Agrochemical Nitrogen Cycles, Photosynthesis Performance of Nitrogen Use Efficiency, and Yield of Maize
Abstract
1. Introduction
2. Materials and Methods
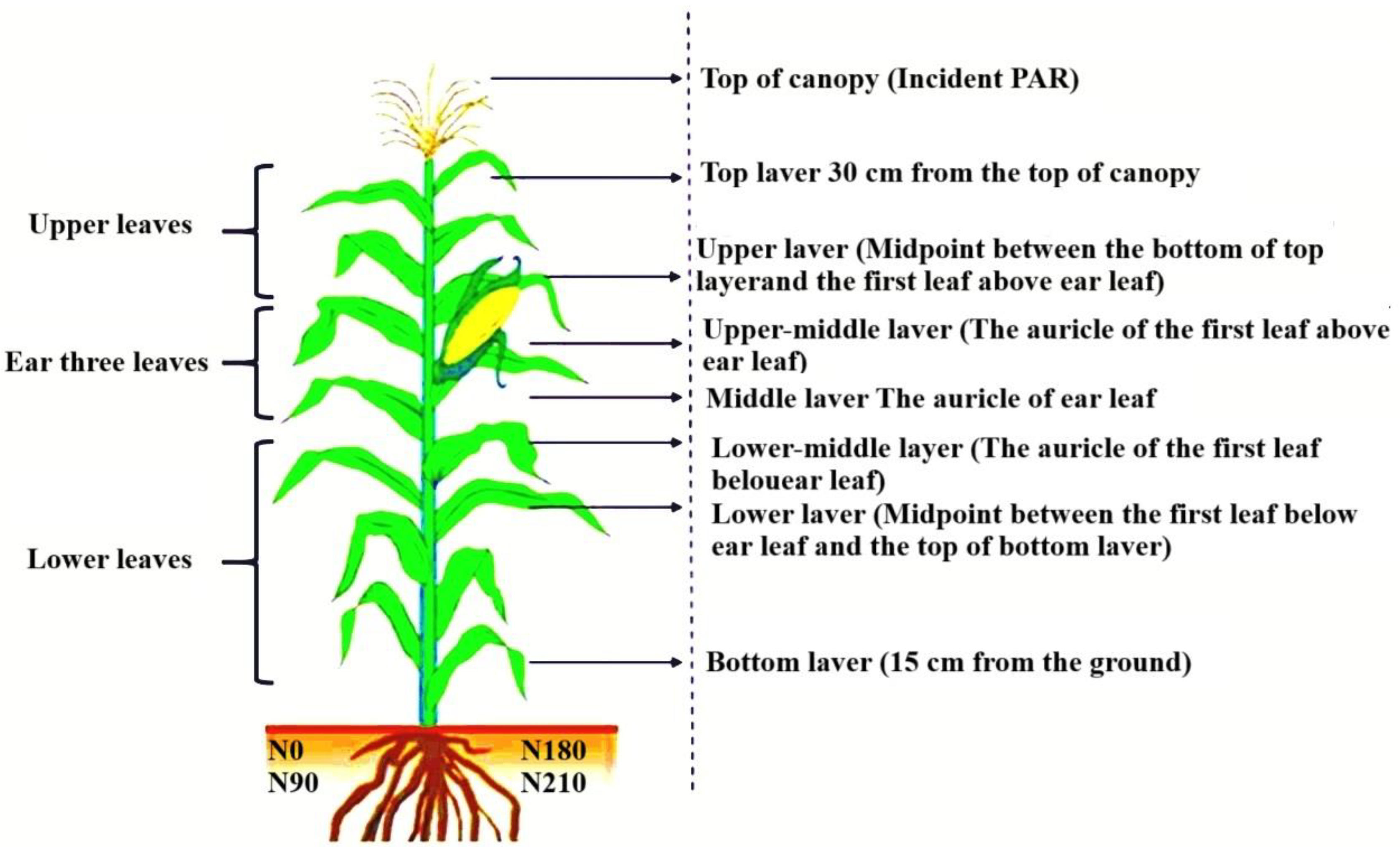
2.1. Description of the Experimental Site
2.2. Experimental Design
2.3. Biomass and Leaf Area
2.4. Photosynthetic Characteristics of Leaves
2.5. Photosynthetically Active Radiation and Light Quality
2.6. Test Yield and Seed
2.7. Parameter Calculation
2.8. Statistical Analysis
3. Results
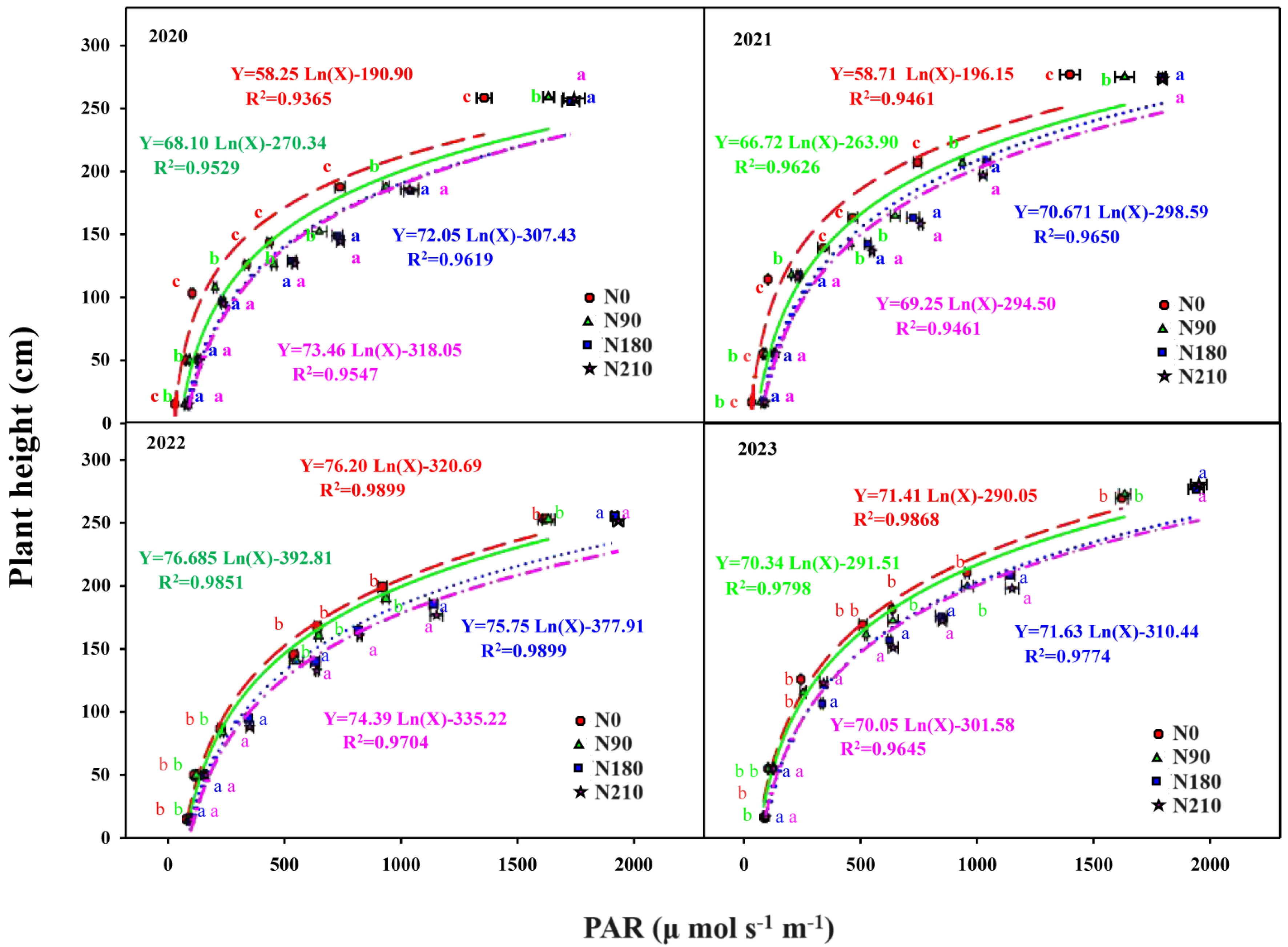
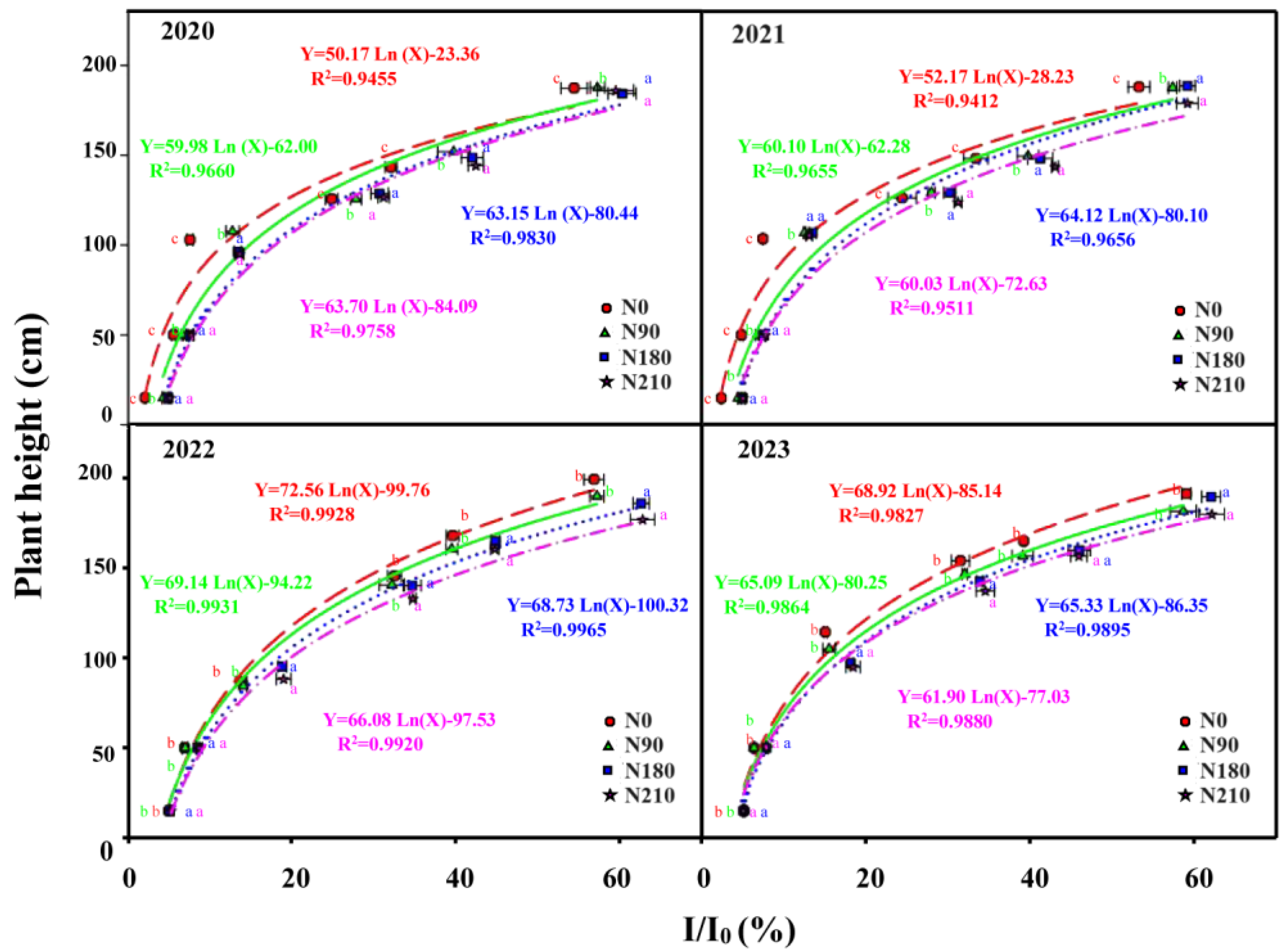
3.1. Effects of N Application on Agronomic Management and Light Distribution
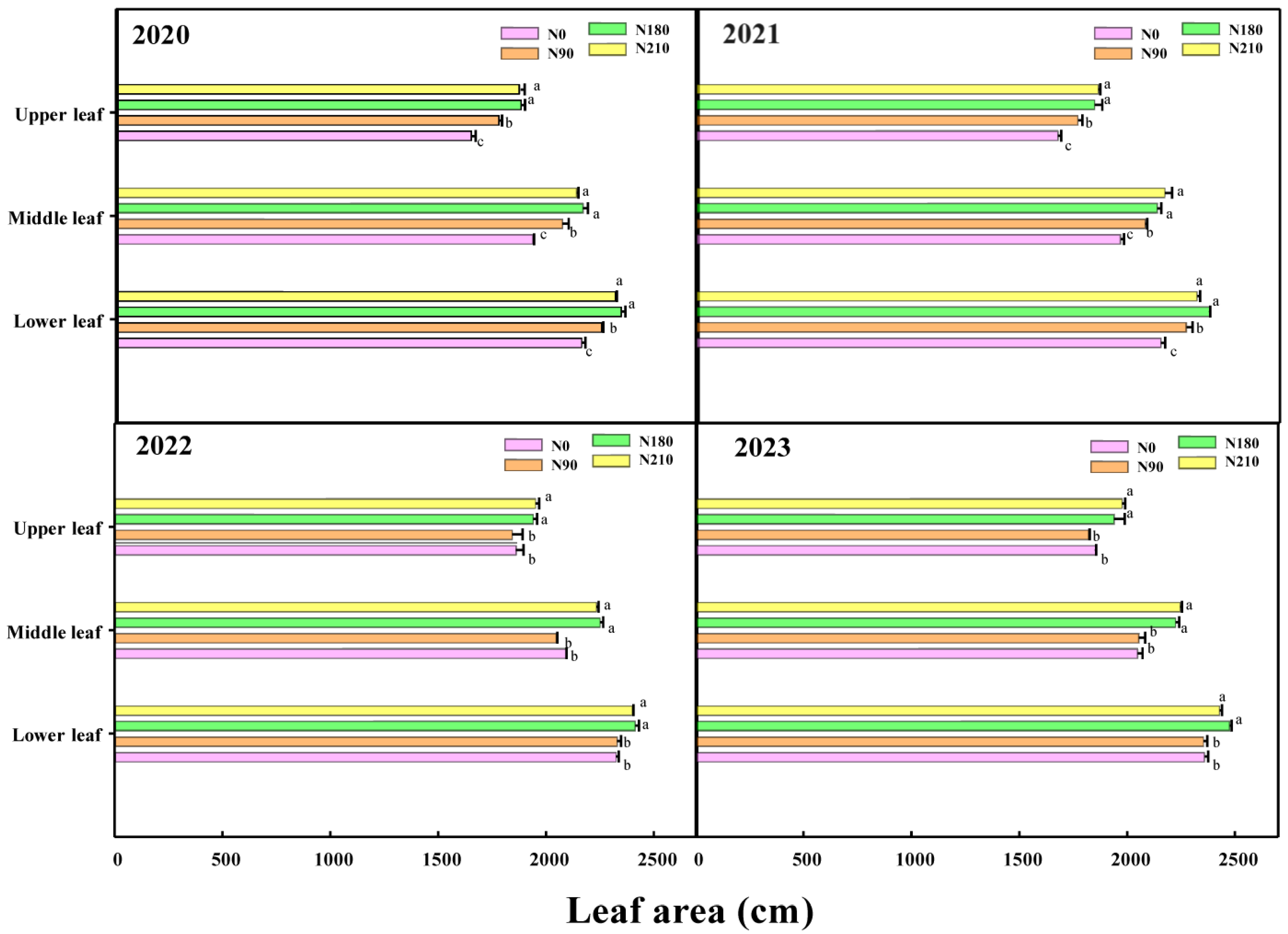
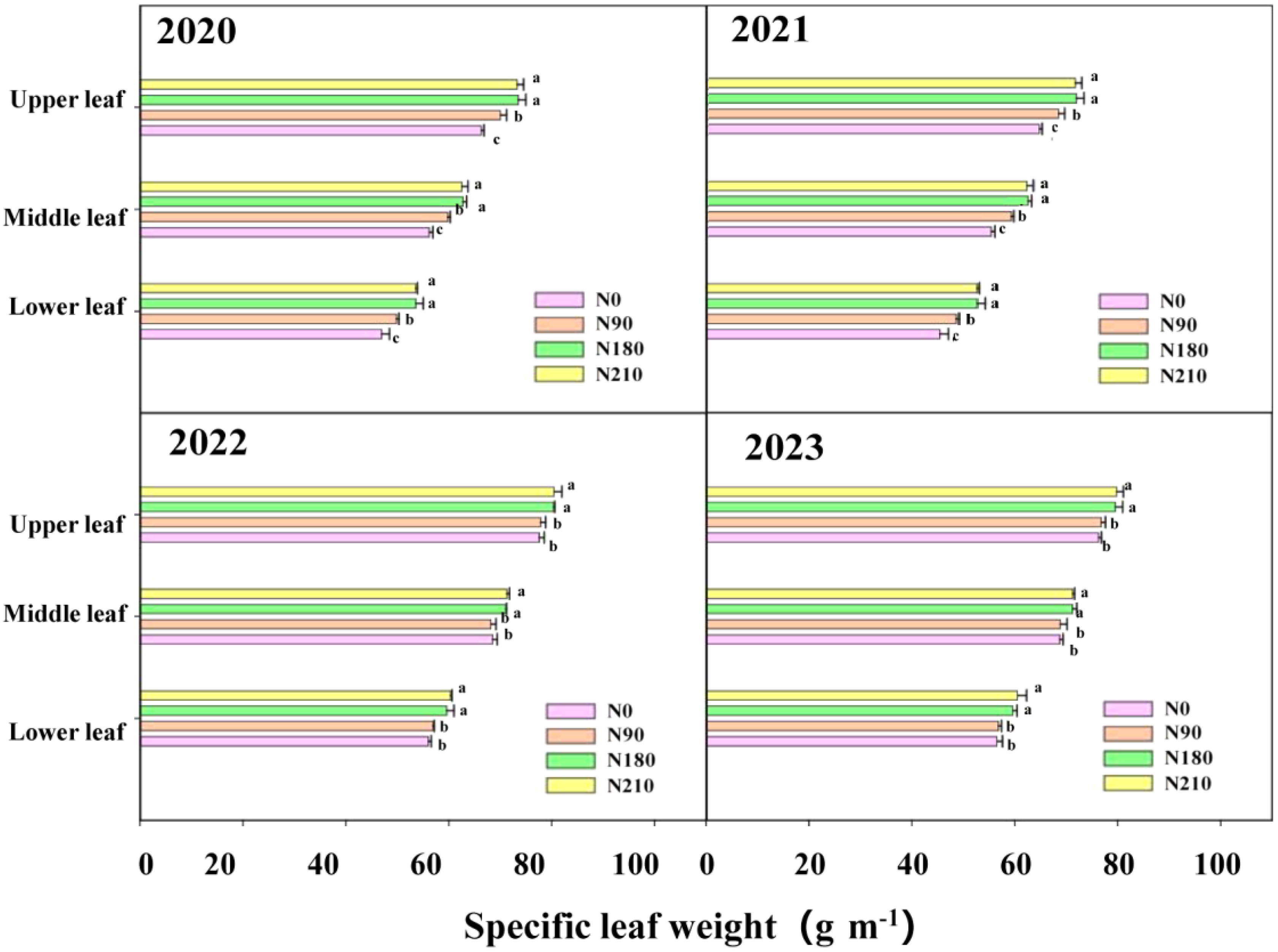
3.2. Effects of Different N Applications on Leaf Area
3.3. Effects of N Application on Photosynthetic Carbon Assimilation
3.4. Effects of Different N Applications on Dry Matter Accumulation
3.5. Effects of N Application on the Grain Yield and Yield Components in the Growth Period of Maize
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noor, H. Morphological and physiological root traits and expression of the gene relationship with nitrogen uptake in Wheat (Triticum aestivum L.). Turk. J. Agric. For. 2024, 48, 1034–1051. [Google Scholar] [CrossRef]
- Bertheiloot, J.; Martre, P.; Andriue, B. Dynamics of light and nitrogen distribution during grain filling within wheat canopy. Plant Physiol. 2008, 148, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chen, Y.; Zhang, H. Canopy light and nitrogen distributions are related to grain yield and nitrogen use efficiency in rice. Field Crops Res. 2017, 206, 74–85. [Google Scholar]
