Chemical Composition and Mixing States of Individual Particles in Indoor and Outdoor Atmospheres
Abstract
1. Introduction
2. Experimental
2.1. Sampling of Individual Particles
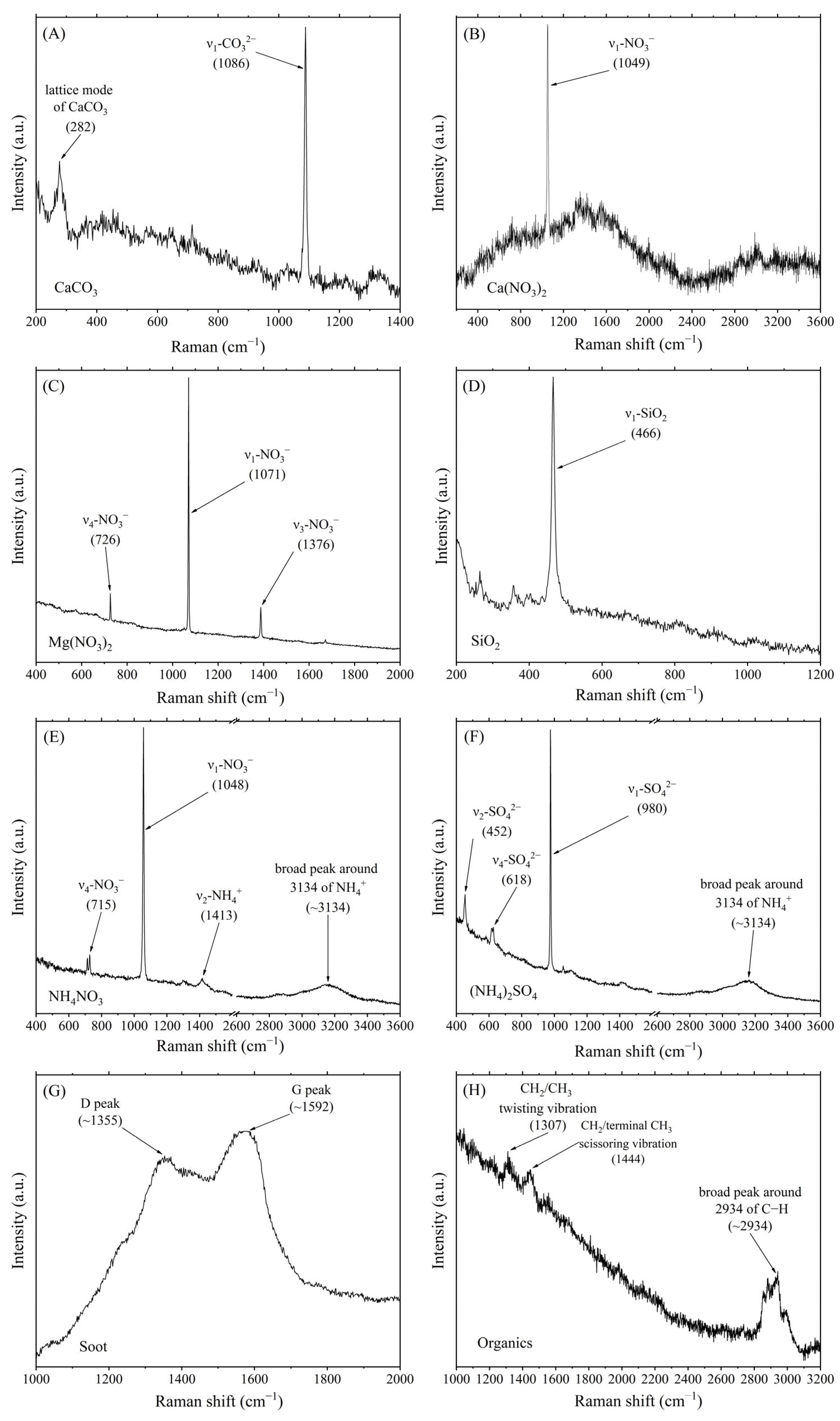
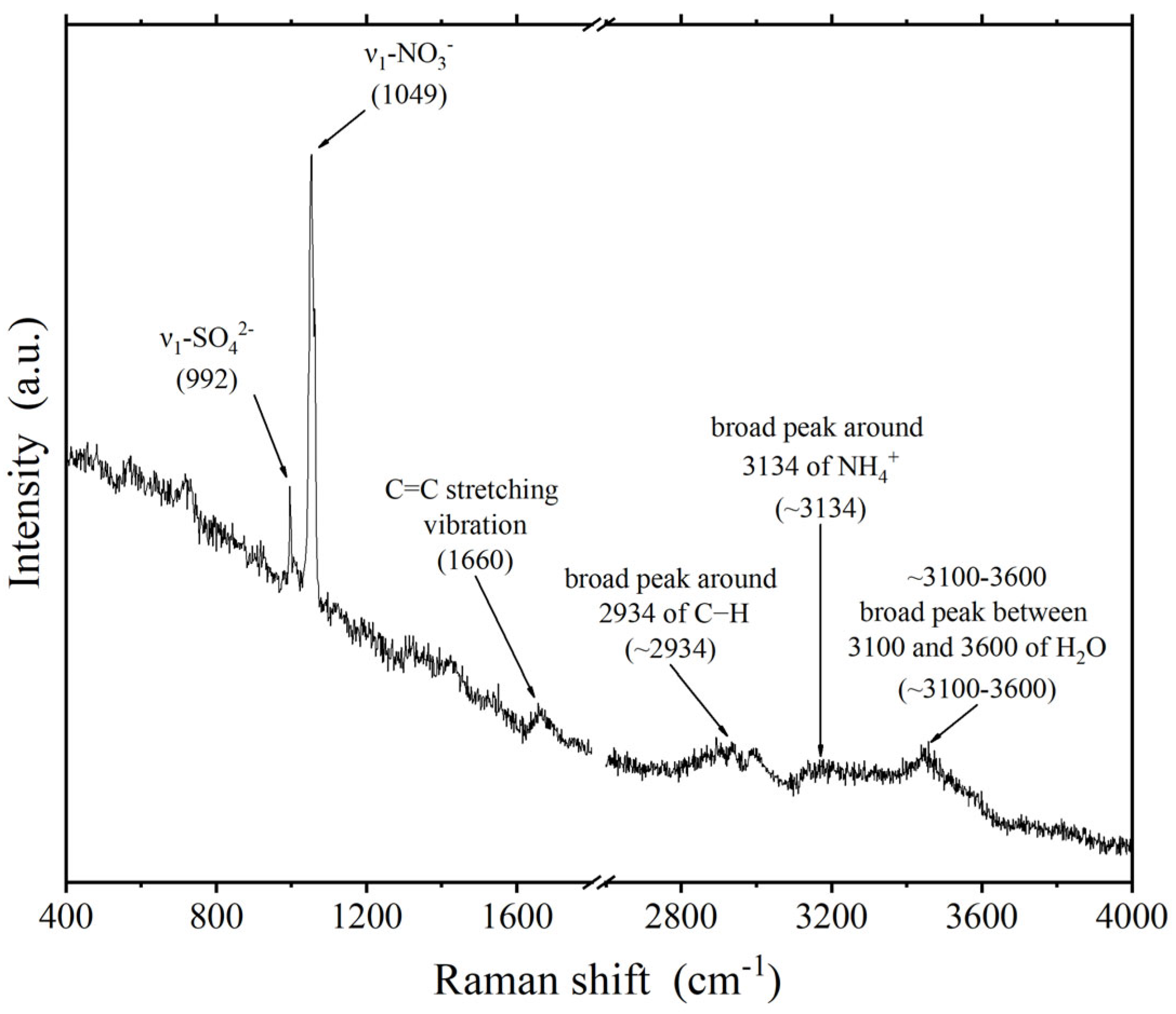
2.2. Raman Spectroscopy Measurements
3. Results and Discussion
3.1. Composition of Individual Particles Indoors and Outdoors
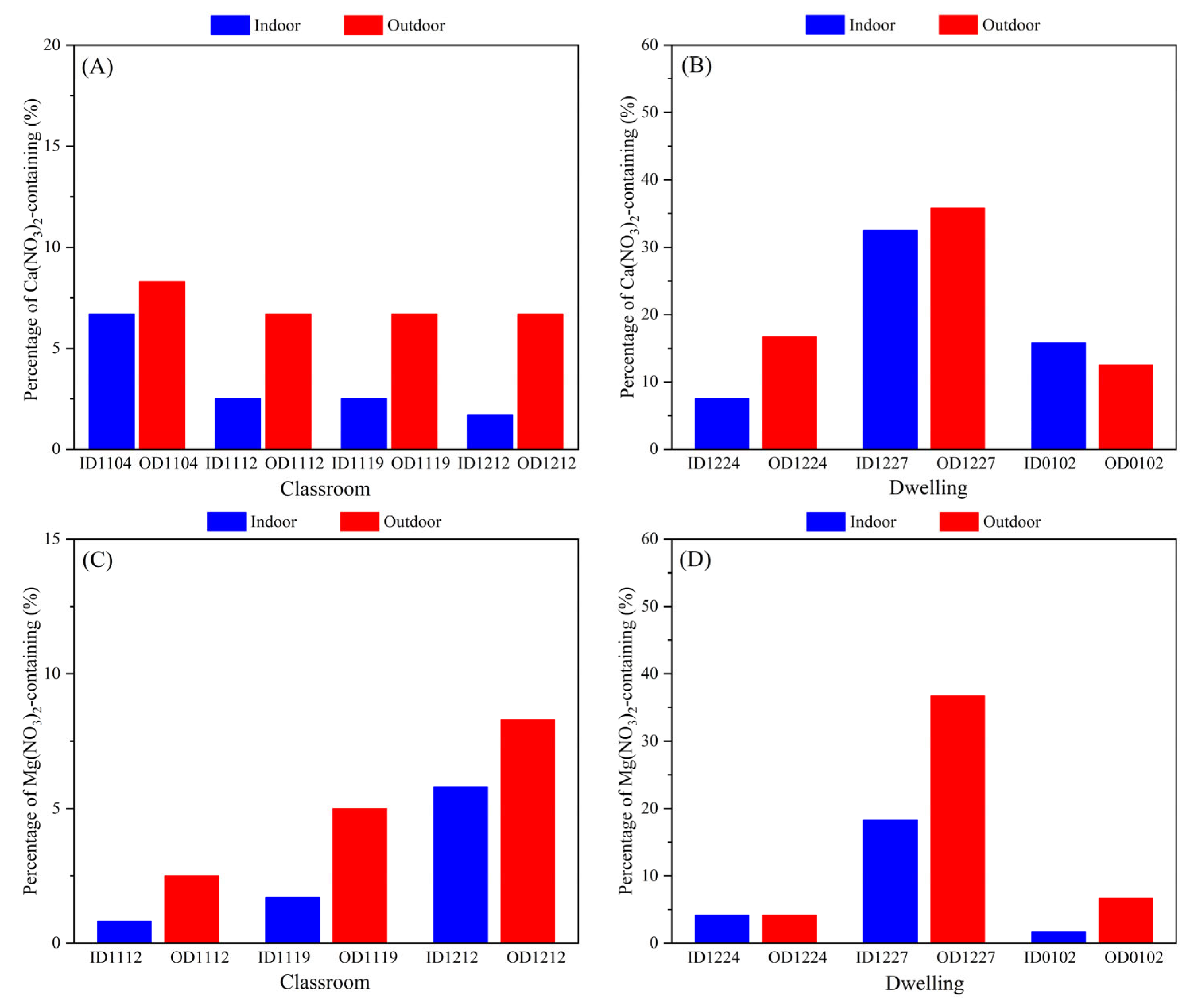
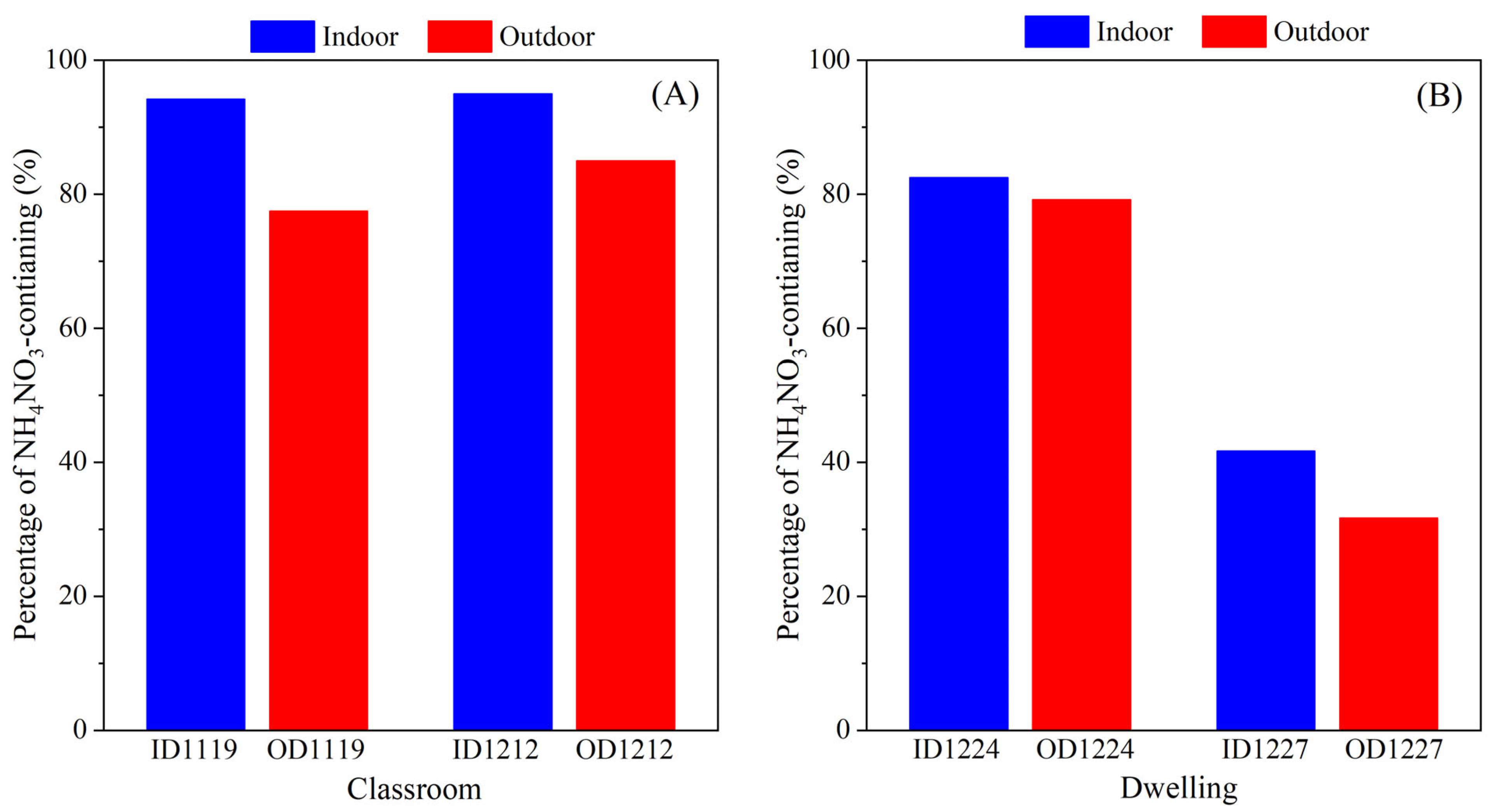
3.2. Comparison of Individual Particle Chemical Composition
3.3. Inorganics with Different Mixing States
3.4. Carbonaceous Matter with Different Mixing States
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jentzsch, P.V.; Bolanz, R.M.; Ciobota, V.; Kampe, B.; Rosch, P.; Majzlan, J.; Popp, J. Raman spectroscopic study of calcium mixed salts of atmospheric importance. Vib. Spectrosc. 2012, 61, 206–213. [Google Scholar] [CrossRef]
- Azimi, P.; Stephens, B. A framework for estimating the US mortality burden of fine particulate matter exposure attributable to indoor and outdoor microenvironments. J. Expo. Sci. Environ. Epidemiol. 2018, 30, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, Y.Q.; Chen, W.; Wang, Y.W.; Qiu, X.H.; Li, W.J.; Hu, M.; Wu, Y.S.; Wang, Q.; Zhang, H.X.Y.; et al. Respiratory inflammation and short-term ambient air pollution exposures in adult Beijing residents with and without prediabetes: A panel study. Environ. Health Perspect. 2020, 128, 067004. [Google Scholar] [CrossRef]
- Block, M.L.; Calderón-Garciduenas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef]
- Zhang, J.K.; Su, Y.F.; Chen, C.Y.; Fu, X.Y.; Long, Y.H.; Peng, X.X.; Huang, X.J.; Wang, G.H.; Zhang, W. Insights into the seasonal characteristics of single particle aerosols in Chengdu based on SPAMS. J. Environ. Sci. 2025, 149, 431–443. [Google Scholar] [CrossRef]
- Zhang, N.; Maung, M.W.; Win, M.S.; Feng, J.L.; Yao, X.H. Carbonaceous aerosol and inorganic ions of PM2.5 in Yangon and Mandalay of Myanmar: Seasonal and spatial variations in composition and sources. Atmos. Pollut. Res. 2022, 13, 101444. [Google Scholar] [CrossRef]
