Interaction Between Radon, Air Ions, and Ultrafine Particles Under Contrasting Atmospheric Conditions in Belgrade, Serbia
Abstract
1. Introduction
1.1. Radon
1.2. Aerosols
1.3. Small Air Ions
2. Materials and Methods
2.1. Instrumentation
- -
- Radon Measurement: a continuous radon monitor Rad-7 (Durridge, Billerica, MA, USA) was deployed to measure radon hourly concentrations in real-time. It is a high-sensitivity electronic radon detector that operates using a solid-state detector in a sealed high voltage counting chamber. RAD7 measures the energy of each alpha particle and uses alpha spectrometry to distinguish between these isotopes based on their discrete energy peaks. This enables the instrument to discriminate radon from other radioactive isotopes like thoron (220Rn) and to reject background radiation. The alpha counts from radon progeny like polonium 218Po and 214Po allow the RAD7 to accurately calculate the radon concentration in Bq/m3.
- -
- Radon in soil gas: a continuous radon monitor RTM1688-2 (Sarad GmbH, Dresden, Germany) and soil gas probe were used to measure radon in soil at 80 cm depth, with integration time set to 15 min. At each measuring location, 4–5 measurements were performed until saturated value of radon in soil was reached.
- -
- Air Ions Measurement: Air ion concentrations were measured using bipolar air ion counter [31], made at the Institute of Physics, Belgrade. Instrument works on the principle of the Gerdien ion counter (or Gerdien condenser) that is a classical instrument used to measure atmospheric ion concentrations and air conductivity. A Gerdien condenser detects air ions by measuring signal of tens of femptoampere electric current (~10−14 A) collected as ions are drawn by a fan through a cylindrical capacitor under the influence of an applied radial electric field within the electrode system. The current, proportional to the ion concentration and mobility, is collected on a central electrode and measured with an electrometer. The instrument is set for detecting class of small air ions (mobility > 0.5 cm2V⁻1s⁻1). The measurement uncertainty of the ion concentration meter is ~5 ions/cm3 under steady-state conditions during indoor measurements and when the measurement is conducted with a sufficiently large number of samples and frequent zeroing [31]. During outdoor measurements, airflow may introduce instability in the air flow through the electrodes. Although the fan speed is feedback-controlled based on the flow rate, disturbances can occur in certain measurements, particularly during system zeroing. In such cases, the measurement uncertainty increases to 20 ions/cm3.
- -
- Nanoparticle size distributions from 10 to 420 nm were measured using NanoScan SMPS™ Nanoparticle Sizer 3910 (TSI Incorporated, Shoreview, MN, USA) for particles from 10 to 420 nm. Instrument operation is based on a unipolar diffusion charging method, where aerosol particles are charged and then classified by electrical mobility using a radial differential mobility analyzer (rDMA). The resulting particle size distribution is determined by measuring the electrical current carried by the particles as a function of their mobility.
- -
- Fine and coarse particles were measured using Optical Particle Sizer 3330 (OPS, TSI Incorporated, Shoreview, MN, USA for particles from 0.3 to 10 μm. OPS measures particle size and concentration using light scattering, where a laser beam illuminates individual particles as they pass through a detection chamber. The intensity and angle of the scattered light are analyzed to determine the particle’s optical diameter based on Mie scattering theory. Using SMPS and OPS together, all particle diameters from 10 nm to 10 µm are covered.
- -
- PM, i.e., cumulative mass concentration of particles, T, RH, and pressure were measured using AirVisual Outdoor monitor. The AirVisual Outdoor monitor by IQAir measures air quality by using laser-based optical sensors (similar to OPS, TSI) to detect particulate matter (PM2.5 and PM10) and electrochemical sensors to measure gaseous pollutants like CO2, temperature, and humidity.
