Abstract
The goal of the study was to determine the concentrations of submicron particulate matter (PM1) and of the mercury contained in it (Hgp) in the air in two teaching rooms in two Polish cities, Gliwice and Warsaw. The levels of atmospheric particulate matter (PM) differ greatly between these two cities. The relations between the indoor (I) and outdoor (O) 24-h concentrations for each PM1 and Hgp were determined and, based on the conclusions, an attempt was made to identify the main sources of the indoor Hgp in both cities. During the whole measuring period (April–June 2015), in both Warsaw and Gliwice, the 24-h outdoor PM1 concentrations were slightly higher than the indoor ones (outdoor and indoor averages were equal to 19.3 µg m−3 and 14.5 µg·m−3, respectively, in Gliwice and to 13.2 µg·m−3 and 9.5 µg·m−3 in Warsaw). In Gliwice, the indoor concentrations of Hgp (2.4 pg·m−3 to 27.7 pg·m−3) were much higher than the outdoor ones (1.1 pg·m−3 to 6.1 pg·m−3); in Warsaw the average concentrations of Hgp were equal to 1.4 pg m−3 indoors and outdoors. The 24-h concentrations of Hgp and the 24-h I/O ratios for Hgp varied more intensely in Gliwice than in Warsaw throughout the whole measuring period. In Warsaw, the teaching room Hgp came mainly from the infiltration of atmospheric (outdoor) Hgp. In Gliwice, a part of the indoor Hgp infiltrated into the teaching room with the outdoor PM1 that most probably was then enriched with gaseous indoor Hg, what resulted in the relatively high indoor Hgp concentrations.
1. Introduction
In comparison with the available number of studies concerning atmospheric air pollution, the problem of indoor air pollution with particulate matter (PM) and its toxic components has hardly been addressed. The concentrations and chemical composition of PM in various rooms (including teaching rooms and laboratories at schools and universities) have been studied in the world for years [1,2,3,4,5] and in Poland PM concentrations inside teaching rooms have already been studied as well [6,7,8,9,10,11,12,13].
It has been established beyond doubt that PM concentrations in the atmospheric air have a significant influence on the PM concentrations inside various buildings [14,15,16,17,18,19,20,21,22]. This impact depends on the internal sources of pollutants and indoor ventilation conditions. Indoors, basic PM sources include human skin, hair, plants, animals, cooking, building materials, tobacco smoking, heating (coal, wood and biomass combustion), cleaning agents and specific activities related to the use of a building [23,24,25,26]. The internal sources also emit other pollutants that may become PM precursors or components due to their deposition on PM particles present in the air [11,25,26].
The I/O ratio is commonly used to define the impact of atmospheric pollutants (e.g., PM) on the indoor pollutant concentration. The I/O is the ratio of the indoor concentration (I) of a compound, element, etc. to its outdoor concentration (O). Based on an analysis of the I/O ratio, it has been estimated that atmospheric air inflow may be accountable for 75% of PM2.5 and 66% of PM10 concentrations [27] in offices located in Upper Silesia, Poland. Observations carried out in kindergartens have shown that the I/O ratio for PM2.5 and PM10 practically depends on the activity of children in the classrooms only, as it determined the intensity of PM resuspension [28]. It has also been proven that in urban areas there is a greater threat from PM2.5-bound metals inside kindergarten rooms than in rural areas (hence a higher I/O ratio) [11]. On the other hand, analysis of the elemental composition of PM1, PM2.5 and PM10, and of the I/O ratio of the elements concerned, has demonstrated that significant contribution to indoor Zn, Pb and S concentrations in fine PM found in a public school in Wrocław was from the penetration of outdoor air [10]. No Polish or other European literature references have been found regarding the content of mercury (Hg) in fine PM in such buildings as schools, kindergartens and universities.
Among other substances, Hg is absorbed on the surface of ambient particles. In general, particulate mercury (mercury bound to ambient PM, Hgp) makes up merely several per cent of the total mass of ambient mercury [29,30,31]. In cities situated in the urbanized southern Poland, the Hgp in the total mass of ambient mercury can range from a few per cent to as much as 14% [30,32]. The proportion of Hgp to total atmospheric mercury varies from one location to another, but also at the same location over time [32,33].
The main anthropogenic source of mercury in the air is the combustion of coal, oil and waste [34]. In Poland, where more than 80% of energy is produced from coal, ambient concentrations of mercury reach high levels, much higher than in other European countries. The ambient concentration of Hgp can reach 600 pg·m−3 [35], 4800 pg·m−3 [32], and 142 pg·m−3 [36] in the central, southern and northern parts of Poland, respectively.
In the air, Hgp may be directly emitted (as a primary pollutant, adsorbed to or constituting PM particles in the primary PM), formed through the adsorption of ambient Hg2+ (bound to compounds) and Hg° vapours to ambient particles, or through the dissolution of ambient Hg2+ in PM-bound water [37,38]. In certain circumstances, the two latter mechanisms are supposed to play as important a role in enriching PM in Hg as the primary emissions do [39].
Although it constitutes just a small contribution to the total mass of ambient mercury, Hgp is an important part of the total Hg and is of particular interest because of its health impact. Mercury is commonly known to be harmful to human health due to its high mobility in the environment, the inability to undergo biodegradation, a high bioaccumulation potential, and a high chemical and biological activity [40,41]. When bound to airborne fine PM, whose particles penetrate deeply into the human respiratory system, and some of them even into the blood flow [42,43], Hgp can pose a major threat to human health.
The aim of this study was to attempt an initial determination of submicron PM (PM1) concentration and the concentration of PM1-bound Hg (Hgp) in two teaching rooms. The indoor (I; both rooms) and outdoor (O; atmospheric air) variability in the 24-h PM1 and Hgp concentrations was discussed. Additionally, the I/O ratio was analysed.
