Short-Term Association between Black Carbon Exposure and Cardiovascular Diseases in Pakistan’s Largest Megacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2.Outcome Definition and Measurement
2.3. Air Sample Collection and Data Acquisition
2.4. Statistical Analysis
3. Results and Discussion
3.1. Overview
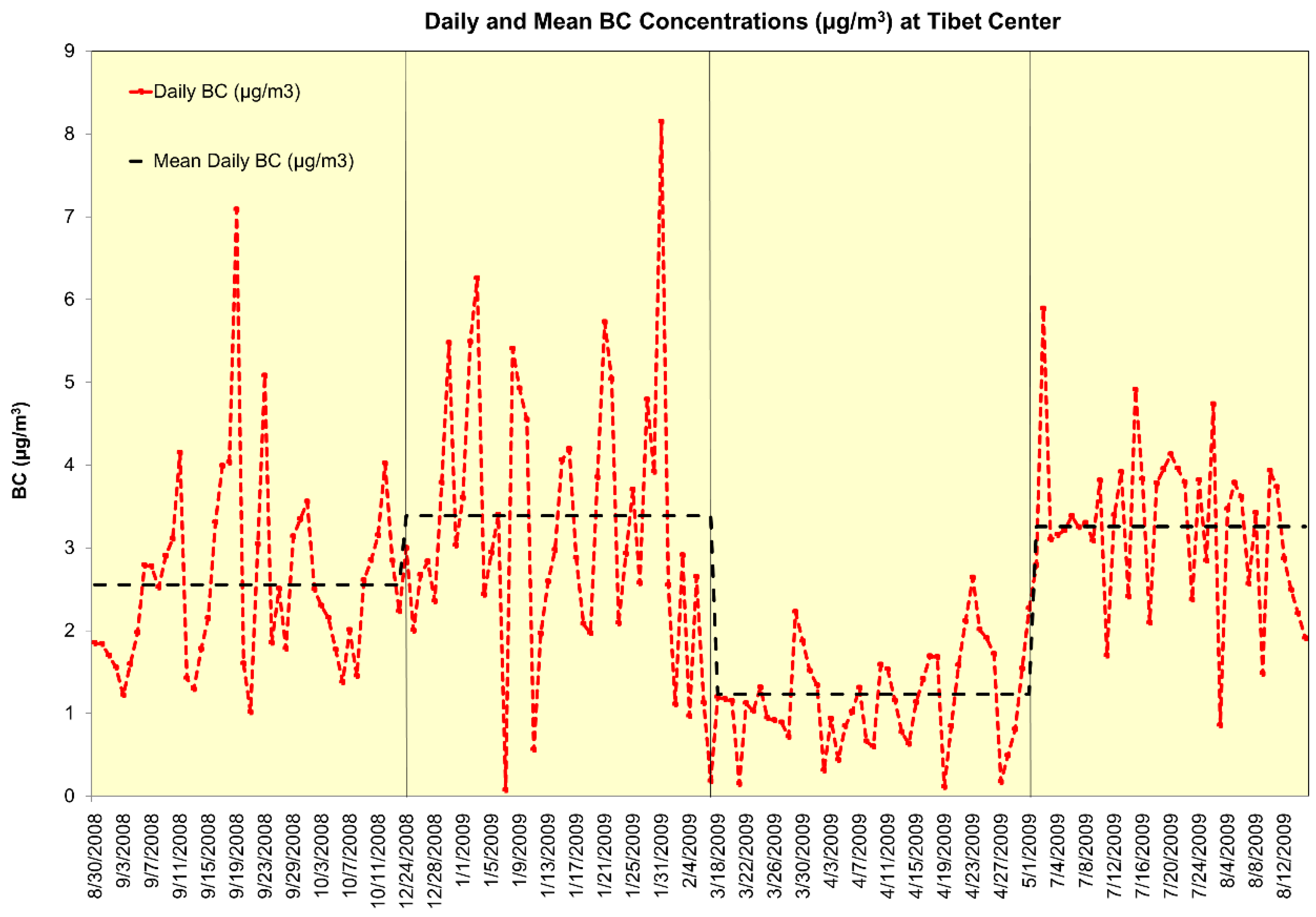
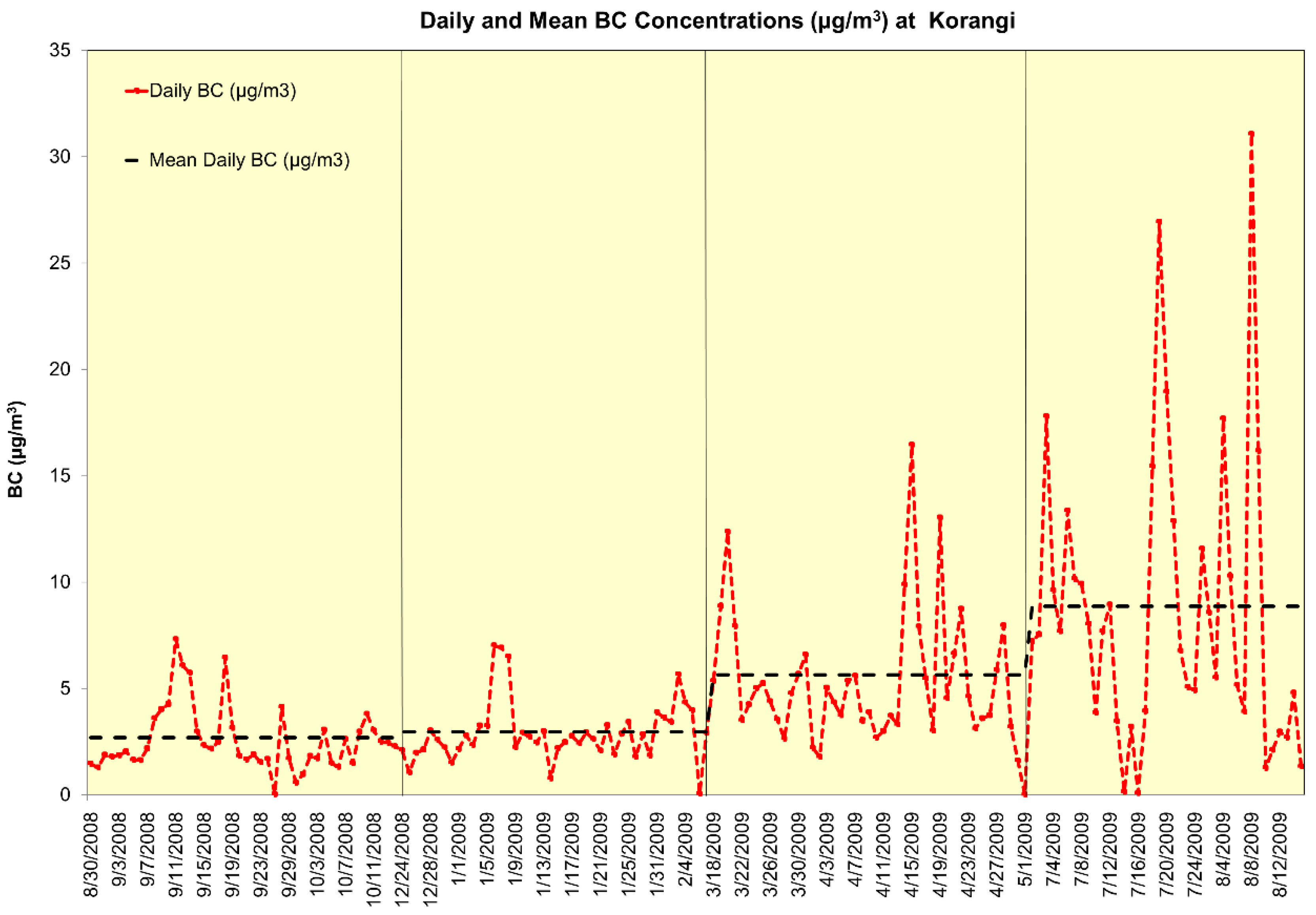
3.2. Daily Mean Black Carbon Concentrations
3.3. Cardiovascular Health Effects of Ambient Black Carbon Pollution
3.4. Strengths and Limitations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molina, M.J.; Molina, L.T. Megacities and atmospheric pollution. J. Air Waste Manag. Assoc. 2004, 54, 644–680. [Google Scholar] [CrossRef] [PubMed]
- Baklanov, A.; Molina, L.T.; Gauss, M. Mesgacities, air quality and climate. Atmos. Environ. 2016, 126, 235–249. [Google Scholar] [CrossRef]
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- WHO. Preventing Disease through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Lippmann, M. Toxicological and epidemiological studies of cardiovascular effects of ambient air fine particulate matter (PM2.5) and its chemical components: Coherence and public health implications. Crit. Rev. Toxicol. 2014, 44, 299–347. [Google Scholar] [CrossRef] [PubMed]
- Rohr, A.C.; Wyzga, R.E. Attributing health effects to individual particulate matter constituents. Atmos. Environ. 2012, 62, 130–152. [Google Scholar] [CrossRef]
