Abstract
Spores of filamentous fungi are common biological particles in indoor air that can negatively impact human health, particularly among immunocompromised individuals and patients with chronic respiratory conditions. Airborne viruses represent an equally pervasive threat, with some carrying the potential for pandemic spread, affecting both healthy individuals and the immunosuppressed alike. This study investigated the abundance and diversity of airborne fungal spores in both hospital and residential environments, using custom designed air samplers with or without the presence of negative air ions (NAIs) inside the sampler. The main purpose of investigation was the assessment of biological effects of NAIs on fungal spore viability, deposition, mycelial growth, and sporulation, as well as airborne viral load. The precise assessment of mentioned biological effects is otherwise difficult to carry out due to low concentrations of studied specimens; therefore, specially devised and designed, ion-bioaerosol interaction air samplers were used for prolonged collection of specimens of interest. The total fungal spore concentrations were quantified, and fungal isolates were identified using cultural and microscopic methods, complemented by MALDI-TOF mass spectrometry. Results indicated no significant difference in overall spore concentration between environments or treatments; however, presence of NAIs induced a delay in the sporulation process of Cladosporium herbarum, Aspergillus flavus, and Aspergillus niger within 72 h. These effects of NAIs are for the first time demonstrated in this work; most likely, they are mediated by oxidative stress mechanisms. A parallel experiment demonstrated a substantially reduced concentration of aerosolized equine herpesvirus 1 (EHV-1) DNA within 10–30 min of exposure to NAIs, with more than 98% genomic load reduction beyond natural decay. These new results on the NAIs interaction with a virus, as well as new findings regarding the fungal sporulation, resulted in part from a novel interaction setup designed for experiments with the bioaerosols. Our findings highlight the potential of NAIs as a possible approach for controlling fungal sporulation and reducing airborne viral particle quantities in indoor environments.
1. Introduction
Filamentous fungi are multicellular microorganisms widely distributed in the environment, where they play a crucial role in the decomposition of organic matter. Most filamentous fungi produce large quantities of spores that are readily released into the air, either naturally or under the influence of various environmental and anthropogenic factors. In indoor environments, fungal spores are typically present in low concentrations; however, certain microclimatic conditions such as increased humidity and temperature, poor ventilation, and insufficient lighting can promote their growth and reproduction. As a result, elevated spore concentrations may occur, potentially affecting air quality and posing risks to human health. In immunocompromised individuals (e.g., hematological patients, those with HIV/AIDS, or organ/tissue transplant recipients), as well as patients with chronic respiratory diseases (such as chronic obstructive pulmonary disease, asthma, and cystic fibrosis) and persons with atopic predisposition, inhalation of fungal spores can contribute to the development of various pathological conditions [1]. Fungi of the genera Alternaria, Cladosporium, Aspergillus, and Penicillium are recognized as major triggers of hypersensitivity reactions [2]. Besides allergic asthma, fungal-induced hypersensitivity may also manifest as allergic fungal rhinosinusitis (AFRS), fungal bronchitis, and allergic bronchopulmonary aspergillosis/mycosis (ABPA/M) [3]. Furthermore, Aspergillus fumigatus and members of the Mucoraceae family (Mucor, Rhizopus, Lichtheimia) are known etiological agents of invasive fungal diseases (IFDs), including invasive pulmonary aspergillosis and mucormycosis [4]. For patients at risk of developing IFDs, prophylactic administration of antifungal agents represents a significant preventive strategy. However, long-term antifungal use can lead to cumulative toxicity, limiting their application and increasing the risk of adverse effects. Additionally, both intrinsic and acquired resistance to antifungals significantly complicates the efficacy of prophylaxis and treatment of IFDs [5].
Regarding the airborne viral pathogens, many of them continue to be leading causes of global morbidity and remain a primary target of vaccination efforts. Viruses transmitted in this manner pose a significant public health threat due to their ease of spread through respiratory droplets or aerosols released when infected individuals cough, sneeze, or even speak. These particles can linger in the air and infect others without direct contact. Some of the most relevant airborne viruses include influenza, SARS-CoV-2 (causing COVID-19) and measles, all of which can spread rapidly in crowded or poorly ventilated spaces. As pointed out in [6], improved ventilation in indoor spaces is essential to minimizing health risks from bioaerosols. Often, human activities in indoor spaces where they spend most of their time contribute to indoor air pollution. A typical example are the combustion processes, such as cooking or burning scented candles [6]. Such activities produce nano-sized pollutant particles, able to interact with the indoor air microbiome, modify its composition, and act as extracellular vesicles to airborne microbes. These fine and ultrafine-size pollutants form, together with the bioaerosols, a combined burden to the air ventilation and filtration systems. The high transmissibility of airborne viruses and their potential for severe illness or outbreaks make both preventive and mitigation strategies critical to controlling their impact on global health. Previous studies have demonstrated that airborne viruses such as influenza and coronaviruses can remain suspended for extended periods, contributing to aerosol transmission in confined indoor spaces [7,8]. For example, the half-lives of viable coronaviruses in aerosols had median estimates of approximately 1.1 to 1.2 h, and virus has been shown to remain viable and infectious in aerosols for hours [9]. Moreover, coronaviruses have remained stable on surfaces, such as plastic and stainless steel, with viable virus detected up to 72 h after application to surfaces [9]. While the saliva droplets produced by coughing and sneezing can be rather large, 1–10 μm in diameter, it has been shown that less than a second is needed for a 10 μm droplet to evaporate [10], leaving carrier particle sizes of submicron range.
It has been shown that filtering and electrostatic precipitation, but also the applied air ions, have the ability to accelerate particle deposition [11,12,13]. Ultraviolet (UV) radiation has been shown to possess microbicidal properties; however, it also produces ozone [14]. High Efficiency Particulate Air (HEPA) filters have proven successful in reducing fungal spore concentrations in air [15,16], limited to periods of active operation. Spores retained on the filters can themselves become secondary sources of spore release. Since the air ionization has been shown to enhance particle deposition [13] and some references indicate microbicidal potential of air ions [17], in this study we focus on the assessment of biological effects of NAIs on microorganisms. We strive to perform accurate quantitative analysis of NAIs effects by comparing air sampling without and with the exposure to NAIs, in both hospital and residential environments.
