Abstract
In Italy, children spend up to 30% of their time in school institutions; for this reason, the evaluation of indoor air quality in schools constitutes a necessary step forward in the direction of child health protection. In this study, we investigated the chemical composition of PM2.5 collected simultaneously indoor and outdoor in three primary schools in Rome. Seasonal variations between winter and spring/summer were evaluated, as well as the role of the main macro-sources of PM (soil, sea, traffic, secondary inorganics and organics). During winter periods, characterized by strong atmospheric stability, the main contributors were organics and combustion products, which accounted for more than 70% of the total mass both indoor and outdoor. Spring/summer period was characterized by very low outdoor concentrations (12 μg/m3 on average) and by a more balanced contribution of organic, traffic and secondary inorganic components. Indoor, the contribution of soil-related species from re-suspension of settled dust and secondary inorganic species from outdoor photochemical reactions became significant. Given that several indoor exceedances of the international air quality standards for PM2.5 were recorded during the most polluted days, the infiltration of outdoor air, due to the inadequate construction characteristics of the buildings and the absence of automated air filtration systems, seemed to be the main causes of the high PM concentrations measured indoor.
1. Introduction
The World Health Organization (WHO) has included air pollution among the key determinants of health [1]. Air pollution, in particular from tobacco smoke and household solid fuel combustion, is, in fact, considered one of the main risk factors for the global burden of disease [2]. It is responsible for several chronic conditions, such as cardiovascular disease [3], asthma [4,5], chronic obstructive pulmonary disease [6], lung cancer [7], and diabetes [8], as well as numerous acute effects, such as airway inflammation [9,10]. The most susceptible to adverse effect are people with preexisting disease, the elderly and children [11]. Indeed, the latter are more likely to develop adverse health outcomes, as they breath higher volumes of air than adults in relation to their body weight and because of their more rapidly growing and developing organs, tissues and immune system [12].
During the past decade many studies that aimed to assess indoor air quality (IAQ) in confined environments have shown that almost 90% of our time is spent in enclosed areas, including private homes, schools, offices, transports and meeting places [13,14]. Given that children spend a consistent part of the day in school buildings, several studies on IAQ in schools have been carried out in European countries in order to improve the knowledge in this field and to produce guidelines on remedial measures. Among others, the results of the SINPHONIE (Schools Indoor Pollution and Health: Observatory Network in Europe) project [15], the HESE (Health Effects of School Environment) project [16] and the SEARCH (School Environment And Respiratory health of Children) project [17] underlined a strong relationship between exposure to indoor pollutants and respiratory and allergic symptoms among students and showed that a significant fraction of the monitored classrooms did not respect the WHO guidelines for indoor levels of PM.
In Italy, compulsory school education covers children up to sixteen years old. According to the school grade (nursery, primary, secondary school), children spend up to 30% of their daytime in school environments. The last updated scenario of Italian air quality and the relative risks for students health [18] highlighted that more than 70% of the Italian schools were built before 1975, following inadequate construction protocols. The “Indoor school” project [19], which addressed 22 primary schools and 22 secondary schools in Italy, showed that indoor PM2.5 exceeded the limit of 25 µg/m3 in 50% of the classrooms. In the final report, it was concluded that building construction characteristics, proximity to pollutant sources (mainly road traffic), inappropriate ventilation and use of cleaning products and procedures were the most important factors influencing air quality in schools.
In 2010, the Central and Regional Italian governments published an agreement [20] in which recommendations are given in order to find the main risk factors for respiratory symptoms in children and to provide measures to reduce their exposure during the school-time. Nevertheless, there are neither current legislation regulating indoor air quality in Italian school buildings nor standardized procedures to monitor indoor pollution.
In the case of particulate matter (PM), health effects related to PM exposure may strongly depend also on the composition of the inhaled dust [21]. Besides determining its mass concentration, it is thus necessary to carry out also its chemical characterization. To this aim, it may be convenient to determine only PM macro-components; that is, the chemical species that individually constitute more than 1% of the overall PM mass. They include some metals (Al, Si, Fe, K, Ca), the main anions (chloride, nitrate, sulfate and carbonate) and cations (sodium, ammonium, potassium, magnesium and calcium), elemental carbon and organic carbon. By determining these species, it is generally possible to obtain the mass closure, which is the correspondence between the gravimetric mass and the sum of the main components, and to estimate the main source contributions to PM mass. This method has been applied successfully in outdoor air quality studies [22] and is also appropriate for indoor studies [23].
The present work was conducted within the framework of LIFE+ Project EXPAH (Population Exposure to Polycyclic Aromatic Hydrocarbons) [24] which was aimed at investigating population exposure to air pollutants in homes, schools, offices and transports. We report here the characterization of indoor air quality in three schools located in the urban area of Rome, Italy. PM2.5 measurements were carried out simultaneously indoor and outdoor in order to evaluate the daily and seasonal variations of PM and of its macro-components. Indoor and outdoor concentrations were compared among sites and with WHO guidelines [25]. Finally, the impact of the main PM macro-sources (soil, sea, secondary inorganics, traffic, and organics) on indoor and outdoor PM concentrations was investigated. To our knowledge, this was the first time that an overall assessment of fine PM components was performed simultaneously in three Italian schools.
2. Experimental
2.1. Site Description
The study was carried out in three primary schools located in the city of Rome, as shown in Figure 1. The first school, labeled IAM, is located in a heavily populated residential area, surrounded by low-traffic roads. The second school, labeled IVI, is located at about 3.5 km from the city center; the front facade is 50 m from a square with high traffic intensity during rush hours. The school labeled IDR is located about 8 km from the city center in the eastern part of the city, in a less populated residential area very close to the Rome orbital highway.
The study was performed during the winter (28 November–22 December, 2011) and the spring/summer (14 May–1 June, 2012), in order to assess the seasonal variability of PM composition. All samplings had the duration of 24 hours (starting from 8:00 a.m.) and were carried out simultaneously inside and outside the building.
The three buildings were built following construction standards that did not take into account the recent energy efficiency and heat dispersion protocols; classrooms, common spaces and corridors were equipped with aluminum windows. No air conditioning system was provided.
In all schools, the sampling devices were located in the corridors at the second floor. The rationale for this choice was to minimize the effect of the main entrance, located at the ground floor and often left open during the school time. Moreover, this positioning also had the advantage of reducing the noise impact of the equipment on learning activities as well as to decrease the risk of accidents to the children. The corridors allowed the access to a maximum of five classrooms, each one occupied by about 25 students. The corridors were used by the students to get to the classrooms before the morning lessons, during the snack time, and after the last lesson, at approximately 1:00 p.m. Only the school personnel were allowed to stay close to the sampling devices.
Figure 1.
Location of the three schools under study.
Natural ventilation through open windows was dominating during the spring/summer period. During the winter, heating was ensured during the daytime by radiators located both in the classrooms and in the corridors; in this season, adequate ventilation was ensured through window openings only during the cleaning activities both in the classrooms and in the corridors. Sampling locations and classrooms were cleaned up every morning before the lessons.
Outdoor samplings were performed in the playgrounds, located in front of the facades of all sites.
2.2. PM2.5 Collection
During the winter period, indoor samplings were performed at each one of the three schools by using two PM2.5 samplers operating at the flow rate of 6 L·min−1 (Bravo Asbesto, TCR Tecora) equipped with Teflon and quartz filters, respectively (TEFLO, 37 mm, 2.0 μm pore size and Tissuquartz 2500QAT, 37 mm, PALL Life Sciences). The sampling flow rate of all six indoor samplers was daily checked before and after each sampling period by using an external flow meter.
At all three schools, outdoor samplings on Teflon and quartz filters were performed by using medium-volume systems (mod. Skypost, TCR Tecora) operating at 38 L·min−1 and equipped with PM2.5 selective inlets. The samplers included a sequential unit able to manage up to eight filters in sequence. The sampling flow rate was automatically controlled by the instruments.
The comparability of the results obtained by using medium-volume (MV) and low-volume (LV) samplers was investigated during the preliminary phase of the EXPAH study [26]. Two LVs and one MV sampler were operated side-by-side for seven days at two indoor locations: the first one was an empty laboratory at the facility of C.N.R. Research Area, at the peri-urban site of Montelibretti; the second one was a classroom in an additional “test” school located a few hundred meters from the IDR site. This preliminary study was addressed to determine organic carbon (OC) and elemental carbon (EC) on quartz filters. The comparison study showed a good consistence of the results obtained by comparing the two co-located LV samplers: the difference between the pairs of values ranged from −2% to +3% for OC and from −18% to +4% for EC, whose concentration was much lower. When comparing LV and MV performance, instead, a good consistence was obtained only for EC, in spite of its very low concentration. The difference between the pairs was on average +3.1% at the test school site and −1.2% at the Montelibretti site. In the case of OC, the values obtained with the LV samplers were in all cases higher than those obtained by using the MV sampler. The difference between LV (average of the two co-located samplers) and MV values ranged from −1% to +13% (mean value: +6.7%) at the school test site and from +0.7% to +25%, (mean value: +11.3%) at Montelibretti site. This bad performance of the LV sampler was ascribed to the well-known positive artifact due to the retention of organic vapors, which may adsorb on the filter media and on the collected dust. The extent of this artifact is in inverse proportion to the face velocity, (13.3 cm/s for the LVs, 54.7 cm/s for the MV). To reduce this artifact, during the regular EXPAH campaigns, sampling on quartz filter was carried out by placing a reduction plate above the filter, so as to decrease the collection surface and to raise the airstream face velocity [27].