2.2. Measuring Places
3. Results and Discussion
3.1. Air Temperature and Relative Humidity
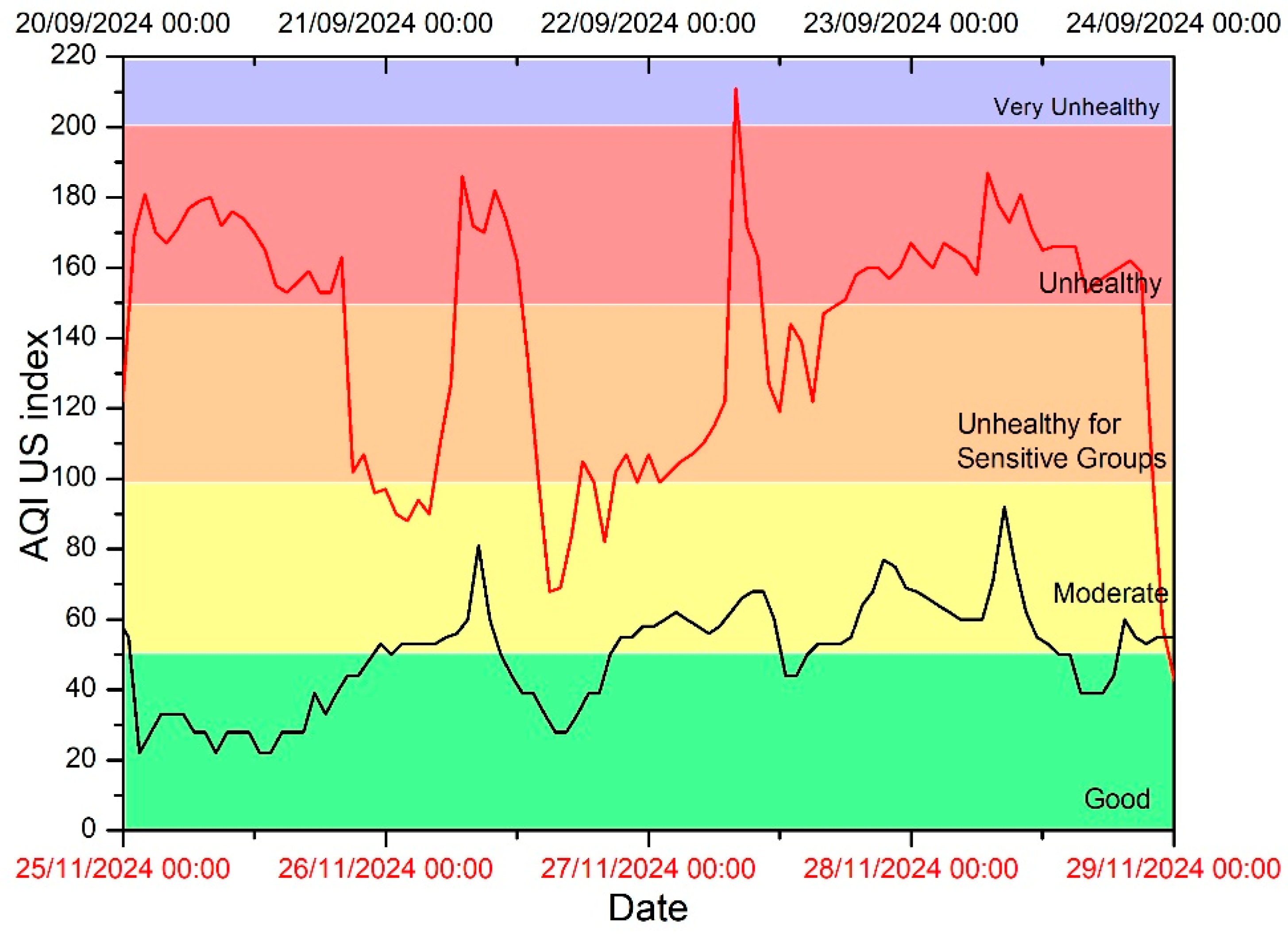
3.2. Air Quality Index
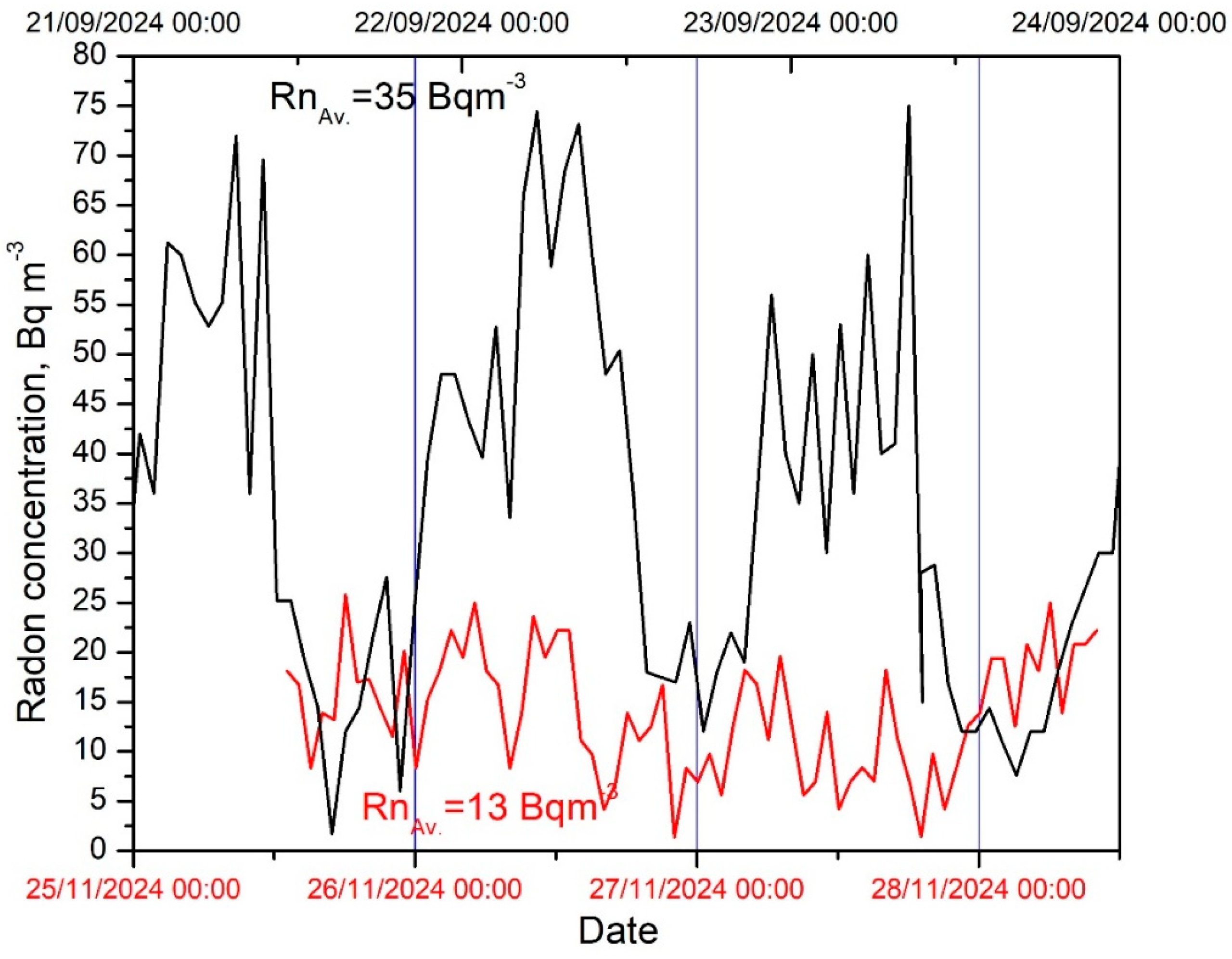
3.3. Radon Concentrations
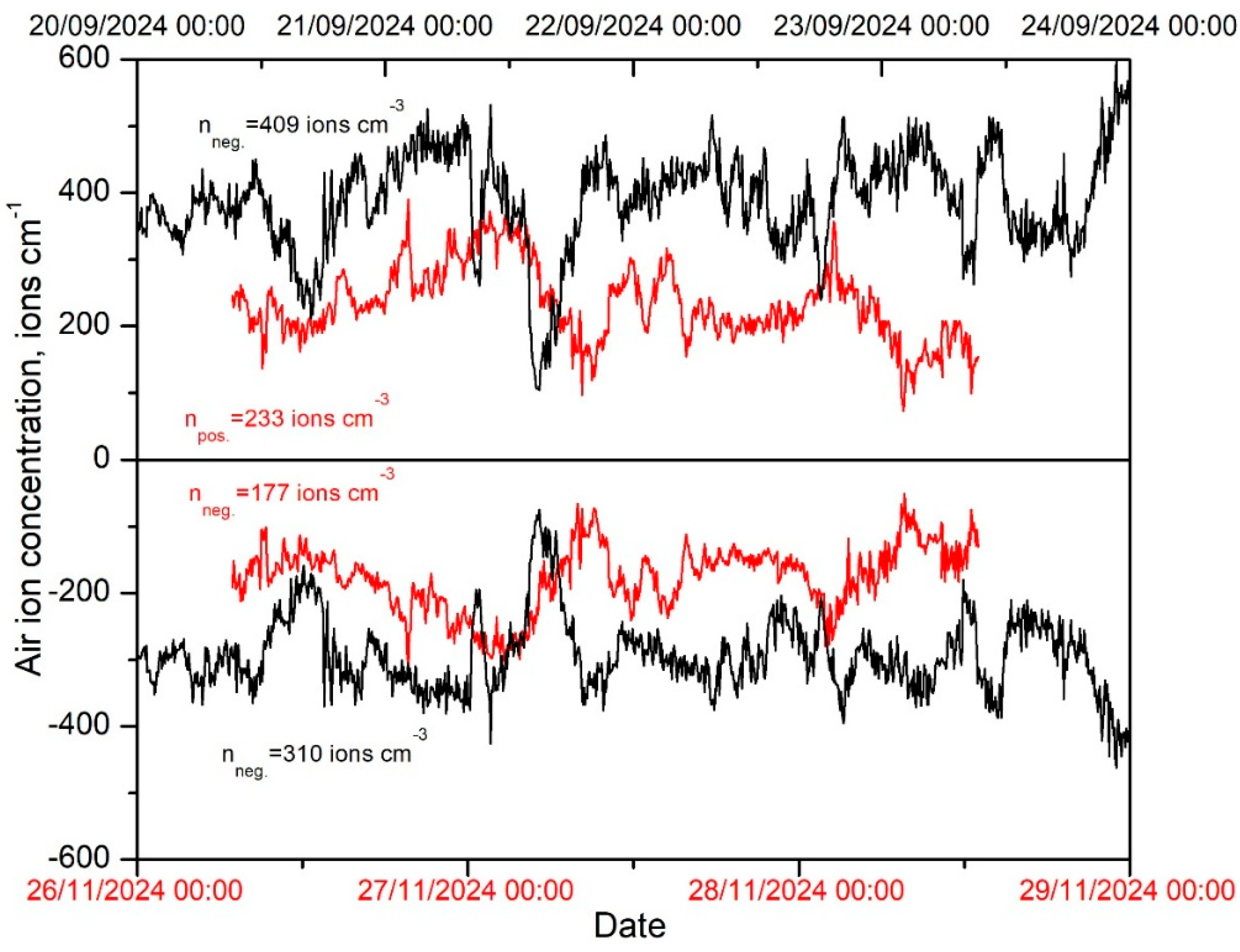
