Particle-Bound Polycyclic Aromatic Hydrocarbon in the Atmosphere of Heavy Traffic Areas in Greater Cairo, Egypt: Status, Source, and Human Health Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
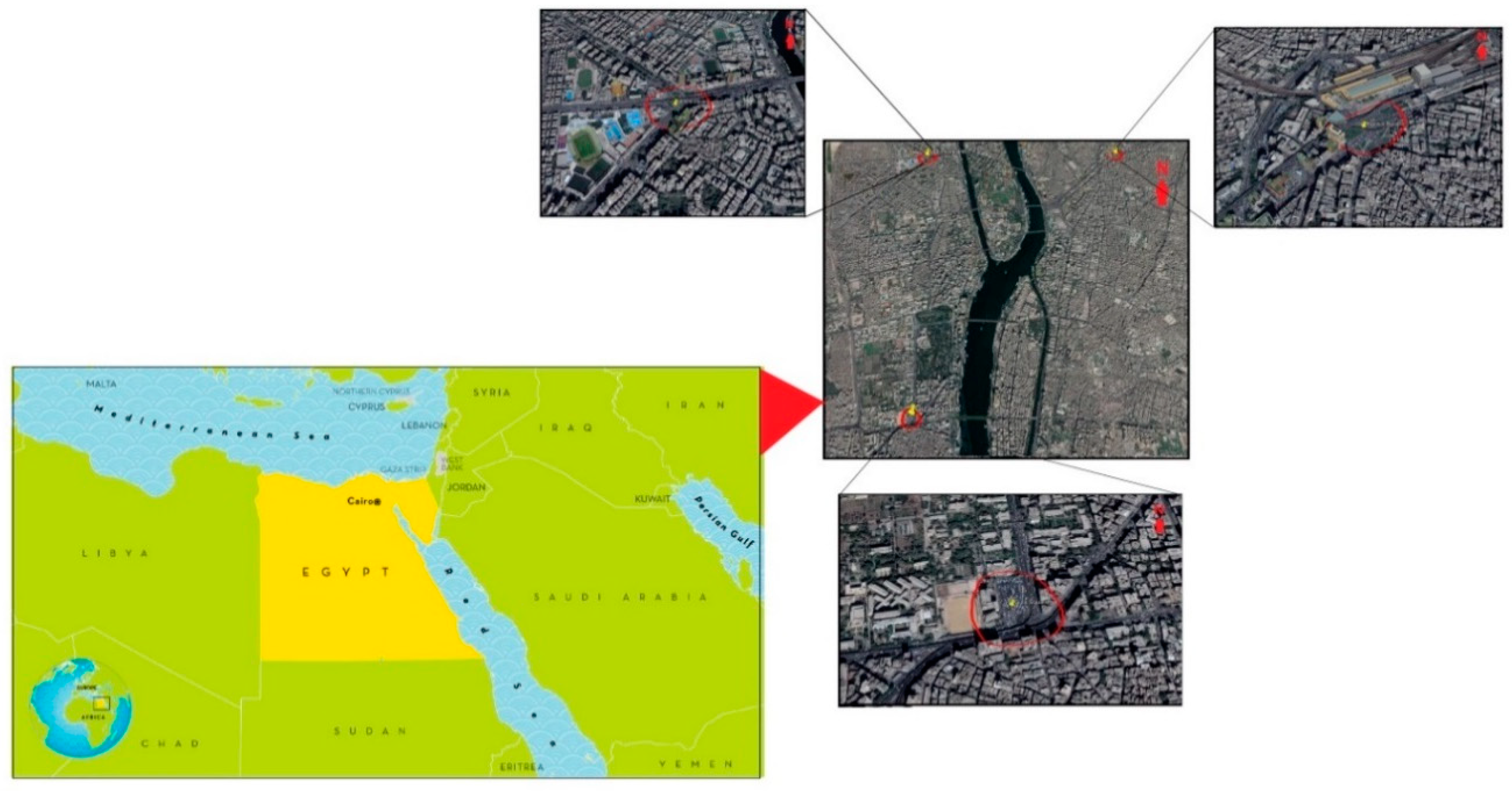
2.1. Sampling Sites and Periods
2.2. Chemical Analysis
3. Results and Discussion
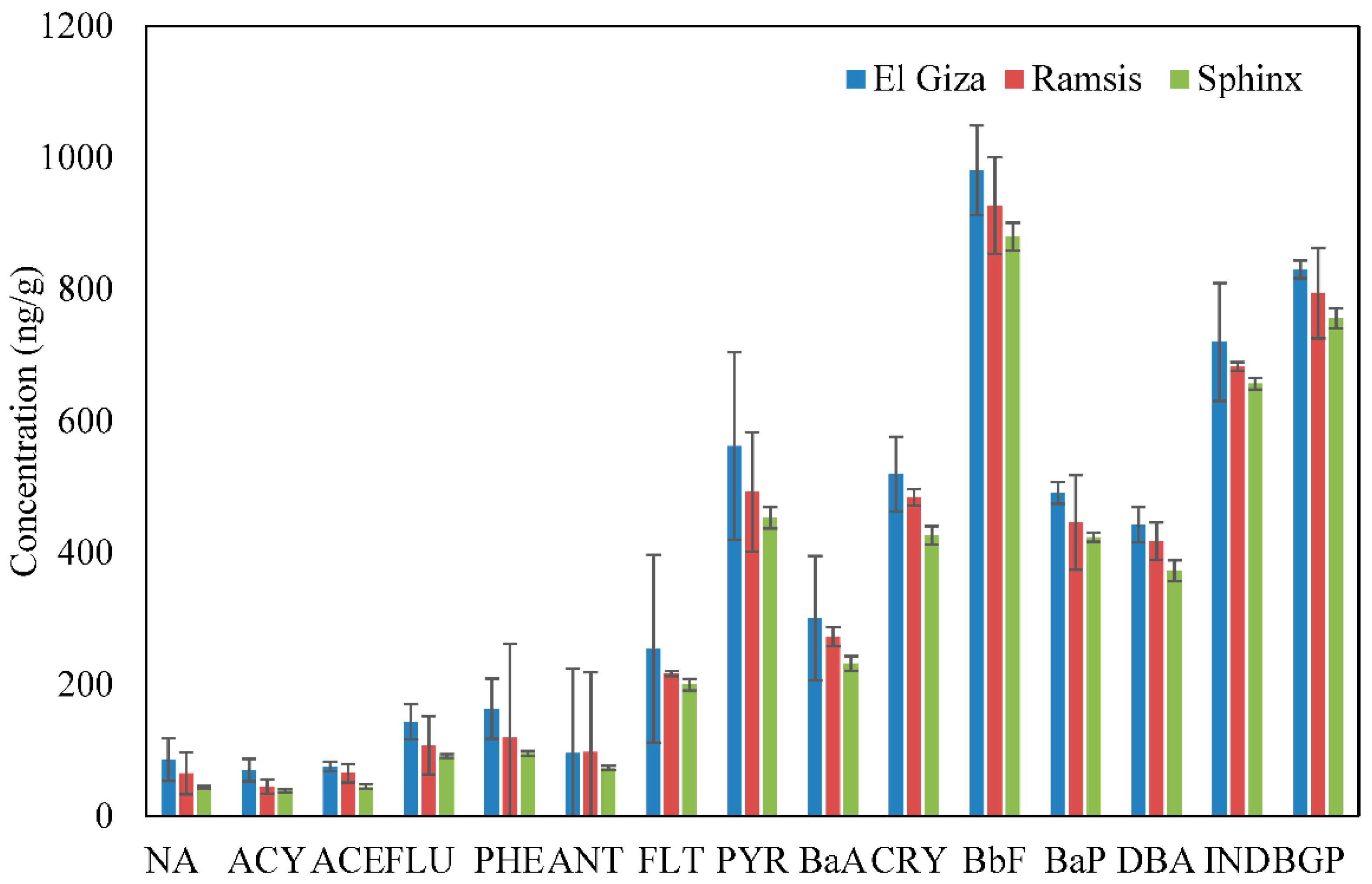
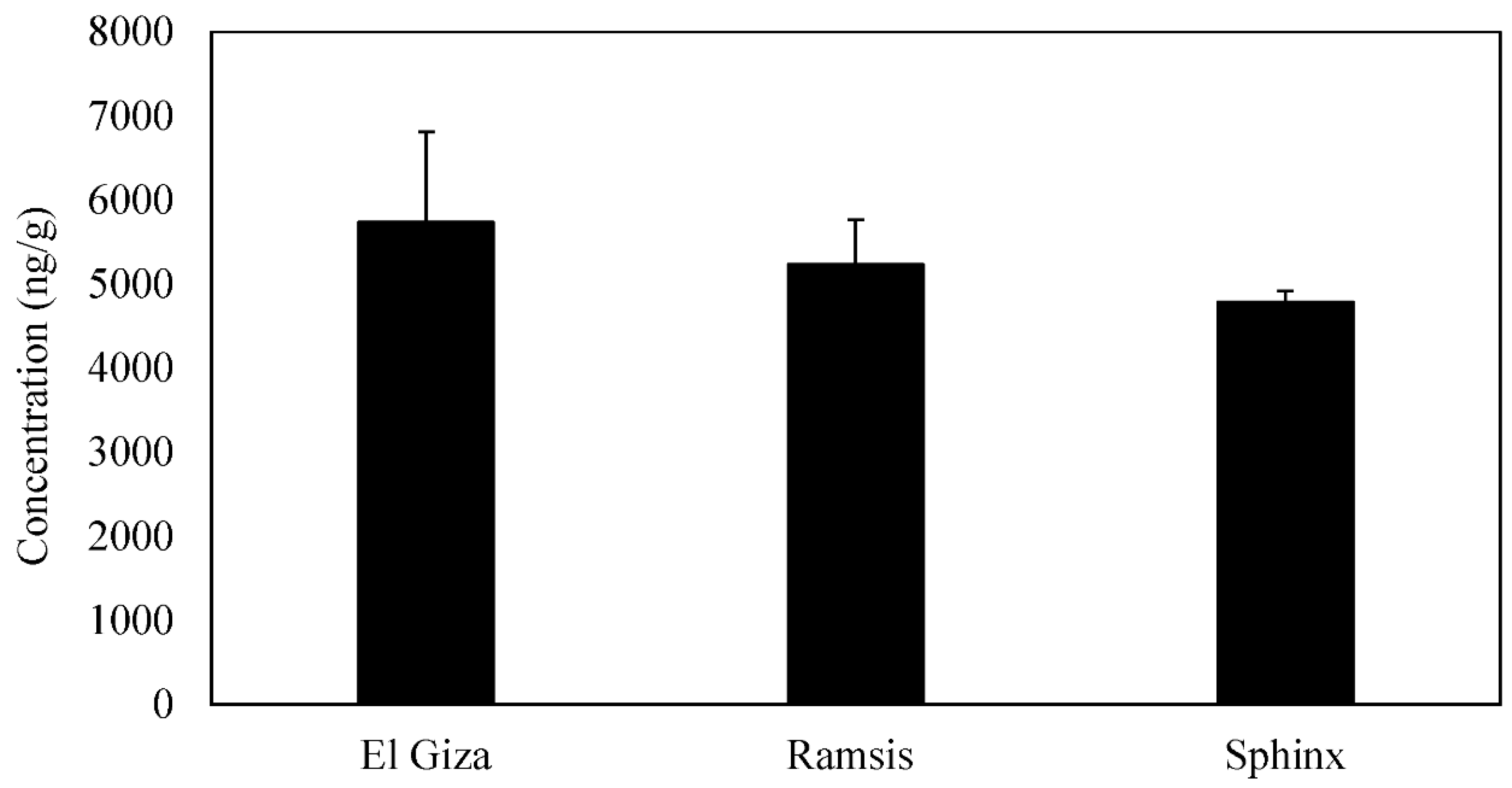
3.1. Spatial Variations of PAHs
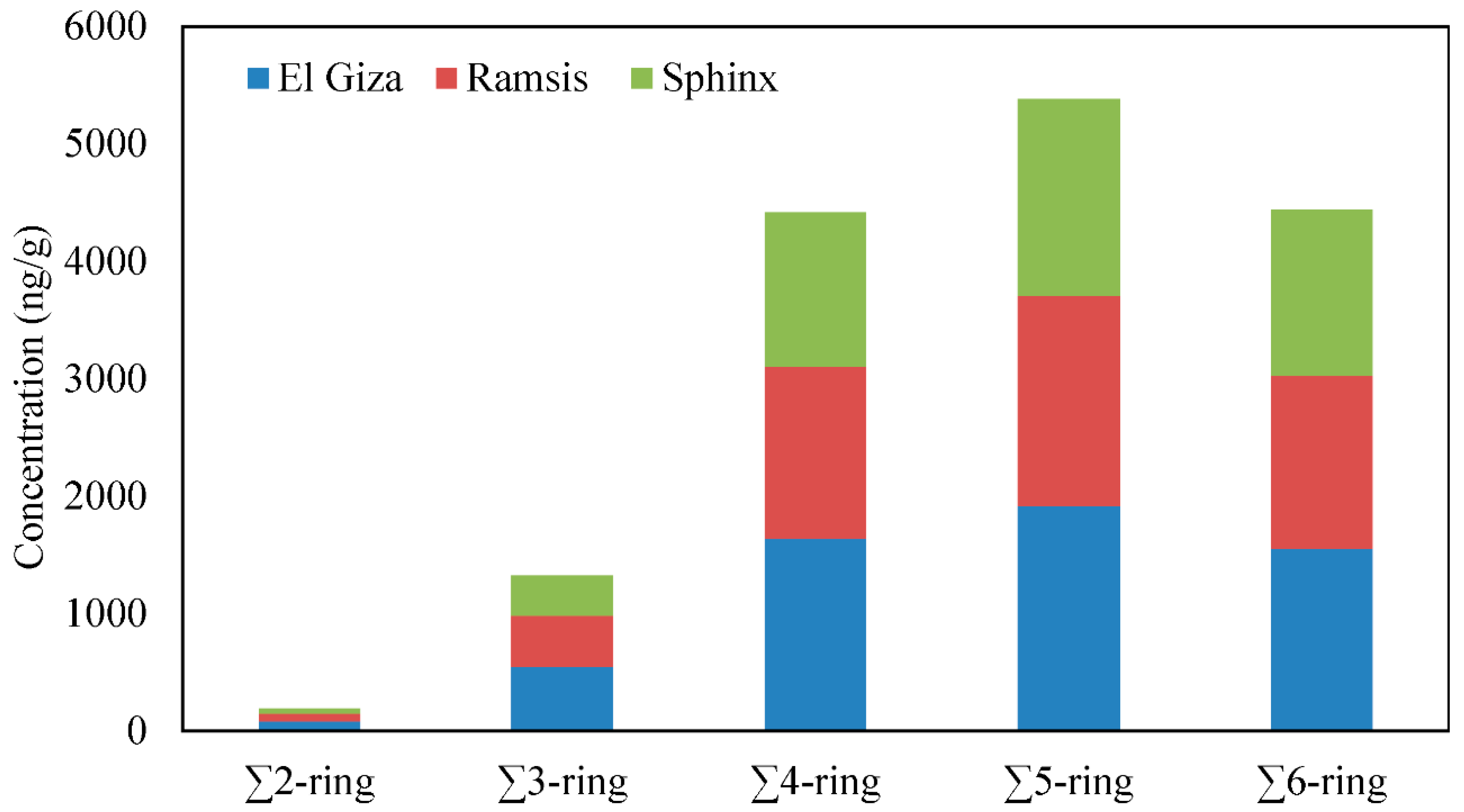
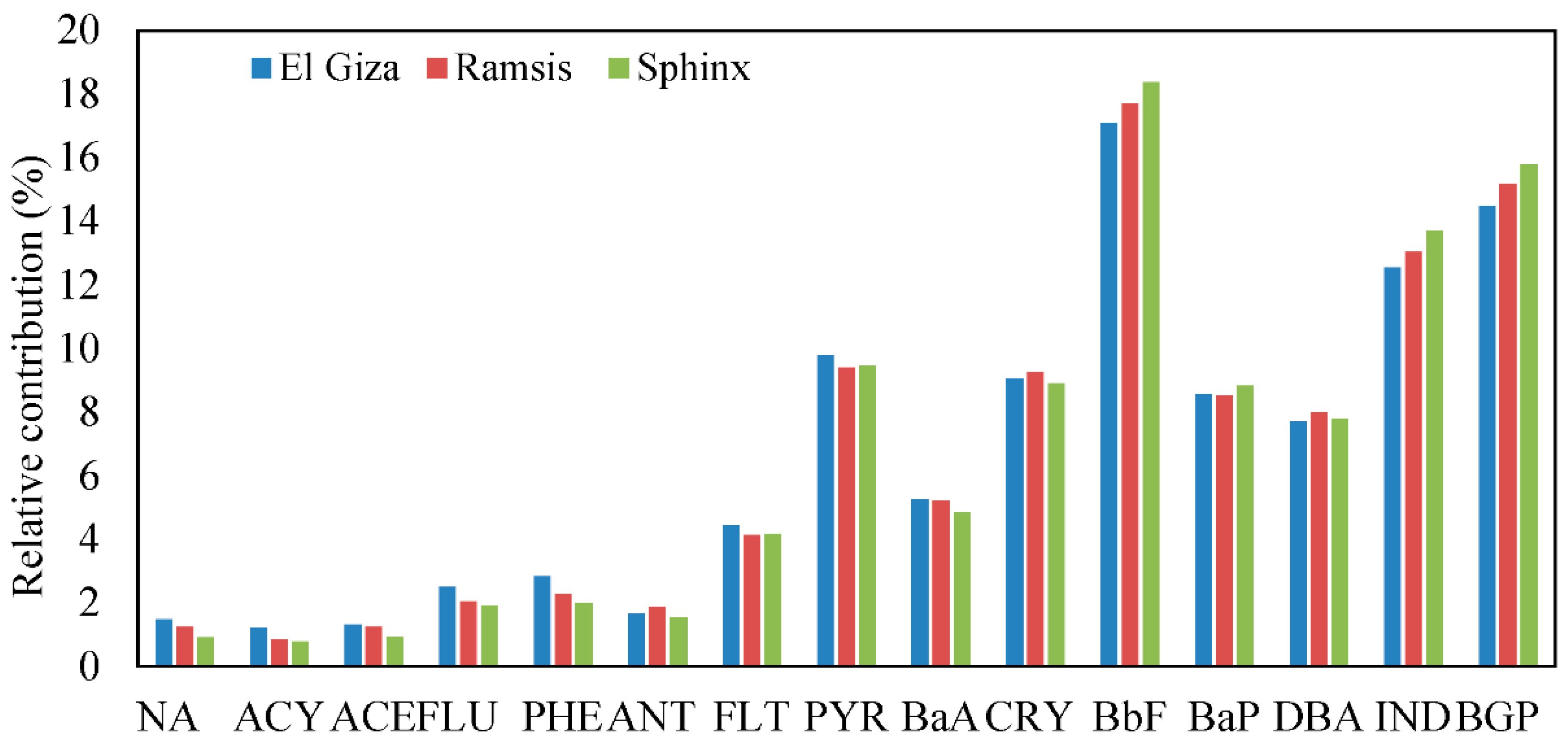
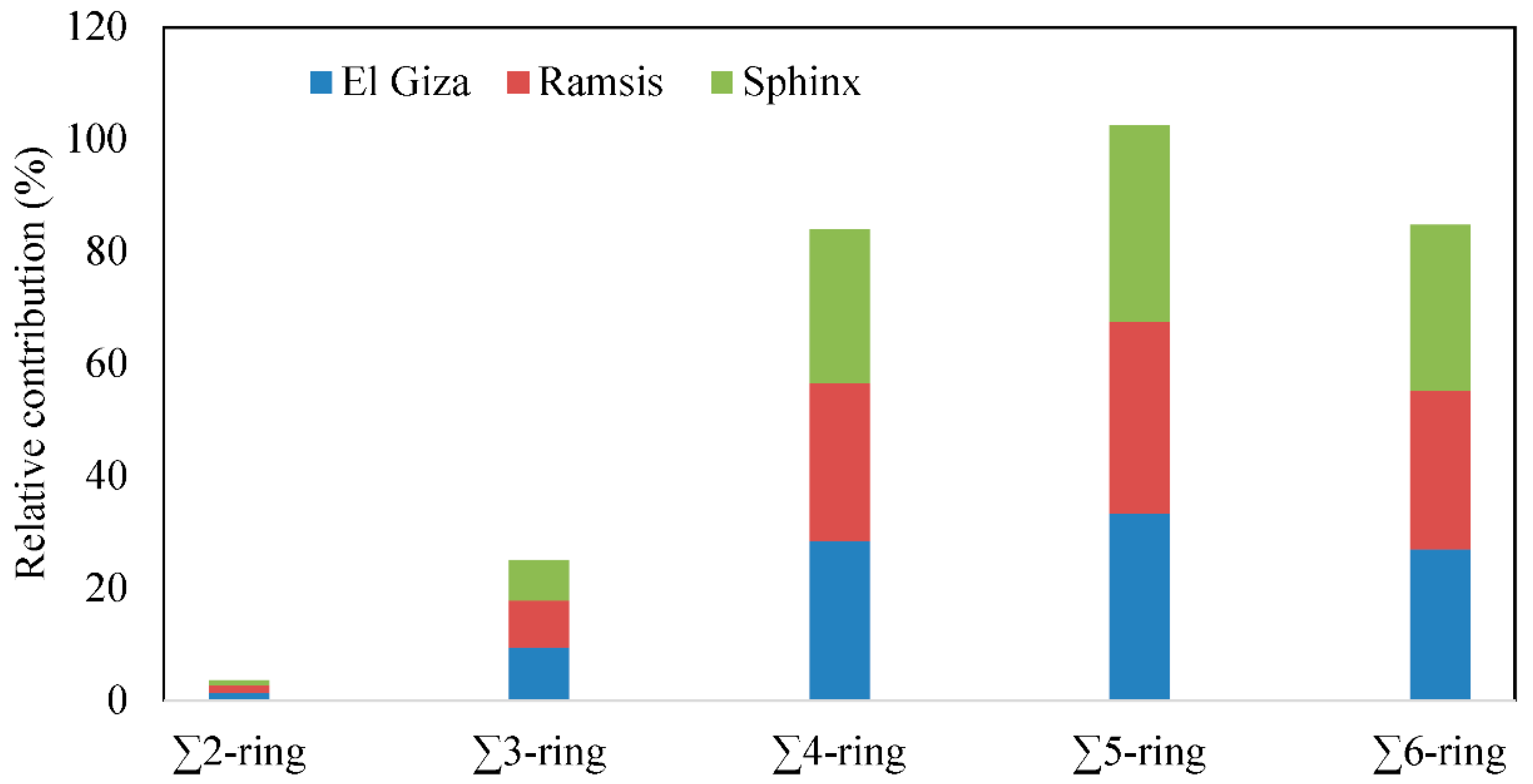
3.2. Distribution of PAH Compounds
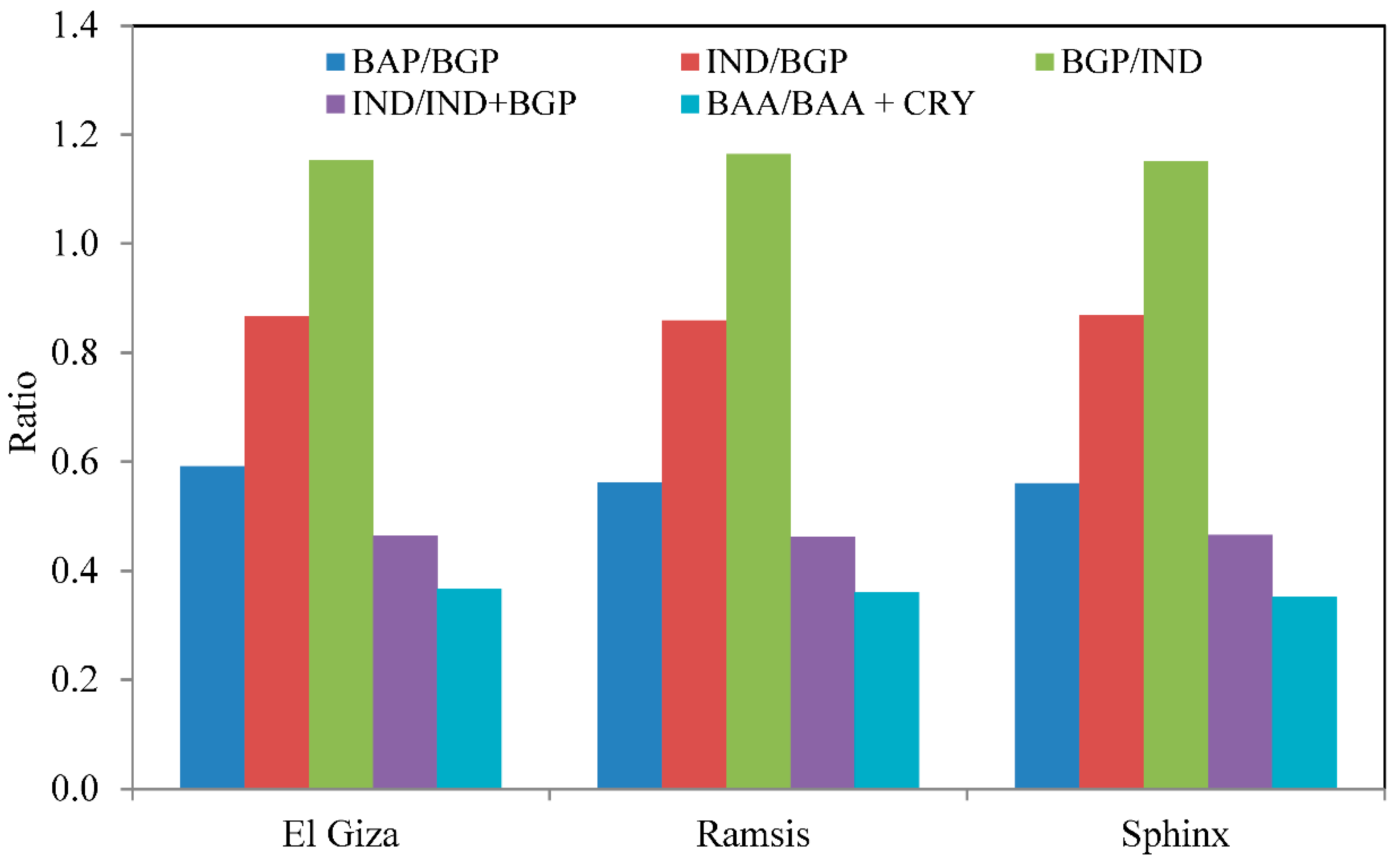
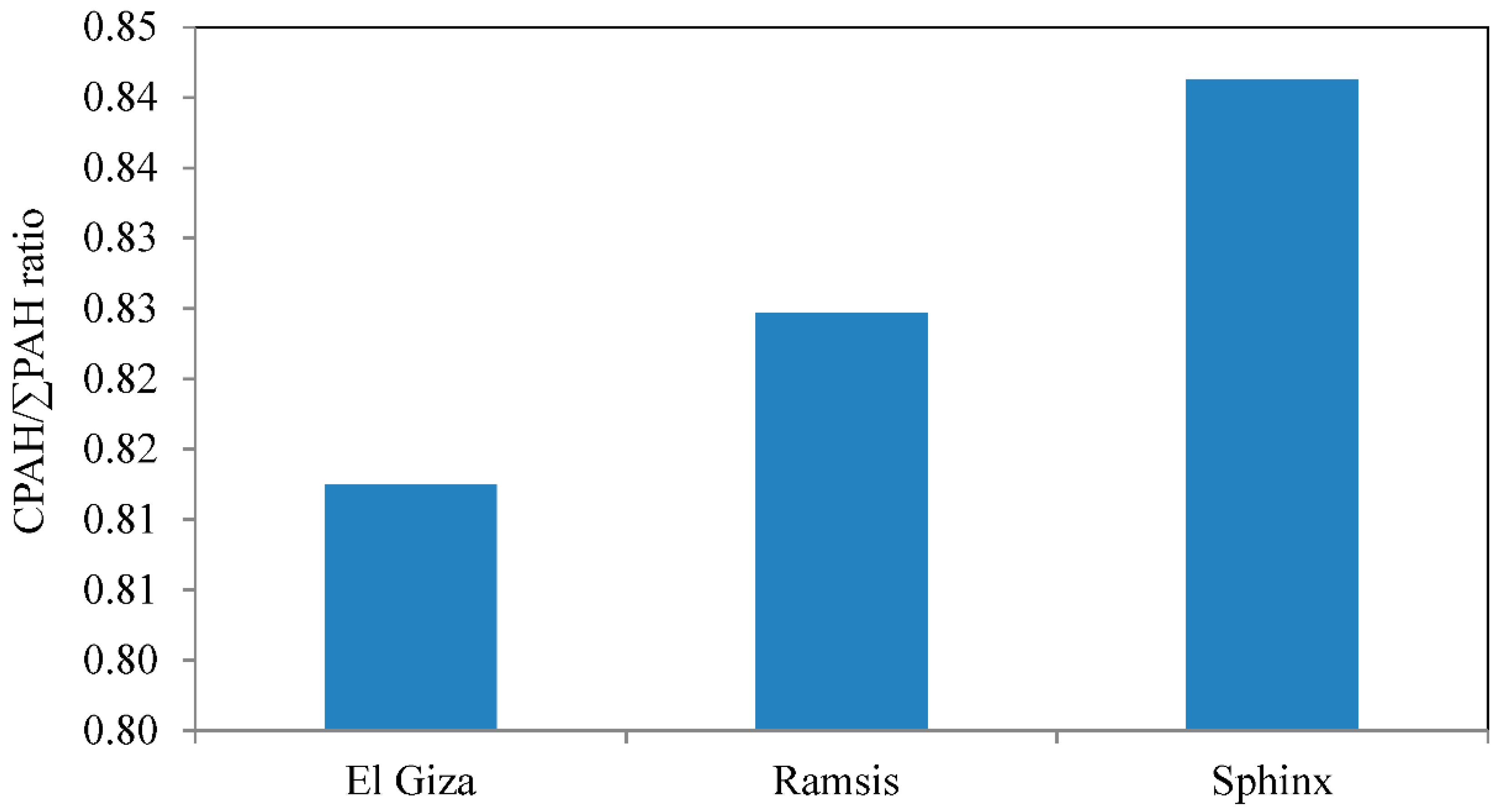
3.3. Source Identification and Evaluation of Diagnostic Ratios
3.4. Health Risk Assessment of Particle-Bound PAH Compounds in the Square Areas
4. Conclusions
Funding
Conflicts of Interest
References
- Vardar, N.; Esen, F.; Tasdemir, Y. Seasonal concentrations and partitioning of PAHs in a suburban site of Bursa, Turkey. Environ. Pollut. 2008, 155, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Esen, F.; Tasdemir, Y.; Vardar, N. Atmospheric concentrations of PAHs, their possible sources and gas to- particle partitioning at a residential site of Bursa, Turkey. Atmos. Res. 2008, 88, 243–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Kamal, A.; Cincinelli, A.; Martellini, T.; Malik, R.N. A review of PAH exposure from the combustion of biomass fuel and their less surveyed effect on the blood parameters. Environ. Sci. Pollut. Res. Int. 2015, 22, 4076–4098. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Outdoor Air Pollution a Leading Environmental Cause of Deaths Accessed in Dec 2015. 2013. Available online: http://www.iarc.fr/en/media-centre/iarcnews/pdf/pr221_E.pdf (accessed on 11 September 2018).
- Hassan, S.K.; Khoder, M.I. Gas-particle concentration, distribution, and health risk assessment of polycyclic aromatic hydrocarbons at a traffic area of Giza, Egypt. Environ. Monit. Assess. 2012, 184, 3593–3612. [Google Scholar] [CrossRef] [PubMed]
- El-Mekawy, A.M.; Mohammed, A.M.F.; Hassan, S.K.M. A review of airborne polycyclic aromatic hydrocarbons (PAHs) in Egypt. Egypt. J. Environ. Res. (EJER) 2016, 5, 53–76. [Google Scholar]
- USEPA (U.S. Environmental Protection Agency). Toxicological Review of Benzene (NONCANCER EFFECTS), CAS No. 71-43-2. In Support of Summary Informationon on the Integrated Risk Information System (IRIS); USEPA: Washington, DC, USA, October 2002. [Google Scholar]
