Perspectives on the Presence of Environmentally Persistent Free Radicals (EPFRs) in Ambient Particulate Matters and Their Potential Implications for Health Risk
Abstract
1. Introduction
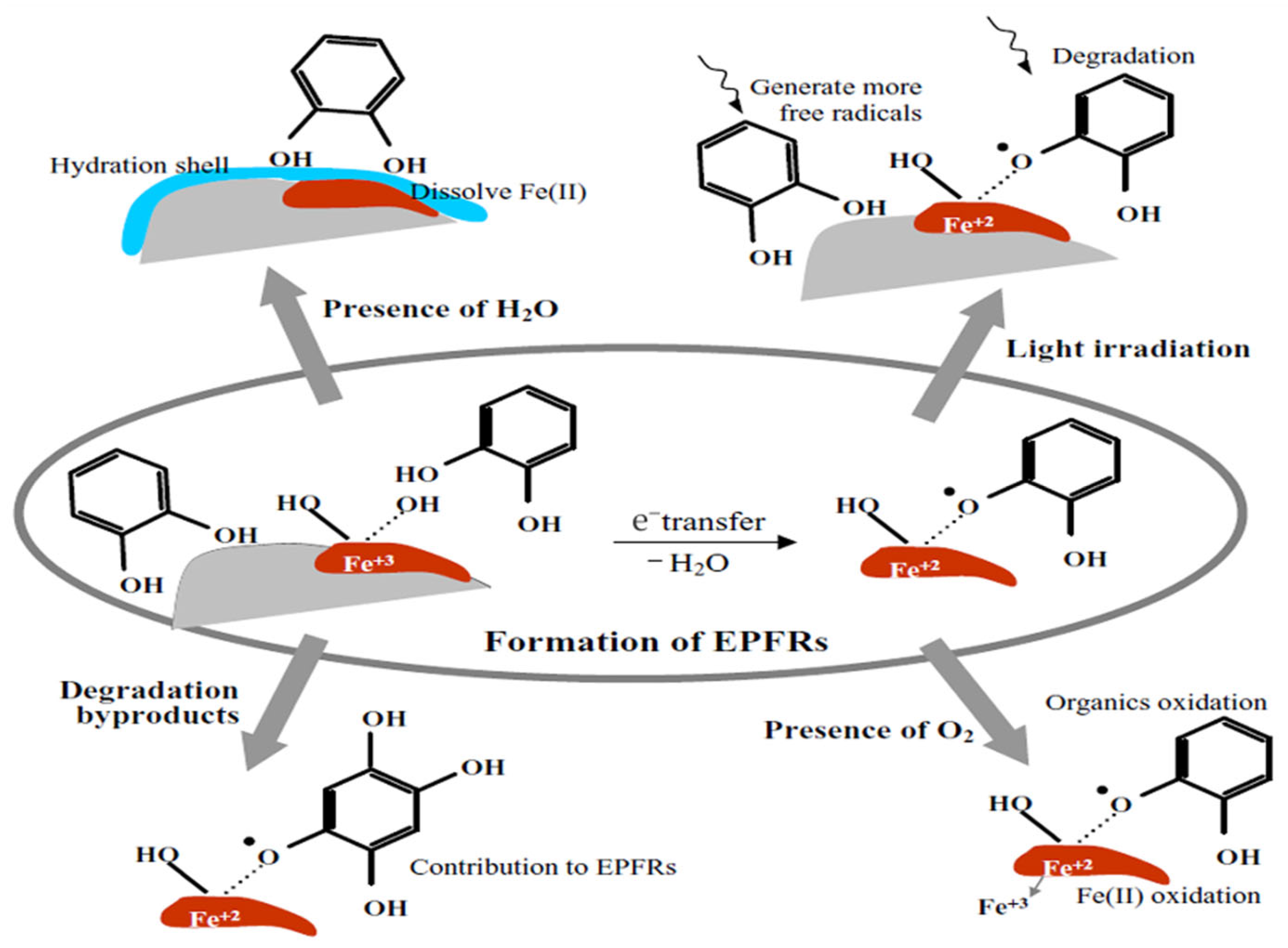
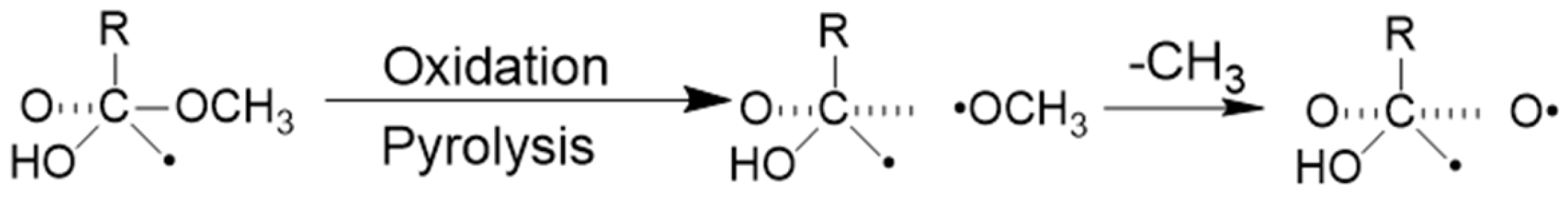
2. Formation Mechanisms of EPFRs
- (1)
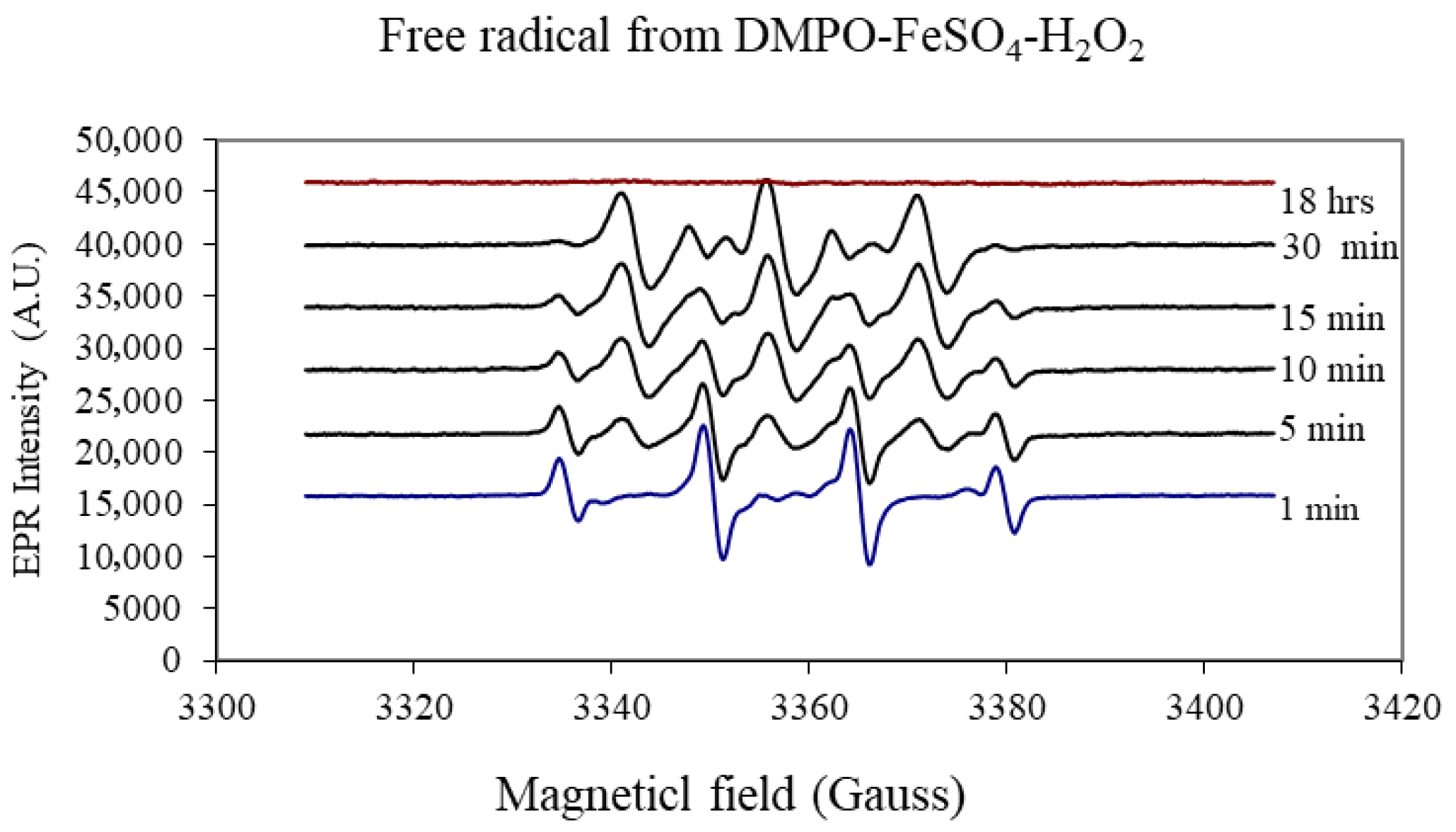
- EPFRs formation mediated by transition metals
| Particles | Organic Adsorbate | Range of g-Values | Half Life | ΔH p-p (Gauss, G) | EPFR Concentration (Spins/g) | Reaction Conditions | Reference |
|---|---|---|---|---|---|---|---|
| Fe(III)2O3/Silica | Phenol | 2.0040–2.0044 | 69.6–111 h | 5.7–6.1 | ~1016–1017 | 150–350 °C At 400 °C: Radical decomposes to cyclopentadienyl species | [25] |
| Catechol | 2.0057–2.0067 | 111 h (max) | Not reported | ~1017(max at 200 °C) | 150–350 °C Maximum EPFRs yield at 200 °C | ||
| Hydroquinone | 2.0053–2.0056 | 69.6 h | Not reported | ~1016 (low yield) | 150–350 °C EPFRs undetectable at 400 °C | ||
| 2Monochlorophenol | 2.0033–2.0052 | 24 h | Not reported | <1016 | 150–400 °C Adsorption via H2O elimination | ||
| 1,2Dichlorobenzene | 2.0057 | Unmeasurable | Not reported | <1015 | 150–400 °C | ||
| Monochlorobenzene | 2.0033 | Unmeasurable | Not reported | <1015 | 150 °C | ||
| Cu(II)O/Silica | Catechol | 2.0061–2.0070 | ~74 min | Not reported | Not reported | 150–400 °C | [27] |
| Hydroquinone | 2.0062–2.0065 | ~4.6 days | Not reported | Not reported | 150–400 °C Stable under vacuum | ||
| Phenol | 2.0032–2.0038 | ~27 min | Not reported | Not reported | 150–400 °C Single radical type | ||
| 2Monochlorophenol | 2.0041–2.0049 | ~24 h | Not reported | Not reported | 150–400 °C | ||
| 1,2Dichlorobenzene | 2.0063–2.0068 | ~1 h | Not reported | Not reported | 150–400 °C | ||
| Monochlorobenzene | 2.0067–2.0070 | ~30 min | Not reported | Not reported | 150–400 °C | ||
| Ni(II)O/SiO2 | Catechol | 2.0051–2.0065 | ~2.8 days | Not reported | Medium yield | 250–300 °C | [34] |
| Hydroquinone | 2.0044–2.0057 | ~2.8 days | Low yield | Up to 400 °C | |||
| Phenol | 2.0035–2.0043 | 5.2 days | Lowest relative yield among all adsorbates | 350 °C | |||
| 2Monochlorophenol(2-MCP) | 2.0042–2.0043 | 3.8 days | Monotonic increase with temperature | 150–400 °C | |||
| Monochlorobenzene | 2.0031–2.0038 | 2.4 days | Higher yield than phenol at >150 °C | 300–400 °C | |||
| 1,2Dichlorobenzene | 2.0034–2.0041 | 1.7 days | Highest yield below 350 °C | 300 °C | |||
| ZnO/Silica | Phenol | 2.0042 | 46–73 | 6.8 | 4.3 × 1018 | 230 °C, 5 min exposure | [35] |
| Monochlorobenzene | 2.0037 | 46–73 | 10.0 | 10.4 × 1018 | 230 °C, 5 min exposure | ||
| 2Monochlorophenol(2-MCP) | 2.0038 | 46–73 | 7.3 | 13.4 × 1018 | 230 °C, 5 min exposure | ||
| 1,2Dichlorobenzene | 2.0038 | 42–60 | 9.1 | 17.5 × 1018 | 230 °C, 5 min exposure | ||
| Hydroquinone | 2.0030 | 42–60 | 10.2 | 32.7 × 1018 | 230 °C, 5 min exposure | ||
| Catechol | 2.0036 | 42–60 | 10.7 | 66.4 × 1018 | 230 °C, 5 min exposure | ||
| CuO | 2,4Dichloro1naphthol | 2.0034 | 81 | 4.17 | 2.38 × 1022 | 25–300 °C,5% CuO on SiO2 | [36] |
| Al2O3 | 2,4Dichloro1naphthol | 2.0036–2.0063 | 108 | 3.96/4.13 | 5.82 × 1022 | 25–300 °C,5% Al2O3 on SiO2 | |
| ZnO | 2,4Dichloro1naphthol | 2.0029–2.0039 | 68 | 4.96/4.52 | 5.42 × 1022 | 25–300 °C,5% ZnO on SiO2 | |
| NiO | 2,4Dichloro1naphthol | 2.0029–2.0039 | 86 | 3.47/3.16 | 3.72 × 1021 | 25–300 °C,5% NiO on SiO2 | |
| Fe(III) | Anthracene | 2.0028–2.0039 | 22.73 | Not reported | Not reported | Room temperature (~23 °C), dark conditions, relative humidity ~7%, clay interlayers | [31] |
| Cu(II) | Anthracene | 2.0028–2.0039 | 21.28 | ~23 °C, dark conditions, relative humidity ~7%, clay interlayers | |||
| Ni(II) | Anthracene | 2.0028–2.0039 | 11.76 | ~23 °C, dark conditions, relative humidity ~7%, clay interlayers | |||
| Co(II) | Anthracene | 2.0028–2.0039 | 18.52 | ~23 °C, dark conditions, relative humidity ~7%, clay interlayers | |||
| Cu(II) | Benzo[a]pyrene | 2.0028–2.0039 | 13.70 | ~23 °C, dark conditions, relative humidity ~7%, clay interlayers | |||