- Li, H.L.; Zhao, C.J.; Huang, W.J. Non-uniform vertical nitrogen distribution within plant canopy and its estimation by remote sensing: A review. Field Crops Res. 2013, 142, 75–84. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.; Lin, X. Radiation Interception and Use Efficiency Contributes to Higher Yields of Newer Maize Hybrids in Northeast China. Agron. J. 2015, 107, 1473–1480. [Google Scholar] [CrossRef]
- Wu, A.; Hammer, G.L.; Doherty, A. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef]
- Dreccer, M.F.; van Oijen, M.; Schapendonk, A.H.; Pot, C.S.; Rabbinge, R. Dynamics of Vertical Leaf Nitrogen Distribution in a Vegetative Wheat Canopy. Impact on Canopy Photosynthesis. Ann. Bot. 2000, 86, 23. [Google Scholar] [CrossRef]
- Drouet, J.-L.; Bonhomme, R. Effect of 3D Nitrogen, Dry Mass per Area and Local Irradiance on Canopy Photosynthesis Within Leaves of Contrasted Heterogeneous Maize Crops. Ann. Bot. 2004, 93, 699–710. [Google Scholar] [CrossRef]
- Tatsuhiko, S.; Sinclair, T.R. Distribution of Nitrogen among Leaves in Soybean Canopies. Crop Sci. 1993, 33, 804–808. [Google Scholar]
- Hirose, T.; Werger, M.J.A.; Pons, T.L.; van Rheenen, J.W.A. Canopy Structure and Leaf Nitrogen Distribution in a Stand of Lysimachia vulgaris L. as Influenced by Stand Density. Oecologia 1988, 77, 145–150. [Google Scholar] [CrossRef]
- Sadras, V.O.; Hall, A.J.; Connor, D.J. Light-associated nitrogen distribution profile in flowering canopies of sunflower. Oecologia 1993, 95, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Q.; Chen, F. Dynamic remobilization of leaf nitrogen components in relation to photosynthetic rate during grain filling in maize. Plant Physiol. Biochem. 2018, 129, 874–882. [Google Scholar] [CrossRef]