Two types of artificial small air ion generation systems are commonly employed. The first is based on dielectric barrier discharge (DBD), which involves electrical discharge between two electrodes separated by a dielectric layer [18], leading to the production of ozone, highly reactive and, in higher concentrations, harmful to any living organisms, as well as the production of ions of unpredictable chemical composition, which can be harmful. The second type utilizes corona discharge (CD), resulting from the strong electric field in the vicinity of the tips of thin and sharp conductors connected to a high-voltage source, considered clean, as it produces electrons which bond with the existing air molecules. Since the use of very high voltage on metal electrodes leads to ozone formation [19], air ionizers with carbon fibers (CFIs), using substantially lower high-voltages, have recently become very popular. Since we wanted to ensure that the observed effects were not a consequence of exposure to ozone, our custom-designed air samplers are equipped with CFIs [20]. The ozone production of CFIs is negligible. Depending on the electrode polarity, corona-based ionizers may be bipolar, producing both positive air ions (PAIs) and negative air ions (NAIs), or unipolar, emitting only one ion type [21]. Most previous studies investigating the antimicrobial effects of NAIs have focused on bacterial and viral models. The antibacterial effect is primarily attributed to oxidative damage of the cell membrane, leading to increased permeability, while the antiviral effect is related to interference with viral adsorption [17,22]. It has been shown, that the electrostatically enhanced deposition of particles can contribute to more than two times shortened particle half-lives in the viral size range of particles [13,23]. In contrast, data on NAIs effects on filamentous fungi remain limited. Available studies suggest that certain NAIs, such as reactive oxygen species (ROS), induce oxidative stress and damage fungal cells [24]. However, it is essential to distinguish between effects caused by NAIs and those due to ozone [25]; therefore, we have employed the CFIs and, additionally, checked ozone levels by the precise measurements.
The effects of NAIs on abundance and distribution of airborne fungal spores in hospital and residential environments, as well as on spore viability and deposition on metal surfaces were determined. Inhibition of fungal growth and sporulation were investigated specifically for mycelium developed from NAI-treated spores. Additionally, we studied the concentration reduction of aerosolized equine herpesvirus 1 (EHV-1), a surrogate viral particle, the pathogenetic properties of which correlate to the features of the most common human respiratory infections [26].
2. Materials and Methods
2.1. Evaluation of the Effect of NAIs on Airborne Fungal Spores
2.1.1. Air Sampling
The study was conducted in three patient rooms within a hospital and three rooms from a residential apartment. In order to precisely control the sampling procedures and guarantee identical conditions of exposure to NAIs in all cases, as well as conditions of propagation of spores within a sampling unit when there is no exposure, we have previously devised and designed customized ion-bioaerosol interaction chambers (air samplers). The newly designed air samplers, shown in Figure 1, provide optimized conditions for efficient interaction of NAIs with the samples. As can be seen, experimental cylindrical air samplers were made of aluminum tubing, 40 cm in height (Figure S1). The precise specimen sampling by direct collection in a Petri dish and control of a quantity of fungal spores deposited on the setup walls, were enabled by the design of customized air samplers. The interior volume of the samplers is not too large, as the concentrations of bioaerosols tend to be moderate to low, making it difficult to precisely measure deposited samples. The carbon fibers producing the NAIs were intentionally placed at the lower end of the long cylindrical samplers. The prolonged sampling was thereby enabled, providing otherwise identical conditions in the samplers with ion-bioaerosol interaction and those without the exposure to NAIs. Prior to the inclusion of CFIs in air samplers, ozone levels were verfied using Aeroqual 500 with O3 Low Sensor Head (0.0–0.5 ppm). Our measurements showed that the ozone concentrations were below the detection limit of this sensor which is 0.001 ppm (1 ppb); thus, ozone concentration was below 5 ppb which is considered the zero ozone emission threshold. Two samplers were deployed in each room: one containing a carbon fiber-based NAIs generator as sleeve attached at the bottom of cylinder (ion-treated air sample—ITAS), and the other serving as a control sampler without an ion source (non-treated air sample—NTAS). All samplers were positioned within the breathing zone to capture airborne particles, including fungal spores, as they settled naturally under the influence of gravity through the sampling cylinders. At the base of each sampler, 90 mm metal Petri dishes containing Sabouraud dextrose agar (SDA) were placed. Air sampling was conducted once per location and lasted for 6 h. Following the sampling, the Petri dishes and air samplers were sealed and transported under controlled conditions for the subsequent mycological analysis.
2.1.2. Determination of Spore Counts in ITS and NTS Air Samples and Evaluation of NAIs Effects on Fungal Growth and Sporulation
To evaluate the effect of NAIs on enhanced spore deposition on sampler walls, the interior surfaces of the cylinders were rinsed with 20 mL of distilled water. The entire volume of the rinse was divided into five test tubes (4 mL each). After centrifugation at 2000× g RPM for 5 min, 3.6 mL of the supernatant was decanted from each tube. The remaining 400 µL of sediment from each tube was inoculated onto two SDA plates. These culture media, as well as those in the original metal Petri dishes from air samplers, were incubated at 26 °C, and fungal growth was monitored after 48 h, 72 h, and 96 h of incubation. At each time point, Petri dishes were opened under aseptic conditions, and fungal growth was documented by photography. To assess the sporicidal effect of NAIs, and their impact on the growth and sporulation of isolated filamentous fungi, the images were analyzed using the ImageJ software ver. 1.54g (National Institutes of Health, Laboratory for Optical and Computational Instrumentation, Madison, WI, USA).
Fungal identification to the genus/species level was performed after 7 days of growth based on cultural and microscopic characteristics, as well as protein profile analysis using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using extended direct transfer procedure [27]. In brief, peripheral front mycelium was harvested from SDA plates with toothpick, smeared on the target plate (MBT Biotarget 96, Bruker Daltonics, Bremen, Germany) and overlaid with 1 µL 70% formic acid and alpha-cyano-4-hydroxycinnamic acid (HCCA) matrix (Bruker Daltonics, Bremen, Germany). After drying at room temperature samples were analyzed using MALDI-TOF MS Biotyper Sirius one IVD System (Bruker Daltonics, Bremen, Germany) in automatic runs operated by flexControl, ver. 3.4.207.20 (Bruker Daltonics, Bremen, Germany). Identification of filamentous fungi was achieved using MBT Compass software ver. 4.1.100 (Bruker Daltonics, Bremen, Germany), based on the comparison of generated mass spectra with database (MBT Filamentous Fungi Library RUO, ver. 4.0, 247 species/species groups). The log score values > 1.7 indicated reliable species identification.
The total number of fungal spores per cubic meter of air, with and without NAIs exposure, was calculated using a modified version of the Omeliansky formula [28]:
where N is the number of spores per cubic meter of air, a is the number of colonies formed in the metal Petri dish from spontaneously suspended spores, b is the total number of colonies in 10 Petri dishes formed from spores deposited on the sampler walls, c is the surface area of the Petri dish/sampler in cm2, t is the sampling duration in minutes. The results are therefore expressed as colony forming units per cubic meter (CFU/m3).
N = 5(a + b) × 104 (ct)−1
To assess the impact of NAIs on growth delay, the diameter of the mycelia (Dm) was measured. The sporulation index (SI) was calculated as follows [29]:
where Dm is the total mycelial diameter and Ds is the diameter of the mycelial area containing spores. This index was used to estimate the NAIs effect on the delay or suppression of fungal sporulation.