| Ni(II) | Benzo[a]pyrene | 2.0028–2.0039 | 43.48 | ~23 °C, dark conditions, relative humidity ~7%, clay interlayers | |||
| Co(II) | Benzo[a]pyrene | 2.0028–2.0039 | 58.82 | ~23 °C, dark conditions, relative humidity ~7%, clay interlayers |
- (2)
- Formation via stabilization in organic matrices
3. Detection Techniques and Methods for EPFRs
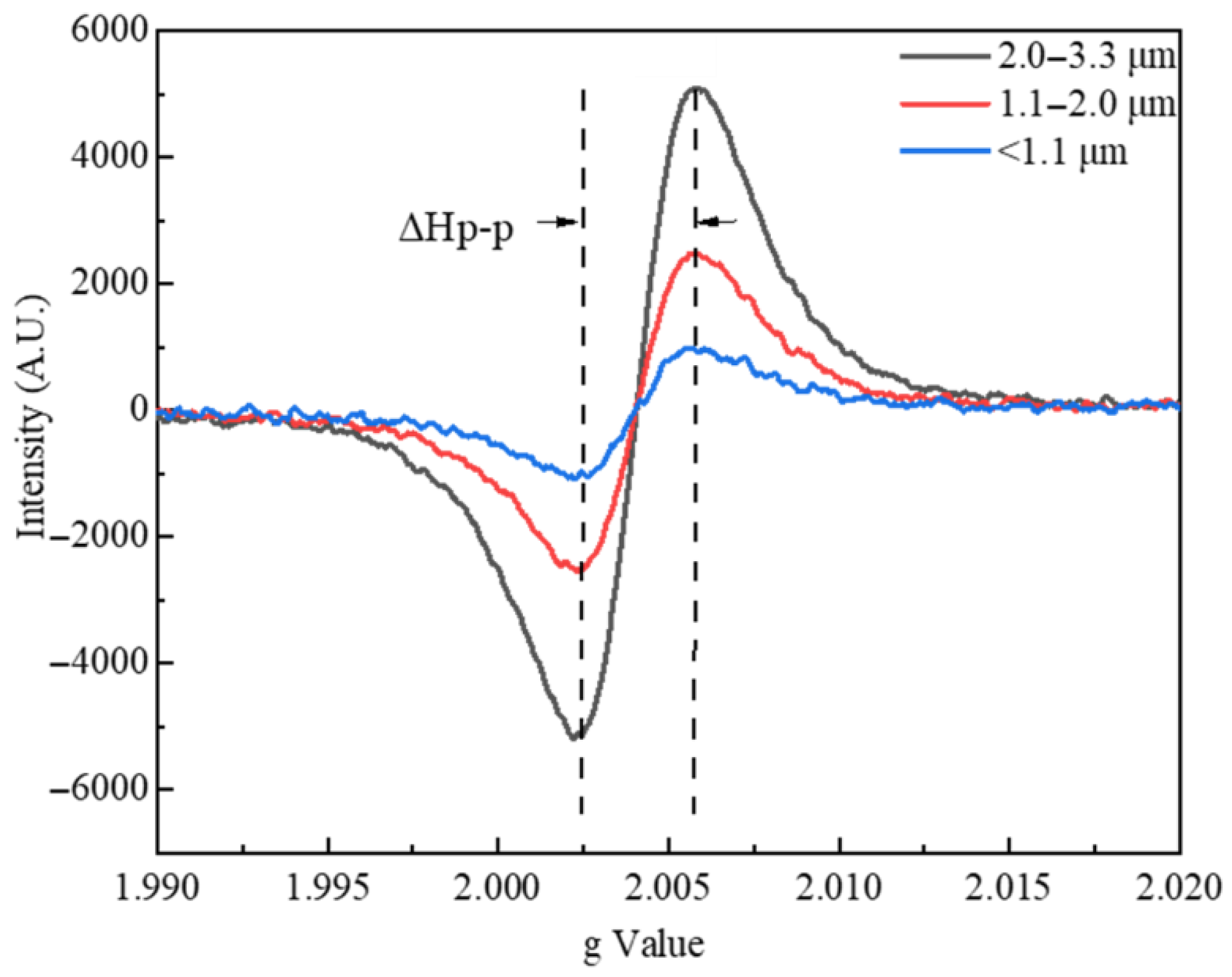
3.1. EPR Spectroscopy
3.2. Gas Chromatography-Mass Spectrometry (GC-MS)
3.3. Spectroscopic Methods
3.4. Complementary Characterization Methods for EPFR Studies
3.5. Chemical Methods (Free Radical Trapping Assays)
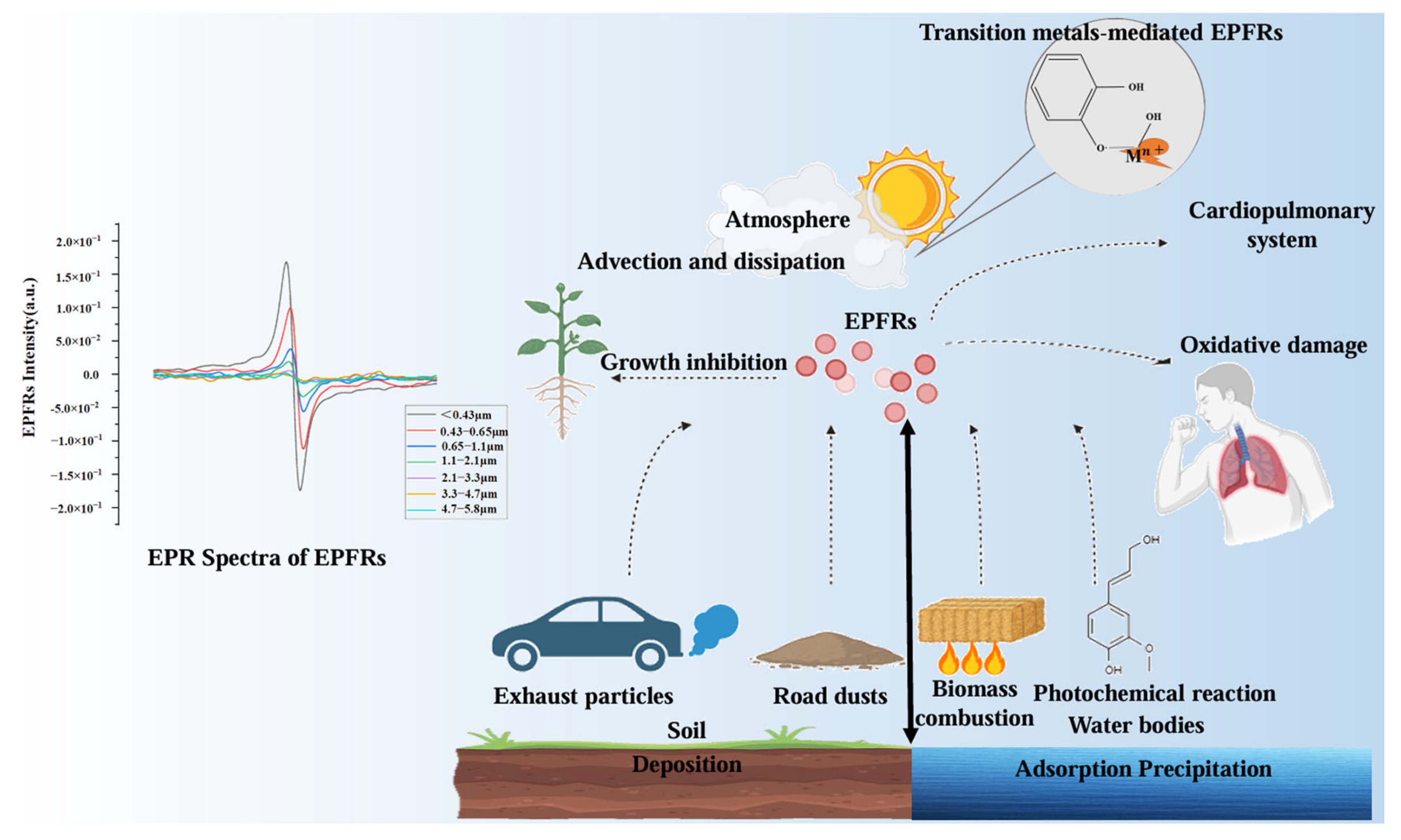
4. Sources and Distribution of EPFRs in Atmospheric Particulate Matter
5. Toxicological Studies of the EPFRs
6. Summary and Perspectives
- Formation Mechanisms
- 2.
- Analytical Method Development
- 3.
- Environmental Transport and Fate
- 4.
- Biological and Health Impacts
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aust, S.; Chignell, C.; Bray, T.; Kalyanaraman, B.; Mason, R. Free radicals in toxicology. Toxicol. Appl. Pharmacol. 1993, 120, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid. Med. Cell Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef]
- Luque-Ceballos, J.C.; Rodríguez-Zamora, P.; López-Olivos, J.C.; Garzón, I.L. Revisiting the Scavenging Activity of Glutathion: Free Radicals Diversity and Reaction Mechanisms. Comput. Theor. Chem. 2023, 1227, 114227. [Google Scholar] [CrossRef]
- Yan, M.; Lo, J.C.; Edwards, J.T.; Baran, P.S. Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714. [Google Scholar] [CrossRef]
- Khamrai, A.; Ganesh, V. How to train free radicals for organic synthesis? A modern approach. J. Chem. Sci. 2021, 133, 5. [Google Scholar] [CrossRef]
- Bergendi, L.; Beneš, L.; Ďuračková, Z.; Ferenčik, M. Chemistry, physiology and pathology of free radicals. Life Sci. 1999, 65, 1865–1874. [Google Scholar] [CrossRef]
- Dellinger, B.; Lomnicki, S.; Khachatryan, L.; Maskos, Z.; Hall, R.W.; Adounkpe, J.; McFerrin, C.; Truong, H. Formation and stabilization of persistent free radicals. Proc. Combust. Inst. 2007, 31, 521–528. [Google Scholar] [CrossRef]
- Katz, M. Photochemistry of air pollution. Am. J. Public Health 1962, 52, 878. [Google Scholar] [CrossRef][Green Version]
- Hawkins, C.L.; Davies, M.J. Detection and characterisation of radicals in biological materials using EPR methodology. Biochim. Biophys. Acta 2014, 1840, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.J.E.; Tapley, J.G.; Jackson, R.; Bond, R.L.; Murnaghan, A.R. Paramagnetic Resonance in Carbonaceous Solids. Nature 1954, 174, 797–798. [Google Scholar] [CrossRef]
- Lyons, M.J.; Gibson, J.F.; Ingram, D.J.E. Free-Radicals produced in Cigarette Smoke. Nature 1958, 181, 1003–1004. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, X.; Su, G.; Chen, X.; Meng, J.; Li, Q.; Wang, C.; Shi, B. Scientific and regulatory challenges of environmentally persistent free radicals: From formation theory to risk prevention strategies. J. Hazard. Mater. 2023, 456, 131674. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Yang, L.; Li, D.; Zheng, M. Critical Influences of Metal Compounds on the Formation and Stabilization of Environmentally Persistent Free Radicals. Chem. Eng. J. 2021, 427, 131666. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Yang, L.; Liu, X.; Wang, M.; Qin, L.; Zheng, M. Metal-Catalyzed Formation of Organic Pollutants Intermediated by Organic Free Radicals. Environ. Sci. Technol. 2022, 56, 14550–14561. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis (Open Access Viewpoint). ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, F.; Fu, H.; Guo, H. Characterisation of environmentally persistent free radicals and their contributions to oxidative potential and reactive oxygen species in sea spray and size-resolved ambient particles. npj Clim. Atmos. Sci. 2025, 8, 27. [Google Scholar] [CrossRef]
- Han, L.; Chen, B. Generation Mechanism and Fate Behaviors of Environmental Persistent Free Radicals. Prog. Chem. 2017, 29, 1008–1020. [Google Scholar]
