An Adiabatic-Expansion-Induced Perturbation Study on Gas–Aerosol Partitioning in Ambient Air—Formation of NH4NO3 and Microdroplet Nitrogen Fixation (2)
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
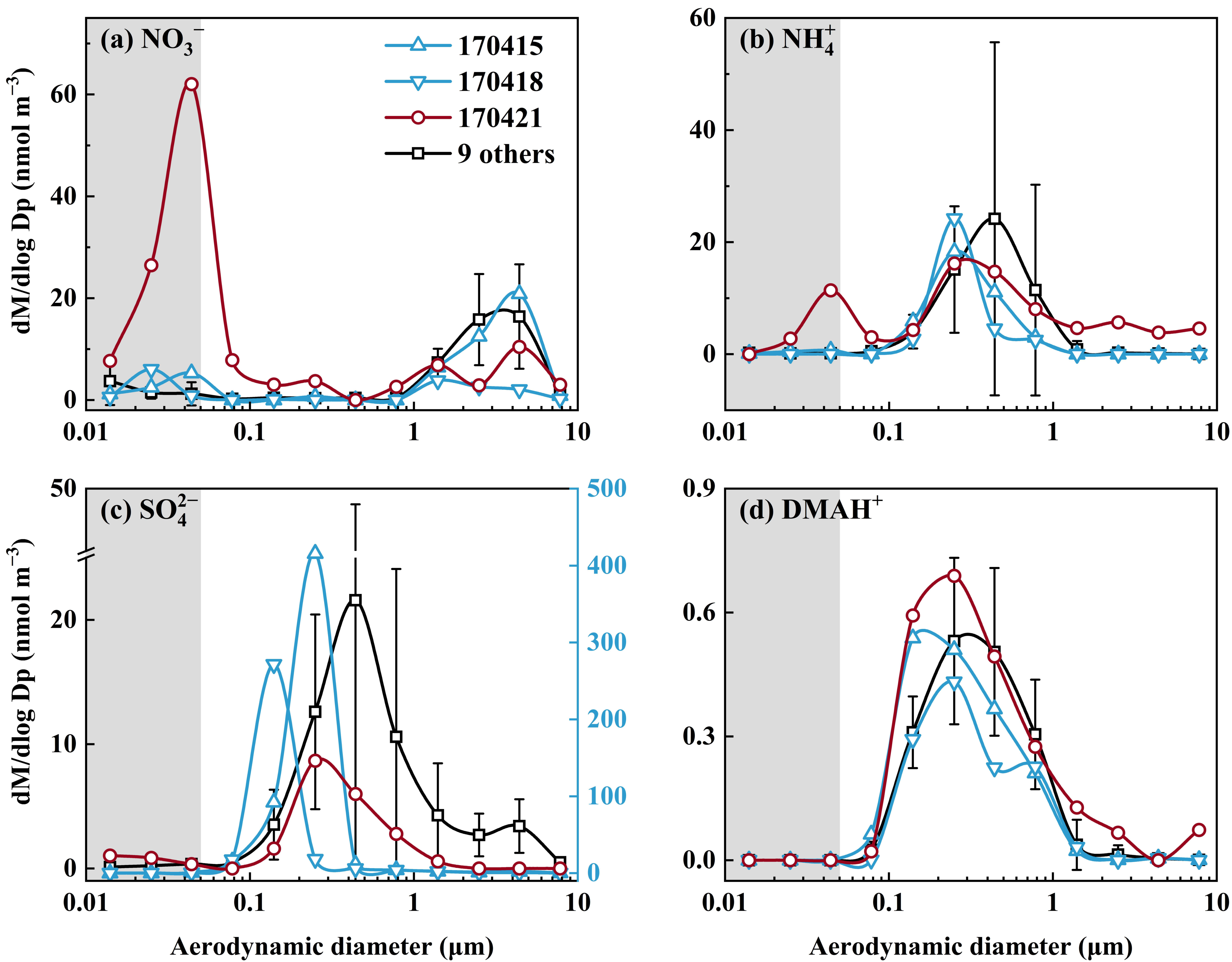
3.1. Perturbation Formation of Comparable NH4NO3 and Organic Nitrate in Campaigns 8 and 9
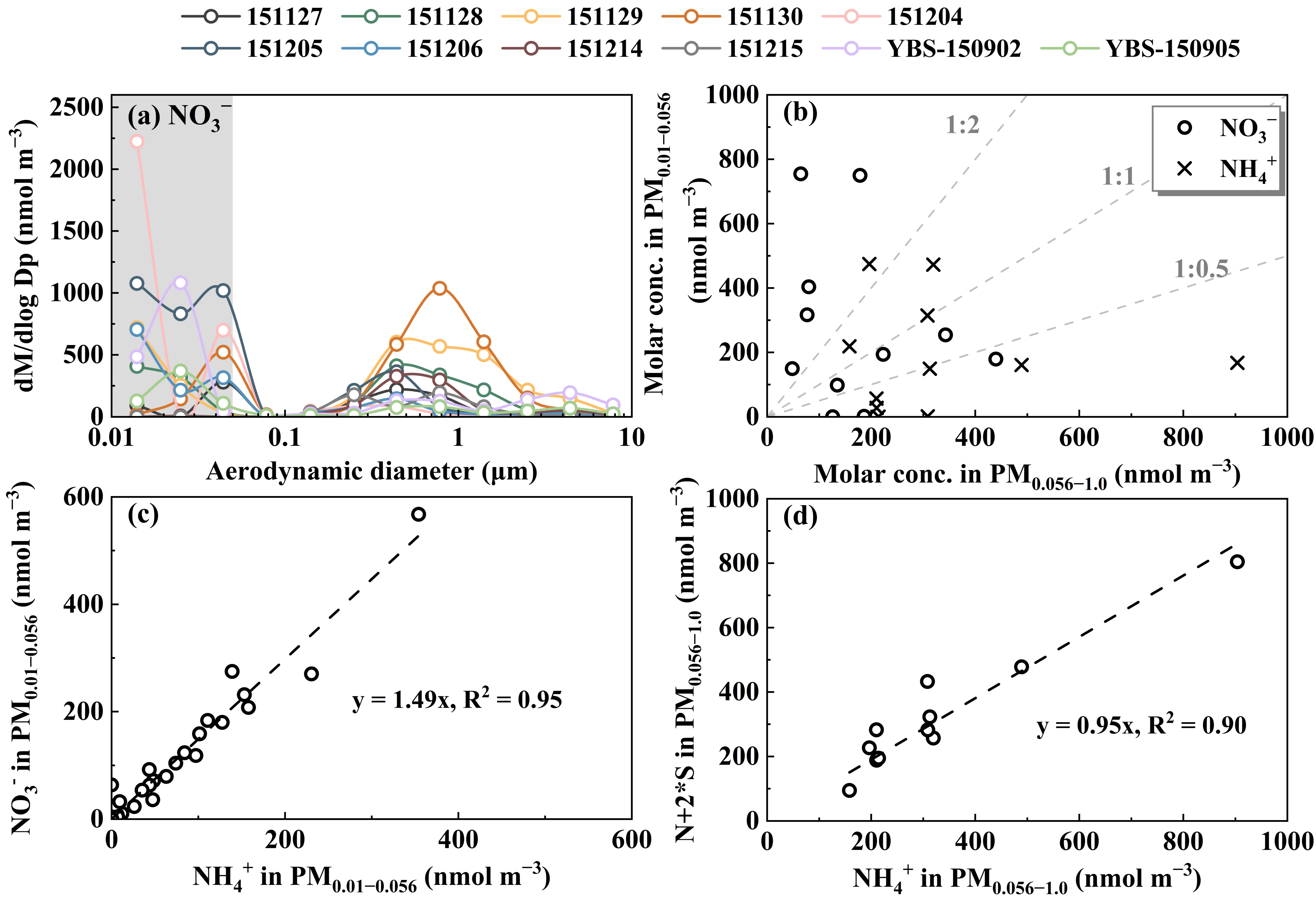