During the summer period, a novel sampling device (SILENT Sequential Air Sampler, FAI Instruments) operating at the flow rate of 10 L·min−1 was employed for indoor particle collection on Teflon filters at all the three sites. The performance of the Silent sampler was also evaluated, according to the European Standard EN 14907 (2005) criteria [28].
All quartz filters were conditioned, before use, at 600 °C for 3 hours to remove adsorbed organic compounds. Teflon filter were weighted before and after sampling by using a 1 μg sensitivity automated microbalance (mod. ME5, Sartorius AG), after conditioning at 50% R.H. and 20 °C for 24 hours.
2.3. Chemical Characterization
At the end of the sampling campaign, all filters were sealed in plastic Petri dishes and stored at 5 °C until the beginning of the analytical procedures. Quartz filters were analyzed for their organic carbon (OC) and elemental carbon (EC) content by thermo-optical analysis (OCEC Carbon Aerosol Analyzer, Sunset Laboratory), applying the NIOSH (National Institute for Occupational Safety and Health)-quartz temperature protocol.
After the gravimetric analysis, particles collected on Teflon filters were analyzed for their elemental content (Na, Al, Si, S, Cl, K, Ca) by X-ray fluorescence (X-Lab2000, SPECTRO Analytical Instruments). Then, the filters were extracted in deionized water and the solution analyzed for their anionic (chloride, nitrate, and sulfate) and cationic (sodium, ammonium, potassium, magnesium, and calcium) content by ion chromatography (ICS1000, Dionex Corporation). The limits of detection (LOD) and the limits of quantification (LOQ) for the considered chemical species are reported in Table A1 (see Appendix).
Field blanks were provided during all the sampling campaigns and the blank values were properly considered in the calculations. Quality control of the analytical phase was performed by comparing XRF and IC results for Cl/Cl−, S/SO42−, Na/Na+.
A complete validation of the overall analytical method had been previously published; detailed information about the performance of the analytical method are reported in Canepari et al. and cited therein [29].
2.4. Mass Reconstruction
The determination of the above species leads to obtaining the mass closure, which is the correspondence between the gravimetric mass and the sum of the main components. The reconstructed mass was obtained by adding elements, ions, carbonate, organic matter and elemental carbon concentrations: elements (Al, Si, Fe, Mg, K, non-soluble Na and Ca,) were calculated as oxides, multiplying each term by a correction factor for oxygen [30]; carbonate was calculated as the sum of magnesium and calcium multiplied by 2.5 and 1.5, respectively; organic matter was obtained multiplying organic carbon by a factor α that takes into account non carbon atoms in organic molecules. According to Turpin and Lin (2001) [31] α was set to 1.6 for urban site. More detailed information about these algorithms are reported in Perrino et al. (2014) [22].
2.5. Macro-Sources
Five macro-sources were considered as the main contributors to the indoor and outdoor PM: soil, sea, secondary inorganics, traffic and organics. The method used to estimate these sources relies on the concentration of individual PM components and it is extensively reported in Perrino et al. (2014) [22]. Briefly, soil contribution is calculated as the sum of metal oxides concentration (Al, Si, Fe, insoluble K, Mg and Ca, magnesium and calcium carbonates) generally associated with mineral dust. These species can be both of natural and of anthropogenic origin, since they may originate either from soil erosion, caused by the wind, or from road dust re-suspension, caused by the movement of motor vehicles. Sea is determined as Na+ content multiplied by 3.27, to take into account other sea-salt components [32]. Secondary inorganic fraction is calculated as the sum of ammonium, nitrate and non-sea-salt sulfate. Traffic is calculated as the sum of elemental carbon plus an equivalent amount of organic carbon, to consider the contribution of primary organic matter to this source [33]; organics is considered as the remaining organic matter contribution, which includes both secondary organic species produced by gas/particle conversion of volatile compounds and primary components, including bio-aerosol.
3. Results and Discussion
3.1. Meteorological Conditions
The meteorological dataset was recorded at the experimental station of C.N.R.—Institute for Atmospheric Pollution Research in Montelibretti, about 25 km from Rome (coordinates: 42.1063°N, 12.6400°E). The data are expressed as one-hour averages (see Appendix, Figure A1, Figure A2 and Figure A3) and are reported together with the daily back-trajectories of the air masses (HYSPLIT model [34]) (see Appendix, Figure A4).
The first week of the winter campaign was characterized by strong atmospheric stability, high temperature difference between day and night (maxima about 15 °C, minima about 2 °C) and very low wind speed, particularly during the night (daytime maxima below 2 m·s−1). A better mixing of the lower atmosphere occurred during the rest of the period: the second week was characterized by higher temperature during the night (minima around 5 °C), while the temperature decreased during the last week (maxima about 10 °C and minima around 0 °C); wind speed was moderate (maxima in the range 3–6 m·s−1) with the only exception of 13 December, when it was very low.
During the spring/summer campaign, temperature was in the range 8–28 °C. Moderate wind speed was registered during the first week, particularly on 14 May (up to 6 m·s−1) and 17 May. During the second and third week, typical summer conditions, characterized by atmospheric stability during the night (very low wind intensity) and better mixing of the air masses during the day (wind velocity up to 3 m·s−1), were registered every day.
3.2. Mass Concentration
Winter and spring/summer indoor and outdoor PM2.5 mass concentration determined gravimetrically (solid lines) and reconstructed by adding the single analytical determinations (dotted lines) are reported in Figure 2.
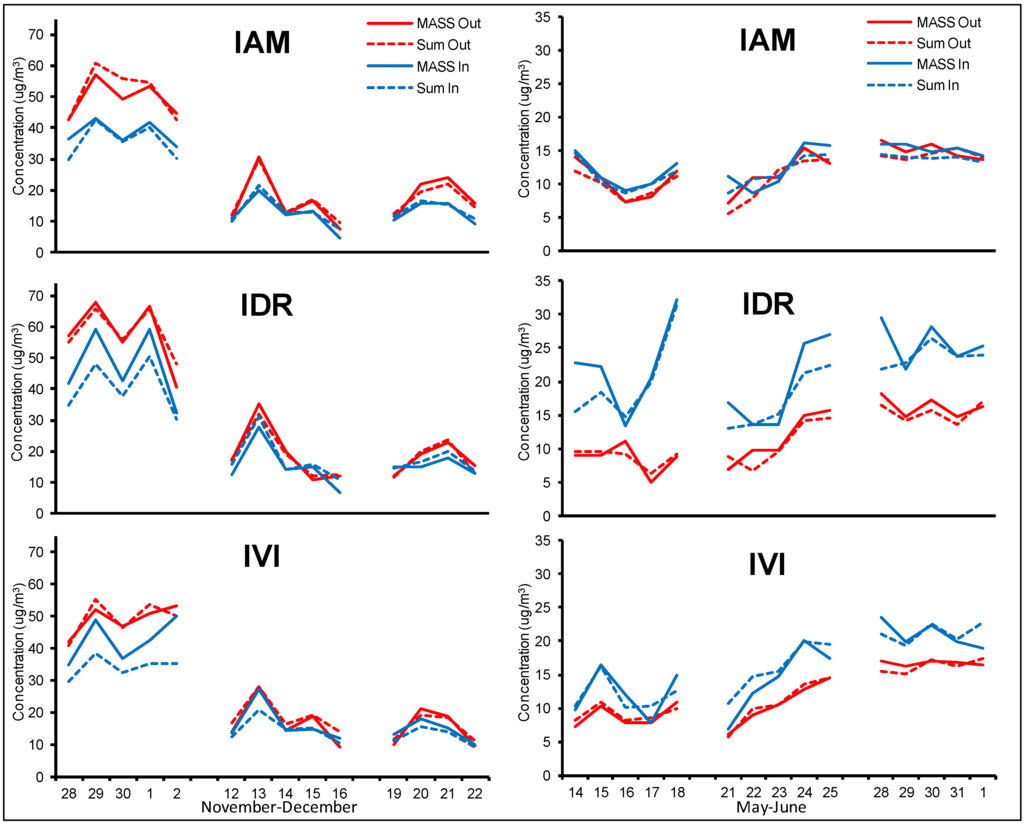
Figure 2.
PM2.5 concentration measured outdoor (red lines) and indoor (blue lines) measured by gravimetry (solid lines) and reconstructed from chemical analyses (dotted lines).
During the winter, the time patterns of outdoor concentrations were highly influenced by the meteorological conditions: during the first week, characterized by very low mixing of the atmosphere, high PM values were measured at all sites, with maximum on 29 November and 1 December; other peaks were registered on 13, 15 and 21 December. IDR was the most polluted site during the first week, while no evident differences among the sites were registered during the second and third week. The regression analysis performed between outdoor concentrations over the whole winter study showed Pearson’s coefficient in the range 0.75–0.93.
Indoor PM concentrations were generally lower than outdoor, but at all the three sites indoor and outdoor concentrations followed the same time pattern. The regression analysis showed Pearson’s coefficient in the range 0.80–0.94. Amato et al. (2014) [35] reported a significant contribution of children activity on particle mass (up to 50%) inside school buildings; however, in our case, the role of children’s movements could have been mitigated by the position of the sampling devices, located in the corridors.