- Baltrėnaitė-Gedienė, E.; Lomnicki, S.; Ogunmusi, O. Research of the impact of environmentally persistent free radicals on chemical element behaviour in the soil-plant system. Chemosphere 2024, 364, 143088. [Google Scholar] [CrossRef]
- Qin, L.; Zhu, W.; Yang, L.; Zheng, M.; Liu, G. Persistent free radicals in the environment. J. Hazard. Mater. 2025, 493, 138332. [Google Scholar] [CrossRef]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.; Li, S.; Gao, Y. Occurrence, formation, environmental fate and risks of environmentally persistent free radicals in biochars. Environ. Int. 2020, 134, 105172. [Google Scholar] [CrossRef]
- Balakrishna, S.; Lomnicki, S.; McAvey, K.M.; Cole, R.B.; Dellinger, B.; Cormier, S.A. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part. Fibre Toxicol. 2009, 6, 11. [Google Scholar] [CrossRef]
- Dugas, T.R.; Lomnicki, S.; Cormier, S.A.; Dellinger, B.; Reams, M. Addressing Emerging Risks: Scientific and Regulatory Challenges Associated with Environmentally Persistent Free Radicals. Int. J. Environ. Res. Public Health 2016, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Vejerano, E.; Lomnicki, S.; Dellinger, B. Formation and stabilization of combustion-generated environmentally persistent free radicals on an Fe(III)2O3/silica surface. Environ. Sci. Technol. 2011, 45, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, R.; Liu, Z.; Wang, W.; Zhang, Q.; Wang, Q. Periodic DFT calculation for the formation of EPFRs from phenol on γ-Al2O3 (110): Site-dependent mechanism and the role of ambient water. J. Environ. Chem. Eng. 2022, 10, 108386. [Google Scholar] [CrossRef]
- Lomnicki, S.; Truong, H.; Vejerano, E.; Dellinger, B. Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ. Sci. Technol. 2008, 42, 4982–4988. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, M.; Wang, Y.; Zhang, L.; Xue, J.; Sun, H.; Mu, Z. Rapid determination of environmentally persistent free radicals (EPFRs) in atmospheric particles with a quartz sheet-based approach using electron paramagnetic resonance (EPR) spectroscopy. Atmos. Environ. 2018, 184, 140–145. [Google Scholar] [CrossRef]
- Runberg, H.L.; Mitchell, D.G.; Eaton, S.S.; Eaton, G.R.; Majestic, B.J. Stability of environmentally persistent free radicals (EPFR) in atmospheric particulate matter and combustion particles. Atmos. Environ. 2020, 240, 117809. [Google Scholar] [CrossRef]
- Feld-Cook, E.E.; Bovenkamp-Langlois, L.; Lomnicki, S.M. Effect of Particulate Matter Mineral Composition on Environmentally Persistent Free Radical (EPFR) Formation. Environ. Sci. Technol. 2017, 51, 10396–10402. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Shi, Y.; Zhu, L.; Wang, C.; Sharma, V.K. Transformation of Polycyclic Aromatic Hydrocarbons and Formation of Environmentally Persistent Free Radicals on Modified Montmorillonite: The Role of Surface Metal Ions and Polycyclic Aromatic Hydrocarbon Molecular Properties. Environ. Sci. Technol. 2018, 52, 5725–5733. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, H.; Li, X.; Bekele, T.G. Characteristics and sources of environmentally persistent free radicals in PM2.5 in Dalian, Northeast China: Correlation with polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. 2022, 29, 24612–24622. [Google Scholar] [CrossRef]
- Pan, B.; Li, H.; Lang, D.; Xing, B. Environmentally persistent free radicals: Occurrence, formation mechanisms and implications. Environ. Pollut. 2019, 248, 320–331. [Google Scholar] [CrossRef]
- Vejerano, E.; Lomnicki, S.M.; Dellinger, B. Formation and stabilization of combustion-generated, environmentally persistent radicals on Ni(II)O supported on a silica surface. Environ. Sci. Technol. 2012, 46, 9406–9411. [Google Scholar] [CrossRef] [PubMed]