- Stanek, L.W.; Sacks, J.D.; Dutton, S.J.; Dubois, J.J.B. Attributing health effects to apportioned components and sources of particulate matter: An evaluation of collective results. Atmos. Environ. 2011, 45, 5655–5663. [Google Scholar] [CrossRef]
- Andreae, M.O.; Gelencsér, A. Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols. Atmos. Chem. Phys. 2006, 6, 3131–3148. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Lamarque, J.F.; Bond, T.C.; Eyring, V. Historical (1850–2000) gridded anthropogenic and biomass burning emissions of reactive gases and aerosols: Methodology and application. Atmos. Chem. Phys. 2010, 10, 7017–7039. [Google Scholar] [CrossRef]
- Schmidt, C.W. Black Carbon: The Dark Horse of Climate Change Drivers. Environ. Health. Perspect. 2011, 119, A172–A175. [Google Scholar] [CrossRef] [PubMed]
- Grahame, T.J.; Klemm, R.; Schlesinger, R.B. Public health and components of particulate matter: The changing assessment of black carbon. J. Air Waste Manag. Assoc. 2014, 64, 620–660. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.H.; Hoek, G.; Simic, L.M. Black Carbon as an Additional Indicator of the Adverse Health Effects of Airborne Particles Compared with PM(10) and PM(2.5). Environ. Health Perspect. 2011, 119, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.H.; Janssen, N.; Gerlofs, N.M. Health Effects of Black Carbon; World Health Organization: Copenhagen, Denmak, 2012. [Google Scholar]
- Alwan, A. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Chen, R.; Chu, C.; Tan, J. Ambient air pollution and hospital admission in Shanghai, China. J. Hazard. Mater. 2010, 181, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.S.; Phalkey, R.; Malik, A.A. A systematic review of air pollution as a risk factor for cardiovascular disease in South Asia: Limited evidence from India and Pakistan. Int. J. Hyg. Environ. Health 2014, 217, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, H.; Fatmi, Z.; Malashock, D. Effect of air pollution on daily morbidity in Karachi, Pakistan. J. Local Glob. Health Sci. 2012, 217, 1–13. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, S.; Fatmi, Z. Assessing the association between fine particulate matter (PM2.5) constituents and cardiovascular diseases in a mega-city of Pakistan. Environ. Health Perspect. 2018. [Google Scholar]
- Malashock, D. Short-Term Associations between PM2.5, Black Carbon, Delta-C, and Cardiovascular Diseases in a Large Developing Megacity. Master’s Thesis, State University of New York, New York, NY, USA, 2012. [Google Scholar]
- NICVD. Our Services: Out-Patients and Emergency. Available online: http://nicvd.org/ (accessed on 5 October 2018).