- Xiong, C.; Chen, X.Y.; Ding, X.L.; Kuang, B.Y.; Pei, X.Y.; Xu, Z.N.; Yang, S.K.; Hu, H.; Wang, Z.B. Reconsideration of surface tension and phase state effects on cloud condensation nuclei activity based on the atomic force microscopy measurement. Atmos. Chem. Phys. 2022, 22, 16123–16135. [Google Scholar] [CrossRef]
- Moorchilot, V.S.; Aravind, U.K.; Menacherry, S.P.M.; Aravindakumar, C.T. Single-particle analysis of atmospheric aerosols: Applications of Raman spectroscopy. Atmosphere 2022, 13, 1779. [Google Scholar] [CrossRef]
- Wang, W.H.; Shao, L.Y.; Zhang, D.Z.; Li, Y.W.; Li, W.J.; Liu, P.J.; Xing, J.P. Mineralogical similarities and differences of dust storm particles at Beijing from deserts in the north and northwest. Sci. Total Environ. 2022, 803, 149980. [Google Scholar] [CrossRef]
- Wang, M.J.; Zheng, N.; Zhao, D.F.; Shang, J.; Zhu, T. Using micro-Raman spectroscopy to investigate chemical composition, mixing states, and heterogeneous reactions of individual atmospheric particles. Environ. Sci. Technol. 2021, 55, 10243–10254. [Google Scholar] [CrossRef]
- Xu, L.; Liu, L.; Zhang, J.; Zhang, Y.; Ren, Y.; Wang, X.; Li, W. Morphology, composition, and mixing state of individual aerosol particles in northeast China during wintertime. Atmosphere 2017, 8, 47. [Google Scholar] [CrossRef]
- Ibrahim, F.; Samsudin, E.Z.; Ishak, A.R.; Sathasivam, J. Hospital indoor air quality and its relationships with building design, building operation, and occupant-related factors: A mini-review. Front. Public Health 2022, 10, 1067764. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Pegues, V.; Van Montfrans, S.; Leng, W.; Marr, L.C. Aerosol Emissions from Fuse-Deposition Modeling 3D Printers in a Chamber and in Real Indoor Environments. Environ. Sci. Technol. 2017, 51, 9516–9523. [Google Scholar] [CrossRef]
- Oeder, S.; Dietrich, S.; Weichenmeier, I.; Schober, W.; Pusch, G.; Jörres, R.A.; Schierl, R.; Nowak, D.; Fromme, H.; Behrendt, H.; et al. Toxicity and elemental composition of particulate matter from outdoor and indoor air of elementary schools in Munich, Germany. Indoor Air 2012, 22, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Lee, C.Y.; Zhang, Y.R.; Bai, H.L. Aerosol-assisted deposition of Mn-Fe oxide catalyst on TiO2 for superior selective catalytic reduction of NO with NH3 at low temperatures. Catal. Commun. 