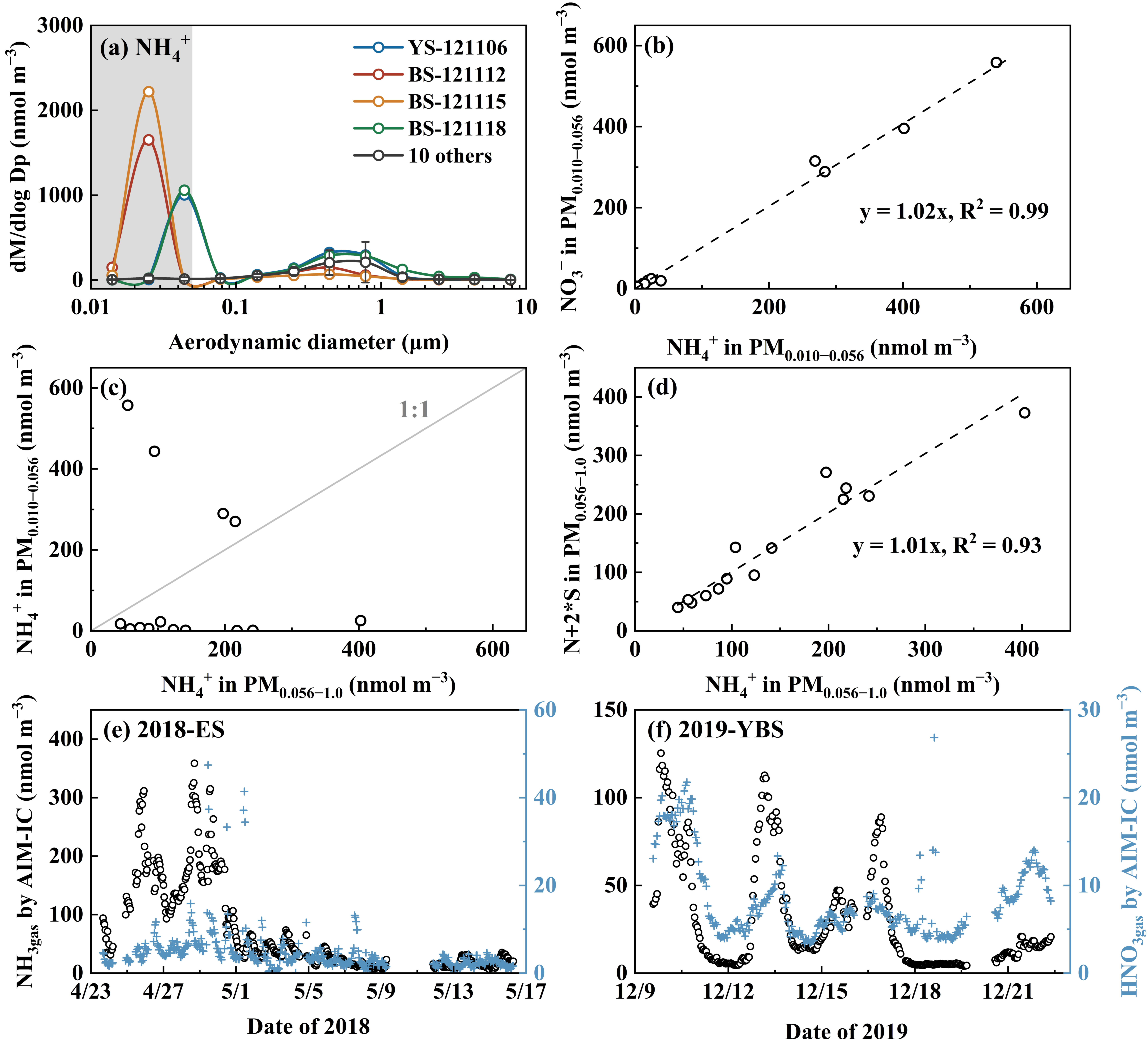
3.2. Is Adiabatic Perturbation Alone Sufficient to Explain NO3− and NH4+ Detected in the Last Three Stages of Nano-MOUDI Sampling in Cold Coastal Atmospheres?