- Huber, D.J.; Nevins, D.J. Autolysis of the cell wall β-D-glucan in corn coleoptiles. Plant Cell Physiol. 1979, 20, 201–212. [Google Scholar]
- Peng, L.; Ma, J.; Chi, W. Low PSII accumulation is involved in efficient assembly of photosystem II in arabidopsis thaliana. Plant Cell Online 2006, 18, 955–969. [Google Scholar]
- Zhang, L.; Paakkarinen, V.; Wijk, K.J.V. Co-Translational Assembly of the D1 Protein into Photosystem II. J. Biol. Chem. 1999, 274, 16062–16067. [Google Scholar]
- Duvick, D.N. Genetic progress in yield of United States maize (Zea mays L.). Maydica 2005, 50, 193–202. [Google Scholar]
- Li, C.; Li, Y.; Shi, Y. Genetic Control of the Leaf Angle and Leaf Orientation Value as Revealed by Ultra-High Density Maps in Three Connected Maize Populations. PLoS ONE 2015, 10, e0121624. [Google Scholar]
- Zhu, L.; Li, M.; Yang, W.; Zhang, J.; Yang, X.; Zhang, Q.; Wang, H. Effects of Different Drying Methods on Drying Characteristics and Quality of Glycyrrhiza uralensis (Licorice). Foods 2023, 12, 1652. [Google Scholar] [CrossRef]
- Li, W.; Fang, H.; Wei, S.; Weiss, M.; Baret, F. Critical analysis of methods to estimate the fraction of absorbed or intercepted photosynthetically active radiation from ground measurements: Application to rice crops. Agric. For. Meteorol. 2021, 297, 108273. [Google Scholar]
- Vazin, F.; Madani, A.; Hassanzadeh, M. Modeling Light Interception and Distribution in Mixed Canopy of Redroot Pigweed (Amaranthus retroflexus) in Competition with Corn (Zea mays L.). Planta Daninha 2010, 28, 455–462. [Google Scholar]
- Austin, R.B. Genetic variation in photosynthesis. J. Agric. Sci. 1989, 112, 287–294. [Google Scholar]