- Kameda, Y.; Shirai, J.; Komai, T.; Nakanishi, J.; Masunaga, S. Atmospheric polycyclic aromatic hydrocarbons: Size distribution, estimation of their risk and their depositions to human respiratory tract. Sci. Total Environ. 2005, 340, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.L.; Warshawsky, D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef] [PubMed]
- USEPA (U.S. Environmental Protection Agency). Documentation for the Final 2002 Mobile National Emissions Inventory; version 3; USEPA: Washington, DC, USA, 2007.
- Hassanien, M.A.; Abdel-Latif, N.M. Polycyclic aromatic hydrocarbons in road dust over Greater Cairo, Egypt. J. Hazard. Mater. 2008, 151, 247–254. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). A review of human carcinogens—Part F. Chemical agents and related occupations. Lancet Oncol. 2009, 10, 1143–1144. [Google Scholar] [CrossRef]
- Hassanien, M.A. Polycyclic aromatic hydrocarbons and carcinogenic risk assessment in developing countries. In Polycyclic Hydrocarbons: Pollution, Health Effects and Chemistry; Editors: Haines, P.A., Hendrickson, M.D., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Park, J.S.; Wada, T.L.; Sweet, S. Atmospheric distribution of polycyclic aromatic hydrocarbons and deposition to Galveston Bay, Texas, USA. Atmos. Environ. 2001, 35, 3241–3249. [Google Scholar] [CrossRef]
- Wang, D.G.; Yang, M.; Jia, H.L.; Zhou, L.; Li, Y.F. Polycyclic aromatic hydrocarbons in urban street dust and surface soil: Comparisons of concentration, profile, and source. Arch. Environ. Contam. Toxicol. 2009, 56, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Lu, B.; Ji, Y.; Bai, Z.; Xu, Y.; Liu, Y.; Jiang, H. Distribution and sources of polycyclic aromatic hydrocarbons in size-differentiated re-suspended dust on building surfaces in an oilfield city, China. Atmos. Environ. 2012, 55, 7–16. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Kim, E.J.; Oh, J.E.; Chang, Y.S. Effects of forest fire on the level and distribution of PCDD/Fs and PAHs in soil. Sci. Total Environ. 2003, 311, 177–189. [Google Scholar] [CrossRef]
- El-Mubarak, A.H.; Rushdi, A.I.; Al-Mutlaq, K.F.; Bazeyad, A.Y.; Simonich, S.L.M.; Simoneit, B.R.T. Identification and source apportionment of polycyclic aromatic hydrocarbons in ambient air particulate matter of Riyadh, Saudi Arabia. Environ. Sci. Pollut. Res. Int. 2014, 21, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Boonyatumanond, R.; Murakami, M.; Wattayakorn, G.; Togo, A.; Takada, H. Sources of polycyclic aromatic hydrocarbons (PAHs) in street dust in a tropical Asian mega-city, Bangkok, Thailand. Sci. Total Environ. 2007, 384, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Binet, S.; Pfohl-Leszkowicz, A.; Brandt, H.; Lafontaine, M.; Castegnaro, M. Bitumen fumes: Review of work on the potential risk to workers and the present knowledge on its origin. Sci. Total Environ. 2002, 300, 37–49. [Google Scholar] [CrossRef]