2018, 111, 36–41. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, B. Indoor sources strongly contribute to exposure of Chinese urban residents to PM2.5 and NO2. J. Hazard. Mater. 2022, 426, 127829. [Google Scholar] [CrossRef]
- Goldstein, A.H.; Nazaroff, W.W.; Weschler, C.J.; Williams, J. How do indoor environments affect air pollution exposure? Environ. Sci. Technol. 2020, 55, 100–108. [Google Scholar] [CrossRef]
- Raju, S.; Woo, H.; Koehler, K.; Fawzy, A.; Liu, C.; Putcha, N.; Balasubramanian, A.; Peng, R.D.; Lin, C.T.; Lemoine, C.; et al. Indoor air pollution and impaired cardiac autonomic function in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2023, 207, 721–730. [Google Scholar] [CrossRef]
- Masoud, C.G.; Li, Y.; Wang, D.S.; Katz, E.F.; DeCarlo, P.F.; Farmer, D.K.; Vance, M.E.; Shiraiwa, M.; Hildebrandt Ruiz, L. Molecular composition and gas-particle partitioning of indoor cooking aerosol: Insights from a FIGAERO-CIMS and kinetic aerosol modeling. Aerosol Sci. Technol. 2022, 56, 1156–1173. [Google Scholar] [CrossRef]
- Pothier, M.A.; Boedicker, E.; Pierce, J.R.; Vance, M.; Farmer, D.K. From the HOMEChem frying pan to the outdoor atmosphere: Chemical composition, volatility distributions and fate of cooking aerosol. Environ. Sci. Process. Impacts 2023, 25, 314–325. [Google Scholar] [CrossRef]
- Patel, S.; Sankhyan, S.; Boedicker, E.K.; DeCarlo, P.F.; Farmer, D.K.; Goldstein, A.H.; Katz, E.F.; Nazaroff, W.W.; Tian, Y.; Vanhanen, J.; et al. Indoor Particulate Matter during HOMEChem: Concentrations, Size Distributions, and Exposures. Environ. Sci. Technol. 2020, 54, 7107–7116. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Shao, L.Y.; Jones, T.; Feng, X.L.; Ge, S.Y.; Yang, C.X.; Cao, Y.X.; BeruBe, K.; Zhang, D.Z. Atmospheric iron particles in PM2.5 from a subway station, Beijing, China. Atmos. Environ. 2022, 283, 119175. [Google Scholar] [CrossRef]
