The Impact of Sampling Medium and Environment on Particle Morphology
Abstract
:1. Introduction
2. Experiments
2.1. Particle Generation, Processing, and Mass-Mobility Analysis
2.2. Substrates, Collection Procedures, and Sample Processing
2.3. Electron Microscopy and Image Processing
3. Results and Discussion
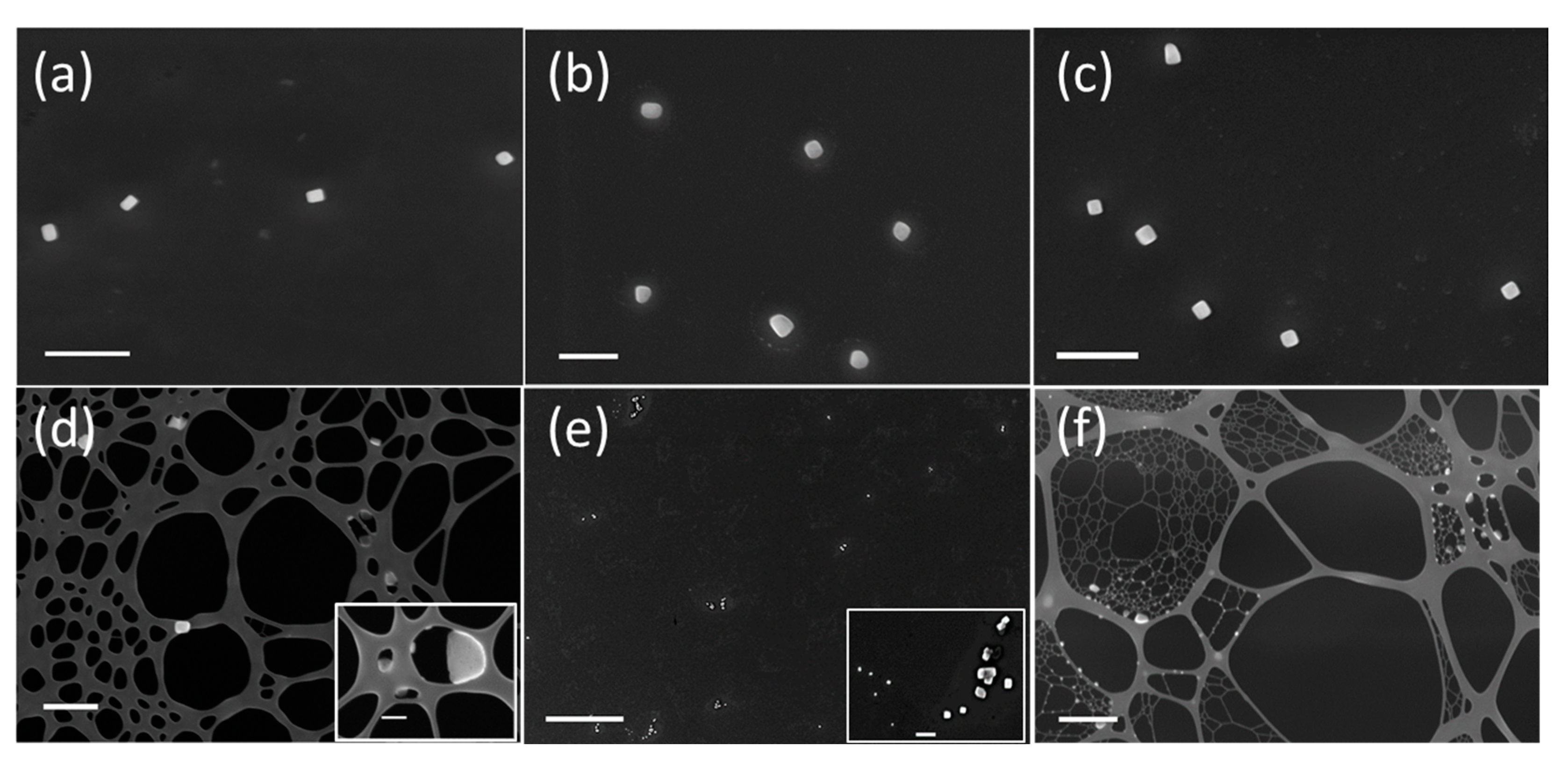
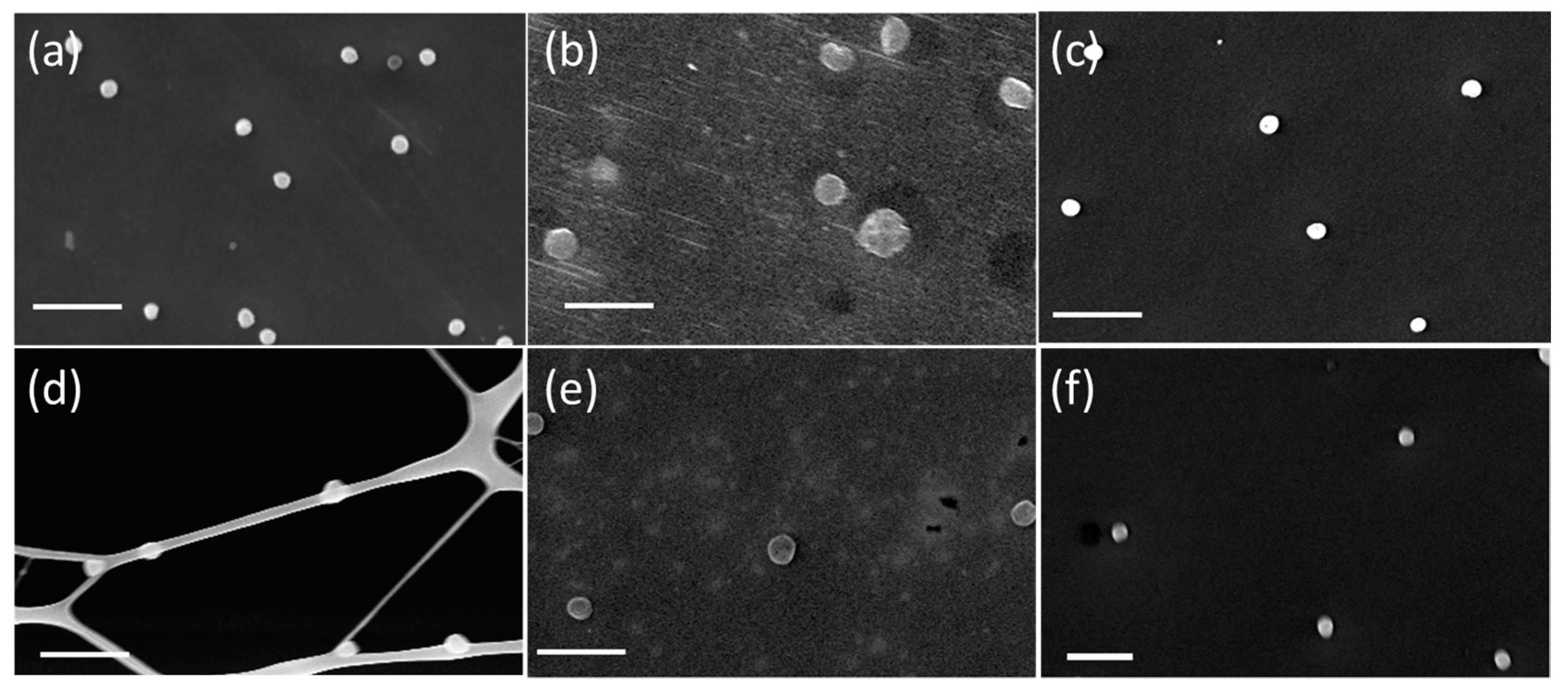
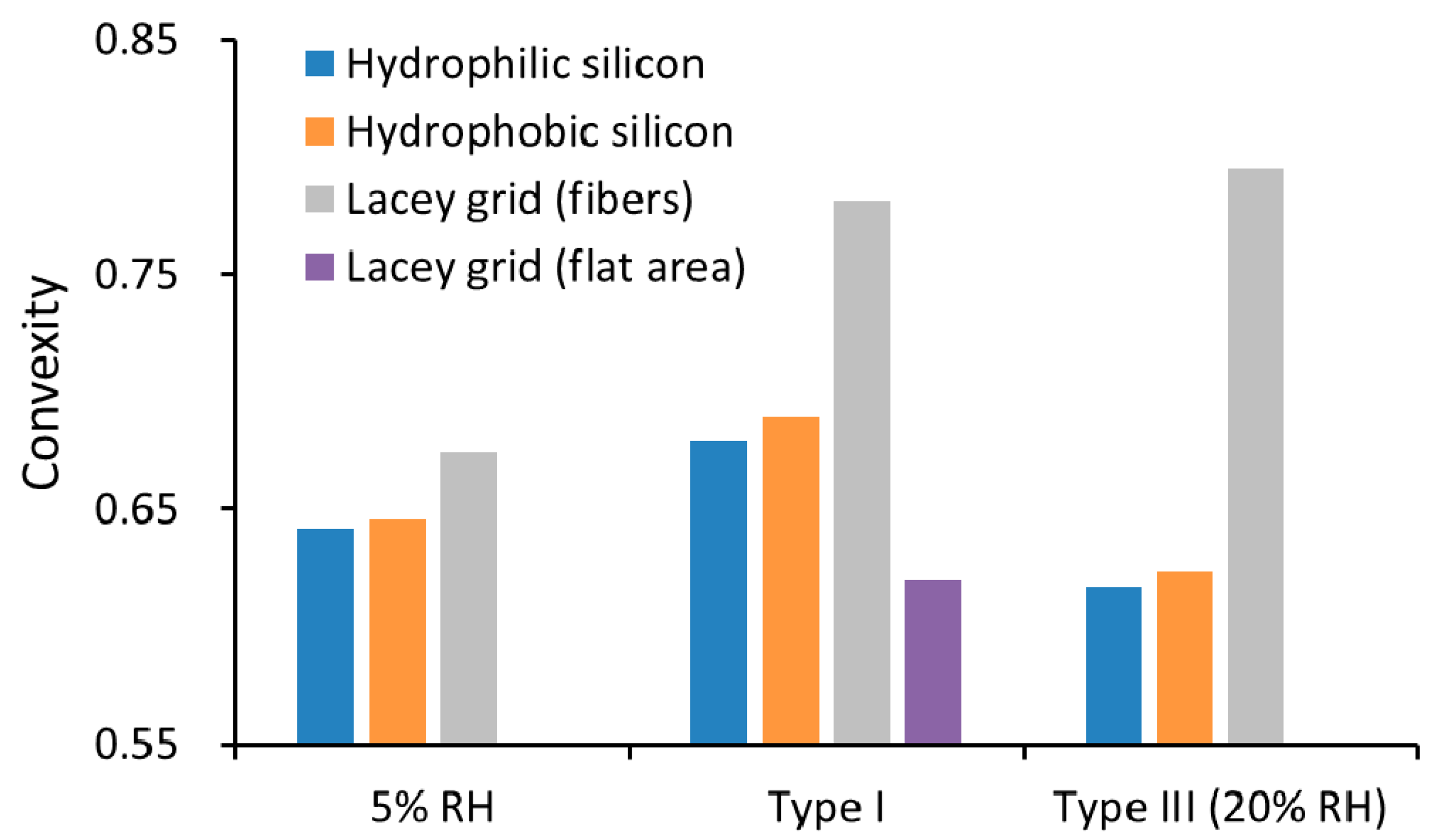
3.1. Sodium Chloride
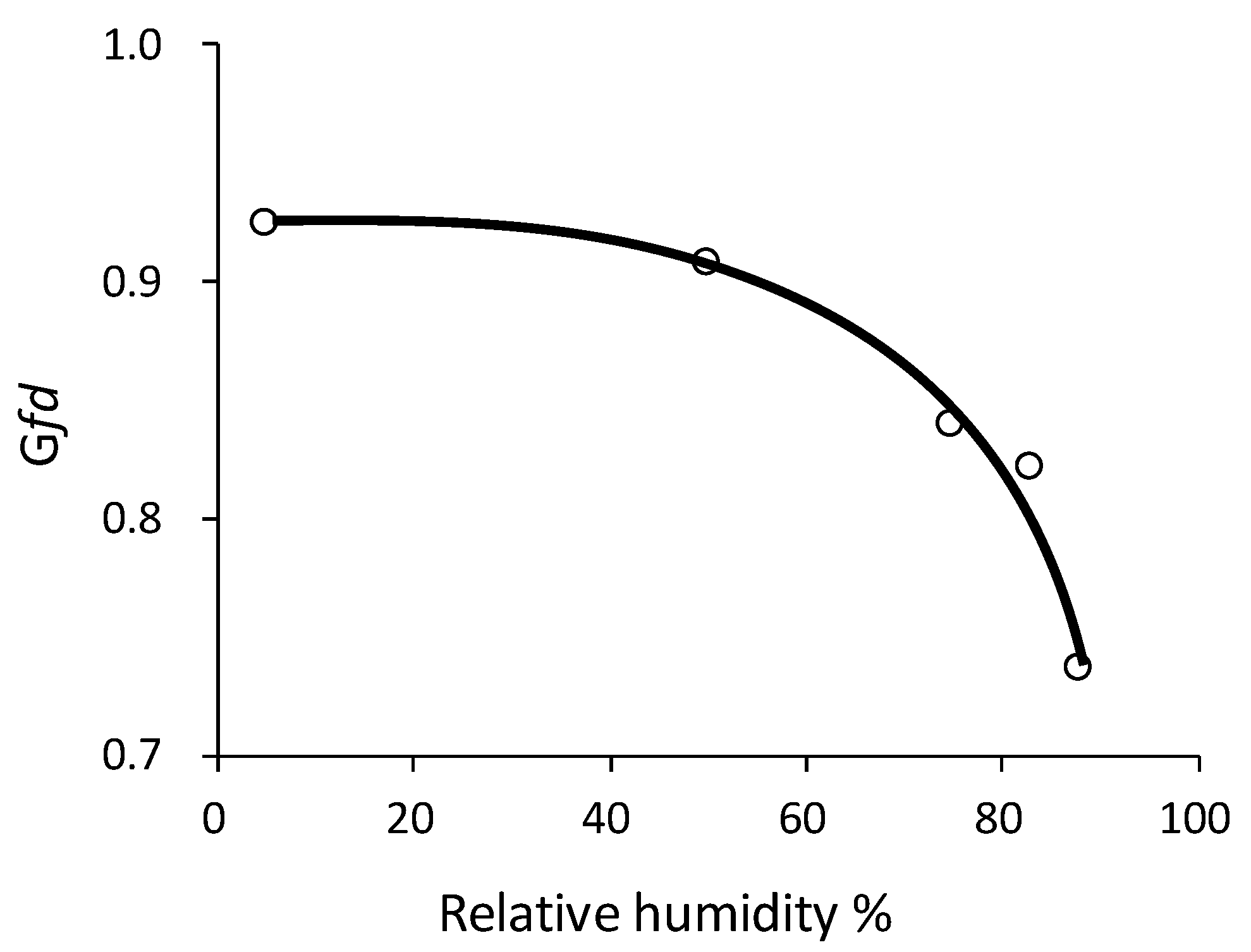
3.2. Sulfuric Acid
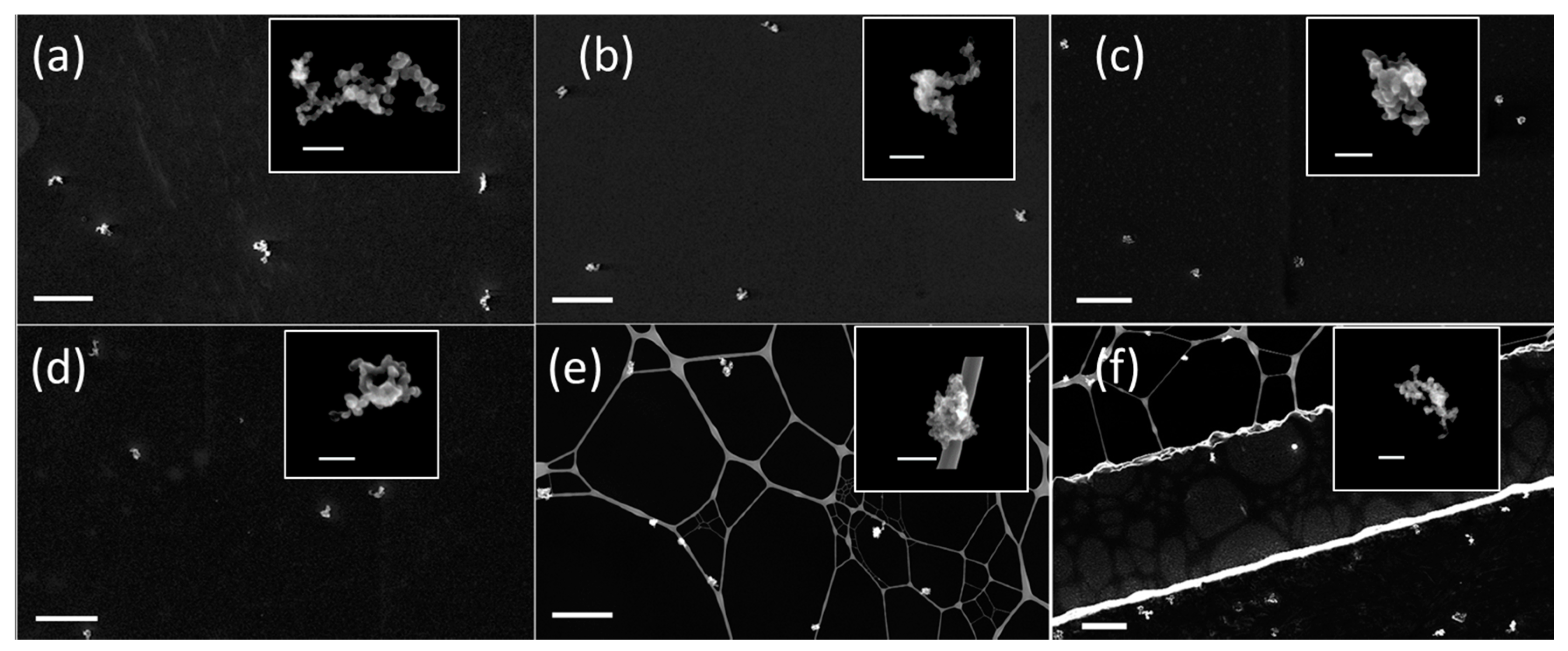
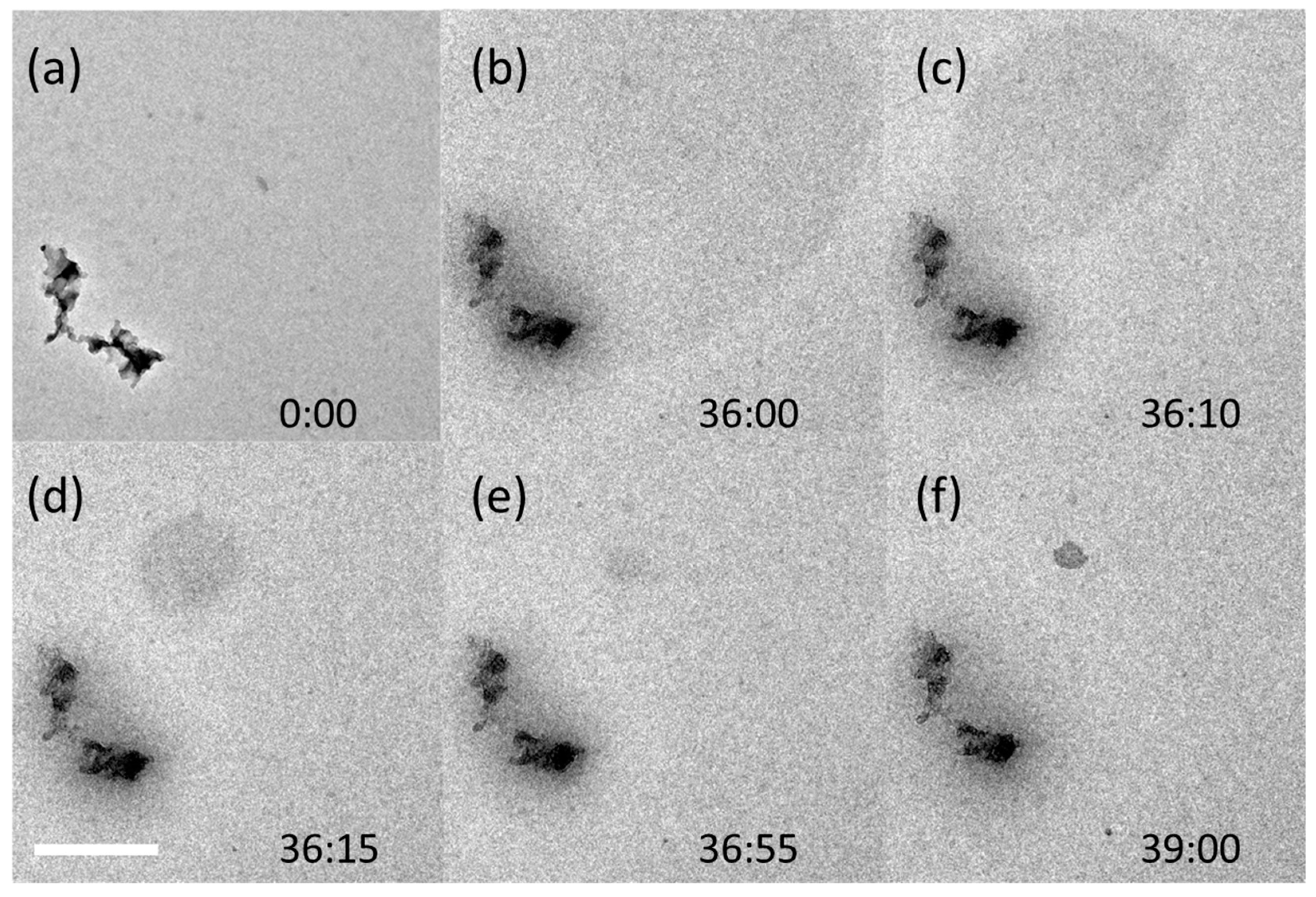
3.3. Soot Aggregates
3.4. In Situ Processing of Soot Aggregates
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lohmann, U.; Feichter, J. Global indirect aerosol effects: A review. Atmos. Chem. Phys. 2005, 5, 715–737. [Google Scholar] [CrossRef]
- Pöschl, U. Atmospheric aerosols: Composition, transformation, climate and health effects. Cheminform 2005, 44, 7520. [Google Scholar] [CrossRef] [PubMed]
- Haywood, J.; Boucher, O. Estimates of the direct and indirect radiative forcing due to tropospheric aerosols: A review. Rev. Geophys. 2000, 38, 513–543. [Google Scholar] [CrossRef]
- Tao, W.K.; Chen, J.P.; Li, Z.; Wang, C.; Zhang, C. Impact of aerosols on convective clouds and precipitation. Rev. Geophys. 2012, 50, RG2001. [Google Scholar] [CrossRef]
- Jayne, J.T.; Leard, D.C.; Zhang, X.F.; Davidovits, P.; Smith, K.A.; Kolb, C.E.; Worsnop, D.R. Development of an aerosol mass spectrometer for size and composition analysis of submicron particles. Aerosol Sci. Technol. 2000, 33, 49–70. [Google Scholar] [CrossRef]
- DeCarlo, P.F.; Kimmel, J.R.; Trimborn, A.; Northway, M.J.; Jayne, J.T.; Aiken, A.C.; Gonin, M.; Fuhrer, K.; Horvath, T.; Docherty, K.S.; et al. Field-deployable, high-resolution, time-of-flight aerosol mass spectrometer. Anal. Chem. 2006, 78, 8281–8289. [Google Scholar] [CrossRef] [PubMed]