- Dyke, P.H.; Foan, C.; Fiedler, H. PCB and PAH releases from power stations and waste incineration processes in the UK. Chemosphere 2003, 50, 469–480. [Google Scholar] [CrossRef]
- Zielinska, B.; Sagebiel, J.; Arnott, W.P.; Rogers, C.F.; Kelly, K.E.; Wagner, D.A.; Lighty, J.S.; Sarofi, A.F.; Palmer, G. Phase and size distribution of polycyclic aromatic hydrocarbons in diesel and gasoline vehicle emissions. Environ. Sci. Technol. 2004, 38, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Miguel, A.H.; Kirchstetter, T.W.; Harley, R.A.; Hering, S. On-road emissions of particulate polycyclic aromatic hydrocarbons and black carbon from gasoline and diesel vehicles. Environ. Sci. Technol. 1998, 32, 450–455. [Google Scholar] [CrossRef]
- Kakimoto, H.; Matsumoto, Y.; Sakai, S.; Kanoh, F.; Arashidani, K.; Tang, N. Comparison of atmospheric polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in an industrialized city (Kitakyushu) and two commercial cities (Sapporo and Tokyo). J. Health Sci. 2002, 48, 370–375. [Google Scholar] [CrossRef]
- Koyano, M.; Mineki, S.; Tsunoda, Y.; Endo, O.; Goto, S.; Ishii, T. Suspended particulate matter in indoor air. J. Health Sci. 2001, 47, 452–459. [Google Scholar] [CrossRef]
- Kume, K.; Ohura, T.; Noda, T.; Amagai, T.; Fusaya, M. Seasonal and spatial trends of suspended-particle associated polycyclic aromatic hydrocarbons in urban Shizuoka, Japan. J. Hazard. Mater. 2007, 144, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.F.J.; de Resends, M.F.; de Furtado, A.L.; Brasil, T.F.; Eberlin, M.N.; Netto, A.D.P. Polycyclic aromatic hydrocarbons (PAHs) in street dust of Rio de Janeiro and Niterói, Brazil: Particle size distribution, sources and cancer risk assessment. Sci. Total Environ. 2017, 599–600, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Pereira Netto, A.D.; Krauss, T.M.; Cunha, I.F.; Rego, E.C.P. Polycyclic aromatic hydrocarbons levels in street dust in the central area of Niterói City, RJ, Brazil. Water Air Soil Pollut. 2006, 176, 57–67. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.; Brown, R.J.C. A review of techniques for the determination of polycyclic aromatic hydrocarbons in air. Trends Anal. Chem. 2011, 30, 1716–1739. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.K. Atmospheric Polycyclic Aromatic Hydrocarbons and Some Heavy Metals in Suspended Particulate Matter in Urban, Industrial and Residential Areas in Greater Cairo. Ph.D. Thesis, Cairo University, Cairo, Egypt, 2006. [Google Scholar]
