Removal of Low-Molecular Weight Aldehydes by Selected Houseplants under Different Light Intensities and CO2 Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
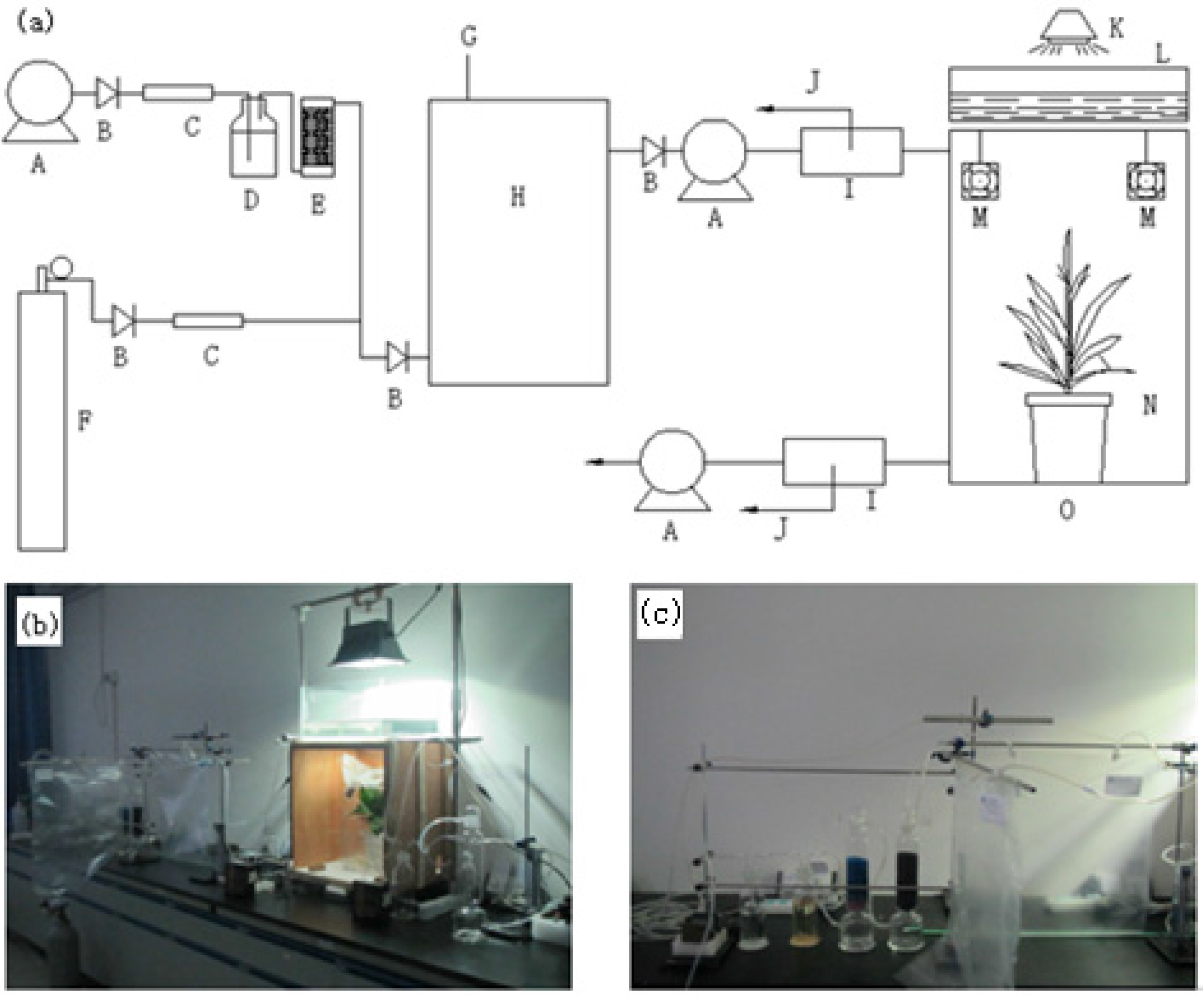
2.2. Reagents and Apparatus
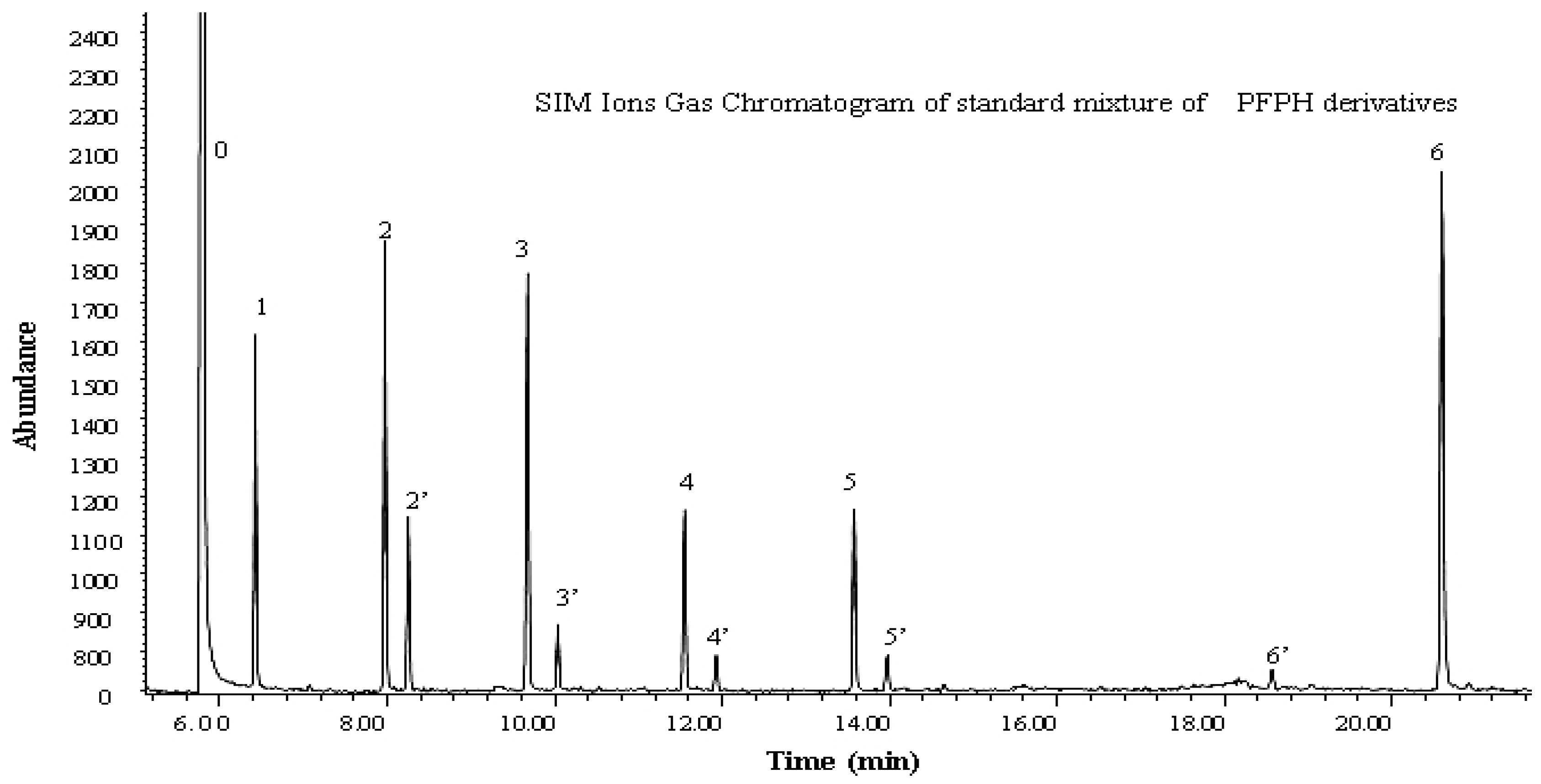
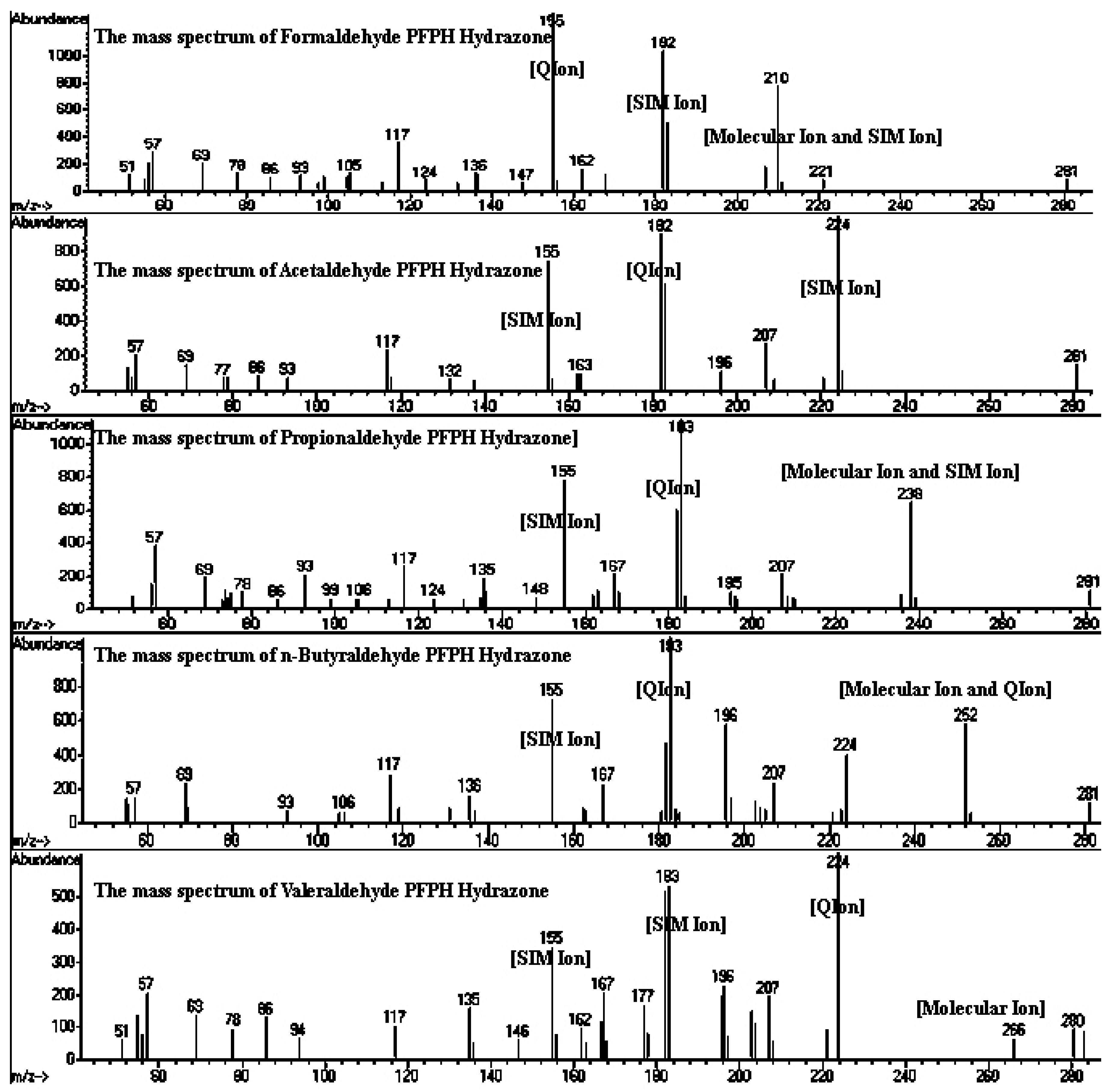
2.3. Sample Collection and Analysis
2.4. Determination of Aldehyde Removal Rate by Houseplants
2.5. Quality Assurance/Quality Control (QA/QC)
3. Results and Discussion
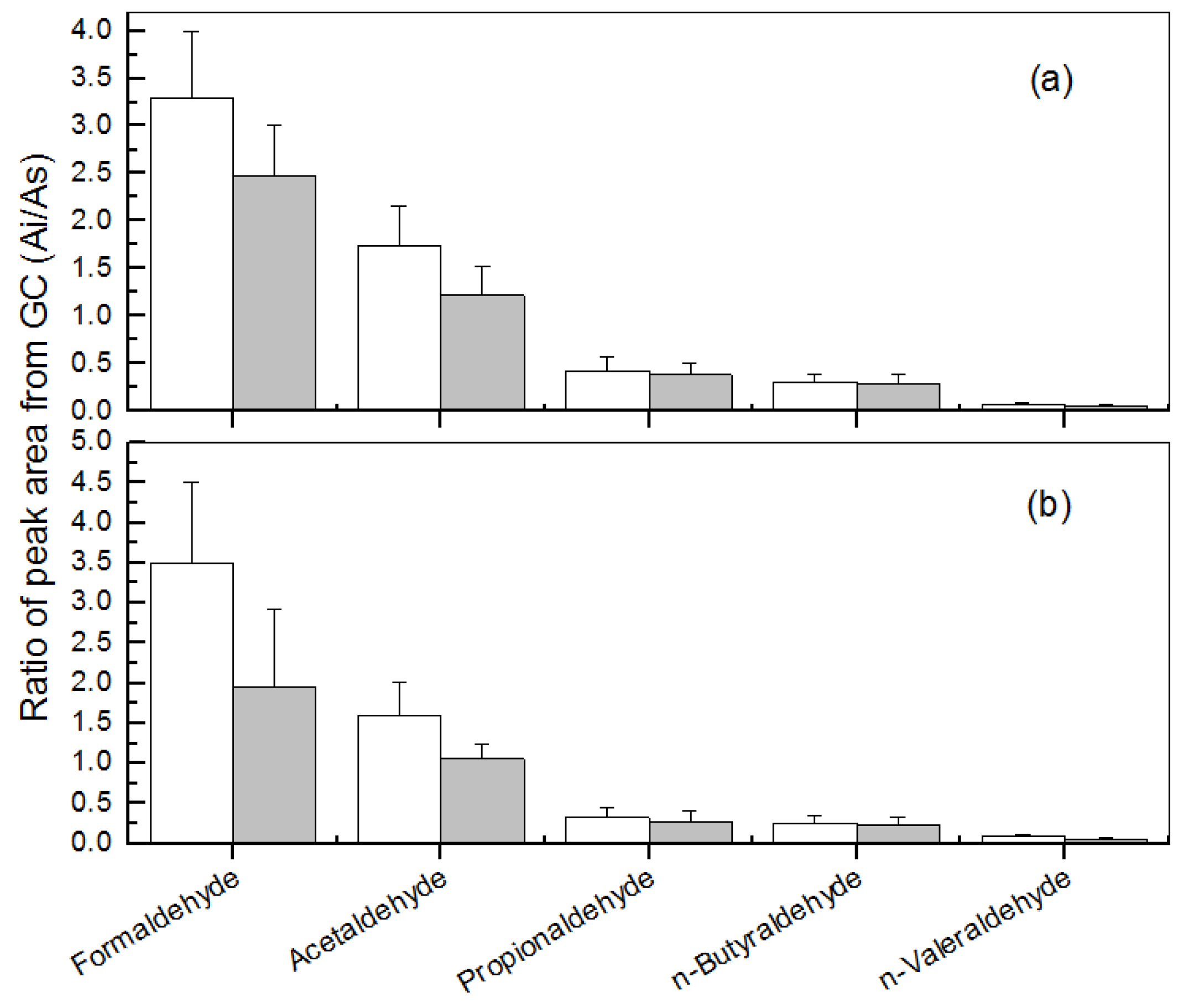
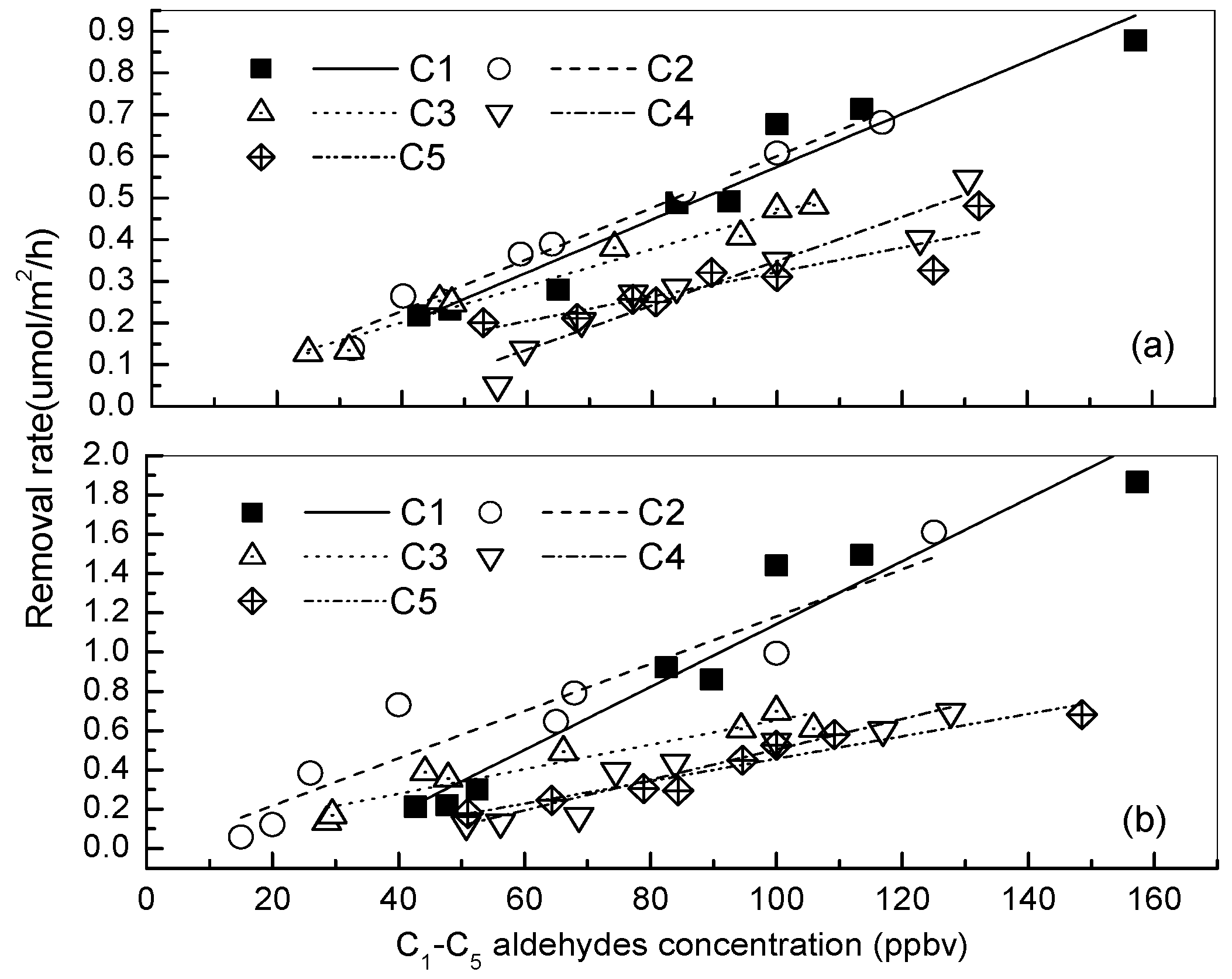
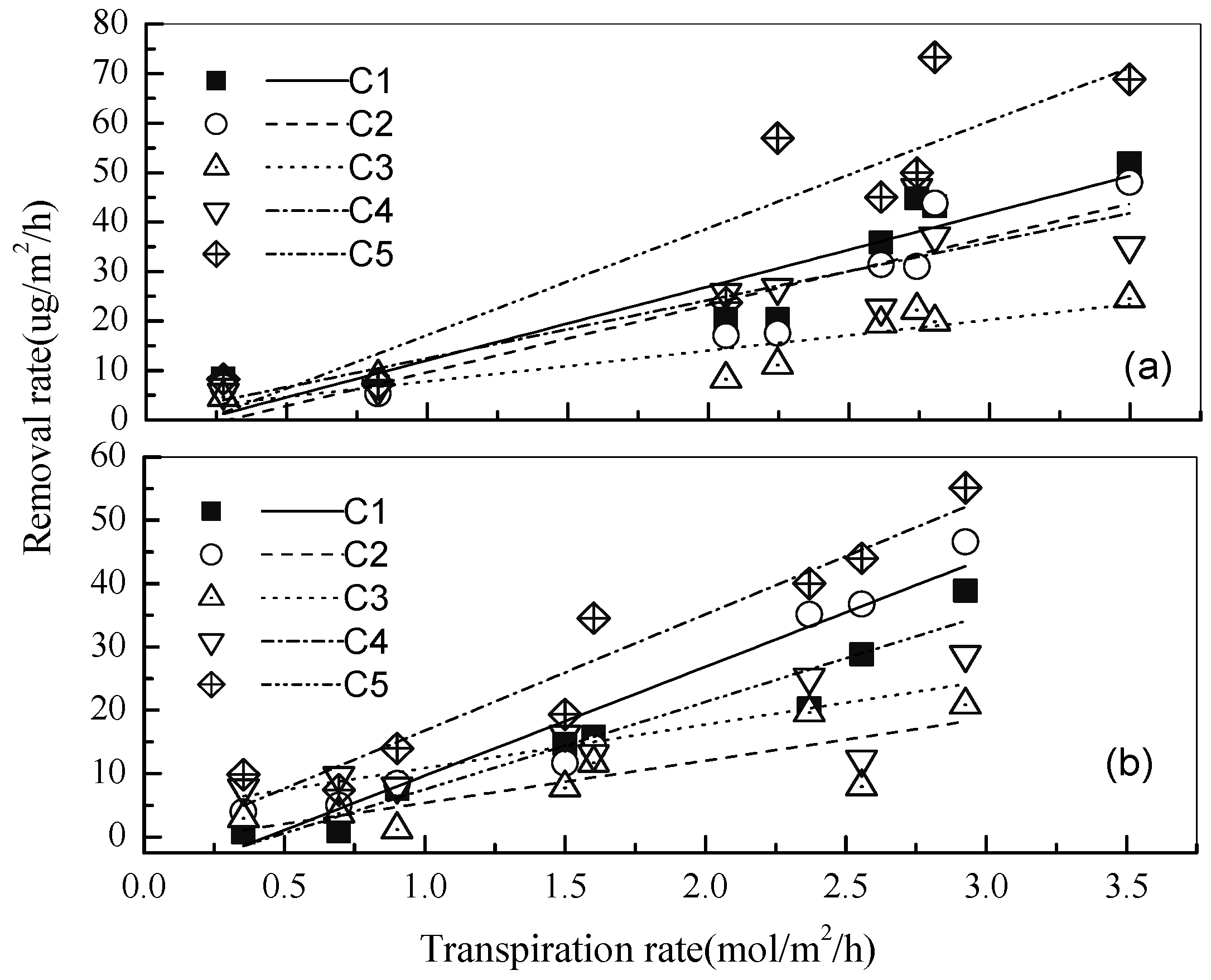
3.1. Removal of Low-Molecular Mass Aldehydes by the Plants under a Normal Indoor Environment
3.2. Effects of Different Light Intensities
