Spatiotemporal Variations and Health Assessment of Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Fine Particles (PM1.1) of a Typical Copper-Processing Area, China
Abstract
1. Introduction
2. Materials and Methods
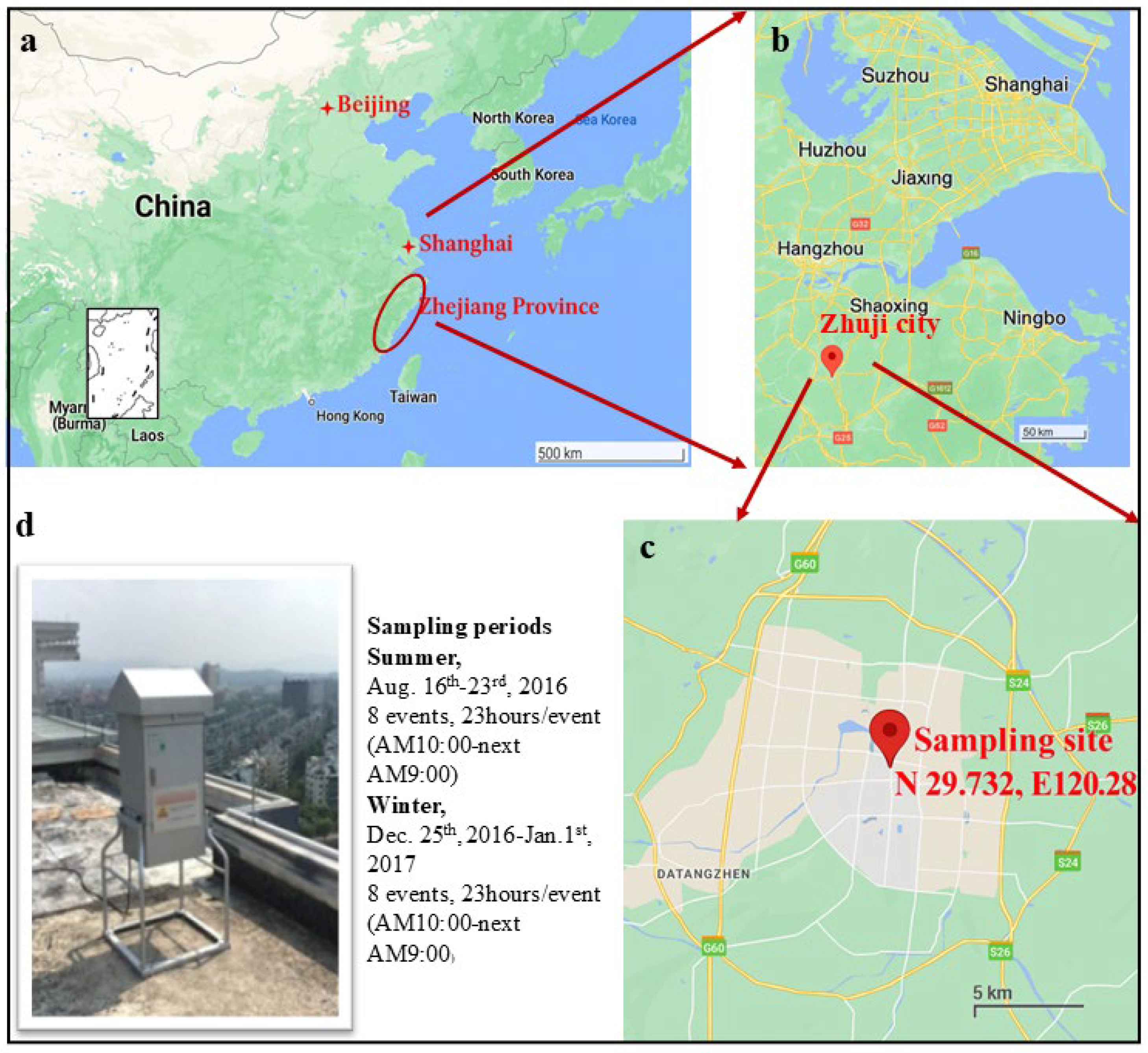
2.1. Ambient Partilce Sampling Site and Sampling Campaigns
2.2. Measurement of Heavy Metal Elements and PAHs in Ambient Particles
2.3. Health Risk Assessment of Heavy Metal Elements and PAHs
2.3.1. Non-Carcinogenic Risks of Heavy Metal Elements
2.3.2. Carcinogenic Risks of Heavy Metal Elements
2.3.3. Carcinogenic Risks of PAHs
2.4. Assessment of Potential Sources of Heavy Metal Elements and PAHs
2.5. Quality Assurance, Quality Control (QA/QC), and Statistical Analysis
3. Results and Discussion
3.1. Variation of Particle Matter (PM), Heavy Metal Elements, and PAHs in PM1.1 and PM2.0
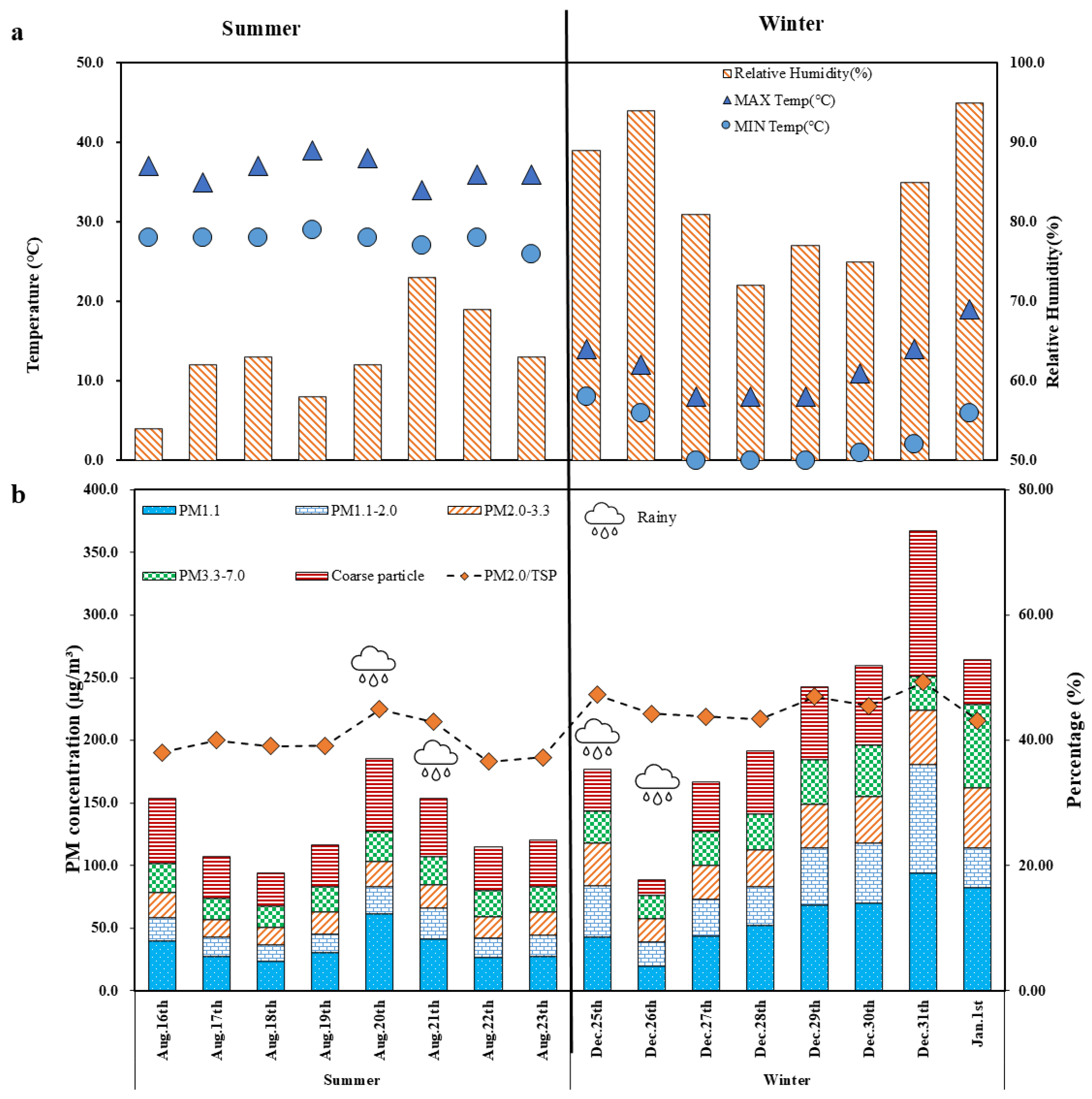
3.1.1. PM in PM1.1 and PM2.0
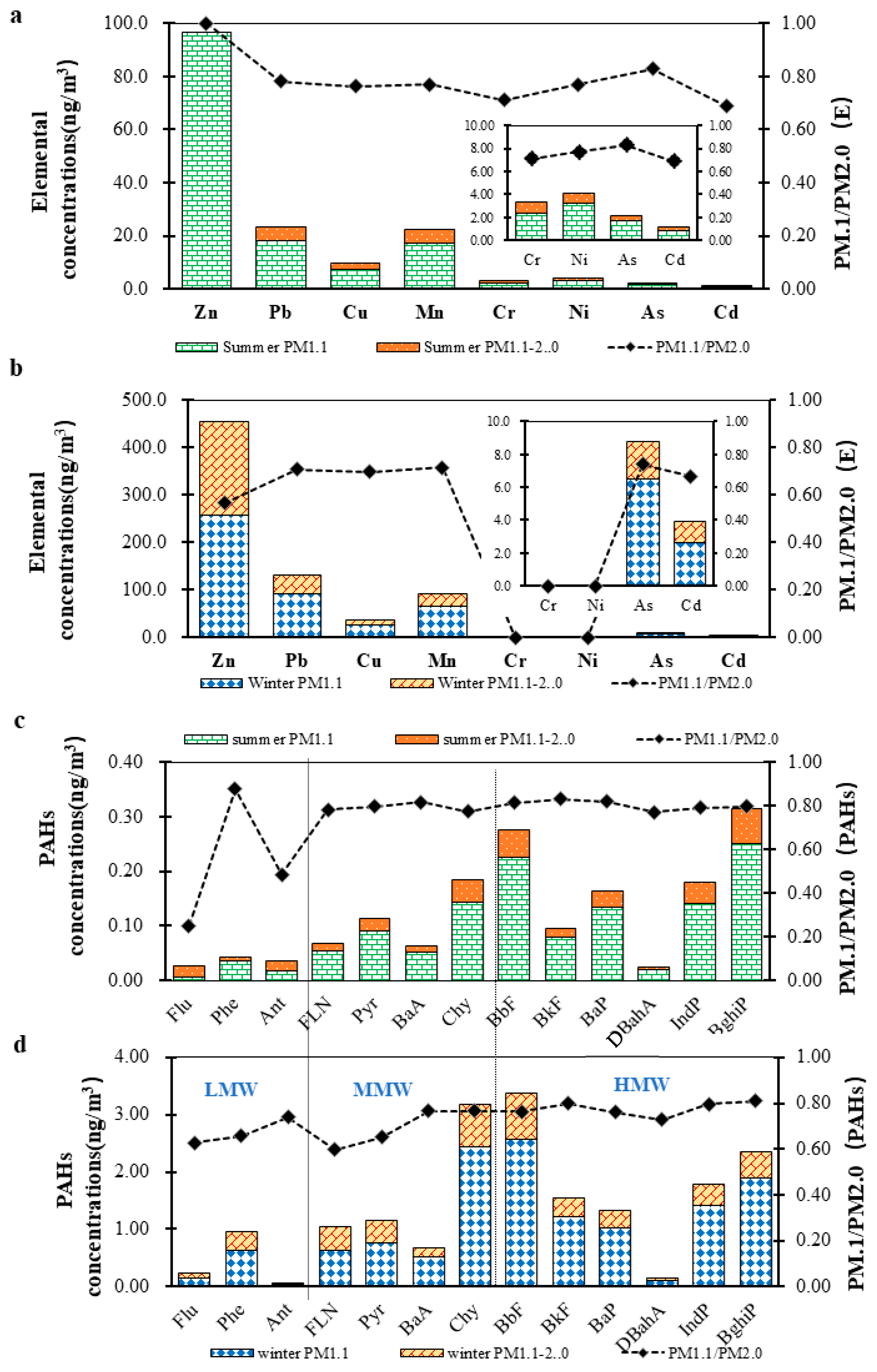
3.1.2. Heavy Metal Elements (HMEs)
3.1.3. PAHs
3.2. Health Risk Assessment of HMEs and PAHs in PM1.1
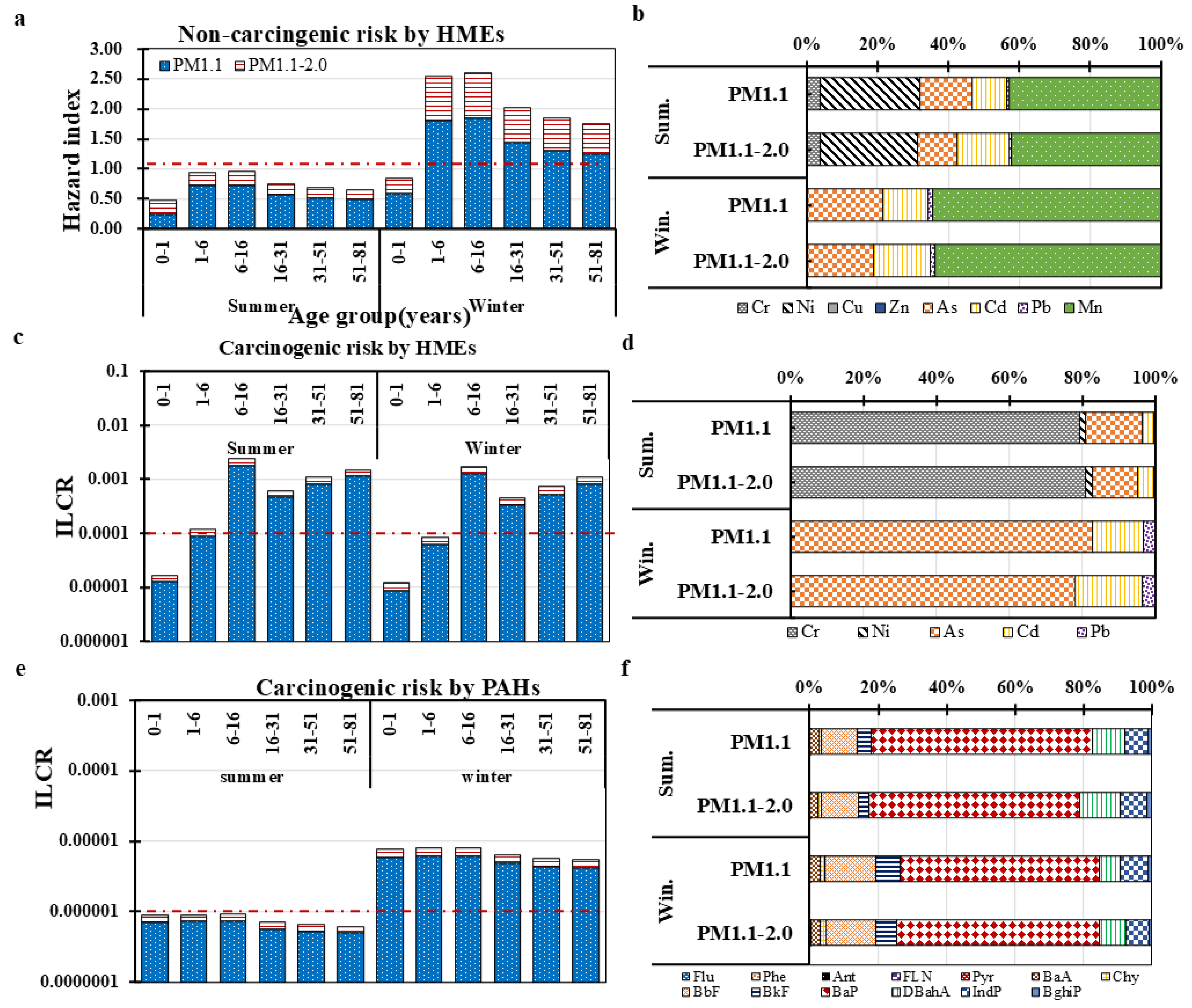
3.2.1. Non-Carcinogenic Risk Assessment of HMEs
3.2.2. Carcinogenic Risk Assessment of HMEs and PAHs
3.3. Assessment of Potential Sources of HMEs and PAHs in PM1.1
3.3.1. Assessment of Potential Sources of HMEs
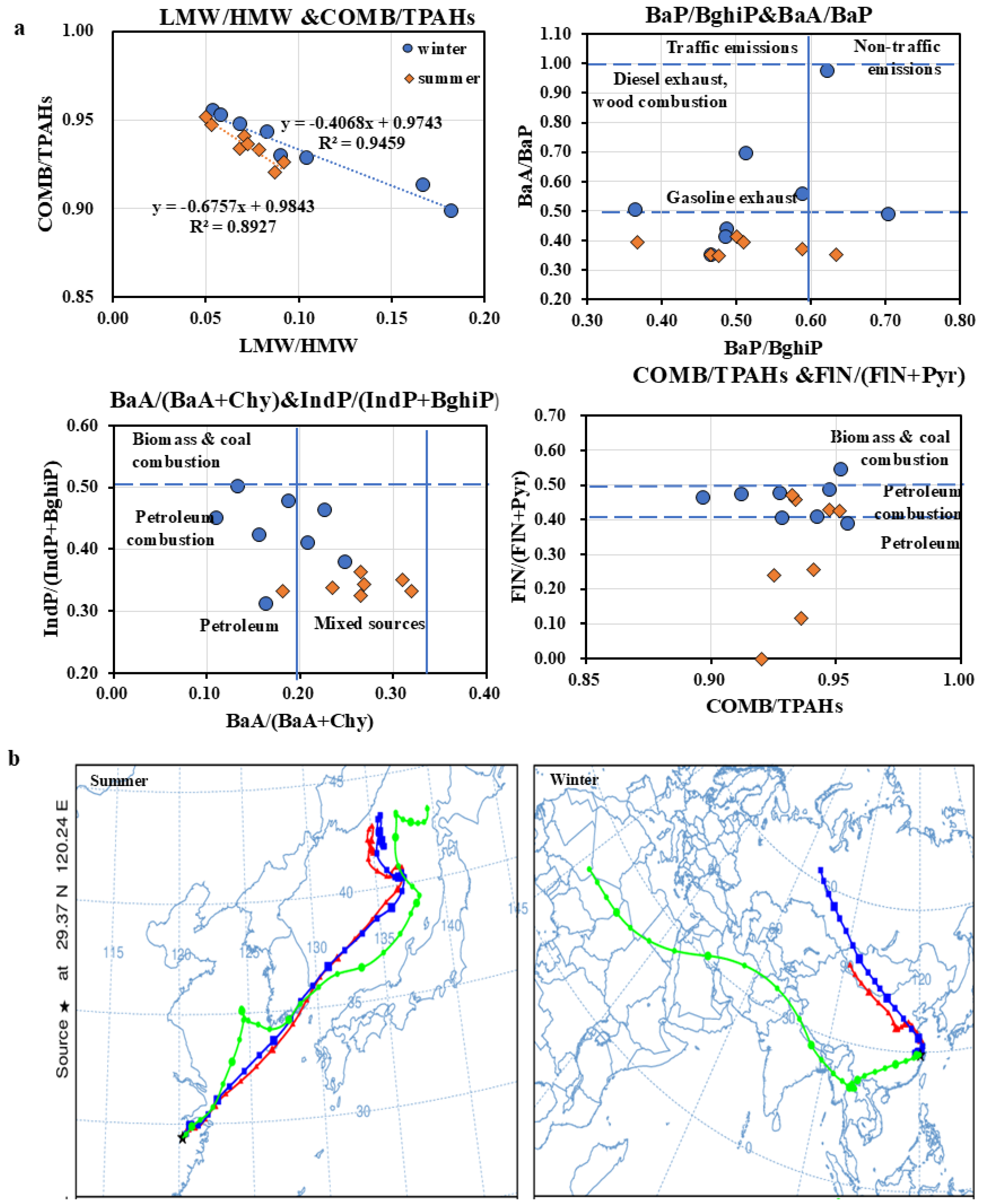
3.3.2. Assessment of Potential Sources of PAHs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Acenaphthene |
| ACY | Acenaphthylene |
| AT | Averaging Time |
| Ant | Anthracene |
| As | Arsenic |
| BW | Body Weight |
| BaA | Benzo[a]anthracene |
| BaP | Benzo[a]pyrene |
| BaPeq | Benzo[a]pyrene equivalent concentration |
| BbF | Benzo[b]fluoranthene |
| BghiP | Benzo[ghi]perylene |
| BkF | Benzo[k]fluoranthene |
| CA | Concentration of Ambient pollutant |
| Cd | Cadmium |
| Chy | Chrysene |
| Cr | Chromium |
| Cu | Copper |
| DBahA | Dibenz[a,h]anthracene |
| ED | Exposure Duration |