- China, S.; Kulkarni, G.; Scarnato, B.; Sharma, N.; Pekour, M.; Shilling, J.; Wilson, J.; Zelenyuk, A.; Chand, D.; Liu, S.; et al. Morphology of diesel soot residuals from supercooled water droplets and ice crystals: Implications for optical properties. Environ. Res. Lett. 2015, 10, 114010. [Google Scholar] [CrossRef]
- Beranek, J.; Imre, D.; Zelenyuk, A. Real-time shape-based particle separation and detailed in situ particle shape characterization. Anal. Chem. 2012, 84, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- McMurry, P.H.; Wang, X.; Park, K.; Ehara, K. The relationship between mass and mobility for atmospheric particles: A new technique for measuring particle density. Aerosol Sci. Technol. 2002, 36, 227–238. [Google Scholar] [CrossRef]
- Levy, M.E.; Zhang, R.; Khalizov, A.F.; Zheng, J.; Collins, D.R.; Glen, C.R.; Wang, Y.; Yu, X.Y.; Luke, W.; Jayne, J.T.; et al. Measurements of submicron aerosols in houston, texas during the 2009 sharp field campaign. J. Geophys. Res. Atmos. 2013, 118, 10518–10534. [Google Scholar] [CrossRef]
- Posfai, M.; Buseck, P.R. Nature and climate effects of individual tropospheric aerosol particles. In Annual Review of Earth and Planetary Sciences; Jeanloz, R., Freeman, K.H., Eds.; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 38, pp. 17–43. [Google Scholar]
- Lee, R.E. The size of suspended particulate matter in air. Science 1972, 178, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Whitby, K.T. Non-ideal collection characteristics of inertial impactors—II. Cascade impactors. J. Aerosol Sci. 1978, 9, 87–100. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Yeh, H.C. Particle bounce in cascade impactors. Environ. Sci. Technol. 1979, 13, 1392–1396. [Google Scholar] [CrossRef]