- Hassan, S.K. Sources and cancer risk of heavyca metals in total suspended particulate in some square areas of Greater Cairo, Egypt. Indian J. Environ. Prot. 2018. accepted. [Google Scholar]
- Fang, G.C.; Wu, Y.S.; Chen, M.H.; Ho, T.T.; Huang, S.H.; Rau, J.Y. Polycyclic aromatic hydrocarbons study in Taichung, Taiwan, during 2002–2003. Atmos. Environ. 2004, 38, 3385–3391. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.P.; Kang, C.H.; Ghim, Y.S.; Kaneyasu, N. Temporal trend and long-range transport of particulate polycyclic aromatic hydrocarbons at Gosan in northeast Asia between 2001 and 2004. J. Geophys. Res. 2006, 111, 1029. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.P.; Kang, C.H. Characteristics of the ambient particulate PAHs at Seoul, a mega city of Northeast Asia in comparison with the characteristics of a background site. Atmos. Res. 2011, 99, 50–56. [Google Scholar] [CrossRef]
- Khillare, P.S.; Balachandran, S.; Hoque, R.R. Profile of PAHs in the diesel vehicle exhaust in Delhi. Environ. Monit. Assess. 2005, 105, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, A.; Egodawatta, P.; Godwin, A.A.; Goonetilleke, A. Role of traffic in atmospheric accumulation of heavy metals and polycyclic aromatic hydrocarbons. Atmos. Environ. 2012, 54, 502–510. [Google Scholar] [CrossRef]
- Bian, Q.; Alharbi, B.; Collett, J., Jr.; Kreidenweis, S.; Pasha, M.J. Measurements and source apportionment of particle-associated polycyclic aromatic hydrocarbons in ambient air in Riyadh, Saudi Arabia. Atmos. Environ. 2016, 137, 186–198. [Google Scholar] [CrossRef]
- Zheng, M.; Fang, M. Particle-associated polycyclic aromatic hydrocarbons in the atmosphere of Hong Kong. Water Air Soil Pollut. 2000, 117, 175–189. [Google Scholar] [CrossRef]
- Cvetkovi, A.; Jovasevic-Stojanovic, M.; Markovic, Z.; Ristovski, D. Concentration and source identification of polycyclic aromatic hydrocarbons in the metropolitan area of Belgrade, Serbia. Atmos. Environ. 2015, 112, 335–343. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, F.G.; Chen, M. Distribution characteristics and source analysis of n-alkanes (C14-C31) and PAHs in total suspended particulates in urban area of Taiyuan city. Rock Miner. Anal. 2003, 22, 206–210. [Google Scholar]