SI = Dm/Ds
2.2. Evaluation of the Effect of NAIs on EHV-1
2.2.1. Experimental Setup
This experiment aimed to assess the impact of ionization on the viral load of aerosolized equine herpesvirus 1 (EHV-1) as a surrogate agent for human pathogens. The study was conducted under controlled conditions using a custom-designed experimental setup tailored for this purpose. In brief, an aqueous solution of viral stock with an initial concentration of 1.38 × 107 viral copies/mL of EHV-1 in minimal essential medium (MEM; Capricorn Scientific, Ebsdorfergrund, Germany) was introduced into a nebulizer NE-U100 (OMRON Healthcare, Kyoto, Japan). The outlet of the nebulizer was positioned below a T branch connector, directing the aerosolized virus through two rubber ducts into separate aluminum cylinders, identical to those used in the fungal spore experiments (denoted as ion-treated viruses—ITV) (Figure S1). A negative control was conducted in a separate cylinder to account for natural viral decay, independent of ionization (non-treated viruses—NTV). At the base of each cylinder, a tightly sealed metal Petri dish was placed to collect aerosol particles that settled over time. Ambient conditions during exposure were approximately 22 °C. The carbon brush ion generator produced an ion concentration over 106 ions/cm3, using a voltage of 220 V based on manufacturer data.
To evaluate the effect of NAIs and natural decay on viral load, two exposure durations were tested independently: 30 min and 10 min. Following each exposure period, aerosol particles deposited in Petri dishes were collected using sterile cotton swabs and suspended in phosphate buffered saline (PBS). The suspensions were briefly vortexed, centrifuged, and subjected to DNA extraction. Viral load was quantified using a standardized assay, and the impact of NAIs was assessed by comparing viral concentrations in the ITV samples to those in the NTV samples expressed as the Cneg/Cion ratio. The experiment was conducted in a biosafety level 2 laminar airflow chamber HERA Safe KSP 12 (Thermo Scientific, Waltham, MA, USA) at all times.
2.2.2. DNA Extraction, EHV-1 Detection and Quantitation
Nucleic acid extraction was performed using the GeneJET Viral DNA/RNA Purification Kit (Thermo Scientific, Waltham, MA, USA), following the manufacturer’s protocol. The method used was the TaqMan Real Time quantitative PCR using a nucleotide probe marked with FAM (fluorophore) and BHQ1 (quencher). The extracted viral DNA was eluted in 50 µL of elution buffer and subsequently analyzed via Real-Time PCR for EHV-1 nucleic acids. The thermocycling and PCR detection were carried out using Gentier mini–Portable Real-Time PCR System (Tianlong, Xi’an, China). The primers, probe, and thermocycling parameters used in this study are all detailed in Table 1, based on the methodology described by Hussey et al. [30]. Quantification was based on a standard curve generated using 10-fold serial dilutions of EHV-1 strain Kentucky (American Bioresearch, Pullman, WA, USA). The initial viral titer was expressed as TCID50/mL and converted to genome copies/mL using a laboratory-established conversion factor derived from parallel titration and qPCR quantification performed in the Laboratory for Virology, Faculty of Veterinary Medicine, University of Belgrade.

Table 1.
Thermocycling conditions, primers, and probe used for Real-Time PCR.
3. Statistical Analysis
To assess differences in spore counts between hospital and residential environments, as well as between ITAS and NTAS samples within the same environment, the Mann–Whitney U test was applied. Fisher’s exact test was used to examine differences in fungal spore distribution between and within environments. Two-way analysis of variance (two-way ANOVA) with post hoc Tukey testing was used to assess differences in Dm and IS during the growth of mycelia formed from NTAS and ITAS spores. Statistical significance was set at p < 0.05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Japan) and Excel (version 16; Microsoft Corp., Redmond, WA, USA) for Windows 10 (Microsoft Corp., Redmond, WA, USA). For the virological dataset, viral load reduction was assessed by comparing DNA copy numbers (copies/mL) between ion-treated (ITV) and non-treated (NTV) groups at two exposure durations (10 and 30 min). Given the small sample size and non-parametric nature of qPCR-based copy number data, viral load values were log10-transformed to enable valid comparisons. Percentage reductions were calculated relative to the initial concentration and to the control group (NTV). All calculations were performed using Microsoft Excel (v16, Microsoft Corp., Redmond, WA, USA).
4. Results
4.1. Effect of NAIs on Airborne Fungal Spores
In the NTAS of the hospital environment, the fungal spore concentration ranged from 9 to 22 CFU/m3 (median of 13 CFU/m3), while in the ITAS of the same environment, it ranged from 11 to 28 CFU/m3 (median of 11 CFU/m3) (p > 0.05) (Figure 1). In the residential NTAS the spore concentration ranged from 9 to 22 CFU/m3 (median 17 CFU/m3), whereas in the ITAS, it ranged from 5 to 11 CFU/m3 (median 9 CFU/m3) (p > 0.05) (Figure 1). No statistically significant differences in fungal spore concentrations were observed between the hospital and residential environments (p > 0.05). The distribution of fungal spores in both settings is presented in Table 2. In the hospital environment, Cladosporium herbarum (41.9%) and Cladosporium cladosporioides (18.6%) spores predominated, while in the residential environment, Aspergillus flavus (24.2%) and Aspergillus niger (18.2%) were dominant (p < 0.05). Within each environment, no significant differences in spore distribution were observed between NTAS and ITAS (p > 0.05).
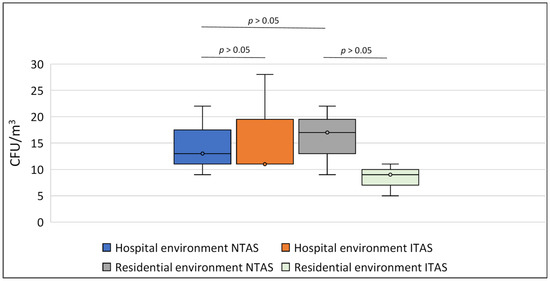
Figure 1.
Number of airborne spores (expressed as CFU/m3) isolated from NTAS and ITAS from hospital and residential environments. Statistical analysis revealed no significant differences in spore counts between NTAS and ITAS within the same environment, nor between the residential and hospital settings (Mann-Whitney U test). NTAS: Non-treated Air Sample; ITAS: Ion-treated Air Sample.

Table 2.
Distribution of fungal spores detected in NTAS and ITAS from hospital and residential environments.