- Vejerano, E.; Lomnicki, S.; Dellinger, B. Lifetime of combustion-generated environmentally persistent free radicals on Zn(II)O and other transition metal oxides. J. Environ. Monit. 2012, 14, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Zheng, M.; Jin, R.; Zhao, Y.; Wu, X.; Xu, Y. Pivotal Roles of Metal Oxides in the Formation of Environmentally Persistent Free Radicals. Environ. Sci. Technol. 2017, 51, 12329–12336. [Google Scholar] [CrossRef]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C.W. Formation, characteristics, and applications of environmentally persistent free radicals in biochars: A review. Bioresour Technol. 2019, 281, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Huo, P.; Zhang, L.; Chen, Q.; Zhang, Y. Pollution characteristics of environmental persistent free radicals and coexisting health risk substances in PM2.5 in Huairou, Beijing. Environ. Chem. 2022, 41, 813–822. [Google Scholar]
- Chen, Q.; Sun, H.; Wang, M.; Mu, Z.; Wang, Y.; Li, Y.; Wang, Y.; Zhang, L.; Zhang, Z. Dominant Fraction of EPFRs from Nonsolvent-Extractable Organic Matter in Fine Particulates over Xi’an, China. Environ. Sci. Technol. 2018, 52, 9646–9655. [Google Scholar] [CrossRef]
- Bešić, E.; Rajić, Z.; Šakić, D. Advancements in electron paramagnetic resonance (EPR) spectroscopy: A comprehensive tool for pharmaceutical research. Acta Pharm. 2025, 74, 551–594. [Google Scholar] [CrossRef]
- Yun, J.; Yang, Q.; Liu, G. Mechanisms of lignin degradation and persistent free radical formation under light or thermal exposure. Cell Rep. Sustain. 2025, 2, 100267. [Google Scholar] [CrossRef]
- Pan, Y.; Nilges, M.J. Electron Paramagnetic Resonance Spectroscopy: Basic Principles, Experimental Techniques and Applications to Earth and Planetary Sciences. Rev. Mineral. Geochem. 2014, 78, 655–690. [Google Scholar] [CrossRef]
- Cummerow, R.L.; Halliday, D.; Moore, G.E. Paramagnetic resonance absorption in salts of the iron group. Phys. Rev. 1947, 72, 1233–1240. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive oxygen and nitrogen species: Impact on endothelial dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, A.; Jeschke, G. Principles of Pulse Electron Paramagnetic Resonance; Oxford University Press (OUP): Oxford, UK, 2001. [Google Scholar]
- Pryor, W.A.; Prier, D.G.; Church, D.F. Electron-spin resonance study of mainstream and sidestream cigarette smoke: Nature of the free radicals in gas-phase smoke and in cigarette tar. Environ. Heal. Perspect. 1983, 47, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, L.; Shi, J.; Li, H.; Lang, D.; Wei, Z.; Li, S.; Pan, B. Distribution of environmentally persistent free radicals in size-segregated PMs emitted from residential biomass fuel combustion. J. Hazard. Mater. 2023, 449, 130956. [Google Scholar] [CrossRef]
- Köhler-Langes, F. The g-Factor—Exploring Atomic Structure and Fundamental Constants. The Electron Mass and Calcium Isotope Shifts; Springer Theses; Springer: Cham, Switzerland, 2017; pp. 5–32. [Google Scholar]
- Kutas, M.; Dale, A. Electrical and magnetic readings of mental functions. In Cognitive Neuroscience; Rugg, M.D., Ed.; Psychology Press: Hove, UK, 1997; pp. 197–242. [Google Scholar]
- Jasniewski, A.; Hu, Y.; Ribbe, M.W. Electron Paramagnetic Resonance Spectroscopy of Metalloproteins. Methods Mol. Biol. 2019, 1876, 197–211. [Google Scholar]
- Ye, S. Probing electronic structures of transition metal complexes using electron paramagnetic resonance spectroscopy. Magn. Reson. Lett. 2023, 3, 43–60. [Google Scholar] [CrossRef]
- Ju, M.; Kim, J.; Shin, J. EPR spectroscopy: A versatile tool for exploring transition metal complexes in organometallic and bioinorganic chemistry. Bull. Korean Chem. Soc. 2024, 45, 835–862. [Google Scholar] [CrossRef]
- Green, U.; Shenberger, Y.; Aizenshtat, Z.; Cohen, H.; Ruthstein, S. Exploring the radical nature of a carbon surface by electron paramagnetic resonance and a calibrated gas flow. J. Vis. Exp. 2014, 86, 51548. [Google Scholar]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Modern Molecular Photochemistry of Organic Molecules. Photochem. Photobiol. 2010, 88, 1033. [Google Scholar] [CrossRef]
- Visconti, G.; Boccard, J.; Feinberg, M.; Rudaz, S. From fundamentals in calibration to modern methodologies: A tutorial for small molecules quantification in liquid chromatography-mass spectrometry bioanalysis. Anal. Chim. Acta 2023, 1240, 340711. [Google Scholar] [CrossRef]