- Sharma, H.; Jain, V.K.; Khan, Z.H. Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in the urban environment of Delhi. Chemosphere 2007, 66, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.; Alam, M.S.; Yin, J.; Stark, C.; Jang, E.-H.; Harrison, R.M.; Shamy, M.; Khoder, M.I.; Shabbaj, I.I. Receptor modelling study of polycyclic aromatic hydrocarbons in Jeddah, Saudi Arabia. Sci. Total Environ. 2015, 506–507, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, M.; Vardar, N.; Sofuoglu, A.; Tasdemir, Y.; Holsn, T.M. Polycyclic aromatic hydrocarbons (PAHs) in Chicago air. Sci. Total Environ. 1999, 27, 57–67. [Google Scholar] [CrossRef]
- Cincinelli, A.; Del Bubba, M.; Martellini, T.; Gambaro, A.; Lepri, L. Gas-particle concentration and distribution of n-alkanes and polycyclic aromatic hydrocarbons in the atmosphere of Prato (Italy). Chemosphere 2007, 68, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Fon, T.Y.W.; Noriatsu, O.; Hiroshi, S. Polycyclic aromatic hydrocarbons (PAHs) in the aerosol of higashi hiroshima, Japan: Pollution scenario and source identification. Water Air Soil Pollut. 2007, 182, 235–243. [Google Scholar] [CrossRef]
- Kishida, M.; Imamura, K.; Takenaka, N.; Maeda, Y.; Viet, P.H.; Bandow, H. Concentrations of atmospheric polycyclic aromatic hydrocarbons in particulate matter and the gaseous phase at roadside sites in Hanoi, Vietnam. Bull. Environ. Contam. Toxicol. 2008, 81, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.C.; Chang, C.N.; Wu, Y.S.; Fu, P.P.C.; Yang, I.L.; Chen, M.H. Characterization, identification of ambient air and road dust polycyclic aromatic hydrocarbons in central Taiwan, Taichung. Sci. Total Environ. 2004, 327, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Hanedar, A.; Alp, K.; Kaynak, B.; Baek, J.; Avsar, E.; Odman, M.T. Concentrations and sources of PAHs at three stations in Istanbul, Turkey. Atmos. Res. 2011, 99, 391–399. [Google Scholar] [CrossRef]
- Mastral, A.M.; Lopez, J.M.; Callen, M.S.; Garcia, T.; Murillo, R.; Navarro, V. Spatial and temporal PAH concentrations in Zaragoza, Spain. Sci. Total Environ. 2003, 307, 111–124. [Google Scholar] [CrossRef]
- Ohura, T.; Amagai, T.; Fusaya, M.; Matsushita, H. Spatial distributions and profiles of atmospheric polycyclic aromatic hydrocarbon in two industrial cities in Japan. Environ. Sci. Technol. 2004, 38, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Rehwagen, M.; Müller, A.; Massolo, L.; Herbarth, O.; Ronco, A. Polycyclic aromatic hydrocarbons associated with particles in ambient air from urban and industrial areas. Sci. Total Environ. 2005, 348, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lammel, G.; Novak, J.; Landlov, A.L.; Dvorsk, A.A.; Klanova, J.; Cupr, P.; Kohoutek, J.; Reimer, E.; Skrdlíkova, L. Sources and distributions of polycyclic aromatic hydrocarbons and toxicity of polluted atmosphere aerosols. In Urban Airborne Particulate Matter: Origins, Chemistry, Fate and Health Impacts; Zereini, F., Wiseman, C.L.S., Eds.; Springer: Berlin, Germany, 2010; pp. 39–62. [Google Scholar]