2. Materials and Methods
2.1. Sampling Sites
The research was conducted in parallel in two Polish cities: Gliwice (where the main sources of air pollution are commercial power generation, household emissions and industries) [44,45,46,47] and Warsaw (where traffic emissions prevail) [48,49,50]. The 24-h samples of PM1 were collected simultaneously in a teaching room in Warsaw and in a student laboratory in Gliwice, as well as outside the two rooms. The rooms were both located on the 2nd floor (approximately 8 to 10 m above the ground level). No air conditioning or air cleaning equipment was used in those rooms. In both rooms, PM samplers were located at 6 to 9 m opposite the windows, more or less 2 m from the nearest wall and 8 to 12 m from the door. At the laboratory, classes are held two-three times a week during which chemical reagents are used, some of which may contain trace amounts of mercury. There are also flow meters, barometers and thermometers, which may be sources of mercury. On the other hand, the lecture room in Warsaw situated in an old building where mercury-in glass thermometers and barometers have been used for years might have had a history of past pollution with mercury. Therefore, before samples were collected, an assessment of short-time concentration of gaseous Hg in the ambient air was carried out at both locations using a Lumex RA915M mercury analyser (Atomic Absorption Spectrometry, AAS, with Zeeman background correction). The instrument was applied at various areas of the rooms, a few times during one day (before classes in the morning, after classes late in the afternoon and after a good airing of the rooms). The measurements were repeated during the PM sampling session, too. The ambient concentration of mercury in the Warsaw room and in the Gliwice laboratory ranged from a few to 24 ng·m−3. The concentrations inside both rooms mainly depended on the duration of airing before measurement, but they did not depend on the duration of the classes, the type of class or the number of people inside the rooms. As a result, it was assumed that no actual internal sources of ambient mercury were identifiable in the rooms and no traces of past mercury pollution were found either.
The outdoor measurements in Warsaw and Gliwice were taken at a height of approximately 4 to 5 m above the ground level. The measurement points outside the buildings in Warsaw and in Gliwice were located in campuses where vehicular traffic is limited and the impact of local pollution from the combustion of fuel is therefore limited as well. Both external measurement points were sited 50 to 70 m—in a straight line—from the internal measurement points (Figure 1). In Warsaw, the distance to the nearest residential buildings where coal is used for heating, was 850 m and in Gliwice it was 350 m. The nearest coal-fired power plants were situated at a distance of 4 km and 2.5 km in Warsaw and Gliwice, respectively. In urban areas, a substantial source of air pollution, including fine PM, is road traffic. Both external measurement points were located about 200 m from a busy road.
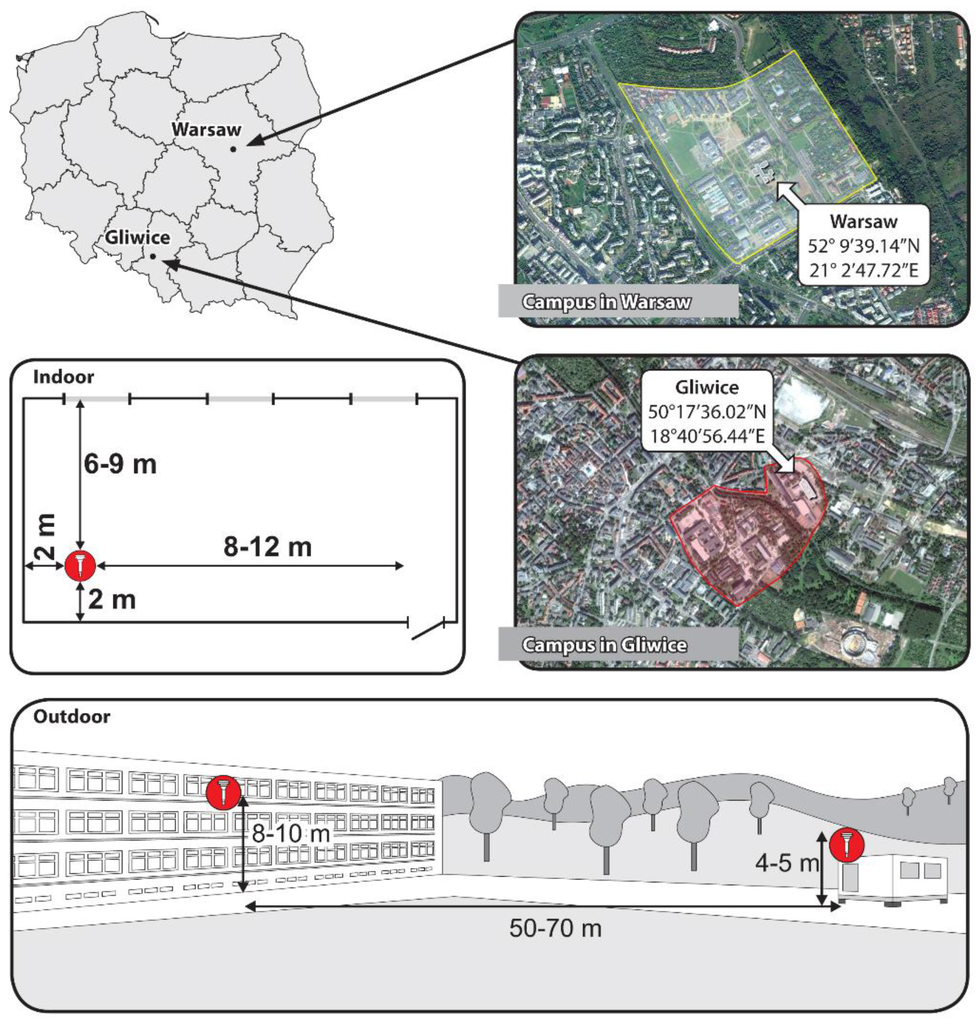
Figure 1.
Measurement points in Warsaw and Gliwice (Poland).
The measurements were carried out from April 2015 to June 2015. The samples were taken from Monday through Friday, which are days when classes or lectures were held in the rooms. The months selected to carry out those initial measurements of Hgp in rooms, were selected specifically to exclude intensive emissions of PM and Hgp from the nearby coal stoves and coal-fired heating and power plants (i.e., the months outside the heating season). At both locations, the emissions may have a strong impact on the pollution of atmospheric air with PM and Hgp, which can also affect the proper analysis of results.
2.2. PM1 Sampling
Altogether, 25 measurements of 24-h concentrations of PM1 were taken (simultaneously indoors and outdoors) in Warsaw and in Gliwice.