- Rothenbacher, S.; Messerer, A.; Kasper, G. Fragmentation and bond strength of airborne diesel soot agglomerates. Part. Fibre Toxicol. 2008, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Khalizov, A.F.; Pagels, J.; Zhang, D.; Xue, H.; McMurry, P.H. Variability in morphology, hygroscopicity, and optical properties of soot aerosols during atmospheric processing. Proc. Natl. Acad. Sci. USA 2008, 105, 10291–10296. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-F.; Turpin, B. Reduction of sampling and analytical errors for electron microscopic analysis of atmospheric aerosols. Atmos. Environ. 1996, 30, 4137–4148. [Google Scholar] [CrossRef]
- Dixkens, J.; Fissan, H. Development of an electrostatic precipitator for off-line particle analysis. Aerosol Sci. Technol. 1999, 30, 438–453. [Google Scholar] [CrossRef]
- Chen, C.; Fan, X.; Shaltout, T.; Qiu, C.; Ma, Y.; Goldman, A.; Khalizov, A.F. An unexpected restructuring of combustion soot aggregates by subnanometer coatings of polycyclic aromatic hydrocarbons. Geophys. Res. Lett. 2016, 43, 11080–11088. [Google Scholar] [CrossRef]
- Soewono, A.; Rogak, S. Morphology and raman spectra of engine-emitted particulates. Aerosol Sci. Technol. 2011, 45, 1206–1216. [Google Scholar] [CrossRef]
- China, S.; Salvadori, N.; Mazzoleni, C. Effect of traffic and driving characteristics on morphology of atmospheric soot particles at freeway on-ramps. Environ. Sci. Technol. 2014, 48, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.K.; Moosmüller, H.; Garro, M.A.; Arnott, W.P.; Walker, J.; Susott, R.A.; Babbitt, R.E.; Wold, C.E.; Lincoln, E.N.; Hao, W.M. Emissions from the laboratory combustion of wildland fuels: Particle morphology and size. J. Geophys. Res. Atmos. 2006, 111, D07204. [Google Scholar] [CrossRef]
- Bambha, R.P.; Dansson, M.A.; Schrader, P.E.; Michelsen, H.A. Effects of volatile coatings and coating removal mechanisms on the morphology of graphitic soot. Carbon 2013, 61, 80–96. [Google Scholar] [CrossRef]