- Cruz, A.L.N.D.; Cook, R.L.; Lomnicki, S.M.; Dellinger, B. Effect of low temperature thermal treatment on soils contaminated with pentachlorophenol and environmentally persistent free radicals. Environ. Sci. Technol. 2012, 46, 5971–5978. [Google Scholar] [CrossRef]
- Nwosu, U.G.; Roy, A.; dela Cruz, A.L.N.; Dellingerab, B.; Cook, R. Formation of environmentally persistent free radical (EPFR) in iron(III) cation-exchanged smectite clay. Environ. Sci.-Process. Impacts 2016, 18, 42–50. [Google Scholar] [CrossRef]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 2016, 109, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sowndarya, S.V.S.; St. John, P.C.; Paton, R.S. A quantitative metric for organic radical stability and persistence using thermodynamic and kinetic features. Chem. Sci. 2021, 12, 13158–13166. [Google Scholar] [CrossRef]
- Bruschi, M.; Limacher, P.A.; Hutter, J.; Lüthi, H.P. A Scheme for the Evaluation of Electron Delocalization and Conjugation Efficiency in Linearly π-Conjugated Systems. J. Chem. Theory Comput. 2009, 5, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Devadiga, D.; Yan, J.; Devadiga, D. Recent Advances in Probing Electron Delocalization in Conjugated Molecules by Attached Infrared Reporter Groups for Energy Conversion and Storage. ACS Appl. Energy Mater. 2025, 8, 1942–1963. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Chen, J.; Saleem, A.R.; Sun, Y.; Ai, J.; Huang, H.; Zhang, L.; Khan, C. Source-oriented pollution characteristics and decay kinetics of environmentally persistent free radicals in PM2.5 and PM10. J. Hazard. Mater. 2025, 495, 139074. [Google Scholar] [CrossRef]
- Baltrėnaitė-Gedienė, E.; Lomnicki, S.; Guo, C. Impact of biochar, fertilizers and cultivation type on environmentally persistent free radicals in agricultural soil. Environ. Technol. Innov. 2022, 28, 102755. [Google Scholar] [CrossRef]
- Zhong, L.; Zhu, B.; Su, W.; Liang, W.; Wang, H.; Li, T.; Cao, D.; Ruan, T.; Chen, J.; Jiang, G. Molecular characterization of diverse quinone analogs for discrimination of aerosol-bound persistent pyrolytic and photolytic radicals. Sci. Bull. 2024, 69, 612–620. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Xue, Y.; Cui, L.; Chen, L.; Ho, K.-F.; Huang, Y. Formation of environmentally persistent free radicals and their risks for human health: A review. Environ. Chem. Lett. 2024, 22, 1327–1343. [Google Scholar] [CrossRef]
- Jia, S.-M.; Chen, M.-H.; Yang, P.-F.; Wang, L.; Wang, G.-Y.; Liu, L.-Y.; Ma, W.-L. Seasonal variations and sources of atmospheric EPFRs in a megacity in severe cold region: Implications for the influence of strong coal and biomass combustion. Environ. Res. 2024, 252, 119067. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Neyman, K.M.; Risse, T.; Sterrer, M.; Fischbach, E.; Freund, H.-J.; Nasluzov, V.A.; Pacchioni, G.; Rösch, N. Density-functional model cluster studies of EPR g tensors of Fs+ centers on the surface of MgO. J. Chem. Phys. 2006, 124, 044708. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Stone, K.; Zang, L.-Y.; Bermúdez, E. Fractionation of aqueous cigarette tar extracts: Fractions that contain the tar radical cause DNA damage. Chem. Res. Toxicol. 1998, 11, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Bau, J.A.; Emwas, A.-H.; Rueping, M. An overview of solid-state electron paramagnetic resonance spectroscopy for artificial fuel reactions. iScience 2022, 25, 105360. [Google Scholar] [CrossRef]
- Yan, X.; Cao, T.; Chen, H.; Wu, J.; Xu, C.; Song, J.; Zhong, Y.; Chen, Y.; Zhang, G.; Peng, P. Formation and evolution of environmentally persistent free radicals in charcoal and soot generated from biomass materials. J. Hazard. Mater. 2025, 488, 137523. [Google Scholar] [CrossRef]
- Xiao, K.; Li, L.; Zhang, Y.; Zhou, Y.; Fu, D.; Luo, Z.; Huang, T.; Lu, S.; Liu, F.; Lu, J.; et al. Environmentally persistent free radicals from residential raw coal combustion and association with chemical components. Emerg. Contam. 2024, 10, 100346. [Google Scholar] [CrossRef]