Since C. herbarum spores were the most prevalent in the hospital environment and A. flavus and A. niger (hereafter presented as A. flavus/A. niger) were predominant in the residential environment, Dm and SI were analyzed only for these fungal species. After 48 h, 72 h, and 96 h, the average Dm of C. herbarum grown from NTAS spores in the hospital environment was 7 mm, 10.7 mm, and 13.5 mm, respectively, while that of C. herbarum grown from ITAS spores was 4.4 mm, 9.1 mm, and 12 mm (p > 0.05) (Figure 2 and Figure 3). During the same time intervals, the average Dm of A. flavus/A. niger grown from NTAS spores in the residential environment was 8.2 mm, 17.1 mm, and 30.7 mm (Figure 2 and Figure 4), while the corresponding diameters for A. flavus/A. niger grown from ITAS spores were 6.1 mm, 15.9 mm, and 31.5 mm (p > 0.05) (Figure 2 and Figure 4).
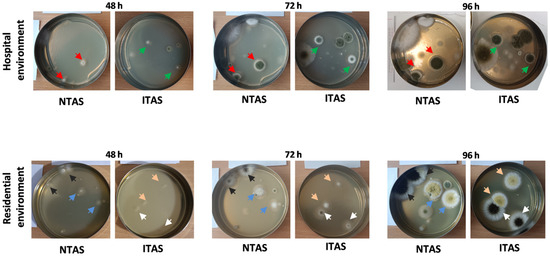
Figure 2.
Representative fungal cultures isolated from NTAS and ITAS in hospital and residential environments after 48, 72, and 96 h of incubation. Red arrows indicate C. herbarum mycelia developed from NTAS spores, while green arrows indicate mycelia originating from ITAS spores. Black and blue arrows mark A. niger and A. flavus mycelia developed from NTAS spores, respectively. White and pink arrows indicate A. niger and A. flavus mycelia formed from ITAS spores, respectively. NTAS: Non-treated Air Sample; ITAS: Ion-treated Air Sample.
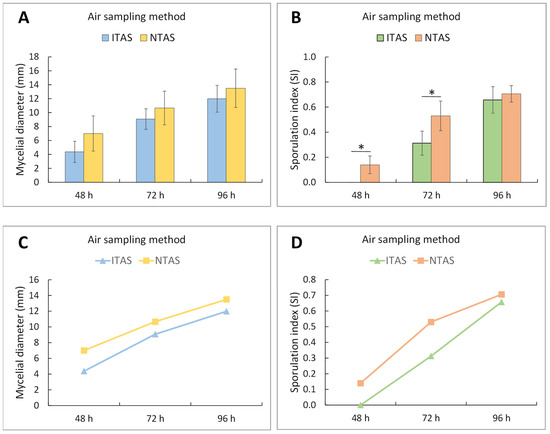
Figure 3.
Two-way ANOVA with post hoc Tukey test for diameter and sporulation index of C. herbarum mycelia after 48, 72, and 96 h of incubation, developed from spores in NTAS and ITAS of hospital environment. (A,C) Mean values of mycelial diameter (±SD). Both incubation time (F = 41.41; p < 0.001) and air sampling method (F = 9.04; p < 0.01) had significant effects on mycelial diameter, while no interaction was observed between these factors (F = 0.32; p = 0.73). No statistically significant differences between groups were found at any individual time point. However, larger mycelial diameters were observed in C. herbarum mycelia derived from NTAS samples. (B,D) Mean values of the sporulation index (±SD). Both incubation time (F = 193.95; p < 0.001) and air sampling method (F = 28.24; p < 0.001) significantly affected sporulation, with a significant interaction between these factors. Statistically significant differences between groups were detected after 48 and 72 h of incubation. NTAS: Non-treated Air Sample; ITAS: Ion-treated Air Sample; SD: Standard Deviation; The asterisk represents p < 0.05.
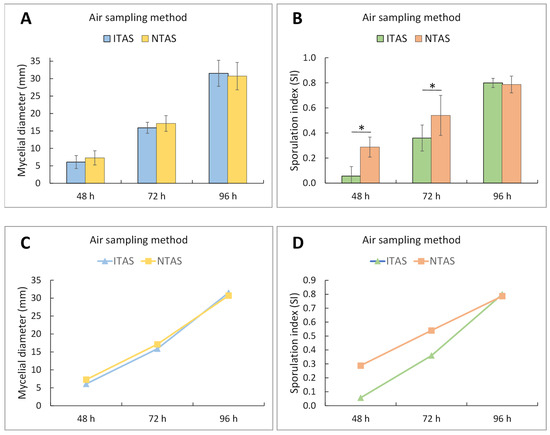
Figure 4.
Two-way ANOVA with post hoc Tukey test for diameter and sporulation index of A. niger/A. flavus mycelia after 48, 72, and 96 h of incubation, developed from spores in NTAS and ITAS of residential environment. (A,C) Mean values of mycelial diameter (±SD). Incubation time had a significant effect (F = 217.31; p < 0.001), while the air sampling method showed no significant influence on mycelial diameter (F = 0.33; p = 0.57). No interaction was observed between these factors. No statistically significant differences between groups were found at any time point. (B,D) Mean values of the sporulation index (±SD). Both incubation time (F = 111.18; p < 0.001) and air sampling method (F = 15.24; p < 0.001) significantly affected sporulation, with a significant interaction between these factors. Statistically significant differences between groups were detected after 48 and 72 h of incubation. NTAS: Non-treated Air Sample; ITAS: Ion-treated Air Sample. SD: Standard Deviation; The asterisk represents p < 0.05.
During the first 72 h of growth, a marked delay in the sporulation process was observed in ITAS mycelia in both hospital and residential environments (C. herbarum SI at 48 h: 0.00; at 72 h: 0.31; A. flavus/A. niger SI at 48 h: 0.06; at 72 h: 0.36) compared to NTAS mycelia (C. herbarum SI at 48 h: 0.14; at 72 h: 0.53; A. flavus/A. niger SI at 48 h: 0.30; at 72 h: 0.54) (Figure 2, Figure 3 and Figure 4). After 96 h, the difference in SI values between ITAS mycelia (C. herbarum SI: 0.66; A. flavus/A. niger SI: 0.80) and NTAS mycelia (C. herbarum SI: 0.71; A. flavus/A. niger SI: 0.79) was not statistically significant (p > 0.05) (Figure 2, Figure 3 and Figure 4).
4.2. Effect of NAIs on Aerosolized EHV-1
The effect of ionization on airborne viral load reduction was assessed by comparing the natural decay of the virus in the control chamber with the reduction observed in the ionized chamber (Figure 5). Therefore, the used experimental method for viral loads was somewhat indirect. It involved the measurement of the concentration of viral copies after a fixed time, with the same initial viral concentration, and with different speeds of decay (natural decay versus the ion-enhanced decay). The result is expressed as an additional percentage of concentration decrease beyond the natural decay. In the control chamber, where no ionization was applied, the viral load naturally declined over time, whereas in the case of applied ionization there was an ion-enhanced decline. After 30 min of natural decline, the concentration of viral copies decreased from an initial 1.38 × 107 copies/mL to 2.089 × 106 copies/mL, corresponding to an 84.86% reduction. In the subsequent shorter, 10-min experiment, the viral load was further reduced to 3.43 × 105 copies/mL, reflecting a 97.51% overall reduction.