- Wang, Y.; Hopke, P.K.; Rattigan, O.V.; Xia, X.; Chalupa, D.C.; Utell, M.J. Characterization of Residential Wood Combustion Particles Using the Two-Wavelength Aethalometer. Environ. Sci. Technol. 2011, 45, 7387–7393. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, V.; Alvi, S.; Ghauri, B.M.; Choudhary, M.; Husain, L. Black carbon aerosols in urban air in South Asia. Atmos. Environ. 2009, 43, 1737–1744. [Google Scholar] [CrossRef]
- Kirchstetter, T.W.; Novakov, T.; Hobbs, P.V. Evidence that the spectral dependence of light absorption by aerosols is affected by organic carbon. J. Geophys. Res. 2004, 109, D21208. [Google Scholar] [CrossRef]
- Bibi, S.; Alam, K.; Chishtie, F.; Bibi, H.; Rahman, S. Temporal variation of Black Carbon concentration using Aethalometer observations and its relationships with meteorological variables in Karachi, Pakistan. J. Atmos. Sol. Terr. Phys. 2017, 157–158, 67–77. [Google Scholar] [CrossRef]
- Li, K.; Liao, H.; Mao, Y.; Ridley, D.A. Source sector and region contributions to concentration and direct radiative forcing of black carbon in China. Atmos. Environ. 2016, 124, 351–366. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Q.; Streets, D.G. Sulfur dioxide and primary carbonaceous aerosol emissions in China and India; 1996–2010. Atmos. Chem. Phys. 2011, 11, 9840–9864. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, H.; Li, J. Impacts of Asian summer monsoon on seasonal and interannual variations of aerosols over eastern China. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Sughis, M.; Nawrot, T.S.; Ihsan, H.S.; Amjad, A.; Nemery, B. Blood pressure and particulate air pollution in schoolchildren of Lahore, Pakistan. BMC Public Health 2012, 12, 378–386. [Google Scholar] [CrossRef] [PubMed]
- PBS. Labour Force Participation Rates and Un-Employment Rates by Age, Sex and Area, 2012–2013. 2013; Statistical Reports and Publications of the Pakistan Bureau of Statistics. Available online: http://www.pbs.gov.pk/sites/default/files/Labour%20Force/publications/lfs_Annual_2012_13/t14-pak.pdf (accessed on 15 August 2018).
- Ali, T.S.; Krantz, G.; Gul, R.; Asad, N.; Johansson, E.; Mogren, I. Gender roles and their influence on life prospects for women in urban Karachi, Pakistan: A qualitative study. Glob. Health Action 2011, 4, 7448. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.S.; Khan, K.; Shaikh, B.T. Gender: Shaping personality, lives and health of women in Pakistan. BMC Womens Health 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Jafar, T.H.; Qadri, Z.; Chaturvedi, N. Coronary artery disease epidemic in Pakistan: More electrocardiographic evidence of ischaemia in women than in men. Heart 2008, 94, 408. [Google Scholar] [CrossRef] [PubMed]