- Laskina, O.; Morris, H.S.; Grandquist, J.R.; Estillore, A.D.; Stone, E.A.; Grassian, V.H.; Tivanski, A.V. Substrate-deposited sea spray aerosol particles: Influence of analytical method, substrate, and storage conditions on particle size, phase, and morphology. Environ. Sci. Technol. 2015, 49, 13447–13453. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.-J.; Gupta, D.; Li, X.; Jung, H.-J.; Kim, H.; Ro, C.-U. Influence of collecting substrates on the characterization of hygroscopic properties of inorganic aerosol particles. Anal. Chem. 2014, 86, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Pang, S.-F.; Wang, Y.; Ma, J.-B.; Zhang, Y.-H. Confocal raman studies of the evolution of the physical state of mixed phthalic acid/ammonium sulfate aerosol droplets and the effect of substrates. J. Phys. Chem. B 2014, 118, 6198–6205. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Bhushan, B. Tribological performance of magnetic thin-film glass disks: Its relation to surface roughness and lubricant structure and its thickness. Wear 1995, 190, 60–75. [Google Scholar] [CrossRef]
- Ghazi, R.; Tjong, H.; Soewono, A.; Rogak, S.N.; Olfert, J.S. Mass, mobility, volatility, and morphology of soot particles generated by a mckenna and inverted burner. Aerosol Sci. Technol. 2013, 47, 395–405. [Google Scholar] [CrossRef]
- Pyrz, W.D.; Buttrey, D.J. Particle size determination using tem: A discussion of image acquisition and analysis for the novice microscopist. Langmuir 2008, 24, 11350–11360. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.B.; Chan, C.; Brown, S.C.; Eschbach, P.; Han, L.; Ensor, D.S.; Stefaniak, A.B.; Bonevich, J.; Vladár, A.E.; Hight Walker, A.R.; et al. Particle size distributions by transmission electron microscopy: An interlaboratory comparison case study. Metrologia 2013, 50, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Stipe, C.B.; Higgins, B.S.; Lucas, D.; Koshland, C.P.; Sawyer, R.F. Inverted co-flow diffusion flame for producing soot. Rev. Sci. Instrum. 2005, 76, 023908. [Google Scholar] [CrossRef]