During the spring/summer campaign, outdoor concentrations were quite low over the entire period (below 20 μg/m3), with maxima during the third week at the three sites. The time patterns were clearly different between sites only during the first, windier week, indicating an effect of local sources on dust content. During the last two weeks, a similar time pattern in the mass concentration at the three sites was recorded (Pearson’s coefficient of the regression analysis was in the range 0.79–0.88). As for winter period, indoor values seemed to be influenced by the outdoor concentration, but some differences between sites lead to hypothesize an influence of students’ movements and activities. IAM site showed very similar indoor and outdoor time pattern over the entire period, with slightly higher indoor concentration only during the first week. On the other hand, indoor IDR and, to a less extent, IVI concentrations were always distinctly higher but in good agreement with outdoor values.
A very good correlation between gravimetric data and reconstructed mass was obtained for both indoor and outdoor environments during the whole study. A satisfactory mass closure was also obtained during the summer campaign, when PM mass concentrations were very low, demonstrating the reliability of this approach also for indoor air quality studies. Pearson’s coefficients of the regression analyses between the data series obtained from gravimetric measurements and reconstructed masses (three sites, indoor and outdoor) were in the range 0.97–0.99 during the winter period and 0.75–0.96 during the spring/summer period.
The comparison of the daily mass concentrations with the recent WHO guidelines (25 μg/m3 over 24-hour sampling) showed that indoor PM frequently exceed the air quality standards. During the winter campaign, 100% of exceedances were recorded during the first week for the three schools; one exceedance was also recorded at IDR and IVI sites on 13 December. During spring/summer campaign, WHO standards were exceeded only at IDR school (14% of the days).
3.3. Chemical Composition—Winter Campaign
To improve the interpretation of PM2.5 mass concentration results, the average PM composition and the daily variations of the main PM macro-sources have been investigated.
The outdoor and indoor daily contribution of the five macro-sources (histograms) and the average composition of PM2.5 (pie charts) are reported in Figure 3. As seen for the mass concentration, the daily variations of the five macro-sources were also divided into three weeks to better understand the effect of meteorology.
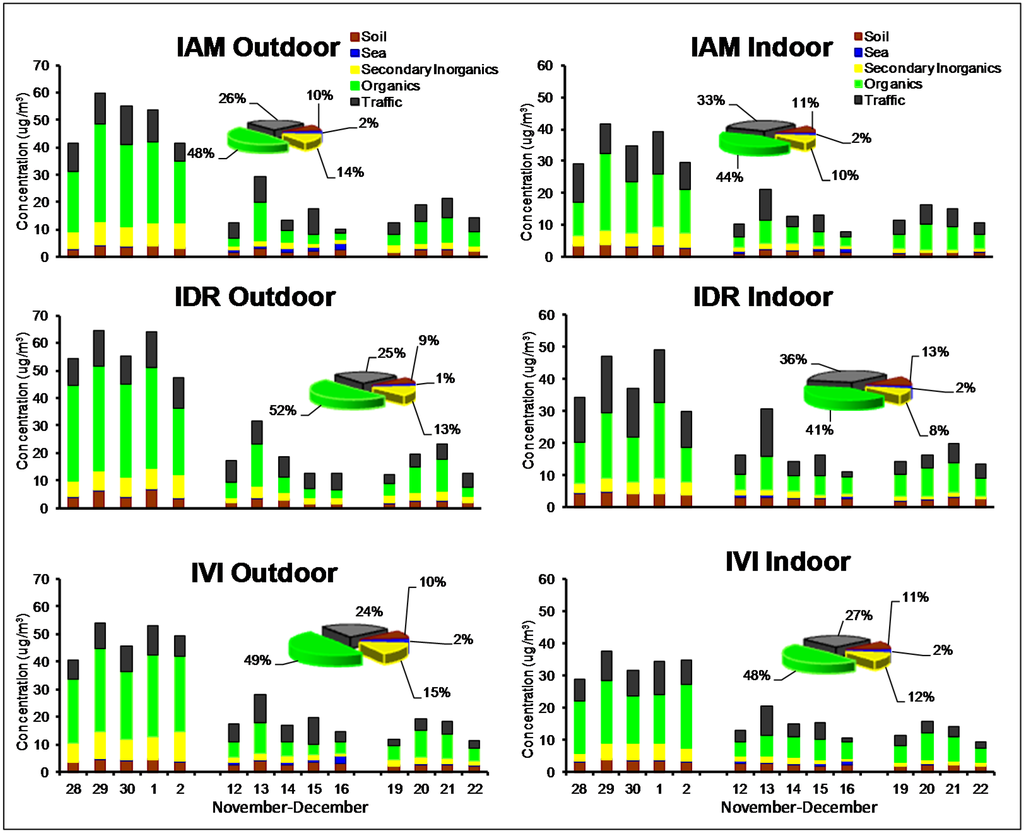
Figure 3.
Outdoor and indoor daily contribution of the five macro-sources (histograms) and average composition of PM2.5 (pie charts) during the winter campaign.
During the winter period, the average composition of outdoor PM was generally similar among the three sites: organics were in the range 48%–52%, traffic between 24% and 26% and secondary inorganics accounted for about 13%–15% of the PM2.5 mass. Soil and sea accounted for about 9%–10% and 1%–2%, respectively.
More information about source intensity can be inferred from the analysis of their daily concentrations.
Table 1 reports the mean concentration and standard deviation of the main PM2.5 components measured during the winter period at the three sites. The data obtained during the first week (atmospheric stability period) are separated from the results obtained during the rest of the winter period. Al and Mg2+ concentrations were not reported because most of the data were below the limit of detection.
During the first week, the most important contribution was due to organic species, with organic carbon component (OC) that accounts, on average, for 21 μg/m3, 25 μg/m3 and 19 μg/m3 for the IAM, IDR and IVI site, respectively (see Table 1). Atmospheric stability observed in this period was responsible for the accumulation of primary organic species originated from typical winter sources, such as domestic heating, and to an enhanced production of organic fraction of secondary origin [36,37]. The role of domestic heating by wood combustion could also explain the increase observed from 28 November to 2 December for soluble potassium (K+), a reliable biomass-burning tracer [38], which showed a time pattern very similar to OC in all sites (see Figure 4 for IAM site and Appendix Figure A5, for IDR and IVI sites). The effect of the ageing of air masses was also responsible for the high contribution of secondary inorganic species [39]: NO3− and SO42- reached concentration as high as 4.7 μg/m3 and 2.5 μg/m3, respectively, and were homogeneously distributed over the sampling areas. EC mean values showed very similar outdoor concentration among the three sites (5.1 μg/m3, 5.5 μg/m3 and 4.2 μg/m3 at IAM, IDR and IVI, respectively), indicating that also combustion species are spatially homogeneous over the sites.

Table 1.
Mean value (M) and standard deviation (σ) of the main chemical components measured indoor and outdoor during the winter period.