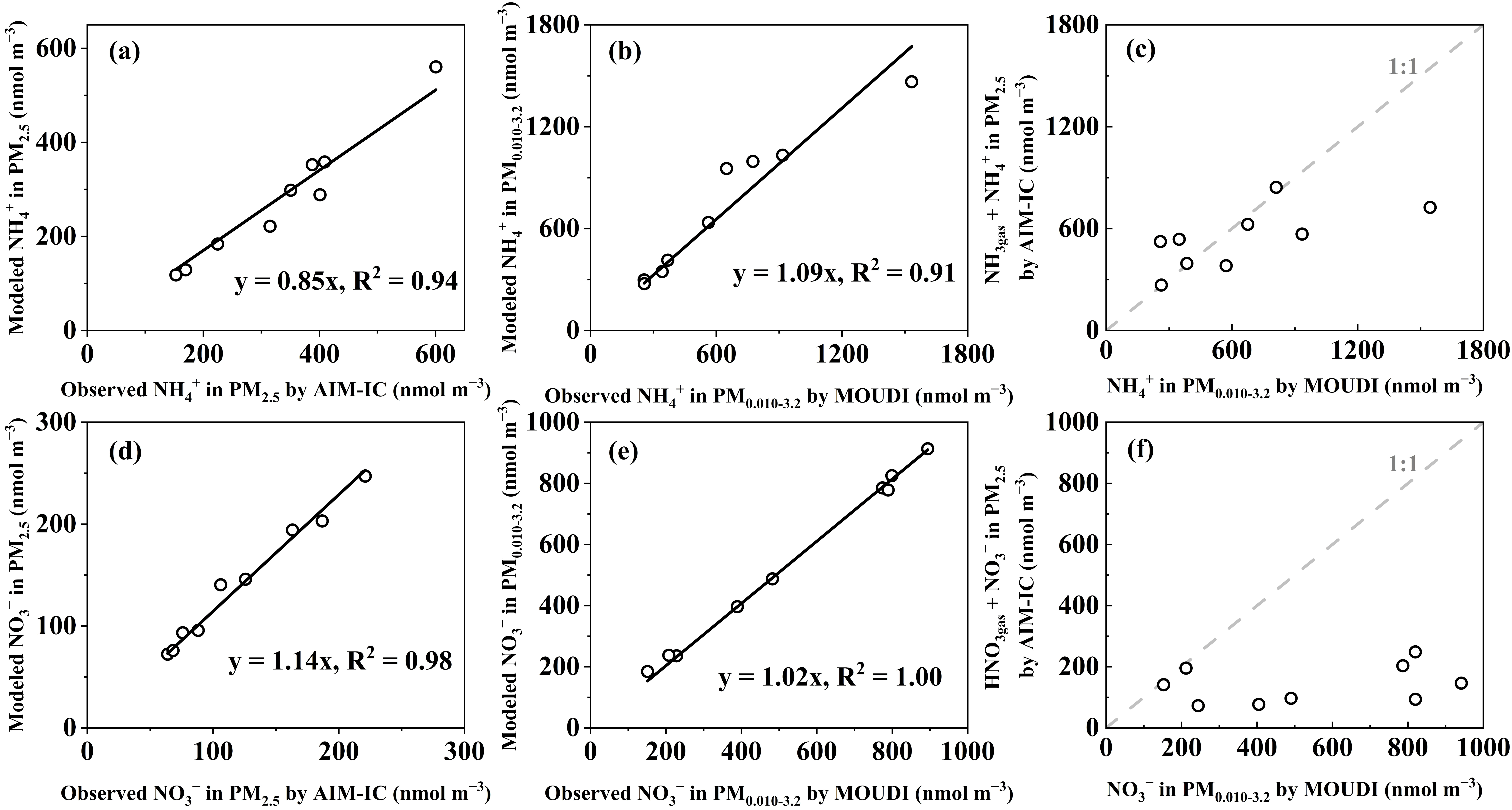
3.3. Adiabatic Perturbation Superimposed Ultrafast Formation of Huge Amounts of NH4NO3 and Organic Nitrate at the Last Three Stages of Nano-MOUDI Sampling in Marine Atmospheres
3.4. Key Factors in Determining Ultrafast Formation of (HNO3 + NH3) and Organic Nitrate: Evidence and Uncertainties
4. Implication
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIM-IC | Ambient Ion Monitor–Ion Chromatograph |
| DMAH+ | particulate dimethylaminium |
| EDS | Energy-Dispersive Spectrometer |
| ES | the East China Sea |
| E-AIM | Extended AIM Aerosol Thermodynamics Model |
| MSA− | particulate methanesulfonic acid |
| Nano MOUDI-II | Nano Micro-Orifice Uniform-Deposit Impactor, second generation |
| N + 2 * S | the sum of the molar concentration of nitrate and twice the molar concentration of sulfate |
| PM2.5 | particulate matter with the aerodynamic diameter below 2.5 μm collected by AIM-IC |
| PM0.010–0.056/PM0.010–3.2/PM0.056–1.0/PM0.056–3.2 | particulate matter with the aerodynamic diameter of 0.010–0.056/0.010–3.2/0.056–1.0/0.056–3.2 μm collected by Nano MOUDI-II |
| SCS | the South China Seat |
| S8/S12 | the 8th or 12th stage of Nano MOUDI-II |
| TEM | Transmission Electron Microscope |
| UTLS | upper troposphere and lower stratosphere |
| YBS | the Yellow Sea and the Bohai Sea |
References
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. R 2013, 20, 8092–8131. [Google Scholar] [CrossRef]
- Peng, J.; Hu, M.; Shang, D.; Wu, Z.; Du, Z.; Tan, T.; Wang, Y.; Zhang, F.; Zhang, R. Explosive secondary aerosol formation during severe haze in the North China Plain. Environ. Sci. Technol. 2021, 55, 2189–2207. [Google Scholar] [CrossRef] [PubMed]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Altieri, K.E.; Fawcett, S.E.; Hastings, M.G. Reactive nitrogen cycling in the atmosphere and ocean. Annu. Rev. Earth Planet. Sci. 2021, 49, 523–550. [Google Scholar] [CrossRef]