| EF | Enrichment Factor |
| EF (exposure) | Exposure Frequency |
| ET | Exposure Time |
| FLN | Fluoranthene |
| Fe | Iron |
| Flu | Fluorene |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HI | Hazard Index |
| HQ | Hazard Quotient |
| HYSPLIT | Hybrid Single-Particle Lagrangian Integrated Trajectory |
| ICP-MS | Inductively Coupled Plasma–Mass Spectrometry |
| ICRF | Inhalation Cancer Risk Factor |
| ILCR | Incremental Lifetime Cancer Risk |
| IndP | Indeno [1,2,3-cd]pyrene |
| InhR | Inhalation Rate |
| Mn | Manganese |
| NAP | Naphthalene |
| Ni | Nickel |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PCA | Principal Component Analysis |
| PM | Particulate Matter |
| PM1.1 | Particulate Matter with aerodynamic diameter less than 1.1 μm |
| PM2.0 | Particulate Matter with aerodynamic diameter less than 2.0 μm |
| Pb | Lead |
| Phe | Phenanthrene |
| Pyr | Pyrene |
| RfC | Reference Concentration |
| TEF | Toxic Equivalency Factor |
| TSP | Total Suspended Particles |
| USEPA | United States Environmental Protection Agency |
| Zn | Zinc |
| ACE | Acenaphthene |
| ACY | Acenaphthylene |
| AT | Averaging Time |
| Ant | Anthracene |
| As | Arsenic |
| BW | Body Weight |
| BaA | Benzo[a]anthracene |
| BaP | Benzo[a]pyrene |
| BaPeq | Benzo[a]pyrene equivalent concentration |
| BbF | Benzo[b]fluoranthene |
| BghiP | Benzo[ghi]perylene |
| BkF | Benzo[k]fluoranthene |
| CA | Concentration of Ambient pollutant |
| Cd | Cadmium |
| Chy | Chrysene |
| Cr | Chromium |
| Cu | Copper |
| DBahA | Dibenz[a,h]anthracene |
| ED | Exposure Duration |
| EF | Enrichment Factor |
| EF (exposure) | Exposure Frequency |
| ET | Exposure Time |
| FLN | Fluoranthene |
| Fe | Iron |
| Flu | Fluorene |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HI | Hazard Index |
| HQ | Hazard Quotient |
| HYSPLIT | Hybrid Single-Particle Lagrangian Integrated Trajectory |
| ICP-MS | Inductively Coupled Plasma–Mass Spectrometry |