- Hoshiko, T.; Yamamoto, K.; Nakajima, F.; Prueksasit, T. Time-series analysis of polycyclic aromatic hydrocarbons and vehicle exhaust in roadside air environment in Bangkok, Thailand. Procedia Environ. Sci. 2011, 4, 87–94. [Google Scholar] [CrossRef]
- Harrison, R.M.; Smith, D.J.T.; Luhana, L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, U.K. Environ. Sci. Technol. 1996, 30, 825–832. [Google Scholar] [CrossRef]
- Bourotte, C.; Forti, M.C.; Taniguchi, S.; Caruso, M.; Lotufo, P.A. A wintertime study of PAHs in fine and coarse aerosols in Sao Paulo City, Brazil. Atmos. Environ. 2005, 39, 3799–3811. [Google Scholar] [CrossRef]
- Lohmann, R.; Northcott, G.L.; Jones, K.C. Assessing the contribution of diffuse domestic burning as a source of PCDD/Fs, PCBs, and PAHs to the U.K. atmosphere. Environ. Sci. Technol. 2000, 34, 2892–2899. [Google Scholar] [CrossRef]
- Ding, X.; Wang, X.M.; Xie, Z.Q.; Xiang, C.H.; Mai, B.X.; Sun, L.G.; Zheng, M.; Sheng, G.Y.; Fu, J.M.; Poschl, U. Atmospheric polycyclic aromatic hydrocarbons observed over the North Pacific Ocean and the Arctic area: Spatial distribution and source identification. Atmos. Environ. 2007, 47, 2061–2072. [Google Scholar] [CrossRef]
- Dickhut, R.M.; Canuel, E.A.; Gustafson, K.E.; Liu, K.; Arzayus, K.M.; Walker, S.E.; Edgecombe, G.; Gaylor, M.O.; Macdonald, E.H. Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay region. Environ. Sci. Technol. 2000, 34, 4635–4640. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, Y.J.; Kang, C.H. Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmos. Environ. 2002, 36, 2917–2924. [Google Scholar] [CrossRef]
- Caricchia, A.M.; Chiavarini, S.; Pessa, M. Polycyclic aromatic hydrocarbons in the urban atmospheric particulate matter in the city of Naples (Italy). Atmos. Environ. 1999, 33, 3731–3738. [Google Scholar] [CrossRef]
- Nassar, H.F.; Tang, N.; Kameda, T.; Toriba, A.; Khoder, M.I.; Hayakawa, K. Atmospheric concentrations of polycyclic aromatic hydrocarbons and selected nitrated derivatives in Greater Cairo, Egypt. Atmos. Environ. 2011, 45, 7352–7359. [Google Scholar] [CrossRef]