- Ahn, K.-H.; Halpern, H.J. Spatially uniform sampling in 4-D EPR spectral-spatial imaging. J. Magn. Reson. 2007, 185, 152–158. [Google Scholar] [CrossRef]
- Spałek, T.; Kruczała, K.; Sojka, Z.; Schlick, S. Deducing 1D concentration profiles from EPR imaging: A new approach based on the concept of virtual components and optimization with the genetic algorithm. J. Magn. Reson. 2007, 189, 139–150. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, L.; Wang, X.; Zheng, M.; Li, C.; Zhang, A.; Fu, J.; Yang, Y.; Qin, L.; Liu, X.; et al. Risk evaluation of environmentally persistent free radicals in airborne particulate matter and influence of atmospheric factors. Ecotoxicol. Environ. Saf. 2020, 196, 110571. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, H.; Li, Q.; Jin, L.; Lyu, R.; Ding, X.; Huo, Y.; Zhao, B.; Jiang, J.; Chen, J.; et al. Toxic potency-adjusted control of air pollution for solid fuel combustion. Nat. Energy 2022, 7, 194–202. [Google Scholar] [CrossRef]
- Lu, S.; Win, M.S.; Zeng, J.; Yao, C.; Zhao, M.; Xiu, G.; Lin, Y.; Xie, T.; Dai, Y.; Rao, L.; et al. A characterization of HULIS-C and the oxidative potential of HULIS and HULIS-Fe(II) mixture in PM2.5 during hazy and non-hazy days in Shanghai. Atmos. Environ. 2019, 219, 117058. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Huang, C.-H.; Shan, G.-Q.; Mao, L.; Kalyanaraman, B.; Qin, H.; Ren, F.-R.; Zhu, B.-Z. The first purification and unequivocal characterization of the radical form of the carbon-centered quinone ketoxy radical adduct. Chem. Commun. 2013, 49, 6436–6438. [Google Scholar] [CrossRef]
- Lin, B.; Yang, L.; Zheng, M.; Qin, L.; Liu, S.; Sun, Y.; Chen, C.; Liu, G. Synergetic promoting/inhibiting mechanisms of copper/calcium compounds in the formation of persistent organic pollutants and environmentally persistent free radicals from anthracene. Chem. Eng. J. 2022, 441, 136102. [Google Scholar] [CrossRef]
- Chang, C.-W.; Chen, I.-Y.; Fittschen, C.; Luo, P.-L. Measurements of absolute line strength of the ν1 fundamental transitions of OH radical and rate coefficient of the reaction OH + H2O2 with mid-infrared two-color time-resolved dual-comb spectroscopy. J. Chem. Phys. 2023, 159, 184203. [Google Scholar] [CrossRef]
- Zhang, F.; Ji, Z.; Zhang, Q.; Shen, R.; Xing, Z.; Wu, G. Electron spin resonance study on free radicals in cyclic olefin copolymers irradiated by gamma rays at cryogenic and room temperatures. Radiat. Phys. Chem. 2023, 202, 110505. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Liu, G.; Zheng, M. Formation of Environmentally Persistent Free Radicals during Thermochemical Processes and their Correlations with Unintentional Persistent Organic Pollutants. Environ. Sci. Technol. 2021, 55, 6529–6541. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; Melin, V.; Irvicelli, K.G.; Murillo-Sierra, J.C.; Canneva, A.; Donadelli, J.A.; Campos, C.H.; Torres, C.C.; Contreras, D.; Celzard, A.; et al. Metal-free photocatalyst based on highly porous activated carbon obtained from agro-industrial residues. Characterization and photocatalytic evaluation. J. Photochem. Photobiol. A Chem. 2025, 462, 116247. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Gamba, L.; Morazzoni, F.; Cosentino, U.; Greco, C.; Lasagni, M.; Pitea, D.; Moro, G.; Cepek, C.; Butera, V.; et al. Experimental and Theoretical Investigation on the Catalytic Generation of Environmentally Persistent Free Radicals from Benzene. J. Phys. Chem. C 2017, 121, 9381–9393. [Google Scholar] [CrossRef]
- Khachatryan, L.; Adounkpe, J.; Maskos, Z.; Dellinger, B. Formation of Cyclopentadienyl Radical from the Gas-Phase Pyrolysis of Hydroquinone, Catechol, and Phenol. Environ. Sci. Technol. 2006, 40, 5071–5076. [Google Scholar] [CrossRef]
- Jezierski, A.; Drozd, J.; Jerzykiewicz, M.; Chen, Y.; Kaye, K.J. EPR in the environmental control: Copper complexes and free radicals in soil and municipal solid waste compost. Appl. Magn. Reason. 1998, 14, 275–282. [Google Scholar] [CrossRef]