- Lan, Z.; Lin, W.; Zhao, G. Sources, Variations, and effects on air quality of atmospheric ammonia. Curr. Pollut. Rep. 2024, 10, 40–53. [Google Scholar] [CrossRef]
- Nair, A.A.; Yu, F. Quantification of atmospheric ammonia concentrations: A review of its measurement and modeling. Atmosphere 2020, 11, 1092. [Google Scholar] [CrossRef]
- Renard, J.J.; Calidonna, S.E.; Henley, M.V. Fate of ammonia in the atmosphere—A review for applicability to hazardous releases. J. Hazard. Mater. 2004, 108, 29–60. [Google Scholar] [CrossRef]
- Parandaman, A.; Tangtartharakul, C.B.; Kumar, M.; Francisco, J.S.; Sinha, A. A computational study investigating the energetics and kinetics of the HNCO + (CH3)2NH reaction catalyzed by a single water molecule. J. Phys. Chem. A 2017, 121, 8465–8473. [Google Scholar] [CrossRef]
- Arathala, P.; Musah, R.A. Catalytic effect of water and formic acid on the reaction of carbonyl sulfide with dimethyl amine under tropospheric conditions. Phys. Chem. Chem. Phys. 2021, 23, 8752–8766. [Google Scholar] [CrossRef]
- Sarkar, S.; Bandyopadhyay, B. Theoretical investigation of the relative impacts of water and ammonia on the tropospheric conversion of N2O5 to HNO3. Phys. Chem. Chem. Phys. 2021, 23, 6651–6664. [Google Scholar] [CrossRef]
- Froyd, K.D.; Murphy, D.M.; Sanford, T.J.; Thomson, D.S.; Wilson, J.C.; Pfister, L.; Lait, L. Aerosol composition of the tropical upper troposphere. Atmos. Chem. Phys. 2009, 9, 4363–4385. [Google Scholar] [CrossRef]
- Ge, C.; Zhu, C.; Francisco, J.S.; Zeng, X.C.; Wang, J. A molecular perspective for global modeling of upper atmospheric NH3 from freezing clouds. Proc. Natl. Acad. Sci. USA 2018, 115, 6147–6152. [Google Scholar] [CrossRef]
- Höpfner, M.; Volkamer, R.; Grabowski, U.; Grutter, M.; Orphal, J.; Stiller, G.; von Clarmann, T.; Wetzel, G. First detection of ammonia (NH3) in the Asian summer monsoon upper troposphere. Atmos. Chem. Phys. 2016, 16, 14357–14369. [Google Scholar] [CrossRef]