- Ghannoum, O.; Evans, J.R.; Chow, W.S.; Andrews, T.J.; Conroy, J.P.; von Caemmerer, S. Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiol. 2005, 137, 638–650. [Google Scholar]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen Utilization1. Agron. J. 1982, 74, 562–564. [Google Scholar]
- Hirel, B.; Le Gouis, J.; Ney, B. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Within-Leaf nitrogen allocation in adaptation to low nitrogen supply in maize during grain-filling stage. Front. Plant Sci. 2016, 7, 699. [Google Scholar]
- Sinclair, T.R.; Horie, T. Leaf Nitrogen, Photosynthesis, and Crop Radiation Use Efficiency: A Review. Crop Sci. 1989, 29, 90–98. [Google Scholar] [CrossRef]
- Vos, J.; Putten, P.E.L.V.D.; Birch, C.J. Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crops Res. 2005, 93, 64–73. [Google Scholar] [CrossRef]
- Boomsma, C.R. Resource availabilty and intra-specific competition in maize: A per-plant and canopy-level morpho- and eco-physiological analysis. Diss. Theses Gradworks 2009, 7, 38–40. [Google Scholar]
- Wu, W.-M.; Chen, H.-J.; Wang, S.-J.; Wei, F.-Z.; Li, J.-C. Effects of Nitrogen Fertilization Application Regime on Dry Matter, Nitrogen Accumulation and Transportation in Summer Maize under Waterlogging at the Seedling Stage. Acta Agron. Sin. 2015, 41, 1246. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Yang, X.; Dong, G.; Palaniappan, K.; Mi, G.; Baskin, T.I. Temperature-compensated cell production rate and elongation zone length in the root of Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 264–276. [Google Scholar] [PubMed]
- Ali, A.A.; Xu, C.; Rogers, A. A global scale mechanistic model of photosynthetic capacity (LUNA V1.0). Geosci. Model Dev. 2016, 9, 587. [Google Scholar]