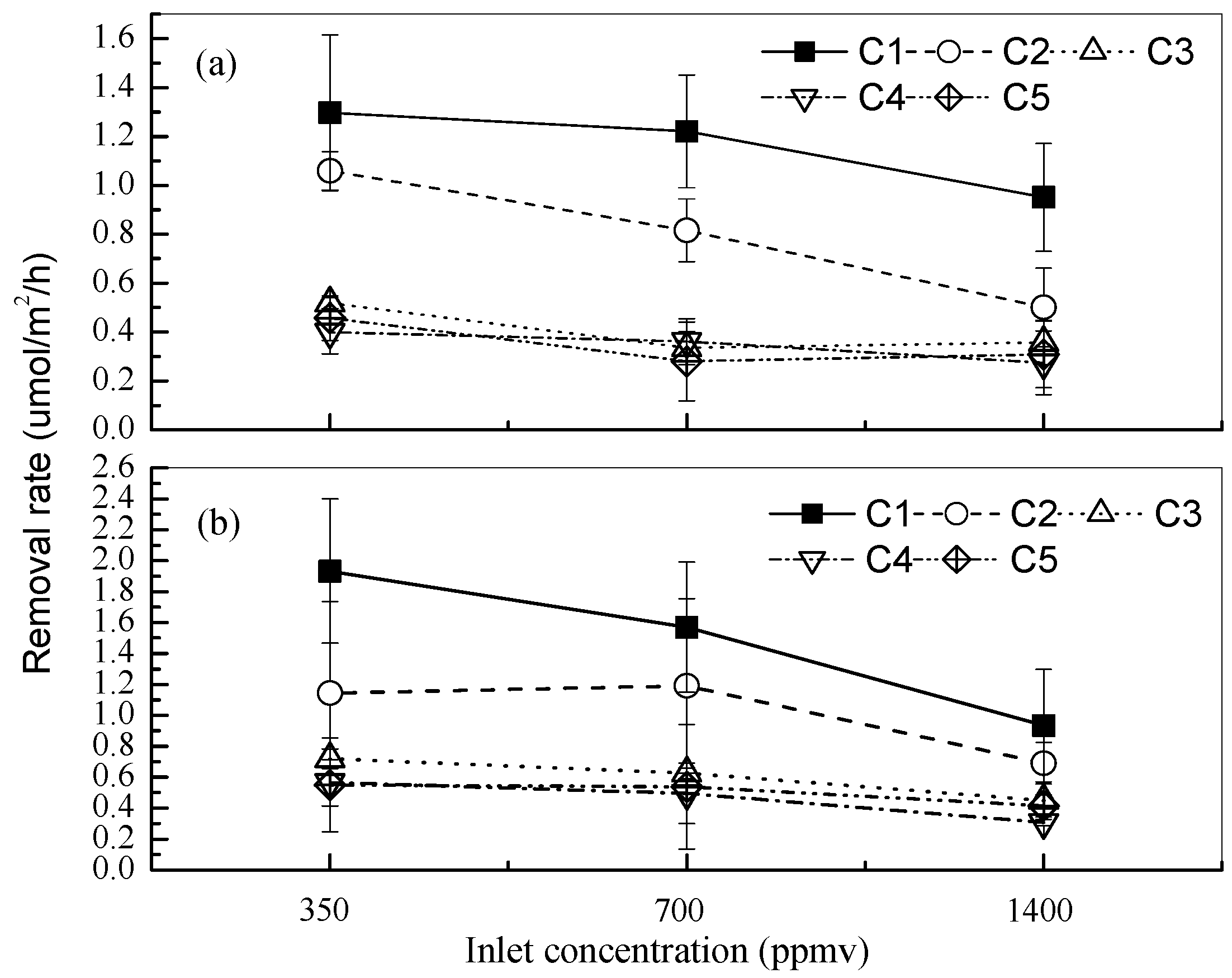
3.3. Effects of Different CO2 Concentrations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): Aresource for assessing exposure to environmental pollutants. J. Exp. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed]
- US Environment Protection Agency. Child-Specific Exposure Factors Handbook; National Center for Environmental Assessment: Washington, DC, USA, 2002.
- Mølhave, L.; Krzyzanowski, M. The right to healthy indoor air: Status by 2002. Indoor Air 2003, 13, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Pennisi, S.V.; Son, K.C.; Kays, S.J. Screening Indoor Plant for Volatile Organic Pollutant Removal Efficiency. HortScience 2009, 44, 1377–1381. [Google Scholar]