- Altieri, K.E.; Spence, K.A.M.; Smith, S. Air-sea ammonia fluxes calculated from high-resolution summertime observations across the Atlantic Southern Ocean. Geophys. Res. Lett. 2021, 48, e2020GL091963. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Lee, D.S.; Asman, W.A.H.; Dentener, F.J.; Van Der Hoek, K.W.; Olivier, J.G.J. A global high-resolution emission inventory for ammonia. Glob. Biogeochem. Cycles 1997, 11, 561–587. [Google Scholar] [CrossRef]
- Chen, D.; Yao, X.; Chan, C.K.; Tian, X.; Chu, Y.; Clegg, S.L.; Shen, Y.; Gao, Y.; Gao, H. Competitive uptake of dimethylamine and trimethylamine against ammonia on acidic particles in marine atmospheres. Environ. Sci. Technol. 2022, 56, 5430–5439. [Google Scholar] [CrossRef] [PubMed]
- Paulot, F.; Jacob, D.J.; Johnson, M.; Bell, T.G.; Baker, A.R.; Keene, W.C.; Lima, I.D.; Doney, S.C.; Stock, C.A. Global oceanic emission of ammonia: Constraints from seawater and atmospheric observations. Glob. Biogeochem. Cycles 2015, 29, 1165–1178. [Google Scholar] [CrossRef]
- Song, X.; Basheer, C.; Zare, R.N. Making ammonia from nitrogen and water microdroplets. Proc. Natl. Acad. Sci. USA 2023, 120, e1993761176. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Li, N.; Tang, B.; Gong, F.; et al. Enhancing electrocatalytic NO reduction to NH3 by the CoS nanosheet with sulfur vacancies. Inorg. Chem. 2022, 61, 8096–8102. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, T.; Luo, Y.; Kong, Q.; Li, T.; Lu, S.; Alshehri, A.A.; Alzahrani, K.A.; Sun, X. Recent advances in strategies for highly selective electrocatalytic N2 reduction toward ambient NH3 synthesis. Curr. Opin. Electrochem. 2021, 29, 100766. [Google Scholar] [CrossRef]
- Gao, Y.; Yao, X. An adiabatic-expansion-induced perturbation study on gas-aerosol partitioning in ambient air—Dimethylamine and trimethylamine (1). 2025. Unpublished work.