The same standard PM1 sampling sets were placed at each measurement point. They comprised pumps (Zambelli, New Castle, PA, USA) and heads equipped with jet impactors (TSI) used to separate particles greater than 1 µm from the air stream. PM1 was sampled at a stable air flow rate (2.3 m3∙h−1) onto 47-mm QMA quartz filters (Whatman, Maidstone, UK). Before the exposure, the clean quartz filters were specially prepared (heated at 650 °C for 2 h), conditioned in a weighing room (48 h; relative air humidity 45% ± 5%; air temperature 20 ± 2 °C) and weighed twice (at 24 h intervals) on a Mettler Toledo AT microbalance (with a resolution of 2 µg) equipped with a Haug U-ionizer. The procedures for conditioning, weighing, storage, and transport of the samples and of the blank sample preparation complied with the QA/QC procedures of the reference method for gravimetric measurements (EN 12341:2014). The weighting accuracy, determined as three standard deviations from the mean obtained from ten weightings of a blank filter (conditioning performed every 48 h), was 20.5 µg.
To prevent Hg re-volatilization, the samples were stored in a refrigerator (2–4 °C) before analysis.
2.3. Hg Analysis
The mercury content of PM1 was determined by applying cold-vapour atomic absorption spectrometry (CVAAS) to thermally decomposed PM1 samples; an MA-2 analyser (Nippon Instr. Co, Tokyo, Japan) was used. The PM1 sample (a 1.5 cm2 section of an exposed filter) and additives were placed in a quartz boat and heated in mercury-free air in the decomposition furnace to 700 °C. Then, the decomposition products were carried to the catalyst furnace and heated to 850 °C. The products of thermal decomposition from the oven, containing elemental mercury, were passed through a buffer solution (pH = 7), and then, after drying, to a gold trap where the amalgam (Hg-Au) is formed. Vaporised Hg°, released upon rapid heating of the trap, was carried to the detector, where the light absorbance of the mixture Hg/carrier gas was measured at the wavelength λ = 253.7 nm.
The calibration curve (0.1–6 ng, R2 = 0.999) was prepared by analysing the calibration standard (Inorganic Ventures; Hg concentration 10 ppm). The limits of detection (LoD, 0.025 ng·Hg) and of quantitation (LoQ, 0.075 ng·Hg) were determined by iteratively analysing 25 blank samples. The method was validated using the standards SRM1633b and SRM2583 (NIST). The repeatability, computed as a standard deviation from 25 measurements, was 4.5% for an actual sample and 3.9% for SRM1633b. The average recovery of SRM1633b was 90% and for SRM2583 was 96% [51]. For each batch of five actual samples, blank samples were analysed (one blank field sample in a batch of five actual samples, separately for the outdoor and indoor measurements). The mass of Hg in a blank field sample was deducted from the mass of Hg determined in actual samples of the given batch.
3. Results and Discussion
3.1. Outdoor Concentrations of PM1 and Hgp—A Comparison with Earlier Studies
The outdoor PM1 concentrations observed in April 2015–June 2015 in Gliwice were similar, and in the case of Warsaw slightly lower than the PM1 concentrations observed earlier in Silesian cities. In Zabrze, situated 15 km west of Gliwice, the ambient concentrations of PM1 were between 16.7 µg·m−3 and 60.2 µg·m−3 (monthly averages), while, in Katowice, 25 km west of Gliwice, 24-h concentrations of PM1 were in the range of 8.0 µg·m−3 to 73.6 µg·m−3 [52,53]. There are no published PM1 data regarding central Poland, where Warsaw is situated, and there are no data of PM1-bound Hg concentrations for that area. Nevertheless, earlier research has shown that in the urban area of Poznań (central Poland, 270 km east of Warsaw), 24-h ambient concentration of PM2.5-bound Hg can reach 77 pg·m−3 [35]. In Poland, the ambient concentration of PM1-bound Hg was measured only in Zabrze before, showing the values of 7.7 pg·m−3 to 186.2 pg·m−3 [52].
Therefore, it can be stated that the average ambient concentrations of PM1-bound Hg in April 2015–June 2015, reaching 3 pg·m−3 in Gliwice and 1.4 pg·m−3 in Warsaw, were very low. Our measurements were taken in a warm season (Table 1) and precipitation was observed on over a dozen measurement days in Warsaw and on a few measurement days in Gliwice. Thus, during the measurement period, emissions of PM and Hg from household heating must have been minimum (in Poland hard coal is mainly used for heating in local boiler plants and household stoves), and the prevailing weather conditions did not support the accumulation of pollution in the bottom layer of the atmosphere, most likely favouring wet (leaching) or dry deposition of the pollutants from the air [54]. In Poland, each summer demonstrates lower PM concentrations than in winter and hence lower concentrations of most PM components including PM-bound Hg [46,51,53]. The presented results were obtained during a short measurement period at the end of spring and the beginning of summer. Since the concentrations of PM and its components in Poland are subject to extensive seasonal fluctuations, the levels shown in this study cannot be considered typical values for the two areas. They are only values of reference to concentrations measured inside teaching rooms and as such demonstrate certain general trends in the PM air pollution in Poland.

Table 1.
Descriptive statistics for the 24-h PM1 and Hgp concentrations, Hgp content in PM1 and meteorological parameters in Gliwice and Warsaw.
The ambient concentrations of PM1 and Hgp and the Hgp content in PM1 were considerably higher in Gliwice than in Warsaw. This applied to the values obtained both outdoors and inside the teaching rooms. The Mann–Whitney and Wilcoxon tests (SPSS) confirmed a statistical significance of the differences between the mean values for both PM1 and Hgp ambient concentrations and for the Hgp content in PM1 in Gliwice and Warsaw (αassump. = 0.05, pcomp. < 0.0002).