| ICRF | Inhalation Cancer Risk Factor |
| ILCR | Incremental Lifetime Cancer Risk |
| IndP | Indeno [1,2,3-cd]pyrene |
| InhR | Inhalation Rate |
| Mn | Manganese |
| NAP | Naphthalene |
| Ni | Nickel |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PCA | Principal Component Analysis |
| PM | Particulate Matter |
| PM1.1 | Particulate Matter with aerodynamic diameter less than 1.1 μm |
| PM2.0 | Particulate Matter with aerodynamic diameter less than 2.0 μm |
| Pb | Lead |
| Phe | Phenanthrene |
| Pyr | Pyrene |
| RfC | Reference Concentration |
| TEF | Toxic Equivalency Factor |
| TSP | Total Suspended Particles |
| USEPA | United States Environmental Protection Agency |
| Zn | Zinc |
References
- Government, Z. Zhuji Government Portal. Available online: https://www.zhuji.gov.cn/art/2022/3/31/art_1370457_10845821.html (accessed on 20 March 2022).
- Wikipedia. Zhuji. Available online: https://en.wikipedia.org/wiki/Zhuji (accessed on 20 March 2022).
- Wesu Ndilila, A.C.C.; McGregor, L.A.; Kalin, R.M.; Hinwood, A.L. Environmental and toenail metals concentrations in copper mining and nonmining communities in Zambia. Int. J. Hyg. Environ. Health 2014, 217, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Heavy metal Pollution in China: Origin, Pattern and Control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W. Size characteristics and health risks of inorganic species in PM(1.1) and PM(2.0) of Shanghai, China, in spring, 2017. Environ. Sci. Pollut. Res. Int. 2020, 27, 14690–14701. [Google Scholar] [CrossRef]
- Sun, H.; Li, J.; Mao, X. Heavy Metals’ Spatial Distribution Characteristics in a Copper Mining Area of Zhejiang Province. J. Geogr. Inf. Syst. 2012, 4, 46–54. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, Z.; Wang, T.; Lian, H.; Sun, Y.; Wu, J. Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China. Atmos. Environ. 2012, 57, 146–152. [Google Scholar] [CrossRef]