- WHO. Indoor Air P Indoor Air Pollution and Child Health in Pollution and Child Health in Pakistan; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Khan, M.S.; Jafary, F.H.; Faruqui, A.M. High prevalence of lack of knowledge of symptoms of acute myocardial infarction in Pakistan and its contribution to delayed presentation to the hospital. BMC Public Health 2007, 7, 284. [Google Scholar] [CrossRef] [PubMed]
- Zia, N.; Shahzad, H.; Baqir, S.M. Ambulance use in Pakistan: An analysis of surveillance data from emergency departments in Pakistan. BMC Emerg. Med. 2015, 15, S9. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, S.; Baumgartner, J.; Weichenthal, S. Impacts of exposure to black carbon, elemental carbon, and ultrafine particles from indoor and outdoor sources on blood pressure in adults: A review of epidemiological evidence. Environ. Res. 2018, 161, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Henze, D.K.; Jack, D.; Henderson, B.H.; Kinney, P.L. Assessing public health burden associated with exposure to ambient black carbon in the United States. Sci. Total Environ. 2016, 539, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Jerrett, M.; Burnett, R.T. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res. Rep. Health Eff. Inst. 2009, 140, 115–136. [Google Scholar]
- Gurjar, B.R.; Butler, T.M.; Lawrence, M.G.; Lelieveld, J. Evaluation of emissions and air quality in megacities. Atmos. Environ. 2008, 42, 1593–1606. [Google Scholar] [CrossRef]
- Gurjar, B.R.; Jain, A.; Sharma, A. Human health risks in megacities due to air pollution. Atmos. Environ. 2010, 44, 4606–4613. [Google Scholar] [CrossRef]
- Ira, T.; Kenneth, D.; Mark, F.; Michael, J.; Frank, K. Traffic-related air pollution: A critical review of the literature on emissions, exposure, and health effects. Res. Rep. Health Eff. Inst. 2010, 17, 386. [Google Scholar]
- Kazmi, J.H.; Zubair, S. Estimation of Vehicle Damage Cost Involved in Road Traffic Accidents in Karachi; Pakistan: A Geospatial Perspective. Procedia Eng. 2014, 77, 70–78. [Google Scholar] [CrossRef]
- Sabzwari, S.R.; Fatmi, Z. Comparison of exhaled carbon monoxide levels among commuters and roadside vendors in an urban and a suburban population in Pakistan. Environ. Monit. Assess. 2011, 180, 399–408. [Google Scholar] [CrossRef] [PubMed]
| Period | ||||
|---|---|---|---|---|
| Variable (Unit) | Mean | SD a | Min | Max |
| Daily Count of Hospital Admissions | ||||
| 0 to 40 years | ||||
| Male (n = 1291) | 4.95 | 3.99 | 1 | 28 |
| Female (n = 1251) | 5.23 | 3.88 | 1 | 25 |
| 41 to 60 years | ||||
| Male (n = 3313) | 10.3 | 9.49 | 1 | 62 |
| Female (n = 2254) | 8.17 | 6.90 | 1 | 51 |
| >61 years | ||||
| Male (n = 1762) | 5.44 | 4.50 | 1 | 31 |
| Female (n = 1152) | 4.01 | 3.79 | 1 | 40 |
| Daily Count of Outpatient/Emergency Room Visits | ||||
| 0 to 40 years | ||||
| Male (n = 2033) | 7.34 | 6.16 | 1 | 39 |
| Female (n = 3879) | 14.37 | 16.16 | 1 | 96 |
| 41 to 60 years | ||||