3.4. Air Ion Concentrations
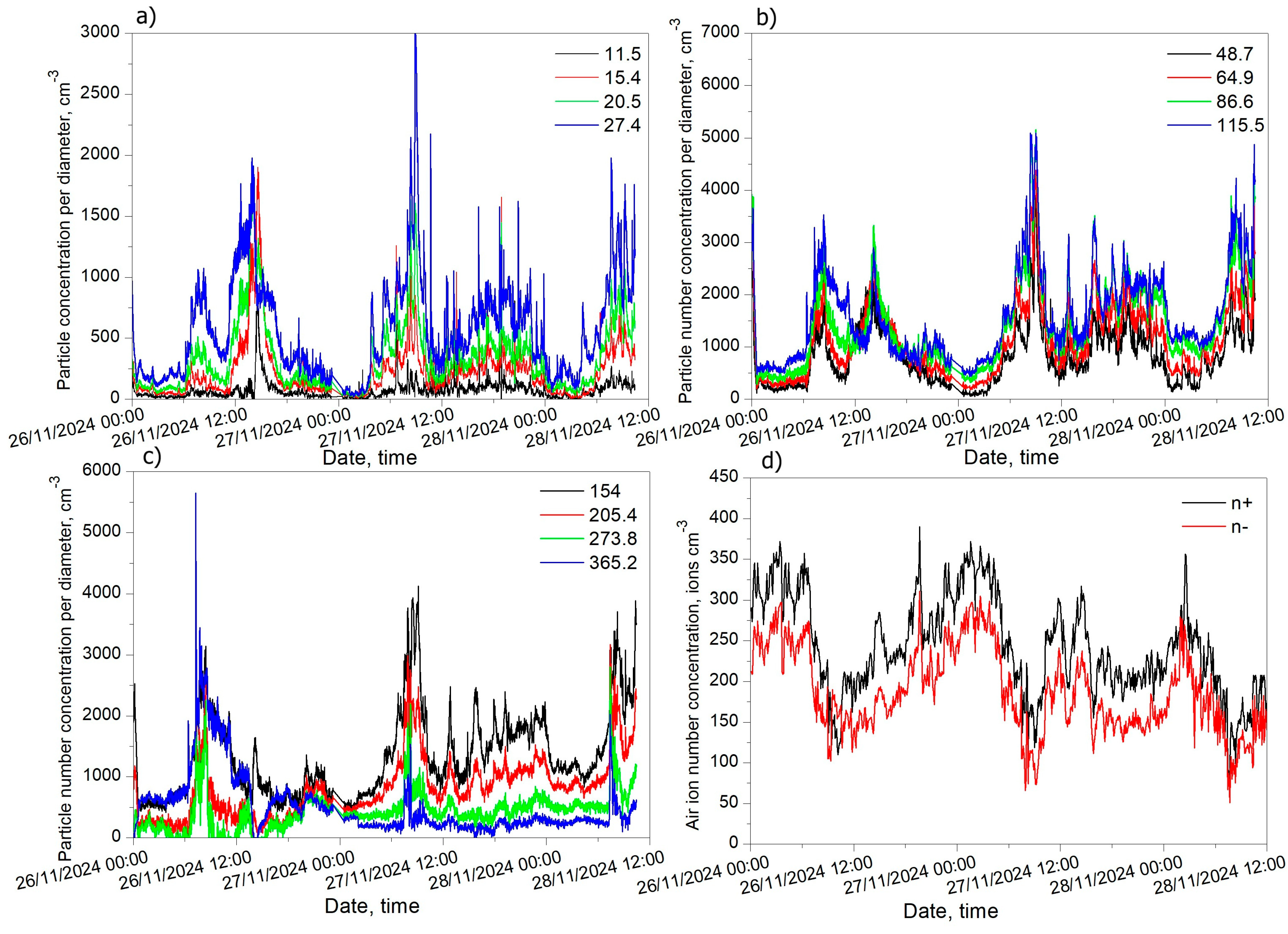
3.5. Nanoparticle Concentration Measured in Highly Polluted Air in the City Canter
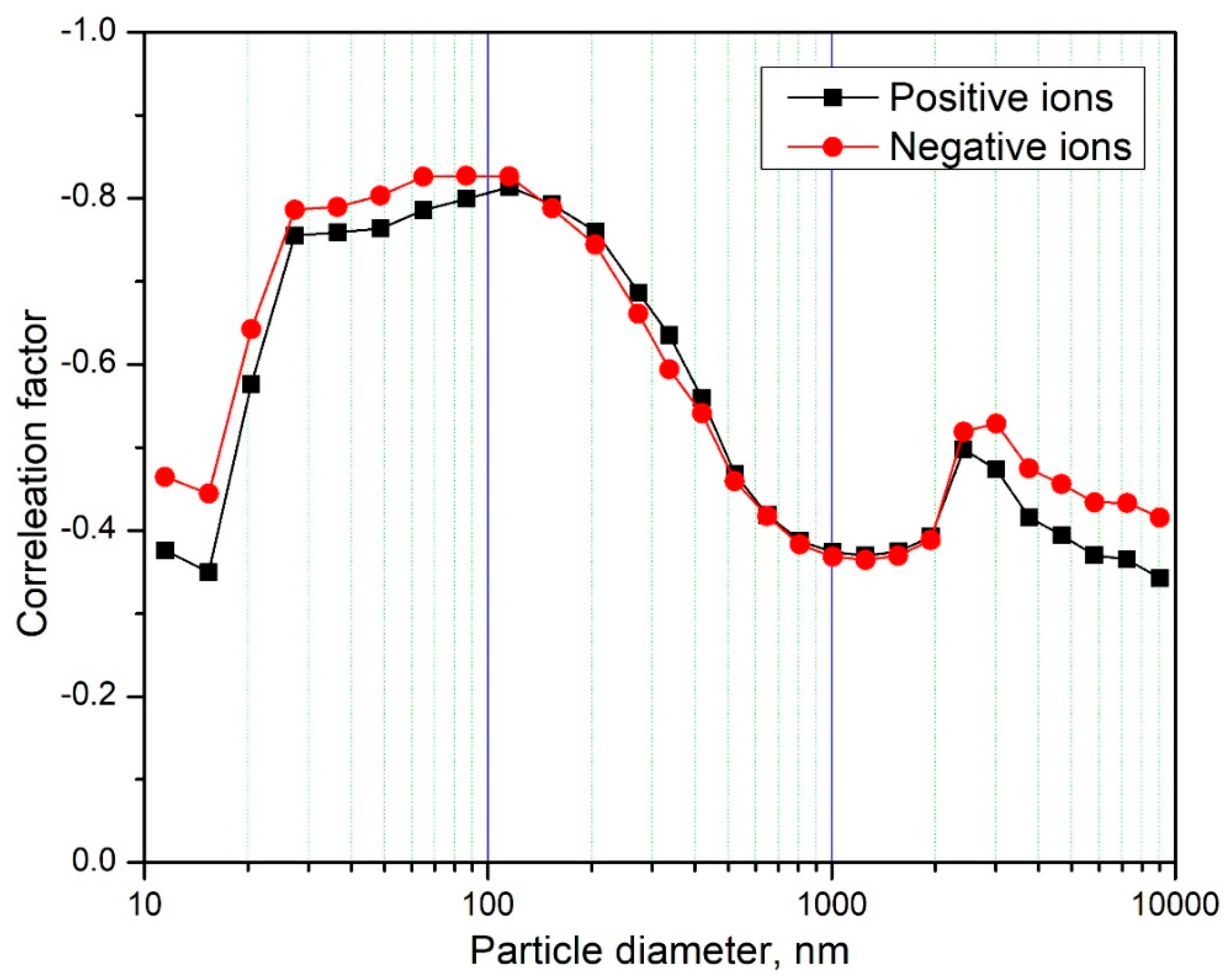
3.6. Correlation Coefficient Between Air Ion Concentration and Particle Concentration by Diameter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.Y.; Ma, A.; Ramachandran, S. Negative Air Ions and Their Effects on Human Health and Air Quality Improvement. Int. J. Mol. Sci. 2018, 19, 2966. [Google Scholar] [CrossRef]
- Majić, M. Sale and Combustion of Tailings as Coal Blamed for Increased Air Pollution in Serbia. Balkan Green Energy News. 2022. Available online: https://balkangreenenergynews.com (accessed on 24 June 2025).