3.2. Indoor–Outdoor PM1 Concentration
In Warsaw, the 24-h PM1 concentrations ranged from 4.0 µg·m−3 to 24.8 µg·m−3 (indoor values) and from 5.7 µg·m−3 to 37.8 µg·m−3 (outdoor values) (Table 1). In Gliwice, the PM1 ranged from 9.9 µg·m−3 to 50.1 µg·m−3 (outdoor values) and from 7.7 µg·m−3 to 26.4 µg·m−3 (indoor values). In both cities, the 24-h PM1 concentrations were generally higher in the atmospheric air than indoors. The differences were statistically significant (Mann–Whitney and Wilcoxon tests; αassump. = 0.05, pcomp. < 0.0001), nevertheless both in Gliwice and Warsaw, the indoor and outdoor 24-h concentrations of PM1 were linearly correlated; the Pearson’s Correlation coefficient was higher for the concentrations in Gliwice (r = 0.9; α = 0.05) than in Warsaw (r = 0.69; α = 0.05) (Table 2).

Table 2.
Pearson’s Correlation coefficients for indoor and outdoor 24-h PM1 and Hgp concentrations and Hgp content in PM1 in Gliwice and Warsaw.
Both in Gliwice and Warsaw (except for one day in Warsaw), the I/O ratio for 24-h PM1 concentrations did not exceed 1 (Figure 2). In Gliwice, its mean value was 0.8 and ranged from 0.5 to 0.9. In Warsaw, the mean value was similar to the mean value in Gliwice (0.7) and ranged from 0.5 to 2.1. Stronger I/O value fluctuations occurred in Warsaw.
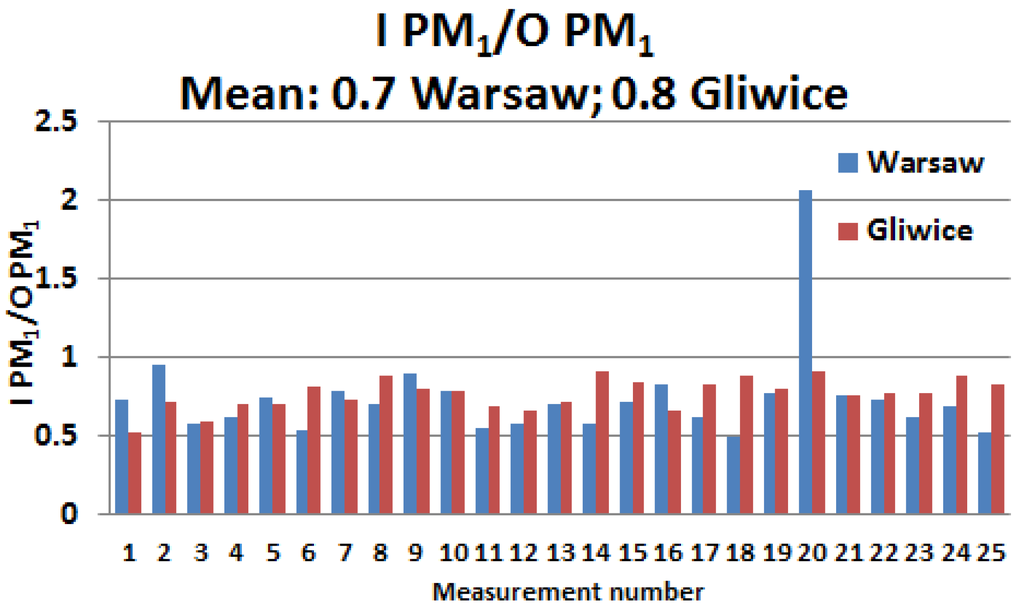
Figure 2.
Ratio of the 24-h indoor (I) and outdoor (O) PM1 concentrations in Gliwice and Warsaw.
The size and properties of particles constituting PM1 suggest that the indoor and outdoor concentrations should be balanced. If effective sources of fine particles were found inside the rooms, the indoor concentration of PM1 could be higher than the outdoor concentrations [1,2,3,4,5,10,11]. Balanced levels of PM1 concentration indoors and outdoors (or higher indoor values at most) are so much more likely, considering that the timing of the measurements fell on a spring/summer season, when the weather conditions were conducive to frequent airing, and the selected rooms were not equipped with an air cleaning or air conditioning system. It seems that the reason for such a surprising relationship between the indoor and outdoor PM1 concentrations at both sites was the location of the measurement points outside the teaching rooms. Perhaps the higher outdoor concentrations of PM1 were a result of the outdoor location of the measuring heads at a lower height (4 m) than in both rooms (2nd floor). Closer to the ground, the influence of resuspension of settled particles on ambient concentrations of PM is possible. Similar phenomena and their reasons have already been reported in previous studies [55,56]. On the other hand, the meteorological conditions present during the spring-summer period, such as a high level of insolation, little breeziness and high ozone concentrations in the air [57], favoured the intensive formation of secondary aerosol in the urban atmosphere [54]. During the summer season, the secondary matter make up to 50% of the PM1 mass [46,52]. Even if gaseous precursors of secondary PM occurred at the same concentration at both rooms as in the outdoor air, the indoor secondary PM formation was definitely limited.
The mentioned observations allow a hypothesis that the migration of PM1 from atmospheric air may have been the main source of indoor air pollution both inside the laboratory in Gliwice and the teaching room in Warsaw. In other words, it can be assumed that there were no significant internal PM and/or PM gaseous precursor sources in either of the rooms.
3.3. Indoor-Outdoor Hgp Concentration
In Warsaw, the mean Hgp concentration over the entire measurement period was the same indoors and outdoors, 1.4 pg·m−3. The 24-h concentrations of Hgp were in the range of 0.9 pg·m−3 to 4.1 pg·m−3 (indoors) and 0.7 pg·m−3 to 2.3 pg·m−3 (outdoors). A different situation occurred in Gliwice, where higher 24-h concentrations of Hgp were observed in the laboratory than outdoors for the most of the measurement period. In Gliwice, the mean indoor Hgp concentrations were twice as high (6.1 pg·m−3) as the outdoor values (3.0 pg·m−3). The range of the 24-h Hgp concentrations was 2.4 pg·m−3 to 27.4 pg·m−3 in the laboratory and 1.1 pg·m−3 to 6.1 pg·m−3 in the atmospheric air. The Mann–Whitney and Wilcoxon tests confirmed a statistical significance of the differences between the mean values of indoor and outdoor Hgp concentrations only in Gliwice (αassump. = 0.05, pcomp. < 0.0002).