- Neuman, J.A.; Gao, R.S.; Fahey, D.W.; Holecek, J.C.; Ridley, B.A.; Walega, J.G.; Grahek, F.E.; Richard, E.C.; McElroy, C.T.; Thompson, T.L.; et al. In situ measurements of HNO3, NOy, NO, and O3 in the lower stratosphere and upper troposphere. Atmos. Environ. 2001, 35, 5789–5797. [Google Scholar] [CrossRef]
- Xu, M.; Kasahara, K.; Sorimachi, A.; Matsuda, K. Nitric acid dry deposition associated with equilibrium shift of ammonium nitrate above a forest by long-term measurement using relaxed eddy accumulation. Atmos. Environ. 2021, 256, 118454. [Google Scholar] [CrossRef]
- Tie, X.; Emmons, L.; Horowitz, L.; Brasseur, G.; Ridley, B.; Atlas, E.; Stround, C.; Hess, P.; Klonecki, A.; Madronich, S.; et al. Effect of sulfate aerosol on tropospheric NOx and ozone budgets: Model simulations and TOPSE evidence. J. Geophys. Res. Atmos. 2003, 108, 8364. [Google Scholar] [CrossRef]
- Takegawa, N.; Kondo, Y.; Koike, M.; Chen, G.; Machida, T.; Watai, T.; Blake, D.R.; Streets, D.G.; Woo, J.H.; Carmichael, G.R.; et al. Removal of NO and NO in Asian outflow plumes: Aircraft measurements over the western Pacific in January 2002. J. Geophys. Res. Atmos. 2004, 109, D23S04. [Google Scholar] [CrossRef]
- Kita, K.; Morino, Y.; Kondo, Y.; Komazaki, Y.; Takegawa, N.; Miyazaki, Y.; Hirokawa, J.; Tanaka, S.; Thompson, T.L.; Gao, R.; et al. A chemical ionization mass spectrometer for ground-based measurements of nitric acid. J. Atmos. Ocean. Tech. 2006, 23, 1104–1113. [Google Scholar] [CrossRef]
- Nojiri, R.; Osada, K.; Kurosaki, Y.; Matsuoka, M.; Sadanaga, Y. Variations in gaseous nitric acid concentrations at Tottori, Japan: Long-range transport from the Asian continent and local production. Atmos. Environ. 2022, 274, 118988. [Google Scholar] [CrossRef]
- Kim, K.; Lee, C.; Choi, D.; Han, S.; Eom, J.; Han, J. A study on the formation reactions and conversion mechanisms of HONO and HNO3 in the atmosphere of Daejeon, Korea. Atmosphere 2024, 15, 267. [Google Scholar] [CrossRef]
- Wespes, C.; Hurtmans, D.; Herbin, H.; Barret, B.; Turquety, S.; Hadji-Lazaro, J.; Clerbaux, C.; Coheur, P. First global distributions of nitric acid in the troposphere and the stratosphere derived from infrared satellite measurements. J. Geophys. Res. Atmos. 2007, 112, D13311. [Google Scholar] [CrossRef]
- Yu, P.; Hu, Q.; Li, K.; Zhu, Y.; Liu, X.; Gao, H.; Yao, X. Characteristics of dimethylaminium and trimethylaminium in atmospheric particles ranging from supermicron to nanometer sizes over eutrophic marginal seas of China and oligotrophic open oceans. Sci. Total Environ. 2016, 572, 813–824. [Google Scholar] [CrossRef]
- Clegg, S.L.; Kleeman, M.J.; Griffin, R.J.; Seinfeld, J.H. Effects of uncertainties in the thermodynamic properties of aerosol components in an air quality model—Part 1: Treatment of inorganic electrolytes and organic compounds in the condensed phase. Atmos. Chem. Phys. 2008, 8, 1057–1085. [Google Scholar] [CrossRef]
- Kittelson, D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998, 29, 575–588. [Google Scholar] [CrossRef]
- Cass, G.R.; Hughes, L.A.; Bhave, P.; Kleeman, M.J.; Allen, J.O.; Salmon, L.G. The chemical composition of atmospheric ultrafine particles. Philos. Trans. R. Soc. A 2000, 358, 2581–2592. [Google Scholar] [CrossRef]