| Male (n = 5673) | 18.85 | 15.12 | 1 | 92 |
| Female (n = 6363) | 21.35 | 21.55 | 1 | 134 |
| >60 years | ||||
| Male (n = 3698) | 12.12 | 7.87 | 1 | 43 |
| Female (n = 2478) | 8.29 | 5.83 | 1 | 32 |
| Daily Pollutant and Meteorological Measurements | ||||
| BC (µg/m3, Korangi) | 4.75 | 4.47 | 0.01 | 31.1 |
| BC (µg/m3, Tibet Center) | 2.53 | 1.43 | 0.07 | 8.15 |
| Maximum Temperature (°C) | 31.97 | 4.90 | 21.7 | 58.9 |
| Mean Temperature (°C) | 27.72 | 4.60 | 17.2 | 35.6 |
| Minimum Temperature (°C) | 23.51 | 5.29 | 10. | 30 |
| Maximum Humidity (%) | 80 | 11.61 | 32. | 100 |
| Mean Humidity (%) | 62.99 | 14.20 | 24 | 90 |
| Minimum Humidity (%) | 42.61 | 17.67 | 6 | 78 |
| Maximum Pressure (Hg) | 29.83 | 0.25 | 29.4 | 31.4 |
| Mean Pressure (Hg) | 29.76 | 0.20 | 29.4 | 30.1 |
| Minimum Pressure (Hg) | 29.71 | 0.20 | 29.3 | 30.1 |
| Relative Risk Estimates (95% Confidence Intervals) | ||||||
|---|---|---|---|---|---|---|
| Pollutant | All Patients | Female | Male | 0–40 years | 41–60 years | >60 years |
| Hospital Admissions | ||||||
| Korangi BC | ||||||
| Lag 0 | 0.9950 (0.9829, 1.0071) | 0.9906 (0.9746, 1.0069) | 0.9986 (0.9811, 1.0163) | 0.9892 (0.9717, 1.0070) | 0.9972 (0.9761, 1.0187) | 0.9939 (0.9751, 1.0130) |
| Lag 1 | 0.9975 (0.9848, 1.0103) | 0.9947 (0.9773, 1.0123) | 0.9998 (0.9818, 1.0181) | 0.9942 (0.9748, 1.0138) | 0.9952 (0.9732, 1.0176) | 1.0025 (0.9835, 1.0218) |
| Lag 2 | 0.9958 (0.9836, 1.0080) | 1.0000 (0.9831, 1.0171) | 0.9917 (0.9748, 1.0090) | 0.9932 (0.9746, 1.0121) | 0.9959 (0.9754, 1.0167) | 0.9946 (0.9755, 1.0140) |
| Lag 3 | 0.9975 (0.9852, 1.0100) | 1.0044 (0.9871, 1.0219) | 0.9908 (0.9737, 1.0083) | 1.0006 (0.9824, 1.0191) | 0.9953 (0.9735, 1.0174) | 1.0007 (0.9820, 1.0197) |
| Tibet Center BC | ||||||
| Lag 0 | 1.0081 (0.9749, 1.0424) | 1.0044 (0.9586, 1.0522) | 1.0119 (0.9653, 1.0609) | 0.9928 (0.9439, 1.0442) | 1.0077 (0.9504, 1.0684) | 1.0162 (0.9658, 1.0691) |
| Lag 1 | 1.0033 (0.9688, 1.0388) | 1.0197 (0.9720, 1.0699) | 0.9889 (0.9412, 1.0391) | 1.0179 (0.9655, 1.0732) | 0.9993 (0.9396, 1.0626) | 0.9945 (0.9444, 1.0471) |
| Lag 2 | 0.9907 (0.9589, 1.0235) | 0.9785 (0.9348, 1.0242) | 0.9975 (0.9529, 1.0441) | 0.9644 (0.9185, 1.0126) | 0.9979 (0.9421, 1.0568) | 1.0017 (0.9538, 1.0519) |
| Lag 3 | 0.9786 (0.9447, 1.0135) | 1.0009 (0.9527, 1.0514) | 0.9581 (0.9120, 1.0065) | 0.9749 (0.9247, 1.0277) | 0.9803 (0.9209, 1.0436) | 0.9891 (0.9390, 1.0418) |
| Outpatient Department/Emergency Room Visits | ||||||
| Korangi BC | ||||||
| Lag 0 | 0.9916 (0.9796, 1.0039) | 0.9893 (0.9710, 1.0081) | 0.9942 (0.9791, 1.0096) | 0.9919 (0.9676, 1.0168) | 0.9900 (0.9699, 1.0105) | 0.9927 (0.9788, 1.0068) |
| Lag 1 | 1.0002 (0.9875, 1.0130) | 0.9963 (0.9759, 1.0170) | 1.0039 (0.9888, 1.0192) | 1.0001 (0.9745, 1.0262) | 1.0017 (0.9803, 1.0234) | 0.9972 (0.9825, 1.0119) |