- Baumard, P.; Budzinski, H.; Michon, Q.; Garrigues, P.; Burgeot, T.; Bellocq, J. Origin and bioavailability of PAHs in the Mediterranean Sea from mussel and sediment. Estuar. Coast. Shelf Sci. 1998, 47, 77–90. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser river basin: A critical appraisal of PAH ratios as indicators of PAH sources and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R. Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environ. Sci. Technol. 1993, 27, 636–651. [Google Scholar] [CrossRef]
- USEPA (U.S. Environmental Protection Agency). National Air Pollutant Emission Trends 1994; Report Number EPA 454/R-95-014; Office of Air Quality Planning and Standards, Research Triangle Park, North Carolina, and USA, USEPA: Washington, DC, USA, 1995.
- Wang, X.L.; Tao, S.; Dawson, R.W.; Xu, F.L. Characterizing and comparing risks of polycyclic aromatic hydrocarbons in a Tianjin wastewater-irrigated area. Environ. Res. 2002, 90, 201–206. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Air Pollution, Part 1: Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Industrial Exposures; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 92: Lyon, France, 2009. [Google Scholar]
- WHO (World Health Organization). Polynuclear Aromatic Hydrocarbons; Air quality Guidelines for Europe; WHO Regional Publications, European Series No. 23; World Health Organization: Geneva, Switzerland, 1987; pp. 105–117. [Google Scholar]
- WHO (World Health Organization). Air Quality Guidelines for Europe; Regional Publications Eur. Ser. No. 91; Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Lin, T.C.; Chang, F.H.; Hsieh, J.H.; Chao, H.R.; Chao, M.R. Characteristics of polycyclic aromatic hydrocarbons and total suspended particulate in indoor and outdoor atmosphere of a Taiwanese temple. J. Hazard. Mater. 2002, 95, 1–12. [Google Scholar] [CrossRef]
- Menichini, E.; Bertolaccini, M.A.; Taggi, F.; Falleni, F.; Monfredini, F. A 3-year study of relationships among atmospheric concentrations of polycyclic aromatic hydrocarbons, carbon monoxide and nitrogen oxides at an urban site. Sci. Total Environ. 1999, 241, 27–37. [Google Scholar] [CrossRef]
- Lim, L.H.; Harrison, R.M.; Harrad, S. The contribution of traffic to atmospheric concentrations of polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 1999, 33, 3538–3542. [Google Scholar] [CrossRef]
- Petry, T.; Schmid, P.; Schlatter, C. The use of toxic equivalency factors in assessing occupational and environmental health risk associated with exposure to airborne mixtures of polycyclic aromatic hydrocarbons (PAHs). Chemosphere 1996, 32, 639–648. [Google Scholar] [CrossRef]
- Nisbet, C.; La Goy, P. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
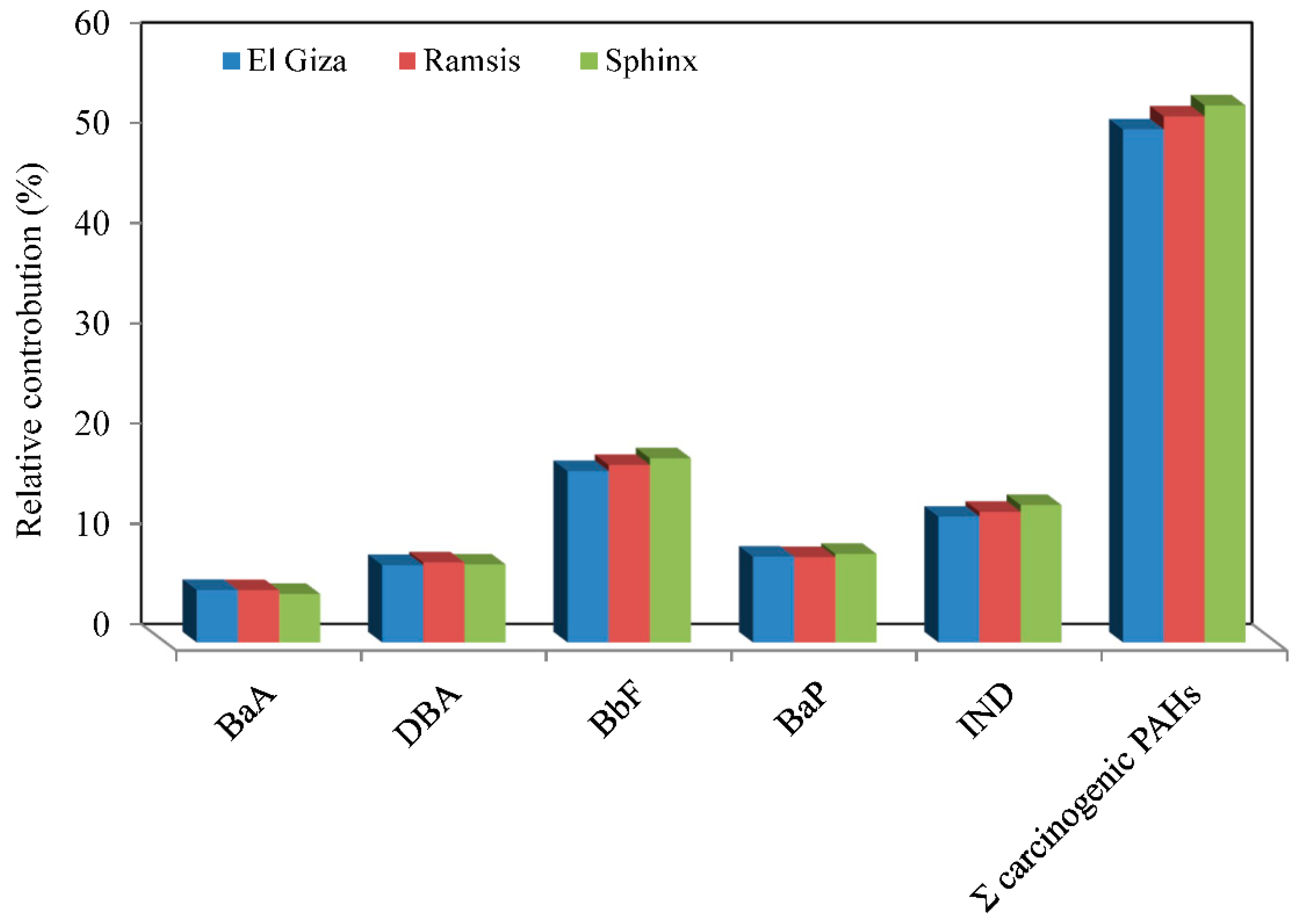
| PAHs | TEF a | El Giza | Ramsis | Sphinx | |||