- Soleimani, M.; Amini, N.; Sadeghian, B.; Wang, D.; Fang, L. Heavy metals and their source identification in particulate matter (PM(2.5)) in Isfahan City, Iran. J. Environ. Sci. 2018, 72, 166–175. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Li, L.; Li, J.; Wei, L.; Chi, W.; Hong, L.; Zhao, Q.; Jiang, J. Seasonal concentration distribution of PM1.0 and PM2.5 and a risk assessment of bound trace metals in Harbin, China: Effect of the species distribution of heavy metals and heat supply. Sci. Rep. 2020, 10, 8160. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Zhu, X.; Wang, A.; Yang, M.; Gu, S.; Wang, X.; Leng, P.; Zierold, K.M.; Li, X.; et al. Seasonal Variations, Source Apportionment, and Health Risk Assessment of Heavy Metals in PM2.5 in Ningbo, China. Aerosol Air Qual. Res. 2019, 19, 2083–2092. [Google Scholar] [CrossRef]
- Talbi, A.; Kerchich, Y.; Kerbachi, R.; Boughedaoui, M. Assessment of annual air pollution levels with PM1, PM2.5, PM10 and associated heavy metals in Algiers, Algeria. Environ. Pollut. 2018, 232, 252–263. [Google Scholar] [CrossRef]
- Zhao, X.L.; Jiang, G.G.; Song, Z.L.; Touseef, B.; Zhao, X.Y.; Huang, Y.Y.; Guo, M.; Bharti, B. Concentrations of heavy metals in PM2.5 and health risk assessment around Chinese New Year in Dalian, China. Open Geosci. 2021, 13, 1366–1374. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, Y.; Wang, Q.; Kang, P.; Zhang, R.; Tang, X. Chemical Composition Characteristics of PM2.5 in Three Cities in Henan, Central China. Aerosol Air Qual. Res. 2017, 17, 2367–2380. [Google Scholar] [CrossRef]
- Pradosh Roy, A.S. Metabolism and toxicity of arsenic: A carcinogen. Curr. Sci. 2002, 82, 38–45. [Google Scholar]
- Badal Kumar Mandal, K.T.S. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Nakajima, D.; Lu, S.; Xiao, K.; Chowdhury, T.; Suzuki, M.; Liu, F. Industrial Source Contributions and Health Risk Assessment of Fine Particle-Bound Polycyclic Aromatic Hydrocarbons (PAHs) during Spring and Late Summer in the Baoshan Area, Shanghai. Processes 2021, 9, 2016. [Google Scholar] [CrossRef]