- Wu, X.M.; Apte, M.G.; Maddalena, R.; Bennett, D.H. Volatile organic compounds in small-and medium-sized commercial buildings in California. Environ. Sci. Technol. 2011, 45, 9075–9083. [Google Scholar] [CrossRef] [PubMed]
- Bakeas, E.B.; Argyris, D.I.; Siskos, P.A. Carbonyl compounds in the urban environment of Athens, Greece. Chemosphere 2003, 52, 805–813. [Google Scholar] [CrossRef]
- Duan, J.; Guo, S.; Tan, J.; Wang, S.; Chai, F. Characteristics of atmospheric carbonyls during haze days in Beijing, China. Atmos. Res. 2012, 114, 17–27. [Google Scholar] [CrossRef]
- Ho, S.H. Analysis of Airborne Organic Compounds Using Thermal Desorption-Gas Chromatography-Mass Spectrometry Methods. Ph.D. Thesis, Hong Kong University of Science and Technology, Hong Kong, China, 2004. [Google Scholar]
- Wang, B.; Lee, S.C.; Ho, K.F. Characteristics of carbonyls: Concentrations and source strengths for indoor and outdoor residential microenvironments in China. Atmos. Environ. 2007, 41, 2851–2861. [Google Scholar] [CrossRef]
- De Bortoli, M.; Knoeppel, H.; Pecchio, E.; Peil, A.; Rogora, L.; Schauenburg, H.; Schlitt, H.; Vissers, H. Concentrations of selected organic pollutants in indoor and outdoor air in northern Italy. Environ. Int. 1986, 12, 343–350. [Google Scholar] [CrossRef]
- Báez, A.P.; Padilla, H.; Garcia, R.; Torres, M.D.C.; Rosas, I.; Belmont, R. Carbonyl levels in indoor and outdoor air in Mexico City and Xalapa, Mexico. Sci. Total Environ. 2003, 302, 211–226. [Google Scholar] [CrossRef]