- Rao, L.; Zhang, L.; Wang, X.; Xie, T.; Zhou, S.; Lu, S.; Liu, X.; Lu, H.; Xiao, K.; Wang, W.; et al. Oxidative Potential Induced by Ambient Particulate Matters with Acellular Assays: A Review. Processes 2020, 8, 1410. [Google Scholar] [CrossRef]
- Xu, M.; Wu, T.; Tang, Y.-T.; Chen, T.; Khachatryan, L.; Iyer, P.R.; Guo, D.; Chen, A.; Lyu, M.; Li, J.; et al. Environmentally persistent free radicals in PM2.5: A review. Waste Dispos. Sustain. Energy 2019, 1, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, H.; Song, W.; Cao, F.; Tian, C.; Zhang, Y.-L. Size-resolved exposure risk of persistent free radicals (PFRs) in atmospheric aerosols and their potential sources. Atmos. Chem. Phys. 2020, 20, 14407–14417. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Wang, C.; Wang, R.; Sha, T.; Yang, X.; Ainur, D. Pollution characteristics of environmental persistent free radicals (EPFRs) and their contribution to oxidation potential in road dust in a large city in northwest China. J. Hazard. Mater. 2023, 442, 130087. [Google Scholar] [CrossRef]
- Künzli, N.; Kaiser, R.; Medina, S.; Studnicka, M.; Chanel, O.; Filliger, P.; Herry, M.; Horak, F., Jr.; Puybonnieux-Texier, V.; Quénel, P.; et al. Public-health impact of outdoor and traffic-related air pollution: A European assessment. Lancet 2000, 356, 795–801. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Shi, Y.; Fan, X.; Wang, T. Formation of environmentally persistent free radicals during the transformation of anthracene in different soils: Roles of soil characteristics and ambient conditions. J. Hazard. Mater. 2019, 362, 214–223. [Google Scholar] [CrossRef]
- Lu, K.; Guo, S.; Tan, Z.; Wang, H.; Shang, D.; Liu, Y.; Li, X.; Wu, Z.; Hu, M.; Zhang, Y. Exploring atmospheric free-radical chemistry in China: The self-cleansing capacity and the formation of secondary air pollution. Natl. Sci. Rev. 2019, 6, 579–594. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Wang, M.; Sun, H.; Mu, Z.; Zhang, L.; Li, Y.; Chen, Q. Source apportionment of environmentally persistent free radicals (EPFRs) in PM2.5 over Xi’an, China. Sci. Total Environ. 2019, 689, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, L.; Li, J.; Ying, Q.; Zhang, H.; Liu, X.; Liao, H.; Li, N.; Liu, Z.; Mao, Y.; et al. Sources of particulate matter in China: Insights from source apportionment studies published in 1987–2017. Environ. Int. 2018, 115, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.-M.; Weng, C.-Y.; Wang, G.-Y.; Ma, W.-L. Pollution characteristics and oxidative potential of atmospheric particles at a typical rural area: A case study during the Chinese Lunar New Year. Atmos. Pollut. Res. 2024, 15, 102251. [Google Scholar] [CrossRef]
- Wang, P.; Pan, B.; Li, H.; Huang, Y.; Dong, X.; Ai, F. The Overlooked Occurrence of Environmentally Persistent Free Radicals in an Area with Low-Rank Coal Burning, Xuanwei, China. Environ. Sci. Technol. 2018, 52, 1054–1061. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.; Zhang, Z.; Cai, Z. Levels, spatial distribution, and source identification of airborne environmentally persistent free radicals from tree leaves. Environ. Pollut. 2020, 257, 113353. [Google Scholar] [CrossRef]
- Shi, Y.; Dai, Y.; Liu, Z.; Nie, X.; Zhao, S.; Zhang, C.; Jia, H. Light-induced variation in environmentally persistent free radicals and the generation of reactive radical species in humic substances. Front. Environ. Sci. Eng. 2020, 14, 106. [Google Scholar] [CrossRef]
- Sadiq, I.Z. Free Radicals and Oxidative Stress: Signaling Mechanisms, Redox Basis for Human Diseases, and Cell Cycle Regulation. Curr. Mol. Med. 2023, 23, 13–35. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, H.; Wang, J.; Shan, M.; Yang, X.; Deng, M.; Wang, Y.; Zhang, L. Long-life type—The dominant fraction of EPFRs in combustion sources and ambient fine particles in Xi’an. Atmos. Environ. 2019, 219, 117059. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Yao, K.; Zheng, H.; Zhang, X.; Li, R.; Wang, N.; Fu, H. The overlooked formation of environmentally persistent free radicals on particulate matter collected from biomass burning under light irradiation. Environ. Int. 2022, 171, 107668. [Google Scholar] [CrossRef]