- Aslam, R.; Sharif, F.; Baqar, M.; Shahzad, L. Source identification and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in air and dust samples of Lahore City. Sci. Rep. 2022, 12, 2459. [Google Scholar] [CrossRef]
- Bulejko, P.; Adamec, V.; Schullerova, B.; Skeril, R. Levels, sources, and health risk assessment of polycyclic aromatic hydrocarbons in Brno, Czech Republic: A 5-year study. Environ. Sci. Pollut. Res. Int. 2016, 23, 20462–20473. [Google Scholar] [CrossRef]
- Agudelo-Castaneda, D.M.; Teixeira, E.C.; Schneider, I.L.; Lara, S.R.; Silva, L.F.O. Exposure to polycyclic aromatic hydrocarbons in atmospheric PM(1.0) of urban environments: Carcinogenic and mutagenic respiratory health risk by age groups. Environ. Pollut. 2017, 224, 158–170. [Google Scholar] [CrossRef]
- ChooChuay, C.; Pongpiachan, S.; Tipmanee, D.; Deelaman, W.; Iadtem, N.; Suttinun, O.; Wang, Q.; Xing, L.; Li, G.; Han, Y.; et al. Effects of Agricultural Waste Burning on PM2.5-Bound Polycyclic Aromatic Hydrocarbons, Carbonaceous Compositions, and Water-Soluble Ionic Species in the Ambient Air of Chiang-Mai, Thailand. Polycycl. Aromat. Compd. 2020, 42, 749–770. [Google Scholar] [CrossRef]
- Jiang, N.; Duan, S.; Yu, X.; Zhang, R.; Wang, K. Comparative major components and health risks of toxic elements and polycyclic aromatic hydrocarbons of PM2.5 in winter and summer in Zhengzhou: Based on three-year data. Atmos. Res. 2018, 213, 173–184. [Google Scholar] [CrossRef]
- USEPA. Exposure Factors Handbook; USEPA: Washington, DC, USA, 2011.
- Nisbet, I.C.T.; LAGoy, P.K. Toxic Equivalency Factors(TEFs) for Polycyclic Aromatic Hydrocarbons(PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Tianqihoubao. Tianqihoubao Zhuji. Available online: http://www.tianqihoubao.com/lishi/zhuji.html (accessed on 1 March 2017).