| µg/m3 | PM2.5 | Na | Si | S | Cl | K | Ca | Cl− | NO3− | SO42− | Na+ | NH4+ | K+ | Ca2+ | OC | EC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAM 1st week (N=5) | Out | M | 49 | 0.13 | 0.30 | 0.69 | 0.15 | 0.81 | 0.32 | 0.13 | 4.2 | 2.5 | 0.10 | 1.4 | 0.71 | 0.48 | 21 | 5.1 |
| σ | 5.7 | 0.011 | 0.034 | 0.11 | 0.025 | 0.13 | 0.064 | 0.019 | 0.98 | 0.56 | 0.016 | 0.26 | 0.11 | 0.10 | 4.2 | 1.3 | ||
| In | M | 38 | 0.19 | 0.32 | 0.70 | 0.13 | 0.73 | 0.50 | 0.11 | 1.6 | 1.9 | 0.086 | 0.96 | 0.64 | 0.41 | 14 | 5.1 | |
| σ | 3.9 | 0.047 | 0.034 | 0.10 | 0.022 | 0.13 | 0.094 | 0.037 | 0.48 | 0.40 | 0.024 | 0.19 | 0.10 | 0.090 | 3.2 | 0.95 | ||
| IDR 1st week (N=5) | Out | M | 56 | 0.14 | 0.34 | 0.67 | 0.19 | 0.91 | 0.37 | 0.16 | 3.5 | 2.5 | 0.13 | 1.3 | 0.87 | 0.73 | 25 | 5.5 |
| σ | 12.9 | 0.040 | 0.060 | 0.14 | 0.065 | 0.25 | 0.11 | 0.061 | 0.46 | 0.58 | 0.019 | 0.38 | 0.23 | 0.35 | 3.5 | 0.73 | ||
| In | M | 47 | 0.23 | 0.47 | 0.69 | 0.27 | 0.85 | 0.65 | 0.18 | 1.4 | 1.8 | 0.093 | 0.75 | 0.73 | 0.35 | 15 | 7.0 | |
| σ | 11.8 | 0.050 | 0.050 | 0.10 | 0.030 | 0.21 | 0.050 | 0.037 | 0.33 | 0.50 | 0.024 | 0.18 | 0.21 | 0.038 | 4.2 | 1.2 | ||
| IVI 1st week (N=5) | Out | M | 50 | 0.13 | 0.32 | 0.70 | 0.13 | 0.90 | 0.35 | 0.10 | 4.7 | 2.5 | 0.10 | 1.6 | 0.83 | 0.44 | 19 | 4.2 |
| σ | 6 | 0.016 | 0.041 | 0.13 | 0.029 | 0.12 | 0.058 | 0.019 | 1.4 | 0.59 | 0.014 | 0.21 | 0.11 | 0.057 | 2.4 | 0.75 | ||
| In | M | 43 | 0.18 | 0.29 | 0.63 | 0.19 | 0.85 | 0.40 | 0.17 | 1.8 | 1.9 | 0.081 | 0.99 | 0.71 | 0.26 | 13 | 4.0 | |
| σ | 6.8 | 0.032 | 0.025 | 0.30 | 0.034 | 0.085 | 0.069 | 0.045 | 0.65 | 0.55 | 0.011 | 0.29 | 0.11 | 0.063 | 1.8 | 0.65 | ||
| IAM 2nd + 3rd week (N=9) | Out | M | 17 | 0.30 | 0.17 | 0.27 | 0.25 | 0.28 | 0.26 | 0.22 | 1.0 | 0.82 | 0.27 | 0.28 | 0.23 | 0.40 | 5.7 | 2.7 |
| σ | 7.6 | 0.36 | 0.054 | 0.10 | 0.33 | 0.15 | 0.11 | 0.34 | 0.41 | 0.31 | 0.24 | 0.13 | 0.13 | 0.13 | 3.1 | 1.3 | ||
| In | M | 12 | 0.17 | 0.18 | 0.23 | 0.26 | 0.26 | 0.28 | 0.15 | 0.36 | 0.57 | 0.13 | 0.20 | 0.20 | 0.23 | 4.8 | 2.3 | |
| σ | 4.4 | 0.13 | 0.038 | 0.11 | 0.19 | 0.11 | 0.066 | 0.14 | 0.078 | 0.29 | 0.10 | 0.095 | 0.10 | 0.072 | 1.8 | 1.1 | ||
| IDR 2nd + 3rd week (N=9) | Out | M | 19 | 0.09 | 0.15 | 0.24 | 0.080 | 0.33 | 0.21 | 0.07 | 1.5 | 0.74 | 0.10 | 0.45 | 0.21 | 0.34 | 6.5 | 3.0 |
| σ | 7.7 | 0.019 | 0.072 | 0.062 | 0.049 | 0.20 | 0.063 | 0.044 | 0.61 | 0.17 | 0.038 | 0.16 | 0.10 | 0.088 | 2.6 | 1.0 | ||
| In | M | 15 | 0.22 | 0.36 | 0.25 | 0.26 | 0.27 | 0.50 | 0.17 | 0.42 | 0.57 | 0.13 | 0.20 | 0.20 | 0.28 | 6.2 | 2.7 | |
| σ | 5.6 | 0.094 | 0.064 | 0.10 | 0.14 | 0.13 | 0.060 | 0.095 | 0.17 | 0.27 | 0.096 | 0.067 | 0.11 | 0.071 | 2.2 | 1.8 | ||
| IVI 2nd + 3rd week (N=9) | Out | M | 17 | 0.27 | 0.18 | 0.29 | 0.22 | 0.27 | 0.27 | 0.19 | 0.87 | 0.86 | 0.25 | 0.28 | 0.23 | 0.46 | 5.9 | 2.6 |
| σ | 8.1 | 0.33 | 0.051 | 0.11 | 0.34 | 0.14 | 0.092 | 0.35 | 0.39 | 0.34 | 0.23 | 0.12 | 0.13 | 0.099 | 2.1 | 1.3 | ||
| In | M | 15 | 0.19 | 0.20 | 0.25 | 0.23 | 0.26 | 0.34 | 0.18 | 0.49 | 0.63 | 0.16 | 0.25 | 0.21 | 0.25 | 5.0 | 1.9 | |
| σ | 4.9 | 0.12 | 0.040 | 0.11 | 0.19 | 0.11 | 0.054 | 0.14 | 0.19 | 0.29 | 0.12 | 0.078 | 0.095 | 0.047 | 1.3 | 1.1 | ||
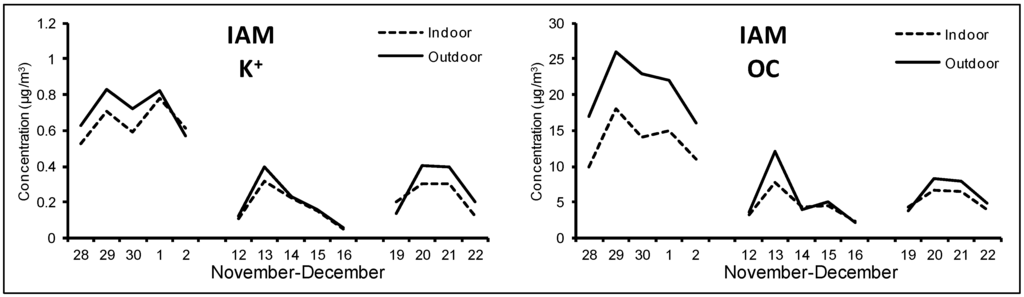
Figure 4.
Outdoor and indoor time pattern of K+ and OC at IAM site during the winter campaign.
The histograms of the second and third week, a period characterized by better mixing conditions, show a general strong decrease for organics and secondary inorganics. An increase of sea-salt was recorded during the second week, when the wind mainly blew from the coast (see Appendix, Figure A4).
The average composition of indoor PM2.5 shows that natural sources (soil and sea) and secondary inorganics did not show consistent variations between sites. Again, organics and traffic were the most important sources, with organics that accounted for 48% at IVI site. Comparing the histograms, it is worth noting that the time pattern of the five main macro-sources was very similar to outdoor, indicating that, in general, indoor values were mainly influenced by outdoor concentration. The effect of the stability conditions of the atmosphere was particularly evident during the first week, when the highest concentrations of all macro-sources were measured.
However, for some components the comparison between indoor and outdoor concentrations seemed to be influenced also by indoor characteristics. Organic carbon was the most abundant component at all sites, with concentration values of 4.8 μg/m3 at IAM, 6.2 μg/m3 at IDR and 5.0 μg/m3 at IVI during the second and third weeks, which strongly increased up to 14 μg/m3, 15 μg/m3 and 13 μg/m3, respectively, during the first week. The analysis of time pattern of OC and K+ confirmed that infiltration from outdoor was probably the main contribution and that, as seen for outdoor, a consistent part of these two species was emitted by the same combustion sources. However, it is worth noting that indoor/outdoor difference measured during the first week was higher for OC than K+, while the same concentration ratio (I/O close to 1) was observed during the residual period for both species. This could be due to a limited contribution of secondary organic aerosol to indoor PM in the examined environment [40,41]; in fact, indoor secondary organic aerosol is generally attributed to cooking activities and to the use of cleaning products, activities that are more specific of homes [42,43]. In addition, according to the fact that indoor samplings were performed in the corridors, the collected particles were only partially influenced by primary organic contribution (textile fibers, skin flakes, hairs, other organic fragments) due to the presence of children [35].
An important decrease in concentration was observed also for some components (NO3− and NH4+) associated to the secondary inorganic fraction. As reported by other authors [44], this decrease is attributable to the depletion of volatile inorganic species at relatively high temperatures occurring indoor during the cold season, which lead to the shift of the solid/gas equilibrium of solid ammonium nitrate towards the gas phase.
As regards components associated to traffic emission (EC), we observed that indoor and outdoor concentrations were very similar and this can be explained by considering that elemental carbon is associated to the submicron fraction of PM, which more easily penetrates inside the building. This result showed that closing the windows scarcely reduce the ability of the finest particles to infiltrate indoor [45].
Re-suspension of the dust deposited indoors, due to the movements of students and school personnel, was observed to be relevant by some authors [46,47]. This can explain the behavior of some crustal components (Si and Ca) that showed higher indoor concentrations at all sites for the entire period of sampling.
As regards sea-spray component (Na+), significant differences between indoor and outdoor values were observed on 16 December when a sea-spray episode occurred (see Appendix, Figure A4).
3.4. Chemical Composition—Spring/Summer Campaign
The daily and average contribution of the investigated macro-sources in indoor and outdoor school environments are shown in Figure 5.
In general, the average composition charts showed a different picture during the spring/summer period, highlighting that the contribution of organics to outdoor PM2.5 was smaller than during the winter period. In addition, an important increase of the secondary inorganics contribution was registered and attributed to the enhanced production of ammonium sulfate [48] by photochemical processes. Traffic source did not show a significant seasonal variation.