Figure 5.
The viral load reduction (%) can be viewed as regards two parameters: exposure time and presence of ionization. Intriguingly, the time of exposure did not affect the reduction of viral concentration.
In contrast, when exposed to air ions, the viral load decline progressed at significantly increased rate. After 30 min of ionization, the viral load decreased from 1.38 × 107 copies/mL to 2.11 × 104 copies/mL, representing a 99.85% total reduction from the initial stock. Similarly, in the additional 10-min experiment, ionization led to a decline to 6.45 × 103 copies/mL, corresponding to a 99.95% total reduction.
To quantify the specific effect of ionization beyond natural decay, the viral loads from the control and ionized chambers were directly compared. In the 30-min experiment, the control chamber retained 2.089 × 106 copies/mL, while the ionized chamber contained only 2.11 × 104 copies/mL. This indicates that ionization alone contributed to an additional 98.99% reduction beyond what was achieved through natural decay. Similarly, in the 10-min experiment, the control chamber retained 3.43 × 105 copies/mL, whereas ionization reduced the viral load further to 6.45 × 103 copies/mL, demonstrating an additional reduction due to ionization beyond natural decay.
These results highlight the marked impact of ionization in reducing airborne viral load, with reductions far exceeding those observed through natural decay alone. 98.12% reduction beyond natural decay.
The outcomes reflect reductions in qPCR-detectable viral genomes, which may not directly correspond to viral infectivity. As formal statistical analysis was not applied and biological replication was not performed, the findings should be regarded as preliminary observations under controlled experimental conditions.
5. Discussion
The presence and concentration of spores of specific fungal species in indoor and outdoor air are largely influenced by the season and climatic zone [31]. The highest concentrations are typically recorded during autumn, while the lowest occur in winter months [31,32,33,34]. The results of our investigation, conducted in Belgrade during January, are partially consistent with findings from previous studies performed in geographic regions with similar climatic conditions [31,32]. It should be emphasized that the method used for spore concentration estimation provides only a rough approximation, as its accuracy is significantly affected by particle size and the dynamics of surrounding air currents [28].
Although our study did not confirm a statistically significant difference in total airborne fungal spore concentration between hospital and residential environments, a difference in their distribution was observed. A higher prevalence of Cladosporium cladosporioides and Cladosporium herbarum spores in hospital environments may be attributed to their ability to thrive under diverse microclimatic conditions, including wide temperature and humidity range. This adaptability enables these species to commonly persist indoors, particularly in areas with elevated moisture levels or in locations prone to dampness, such as bathrooms, basements, and window surroundings. In addition, an important source of Cladosporium spp. spores in indoor air is the ventilation system, as fungal mycelia are frequently found on fan surfaces, within air ducts, and on the fins of cooling evaporators [35]. It is important to note that the hospital included in the study is not fully conventionally air conditioned and allows for natural ventilation through openable windows. Additionally, in two out of three patient rooms included in our study, air samplers were placed near windows, which may further explain the higher frequency of Cladosporium spp. spores in the hospital environment.
In contrast, spores of Aspergillus flavus and Aspergillus niger were predominantly detected in the residential setting. House plants represent a significant ecological niche for the growth of these fungi [36]. They also contribute to the decomposition of organic matter such as food residues, cellulose-based materials (e.g., paper, cardboard), and fabrics [37]. Given that air samplers in the apartment were placed in the living room, bedroom, and kitchen, the dominance of Aspergillus spp. spores in the air of the residential environment was expected.
The use of modern carbon fiber-based ionizers results in negligible or entirely absent ozone production. The absence of ozone in case of the CFIs we used was confirmed by the ozone concentration measurements. A voltage of 5 kV applied to carbon fibers forms a strong electric field at the fiber tips, leading to the emission of electrons into the immediate surroundings. These free electrons, accelerated by the electric field, collide with ambient gas molecules, producing various negative air ions (NAIs), including superoxide (O2−), nitrate (NO2−), nitrite (NO3−), and bicarbonate (HCO3−) anions [38].
Subsequent evolution of NAIs involves hydration through the attachment of 20–30 water molecules, resulting in the formation of cluster anions—stable structures that significantly prolong the lifespan of NAIs. These hydrated cluster ions can bind to airborne particles [39,40], facilitating their more rapid sedimentation. Aligned with the previous observations, ionization leadsto increasing particle deposition through electrostatic interactions [23]. Specifically, ions attach to airborne particles and induce electrostatic chargesthat facilitate interactions between particles, and particle interactions with surroundings. Recent studiesdelivered practical evidence in support of this mechanism. For example, Čereška et al. [41] indicated that introducing NAIs into a controlled chamber leads to considerableincrease in agglomeration of particulate matter (PM), especially for particles smaller than 1 μm. The authors found that using ion generators resulted in up to a 5% improvement in particle agglomeration outcomes, while ion concentration and airflow rate influenced the efficiency. Furthermore, Grinshpun et al. [13] studied the use of NAIs to improve submicron aerosol particle deposition rates. Campler et al. [42] observed numerical trends indicating that ionization enhanced particle deposition. Insignificant spore deposition on the inner surfaces of air sampling devices is attributed to their considerable size, which resulted in their prompt settling at the bottom of the samplers. However, NAIs affected spore germination and growth.
Although there was no substantial overall difference in total airborne fungal spore counts between NTAS and ITAS in both hospital and residential settings, a paradoxical observation was made in one hospital room, where the concentration of spores was higher in the ITAS compared to the NTAS. Considering that Cladosporium spp. spores are generally larger and heavier, their higher rate of deposition in comparison with the smaller and lighter Aspergillus spp. spores probably contributed to the observed outcome.
Airborne fungi and viruses are key bioaerosol components that can significantly impact indoor and outdoor air quality. Their presence is influenced by environmental conditions such as humidity, temperature, and human activity. Both entities pose their respective risks on human health, acting as allergens or respiratory irritants, or frank infectious risks. Importantly, interactions between fungi and viruses may modulate their persistence or pathogenicity; e.g., when viruses attach to larger fungal particles, the effective droplet or particle size increases, potentially influencing how long they remain airborne and where they deposit in the respiratory tract. Together, these agents contribute to the biological load of the air and are important indicators of bioaerosol-related health risks.