- Hida, J.; Tanaka, Y.; Yamada, M.; Taki, K.; Yoshia, H. Field measurement of carbonyl compound concentration in indoor air of wooden residential houses newly constructed from 2001 to 2002. J. Jpn. Wood Res. Soc. 2006, 52, 215–220. [Google Scholar] [CrossRef]
- Báez, A.P.; Padilla, H.; Garcia, R.; Belmont, R.; Torres, M.D. Measurement of indoor-outdoor carbonyls at four residential homes in Mexico city metropolitan area. Int. J. Environ. Pollut. 2006, 26, 90–105. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Liu, Y.J.; Zhang, M.G. Personal exposure and source characteristics of carbonyl compounds and BTEXs within homes in Beijing, China. Build. Environ. 2013, 61, 210–216. [Google Scholar] [CrossRef]
- Weng, M.L.; Zhu, L.Z.; Yang, K.; Chen, S. Levels, sources, and health risks of carbonyls in residential indoor air in Hangzhou, China. Environ. Monit. Assess. 2010, 163, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Pegas, P.N.; Alves, C.A.; Nunes, T.; Bate-Epey, E.F.; Evtyugina, M.; Pio, C.A. Could houseplants improve indoor air quality in schools? J. Toxicol. Environ. Health A 2012, 75, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A.; Orwell, R.L.; Tarran, J.; Torpy, F.; Burchett, M. Potted-plant/growth media interactions and capacities for removal of volatiles from indoor air. In Proceedings of the Health Buildings 2003—HB2003, Singapore, 7–11 December 2003; pp. 441–445.