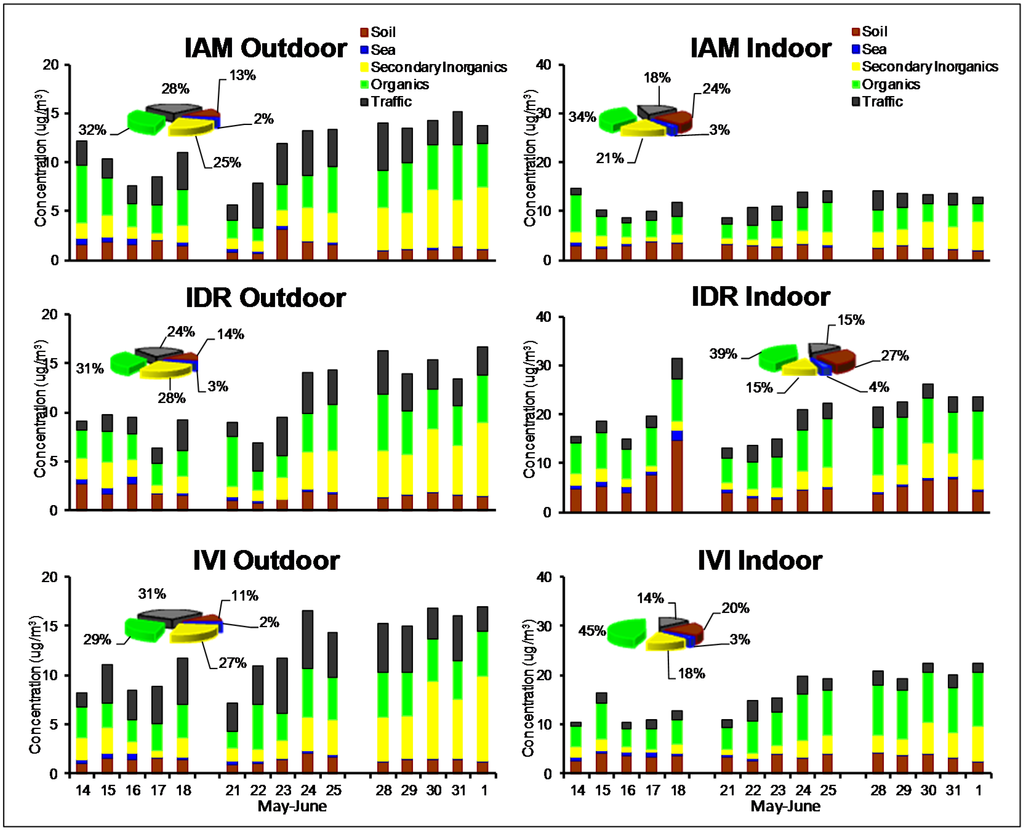
Figure 5.
Outdoor and indoor daily contributions of the five macro-sources (histograms) and average composition of PM2.5 (pie charts) during the spring/summer campaign.
Indoors, the contribution of soil accounted as the second most important source after organics at all sites. Outdoor and indoor daily composition showed a significant difference between the first and second half of the campaign, when a change in the meteorological conditions occurred. The effect of stable conditions is clear on that species produced by secondary processes (organics and secondary inorganics), which showed a concentration increase both outdoors and indoors.
The mean concentration and standard deviations reported in Table 2 for the main components (average of the three weeks) also showed some clear winter-summer differences. Despite the contribution of organics outdoor was between 29% and 32%, a strong decrease was observed in comparison to winter, when it was 48%–52%. Spring/summer outdoor OC was practically identical at all sites (3.3 μg/m3 at IAM, 3.2 μg/m3 at IVI and 3.6 μg/m3 at IDR), while indoor OC concentration exceeded outdoor concentration at IDR and IVI, indicating a clear role of the presence of children and school personnel. This contribution, probably of primary origin and due to re-suspension of settled dust, textile fibers, skin flakes and other biological organic fragments [35], was more evident during the spring/summer campaign, when outdoor OC was lower than during the winter (daily concentrations were in the range 1.7–5.0 μg/m3 during the spring/summer and 3.5–28 μg/m3 during the winter) and thus a lower contribution from outdoor particles is expected.

Table 2.
Mean value (M) and standard deviation (σ) of the main chemical components measured indoor and outdoor during the spring/summer period.
| µg/m3 | PM2.5 | Na | Si | S | Cl | K | Ca | Cl− | NO3− | SO42− | Na+ | NH4+ | K+ | Ca2+ | OC | EC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAM (N=15) | Out | M | 12 | 0.093 | 0.22 | 0.61 | 0.044 | 0.10 | 0.28 | 0.027 | 0.37 | 1.9 | 0.084 | 0.73 | 0.072 | 0.21 | 3.3 | 1.5 |
| σ | 3.1 | 0.081 | 0.078 | 0.40 | 0.010 | 0.033 | 0.12 | 0.011 | 0.24 | 1.2 | 0.051 | 0.54 | 0.024 | 0.075 | 0.89 | 0.53 | ||
| In | M | 13 | 0.14 | 0.38 | 0.67 | 0.10 | 0.14 | 0.76 | 0.063 | 0.22 | 1.8 | 0.10 | 0.67 | 0.088 | 0.48 | 3.3 | 1.1 | |
| σ | 2.8 | 0.038 | 0.061 | 0.38 | 0.012 | 0.025 | 0.19 | 0.017 | 0.070 | 1.1 | 0.039 | 0.48 | 0.022 | 0.16 | 0.86 | 0.42 | ||
| IDR (N=15) | Out | M | 12 | 0.091 | 0.24 | 0.65 | 0.044 | 0.15 | 0.27 | 0.033 | 0.49 | 2.1 | 0.086 | 0.83 | 0.11 | 0.21 | 3.2 | 1.3 |
| σ | 4.1 | 0.052 | 0.079 | 0.38 | 0.016 | 0.11 | 0.089 | 0.018 | 0.32 | 1.3 | 0.064 | 0.61 | 0.12 | 0.059 | 0.91 | 0.53 | ||
| In | M | 22 | 0.22 | 0.69 | 0.73 | 0.21 | 0.26 | 1.4 | 0.21 | 0.55 | 2.0 | 0.24 | 0.68 | 0.20 | 1.0 | 5.9 | 1.4 | |
| σ | 5.9 | 0.12 | 0.36 | 0.43 | 0.11 | 0.11 | 0.83 | 0.15 | 0.24 | 1.3 | 0.13 | 0.44 | 0.094 | 0.55 | 1.2 | 0.4 | ||
| IVI (N=15) | Out | M | 12 | 0.085 | 0.18 | 0.69 | 0.033 | 0.10 | 0.23 | 0.019 | 0.43 | 2.2 | 0.082 | 0.91 | 0.069 | 0.20 | 3.6 | 1.9 |
| σ | 4.1 | 0.035 | 0.043 | 0.47 | 0.009 | 0.025 | 0.043 | 0.006 | 0.34 | 1.6 | 0.054 | 0.76 | 0.019 | 0.069 | 0.87 | 0.57 | ||
| In | M | 16 | 0.17 | 0.42 | 0.73 | 0.11 | 0.16 | 0.74 | 0.067 | 0.38 | 2.0 | 0.13 | 0.71 | 0.10 | 0.67 | 5.3 | 1.1 | |
| σ | 5.2 | 0.058 | 0.092 | 0.44 | 0.028 | 0.028 | 0.17 | 0.026 | 0.18 | 1.4 | 0.068 | 0.54 | 0.022 | 0.21 | 1.8 | 0.42 | ||
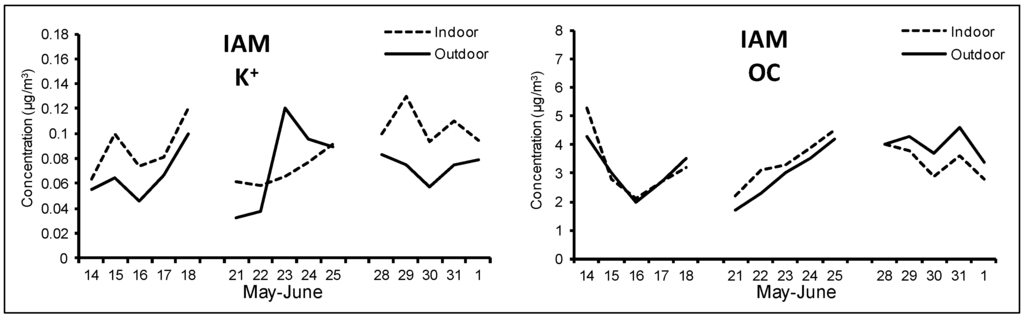
Figure 6.
Outdoor and indoor time pattern for K+ and OC at IAM site during the spring/summer campaign.
Soil also showed higher concentrations indoors, in agreement with a greater dryness of earth crust during the warm season and a consequent more intense re-suspension caused by people movements [49]. Typical components of soil were highly correlated (e.g., Pearson’s coefficient for outdoor Ca and Si was higher than 0.8) and the same behavior was registered for K+. As reported in Figure 6 for IAM site (see also Figure A6 in Appendix for IDR and IVI sites), K+ showed a different time pattern compared to OC, with respect to the winter campaign; this indicate that during the spring/summer season it was mainly of crustal origin, while during the winter the combustion source prevailed. Secondary inorganic components, constituted almost exclusively by ammonium sulfate, showed very similar concentrations both outdoor and indoor. In each site, Pearson coefficient (R2) between these species was always above 0.95, since they are exclusively of outdoor origin and typically associated to the finer fraction of PM. This suggests the occurrence of an effective infiltration process due to a very frequent opening of the windows. Ammonium, also of secondary origin, did not show significant concentration variations in comparison to winter, as this component was in the form of ammonium nitrate during the winter and of ammonium sulfate during the spring/summer.
4. Conclusions
The chemical characterization of PM2.5 collected in three school buildings in Rome showed that, during the cold season, the main contributors to the collected particles, both in indoor and outdoor environments, were organic species and combustion products (mainly from domestic heating and vehicular traffic). During the spring/summer period, outdoor PM was characterized by a more balanced contribution of organic, traffic and secondary inorganic components and by very low concentrations; indoor, the contribution of soil-related species from earth crust erosion and re-suspension of settled dust and components from secondary reactions in atmosphere became more important.