- Yan, L.; Cheng, Y.; Wu, F.; Chang, H.; Liu, S.; Wang, Y.L.; Yu, C.W.; Zhang, Y.W. Microscopic morphology and elemental composition of atmospheric particles from seven micro-environments in Xi’an. Indoor Built Environ. 2019, 28, 1237–1253. [Google Scholar] [CrossRef]
- Li, J.Y.; Xu, W.Q.; Li, Z.J.; Duan, M.Z.; Ouyang, B.; Zhou, S.; Lei, L.; He, Y.; Sun, J.X.; Wang, Z.F.; et al. Real-time characterization of aerosol particle composition, sources and influences of increased ventilation and humidity in an office. Indoor Air 2021, 31, 1364–1376. [Google Scholar] [CrossRef]
- Perrino, C.; Pelliccioni, A.; Tofful, L.; Canepari, S. Indoor PM10 in university classrooms: Chemical composition and source behavior. Atmos. Environ. 2022, 287, 119260. [Google Scholar] [CrossRef]
- Sheu, R.; Fortenberry, C.F.; Walker, M.J.; Eftekhari, A.; Stonner, C.; Bakker, A.; Peccia, J.; Williams, J.; Morrison, G.C.; Williams, B.J.; et al. Evaluating indoor air chemical diversity, indoor-to-outdoor emissions, and surface reservoirs using high-resolution mass spectrometry. Environ. Sci. Technol. 2021, 55, 10255–10267. [Google Scholar] [CrossRef]
- Xing, F.Y.; Xu, X.M.; Hu, Z.M.; Wen, X.; Duan, W.M.; Pang, X.B.; Fan, M.K. Characterization of PM2.5 composition and mixing state during haze events in Chengdu using Micro-Raman spectroscopy. Atmos. Pollut. Res. 2025, 16, 102411. [Google Scholar] [CrossRef]
- Zhao, D.F.; Zhu, T.; Chen, Q.; Liu, Y.J.; Zhang, Z.F. Raman micro-spectrometry as a technique for investigating heterogeneous reactions on individual atmospheric particles. Sci. China Chem. 2011, 54, 154–160. [Google Scholar] [CrossRef]
- Geng, H.; Jin, C.S.; Zhang, D.P.; Wang, S.R.; Xu, X.T.; Wang, S.R.; Xu, X.T.; Wang, X.R.; Zhang, Y.; Wu, L.; et al. Characterization of size-resolved urban haze particles collected in summer and winter at Taiyuan City, China using quantitative electron probe X-ray microanalysis. Atmos. Res. 2017, 190, 29–42. [Google Scholar] [CrossRef]
- Eom, H.J.; Gupta, D.; Cho, H.R.; Hwang, H.J.; Do Hur, S.; Gim, Y.; Ro, C.U. Single-particle investigation of summertime and wintertime Antarctic Sea spray aerosols using low-Z particle EPMA, Raman microspectrometry, and ATR-FTIR imaging techniques. Atmos. Chem. Phys. 2016, 16, 13823–13836. [Google Scholar] [CrossRef]