|---|---|---|---|---|---|---|---|
| Average Concentration (ng/m3) | BaPaquiv. Concentration (ng/m3) | Average Concentration (ng/m3) | BaPaquiv. Concentration (ng/m3) | Average Concentration (ng/m3) | BaPaquiv. Concentration (ng/m3) | ||
| NA | 0.001 | 85.7 | 0.09 | 64.9 | 0.06 | 43.7 | 0.04 |
| ACY | 0.001 | 70.1 | 0.07 | 45.2 | 0.05 | 38.3 | 0.04 |
| ACE | 0.001 | 75.2 | 0.08 | 65.3 | 0.07 | 44.7 | 0.04 |
| FLU | 0.001 | 143.2 | 0.14 | 107.6 | 0.11 | 91.4 | 0.09 |
| PHE | 0.001 | 163.1 | 0.16 | 119.2 | 0.12 | 95.8 | 0.10 |
| ANT | 0.010 | 95.6 | 0.96 | 97.5 | 0.98 | 73.4 | 0.73 |
| FLT | 0.001 | 254.5 | 0.25 | 216.7 | 0.22 | 199.7 | 0.20 |
| PYR | 0.001 | 562.0 | 0.56 | 492.3 | 0.49 | 453.2 | 0.45 |
| BaA | 0.100 | 300.6 | 30.06 | 272.9 | 27.29 | 232.0 | 23.20 |
| CRY | 0.010 | 519.7 | 5.20 | 484.3 | 4.84 | 426.5 | 4.27 |
| BbF | 0.100 | 981.0 | 98.10 | 927.3 | 92.73 | 880.2 | 88.02 |
| BaP | 1.00 | 491.2 | 491.17 | 445.9 | 445.91 | 423.4 | 423.38 |
| DBA | 1.00 | 442.7 | 442.68 | 418.0 | 418.00 | 372.7 | 372.68 |
| IND | 0.100 | 720.1 | 72.01 | 682.5 | 68.25 | 656.7 | 65.67 |
| BGP | 0.010 | 830.5 | 8.30 | 794.6 | 7.95 | 756.1 | 7.56 |
| Total carcinogenicity activity (TCA) | 1149.8 | 1067.1 | 986.5 | ||||
| Contribution of BaA to the TCA (%) | 2.61 | 2.56 | 2.35 | ||||
| Contribution of Bbf to the TCA (%) | 8.53 | 8.69 | 8.92 | ||||
| Contribution of BaP to the TCA (%) | 42.72 | 41.79 | 42.92 | ||||
| Contribution of DBA to the TCA (%) | 38.50 | 39.17 | 37.78 | ||||
| Contribution of IND to the TCA (%) | 6.26 | 6.40 | 6.66 | ||||
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.K. Particle-Bound Polycyclic Aromatic Hydrocarbon in the Atmosphere of Heavy Traffic Areas in Greater Cairo, Egypt: Status, Source, and Human Health Risk Assessment. Atmosphere 2018, 9, 368. https://doi.org/10.3390/atmos9100368
Hassan SK. Particle-Bound Polycyclic Aromatic Hydrocarbon in the Atmosphere of Heavy Traffic Areas in Greater Cairo, Egypt: Status, Source, and Human Health Risk Assessment. Atmosphere. 2018; 9(10):368. https://doi.org/10.3390/atmos9100368
Chicago/Turabian StyleHassan, Salwa Kamal. 2018. "Particle-Bound Polycyclic Aromatic Hydrocarbon in the Atmosphere of Heavy Traffic Areas in Greater Cairo, Egypt: Status, Source, and Human Health Risk Assessment" Atmosphere 9, no. 10: 368. https://doi.org/10.3390/atmos9100368
APA StyleHassan, S. K. (2018). Particle-Bound Polycyclic Aromatic Hydrocarbon in the Atmosphere of Heavy Traffic Areas in Greater Cairo, Egypt: Status, Source, and Human Health Risk Assessment. Atmosphere, 9(10), 368. https://doi.org/10.3390/atmos9100368