- Ling, X.; Jayaratne, R.; Morawska, L. The relationship between airborne small ions and particles in urban environments. Atmos. Environ. 2013, 79, 1–6. [Google Scholar] [CrossRef]
- Hartmann, A.; Kriegel, M. The influence of air ions on the particle concentration in indoor environments—A systematic literature review. Results Eng. 2022, 15, 100528. [Google Scholar] [CrossRef]
- Kukolj, S.; Stauffer, A.; Matković, V.; Zander, E. The Health Harm of Air Pollution in Belgrade; Health and Environment Alliance (HEAL): Brussels, Belgium, 2019. [Google Scholar]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Stojanovska, Z.; Boev, B.; Zunic, Z.S.; Ivanova, K.; Ristova, M.; Tsenova, M.; Stojanovska, M. Variation of indoor radon concentration and ambient dose equivalent rate in different outdoor and indoor environments. Radiat. Environ. Biophys. 2016, 55, 171–183. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources, Effects and Risks of Ionizing Radiation: Report to the General Assembly, with Scientific Annexes; UNSCEAR: New York, NY, USA, 2016. [Google Scholar]
- International Commission on Radiological Protection (ICRP). Lung cancer risk from radon and progeny and statement on radon. ICRP Publication 115. Ann. ICRP 2010, 40, 1–64. [Google Scholar] [CrossRef]
- Hirsikko, A.; Nieminen, T.; Gagné, S.; Lehtipalo, K.; Manninen, H.E.; Ehn, M.; Hõrrak, U.; Kerminen, V.-M.; Laakso, L.; McMurry, P.H.; et al. Atmospheric ions and nucleation: A review of observations. Atmos. Chem. Phys. 2011, 11, 767–798. [Google Scholar]
- Hoppel, W.A.; Anderson, R.V.; Willett, J.C. Atmospheric Electricity and the Earth’s Environment; Naval Research Laboratory Report 8964; The National Academies Press: Washington, DC, USA, 1986. [Google Scholar]
- Jiang, K.; Pan, Z.; Pan, F.; Teuling, A.J.; Han, G.; An, P.; Chen, X.; Wang, J.; Song, Y.; Cheng, L.; et al. Combined influence of soil moisture and atmospheric humidity on land surface temperature under different climatic background. iScience 2023, 26, 106837. [Google Scholar] [CrossRef]
- Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP). Measurement of Radon and Radon Daughters in Air; NCRP Report No. 97; National Council on Radiation Protection and Measurements: Bethesda, MD, USA, 1988. [Google Scholar]
- Israel, H. Atmospheric Electricity; Israel Program for Scientific Translations: Jerusalem, Israel, 1970; Volume 2. [Google Scholar]
- Harrison, R.G.; Carslaw, K.S. Ion-aerosol-cloud processes in the lower atmosphere. Rev. Geophys. 2003, 41, 1012. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- McMurry, P.H. A review of atmospheric aerosol measurements. Atmos. Environ. 1999, 34, 1959–1999. [Google Scholar] [CrossRef]
- Kulmala, M.; Vehkamäki, H.T.K.; Petäjä, T.; Dal Maso, M.; Lauri, A.; Kerminen, V.-M.; Birmili, W.; McMurry, P.H. Formation and growth rates of ultrafine atmospheric particles: A review of observations. J. Aerosol Sci. 2004, 35, 143–176. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Harder, V.; Ibald-Mulli, A.; Khandoga, A.; Koenig, W.; Krombach, F.; Radykewicz, R.; Stampfl, A.; Thorand, B.; Peters, A. Cardiovascular effects of fine and ultrafine particles. J. Aerosol Med. 2005, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Leikauf, G.D.; Kim, S.H.; Jang, A.S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef]
- Tammet, H. Size and mobility of nanometer particles, clusters and ions. J. Aerosol Sci. 1995, 26, 459–476. [Google Scholar] [CrossRef]