- Ghazi, R.; Olfert, J. Coating mass dependence of soot aggregate restructuring due to coatings of oleic acid and dioctyl sebacate. Aerosol Sci. Technol. 2013, 47, 192–200. [Google Scholar] [CrossRef]
- Khalizov, A.F.; Zhang, R.; Zhang, D.; Xue, H.; Pagels, J.; McMurry, P.H. Formation of highly hygroscopic soot aerosols upon internal mixing with sulfuric acid vapor. J. Geophys. Res. Atmos. 2009, 114, D05208. [Google Scholar] [CrossRef]
- Chakrabarty, R.K.; Moosmüller, H.; Arnott, W.P.; Garro, M.A.; Walker, J. Structural and fractal properties of particles emitted from spark ignition engines. Environ. Sci. Technol. 2006, 40, 6647–6654. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.L.; Littlejohn, D.; Lunden, M.M.; Brown, N.J. Automated measurements of ammonia and nitric acid in indoor and outdoor air. Environ. Sci. Technol. 2003, 37, 2114–2119. [Google Scholar] [CrossRef] [PubMed]
- Tidy, G.; Neil Cape, J. Ammonia concentrations in houses and public buildings. Atmos. Environ. A Gen. Top. 1993, 27, 2235–2237. [Google Scholar] [CrossRef]
- Peng, J.; Hu, M.; Guo, S.; Du, Z.; Zheng, J.; Shang, D.; Levy Zamora, M.; Zeng, L.; Shao, M.; Wu, Y.-S.; et al. Markedly enhanced absorption and direct radiative forcing of black carbon under polluted urban environments. Proc. Natl. Acad. Sci. USA 2016, 113, 4266–4271. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Chung, S.H.; Buseck, P.R. Shapes of soot aerosol particles and implications for their effects on climate. J. Geophys. Res. 2010, 115, D15. [Google Scholar] [CrossRef]