- Deng, C.H.; Brooks, S.D.; Vidaurre, G.; Thornton, D.C.O. Using Raman microspectroscopy to determine chemical composition and mixing state of airborne marine aerosols over the Pacific Ocean. Aerosol Sci. Technol. 2013, 48, 193–206. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, D.F.; Song, X.J.; Zhu, T. NO2-initiated multiphase oxidation of SO2 by O2 on CaCO3 particles. Atmos. Chem. Phys. 2018, 18, 6679–6689. [Google Scholar] [CrossRef]
- Sobanska, S.; Hwang, H.; Choel, M.; Jung, H.J.; Eom, H.J.; Kim, H.; Barbillat, J.; Ro, C.U. Investigation of the chemical mixing state of individual Asian dust particles by the combined use of electron probe X-ray microanalysis and Raman microspectrometry. Anal. Chem. 2012, 84, 3145–3154. [Google Scholar] [CrossRef]
- Guo, X.; Tan, S.H.; Shang, Z.J.; Guo, Y.C.; Zhang, Y.H. Confocal Raman spectroscopy studies on the interactions between NH4+, NO3− and H2O in supersaturated NH4NO3 droplets. Acta Phys.-Chim. Sin. 2012, 28, 766–772. [Google Scholar] [CrossRef]
- Chu, Y.X.; Chan, C.K. Reactive uptake of dimethylamine by ammonium sulfate and ammonium sulfate-sucrose mixed particles. J. Phys. Chem. A 2016, 121, 206–215. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Liu, L.; Yang, Y.; Deng, Y.; Li, K.J.; Cheng, H.Y.; Dong, X.; Li, W.J.; Zhang, L.W. The application of Raman spectroscopy combined with multivariable analysis on source apportionment of atmospheric black carbon aerosols. Sci. Total Environ. 2019, 685, 189–196. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2014, 46, 4–20. [Google Scholar] [CrossRef]
- Mbazima, S.J.; Moolla, R.; Joseph, J.S. Release and health outcomes of exposure to chalk particles in classrooms: A systematic literature review. Int. J. Environ. Health Res. 2024, 34, 3515–3539. [Google Scholar] [CrossRef]
- Lunden, M.M.; Revzan, K.L.; Fischer, M.L.; Thatcher, T.L.; Littlejohn, D.; Hering, S.V.; Brown, N.J. The transformation of out-door ammonium nitrate aerosols in the indoor environment. Atmos. Environ. 2003, 37, 5633–5644. [Google Scholar] [CrossRef]