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.C.; Wang, G.; Cao, J.; Lee, C.S.L.; Zhu, L.; Chen, Z.; Zhao, Y.; et al. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. 2013, 13, 7053–7074. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Vangrieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Fan, C.C.; Scott, A.J. Industrial Agglomeration and Development: A Survey of Spatial Economic Issues in East Asia and a Statistical Analysis of Chinese Regions. Econ. Geogr. 2009, 79, 295–319. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Dong, S.; Yonemachi, S.; Lu, S.; Wang, Q. Characterization, Pollution Sources, and Health Risk of Ionic and Elemental Constituents in PM2.5 of Wuhan, Central China. Atmosphere 2020, 11, 760. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Terzi, E.; Cai, Q.Y. On the use of PAH molecular diagnostic ratios in sewage sludge for the understanding of the PAH sources. Is this use appropriate? Chemosphere 2007, 69, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Cheng Kang Li, R.M.K. The use of polycyclic aromatic hydrocarbons as source signatures in receptor modeling. Atmos. Environ. 1993, 27, 523–532. [Google Scholar]
- Kappos, A.D.; Bruckmann, P.; Eikmann, T.; Englert, N.; Heinrich, U.; Höppe, P.; Koch, E.; Krause, G.H.M.; Kreyling, W.G.; Rauchfuss, K.; et al. Health effects of particles in ambient air. Int. J. Hyg. Environ. Health 2004, 207, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Hien, P.D.; Bac, V.T.; Thinh, N.T.H.; Anh, H.L.; Thang, D.D.; Nghia, N.T. A Comparison Study of Chemical Compositions and Sources of PM1.0 and PM2.5 in Hanoi. Aerosol Air Qual. Res. 2021, 21, 210056. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of People’s Republic of China. Ambient Air Quality Standards. 2016. Available online: https://english.mee.gov.cn/Resources/standards/Air_Environment/quality_standard1/201605/t20160511_337502.shtml (accessed on 8 May 2023).