- Cappa, C.D.; Onasch, T.B.; Massoli, P.; Worsnop, D.R.; Bates, T.S.; Cross, E.S.; Davidovits, P.; Hakala, J.; Hayden, K.L.; Jobson, B.T.; et al. Radiative absorption enhancements due to the mixing state of atmospheric black carbon. Science 2012, 337, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Whitehead, J.; Alfarra, M.R.; Reyes-Villegas, E.; Spracklen, D.V.; Reddington, C.L.; Kong, S.; Williams, P.I.; Ting, Y.-C.; Haslett, S.; et al. Black-carbon absorption enhancement in the atmosphere determined by particle mixing state. Nat. Geosci. 2017, 10, 184–188. [Google Scholar] [CrossRef]
- Liu, S.; Aiken, A.C.; Gorkowski, K.; Dubey, M.K.; Cappa, C.D.; Williams, L.R.; Herndon, S.C.; Massoli, P.; Fortner, E.C.; Chhabra, P.S.; et al. Enhanced light absorption by mixed source black and brown carbon particles in UK winter. Nat. Commun. 2015, 6, 8435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- China, S.; Scarnato, B.; Owen, R.C.; Zhang, B.; Ampadu, M.T.; Kumar, S.; Dzepina, K.; Dziobak, M.P.; Fialho, P.; Perlinger, J.A.; et al. Morphology and mixing state of aged soot particles at a remote marine free troposphere site: Implications for optical properties. Geophys. Res. Letts. 2015, 42, 1243–1250. [Google Scholar] [CrossRef]
- Mikhailov, E.F.; Vlasenko, S.S.; Kiselev, A.A.; Ryshkevich, T.I. Restructuring factors of soot particles. Izv. Atmos. Ocean Phys. 1998, 34, 307–317. [Google Scholar]
- Ma, X.; Zangmeister, C.D.; Gigault, J.; Mulholland, G.W.; Zachariah, M.R. Soot aggregate restructuring during water processing. J. Aerosol Sci. 2013, 66, 209–219. [Google Scholar] [CrossRef]
- Huang, P.F.; Turpin, B.J.; Pipho, M.J.; Kittelson, D.B.; McMurry, P.H. Effects of water condensation and evaporation on diesel chain-agglomerate morphology. J. Aerosol Sci. 1994, 25, 447–459. [Google Scholar] [CrossRef]
- Semeniuk, T.A.; Wise, M.E.; Martin, S.T.; Russell, L.M.; Buseck, P.R. Hygroscopic behavior of aerosol particles from biomass fires using environmental transmission electron microscopy. J. Atmos. Chem. 2007, 56, 259–273. [Google Scholar] [CrossRef]