- Wood, R.A.; Burchett, M.D.; Alquezar, R.; Orwell, R.L.; Tarran, J.; Torpy, F. The potted-plant microsm substantially reduces indoor air VOC pollution: I. office field-study. Water Air Soil Pollut. 2006, 175, 163–180. [Google Scholar] [CrossRef]
- Dingle, P.; Tapsell, P.; Hu, S. Reducing formaldehyde exposure in office environments using plants. Bull. Environ. Contam. Toxicol. 2000, 64, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.R. Foliar sorption of atmospheric sulfur dioxide by woody plants. Environ. Pollut. 1974, 7, 133–140. [Google Scholar] [CrossRef]
- Fujii, S.; Cha, H.; Kagi, N.; Miyamura, H.; Kim, Y.S. Effects on air pollutant removal by plant absorption and adsorption. Build. Environ. 2005, 40, 105–112. [Google Scholar] [CrossRef]
- Barber, J.L.; Kurt, P.B.; Thomas, G.O.; Kerstiens, G.; Jones, K.C. Investigation into the importance of the stomatal pathway in the exchange of PCBs between air and plants. Environ. Sci. Technol. 2002, 36, 4282–4287. [Google Scholar] [CrossRef] [PubMed]
- Treesubsuntorn, C.; Thiravetyan, P. Removal of benzene from indoor air by Dracaena sanderiana: Effect of wax and stomata. Atmos. Environ. 2012, 57, 317–321. [Google Scholar] [CrossRef]
- Kacálková, K.P.; Tlustoš, P. The uptake of persistent organic pollutants by plants. Cent. Eur. J. Biol. 2011, 6, 223–235. [Google Scholar] [CrossRef]
- Collins, C.; Fryer, M.; Grosso, A. Plant uptake of non-ionic organic chemicals. Environ. Sci. Technol. 2006, 40, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Omasa, K.; Tobe, K.; Hosomi, M.; Kobayashi, C. Absorption of ozone and seven organic pollutants by Populus nigra and Camellia sasanqua. Environ. Sci. Technol. 2000, 34, 2498–2500. [Google Scholar] [CrossRef]
- Seco, R.; Penuelas, J.; Filella, I. Formaldehyde emission and uptake by Mediterranean trees Quercus ilex and Pinus halepensis. Atmos. Environ. 2008, 42, 7907–7914. [Google Scholar] [CrossRef]
- Kvesitadze, E.; Sadunishvili, T.; Kvesitadze, G. Mechanisms of organic contaminants uptake and degradation in plants. World Acad. Sci. Eng. Technol. 2009, 55, 458–468. [Google Scholar]
- Schreiber, L. Polar paths of diffusion across plant cuticles: New evidence for an old hypothesis. Ann. Bot. 2005, 95, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, J. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. J. Exp. Bot. 2006, 57, 2471–2491. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, H.; Hilgers, U.; Weidner, M. Assimilation and metabolism of formaldehyde by leaves appear unlikely to be of value for indoor air purification. New Phytol. 2000, 147, 307–315. [Google Scholar] [CrossRef]
- Kondo, T.; Hasegawa, K.; Uchida, R.; Onishi, M. Absorption of atmospheric C2–C5 aldehydes by various trees pecies and their tolerance to C2–C5 aldehydes. Sci. Total Environ. 1998, 224, 121–132. [Google Scholar] [CrossRef]
- Rottenberger, S.; Kuhn, U.; Wolf, A.; Schebeske, G.; Oliva, S.T.; Tavares, T.M.; Kesselmeier, J. Exchange of short-chain aldehydes between Amazonian vegetation and the atmosphere. Ecol. Appl. 2004, 14, 247–262. [Google Scholar] [CrossRef]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology; Academic Press: Oxford, UK, 2009. [Google Scholar]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; et al. The global carboncycle: A test of our knowledge of Earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; vander Linden, P.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change 2001: The Scientific Basis; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Ignatova, L.K.; Novichkova, N.S.; Mudrik, V.A.; Lyubimov, V.Y.; Ivanov, B.N.; Romanova, A.K. Growth, photosynthesis, and metabolism of sugar beet at an early stage of exposure to elevated CO2. Russ. J. Plant Physiol. 2005, 52, 158–164. [Google Scholar] [CrossRef]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr. Sci. 2010, 99, 46–57. [Google Scholar]