- Zhu, X.G.; Long, S. Optimizing the Distribution of Resources between Enzymes of Carbon Metabolism Can Dramatically Increase Photosynthetic Rate: A Numerical Simulation Using an Evolutionary Algorithm. Plant Physiol. 2007, 145, 513–526. [Google Scholar] [PubMed]
- Makino, A.; Sakuma, H.; Sudo, E. Differences between maize and rice in N-use efficiency for photosynthesis and protein allocation. Plant Cell Physiol. 2003, 44, 952–956. [Google Scholar]
- Echarte, L.; Rothstein, S.; Tollenaar, M. The Response of Leaf Photosynthesis and Dry Matter Accumulation to Nitrogen Supply in an Older and a Newer Maize Hybrid. Crop Sci. 2008, 48, 656–665. [Google Scholar]
- Paponov, I.A.; Engels, C. Effect of nitrogen supply on leaf traits related to photosynthesis during grain filling in two maize genotypes with different N efficienc. J. Plant Nutr. Soil Sci. 2003, 166, 756–763. [Google Scholar]
- Ding, L.; Wang, K.; Jiang, G. Effects of Nitrogen Deficiency on Photosynthetic Traits of Maize Hybrids Released in Different Years. Ann. Bot. 2005, 96, 925–930. [Google Scholar]
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar]
- Liang, S.; Yoshihira, T.; Sato, C. Grain yield responses to planting density in twin and narrow row cultivation of early cultivars in maize. Grassl. Sci. 2019, 66, 183–193. [Google Scholar]
- Chen, X.P.; Cui, Z.L.; Vitousek, P.M.; Cassman, K.G.; Matson, P.A.; Bai, J.S.; Meng, Q.F.; Hou, P.; Yue, S.C.; Römheld, V.; et al. Integrated soil-crop system management for food security. Proc. Natl. Acad. Sci. USA 2011, 108, 6399–6404. [Google Scholar]