- Deshmukh, D.K.; Deb, M.K.; Ying, I.T.; Mkoma, S.L. Water Soluble Ions in PM2.5 and PM1 Aerosols in Durg City, Chhattisgarh, India. Aerosol Air Qual. Res. 2011, 11, 696–708. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Li, H.; Wu, N.; Zhang, Q. Chemical characteristics and source apportionment of ambient PM1.0 and PM2.5 in a polluted city in North China plain. Atmos. Environ. 2020, 242, 117867. [Google Scholar] [CrossRef]
- Zhang, N.; Cao, J.; Xu, H.; Zhu, C. Elemental compositions of PM2.5 and TSP in Lijiang, southeastern edge of Tibetan Plateau during pre-monsoon period. Particuology 2013, 11, 63–69. [Google Scholar] [CrossRef]
- Morakinyo, O.M.; Mukhola, M.S.; Mokgobu, M.I. Health Risk Analysis of Elemental Components of an Industrially Emitted Respirable Particulate Matter in an Urban Area. Int. J. Environ. Res. Public Health 2021, 18, 3653. [Google Scholar] [CrossRef]
- Yu, Q.; Ding, X.; He, Q.; Yang, W.; Zhu, M.; Li, S.; Zhang, R.; Shen, R.; Zhang, Y.; Bi, X.; et al. Nationwide increase of polycyclic aromatic hydrocarbons in ultrafine particles during winter over China revealed by size-segregated measurements. Atmos. Chem. Phys. 2020, 20, 14581–14595. [Google Scholar] [CrossRef]
- Lao, J.Y.; Wang, S.Q.; Chen, Y.Q.; Bao, L.J.; Lam, P.K.S.; Zeng, E.Y. Dermal exposure to particle-bound polycyclic aromatic hydrocarbons from barbecue fume as impacted by physicochemical conditions. Environ. Pollut. 2020, 260, 114080. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.; Syed, J.H.; Junaid, M.; Mahmood, A.; Li, J.; Zhang, G.; Malik, R.N. Elucidating the urban levels, sources and health risks of polycyclic aromatic hydrocarbons (PAHs) in Pakistan: Implications for changing energy demand. Sci. Total Environ. 2018, 619–620, 165–175. [Google Scholar] [CrossRef]
- Gao, P.; Lei, T.; Jia, L.; Song, Y.; Lin, N.; Du, Y.; Feng, Y.; Zhang, Z.; Cui, F. Exposure and health risk assessment of PM(2.5)-bound trace metals during winter in university campus in Northeast China. Sci. Total Environ. 2017, 576, 628–636. [Google Scholar] [CrossRef]
- Luo, L.; Bai, X.; Liu, S.; Wu, B.; Liu, W.; Lv, Y.; Guo, Z.; Lin, S.; Zhao, S.; Hao, Y.; et al. Fine particulate matter (PM(2.5)/PM(1.0)) in Beijing, China: Variations and chemical compositions as well as sources. J. Environ. Sci. China 2022, 121, 187–198. [Google Scholar] [CrossRef]
| Parameter | Definition | Values for Age Categories (Years) | References | |||||
|---|---|---|---|---|---|---|---|---|
| Infant (0–1) | Young Children | Children (6–16) | Adult1 (16–31) | Adult2 (31–51) | Adult3 (51–81) | |||
| (1–6) | ||||||||
| C(e) | Mean concentration in airborne particle (mg/m3) | C (element) | [5,25,26] | |||||
| CA(PAHs) | Converted BaP toxic equivalent (TEQ BaP) by the toxic equivalency factors (mg/m3) | CA | ||||||
| InhR | Inhalation rate (m3/day) | 5.4 | 9 | 13.6 | 16 | 16 | 15 | |
| ED | Exposure duration (year) | 0.5 | 3.5 | 11 | 23.5 | 41 | 66 | |
| EF | Exposure frequency (days/year) | 350 | 350 | 350 | 350 | 350 | 350 | |
| ET | Exposure time (hours/day) | 1.5 | 1.5 | 3 | 3 | 3 | 3 | |
| BW | Body weight (kg) | 8.8 | 14.5 | 43 | 65 | 71.2 | 70 | |
| AT | Average time (h) | ED*ET*365 | ||||||
| Atn | Average time for carcinogens (h) | 70*24*365 | ||||||
| Cr | Ni | Cu | Zn | As | Cd | Pb | Mn | |
|---|---|---|---|---|---|---|---|---|
| RfC (mg/m3) | 0.0001 | 0.000014 | 0.000402 | 0.301 | 2 × 10−5 | 0 | 0.00325 | 0 |
| IUR (ug/m3.day)−1 | 0.012 | 0.00026 | 0.0043 | 0 | 1.2 × 10−5 |
| Diagnostic Ratios | Value | Potential Source | References |
|---|---|---|---|
| LMW/HMW | <1 | pyrogenic sources | [16,20] |
| Low (high)-molecular-weight (L/HMW) | >1 | petrogenic sources | |
| COMB/TPAHs | ~1 | dominance of combustion-derived PAHs (COMB) | [26] |
| BaP/BghiP | <0.6 | non-traffic emissions | [29] |
| >0.6 | traffic-related sources | ||
| IndP/(IndP + BghiP) | <0.2 | petrogenic sources | [29] |
| 0.2–0.5 | petroleum combustion | ||
| >0.5 | biomass or coal combustion | ||
| BaA/BaP | ~0.5 | gasoline exhaust | [30] |
| ~1.0 | diesel exhaust | ||
| FLN/(FLN + PYR) | <0.4 | petrogenic sources | [18] |
| 0.4–0.5 | fossil fuel combustion | ||
| >0.5 | biomass or coal combustion |
| Sampling City | Sampling Season | PM1.0/PM2.5 | PM1.1/PM2.0 | Sampling Periods | Literature |
|---|---|---|---|---|---|
| Zhuji (China) | Summer | 0.401 (PM2.0/TSP) | 0.664 | August 2016 | This study |
| Winter | 0.458 (PM2.0/TSP) | 0.587 | December 2016 | This study | |
| Shanghai (China) | Spring | 0.21 (PM1.1/TSP) | 21 March–11 April 2017 | [5] | |