The study also highlighted that air quality in school buildings is strongly dependent from infiltration processes of outdoor air. An easy penetration was registered both during winter and spring/summer period for those components associated with the finest particles (K+, EC, SO42−): this finding was ascribed to building construction characteristics and to the absence of automated air filtration systems. Forty-percent exceedances of WHO PM2.5 standards were recorded during the winter period, which grew up to 100% during the most polluted days. The spring/summer campaign registered a lower number of exceedances (13%), all measured at one of the three sites.
Although adverse health effects of the exposure to air pollutants have been widely studied, it is important to note that levels of most health-related chemical species are not regulated in the current Italian and EU legislations. Given that children spend an important part of their time in enclosed areas (homes and schools) and that children are more sensitive to air pollution effects than adults, more research about air quality in school environment is needed. These results suggest as a first strategy the refurbishment of older school buildings. Secondly, more severe regulatory standards are needed for the reduction of those species recognized to cause the major health risks among the population (e.g., combustion- and traffic-related pollutants).
Acknowledgments
This study was conducted in the framework of LIFE + Project EXPAH (Population Exposure to PAH) funded by the European Union.
The authors would also like to thank the working team of the CNR-IIA: M. Catrambone and T. Sargolini.
Author Contributions
Luca Tofful performed the experiments, analyzed the data and wrote the manuscript. Cinzia Perrino designed the study, coordinated the data-analysis and revised the paper.
Appendix

Table A1.
Limits of detection (LOD) and limits of quantification (LOQ), expressed in µg/m3, of the chemical species analyzed during the study.
| µg/m3 | Medium-Volume | Low-Volume | ||
|---|---|---|---|---|
| LOD | LOQ | LOD | LOQ | |
| Na | 0.02 | 0.07 | 0.09 | 0.28 |
| Si | 0.02 | 0.07 | 0.09 | 0.28 |
| S | 0.02 | 0.07 | 0.09 | 0.28 |
| Cl | 0.02 | 0.07 | 0.09 | 0.28 |
| K | 0.01 | 0.04 | 0.05 | 0.14 |
| Ca | 0.01 | 0.04 | 0.05 | 0.14 |
| Cl- | 0.002 | 0.006 | 0.013 | 0.038 |
| NO3− | 0.005 | 0.015 | 0.031 | 0.094 |
| SO42− | 0.01 | 0.03 | 0.063 | 0.19 |
| Na+ | 0.002 | 0.006 | 0.013 | 0.038 |
| NH4+ | 0.002 | 0.006 | 0.013 | 0.038 |
| K+ | 0.005 | 0.015 | 0.031 | 0.094 |
| Ca2+ | 0.005 | 0.015 | 0.031 | 0.094 |
| OC | 0.4 | 1.2 | 1.6 | 4.8 |
| EC | 0.1 | 0.3 | 0.4 | 1.2 |
Figure A1.
Time pattern of temperature during the winter and spring/summer campaigns.
Figure A2.
Time pattern of atmospheric pressure during the winter and spring/summer campaigns.
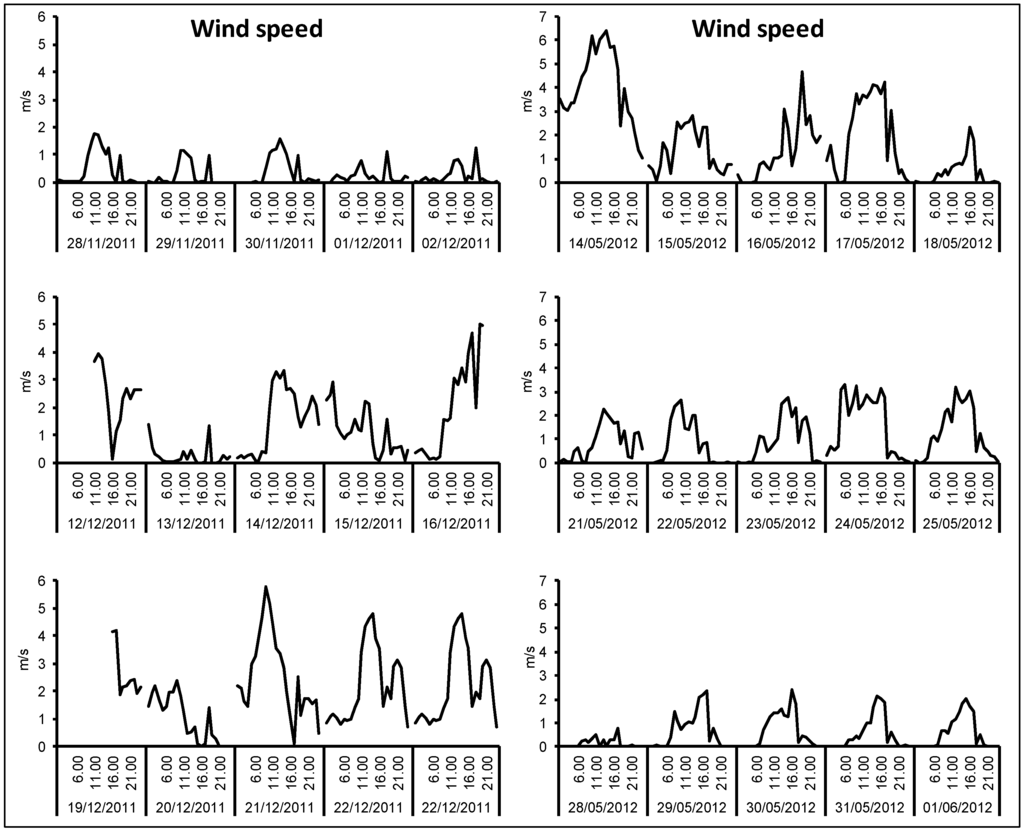
Figure A3.
Time pattern of wind speed during the winter and spring/summer campaigns.
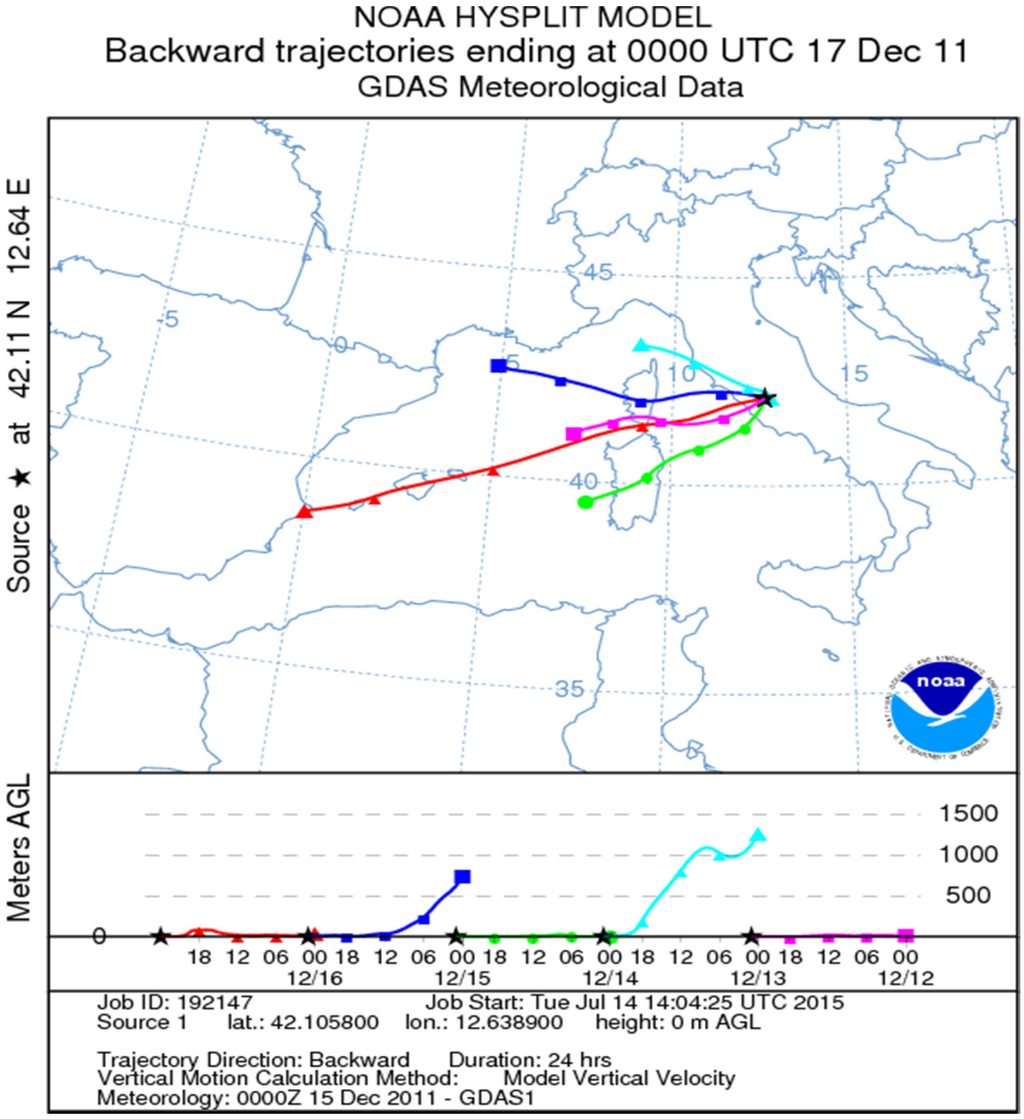
Figure A4.
Backward trajectories of the air masses during 12, 13, 14, 15 and 16 December (duration 24 h). HYSPLIT model [34].