- Grindlay, D.J.C. Towards an explanation of crop nitrogen demand based on the optimization of leaf nitrogen per unit leaf area. J. Agric. Sci. Camb. 1997, 128, 377–396. [Google Scholar] [CrossRef]
- Noor, H. Responses of Photosynthetic Characteristics, and Agronomic Attributes to Sowing Methods of Winter Wheat. Russ. J. Plant Physiol. 2024, 71, 147. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, C.; Chen, X. Characterization of the plant traits contributed to high grain yield and high grain nitrogen concentration in maize. Field Crops Res. 2014, 159, 1–9. [Google Scholar] [CrossRef]
- Baki, A.E.; Siefritz, F.; Man, H.M. Nitrate reductase in (Zea mays L.) under salinity. Plant Cell Environ. 2000, 23, 515–521. [Google Scholar] [CrossRef]
- Peng, S.; Garcia, F.V.; Laza, R.C.; Cassman, K.G. Adjustment for Specific Leaf Weight Improves Chlorophyll Meter’s Estimate of Rice Leaf Nitrogen Concentration. Agron. J. 1993, 85, 85–90. [Google Scholar] [CrossRef]
- Markus, L.; Katharina, S.; Hans, S. Vertical leaf nitrogen distribution in relation to nitrogen status in grassland plants. Ann. Bot. 2003, 92, 679–688. [Google Scholar]
- Noor, H.; Min, S.; Yu, S.; Lin, W. Different sowing methods increase the yield and quality of soil water consumption of dryland Winter wheat on the loess plateau China. Appl. Ecol. Environ. Res. 2020, 18, 8285–8308. [Google Scholar] [CrossRef]
- Noor, H.; Shah, A.A.; Ding, P.; Ren, A.; Sun, M.; Gao, Z. Long-Term Nutrient Cycle in Improved Grain Yield of Dryland Winter Wheat (Triticum aestivum L.) under Hydrological Process of Plant Ecosystem Distribution in the Loess Plateau of China. Plants 2023, 12, 2369. [Google Scholar] [CrossRef]
- Noor, H.; Wang, Q.; Fida, N. Effects of sowing methods and nitrogen rates on photosynthetic characteristics, yield and quality of winter wheat. Photosynthetica 2021, 59, 277–285. [Google Scholar] [CrossRef]