- Kumar, S.; Dwivedi, S.K. Chemical and biological components of atmospheric particulate matter and their impacts on human health and crops: A review. Aerobiologia 2022, 38, 287–327. [Google Scholar] [CrossRef]
- Kristensen, K.; Lunderberg, D.M.; Liu, Y.J.; Misztal, P.K.; Tian, Y.L.; Arata, C.; Nazaroff, W.W.; Goldstein, A.H. Gas-Particle partitioning of semivolatile organic compounds in a residence: Influence of particles from candles, cooking, and outdoors. Environ. Sci. Technol. 2023, 57, 3260–3269. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, K.L.; Delgado-Saborit, J.M.; Harrison, R.M. Emissions and indoor concentrations of particulate matter and its spe-cific chemical components from cooking: A review. Atmos. Environ. 2013, 71, 260–294. [Google Scholar] [CrossRef]
- Fankhauser, A.M.; Butman, J.L.; Cooke, M.E.; Fyodorova, Y.; Liu, Y.D.L.; O’Brien, R.E.; Mcneill, V.F.; Geiger, F.M.; Grassian, V.H.; Ault, A.P. Indoor surface chemistry variability: Microspectroscopic analysis of deposited particles in dwellings across the United States. Environ. Sci. Process. Impacts 2025. [Google Scholar] [CrossRef] [PubMed]
- Lunderberg, D.M.; Kristensen, K.; Liu, Y.J.; Misztal, P.K.; Tian, Y.L.; Arata, C.; Wernis, R.; Kreisberg, N.; Nazaroff, W.W.; Goldstein, A.H. Characterizing airborne phthalate concentrations and dynamics in a normally occupied residence. Environ. Sci. Technol. 2019, 53, 7337–7346. [Google Scholar] [CrossRef]
- Lunderberg, D.M.; Kristensen, K.; Tian, Y.L.; Arata, C.; Misztal, P.K.; Liu, Y.J.; Kreisberg, N.; Katz, E.F.; DeCarlo, P.F.; Patel, S.; et al. Surface emissions modulate indoor SVOC concentrations through volatility-dependent partitioning. Environ. Sci. Technol. 2020, 54, 6751–6760. [Google Scholar] [CrossRef]
- Nazaroff, W.W.; Weschler, C.J. Indoor acids and bases. Indoor Air 2020, 30, 559–644. [Google Scholar] [CrossRef]