In this study, the EHV-1 was used as a proxy for the common airborne transmitted viruses, as it possesses a swathe of similar pathogenetic properties to corresponding illness in humans [43], which correlate to the features of the most common respiratory human infections [26]. As observed in the fungal spore experiments, we propose that, in our experimental setup NAIs effect has impinged on lingering virus aerosol, enhancing particulate settling. We would like to emphasize that experimental conditions in virological analyses differed from real-world scenarios. Assessing the viability of viral particles would provide more meaningful insights into infectivity in future studies which should include plaque assays or TCID50 evaluations to determine the infectious potential of viruses. Although, the results may vary depending on the investigated viral pathogen.
Our findings suggest that NAIs have a limited effect on inhibiting spore germination and slowing mycelial growth. Although a delay in the growth of C. herbarum, A. flavus, and A. niger mycelia was observed within the first 48 h, this effect was not sustained during the following 24-h period. In fact, after 96 h, the diameters of C. herbarum mycelia grown from the ITAS were approximately equal to those in the NTAS, while A. flavus and A. niger mycelia from the ITAS even showed greater diameters compared to those in the NTAS. It is plausible that exposure to stress factors such as NAIs activates adaptive mechanisms in fungi, thereby accelerating their growth processes.
Although NAIs did not significantly inhibit mycelial growth, our results indicate that they delay the sporulation process in both black pigmented fungi (Cladosporium herbarum) and hyaline filamentous fungi (A. flavus and A. niger). However, it is important to emphasize that no inhibitory effects on sporulation were observed for C. cladosporioides, nor for other species that were isolated in significantly smaller numbers, such as Aspergillus versicolor, Aspergillus calidoustus, and Penicillium chrysogenum. Interestingly, spores of C. cladosporioides predominantly contain dihydroxynaphthalene (DHN) melanin, a molecule that provides protection against various environmental stressors, including UV radiation and reactive oxygen species (ROS) [44,45]. In contrast, the spores of C. herbarum, A. flavus, and A. niger contain significantly higher concentrations of dihydroxyphenylalanine (DOPA) melanin [46]. Based on these findings, it may be hypothesized that NAIs promote the oxidation of DOPA melanin, resulting in the generation of ROS that damage and/or disrupt the normal metabolic processes of fungal cells, ultimately leading to the inhibition of sporulation observed in our study [47]. An alternative mechanism through which NAIs may inhibit sporulation in A. flavus and A. niger is via modulation of gene expression involved in conidiogenesis. It has been demonstrated that ROS, including H2O2 formed enzymatically and/or non-enzymatically from certain NAIs (e.g., O2−), induce enhanced expression of antioxidant genes (CAT, GTX, and SOD) and their regulators (Skn7 and Yap1), as part of the oxidative stress response in some Aspergillus spp. [48]. The transcription factor brlA has been identified as a central regulator of conidiogenesis, particularly during the initiation of conidiophore formation, when its expression reaches a peak [49,50]. To summarize, the delayed sporulation after NAI exposure was observed in Aspergillus flavus, Aspergillus niger, and Cladosporium herbarum, during the 72-h growth period following spore treatment. This indicates that NAIs can temporarily inhibit sporulation in these fungal species.
It is plausible that this adaptive response leads to suppression of the expression of conidiogenesis-related genes, including brlA, as the cell prioritizes the activation of detoxification pathways and maintenance of redox homeostasis. Consequently, transcription of factors involved in differentiation and sporulation may be delayed or inhibited until oxidative stress is mitigated. Furthermore, oxidative stress has been shown to induce elevated expression of the antioxidant transcription factor mtfA [51], which, according to previous reports, results in downstream repression of brlA gene expression—another potential explanation for our observed outcomes [49].
Although our results indicate a delay in sporulation and, to some extent, inhibited mycelial growth of certain filamentous fungi developed from NAIs-treated spores, several limitations of this study should be considered. First, the air sampling was performed over a relatively short period and under static indoor conditions, which may not fully capture the variability of airborne fungal concentrations over time or in dynamic environments with frequent human activity or ventilation changes. Second, the study was limited to a small number of rooms and locations, which constrains the generalizability of the findings across broader building types, climates, and geographic settings. Third, the quantification of airborne spores using passive sedimentation and the modified Omeliansky method provides only an approximate estimation of spore concentrations, as this technique is sensitive to particle size, sedimentation velocity, and ambient air currents. Fourth, the identification of fungi was conducted using morphological and MALDI-TOF-based techniques, which, although acceptable, may lack the resolution of full genomic sequencing for accurate species-level differentiation, particularly for cryptic or morphologically similar taxa. Additionally, while the observed delay in sporulation is suggestive of a biological response to NAIs exposure, the exact cellular and molecular mechanisms remain unclear. No direct measurements of oxidative stress markers or gene expression were performed to validate the hypothesized pathways involving ROS production and transcriptional regulation. Lastly, potential interactions between NAIs and other indoor air pollutants (e.g., VOCs, particulates) were not explored and may influence the effectiveness of ionization-based interventions in real-world applications.
6. Conclusions
The effects of NAIs on the germination of fungal spores exhibited a measurable inhibitory result in both hospital and residential environments. The most pronounced effect was the inhibition of sporulation in mycelia developed from NAIs-treated spores. The most plausible mechanism is ROS-mediated, resulting from ROS produced by NAIs. However, while NAIs can affect fungal development, their impact on overall airborne spore concentration was not significant under the tested conditions.
Regarding ion effect on aerosolized viruses, overall, ionization resulted in a substantial reduction in airborne EHV-1 genomic content under controlled conditions. A 10-min ionization treatment may be sufficient for significant viral reduction, whereas a 30-min treatment ensures near-total elimination of viral particles. However, studying various other timeframes would provide a more comprehensive understanding of the optimal duration for ionization, as different conditions and viral loads may require adjustments to maximize efficiency.
Future research should aim to further investigate the influence of NAIs on the morphological, structural, and genetic characteristics of fungi. Such studies could clarify the underlying mechanisms of action and evaluate the potential for broader antimicrobial applications of NAIs. Further work is needed to confirm viral inactivation, optimize exposure durations, and assess broader applicability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos16080896/s1, Figure S1: Experimental air samplers without (left) and with (right) a source of NAIs.