The I/O ratios of the 24-h concentrations of Hgp in both cities are presented in Figure 3. In Warsaw, half of the 24-h I/O values yielded a result slightly lower than 1. In Gliwice, the ratio was high (only in three cases was it less than 1) and the maximum and mean I/O values there were 9.1 and 2.3, respectively. In Warsaw, the highest I/O value slightly exceeded 3, while the mean ratio was 1.1.
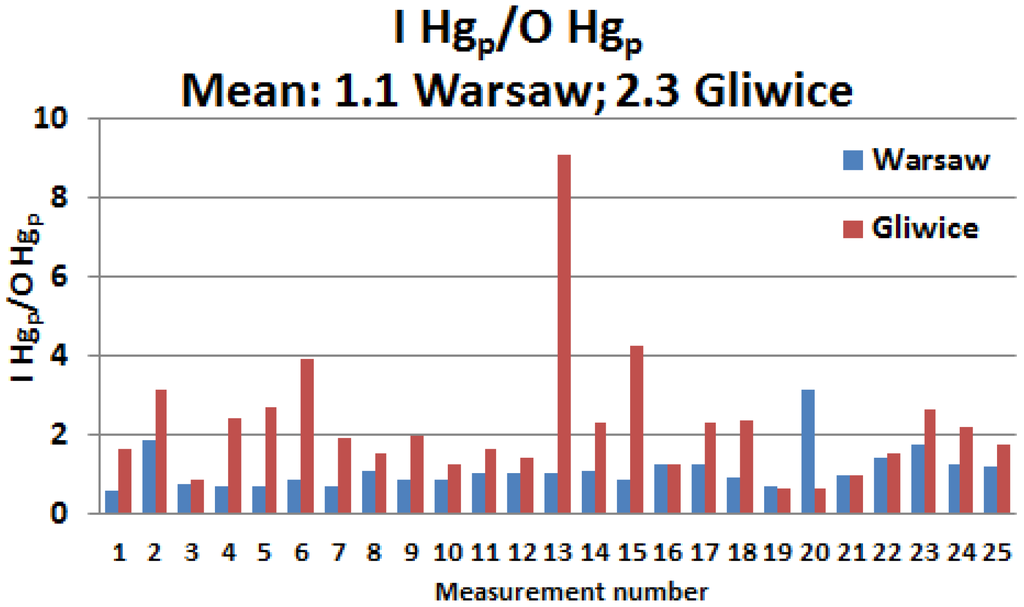
Figure 3.
Ratio of the 24-h indoor (I) and outdoor (O) Hgp concentrations in Gliwice and Warsaw.
The 24-h indoor and outdoor concentrations of Hgp were significantly correlated in Warsaw; the 24-h indoor concentrations of Hgp were also correlated with the PM1 concentrations inside the teaching room (Table 2). The Mann–Whitney and Wilcoxon tests confirmed that the mean values of indoor and outdoor Hgp concentrations in Warsaw were equal (αassump. = 0.05, pcomp. < 0.93). Therefore, it can be concluded that in Warsaw, the infiltration of Hgp from atmospheric air must have had an expected and obvious impact on the indoor Hgp concentrations. In Gliwice, however the situation was different. There were no significant indoor and outdoor correlations between 24-h Hgp concentrations; the 24-h concentrations of indoor Hgp were not significantly correlated with 24-h PM1 concentrations.
The authors believe that the indoor PM1 in Gliwice was enriched with gaseous Hg. The outdoor Hgp concentrations were twice as low as the indoor Hgp concentrations, so the PM1 particles that were transported into the room were not very rich in Hg. On the other hand, when the observations were carried out, the laboratory in Gliwice was not as often aired as the teaching room in Warsaw, therefore the concentrations of gaseous Hg could be maintained at a higher level longer than in the teaching room. Although the concentrations of gaseous Hg in both rooms, measured periodically, varied significantly, their higher values (10 to 20 ng·m−3) were more frequently observed in Gliwice. Considering that in urban areas ambient concentrations of gaseous Hg usually remain below 2 or 3 ng·m−3 [32,58,59], the valued measured at the rooms were high, which indicates their possible correlation with the Hgp levels. It seems that much higher concentrations of indoor PM1 were observed in Gliwice, and the differences in the meteorological conditions present at both sites over the measurement period were the reason that the PM1 particles inside the laboratory room in Gliwice adsorbed gaseous Hg much more effectively than in the case of the Warsaw teaching room. However, a categorical confirmation of these conclusions will only be possible when parallel continuous measurements of gaseous Hg and Hgp in both rooms are carried out.
In any case, the fact that the PM1 particles infiltrated from the outside were enriched with mercury inside both rooms is also supported by the analysis of Hgp content in the PM1 (Table 1; Figure 4). Although during the measurement period the average indoor and outdoor concentration of Hgp in Warsaw was equal, the average Hgp content in PM1 (ppm) was twice as high indoors as outdoors. Such a twofold increase in Hgp in the indoor PM1 as compared with the Hgp content in the outdoor PM1 also occurred in Gliwice. In both cities, the differences (indoor–outdoor) were statistically significant (Mann–Whitney and Wilcoxon tests; αassump. = 0.05, pcomp. < 0.0001). The average ratio of the 24-h Hgp content in the indoor/outdoor PM1 was twice as high in Gliwice as in Warsaw, amounting to approximately 3 and 1.5, respectively (Figure 4).