- Hiranuma, N.; Brooks, S.D.; Auvermann, B.W.; Littleton, R. Using environmental scanning electron microscopy to determine the hygroscopic properties of agricultural aerosols. Atmos. Environ. 2008, 42, 1983–1994. [Google Scholar] [CrossRef]
- Köllensperger, G.; Friedbacher, G.; Kotzick, R.; Niessner, R.; Grasserbauer, M. In-situ atomic force microscopy investigation of aerosols exposed to different humidities. Anal. Bioanal. Chem. 1999, 364, 296–304. [Google Scholar] [CrossRef]
- Ebert, M.; Inerle-Hof, M.; Weinbruch, S. Environmental scanning electron microscopy as a new technique to determine the hygroscopic behaviour of individual aerosol particles. Atmos. Environ. 2002, 36, 5909–5916. [Google Scholar] [CrossRef]

| Chemical | Wetting Angle, Degrees | |||
|---|---|---|---|---|
| Silicon | Silicon Nitride | Graphite | ||
| Hydrophilic | Hydrophobic | |||
| Water | 45 | 92 | 86 | |
| Sulfuric acid (20%) | 69 | 73 | ||
| Sulfuric acid (50%) | 61 | |||
| Sulfuric acid (80%) | 20 1 | 92 | 16 | |
| Sulfuric acid (98%) | 20 1 | |||
| Environment | Description |
|---|---|
| Type I (Humidified) | Exposed to a 92–96% relative humidity (RH) |
| Type II (Cold storage) | Exposed to a 55% RH at room temperature, sealed in a container, chilled in a freezer to −20 °C, removed from the freezer, brought to room temperature, and then exposed to ambient air (RH 16 to 50%) |
| Type IIIa (Moderately flooded) | Exposed to a 55% RH at room temperature, sealed in a container, chilled in a freezer to −20 °C, removed from the freezer and exposed to ambient air while still cold; ambient air RH is 16–20%. |
| Type IIIb (Severely flooded) | Exposed to a 55% RH at room temperature, sealed in a container, chilled in a freezer to −20 °C, removed from the freezer and exposed to ambient air while still cold; ambient air RH is 50%. |