- Anten, N.; Schieving, F.; Werger, M. Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 monoand dicotyledonous species. Oecologia 1995, 101, 504–513. [Google Scholar] [CrossRef]
- Noor, H.; Ding, P.; Li, L.; Ren, A.; Sun, M.; Gao, Z. Effects of Nitrogen on Photosynthetic Productivity and Yield Quality of Wheat (Triticum aestivum L.). Agronomy 2023, 13, 1448. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, D.; Mu, X.; Xiao, C.; Chen, F.; Yuan, L.; Mi, G. Vertical Distribution of Photosynthetic Nitrogen Use Efficiency and Its Response to Nitrogen in Field-Grown Maize. Crop Sci. 2016, 56, 397–399. [Google Scholar] [CrossRef]
- Noor, H.; Sun, M.; Algwaiz, H.I.M.; Sher, A.; Fiaz, S.; Attia, K.A.; Wani, S.H.; AlKahtani, M.D.F.; Al Husnain, L.; Lin, W.; et al. Chlorophyll fluorescence and grain filling characteristic of wheat (Triticum aestivum L.) in response to nitrogen application level. Mol. Biol. Rep. 2022, 49, 7157–7172. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Grain Yield (kg ha−1) | PFPN (kg kg−1) | NUE (kg kg−1) | NIE (kg kg−1) | NRE (kg kg−1) | |
|---|---|---|---|---|---|---|
| 2020 | N0 | 13.01 b | 43.37 b | 6.49 b | 29.98 b | 0.22 a |
| N90 | 13.15 b | 43.83 b | 6.59 b | 31.96 b | 0.22 a | |
| N180 | 13.85 a | 46.32 a | 9.44 a | 38.83 a | 0.24 a | |
| N210 | 13.85 a | 46.16 a | 9.28 a | 38.48 a | 0.24 a | |
| 2021 | N0 | 12.34 c | 41.44 c | 7.87 c | 22.99 c | 0.34 a |
| N90 | 13.05 b | 43.48 b | 9.92 b | 27.96 b | 0.35 a | |
| N180 | 14.17 a | 47.25 a | 13.68 a | 36.51 a | 0.37 a | |
| N210 | 14.26 a | 47.54 a | 13.98 a | 37.61 a | 0.37 a | |