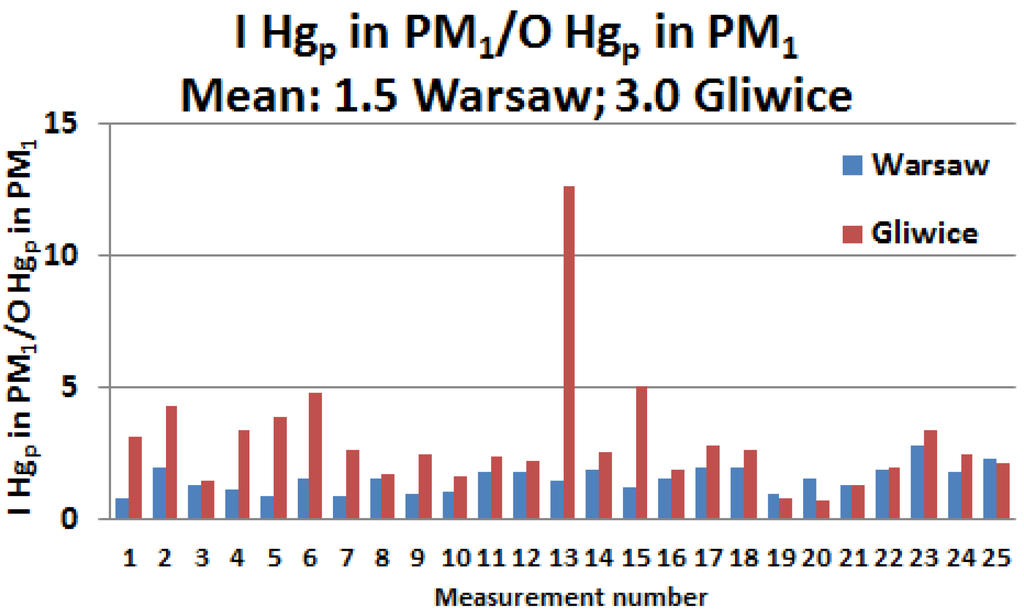
Figure 4.
Ratio of the 24-h indoor (I) and outdoor (O) Hgp contents of PM1 in Gliwice and Warsaw.
These observations lead to the conclusion that the indoor air pollution, even if there are no additional sources of fine PM and PM-bound Hg, might pose a greater threat to the health of people staying inside such a room, than hazards caused by external air pollution. The conditions inside rooms of buildings, such as teaching rooms, i.e., a constant temperature, slight air movements and limited air exchange, can favour the sorption of air gaseous components on the surfaces of PM particles. This process might be especially effective on a well-developed surface area of fine PM particles. Certainly, the effectiveness of this phenomenon depends on the chemical composition of the PM particles. In Poland, fine atmospheric particles are richer in elemental carbon (large surface area) and inorganic salts (e.g., NaCl and KCl with strong sorption properties) in winter than in summer [46]. Therefore, it can be suspected that during the winter, the phenomenon described in the presented paper may have a great impact on the level of the pollution of indoor air with Hgp.
4. Conclusions
In Warsaw and Gliwice, the PM1 concentration inside the teaching room during the summer was mainly influenced by the intensity of the atmospheric PM1 infiltration. Therefore, the PM1 reaching the indoor premises had the same composition and properties as the atmospheric PM1. On the other hand, gaseous Hg could have been more strongly absorbed on the indoor PM particles than in the atmospheric air. One of the reasons for such a phenomenon is the occurrence of much higher concentrations of gaseous Hg indoors than outdoors. This is why, inside the rooms, toxic properties of PM1 can be more harmful than the properties of the atmospheric PM1. The enrichment of indoor PM1 particles with mercury suggests that in the case of such PM components as Hg the influence of the polluted atmospheric air on the indoor air quality was more evident than in the case of PM1.
Taking into account the length of time when students and academic staff stayed inside the rooms, despite generally identified low levels of mercury concentration outside of the buildings and intensive efforts on straining the presence of any significant mercury sources inside the examined premises, during the last couple of years the mercury exposure has been found to be a real threat and must be properly dealt with. It is worth emphasizing that the aforementioned conclusions pertain to the mercury associated with very fine PM particles, and therefore, to the ones which can easily be transferred into the lungs and other parts of the human body together with the adsorbed compounds.
Acknowledgments
The work was financed within two projects of the National Science Centre (No. DEC-2013/09/N/ST10/04224 and No. 2012/07/D/ST10/02895). This research was supported by The Faculty of Civil and Environmental Engineering basic (statutory) research projects.
Author Contributions
The study was completed with cooperation between all authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, C.C.; Peng, C.K. Characterization of indoor PM10, PM2.5, and ultrafine particles in elementary school classrooms: A review. Environ. Eng. Sci. 2010, 27, 915–922. [Google Scholar] [CrossRef]
- Morawska, L.; Afshari, A.; Bae, G.N.; Buonanno, G.; Chao, C.Y.H.; Hänninen, O.; Hofmann, W.; Isaxon, C.; Jayaratne, E.R.; Salthammer, T.; et al. Indoor aerosols: From personal exposure to risk assessment. Indoor Air 2013, 23, 462–487. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Duarte, M.; Ferreira, M.; Alves, A.; Almeida, A.; Cunha, Â. Air quality in a school with dampness and mould problems. Air Qua. Atmos. Health 2016, 9, 107–115. [Google Scholar] [CrossRef]
- Wangchuk, T.; He, C.; Dudzinska, M.R.; Morawska, L. Seasonal variations of outdoor air pollution and factors driving them in the school environment in rural Bhutan. Atmos. Environ. 2015, 113, 151–158. [Google Scholar] [CrossRef]
- Romagnoli, P.; Balducci, C.; Perilli, M.; Vichi, F.; Imperiali, A.; Cecinato, A. Indoor air quality at life and work environments in Rome, Italy. Environ. Sci. Pollut. Res. 2016, 23, 3503–3516. [Google Scholar] [CrossRef] [PubMed]
- Dumała, S.M.; Dudzińska, M.R. Microbiological indoor air quality in Polish schools. Rocz. Ochr. Sr. 2013, 15, 231–244. [Google Scholar]
- Polednik, B. Particulate matter and student exposure in school classrooms in Lublin, Poland. Environ. Res. 2013, 120, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Polednik, B. Variations in particle concentrations and indoor air parameters in classrooms in the heating and summer season. Arch. Environ. Prot. 2013, 39, 15–28. [Google Scholar] [CrossRef]
- Zwoździak, A.; Sówka, I.; Fortuna, M. Influence of PM1, PM2.5, PM10 Concentrations in indoor school environment on spirometric parameters in schoolchildren. Rocz. Ochr. Sr. 2013, 15, 2022–2038. [Google Scholar]
- Zwoździak, A.; Sówka, I.; Krupińska, B.; Zwoździak, J.; Nych, A. Infiltration or indoor sources as determinants of the elemental composition of particulate matter inside a school in Wroclaw, Poland? Build. Environ. 2013, 66, 173–180. [Google Scholar] [CrossRef]