- Barbehenn, R.V.; Karowe, D.N.; Chen, Z. Performance of a generalist grasshopper on a C3 and a C4 grass: Compensation for the effects of elevated CO2 on plant nutritional quality. Oecologia 2004, 140, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Roumet, C.; Garnier, E.; Suzor, H.; Salager, J.L.; Roy, J. Short and long-term responses of whole-plant gas exchange to elevated CO2 in four herbaceous species. Environ. Exp. Bot. 2000, 43, 155–169. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell. Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, M.; Furukawa, A. Responses of individual stomata in Ipomoea pes-caprae to various CO2 concentrations. Physiol. Plant. 2008, 132, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Broadmeadow, M.S.J.; Heath, J.; Randle, T.J. Environmental Limitations to O3 Uptake—Some Key Results from Young Trees Growing at Elevated CO2 Concentrations. Water Air Soil Pollut. 1999, 116, 299–310. [Google Scholar] [CrossRef]
- Hu, Z.H.; Shen, Y.B.; Su, X.H. Saturated Aldehydes C6–C10 Emitted from Ashleaf Maple (Acer negundo L.) Leaves at Different Levels of Light Intensity, O2, and CO2. J. Plant Biol. 2009, 52, 289–297. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.L.; Xie, C.J.; Huang, J.; Yu, J.Z.; Feng, J.L.; Sheng, G.Y.; Fu, J.M.; Wu, M.H. Determination of gaseous carbonyl compounds by their pentafluorophenyl hydrazones with gas chromatography/mass spectrometry. Anal. Chim. Acta 2009, 635, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hasegawa, K.; Uchida, R.; Onishi, M.; Mizukami, A.; Omasa, K. Absorption of Atmospheric Formaldehyde by Deciduous Broad-Leaved, Evergreen Broad-Leaved, and Coniferous Tree Species. Bull. Chem. Soc. Jpn. 1996, 69, 3673–3679. [Google Scholar] [CrossRef]
- Seco, R.; Penuelas, J.; Filella, I. Short-chain oxygenated VOCs: Emission and uptake by plants and atmospheric sources, sinks, and concentrations. Atmos. Environ. 2007, 41, 2477–2499. [Google Scholar] [CrossRef]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Annu. Rev. Plant Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hasegawa, K.; Uchida, R. Absorption of formaldehyde by oleander (Nerium indicum). Environ. Sci. Technol. 1995, 29, 2901–2903. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light Regulation of Stomatal Movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, T.A. Porosity at a price: The control of stomatal conductance in relation to photosynthesis. Top. Photosynth. 1985, 6, 419–452. [Google Scholar]
- Herrick, J.D.; Maherali, H.; Thomas, R.B. Reduced stomatal conductance in sweetgum (Liquidambar styaciflua) sustained over long-term CO2 enrichment. New Phytol. 2004, 162, 387–396. [Google Scholar] [CrossRef]

| Carbonyl Name | Removal Rate (μmol/m2/h) a | |
|---|---|---|
| Schefflera octophylla (Lour.) Harms | Chamaedorea elegans (Lour.) Harms | |
| Formaldehyde | 0.677 ± 0.105 | 1.440 ± 0.270 |
| Acetaldehyde | 0.608 ± 0.074 | 0.995 ± 0.131 |
| Propionaldehyde | 0.474 ± 0.053 | 0.698 ± 0.091 |
| n-butyraldehyde | 0.350 ± 0.063 | 0.544 ± 0.041 |
| n-valeraldehyde | 0.311 ± 0.100 | 0.526 ± 0.143 |
| Total | 2.420 ± 0.395 | 4.203 ± 0.676 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xie, C.-J.; Cai, J.; Yan, L.-S.; Lu, M.-M. Removal of Low-Molecular Weight Aldehydes by Selected Houseplants under Different Light Intensities and CO2 Concentrations. Atmosphere 2016, 7, 144. https://doi.org/10.3390/atmos7110144
Li J, Xie C-J, Cai J, Yan L-S, Lu M-M. Removal of Low-Molecular Weight Aldehydes by Selected Houseplants under Different Light Intensities and CO2 Concentrations. Atmosphere. 2016; 7(11):144. https://doi.org/10.3390/atmos7110144
Chicago/Turabian StyleLi, Jian, Chun-Juan Xie, Jing Cai, Liu-Shui Yan, and Ming-Ming Lu. 2016. "Removal of Low-Molecular Weight Aldehydes by Selected Houseplants under Different Light Intensities and CO2 Concentrations" Atmosphere 7, no. 11: 144. https://doi.org/10.3390/atmos7110144
APA StyleLi, J., Xie, C.-J., Cai, J., Yan, L.-S., & Lu, M.-M. (2016). Removal of Low-Molecular Weight Aldehydes by Selected Houseplants under Different Light Intensities and CO2 Concentrations. Atmosphere, 7(11), 144. https://doi.org/10.3390/atmos7110144