| 2022 | N0 | 14.71 b | 49.04 b | 8.25 b | 31.22 b | 0.27 a |
| N90 | 14.85 b | 49.50 b | 8.81 b | 32.17 b | 0.27 a | |
| N180 | 15.42 a | 51.39 a | 10.70 a | 36.89 a | 0.29 a | |
| N210 | 15.45 a | 51.51 a | 10.82 a | 37.31 a | 0.29 a | |
| 2023 | N0 | 14.02 c | 46.75 c | 8.37 c | 24.15 c | 0.35 a |
| N90 | 14.62 b | 48.75 b | 10.37 b | 29.03 b | 0.36 a | |
| N180 | 15.74 a | 52.48 a | 14.10 a | 37.65 a | 0.37 a | |
| N210 | 15.76 a | 52.53 a | 14.15 a | 37.79 a | 0.37 a | |
| F-value | ||||||
| Y | ns | ** | ns | ns | ns | |
| N | ns | ** | ns | ns | ns | |
| Y × N | ns | ns | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Noor, H.; Lin, C.; Feng, Y.; Luo, Z.; Hou, Y.; Seleiman, M.F.; Noor, F. Agrochemical Nitrogen Cycles, Photosynthesis Performance of Nitrogen Use Efficiency, and Yield of Maize. Atmosphere 2025, 16, 373. https://doi.org/10.3390/atmos16040373
Zheng H, Noor H, Lin C, Feng Y, Luo Z, Hou Y, Seleiman MF, Noor F. Agrochemical Nitrogen Cycles, Photosynthesis Performance of Nitrogen Use Efficiency, and Yield of Maize. Atmosphere. 2025; 16(4):373. https://doi.org/10.3390/atmos16040373
Chicago/Turabian StyleZheng, Haixia, Hafeez Noor, Changchun Lin, Yu Feng, Zhengming Luo, Yanjun Hou, Mahmoud F. Seleiman, and Fida Noor. 2025. "Agrochemical Nitrogen Cycles, Photosynthesis Performance of Nitrogen Use Efficiency, and Yield of Maize" Atmosphere 16, no. 4: 373. https://doi.org/10.3390/atmos16040373
APA StyleZheng, H., Noor, H., Lin, C., Feng, Y., Luo, Z., Hou, Y., Seleiman, M. F., & Noor, F. (2025). Agrochemical Nitrogen Cycles, Photosynthesis Performance of Nitrogen Use Efficiency, and Yield of Maize. Atmosphere, 16(4), 373. https://doi.org/10.3390/atmos16040373








