± 16.7 | 36.4 ± 15.0 |
| Meteorological Parameters | Correlation with PM2.5 Mass | |
|---|---|---|
| Temperature (°C) | 17.7 ± 11.2 a | −0.47 |
| Pressure (hPa) | 101.6 ± 1.1 | 0.33 |
| Relative humidity (%) | 74.7 ± 10.7 | −0.23 |
| Wind speed (m/s) | 3.0 ± 0.7 | −0.16 |
| Winter | Spring | Summer | Autumn | Industrial | Urban | Clean | Haze | Non-Haze | |
|---|---|---|---|---|---|---|---|---|---|
| straw burning | 1.03 | 0.73 | 0.37 | 0.71 | 0.76 | 0.75 | 0.51 | 1.11 | 0.64 |
| coal combustion | 7.34 | 6.38 | 2.25 | 3.63 | 5.53 | 5.09 | 3.37 | 8.56 | 4.48 |
| construction | 1.09 | 1.92 | 1.51 | 0.99 | 1.20 | 1.76 | 1.35 | 0.79 | 1.31 |
| soil | 2.05 | 2.40 | 0.54 | 1.49 | 1.60 | 1.85 | 1.32 | 2.47 | 1.46 |
| steel | 3.87 | 2.74 | 1.40 | 2.09 | 2.82 | 2.80 | 1.90 | 4.13 | 2.38 |
| fugitive | 3.14 | 4.55 | 0.87 | 1.90 | 2.85 | 2.49 | 2.09 | 3.62 | 2.38 |
| cooking | 0.52 | 0.43 | 0.22 | 0.48 | 0.41 | 0.49 | 0.30 | 0.60 | 0.37 |
| gasoline vehicle | 2.99 | 2.57 | 1.83 | 3.09 | 1.98 | 3.81 | 2.04 | 3.77 | 2.23 |
| diesel vehicle | 3.35 | 2.86 | 1.98 | 3.13 | 2.13 | 4.16 | 2.25 | 3.81 | 2.43 |
| sulfate | 7.65 | 12.40 | 11.10 | 4.77 | 9.54 | 8.53 | 8.00 | 24.70 | 7.65 |
| nitrate | 6.60 | 15.27 | 1.53 | 5.50 | 7.87 | 7.27 | 5.27 | 20.19 | 6.10 |
| Sea salt | 1.18 | 0.83 | 0.43 | 0.81 | 0.87 | 0.85 | 0.58 | 1.66 | 0.71 |
| SOA | 15.09 | 6.44 | 3.52 | 4.49 | 7.69 | 7.99 | 5.38 | 20.22 | 7.76 |
| ceramic | 0.63 | 0.55 | 0.14 | 0.23 | 1.49 | -- | -- | 1.76 | 0.71 |
| cement | 0.67 | 0.59 | 0.15 | 0.18 | 1.52 | -- | -- | 1.88 | 0.72 |
| textile | 0.14 | 0.35 | 0.40 | 0.10 | 0.93 | -- | -- | 0.31 | 0.48 |
| others | 12.25 | 4.02 | 4.21 | 2.30 | 1.43 | 7.36 | 9.66 | 8.02 | 4.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Wang, T.; Kasoar, M.; Xie, M.; Li, S.; Zhuang, B.; Li, M. Source Apportionment of PM2.5 during Haze and Non-Haze Episodes in Wuxi, China. Atmosphere 2018, 9, 267. https://doi.org/10.3390/atmos9070267
Chen P, Wang T, Kasoar M, Xie M, Li S, Zhuang B, Li M. Source Apportionment of PM2.5 during Haze and Non-Haze Episodes in Wuxi, China. Atmosphere. 2018; 9(7):267. https://doi.org/10.3390/atmos9070267
Chicago/Turabian StyleChen, Pulong, Tijian Wang, Matthew Kasoar, Min Xie, Shu Li, Bingliang Zhuang, and Mengmeng Li. 2018. "Source Apportionment of PM2.5 during Haze and Non-Haze Episodes in Wuxi, China" Atmosphere 9, no. 7: 267. https://doi.org/10.3390/atmos9070267
APA StyleChen, P., Wang, T., Kasoar, M., Xie, M., Li, S., Zhuang, B., & Li, M. (2018). Source Apportionment of PM2.5 during Haze and Non-Haze Episodes in Wuxi, China. Atmosphere, 9(7), 267. https://doi.org/10.3390/atmos9070267