- Mainka, A.; Zajusz-Zubek, E.; Kaczmarek, K. PM2.5 in urban and rural nursery schools in Upper Silesia, Poland: Trace elements analysis. Int. J. Environ. Res. Public Health 2015, 12, 7990–8008. [Google Scholar] [CrossRef] [PubMed]
- Mainka, A.; Zajusz-Zubek, E. Indoor air quality in urban and rural preschools in Upper Silesia, Poland: Particulate matter and carbon dioxide. Int. J. Environ. Res. Public Health 2015, 12, 7697–7711. [Google Scholar] [CrossRef] [PubMed]
- Brągoszewska, E.; Mainka, A.; Pastuszka, J.S. Bacterial aerosols in an urban nursery school in Gliwice, Poland: A case study. Aerobiologia 2015. [Google Scholar] [CrossRef]
- Pastuszka, J.S.; Paw, U.K.T.; Kabała-Dzik, A.; Kohyama, N.; Sokal, J.A. Respirable airborne fibers in the home environment in Upper Silesia, Poland, compared with Davis, California. J. Aerosol Sci. 2000, 31, 484–485. [Google Scholar] [CrossRef]
- Colbeck, I.; Nasir, Z.A.; Ali, Z. Characteristics of indoor/outdoor particulate pollution in urban and rural residential environment of Pakistan. Indoor Air 2010, 20, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.; Lind, T.; Nilsson, M.A.M.; Bellander, T. PM2.5, soot and NO2 indoor–outdoor relationships at homes, pre-schools and schools in Stockholm, Sweden. Atmos. Environ. 2010, 44, 4536–4544. [Google Scholar] [CrossRef]
- Worobiec, A.; Samek, L.; Krata, A.; Van Meel, K.; Krupinska, B.; Stefaniak, E.A.; Karaszkiewicz, P.; Grieken, R.V; Van Grieken, R. Transport and deposition of airborne pollutants in exhibition areas located in historical buildings–study in Wawel Castle Museum in Cracow, Poland. J. Cult. Herit. 2010, 11, 354–359. [Google Scholar] [CrossRef]
- Hänninen, O.; Hoek, G.; Mallone, S.; Chellini, E.; Katsouyanni, K.; Gariazzo, C.; Cattani, G.; Marconi, A.; Molnar, P.; Bellander, T.; et al. Seasonal patterns of outdoor PM infiltration into indoor environments: Review and meta-analysis of available studies from different climatological zones in Europe. Air Qual. Atmos. Health 2011, 4, 221–233. [Google Scholar]
- Goyal, R.; Kumar, P. Indoor–outdoor concentrations of particulate matter in nine microenvironments of a mix-use commercial building in megacity Delhi. Air Qual. Atmos. Health 2013, 6, 747–757. [Google Scholar] [CrossRef]
- Che, W.W.; Frey, H.C.; Lau, A.K. Comparison of sources of variability in school age children exposure to ambient PM2.5. Environ. Sci. Technol. 2015, 49, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.; Latif, M.T.; Mohamed, A.F. The PM10 compositions, sources and health risks assessment in mechanically ventilated office buildings in an urban environment. Air Qual. Atmos. Health 2016, 9, 597–612. [Google Scholar] [CrossRef]
- Rogula-Kopiec, P.; Pastuszka, J.S.; Rogula-Kozłowska, W.; Majewski, G. Particulate matter in indoor spaces: Known facts and the knowledge gaps. Ann. Warsaw Univ. Life Sci. – SGGW, Land Reclam. 2015, 47, 43–54. [Google Scholar] [CrossRef]
- Pastuszka, J.S.; Paw, U.K.T.; Lis, D.O.; Wlazło, A.; Ulfig, K. Bacterial and fungal aerosol in indoor environment in Upper Silesia, Poland. Atmos. Environ. 2000, 34, 3833–3842. [Google Scholar] [CrossRef]
- Lippman, M. Environmental Toxicants: Human Exposures and Their Health Effects; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Spellman, F.R. The Science of Air Concepts and Applications; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Rogula-Kopiec, P.; Rogula-Kozłowska, W.; Kozielska, B.; Sówka, I. PAH concentrations inside a wood processing plant and the indoor effects of outdoor industrial emissions. Pol. J. Environ. Stud. 2015, 24, 11–17. [Google Scholar] [CrossRef]
- Rogula-Kopiec, P.; Pastuszka, J.S.; Rogula-Kozłowska, W.; Czechowski, P.O.; Majewski, G. Suspended dust in office and laboratory-the effects of selected factors on the concentrations and the respirable fractions content. In Air Protection in Theory and Practice; Konieczyński, J., Ed.; Instytut Podstaw Inżynierii Środowiska Polskiej Akademii Nauk: Zabrze, Poland, 2014; pp. 231–242. [Google Scholar]
- Mainka, A.; Brągoszewska, E.; Kozielska, B.; Pastuszka, J.S.; Zajusz-Zubek, E. Indoor air quality in urban nursery schools in Gliwice, Poland: Analysis of the case study. Atmos. Pollut. Res. 2015, 6, 1098–1104. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Stratton, W.J. Atmospheric mercury speciation: Concentrations and behavior of reactive gaseous mercury in ambient air. Environ. Sci. Technol. 1998, 32, 49–57. [Google Scholar] [CrossRef]
- Pyta, H.; Rosik-Dulewska, C.; Czaplicka, M. Speciation of ambient mercury in the Upper Silesia region, Poland. Water Air Soil Soil. Pollut. 2009, 197, 233–240. [Google Scholar] [CrossRef]
- Hladikova, V.; Petrik, J.; Jursa, S.; Ursinyova, M.; Koèan, J. Atmospheric mercury levels in the Slovak Republic. Chemosphere 2001, 45, 801–806. [Google Scholar] [CrossRef]
- Pyta, H. Ambient air pollution by mercury species at the urban station in Zabrze, Southern Poland; EDP Sciences: Roma, Italy, 2013. [Google Scholar]
- Schleicher, N.J.; Schäfer, J.; Blanc, G.; Chen, Y.; Chai, F.; Cen, K.; Norra, S. Atmospheric particulate mercury in the megacity Beijing: Spatio-temporal variations and source apportionment. Atmos. Environ. 2015, 109, 251–261. [Google Scholar] [CrossRef]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Telmer, K. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef]