- Pandit, S.; Mora Garcia, S.L.M.; Grassian, V.H. HONO production from gypsum surfaces following exposure to NO2 and HNO3,Roles of relative humidity and light source. Environ. Sci. Technol. 2021, 55, 9761–9772. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Ma, Q.X.; Chu, B.W.; He, H. Homogeneous and heterogeneous photolysis of nitrate in the atmosphere: State of the science, current research needs, and future prospects. Front. Environ. Sci. Eng. 2022, 17, 48. [Google Scholar] [CrossRef]
- Dong, Z.W.; Qin, D.H.; Li, K.M.; Kang, S.C.; Wei, T.; Lu, J.F. Spatial variability, mixing states and composition of various haze particles in atmosphere during winter and summertime in northwest China. Environ. Pollut. 2019, 246, 79–88. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]

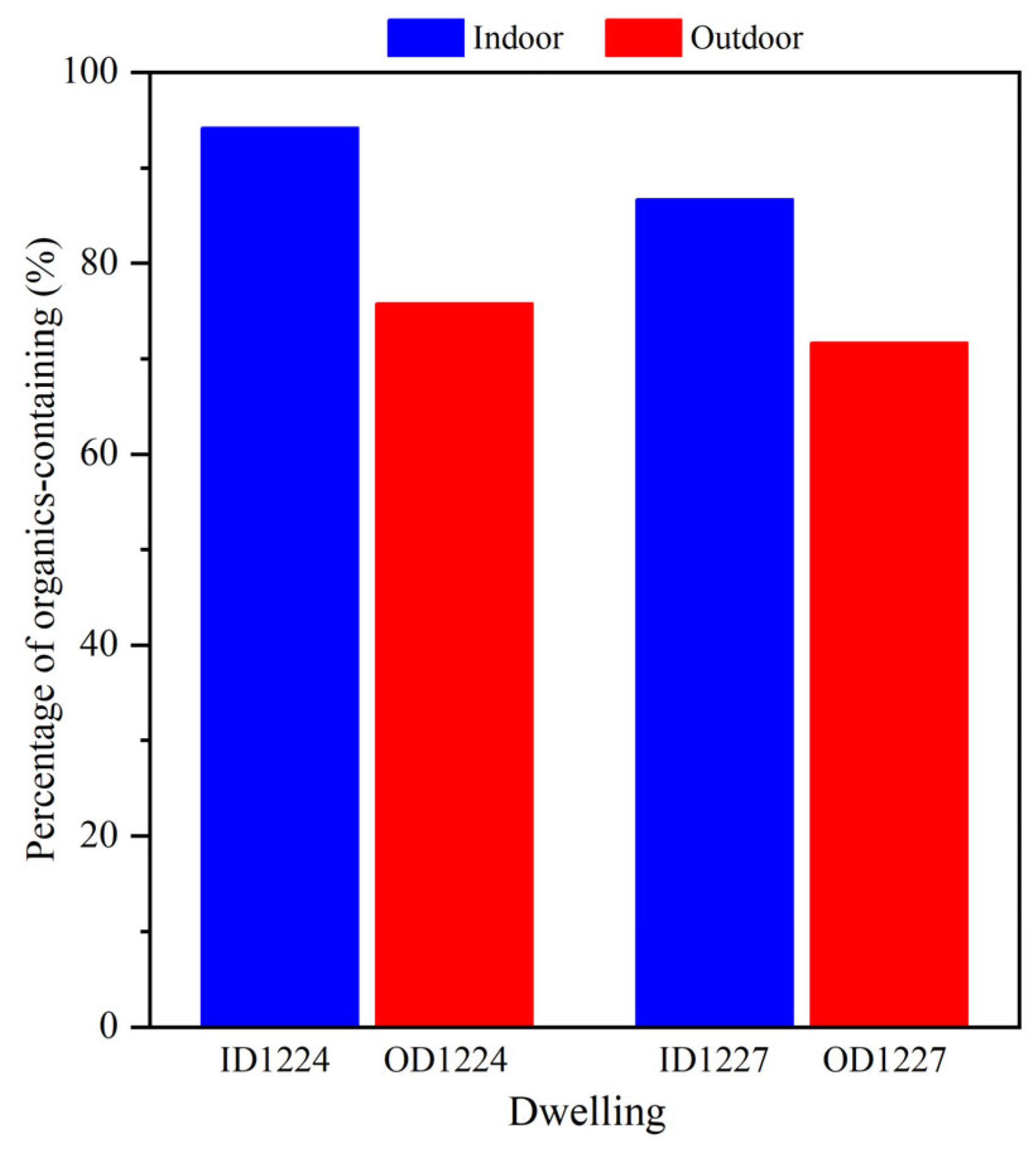

| ID 1104 3 | OD 1104 | ID 1112 | OD 1112 | ID 1119 | OD 1119 | ID 1212 | OD 1212 | ID 1224 | OD 1224 | ID 1227 | OD 1227 | ID 0102 | OD 0102 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca-Salt Particles | |||||||||||||||
| Ca(NO3)2 2 | 24 | 17 | 4 | 1 | 3 | 1 | 2 | 2 | 5 | 2 | 61 | ||||
| CaSO4 | 1 | 1 | 1 | 3 | |||||||||||
| CaCO3 | 1 | 2 | 1 | 4 | |||||||||||
| Ca(NO3)2/CaSO4 | 6 | 8 | 3 | 4 | 2 | 4 | 1 | 2 | 4 | 15 | 22 | 11 | 14 | 7 | 103 |
| CaCO3/Ca(NO3)2 1 | 2 | 1 | 2 | 1 | 1 | 2 | 2 | 4 | 1 | 16 | |||||
| CaCO3/Ca(NO3)2/CaSO4 | 1 | 1 | 1 | 3 | |||||||||||
| GaMg-Salt and SiO2 Particles | |||||||||||||||
| Mg(NO3)2 | 1 | 1 | 1 | 2 | 1 | 1 | 7 | 13 | 2 | 1 | 30 | ||||
| CaMg(CO3)2/Ca(NO3)2 | 1 | 1 | |||||||||||||
| CaMg(CO3)2/Ca(NO3)2/CaSO4 | 1 | 1 | 2 | ||||||||||||
| Ca(NO3)2/Mg(NO3)2 | 2 | 1 | 1 | 4 | 2 | 3 | 13 | 24 | 7 | 57 | |||||
| CaSO4/Mg(NO3)2 | 2 | 1 | 3 | ||||||||||||
| Ca(NO3)2/Mg(NO3)2//CaSO4 | 2 | 2 | 1 | 1 | 2 | 5 | 13 | ||||||||
| SiO2 | 1 | 1 | |||||||||||||
| NH3-Salt Particles | |||||||||||||||
| (NH4)2SO4 | 1 | 1 | |||||||||||||
| NH4NO3 | 52 | 69 | 60 | 58 | 24 | 40 | 61 | 54 | 86 | 74 | 44 | 29 | 84 | 97 | 832 |
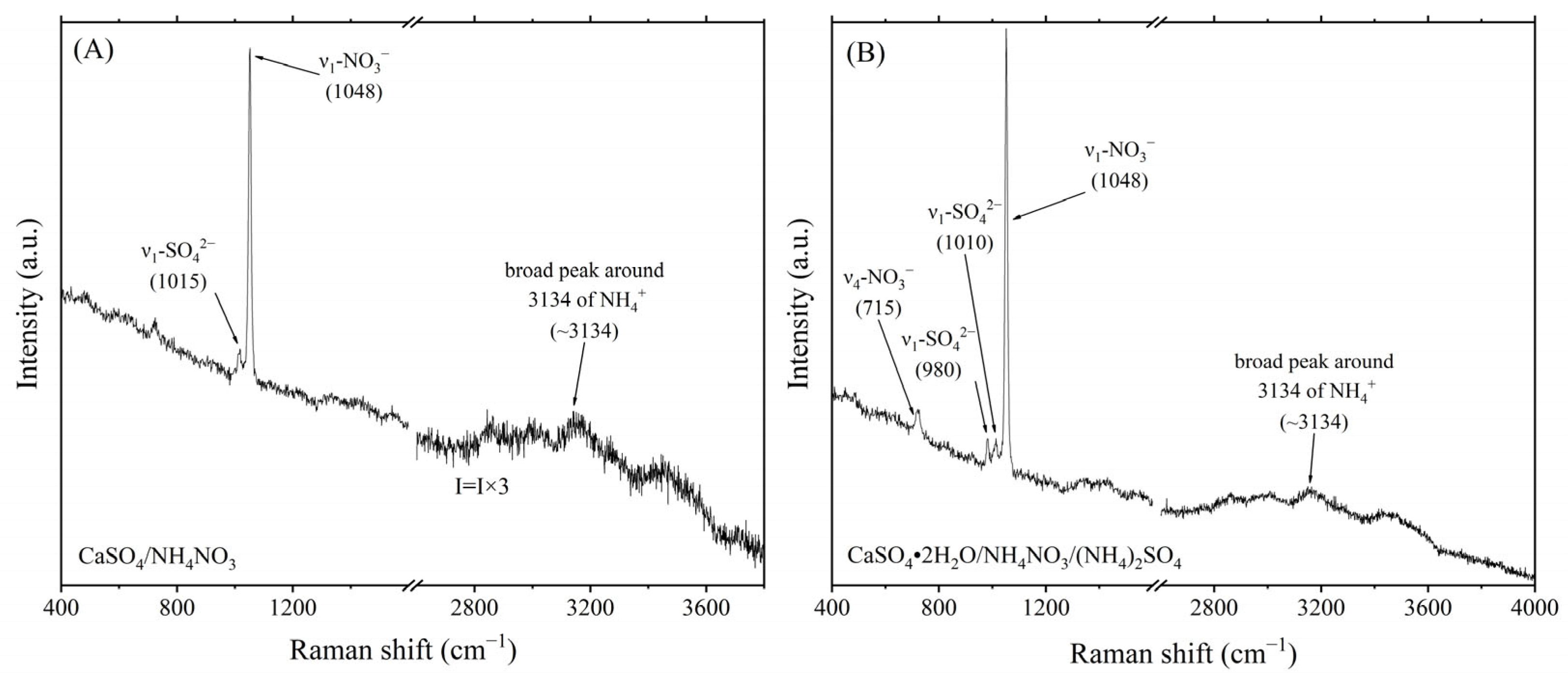
| NH4NO3/(NH4)2SO4 | 13 | 8 | 32 | 39 | 3 | 1 | 10 | 9 | 8 | 10 | 5 | 4 | 9 | 1 | 152 |
| Ca-NH3-Salt Particles | |||||||||||||||
| CaCO3/NH4NO3 | 2 | 1 | 1 | 1 | 5 | ||||||||||
| CaSO4/CaCO3/NH4NO3 | 1 | 1 | |||||||||||||
| CaSO4/NH4NO3 | 13 | 8 | 18 | 9 | 72 | 34 | 35 | 23 | 5 | 11 | 2 | 230 | |||