Author Contributions
Conceptualization, S.M., A.R. and P.K.; methodology, S.M., A.I. and M.J.; validation, P.K., A.I. and I.A.; formal analysis, S.M., A.R. and M.J.; investigation, S.M., A.R., M.J. and P.K.; resources, B.G., A.S., G.P. and M.K.; writing—original draft preparation, S.M., A.R. and M.J.; writing—review and editing, A.I., I.A. and J.A.S.; visualization, S.M.; supervision, P.K. and I.A.; project administration, J.T. and S.D.; funding acquisition, P.K. and J.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Fund of the Republic of Serbia, Green program of cooperation between science and industry, grant no. 5661, acronym: IonCleanTech, and by the Ministry of Science, Technological Development, and Innovations of the Republic of Serbia through two grant agreements with the University of Belgrade–Faculty of Pharmacy, no. 451-03-136/2025-03/200161 and no 451-03-137/2025-03/200161. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funder.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The authors also acknowledge funding provided by the Institute of Physics Belgrade, University of Belgrade, the Faculty of Medicine, University of Belgrade, and the Faculty of Veterinary Medicine, University of Belgrade, through the grants by the Ministry of Science, Technological Development, and Innovations of the Republic of Serbia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, A.H.; Martin, S.; Phan, B.; Benigno, M.; Stephens, J.; Chambers, R.; Aram, J.A. Patient characteristics and risk factors in invasive mold infections: Comparison from a systematic review and database analysis. Clinicoecon. Outcomes Res. 2021, 13, 593–602. [Google Scholar] [CrossRef]
- Kraft, S.; Buchenauer, L.; Polte, T. Mold, mycotoxins and a dysregulated immune system: A combination of concern? Int. J. Mol. Sci. 2021, 22, 12269. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Oliveira, D.; Lisboa, C.; Boechat, J.L.; Delgado, L. Clinical manifestations of human exposure to fungi. J. Fungi 2023, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O. Diagnosis and treatment of invasive mold diseases. Infect. Chemother. 2023, 55, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Paiva Macedo, J.; Dias, V.C. Antifungal resistance: Why are we losing this battle? Future Microbiol. 2024, 19, 1027–1040. [Google Scholar] [CrossRef]
- Yun, H.; Seo, J.H.; Kim, Y.G.; Yang, J. Impact of scented candle use on indoor air quality and airborne microbiome. Sci. Rep. 2025, 15, 10181. [Google Scholar] [CrossRef]
- Azuma, K.; Yanagi, U.; Kagi, N.; Kim, H.; Ogata, M.; Hayashi, M. Environmental factors involved in SARS-CoV-2 transmission: Effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ. Health Prev. Med. 2020, 25, 66. [Google Scholar] [CrossRef]
- Rawat, M.S.; Roberts, A.D.; Brown, D.M.; Ferro, A.R. Resuspension of Seeded Particles Containing Live Influenza A Virus in a Full-Scale Laboratory. Buildings 2023, 13, 1734. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Morawska, L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 2006, 16, 335–347. [Google Scholar] [CrossRef]
- Feng, Z.; Cao, S.-J.; Haghighat, F. Removal of SARS-CoV-2 Using UV+Filter in Built Environment. Sustain. Cities Soc. 2021, 74, 103226. [Google Scholar] [CrossRef]
- Park, D.H.; An, S.-H.; Lee, Y.; Kim, Y.-J.; Han, B.; Kim, H.-J. Development of On-Demand Antiviral Electrostatic Precipitators with Electrothermal-Based Antiviral Surfaces against Airborne Virus Particles. Toxics 2022, 10, 601. [Google Scholar] [CrossRef]
- Grinshpun, S.A.; Mainelis, G.; Trunov, M.; Adhikari, A.; Reponen, T.; Willeke, K. Evaluation of Ionic Air Purifiers for Reducing Aerosol Exposure in Confined Indoor Spaces. Indoor Air 2005, 15, 235–245. [Google Scholar] [CrossRef]
- Claus, H. Ozone generation by ultraviolet lamps. Photochem. Photobiol. 2021, 97, 471–476. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kawakami, Y. Effectiveness of airborne fungi removal by using a HEPA air purifier fan in houses. Biocontrol Sci. 2018, 23, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L. HEPA filters and airborne viruses, bacteria, and fungi. Otolaryngol. Head Neck Surg. 2022, 166, 1005. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.A.; Gaunt, L.F.; Beggs, C.B.; Shepherd, S.J.; Sleigh, P.A.; Noakes, C.J.; Kerr, K.G. Bactericidal action of positive and negative ions in air. BMC Microbiol. 2007, 7, 32. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Zhu, S.; Yu, F.; Dai, B.; Yang, D. A review of recent advances of dielectric barrier discharge plasma in catalysis. Nanomaterials 2019, 9, 1428. [Google Scholar] [CrossRef]
- Altamimi, G.; Illias, H.A.; Mokhtar, N.; Mokhlis, H.; Bakar, A.H.A. Corona discharges under various types of electrodes. In Proceedings of the IEEE International Conference on Power and Energy (PECon), Kuching, Malaysia, 1–3 December 2014. [Google Scholar]
- Radalj, A.; Nikšić, A.; Trajković, J.; Knezević, T.; Janković, M.; De Luka, S.; Djoković, S.; Mijatović, S.; Ilić, A.; Arandjelović, I.; et al. Combating Pathogens Using Carbon-Fiber Ionizers (CFIs) for Air Purification: A Narrative Review. Appl. Sci. 2024, 14, 7311. [Google Scholar] [CrossRef]
- Panich, I.; Tippayawong, N. Effect of needle cone angle and air flow rate on electrostatic discharge characteristics of a corona-needle ionizer. J. Electrostat. 2010, 68, 254–260. [Google Scholar]
- Han, B.; Kim, H.J.; Kim, Y.J.; Sioutas, C. Unipolar charging of fine and ultra-fine particles using carbon fiber ionizers. Aerosol Sci. Technol. 2008, 42, 793–800. [Google Scholar] [CrossRef]
- Kolarž, P.; Ilić, A.Ž.; Janković, M.; Janićijević, A.; Trbovich, A.M. Estimating aerosol particle removal in indoor air by ion-enhanced deposition. J. Aerosol Sci. 2023, 173, 106199. [Google Scholar] [CrossRef]
- Yang, G.; Niu, B.; Zong, Z.; Wu, W.; Fang, X.; Chen, H.; Zhang, Y.; Mu, H.; Gao, H. Microbicidal effect of negative air ion against Penicillium citrinum and quality control of Chinese bayberry. Food Control. 2024, 162, 110476. [Google Scholar] [CrossRef]