- Tammet, H.; Hõrrak, U.; Laakso, L.; Kulmala, M. Factors of air ion balance in a coniferous forest according to measurements in Hyytiälä, Finland. Atmos. Chem. Phys. 2006, 6, 3377–3390. [Google Scholar] [CrossRef]
- Laakso, L.; Petäjä, T.; Lehtinen, K.E.J.; Kulmala, M.; Paatero, J.; Hõrrak, U.; Tammet, H.; Joutsensaari, J. Ion production rate in a boreal forest based on ion, particle and radiation measurements. Atmos. Chem. Phys. 2004, 4, 1933–1943. [Google Scholar] [CrossRef]
- Hõrrak, U.; Mirme, A.; Salm, J.; Tamm, E.; Tammet, H. Air ion measurements as a source of information about atmospheric aerosols. Atmos. Res. 1998, 46, 233–242. [Google Scholar] [CrossRef]
- Harrison, R.G. Atmospheric Electricity and Cloud Microphysics; Springer: Cham, Switzerland, 2023; pp. 15–20. [Google Scholar]
- Kolarž, P.; Ilić, A.; Janković, M.; Janićijević, A.; Trbovich, A.M. Estimating aerosol particle removal in indoor air by ion-enhanced deposition. J. Aerosol Sci. 2023, 173, 106199. [Google Scholar] [CrossRef]
- Xiao, S.; Wei, T.; Petersen, J.D.; Zhou, J.; Lu, X. Biological effects of negative air ions on human health and integrated multiomics to identify biomarkers: A literature review. Environ. Sci. Pollut. Res. 2023, 30, 69824–69836. [Google Scholar] [CrossRef]
- Krueger, A.P.; Reed, E.J. Biological impact of small air ions. Science 1976, 193, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Kolarž, P.; Miljković, B.; Ćurguz, Z. Air-ion counter and mobility spectrometer. Nucl. Instrum. Methods B 2011, 279, 219–222. [Google Scholar] [CrossRef]
- Republic Hydrometeorological Service of Serbia (RHMSS). Seasonal Bulletin for Serbia: Autumn 2024; Division for Climate Monitoring and Climate Forecast: Belgrade, Serbia, 2024. [Google Scholar]
- Sesana, L.; Caprioli, E.; Marcazzan, G.M. Long period study of outdoor radon concentration in Milan and correlation with atmospheric dispersion. J. Environ. Radioact. 2003, 65, 147–160. [Google Scholar] [CrossRef]
- Porstendörfer, J. Properties and behaviour of radon and thoron and their decay products in the air. J. Aerosol Sci. 1994, 25, 219–263. [Google Scholar] [CrossRef]
- Čeliković, I.; Pantelić, G.; Vukanac, I.; Nikolić, J.K.; Živanović, M.; Cinelli, G.; Gruber, V.; Baumann, S.; Ciotoli, G.; Poncela, L.S.Q.; et al. Overview of Radon Flux Characteristics, Measurements, Models and Its Potential Use for the Estimation of Radon Priority Areas. Atmosphere 2022, 13, 2005. [Google Scholar] [CrossRef]
- Whiteman, C.D.; Hoch, S.W.; Horel, J.D.; Charland, A. Relationship between particulate air pollution and meteorological variables in Utah’s Salt Lake Valley. Atmos. Environ. 2014, 94, 742–753. [Google Scholar] [CrossRef]
- Kumar, K.C.; Kamsali, N. One year of 222Rn concentration at a typical rural site in South India. Radiat. Prot. Environ. 2021, 44, 73–78. [Google Scholar] [CrossRef]
- Stranden, E.; Kolstad, A.K.; Lind, B. The influence of moisture and temperature on radon exhalation. Radiat. Prot. Dosim. 1984, 7, 55–58. [Google Scholar] [CrossRef]
- Rahman, S.; Matiullah Ghauri, B. Effect of moisture on the radon exhalation rate from soil, sand and brick samples. Radiat. Prot. Dosim. 2008, 130, 172–177. [Google Scholar] [CrossRef]
- Kolarž, P.; Ćurguz, Z. Air ions as indicators of short-term indoor radon variations. Appl. Radiat. Isot. 2015, 99, 179–185. [Google Scholar] [CrossRef]
- Kolarž, P.M.; Filipović, D.M.; Marinković, B.P. Daily variations of indoor air-ion and radon concentrations. Appl. Radiat. Isot. 2009, 67, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Tiitta, P.; Miettinen, P.; Vaattovaara, P.; Laaksonen, A.; Joutsensaari, J.; Hirsikko, A.; Aalto, P.; Kulmala, M. Road-side measurement of aerosol and ion number size distributions. Boreal Environ. Res. 2007, 12, 311–321. [Google Scholar]