- Gaffney, J.S.; Marley, N.A. The impacts of peroxyacetyl nitrate in the atmosphere of megacities and large urban areas: A historical perspective. Acs Earth Space Chem. 2021, 5, 1829–1841. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Cai, H.; Wang, H.; Zhang, C.; Chen, J.; Dai, Y.; Zhao, W.; Li, J.; Gong, D.; et al. Complexities of peroxyacetyl nitrate photochemistry and its control strategies in contrasting environments in the Pearl River Delta region. Npj Clim. Atmos. Sci. 2024, 7, 116. [Google Scholar] [CrossRef]
- Heindel, J.P.; LaCour, R.A.; Head-Gordon, T. The role of charge in microdroplet redox chemistry. Nat. Commun. 2024, 15, 3670. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, D.; Liang, C.; Zhang, X. Spontaneous reduction of transition metal ions by one electron in water microdroplets and the atmospheric implications. J. Am. Chem. Soc. 2023, 145, 2800–2805. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wingen, L.M.; Finlayson-Pitts, B.J. Toward a molecular understanding of the surface composition of atmospherically relevant organic particles. Proc. Natl. Acad. Sci. USA 2022, 119, e2085833177. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, X.; Wang, B.; Ding, X.; Deng, W.; Lü, S.; Zhang, Y. Emission factor of ammonia (NH3) from on-road vehicles in China: Tunnel tests in urban Guangzhou. Environ. Res. Lett. 2014, 9, 64027. [Google Scholar] [CrossRef]
- Lee, J.K.; Samanta, D.; Nam, H.G.; Zare, R.N. Micrometer-sized water droplets induce spontaneous reduction. J. Am. Chem. Soc. 2019, 141, 10585–10589. [Google Scholar] [CrossRef]
- Chen, D.; Shen, Y.; Wang, J.; Gao, Y.; Gao, H.; Yao, X. Mapping gaseous dimethylamine, trimethylamine, ammonia, and their particulate counterparts in marine atmospheres of China’s marginal seas—Part 1: Differentiating marine emission from continental transport. Atmos. Chem. Phys. 2021, 21, 16413–16425. [Google Scholar] [CrossRef]
- Xiong, H.; Lee, J.K.; Zare, R.N.; Min, W. Strong electric field observed at the interface of aqueous microdroplets. J. Phys. Chem. Lett. 2020, 11, 7423–7428. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Fan, Q.; Zhu, Y.; Shen, H.; Yuan, Q.; Gao, Y.; Gao, H.; Yao, X. An Adiabatic-Expansion-Induced Perturbation Study on Gas–Aerosol Partitioning in Ambient Air—Formation of NH4NO3 and Microdroplet Nitrogen Fixation (2). Atmosphere 2025, 16, 544. https://doi.org/10.3390/atmos16050544
Gao Y, Fan Q, Zhu Y, Shen H, Yuan Q, Gao Y, Gao H, Yao X. An Adiabatic-Expansion-Induced Perturbation Study on Gas–Aerosol Partitioning in Ambient Air—Formation of NH4NO3 and Microdroplet Nitrogen Fixation (2). Atmosphere. 2025; 16(5):544. https://doi.org/10.3390/atmos16050544
Chicago/Turabian StyleGao, Yating, Qinchu Fan, Yujiao Zhu, Hengqing Shen, Qi Yuan, Yang Gao, Huiwang Gao, and Xiaohong Yao. 2025. "An Adiabatic-Expansion-Induced Perturbation Study on Gas–Aerosol Partitioning in Ambient Air—Formation of NH4NO3 and Microdroplet Nitrogen Fixation (2)" Atmosphere 16, no. 5: 544. https://doi.org/10.3390/atmos16050544
APA StyleGao, Y., Fan, Q., Zhu, Y., Shen, H., Yuan, Q., Gao, Y., Gao, H., & Yao, X. (2025). An Adiabatic-Expansion-Induced Perturbation Study on Gas–Aerosol Partitioning in Ambient Air—Formation of NH4NO3 and Microdroplet Nitrogen Fixation (2). Atmosphere, 16(5), 544. https://doi.org/10.3390/atmos16050544