- Siudek, P.; Frankowski, M.; Siepak, J. Atmospheric particulate mercury at the urban and forest sites in central Poland. Environ. Sci. Pollut. Res. 2016, 23, 2341–2352. [Google Scholar] [CrossRef] [PubMed]
- Bełdowska, M.; Saniewska, D.; Falkowska, L.; Lewandowska, A. Mercury in particulate matter over Polish zone of the southern Baltic Sea. Atmos. Environ. 2012, 46, 397–404. [Google Scholar] [CrossRef]
- Forlano, L.; Hedgecock, I.M.; Pirrone, N. Elemental gas phase atmospheric mercury as it interacts with the ambient aerosol and its subsequent speciation and deposition. Sci. Total Environ. 2000, 259, 211–222. [Google Scholar] [CrossRef]
- Malcolm, E.G.; Keeler, G.J. Evidence for a sampling artifact for particulate-phase mercury in the marine atmosphere. Atmos. Environ. 2007, 41, 3352–3359. [Google Scholar] [CrossRef]
- Xiu, G.L.; Jin, Q.; Hang, D.; Shi, S.; Huang, X.; Zghang, W.; Bao, L.; Gao, P.; Chen, B. Characterization of size fractionated particulate mercury in Shanghai ambient air. Atmos. Environ. 2005, 39, 419–427. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Review: Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003, 18, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W. The three modern faces of mercury. Environ. Health Perspect. 2002, 110, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.M.; Yin, J. Particulate matter in the atmosphere: Which particle properties are important for its effects on health? Sci. Total Environ. 2000, 249, 85–101. [Google Scholar] [CrossRef]
- Englert, N. Fine particles and human health—A review of epidemiological studies. Toxicol. Lett. 2004, 149, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pastuszka, J.S.; Wawroś, A.; Talik, E.; Paw, U.K.T. Optical and chemical characteristics of the atmospheric aerosol in four towns in southern Poland. Sci. Total. Environ. 2003, 309, 237–251. [Google Scholar] [CrossRef]
- Pastuszka, J.S.; Rogula-Kozłowska, W.; Zajusz-Zubek, E. Characterization of PM10 and PM2.5 and associated heavy metals at the crossroads and urban background site in Zabrze, Upper Silesia, Poland, during the smog episodes. Environ. Monit. Assess. 2010, 168, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kozłowska, W.; Klejnowski, K.; Rogula-Kopiec, P.; Ośródka, L.; Krajny, E.; Błaszczak, B.; Mathews, B. Spatial and seasonal variability of the mass concentration and chemical composition of PM2.5 in Poland. Air Qual. Atmos. Health 2014, 7, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kozłowska, W.; Majewski, G.; Czechowski, P.O. The size distribution and origin of elements bound to ambient particles: A case study of a Polish urban area. Environ. Monit. Assess. 2015, 187, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Majewski, G.; Rogula-Kozłowska, W. The elemental composition and origin of fine ambient particles in the largest Polish conurbation: First results from the short-term winter campaign. Theor. Appl. Climatol. 2016, 125, 79–92. [Google Scholar] [CrossRef]
- Majewski, G.; Rogula-Kozłowska, W.; Czechowski, P.O.; Badyda, A.; Brandyk, A. The impact of selected parameters on visibility: First results from a long-term campaign in Warsaw, Poland. Atmosphere 2015, 6, 1154–1174. [Google Scholar] [CrossRef]
- Badyda, A.J.; Dabrowiecki, P.; Lubinski, W.; Czechowski, P.O.; Majewski, G. Exposure to traffic-related air pollutants as a risk of airway obstruction. Adv. Exp. Med. Biol. 2013, 755, 35–45. [Google Scholar] [PubMed]
- Pyta, H.; Rogula-Kozłowska, W. Determination of mercury in size-segregated ambient particulate matter using CVAAS. Microchem. J. 2016, 124, 76–81. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Klejnowski, K. Submicrometer aerosol in rural and urban backgrounds in southern Poland: Primary and secondary components of PM1. Bull. Environ. Contam. Tox. 2013, 90, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kozielska, B.; Rogula-Kozłowska, W.; Klejnowski, K. Seasonal variations in health hazards from polycyclic aromatic hydrocarbons bound to submicrometer particles at three characteristic sites in the heavily polluted Polish region. Atmosphere 2014, 6, 1–20. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Huang, H.; Cao, J.J.; Lee, S.C.; Zou, C.W.; Chen, X.G.; Fan, S.J. Spatial variation and relationship of indoor/outdoor PM2.5 at residential homes in Guangzhou city, China. Aerosol Air Qual. Res. 2007, 7, 518–580. [Google Scholar]
- Cao, J.J.; Huang, H.; Lee, S.C.; Chow, J.C.; Zou, C.W.; Ho, K.F.; Watson, J.G. Indoor/outdoor relationships for organic and elemental carbon in PM2.5 at residential homes in Guangzhou, China. Aerosol Air Qual. Res. 2012, 12, 902–910. [Google Scholar] [CrossRef]
- Rozbicka, K.; Majewski, G.; Rozbicki, T. Seasonal variation of air pollution in Warsaw conurbation. Meteorol. Z. 2014, 23, 175–179. [Google Scholar]
- Majewski, G.; Czechowski, P.O.; Badyda, A.J.; Rogula-Kozłowska, W. The estimation of total gaseous mercury concentration (TGM) using exploratory and stochastic methods. Pol. J. Environ. Stud. 2013, 22, 759–771. [Google Scholar]
- Majewski, G.; Czechowski, P.O.; Badyda, A.; Kleniewska, M.; Brandyk, A. Ocena stężenia całkowitej rtęci gazowej (TGM) na terenie stacji tła regionalnego Granica-KPN (województwo mazowieckie, Polska) w latach 2010–2011. Rocz. Ochr. Środ. 2013, 15, 1302–1317. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).