- Pratt, R.; Barnard, R.W. Some effects of ionized air on Penicillium notatum. J. Am. Pharm. Assoc. Sci. Ed. 1960, 49, 643–646. [Google Scholar] [CrossRef]
- Pan, M.; Lednicky, J.A.; Wu, C.Y. Collection, Particle Sizing and Detection of Airborne Viruses. J. Appl. Microbiol. 2019, 127, 1596–1611. [Google Scholar] [CrossRef] [PubMed]
- Honsig, C.; Selitsch, B.; Hollenstein, M.; Vossen, M.G.; Spettel, K.; Willinger, B. Identification of Filamentous Fungi by MALDI-TOF Mass Spectrometry: Evaluation of Three Different Sample Preparation Methods and Validation of an In-House Species Cutoff. J. Fungi 2022, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.H.; Mawla, H.A. Sedimentation with the Omeliansky formula as an accepted technique for quantifying airborne fungi. Pol. J. Environ. Stud. 2012, 21, 1539–1541. [Google Scholar]
- Amaga, I.; Takahashi, T.; Ishii, K.; Kato, M.; Kobayashi, Y. Antifungal effect of blue LED irradiation on the blue mold, Penicillium italicum, in Satsuma mandarin fruits. Hortic. Res. Jpn. 2015, 14, 83–87. [Google Scholar]
- Hussey, S.B.; Clark, R.; Lunn, K.F.; Breathnach, C.; Soboll, G.; Whalley, J.M.; Lunn, D.P. Detection and quantification of equine herpesvirus-1 viremia and nasal shedding by real-time polymerase chain reaction. J. Vet. Diagn. Investig. 2006, 18, 335–342. [Google Scholar] [CrossRef]
- Anees-Hill, S.; Douglas, P.; Pashley, C.; Hansell, A.; Marczylo, E. A systematic review of outdoor airborne fungal spore seasonality across Europe and the implications for health. Sci. Total Environ. 2021, 818, 151716. [Google Scholar] [CrossRef]
- Sautour, M.; Sixt, N.; Dalle, F.; L’Ollivier, C.; Fourquenet, V.; Calinon, C.; Paul, K.; Valvin, S.; Maurel, A.; Aho, S.; et al. Profiles and seasonal distribution of airborne fungi in indoor and outdoor environments at a French hospital. Sci. Total Environ. 2009, 407, 3766–3771. [Google Scholar] [CrossRef]
- Hao, Z.F.; Ao, J.H.; Hao, F.; Yang, R.Y.; Zhu, H.; Zhang, J. Environment surveillance of filamentous fungi in two tertiary care hospitals in China. Chin. Med. J. 2011, 124, 1970–1975. [Google Scholar] [PubMed]
- Priyamvada, H.; Singh, R.K.; Akila, M.; Ravikrishna, R.; Verma, R.S.; Gunthe, S.S. Seasonal variation of the dominant allergenic fungal aerosols—One year study from southern Indian region. Sci. Rep. 2017, 7, 11171. [Google Scholar] [CrossRef] [PubMed]
- Bensch, K.; Groenewald, J.Z.; Meijer, M.; Dijksterhuis, J.; Jurjević, Ž.; Andersen, B.; Houbraken, J.; Crous, P.W.; Samson, R.A. Cladosporium species in indoor environments. Stud. Mycol. 2018, 89, 177–301. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, B.; Hedayati, M.T.; Hedayati, N.; Ilkit, M.; Syedmousavi, S. Aspergillus species in indoor environments and their possible occupational and public health hazards. Curr. Med. Mycol. 2016, 2, 36–42. [Google Scholar] [CrossRef]
- Shamim, M.; Kumar, M.; Kumar, R.R.; Pandey, P.; Srivastava, D.; Kumar, D.; Khan, N.A.; Kumar, R.R.; Singh, K.N. Understanding the diversity of Aspergillus by next-generation sequencing. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 2; pp. 29–40. [Google Scholar]
- Jiang, S.-Y.; Ma, A.; Ramachandran, S. Negative Air Ions and Their Effects on Human Health and Air Quality Improvement. Int. J. Mol. Sci. 2018, 19, 2966. [Google Scholar] [CrossRef]
- Secretariat, M.A. Air cleaning technologies: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2005, 5, 1–52. [Google Scholar]
- Weng, H.; Zhang, Y.; Huang, X.; Liu, X.; Tang, Y.; Yuan, H.; Xu, Y.; Li, K.; Zhang, Y. Pilot study on the production of negative oxygen ions based on lower voltage ionization method and application in air purification. Atmosphere 2024, 15, 860. [Google Scholar] [CrossRef]
- Čereška, A.; Tetsmann, I.; Bareikis, R.; Jasevičius, R. Research of Air Purification Using Ion Energy Effect on Particulate Matter Agglomeration. Atmosphere 2024, 15, 915. [Google Scholar] [CrossRef]
- Campler, M.R.; Shen, Y.-F.; Klüppel, L.M.; Arruda, A.G. Assessing the Impact of a Negative Air Ionization System on Particulate Matter and Gaseous Pollutants in the Swine Farrowing Environment. PLoS ONE 2025, 20, e0316914. [Google Scholar] [CrossRef]
- Slater, J. Equine Herpesviruses. In Equine Infectious Diseases; Sellon, D.C., Long, M.T., Eds.; WB Saunders: St. Louis, MO, USA, 2014; pp. 151–168. [Google Scholar]
- Pilawa, B.; Buszman, E.; Gondzik, A.; Wilczynski, S.; Zdybel, M.; Witoszynska, T.; Wilczok, T. Effect of pH on paramagnetic centers in Cladosporium cladosporioides melanin. Acta Phys. Pol. A 2005, 108, 147–150. [Google Scholar] [CrossRef]
- Llorente, C.; Bárcena, A.; Vera Bahima, J.; Saparrat, M.C.; Arambarri, A.M.; Rozas, M.F.; Mirífico, M.V.; Balatti, P.A. Cladosporium cladosporioides LPSC 1088 produces the 1,8-dihydroxynaphthalene-melanin-like compound and carries a putative pks gene. Mycopathologia 2012, 174, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.K.; Gajjar, D.U.; Vasavada, A.R. DOPA and DHN pathway orchestrate melanin synthesis in Aspergillus species. Med. Mycol. 2014, 52, 10–18. [Google Scholar]
- Hachinohe, M.; Matsumoto, H. Involvement of melanin synthesis and reactive oxygen species in phytotoxic action of L-DOPA in carrot cells. Crop Prot. 2007, 26, 294–298. [Google Scholar] [CrossRef]
- Shao, H.; Tu, Y.; Wang, Y.; Jiang, C.; Ma, L.; Hu, Z.; Wang, J.; Zeng, B.; He, B. Oxidative stress response of Aspergillus oryzae induced by hydrogen peroxide and menadione sodium bisulfite. Microorganisms 2019, 7, 225. [Google Scholar] [CrossRef]
- Cho, H.-J.; Son, S.-H.; Chen, W.; Son, Y.-E.; Lee, I.; Yu, J.-H.; Park, H.-S. Regulation of conidiogenesis in Aspergillus flavus. Cells 2022, 11, 2796. [Google Scholar] [CrossRef]
- Yu, J.-H. Regulation of development in Aspergillus nidulans and Aspergillus fumigatus. Mycobiology 2010, 38, 229–237. [Google Scholar] [CrossRef]
- Smith, T.D.; Calvo, A.M. The mtfA transcription factor gene controls morphogenesis, gliotoxin production, and virulence in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot. Cell 2014, 13, 766–775. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).