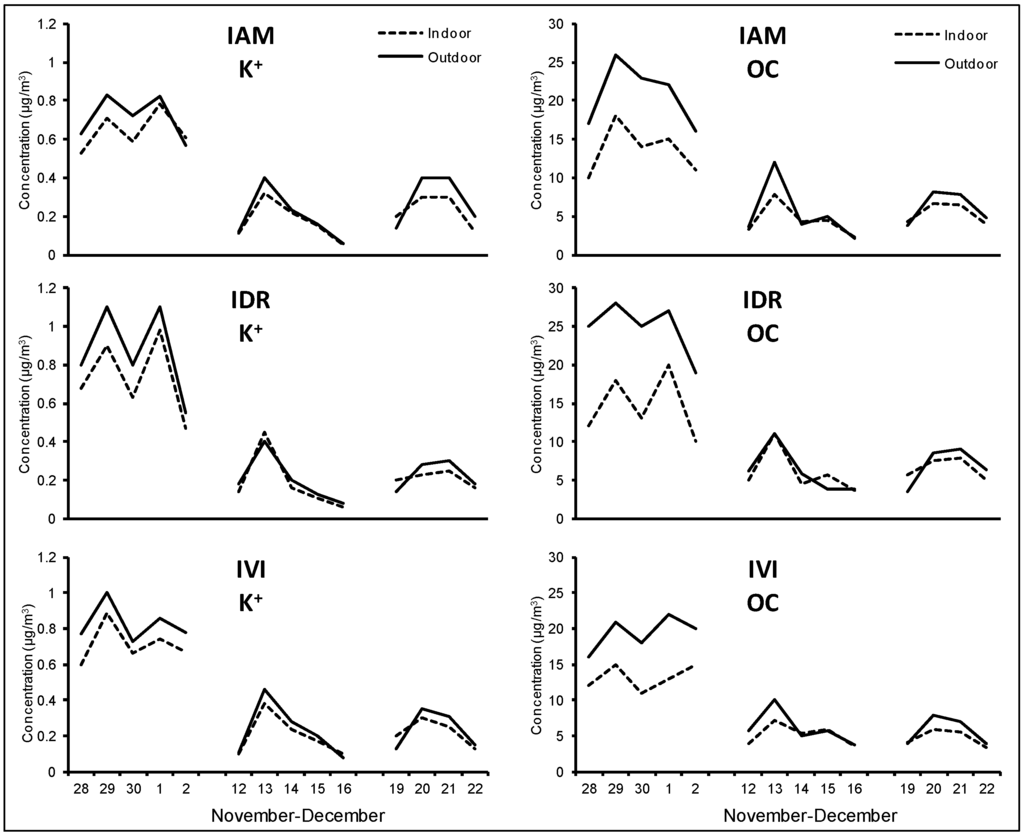
Figure A5.
K+ and OC daily concentrations measured outdoor (solid lines) and indoor (dotted lines) at the three schools during the winter period.
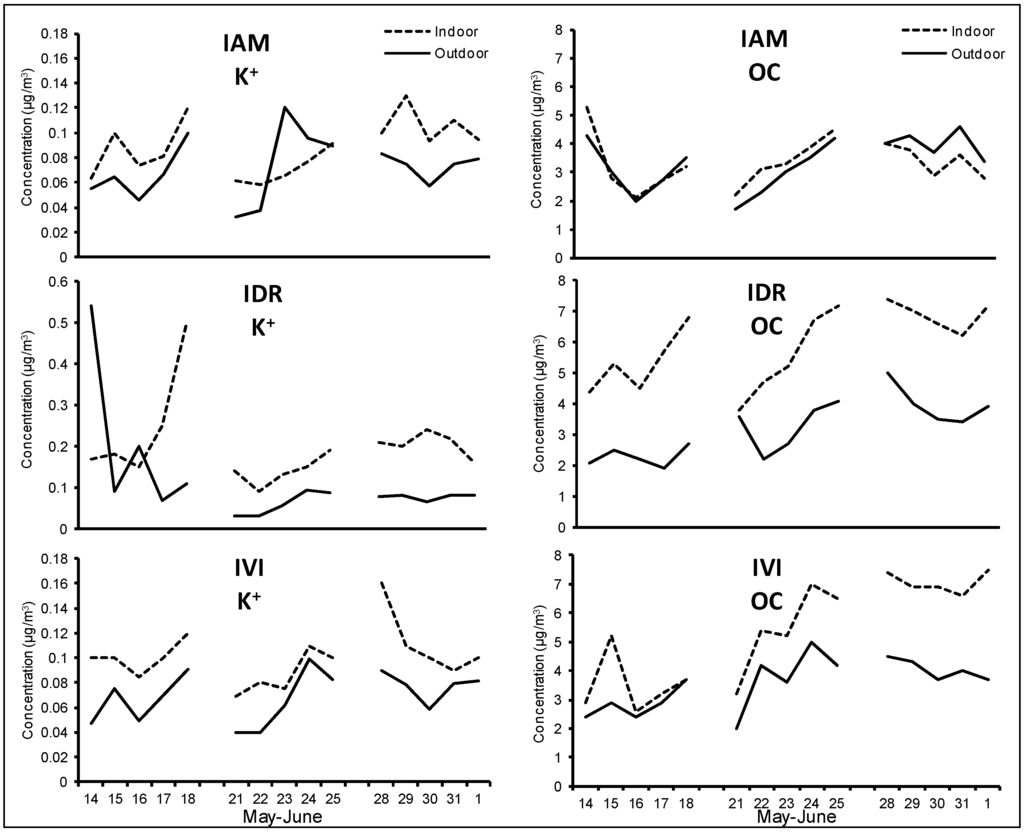
Figure A6.
K+ and OC daily concentrations measured outdoor (solid lines) and indoor (dotted lines) at the three schools during the spring/summer period.
References
- Regional Office for Europe and World Health Organization. Air Quality Guidelines: Global Update 2005. Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide; World Health Organization: Copenaghen, Denmark, 2006. [Google Scholar]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013, 380, 2224–2260. [Google Scholar] [CrossRef]
- Bauer, M.; Moebus, S.; Möhlenkamp, S.; Dragano, N.; Nonnemacher, M.; Fuchsluger, M.; Kessler, C.; Jakobs, H.; Memmesheimer, M.; Erbel, R.; et al. Urban particulate matter air pollution is associated with subclinical atherosclerosis: Results from the HNR (Heinz Nixdorf Recall) study. J. Am. Coll. Cardiol. 2010, 56, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Salam, M.T.; Eckel, S.P.; Breton, C.V.; Gilliland, F.D. Chronic effects of air pollution on respiratory health in Southern California children: Findings from the Southern California children’s health study. J. Thorac. Dis. 2015, 7, 46–58. [Google Scholar] [PubMed]
- Bui, D.S.; Burgess, J.A.; Matheson, M.C.; Erbas, B.; Perret, J.; Morrison, S.; Giles, G.G.; Hopper, J.L.; Thomas, P.S.; Markos, J.; et al. Ambient wood smoke, traffic pollution and adult asthma prevalence and severity. Respirology 2013, 18, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Mills, I.C.; Anderson, H.R.; Cohen, A.; Hansell, A.; Kauffmann, F.; Krämer, U.; Marcon, A.; Perez, L.; Sunyer, J.; et al. Ambient air pollution: A cause for COPD? Eur. Respir. J. 2014, 43, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European study of cohorts for air pollution effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Eze, I.C.; Hemkens, L.G.; Bucher, H.C.; Hoffmann, B.; Schindler, C.; Künzli, N.; Schikowski, T.; Probst-Hensch, N.M. Association between ambient air pollution and diabetes mellitus in Europe and North America: Systematic review and meta-analysis. Environ. Health Persp. 2015, 123, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Berhane, K.; Zhang, Y.; Linn, W.S.; Rappaport, E.B.; Bastain, T.M.; Salam, M.T.; Islam, T.; Lurmann, F.; Gilliland, F.D. The effect of ambient air pollution on exhaled nitric oxide in the children’s health study. Eur. Respir. J. 2011, 37, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Eckel, S.P.; Berhane, K.; Salam, M.T.; Rappaport, E.B.; Linn, W.S.; Bastain, T.M.; Zhang, Y.; Lurmann, F.; Avol, E.L.; Gilliland, F.D. Residential traffic related pollution exposures and exhaled nitric oxide in the children’s health study. Environ. Health Persp. 2011, 119, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III. Epidemiology of fine particulate air pollution and human health: Biologic mechanisms and who’s at risk? Environ. Health Persp. 2000, 108, 713–723. [Google Scholar] [CrossRef]
- Mendell, M.J.; Heath, G.A. Do indoor pollutants and thermal conditions in schools influence student performance? A critical review of the literature. Indoor Air 2005, 15, 27–52. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, C.; Edwards, R.D.; Bayer-Oglesby, L.; Gauderman, W.J.; Ilacqua, V.; Jantunen, M.J.; Lai, H.K.; Nieuwenhuijsen, M.; Kunzli, N. Indoor time-microenvironment-activity patterns in seven regions of Europe. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Schools Indoor Pollution and Health: Observatory Network in Europe (SINPHONIE Project). Available online: http://www.webcitation.org/6aNhsDNde (accessed on 29 July 2015).