| Aerosol | Environment 1 | Untreated Silicon | Hydrophobic Silicon | Lacey Grid | |
|---|---|---|---|---|---|
| Fiber | Flat | ||||
| Coated Soot | Type I (Humidified) | Minimal | Minimal | Major | Minimal |
| Type II (Cold storage) | Minimal | Minimal | Minimal | Minimal | |
| Type IIIa (Moderately flooded) | Minimal | Minimal | Major | - | |
| Type IIIb (Severely flooded) | Major | Major | Major | - | |
| Sodium Chloride | Type I (Humidified) | Moderate | Minimal | Moderate | - |
| Type II (Frozen) | Minimal | Minimal | Minimal | - | |
| Type IIIa (Moderately flooded) | Major | Minimal | Moderate | - | |
| Type IIIb (Severely flooded) | Major | Major | Major | - | |
| Sulfuric Acid | Untreated | Minimal | Minimal | Minimal | Moderate |
| Type I (Humidified) | Major | Moderate | Moderate | Major | |
| Type II (Frozen) | Major | Minimal | Minimal | - | |
| Type IIIa (Moderately flooded) | Major | Major | Major | - | |
| Type IIIb (Severely flooded) | Major | Major | Major | - | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Enekwizu, O.Y.; Ma, Y.; Zakharov, D.; Khalizov, A.F. The Impact of Sampling Medium and Environment on Particle Morphology. Atmosphere 2017, 8, 162. https://doi.org/10.3390/atmos8090162
Chen C, Enekwizu OY, Ma Y, Zakharov D, Khalizov AF. The Impact of Sampling Medium and Environment on Particle Morphology. Atmosphere. 2017; 8(9):162. https://doi.org/10.3390/atmos8090162
Chicago/Turabian StyleChen, Chao, Ogochukwu Y. Enekwizu, Yan Ma, Dmitry Zakharov, and Alexei F. Khalizov. 2017. "The Impact of Sampling Medium and Environment on Particle Morphology" Atmosphere 8, no. 9: 162. https://doi.org/10.3390/atmos8090162
APA StyleChen, C., Enekwizu, O. Y., Ma, Y., Zakharov, D., & Khalizov, A. F. (2017). The Impact of Sampling Medium and Environment on Particle Morphology. Atmosphere, 8(9), 162. https://doi.org/10.3390/atmos8090162