| Lag 2 | 0.9837 (0.9715, 0.9961) | 0.9779 (0.9588, 0.9975) | 0.9893 (0.9745, 1.0045) | 0.9857 (0.9619, 1.0100) | 0.9790 (0.9581, 1.0003) | 0.9898 (0.9759, 1.0040) |
| Lag 3 | 0.9919 (0.9803, 1.0036) | 0.9912 (0.9732, 1.0096) | 0.9921 (0.9780, 1.0064) | 0.9997 (0.9776, 1.0223) | 0.9865 (0.9668, 1.0066) | 0.9937 (0.9803, 1.0073) |
| Tibet Center BC | ||||||
| Lag 0 | 1.0251 (0.9909, 1.0604) | 1.0340 (0.9804, 1.0905) | 1.0165 (0.9754, 1.0593) | 1.0227 (0.9559, 1.0940) | 1.0392 (0.9829, 1.0987) | 1.0023 (0.9629, 1.0434) |
| Lag 1 | 1.0255 (0.9917, 1.0604) | 1.0365 (0.9837, 1.0923) | 1.0125 (0.9717, 1.0551) | 1.0162 (0.9522, 1.0845) | 1.0422 (0.9860, 1.1016) | 1.0164 (0.9760, 1.0583) |
| Lag 2 | 0.9873 (0.9555, 1.0203) | 0.9810 (0.9318, 1.0329) | 0.9928 (0.9537, 1.0334) | 0.9893 (0.9283, 1.0544) | 0.9928 (0.9403, 1.0483) | 0.9860 (0.9479, 1.0259) |
| Lag 3 | 0.9966 (0.9632, 1.0309) | 0.9952 (0.9442, 1.0488) | 0.9971 (0.9560, 1.0398) | 1.0031 (0.9387, 1.0718) | 0.9927 (0.9394, 1.0489) | 1.0060 (0.9649, 1.0488) |
| Relative Risk Estimates (95% Confidence Intervals) | ||
|---|---|---|
| Pollutant | Hospital Admissions | Outpatient Department & Emergency Room Visits |
| Korangi BC | ||
| Lag 0 | 1.0136 (0.9875, 1.0404) | 0.9545 (0.9076, 1.0037) |
| Lag 1 | 1.0197 (0.9755, 1.0659) | 0.9602 (0.9118, 1.0111) |
| Lag 2 | 1.0084 (0.9851, 1.0322) | 0.9658 (0.9193, 1.0147) |
| Lag 3 | 1.0089 (0.9637, 1.0562) | 0.9867 (0.9456, 1.0295) |
| Tibet Center BC | ||
| Lag 0 | 1.0129 (0.9869, 1.0397) | 1.0074 (0.9335, 1.0873) |
| Lag 1 | 0.9896 (0.9476, 1.0333) | 1.0477 (0.9751, 1.1256) |
| Lag 2 | 1.0226 (1.0008, 1.0448) | 0.9778 (0.9058, 1.0557) |
| Lag 3 | 0.981 (0.9365, 1.0275) | 1.0234 (0.9444, 1.1089) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malashock, D.; Khwaja, H.A.; Fatmi, Z.; Siddique, A.; Lu, Y.; Lin, S.; Carpenter, D. Short-Term Association between Black Carbon Exposure and Cardiovascular Diseases in Pakistan’s Largest Megacity. Atmosphere 2018, 9, 420. https://doi.org/10.3390/atmos9110420
Malashock D, Khwaja HA, Fatmi Z, Siddique A, Lu Y, Lin S, Carpenter D. Short-Term Association between Black Carbon Exposure and Cardiovascular Diseases in Pakistan’s Largest Megacity. Atmosphere. 2018; 9(11):420. https://doi.org/10.3390/atmos9110420
Chicago/Turabian StyleMalashock, Daniel, Haider A. Khwaja, Zafar Fatmi, Azhar Siddique, Yi Lu, Shao Lin, and David Carpenter. 2018. "Short-Term Association between Black Carbon Exposure and Cardiovascular Diseases in Pakistan’s Largest Megacity" Atmosphere 9, no. 11: 420. https://doi.org/10.3390/atmos9110420
APA StyleMalashock, D., Khwaja, H. A., Fatmi, Z., Siddique, A., Lu, Y., Lin, S., & Carpenter, D. (2018). Short-Term Association between Black Carbon Exposure and Cardiovascular Diseases in Pakistan’s Largest Megacity. Atmosphere, 9(11), 420. https://doi.org/10.3390/atmos9110420