- Yang, J.; Busen, H.; Scherb, H.; Hürkamp, K.; Guo, Q.; Tschiersch, J. Modeling of radon exhalation from soil influenced by environmental parameters. Sci. Total Environ. 2019, 656, 1304–1311. [Google Scholar] [CrossRef]
- Čeliković, I.; Pantelić, G.; Vukanac, I.; Krneta Nikolić, J.; Živanović, M.; Cinelli, G.; Gruber, V.; Baumann, S.; Quindos Poncela, L.S.; Rabago, D. Outdoor radon as a tool to estimate radon priority areas: A literature overview. Int. J. Environ. Res. Public Health 2022, 19, 662. [Google Scholar] [CrossRef]
- Hõrrak, U.; Salm, J.; Tammet, H. Diurnal variation in the concentration of air ions and aerosol particles. J. Geophys. Res. 2000, 105, 9291–9302. [Google Scholar] [CrossRef]
- Dos Santos, V.N.; Herrmann, E.; Manninen, H.E.; Hussein, T.; Hakala, J.; Nieminen, T.; Aalto, P.P.; Merkel, M.; Wiedensohler, A.; Kulmala, M.; et al. Variability of air ion concentrations in urban Paris. Atmos. Chem. Phys. 2015, 15, 13717–13737. [Google Scholar] [CrossRef]
- Rowell, A.; Brean, J.; Beddows, D.C.S.; Shi, Z.; Kumar, A.; Rissanen, M.; Dal Maso, M.; Mettke, P.; Weinhold, K.; Merkel, M.; et al. The behaviour of charged particles (ions) during new particle formation events in urban Leipzig, Germany. Atmos. Chem. Phys. 2024, 24, 10349–10361. [Google Scholar] [CrossRef]
- Cheung, H.C.; Chou, C.C.K.; Jayaratne, E.R.; Morawska, L. Impact of particle formation on atmospheric ions and particle number concentrations in an urban environment. Atmos. Res. 2015, 157, 127–136. [Google Scholar] [CrossRef]
- Harrison, R.M. Urban atmospheric chemistry: A review of current understanding. Environ. Pollut. 2000, 110, 3–25. [Google Scholar]
- Weber, R.J.; McMurry, P.H.; Mauldin, R.L.; Tanner, D.J.; Eisele, F.L.; Clarke, A.D.; Kapustin, V.N. New particle formation in the remote troposphere: A comparison of observations at various sites. Geophys. Res. Lett. 1999, 26, 307–310. [Google Scholar] [CrossRef]
- Graeffe, G.; Keskinen, J.; Lehtimäki, M. Small ion concentration in houses with enhanced radon concentration. Environ. Int. 1989, 15, 309–313. [Google Scholar] [CrossRef]
- Akinloye, M.K.; Olomo, J.B.; Oladapo, O. Effect of soil moisture on radon exhalation rate from soils. Radiat. Prot. Dosim. 2020, 190, 152–159. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabek, F.; Kolarž, P.; Čeliković, I.; Ćurčić, M.; Janičijević, A. Interaction Between Radon, Air Ions, and Ultrafine Particles Under Contrasting Atmospheric Conditions in Belgrade, Serbia. Atmosphere 2025, 16, 808. https://doi.org/10.3390/atmos16070808
Shabek F, Kolarž P, Čeliković I, Ćurčić M, Janičijević A. Interaction Between Radon, Air Ions, and Ultrafine Particles Under Contrasting Atmospheric Conditions in Belgrade, Serbia. Atmosphere. 2025; 16(7):808. https://doi.org/10.3390/atmos16070808
Chicago/Turabian StyleShabek, Fathya, Predrag Kolarž, Igor Čeliković, Milica Ćurčić, and Aco Janičijević. 2025. "Interaction Between Radon, Air Ions, and Ultrafine Particles Under Contrasting Atmospheric Conditions in Belgrade, Serbia" Atmosphere 16, no. 7: 808. https://doi.org/10.3390/atmos16070808
APA StyleShabek, F., Kolarž, P., Čeliković, I., Ćurčić, M., & Janičijević, A. (2025). Interaction Between Radon, Air Ions, and Ultrafine Particles Under Contrasting Atmospheric Conditions in Belgrade, Serbia. Atmosphere, 16(7), 808. https://doi.org/10.3390/atmos16070808