- Vejerano, E.P.; Rao, G.; Khachatryan, L.; Cormier, S.A.; Lomnicki, S. Environmentally Persistent Free Radicals: Insights on a New Class of Pollutants. Environ. Sci. Technol. 2018, 52, 2468–2481. [Google Scholar] [CrossRef]
- Harmon, A.C.; Noël, A.; Subramanian, B.; Perveen, Z.; Jennings, M.H.; Chen, Y.-F.; Penn, A.L.; Legendre, K.; Paulsen, D.B.; Varner, K.J.; et al. Inhalation of particulate matter containing free radicals leads to decreased vascular responsiveness associated with an altered pulmonary function. Am. J. Physiol. Circ. Physiol. 2021, 321, H667–H683. [Google Scholar] [CrossRef]
- Cormier, S.A.; Lomnicki, S.; Backes, W.; Dellinger, B. Origin and health impacts of emissions of toxic by-products and fine particles from combustion and thermal treatment of hazardous wastes and materials. Environ. Health Perspect. 2006, 114, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, J. Air pollution, health status and public awareness of environmental problems in China. Sci. Rep. 2024, 14, 19861. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, J.; Ferrara, F.; Vallese, A.; Guiotto, A.; Colella, S.; Pecorelli, A.; Valacchi, G. Comparison of Pollutant Effects on Cutaneous Inflammasomes Activation. Int. J. Mol. Sci. 2023, 24, 16674. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, P.T. The skin, free radicals, and oxidative stress. Dermatol. Nurs. 1995, 7, 361–369. [Google Scholar]
- Zhou, X.-L.; Yang, F.; Sun, H.-L.; Yin, Y.-N.; Ye, W.-T.; Zhu, R. Cobalt-Catalyzed Intermolecular Hydrofunctionalization of Alkenes: Evidence for a Bimetallic Pathway. J. Am. Chem. Soc. 2019, 141, 7250–7255. [Google Scholar] [CrossRef]
- Sarmiento, D.J.; Majestic, B.J. The Photochemistry of Quinones and Combustion-derived Particles in Forming Hydroxyl Radicals and Singlet Oxygen in the Atmosphere. Atmos. Environ. 2025, 351, 121189. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, W.; Ma, Z.; Liu, Y.; Ru, H.; Zhou, J.; Zang, Y.; Xu, Z.; Qian, G. Short-term exposure to ZnO/MCB persistent free radical particles causes mouse lung lesions via inflammatory reactions and apoptosis pathways. Environ. Pollut. 2020, 261, 114039. [Google Scholar] [CrossRef]
- Drew, L. Air pollution and brain damage: What the science says. Nature 2025, 637, 536–538. [Google Scholar] [CrossRef]
- Perera, F.P.; Rauh, V.; Tsai, W.Y.; Kinney, P.; Camann, D.; Barr, D.; Bernert, T.; Garfinkel, R.; Tu, Y.H.; Diaz, D.; et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ. Health Perspect. 2003, 111, 201–205. [Google Scholar] [CrossRef]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef]
- Ma, Y.; He, X.; Qi, K.; Wang, T.; Qi, Y.; Cui, L.; Wang, F.; Song, M. Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. 2019, 77, 210–217. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Lu, J.; Wang, X.; Xiao, K.; Niuhe, J.; Liu, X.; Yonemochi, S. Perspectives on the Presence of Environmentally Persistent Free Radicals (EPFRs) in Ambient Particulate Matters and Their Potential Implications for Health Risk. Atmosphere 2025, 16, 876. https://doi.org/10.3390/atmos16070876
Lu S, Lu J, Wang X, Xiao K, Niuhe J, Liu X, Yonemochi S. Perspectives on the Presence of Environmentally Persistent Free Radicals (EPFRs) in Ambient Particulate Matters and Their Potential Implications for Health Risk. Atmosphere. 2025; 16(7):876. https://doi.org/10.3390/atmos16070876
Chicago/Turabian StyleLu, Senlin, Jiakuan Lu, Xudong Wang, Kai Xiao, Jingying Niuhe, Xinchun Liu, and Shinichi Yonemochi. 2025. "Perspectives on the Presence of Environmentally Persistent Free Radicals (EPFRs) in Ambient Particulate Matters and Their Potential Implications for Health Risk" Atmosphere 16, no. 7: 876. https://doi.org/10.3390/atmos16070876
APA StyleLu, S., Lu, J., Wang, X., Xiao, K., Niuhe, J., Liu, X., & Yonemochi, S. (2025). Perspectives on the Presence of Environmentally Persistent Free Radicals (EPFRs) in Ambient Particulate Matters and Their Potential Implications for Health Risk. Atmosphere, 16(7), 876. https://doi.org/10.3390/atmos16070876