| Beijing (China) | Winter | 0.55 | 26 December 2018–11 January 2019 | [28] | |
| Durg (India) | Annual | 0.48 | July 2009–June 2010 | [35] | |
| Algiers (Algeria) | Annual | 0.566 | 1 January–30 September 2015; 4 March–30 November 2016 | [11] | |
| Harbin (China) | Heat supply periods | 0.832 | November 2014–February 2015 | [9] | |
| No heat supply | 0.769 | February 2015–October 2015 | [9] | ||
| Hanoi (Vietnam) | 0.676 | November 2015–June 2016 | [32] | ||
| Handan (China) | Winter | 0.75 | November 2015 | [36] | |
| Beijing (China) | Annual | 0.533 | 2013–2017 | [19] | |
| Incheon (Korea) | Summer | 0.807 | August, 2022 | [35] | |
| Winter | 0.677 | 21 November–11 December 2022 |
| Summer PM1.1 | Winter PM1.1 | ||||||
|---|---|---|---|---|---|---|---|
| Element | EFs | F1 | F2 | F3 | EFs | F1 | F2 |
| Cr | 6.61 | 0.03 | 0.65 | −0.03 | - | - | - |
| Mn | 5.69 | 0.10 | −0.29 | 0.76 | 19.77 | 0.27 | 0.82 |
| Ni | 17.12 | 0.00 | 0.64 | 0.10 | - | - | - |
| Cu | 66.28 | 0.33 | −0.22 | −0.48 | 211.5 | 0.35 | −0.32 |
| Zn | 364.6 | 0.44 | 0.09 | 0.08 | 892.5 | 0.40 | −0.13 |
| As | 92.58 | 0.42 | 0.02 | 0.24 | 316.3 | 0.40 | 0.24 |
| Cd | 2238.5 | 0.36 | −0.14 | −0.31 | 6727.0 | 0.38 | −0.36 |
| Pb | 273.0 | 0.43 | 0.07 | 0.02 | 1267.4 | 0.41 | −0.11 |
| % of variance | --- | 54.4 | 25.0 | 13.8 | --- | 82.0 | 11.7 |
| % of cumulative | --- | 54.4 | 79.4 | 93.3 | --- | 82.0 | 93.7 |
| Source | steel industry activities; vehicle emissions | stainless steel manufacture | coal combustion; road particles | steel industry activities; vehicle emissions | coal combustion | ||
| Cr | Mn | Co | Ni | Cu | Zn | As | Cd | Pb | Fe | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Summer PM1.1 | Cr | 1.000 | −0.421 | −0.076 | 0.884 | −0.233 | 0.173 | 0.108 | −0.137 | 0.190 | 0.124 |
| Mn | −0.421 | 1.000 | −0.013 | −0.292 | −0.135 | 0.216 | 0.379 | 0.002 | 0.156 | 0.122 | |
| Co | −0.076 | −0.013 | 1.000 | 0.141 | 0.417 | 0.274 | 0.018 | 0.009 | 0.275 | 0.141 | |
| Ni | 0.884 | −0.292 | 0.141 | 1.000 | −0.358 | 0.181 | 0.014 | −0.182 | 0.053 | −0.062 | |
| Cu | −0.233 | −0.135 | 0.417 | −0.358 | 1.000 | 0.608 | 0.485 | 0.757 | 0.673 | 0.680 | |
| Zn | 0.173 | 0.216 | 0.274 | 0.181 | 0.608 | 1.000 | 0.925 | 0.729 | 0.921 | 0.880 | |
| As | 0.108 | 0.379 | 0.018 | 0.014 | 0.485 | 0.925 | 1.000 | 0.639 | 0.926 | 0.928 | |
| Cd | −0.137 | 0.002 | 0.009 | −0.182 | 0.757 | 0.729 | 0.639 | 1.000 | 0.623 | 0.624 | |
| Pb | 0.190 | 0.156 | 0.275 | 0.053 | 0.673 | 0.921 | 0.926 | 0.623 | 1.000 | 0.977 | |
| Fe | 0.124 | 0.122 | 0.141 | −0.062 | 0.680 | 0.880 | 0.928 | 0.624 | 0.977 | 1.000 | |
| Winter PM1.1 | Cr | - | - | - | - | - | - | - | - | - | - |
| Mn | - | 1.000 | 0.172 | - | 0.367 | 0.541 | 0.770 | 0.340 | 0.570 | 0.733 | |
| Co | - | 0.172 | 1.000 | - | 0.504 | 0.699 | 0.505 | 0.810 | 0.760 | 0.578 | |
| Ni | - | - | - | - | - | - | - | - | - | - | |
| Cu | - | 0.367 | 0.504 | - | 1.000 | 0.807 | 0.702 | 0.752 | 0.798 | 0.410 | |
| Zn | - | 0.541 | 0.699 | - | 0.807 | 1.000 | 0.870 | 0.897 | 0.966 | 0.621 | |
| As | - | 0.770 | 0.505 | - | 0.702 | 0.870 | 1.000 | 0.816 | 0.906 | 0.755 | |
| Cd | - | 0.340 | 0.810 | - | 0.752 | 0.897 | 0.816 | 1.000 | 0.956 | 0.669 | |
| Pb | - | 0.570 | 0.760 | - | 0.798 | 0.966 | 0.906 | 0.956 | 1.000 | 0.742 | |
| Fe | - | 0.733 | 0.578 | - | 0.410 | 0.621 | 0.755 | 0.669 | 0.742 | 1.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Ruan, J.; Wang, Q. Spatiotemporal Variations and Health Assessment of Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Fine Particles (PM1.1) of a Typical Copper-Processing Area, China. Atmosphere 2025, 16, 674. https://doi.org/10.3390/atmos16060674
Wang W, Ruan J, Wang Q. Spatiotemporal Variations and Health Assessment of Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Fine Particles (PM1.1) of a Typical Copper-Processing Area, China. Atmosphere. 2025; 16(6):674. https://doi.org/10.3390/atmos16060674
Chicago/Turabian StyleWang, Weiqian, Jie Ruan, and Qingyue Wang. 2025. "Spatiotemporal Variations and Health Assessment of Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Fine Particles (PM1.1) of a Typical Copper-Processing Area, China" Atmosphere 16, no. 6: 674. https://doi.org/10.3390/atmos16060674
APA StyleWang, W., Ruan, J., & Wang, Q. (2025). Spatiotemporal Variations and Health Assessment of Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Fine Particles (PM1.1) of a Typical Copper-Processing Area, China. Atmosphere, 16(6), 674. https://doi.org/10.3390/atmos16060674