| CaSO4/NH4NO3/(NH4)2SO4 | 1 | 2 | 1 | 2 | 1 | 7 | |||||||||
| Mg-NH3-Salt Particles | |||||||||||||||
| Mg(NO3)2/NH4NO3 | 1 | 7 | 3 | 1 | 12 | ||||||||||
| Na-NH3-Salt Particles | |||||||||||||||
| Na2SO4/NH4NO3 | 1 | 15 | 27 | 1 | 12 | 3 | 1 | 60 | |||||||
| Total measured particles | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 1680 |
| ID 1104 2 | OD 1104 | ID 1112 | OD 1112 | ID 1119 | OD 1119 | ID 1212 | OD 1212 | ID 1224 | OD 1224 | ID 1227 | OD 1227 | ID 0102 | OD 0102 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soot | 1 | 1 | 1 | 3 | 1 | 1 | 2 | 2 | 12 | ||||||
| Soot/inorganics | 4 | 3 | 10 | 4 | 2 | 3 | 2 | 2 | 5 | 2 | 1 | 1 | 39 | ||
| Soot/organics | 3 | 1 | 1 | 1 | 2 | 1 | 5 | 1 | 1 | 16 | |||||
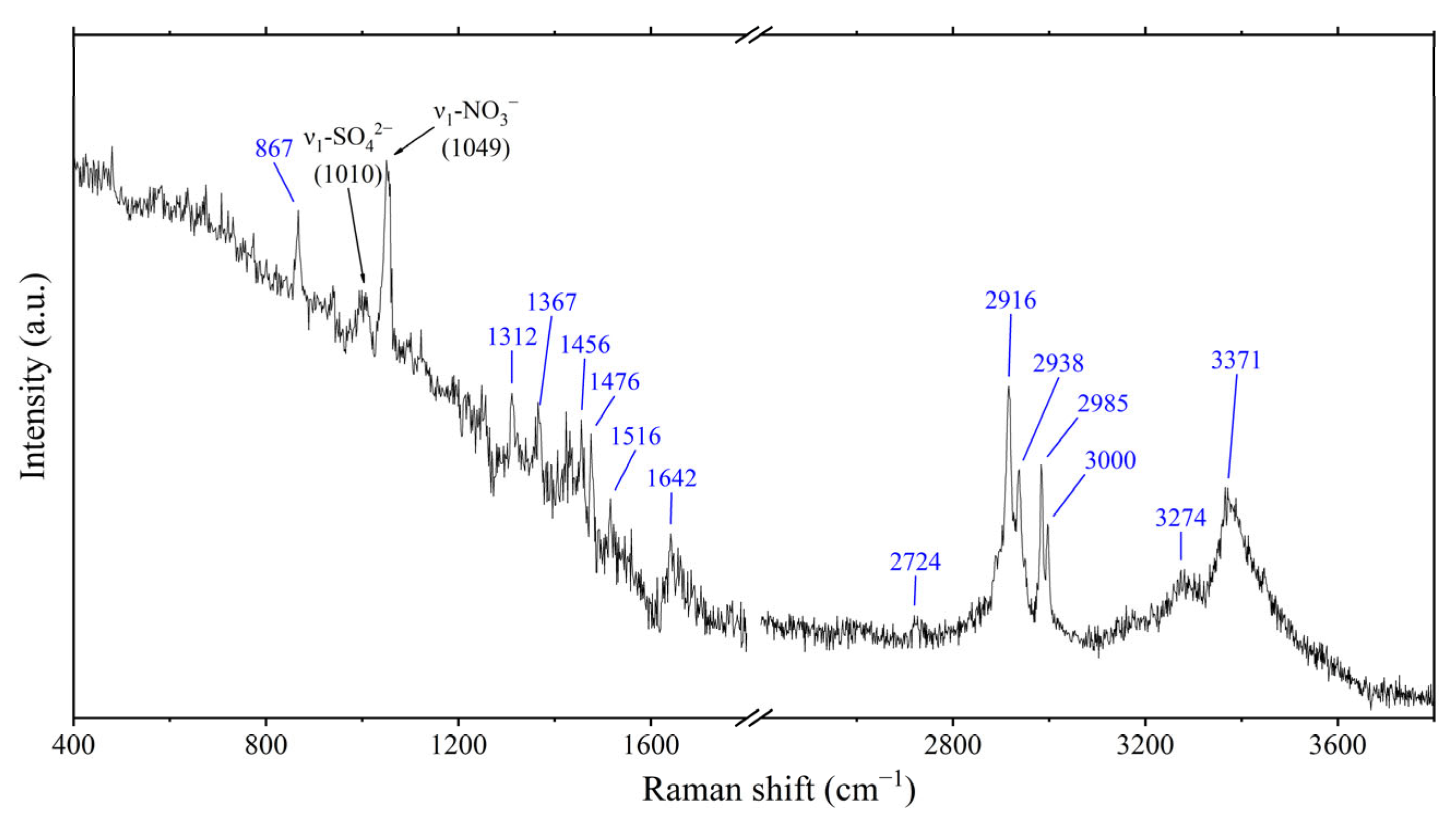
| Soot/organics/inorganics | 17 | 14 | 10 | 4 | 2 | 17 | 16 | 12 | 12 | 14 | 17 | 20 | 34 | 189 | |
| Organics | 1 | 2 | 1 | 1 | 6 | 5 | 1 | 17 | |||||||
| Organics/inorganics 1 | 76 | 63 | 53 | 76 | 98 | 96 | 83 | 85 | 74 | 38 | 84 | 58 | 91 | 75 | 1050 |
| Total measured particles | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 1680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, Q.; Wang, J.; Ye, L.; Zhang, L.; Xu, P.; Wang, M. Chemical Composition and Mixing States of Individual Particles in Indoor and Outdoor Atmospheres. Atmosphere 2025, 16, 707. https://doi.org/10.3390/atmos16060707
Huang Y, Li Q, Wang J, Ye L, Zhang L, Xu P, Wang M. Chemical Composition and Mixing States of Individual Particles in Indoor and Outdoor Atmospheres. Atmosphere. 2025; 16(6):707. https://doi.org/10.3390/atmos16060707
Chicago/Turabian StyleHuang, Yan, Qingcheng Li, Jingjing Wang, Linlin Ye, Linfeng Zhang, Panya Xu, and Mingjin Wang. 2025. "Chemical Composition and Mixing States of Individual Particles in Indoor and Outdoor Atmospheres" Atmosphere 16, no. 6: 707. https://doi.org/10.3390/atmos16060707
APA StyleHuang, Y., Li, Q., Wang, J., Ye, L., Zhang, L., Xu, P., & Wang, M. (2025). Chemical Composition and Mixing States of Individual Particles in Indoor and Outdoor Atmospheres. Atmosphere, 16(6), 707. https://doi.org/10.3390/atmos16060707