- Simoni, M.; Annesi-Maesano, I.; Sigsgaard, T.; Norback, D.; Wieslander, G.; Nystad, W.; Canciani, M.; Sestini, P.; Viegi, G. School air quality related to dry cough, rhinitis, and nasal patency in children. Eur. Respir. J. 2010, 35, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Zauli, S.S.; Colaiacomo, E.; de Maio, F.; Lauriola, P.; Sinisi, L. School environment and children respiratory health: The SEARCH project. Epidemiol. Prev. 2009, 33, 239–241. [Google Scholar]
- GARD-Italy Progetto n°1 (Programma di prevenzione per le scuole dei rischi indoor per malattie respiratorie e allergiche). Available online: http://www.webcitation.org/6aNiRfXZY (accessed on 29 July 2015).
- Progetto CCM Indoor School (Esposizione ad inquinanti indoor: linee guida per la valutazione dei fattori di rischio in ambiente scolastico e definizione delle misure per la tutela della salute respiratoria degli scolari e degli adolescenti). Available online: http://www.webcitation.org/6aNiYEaK7 (accessed on 29 July 2015).
- Linee di indirizzo per la prevenzione nelle scuole dei fattori di rischio indoor per allergie ed asma. Available online: http://www.webcitation.org/6aNihXX2s (accessed on 29 July 2015).
- Lazaridis, M.; Aleksandropoulou, V.; Hanssen, J.E.; Dye, C.; Eleftheriadis, K.; Katsivela, E. Inorganic and carbonaceous components in indoor/outdoor particulate matter in two residential houses in Oslo, Norway. J. Air Waste Manage 2012, 58, 346–356. [Google Scholar] [CrossRef]
- Perrino, C.; Catrambone, M.; Dalla Torre, S.; Rantica, E.; Sargolini, T.; Canepari, S. Seasonal variations in the chemical composition of particulate matter: A case study in the Po Valley. Part I: macro-components and mass closure. Environ. Sci. Pollut. Res. 2014, 21, 3999–4009. [Google Scholar] [CrossRef] [PubMed]
- Perrino, C.; Tofful, L.; Canepari, S. Chemical characterization of indoor and outdoor fine particulate matter in an occupied apartment in Rome, Italy. Indoor Air 2015. [Google Scholar] [CrossRef]
- Population Exposure to PAH (EXPAH project). Available online: https://ricercascientifica.inail.it/expah/ (accessed on 20 July 2015).
- Regional Office for Europe and World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Copenaghen, Denmark, 2010. [Google Scholar]
- Technical Report on Activities Carried Out by CNR-IIA and INAIL - ex ISPESL in the Frame of the EXPAH Project (Actions 3.2 and 3.3). Available online: https://ricercascientifica.inail.it/expah/pblTechRep.asp (accessed on 20 July 2015).
- Viana, M.; Chi, X.; Maenhaut, W.; Cafmeyer, J.; Querol, X.; Alastuey, A.; Mikuška, P.; Večeřa, Z. Influence of sampling artefacts on measured PM, OC, and EC levels in carbonaceous aerosols in an urban area. Aerosol Sci. Tech. 2006, 40, 107–117. [Google Scholar] [CrossRef]
- European Standard EN 14907. Ambient Air Quality-Standard Gravimetric Measurement Method for the Determination of the PM2.5 Mass Fraction of Suspended Particulate Matter. Available online: http://standards.cen.eu/dyn/www/f?p=CENWEB:110:0::::FSP_PROJECT:20497&cs=15F8D99DB6F7D293AEB4A640619A17FB5 (accessed on 20 July 2015).
- Canepari, S.; Perrino, C.; Astolfi, M.L.; Catrambone, M.; Perret, D. Determination of soluble ions and elements in suspended particulate matter: inter-technique comparison of XRF, IC and ICP for sample-by-sample quality control. Talanta 2009, 77, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Marcazzan, G.M.; Vaccaro, S.; Valli, G.; Vecchi, R. Characterisation of PM10 and PM2.5 particulate matter in the ambient air of Milan (Italy). Atmos. Environ. 2001, 35, 4639–4650. [Google Scholar] [CrossRef]
- Turpin, B.J.; Lim, H.J. Species contributions to PM2.5 mass concentrations: Revisiting common assumptions for estimating organic mass. Aerosol Sci. Tech. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Theodosi, C.; Grivas, G.; Zarmpas, P.; Chaloulakou, A.; Mihalopoulos, N. Mass and chemical composition of size-segregated aerosols (PM1, PM2.5, PM10) over Athens, Greece: Local versus regional sources. Atmos. Chem. Phys. 2011, 11, 11895–11911. [Google Scholar]
- Castro, L.M.; Pio, C.A.; Harrison, R.M.; Smith, D.J.T. Carbonaceous aerosol in urban and rural European atmospheres: Estimation of secondary organic carbon concentrations. Atmos. Environ. 1999, 33, 2771–2781. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration (NOAA). Air Resources Laboratory (ARL)—Hybrid Single Particle Lagrangian Integrated Trajectory Model (HYSPLIT). Available online: https://ready.arl.noaa.gov/HYSPLIT.php (accessed on 20 July 2015).
- Amato, F.; Rivas, I.; Viana, M.; Moreno, T.; Bouso, L.; Reche, C.; Querol, X. Sources of indoor and outdoor PM2.5 concentrations in primary schools. Sci. Total Environ. 2014, 490, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Liu, Z.R.; Zhang, J.K.; Hu, B.; Ji, D.S.; Yu, Y.C.; Wang, Y.S. Aerosol physicochemical properties and implications for visibility during an intense haze episode during winter in Beijing. Atmos. Chem. Phys. 2015, 15, 3205–3215. [Google Scholar] [CrossRef]
- Xu, H.; Guinot, B.; Shen, Z.; Ho, K.F.; Niu, X.; Xiao, S.; Huang, R.; Cao, J. Characteristics of organic and elemental carbon in PM2.5 and PM0.25 in indoor and outdoor environments of a middle school: Secondary formation of organic carbon and sources identification. Atmosphere 2015, 6, 361–379. [Google Scholar] [CrossRef]
- Pio, C.A.; Legrand, M.; Alves, C.A.; Oliveira, T.; Afonso, J.; Caseiro, A.; Puxbaum, H.; Sanchez-Ochoa, A.; Gelencsér, A. Chemical composition of atmospheric aerosols during the 2003 summer intense forest fire period. Atmos. Environ. 2008, 42, 7530–7543. [Google Scholar] [CrossRef]
- Perrino, C.; Catrambone, M.; Pietrodangelo, A. Influence of atmospheric stability on the mass concentration and chemical composition of atmospheric particles: A case study in Rome, Italy. Environ. Int. 2008, 34, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Morawska, L.; He, C.R.; Zhang, Y.L.; Ayoko, G.; Cao, M. Characterization of particle number concentrations and PM2.5 in a school: Influence of outdoor air pollution on indoor air. Environ. Sci. Pollut. Res. Int. 2010, 17, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Spengler, J.D. Indoor-outdoor relationships of respirable sulfates and particles. Atmos. Environ. 1981, 15, 335–343. [Google Scholar] [CrossRef]
- Nunes, R.A.O.; Branco, P.T.B.S.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Sousa, S.I.V. Particulate matter in rural and urban nursery schools in Portugal. Environ. Pollut. 2015, 202, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.T.B.S.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Sousa, S.I.V. Indoor air quality in urban nurseries at Porto city: Particulate matter assessment. Atmos. Environ. 2014, 84, 133–143. [Google Scholar] [CrossRef]
- Lunden, M.M.; Revzan, K.L.; Fischer, M.L.; Thatcher, T.L.; Littlejohn, D.; Hering, S.V.; Brown, N.J. The transformation of outdoor ammonium nitrate aerosols in the indoor environment. Atmos. Environ. 2003, 37, 5633–5644. [Google Scholar] [CrossRef]
- Rivas, I.; Viana, M.; Moreno, T.; Bouso, L.; Pandolfi, M.; Alvarez-Pedrerol, M.; Forns, J.; Alastuey, A.; Sunyer, J.; Querol, X. Outdoor infiltration and indoor contribution of UFP and BC, OC, secondary inorganic ions and metals in PM2.5 in schools. Atmos. Environ. 2015, 106, 129–138. [Google Scholar] [CrossRef]
- Tran, D.T.; Alleman, L.Y.; Coddeville, P.; Galloo, J.C. Elemental characterization and source identification of size resolved atmospheric particles in French classrooms. Atmos. Environ. 2012, 54, 250–259. [Google Scholar] [CrossRef]
- Almeida, S.M.; Canha, N.; Silva, A.; do Carmo Freitas, M.; Pegas, P.; Alves, C.; Pio, C.A. Children exposure to atmospheric particles in indoor of Lisbon primary schools. Atmos. Environ. 2011, 45, 7594–7599. [Google Scholar] [CrossRef]
- Eatough, D.J.; Caka, F.M.; Farber, R.J. The conversion of SO2 to sulfate in the atmosphere. Isr. J. Chem. 1994, 34, 301–314. [Google Scholar] [CrossRef]
- Castillo, S.; de la Rosa, J.D.; Sánchez de la Campa, A.M.; González-Castanedo, Y.; Fernández-Caliani, J.C.; Gonzalez, I.; Romero, A. Contribution of mine wastes to atmospheric metal deposition in the surrounding area of an abandoned heavily polluted mining district (Rio Tinto mines, Spain). Sci. Total Environ. 2013, 449, 363–372. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).